
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
OPINION article
Front. Endocrinol. , 09 December 2022
Sec. Cardiovascular Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1097968
This article is part of the Research Topic Insights in Cardiovascular Endocrinology: 2023 View all 8 articles
 Imma Forzano1
Imma Forzano1 Pasquale Mone1
Pasquale Mone1 Fahimeh Varzideh1
Fahimeh Varzideh1 Stanislovas S. Jankauskas1
Stanislovas S. Jankauskas1 Urna Kansakar1
Urna Kansakar1 Antonio De Luca2
Antonio De Luca2 Gaetano Santulli1,3*
Gaetano Santulli1,3*Resistant hypertension is defined by blood pressure (BP) targets not achieved despite the use of at least 3 anti-hypertensive drugs of different classes, including a diuretic (1). Diagnosed in more than 10% of hypertensive patients, it represents a high-risk phenotype, leading to an increased risk of cardiovascular disease and all-cause mortality (2). A BP that cannot be controlled with the use of at least 5 antihypertensive agents of different classes, including a long-acting thiazide-like diuretic such as chlorthalidone, and spironolactone is defined refractory hypertension. Substantial evidence indicates that aldosterone excess is very common in patients with resistant hypertension and primary aldosteronism is present in ~20% of patients with confirmed resistant hypertension; intriguingly a positive relationship (more pronounced in men) between weight gain and aldosterone levels has also been demonstrated (3, 4). Despite its side effects (5), the mineralocorticoid receptor antagonist spironolactone remains the preferred 4th line add-on therapy in patients with resistant hypertension. The adverse effects of spironolactone (which include reduced testosterone synthesis, hyperkalemia, gynecomastia, breast tenderness, menstrual irregularities and postmenopausal bleeding) are essentially due to the off-target blockade of several steroid hormone receptors (5). To counteract these obstacles, a different approach has been applied, i.e. directly targeting the synthesis of aldosterone instead of blocking its receptor. However, Osilodrostat, the first inhibitor of the enzyme aldosterone synthase, was associated with off-target inhibition of cortisol synthesis (6), an effect explained by the >90% sequence similarity between 11β-hydroxylase (the final enzyme required for cortisol synthesis, encoded by the gene CYP11B1) and aldosterone synthase (encoded by the gene CYP11B2) (7).
Baxdrostat, a drug originally developed by Roche (RO6836191) (8) and subsequently licensed to CinCor Pharma, Inc (CIN-107) (9), embodies an exquisite example of selective inhibition of aldosterone synthase, without affecting 11β-hydroxylase. Preclinical studies conducted in cynomolgus monkeys demonstrated that this molecule inhibited aldosterone synthesis without affecting the adrenocorticotropic hormone–induced rise in cortisol (8); these findings were also confirmed in healthy subjects (Clinicaltrials.gov Identifier: NCT01995383) (8). Safety, pharmacokinetics, and pharmacodynamics of multiple ascending doses of Baxdrostat were later tested in a Phase I trial, which confirmed that Baxdrostat was safe and well tolerated and induced a dose-dependent reduction in plasma aldosterone but not on cortisol.
Baxdrostat has been tested in a randomized, double-blind, placebo-controlled, dose-ranging Phase II trial: A Study of CIN-107 in Adults with Treatment-Resistant Hypertension (rHTN) (BrigHTN, Clinicaltrials.gov Identifier: NCT04519658). The results of this clinical trial have been presented by Dr. Mason W. Freeman at the latest Scientific Sessions of the American Heart Association in Chicago (Session “Late-Breaking Science: Resistant Hypertension: A Pressure Cooker”) and simultaneously published in The New England Journal of Medicine (10).
The BrigHTN trial was conducted from 30 July 2020 to 14 June 2022, screening 779 individuals, of which 274 were randomly assigned to receive placebo (69 patients), 0.5 mg Baxdrostat (69 patients), 1 mg Baxdrostat (69 patients) or 2 mg Baxdrostat (67 patients). Before randomization, the design of the study included a screening period (up to 8 weeks) and a 2-week run-in period to assess medication adherence (Figure 1). At baseline, the main characteristics were similar across all treatment groups. Black patients represented 28% of all participants, 29-46% had diabetes.
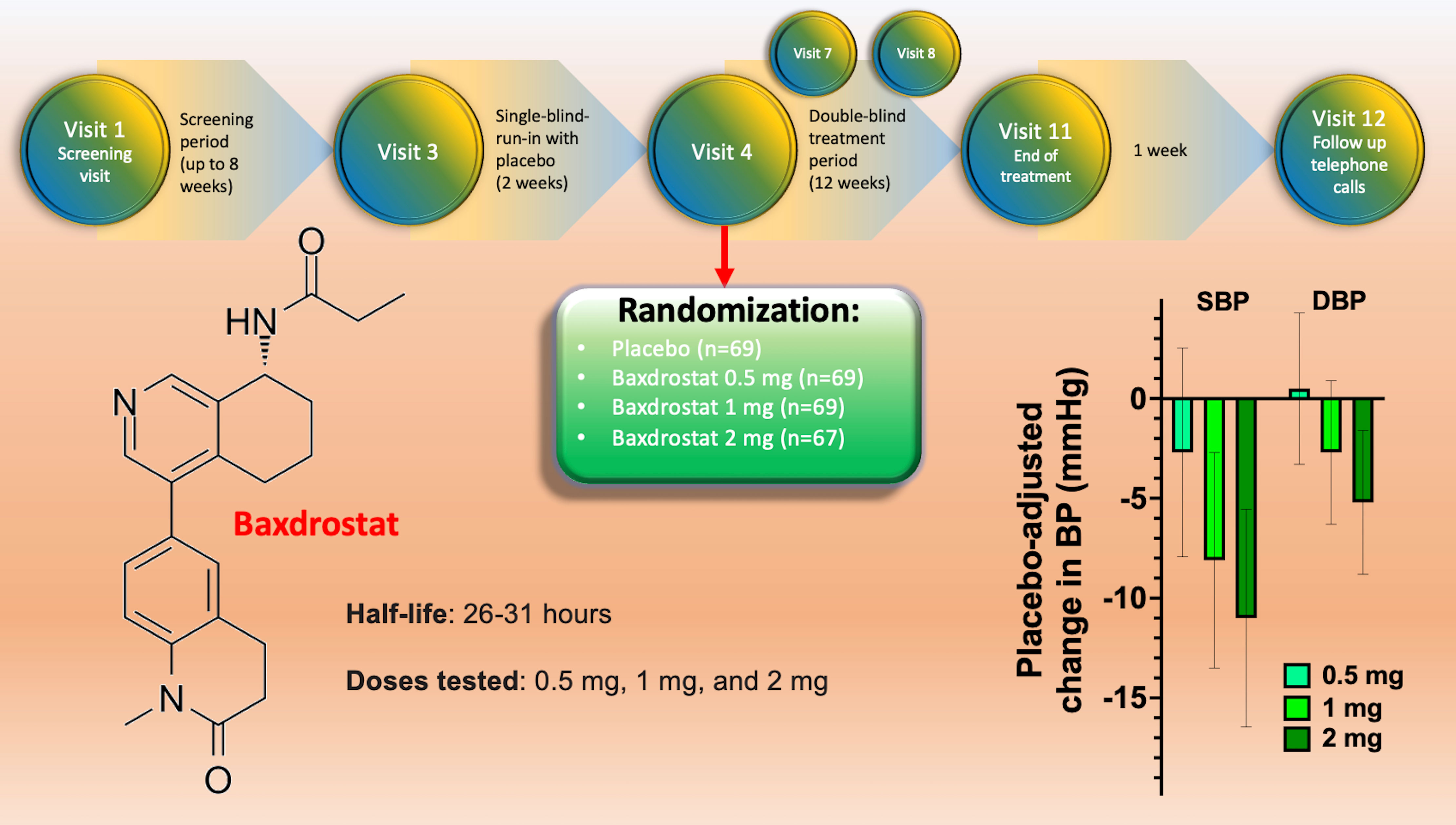
Figure 1 Design of the BrigHTN trial (top), structure of Baxdrostat (right), and representation of the main results in terms of blood pressure reduction at 4 weeks for the three dose groups; SBP, systolic blood pressure; DBP, diastolic blood pressure.
The trial was stopped early for overwhelming efficacy of the drug: indeed, twelve weeks after randomization, Baxdrostat at 1 and 2 mg significantly lowered systolic BP compared to placebo (meeting the primary outcome of the study). The secondary outcome (differences in diastolic BP), was met at the 2 mg dose (10). Exploratory end points included the demonstration that Baxdrostat reached a maximum plasma level in <4h, leading to a dose-dependent decrease in serum aldosterone, without affecting cortisol levels.
In terms of side effects, none of the serious adverse events observed were deemed by the investigators to be related to Baxdrostat. Moreover, none of the patients had to discontinue the trial because of hyperkalemia, which is remarkable: the cases of hyperkalemia observed in a few patients receiving Baxdrostat resolved rapidly with “routine dietary advice” (10); it has to be noted, though, that patients with an estimated glomerular filtration rate >45 ml/min/1.73m2, had been excluded. Another noteworthy exclusion criterion, which reduces the generalization of the results of the BrigHTN trial, is having a mean seated systolic BP ≥180 mmHg or diastolic BP ≥110 mmHg. An important limitation of the trial that needs to be highlighted is that the effect of this new drug was only compared to placebo and not to other anti-hypertensive drugs; further investigations in this sense, including phase III trials, are warranted.
In summary, the selective aldosterone synthase inhibitor Baxdrostat leads to significant reduction in both systolic and diastolic BP in patients with resistant hypertension, representing a new powerful tool to treat resistant hypertension.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The Santulli’s Lab is supported in part by the National Institutes of Health (NIH): National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Center for Advancing Translational Sciences (NCATS: UL1TR002556-06) to GS, by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to GS), and by the Diabetes Action Research and Education Foundation (to GS). FV is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-22POST995561). SSJ is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-21POST836407).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Ruilope LM, Rodriguez-Sanchez E, Navarro-Garcia JA, Segura J, Ortiz A, Lucia A, et al. Resistant hypertension: new insights and therapeutic perspectives. Eur Heart J Cardiovasc Pharmacother (2020) 6:188–93. doi: 10.1093/ehjcvp/pvz057
2. Trimarco V, Izzo R, Mone P, Lembo M, Manzi MV, Pacella D, et al. Therapeutic concordance improves blood pressure control in patients with resistant hypertension. Pharmacol Res (2022) In press. doi: 10.1016/j.phrs.2022.106557
3. Dudenbostel T, Ghazi L, Liu M, Li P, Oparil S, Calhoun DA. Body mass index predicts 24-hour urinary aldosterone levels in patients with resistant hypertension. Hypertension (2016) 68:995–1003. doi: 10.1161/HYPERTENSIONAHA.116.07806
4. Williams B, MacDonald TM, Morant S, Webb DJ, Sever P, McInnes G, et al. British Hypertension society's PSG: Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet (2015) 386:2059–68. doi: 10.1016/S0140-6736(15)00257-3
5. Lainscak M, Pelliccia F, Rosano G, Vitale C, Schiariti M, Greco C, et al. Safety profile of mineralocorticoid receptor antagonists: Spironolactone and eplerenone. Int J Cardiol (2015) 200:25–9. doi: 10.1016/j.ijcard.2015.05.127
6. Duggan S. Osilodrostat: First approval. Drugs (2020). 80:495–500. doi: 10.1007/s40265-020-01277-0
7. MacKenzie SM, Davies E, Alvarez-Madrazo S. Analysis of the aldosterone synthase (CYP11B2) and 11beta-hydroxylase (CYP11B1) genes. Methods Mol Biol (2017) 1527:139–50. doi: 10.1007/978-1-4939-6625-7_11
8. Bogman K, Schwab D, Delporte ML, Palermo G, Amrein K, Mohr S, et al. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone synthase (CYP11B2). Hypertension (2017) 69:189–96. doi: 10.1161/HYPERTENSIONAHA.116.07716
9. Freeman MW, Bond M, Murphy B, Hui J, Isaacsohn J. Results from a phase 1, randomized, double-blind, multiple ascending dose study characterizing the pharmacokinetics and demonstrating the safety and selectivity of the aldosterone synthase inhibitor baxdrostat in healthy volunteers. Hypertens Res (2022). doi: 10.1038/s41440-022-01070-4
Keywords: aldosterone, baxdrostat, blood pressure, BrigHTN, CIN-107, clinical trial, hypertension, resistant hypertension
Citation: Forzano I, Mone P, Varzideh F, Jankauskas SS, Kansakar U, De Luca A and Santulli G (2022) The selective aldosterone synthase inhibitor Baxdrostat significantly lowers blood pressure in patients with resistant hypertension. Front. Endocrinol. 13:1097968. doi: 10.3389/fendo.2022.1097968
Received: 14 November 2022; Accepted: 23 November 2022;
Published: 09 December 2022.
Edited by:
Guanghong Jia, University of Missouri, United StatesReviewed by:
Bruno Trimarco, University of Naples Federico II, ItalyCopyright © 2022 Forzano, Mone, Varzideh, Jankauskas, Kansakar, De Luca and Santulli. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gaetano Santulli, Z3NhbnR1bGxpMDAxQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.