
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol., 15 December 2022
Sec. Gut Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1083707
This article is part of the Research TopicCan Traditional Chinese Medicines affect Endocrine Diseases via Effects on the Intestinal Flora?View all 21 articles
 Xingjian Zhou1,2†
Xingjian Zhou1,2† Xingyu Peng1,2†
Xingyu Peng1,2† Huan Pei1,2†
Huan Pei1,2† Yuhan Chen1,2
Yuhan Chen1,2 Hui Meng1,2
Hui Meng1,2 Jiali Yuan1,2
Jiali Yuan1,2 Haijing Xing1,2*
Haijing Xing1,2* Yueying Wu1,2*
Yueying Wu1,2*The plant-based refers to plant-based raw materials or products that are available as the source of protein and fat. Utilization and development of walnuts as a plant-based, resulting in a high-quality protein-rich walnut plant-based product: walnut protein powder and walnut peptides. Progress in research on the application of walnuts as a plant-based has been advanced, solving the problem of wasted resources and environmental pollution caused by the fact that walnut residue, a product of walnuts after oil extraction, is often thrown away as waste, or becomes animal feed or compost. This paper reviews and summarizes the research and reports on walnut plant-based at home and abroad, focusing on the application of walnut plant-based in the preparation process (enzymatic and fermentation methods) and the biological activity of the walnut protein and walnut peptide, to provide a theoretical basis for the further processing of walnuts as a walnut plant-based. It can make full use of walnut resources and play its nutritional and health care value, develop and build a series of walnut plant-based products, improve the competitiveness of walnut peptide products, turn them into treasure, and provide more powerful guidance for the development of food and medicine health industry in Yunnan.
Walnut (Juglans regia L.), as the medicinal and food biological resource in China, belongs to the genus Walnut in the Walnut family, rich in oleic acid, linoleic acid, α-linolenic acid, and other unsaturated fatty acids, vitamins, and proteins. It has a high nutritional value (1, 2), is known as the “longevity fruit” and the “educational fruit,” and is an important economic forest tree in China. Yunnan province is the largest walnut-producing area in China (accounting for 27.17% of the national walnut production), and Fengqing County is the main walnut-producing area in Yunnan province (3). By 2020, Fengqing County’s walnut cultivation area reaches 1,718,000 mu (mu is a municipal unit of land area in China, about 666.667 square meters), with an annual output of about 103,500 tons and an annual output value of up to 2 billion yuan, ranking first in the country for many years in terms of cultivation area and annual output (4). Walnuts are commonly used to make walnut oil because they contain 65% to 70% oil (5). However, the large number of by-products produced after their oil extraction, walnut residues, are often abandoned and even pollute the environment (6). Recently, plenty of researches have shown that walnut residues can still improve learning and memory as well as antioxidant function (7, 8), therefore, the secondary development and utilization of walnut residues need to be addressed urgently.
In recent years, there has been a boom in “plant-based products” at home and abroad. The term “plant-based products” is derived from the American Plant-Based Foods Association’s concept of “finished food products consisting of ingredients obtained from plants such as vegetables, fruits, grains, nuts, seeds and/or legumes (9)”T/CIFST 002-2021, proposed by China’s Chinese Society of Food Science and Technology in 2021, means “foods made from plant materials (including algae and fungi) or their products as a source of protein and fat, with or without the addition of other ingredients, and made by a certain process with similar texture, flavor, morphology, and other quality characteristics to those of certain foods of animal origin.”.Thus, this paper is intended to review the “walnut plant-based+” series of products using walnuts as the main raw material for plant-based food products, to provide a basis and support for increasing the added value of walnut residues and contributing to Yunnan’s biomedical industry.
According to the definition of “plant-based food” by the American Plant-Based Food Association and the Chinese Society of Food Science and Technology, we consider that “walnut plant-based” can be understood as: the raw material of the walnut plant or its products that serve as a source of protein and fat, including walnut kernels and their Walnuts and their by-products after oil extraction (walnut residue). One of them, walnut kernels, is commonly used in the preparation of walnut oil and nuts and is widely used, while walnut residues are often abandoned as a by-product of walnut oil extraction, and even pollute the environment. Hence, the secondary use and exploitation of walnut residues as the main raw material for “walnut plant-based+” products is promising.
It’s confirmed that walnut residues contain a variety of nutrients such as protein, fat, inorganic salts, vitamins, and fiber (10). The crude fat, crude fiber, and crude fiber in walnut residues are higher than those of similar nut residues, and of these, 143.4% and 334.8% are higher respectively compared to soybean residue (Figure 1A). The vitamin D3 content is up to 139,000 IU/kg, much higher than most nut residue (11). Besides, walnut residues have 18 amino acids, glutamic acid (21.30%~21.70%), arginine (13.60%~15.20%), and aspartic acid (10.20%~10.50%) are the main amino acids (12) (Figure 1B), which shows that walnut residues have high nutritional value and is a more promising “walnut plant-based +”. It can be applied in the development of walnut plant-based foods.
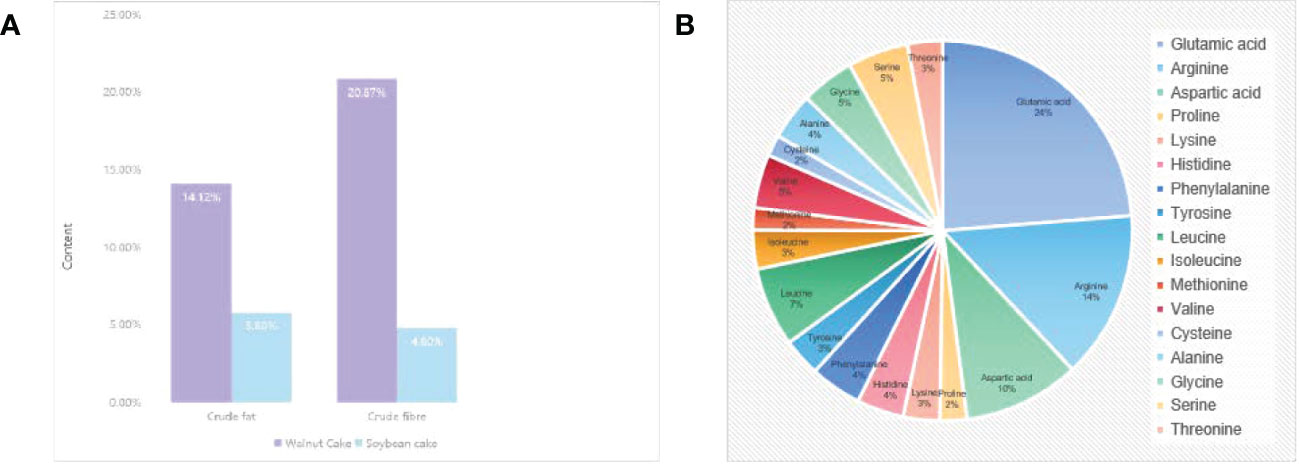
Figure 1 The main component of walnut residue. (A) Comparison of crude fat and crude fiber in walnut residue and soya residue. (B) Composition and content of amino acids in walnut residue.
At present, the major methods of walnut oil extraction in China include pressing and solvent leaching (13). The pressing method (14) uses mechanical pressing to squeeze the oil out of the walnut kernels. The pressing methods are divided into cold pressing and hot pressing, the cold pressing method is carried out at a low temperature, without the use of chemical materials to refine the oil and meet the edible standard (13, 15). It is currently the most used method in China (16), and the obtained walnut residues can better maintain the physical properties and nutrients of walnut residues, and also has a higher protein extraction rate (6), and the protein extracted from walnut cake dross has better solubility, emulsification, and water absorption, and yet requires higher cost and lower oil output than the hot pressing method, and is prone to oxidative rancidity. The oil output of the hot pressing method is higher, and the oil absorption and emulsion stability of the protein extracted from the walnut residues are better, while the oil extraction temperature is higher, and 30% of the walnut shells are also present during the extraction process, making the composition of the obtained walnut residues complex in composition and serious protein denaturation. The organic solvent leaching method (17) is a way to extract the oil in walnut kernels with organic solvents using the extraction principle. The extraction effect is better and suitable for large-scale production, but the equipment is complicated and the solvent residue leads to protein denaturation and the loss of unsaturated fatty acids. In summary, pressing is the most commonly used method for extracting walnut oil. The properties of the walnut residues obtained from different extraction methods vary, and the researcher selects the extraction method according to the purpose of the study and the actual needs of the product development (Figure 2).
As a plant-derived protein powder, walnut protein powder is now widely used in the food sector. Walnut protein powder is mainly a high-protein product obtained by drying and micronizing the by-products produced after the extraction of oils and fats. Walnut powder is mainly divided into two types, which include full-fat and low-fat. The low-fat walnut powder contains less oil and therefore has a longer shelf life, while the full-fat walnut powder has a high oil content and therefore has a shorter shelf life. Therefore, low-fat walnut powder is popular in the market (18). There are two main ways of preparing low-fat walnut protein powder, such as using the cold-pressed walnut residue and Xinjiang high-quality walnut defatted residue as raw material (Figure 3) (19). Walnut phycobilisome is weakly soluble (5) and its emulsification is positively correlated with solubility and is also influenced by the concentration of walnut phycobilisome, temperature, pH, and salt ion levels (10). Yi’s study showed (20) that the solubility, emulsification, and emulsion stability of Walnut vegetable proteins were lower at around pH 5.0. Gao’s study (21) confirmed that Walnut vegetable proteins have low emulsification near the isoelectric point and rise in emulsification away from the isoelectric point. Based on the different properties, Walnut protein powders are divided into different categories (Table 1).
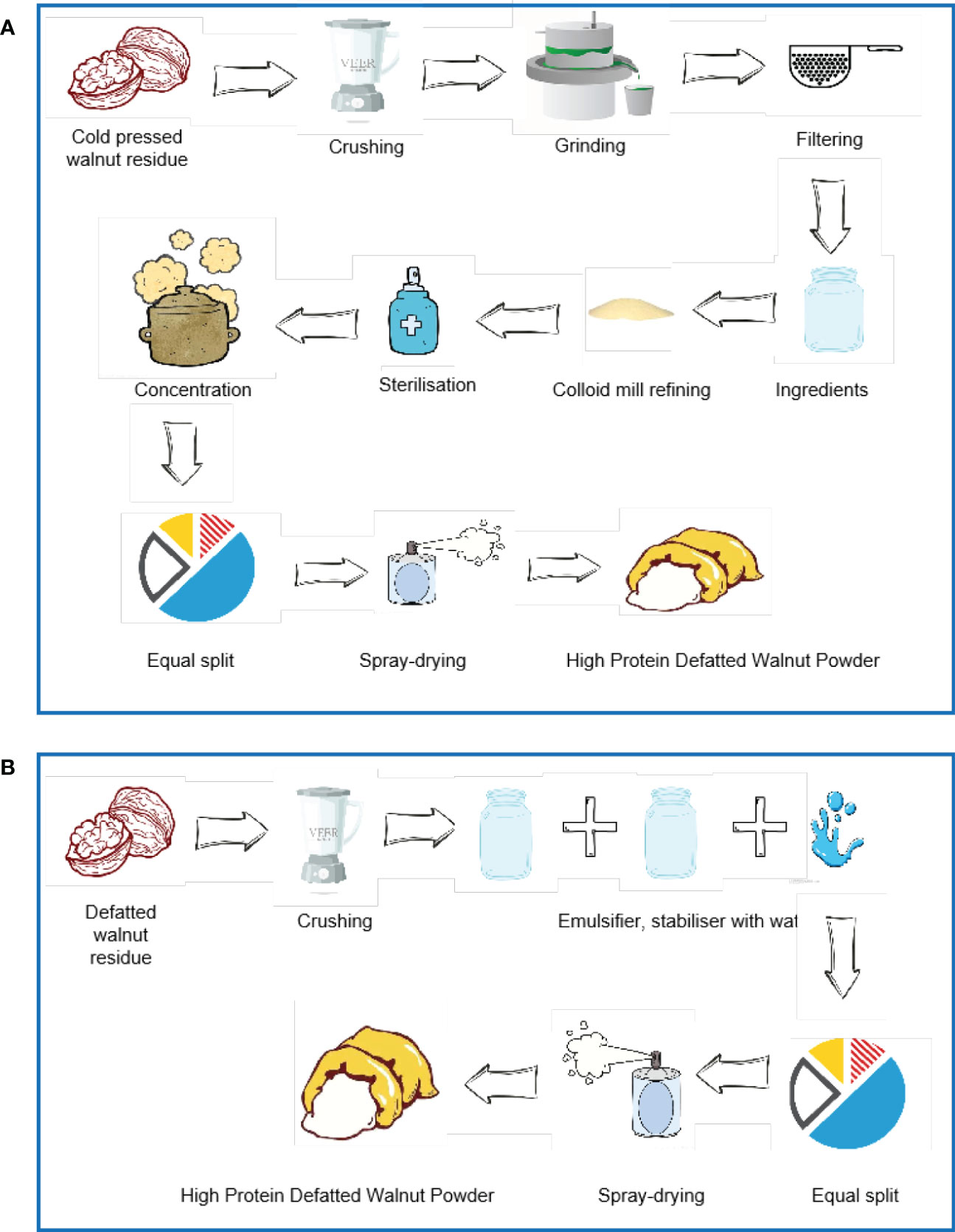
Figure 3 There are two main ways of preparing low-fat walnut protein powder. (A) The method of using the cold-pressed walnut residue as raw material. (B) The method of using Xinjiang high-quality walnut defatted residue as raw material.
Walnut protein powder is mainly prepared using walnut residues. Commonly used methods for the preparation of Walnut protein powder include salt-soluble acid precipitation, dilute acid precipitation, alkali-soluble acid precipitation, enzymatic digestion, reverse micellar method, membrane separation, ion exchange, and physically assisted methods (24) (Table 2). Among all these methods, the alkali-solution and acid-isolation method has the advantages of high purity of the isolated protein and good quality of the product and is the main and commonly used preparation method, which has been widely used in the preparation and practical production of walnut isolated protein at domestic and overseas (Figure 4). However, the effect of acid and alkali made the prepared Walnut protein poor in organoleptic properties, showing a brownish color and requiring further decolorization (5, 24). Yang (28) investigated the effects of four factors on the protein extraction rate, NaOH solution concentration, material-to-liquid ratio, extraction temperature, and extraction time using the alkali-solution and acid-isolation methods. The optimum extraction conditions for alkali-soluble pecan kernel protein were determined as “NaOH concentration 0.02 mol/L, material-to-liquid ratio 1:30 (g/mL), extraction temperature 50°C, extraction time 1.5 h, and an isoelectric point 4.5”.To improve the yield of Walnut vegetable proteins, auxiliary extraction techniques such as ultrasonic technology and subcritical water can be used, with mild reaction conditions and easy operation (27, 29, 30). As can be seen, each of the methods for preparing Walnut protein powder has its advantages and disadvantages, so the characteristics of the raw material and the actual production requirements should be taken into account when selecting a method for preparing the protein.
Walnut protein has biological activities such as antioxidant and anti-inflammatory. Han Haitao et al. showed that the main components of Walnut protein had good antioxidant activity, and the DPPH radical scavenging ability of the clear protein, alcoholic protein, and gluten-2 in walnut protein could reach 97.15%, 93.35%, and 90.58%, respectively (24). Meanwhile, Walnut protein delayed the onset of acute colitis induced by sodium dextran sulfate, slowed down the weight loss in mice caused by colitis, and had significant anti-inflammatory activity due to the hydrolysis of walnut protein into small molecule peptides with anti-colitis activity by the action of intestinal digestive enzymes, which exerted anti-inflammatory effects, which inspired us to focus our research on the biological activity of walnut peptides (12).
Numerous studies have confirmed that walnut peptides have a strong antioxidant capacity both in vitro and in vivo, which is closely related to the amino acid sequence and composition of the peptides (31, 32). Walnut peptides are mostly composed of two to several dozen amino acids through peptide bonds and their relative molecular mass is generally less than 6000 Da (30). As a natural active peptide, the walnut peptide has good characteristics such as high concentration, low viscosity, good solubility, and relative stability to pH changes, and it is better than Walnut protein in terms of foaming, emulsification, and oil absorption, with high safety and excellent application prospects (Figure 5).
Walnut peptides have a variety of biological activities, however, whether the peptides can perform normally or maximize their biological activity requires the selection of a suitable preparation and extraction method according to the characteristics of the raw material, the actual production needs, and the available conditions. The preparation of walnut peptides refers to the process of hydrolysis of walnut vegetable proteins into small molecular peptides with molecular weights between amino acids and proteins using biological or chemical methods. Bioactive peptides are prepared from plants by enzymatic, fermentation, and chemical methods (33). Enzymatic and fermentation methods are more commonly used (34, 35) (Table 3), while enzymatic digestion is the most dominant method of preparation (39).
Walnut residue has 15% to 20% residual oil, which leads to protein denaturation unfavorable to storage, and how to degrease it is an urgent technical problem to be solved (40). Leaching is the main method used to degrease walnut residues; however, this method has solvent residues and is complex to operate. In recent years, subcritical extraction techniques have been increasingly used in the extraction of oil and fats, and are available on a large scale for industrial production (41).
In the preparation of walnut peptides, the choice of enzymes is crucial. The commonly used enzymes and their enzymatic effects are alkaline proteases> papain > trypsin > neutral protease > flavor protease > pepsin (34, 42, 43). Wang Duan (44) et al. used neutral protease to hydrolyze defatted walnut residue powder to prepare peptides and optimized the extraction process of walnut peptides, and the peptide yield was 0.45 g/g under the optimal process conditions. Lu Xiaodan (45) showed that microwave and ultrasonic treatment resulted in significantly higher yields of walnut peptides. Chen Shujun (46) et al. used a complex protease enzyme to optimize the complex protease enzymatic digestion process, and the peptide mass concentration was 10.01 mg/mL and the degree of hydrolysis was 11.45% under the optimal enzymatic conditions.
The above studies illustrate that when preparing walnut peptides by enzymatic digestion, the preparation efficiency and biological activity are influenced by the conditions of enzymatic digestion, the type of complex enzyme, and the sequence of enzymatic digestion and pretreatment. Enzymatic digestion is still the most important method for the preparation of walnut peptides now. To obtain good biologically active walnut peptides efficiently, suitable proteases should be selected according to the actual production needs, providing a favorable guide for the industrial production of walnut peptides.
The exposure of hydrophobic amino acids of the protein after enzymatic digestion causes bitterness limiting its application in food (47). Choosing the right method of de-bittering can improve the taste and flavor of the product and increase its usefulness in processing and production. Three methods are currently commonly used to remove the bitterness of proteinaceous peptides (41) (Table 4).
Fermentation methods are divided into solid and liquid fermentation, with solid fermentation being a microbial fermentation process that takes place on a solid substrate feedstock with little to no free-flowing water in the fermentation substrate (48). Compared to liquid fermentation, solid-state fermentation is less costly, has a wider source of substrates, is less polluting to the environment, is less technically and environmentally demanding, and is more operational (49). Liquid fermentation, with its high level of free water, is a relatively new fermentation technology that began in 1995 (50). Liquid fermentation makes full use of raw materials, and has a short fermentation cycle and a stable product quality, but requires advanced and well-controlled production equipment and a large investment in equipment.
Walnut peptides were mostly prepared by solid-state fermentation of walnut residues. He Ying (51) et al. used lactic acid fermentation of walnut meal to prepare walnut peptides, and the yield of walnut peptides reached 12.84 mg/g and had a powerful free radical scavenging rate and reducing ability. Liu Xiao (52) et al. used Bacillus subtilis and Aspergillus niger for solid-state fermentation of walnut residues, and the result was that Bacillus subtilis fermentation produced a significantly higher content of walnut peptides than Aspergillus niger at 243.97 mg/g. Wu Wanxing (53) used fungal and bacterial microorganisms for solid-state fermentation of walnut residues and concluded that the bacterial peptide yield was significantly higher than the fungal at 30.20% at the respective optimum fermentation temperatures.
Liquid fermentation has the advantage of a shorter fermentation cycle than solid fermentation, faster cell appreciation, more uniform cell development, and so on. Liang Heng (54) et al. screened Bacillus subtilis, Bacillus natto, and Bacillus licheniformis from Bacillus subtilis for liquid fermentation of walnut meal, The fermentation process was also optimized to verify the inhibitory effect of the polyphenolic substances in walnut residues on the growth of Bacillus subtilis and showed a concentration dependence. Using liquid fermentation technology, Xu Dian (55) investigated the optimal process parameters for the fermentation of Aspergillus niger and determined the antioxidant capacity of four constituents of walnut peptides with different molecular weights. The experimental results showed that the highest peptide content of 7.20 mg/mL was achieved at the optimum process parameters, and the antioxidant capacity of the fermentation liquid was significant, with walnut peptides with a molecular weight of <5 KDa having the strongest antioxidant capacity.
Studies have shown that the antioxidant activity of peptides is closely related to their molecular weight (56, 57), and the smaller the molecular weight, the stronger the antioxidant activity of short peptides (32, 46). Therefore, increasing the degree of hydrolysis when carrying out hydrolysis, adding small molecular weight peptides, and separating and purifying the enzymatic digest maximize the biological activity of walnut peptides.Common active peptide isolation methods (Table 5) (33, 58).
Several methods are available for the isolation of bioactive peptides, each with its advantages and disadvantages, and often a single isolation method does not meet the requirements. Thus, to obtain highly active, high-purity target peptides, multiple isolation methods are often used in combination.
Walnut seed coat is rich in polyphenols, which can be reacted with browning under the action of polyphenol oxidase, forming brown compounds that seriously affect the quality of walnut further processed products. Cheng Jing (59) used 2% citric acid for pretreatment in the preparation of walnut peptides from walnut residues, which reduced the browning reaction. Decolourisation also plays a crucial role in the preparation of walnut peptides. Wang Wei (60) et al. explored the optimal conditions for the decolorization of enzymatic hydrolysate of walnut protein by activated carbon, and the decolorization rate was 78.05% under these conditions. According to the actual needs of the selection of suitable methods of polyphenol removal and decolorization, the process can improve the quality and market competitiveness of the product and broaden the development of walnut peptides.
The conventional concept is that proteins are ingested and absorbed by the body after being hydrolyzed into amino acids by various proteases, however, the discovery of peptides and peptide absorption channels in the small intestine suggest that proteins are not necessarily absorbed as free amino acids after digestion and degradation, rather they are mainly in the form of oligopeptides (61), that is, small molecules of peptides are more easily absorbed than proteins or amino acids (42). The biological activities and mechanisms of action of walnut peptides in gut microbiota regulation, antioxidants (8, 34, 42), anti-fatigue, memory improvement, anti-epilepsy, and improvement of metabolic diseases have been investigated by domestic and foreign researchers.
It’s shown that the walnut-derived peptide leucine-proline-phenylalanine (LPF) has a protective and restorative effect on dextran sodium sulfate-induced colitis in mice. Besides, the walnut peptide regulated the gut microbiota disorders in mice by increasing the relative abundance of beneficial genera and decreasing the relative abundance of potentially harmful genera (62). Wang et al. showed the effect of walnut-derived peptide PW 5 on Beta-amyloid protein and intestinal microbiota (63). The above studies have verified the role of walnut peptides in gut microbiota regulation.
Qian Du (64) et al. proposed the hypothesis that walnut peptides may have functions such as promoting brain development and improving learning and memory by significantly increasing the antioxidant properties of tissues and enhancing the ability for free radical scavenging. The mechanisms of protective effects of three novel nucleotides, LVRL, LRYL, and VLLALVLLR, on high glucose-induced insulin resistance (IR) and oxidative stress in HepG 2 cells were elucidated by Wang et al (65). Walnut-derived peptides significantly reduce reactive oxygen species (ROS). Walnut-derived peptides LVRL and LRYL increase antioxidant enzyme activity pathways by activating Nrf2/HO-1 signaling, thereby inhibiting high glucose-induced ROS production and MAPK activation and improving glucose uptake and IR in HepG 2 cells, resulting in alleviating effects on hepatic IR, probably due to the reduced oxidative stress characteristics of walnut residues.
Alzheimer’s disease(AD) is one of the major neurodegenerative diseases in the elderly, with Aβ-induced oxidative stress and neuroinflammation in the brain attributing to the pathogenesis of AD. Zhao et al. showed (66) that the walnut peptide YVLLPSPK improved learning and memory in scopolamine-induced cognitive impairment in mice through a mechanism related to the NF2/KEAP1/HO-1 pathway, providing a deeper theoretical basis for the research on the mechanisms by which walnut peptides improve learning memory and cognitive impairment. Zou et al. (67) demonstrated that the addition of walnut peptides was effective in improving cognitive impairment and memory disorder in mice and that supplementation with walnut peptides was effective in restoring the levels of antioxidant enzymes and inflammatory mediators, thereby reducing the inflammatory response and regulating the antioxidant systems et al. (67) demonstrated that the addition of walnut peptides was effective in improving cognitive impairment and memory deficits in mice, and that supplementation with walnut peptides was effective in restoring levels of antioxidant enzymes and inflammatory mediators, thereby reducing inflammatory responses and modulating the antioxidant system which has a protective effect on AD.
It’s proved that walnut peptide alleviates fatigue by promoting the synthesis of red blood cells in the animal body so that it could reduce the production of lactic acid and urea ammonia during strenuous exercise and delay fatigue. At the same time, it can rapidly decompose lactic acid and urea ammonia and expel them out of the body after exercise, thus speeding up fatigue recovery (68, 69). Liu et al. showed (8) that walnut oligopeptide significantly inhibited fatigue-induced oxidative stress, improved pyruvate kinase, and succinate dehydrogenation activities in mouse skeletal muscle increased mitochondrial biogenesis factor mRNA expression and mitochondrial content and exerted anti-fatigue effects in mice. Uran et al. (47) measured the time to exhaustion of weighted swimming, serum urea nitrogen and lactate dehydrogenase activity in each group of mice, the blood lactate level in each group of mice, and the liver and muscle glycogen content in each group of mice. The results showed that walnut peptides increased lactate dehydrogenase activity, reduced blood lactate and serum urea nitrogen levels, increased muscle glycogen reserves, and significantly prolonged weight-bearing swimming time, thus having a better anti-fatigue effect. It’s shown that, as a plant-derived active peptide, walnut relieve fatigue to some extent.
Walnut peptides still have other functions, such as antiepileptic activity, improving hyperlipidemia as well as hepatic lipid metabolism, and so on (Table 6).
The two applications of walnut plant-based are considered in terms of economic benefits, nutritional value, and environmental impact, each with its focus. Walnut plant protein powder is cheaper to produce but has less nutritional and food applications than walnut peptides, while walnut peptides, although slightly more costly than walnut plant protein, have a variety of biological activities and are widely used in food and pharmaceuticals, and researchers have focused on walnut peptides. In recent years, the rich walnut resources of Yunnan have received a great deal of attention from researchers. The application of plant-based products in food has also created a boom in international and domestic markets. Plant-based dairy products, plant-based meat, and other plant-based products are entering the market. Research on walnut plant protein powder and walnut peptides has also focused on their active functions such as antioxidant, anti-fatigue, and memory improvement, and the subsequent development of healthy food and functional food with various functions by combining walnut plant protein powder and walnut peptides with herbs in Yunnan. For example, walnut peptides can be compounded with herbs in Yunnan such as Gastrodia elata Bl., Polygonati Rhizoma, and Radix Puerariae (76–78) (Table 7) to develop healthy foods and functional foods with anti-fatigue, immunity enhancement, and unique flavors. However, due to Yunnan’s backward economic and technological level, the walnut resources are mostly in primary processing products, the walnut plant-based resources are discarded and wasted, and the visibility is low. Consequently, it is not only necessary to combine walnut plant-based products with Yunnan’s authentic medicinal herbs to improve their innovation, but also to actively improve the production methods to open up the visibility of Yunnan’s walnut plant-based products by joining forces with the government, the public and the media, so that Yunnan’s walnut resources and walnut plant-based products go out of Yunnan and out of China and that the walnut plant-based products of Yunnan have a place in the plant-based market at home and abroad.
Author contributions were as follows: study design YW and HX, data collection XZ, XP and HP, data interpretation YC, HM and JY, manuscript preparation XZ, XP and HP, and funds collection JY. All authors contributed to the article and approved the submitted version.
This work was funded in part by a grant from the Yunnan Provincial Science and Technology Department (202102AE090031), Yunnan Science and technology planning project-joint major project of traditional Chinese medicine(2019FF002(-002)), the Provincial Innovation Team of Yunnan University of Chinese Medicine for Traditional Chinese Medicine to Regulate Human Microecology (No. 2018HC011).
We thank all the scholars who provided relevant guidance for the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Zhong L, Bornman JF, Wu G, Hornoff A, Dovi KAP, Al-Ali H, et al. The nutritional and phytochemical composition of the indigenous Australian pindan walnut (Terminalia cunninghamii) kernels. Plant Foods Hum Nutr (2018) 73(1):40–6. doi: 10.1007/s11130-017-0647-9
2. Zhang Y, Ma J, Tang Y, Liu Y, Lan H, Zhang H. Research overview and development analysis of walnut shelling equipment in China. J Chinese Agricultural Mechanization (2020) 43(09):95–101. doi: 10.13733/j.jcam.issn.2095-5553.2022.09.013
3. Hao Y. Research on germplasm resources and breeding of walnut. (Beijing: Beijing Forestry University) (2008).
4. Yang X. Fengqing county, China: Building the hometown of walnuts . (Yunnan: World of Wealth) (2020) 9, p. 46–7.
5. Martinez ML, Labuckas DO, Lamarque AL, Maestri DM. Walnut (Juglans regia l.): genetic resources, chemistry, by-products. J Sci Food Agric (2010) 90(12):1959–67. doi: 10.1002/jsfa.4059
6. Burbano JJ, Correa MJ. Composition and physicochemical characterization of walnut flour, a by-product of oil extraction. Plant Foods Hum Nutr (2021) 76(2):233–9. doi: 10.1007/s11130-021-00898-4
7. Gao P, Liu R, Jin Q, Wang X. Comparative study of chemical compositions and antioxidant capacities of oils obtained from two species of walnut: Juglans regia and juglans sigillata. Food Chem (2019) 279:279–87. doi: 10.1016/j.foodchem.2018.12.016
8. Chauhan A, Chauhan V. Beneficial effects of walnuts on cognition and brain health. Nutrients (2020) 12(2):550. doi: 10.3390/nu12020550
9. McClements DJ, Grossmann L. The science of plant-based foods: Constructing next-generation meat, fish, milk, and egg analogs. Compr Rev Food Sci Food Saf (2021) 20(4):4049–100. doi: 10.1111/1541-4337.12771
10. Li X, Guo M, Chi J, Ma J. Bioactive peptides from walnut residue protein. Molecules (2020) 25(6):1285. doi: 10.3390/molecules25061285
11. Sun F, Li Xi, Mo F, Li Y, Liu R, Zheng C, et al. Analysis of the nutritional composition of walnut cake for forage. Feed Res (2010) 10):37–8+41. doi: 10.13557/j.cnki.issn1002-2813.2010.10.010
12. Mao X, Hua Y, Chen G. Amino acid composition, molecular weight distribution and gel electrophoresis of walnut (Juglans regia l.) proteins and protein fractionations. Int J Mol Sci (2014) 15(2):2003–14. doi: 10.3390/ijms15022003
13. Ghafoor K, Juhaimi FA, Geçgel Ü, Babiker EE, Özcan MM. Influence of roasting on oil content, bioactive components of different walnut kernel. J Oleo Sci (2020) 69(5):423–8. doi: 10.5650/jos.ess19205
14. Subra-Paternault P, Garcia-Mendoza MDP, Savoire R, Harscoat-Schiavo C. Impact of hydro-alcoholic solvents on the oil and phenolics extraction from walnut (Juglans regia l.) press-cake and the self-emulsification of extracts. Foods (2022) 11(2):186. doi: 10.3390/foods11020186
15. Ahmed IAM, Al-Juhaimi FY, Özcan MM, Osman MA, Gassem MA, Salih HAA. Effects of cold-press and soxhlet extraction systems on antioxidant activity, total phenol contents, fatty acids, and tocopherol contents of walnut kernel oils. J Oleo Sci (2019) 68(2):167–73. doi: 10.5650/jos.ess18141
16. Gao P, Ding Y, Chen Z, Zhou Z, Zhong W, Hu C, et al. Characteristics and antioxidant activity of walnut oil using various pretreatment and processing technologies. Foods (2022) 11(12):1698. doi: 10.3390/foods11121698
17. Xu Y, Bi S, Xiong C, Dai Y, Zhou Q, Liu Y. Identification of aroma active compounds in walnut oil by monolithic material adsorption extraction of RSC18 combined with gas chromatography-olfactory-mass spectrometry. Food Chem (2022) :402:134303. doi: 10.1016/j.foodchem.2022.134303
18. Huo YQ, Liu CJ, Nie RZ, Zhou R, Bao HH, Tang SW. Research progress on the composition, preparation and properties of walnut protein. J Chin Cereals Oils (2020) 35(12):191–7. doi: 10.3969/j.issn.1003-0174.2020.12.030
19. Jin ZC, Zhang RG, Han JQ, Ma L, Yang X, Wang XL, et al. Walnut protein and its development and utilization. Food Ferment Ind (2016) 42(06):265–70. doi: 10.13995/j.cnki.11-1802/ts.201606046
20. Yi J, Cao C, Zhu Z. Study on factors influencing solubility and emulsification of walnut isolated protein. J Shaanxi Univ Sci Technol (2017) 35(05):128–32+38. doi: 10.19481/j.cnki.issn2096-398x.2017.05.023
21. Gao P, Li H, Chen Z, Yang X, Hu C, He D, et al. Optimization of the preparation process and functional properties of walnut isolated protein. China Oils Fats (2022) 47(08):34–9. doi: 10.19902/j.cnki.zgyz.1003-7969.210795
22. Sze-Tao KWC, Sathe SK. Walnuts (Juglans regia l): proximate composition, protein solubility, protein amino acid composition and protein in vitro digestibility. J Sci Food Agric (2000) 80(9):1393–401. doi: 10.1002/1097-0010(200007)80:9<1393::aid-jsfa653>3.0.co;2-f
23. Li M, Zhang Y, You X, Zhou K, Wang Y, Wei P, et al. Processing Technology Optimization of Walnut Beverage. Storage and Process (2020) 20 (04):158–164. doi: 10.3969/j.issn.1009-6221.2020.04.025
24. Liu MC, Yang SJ, Hong D, Yang JP, Liu M, Lin Y, et al. A simple and convenient method for the preparation of antioxidant peptides from walnut (Juglans regia l.) protein hydrolysates. Chem Cent J (2016) 10:39. doi: 10.1186/s13065-016-0184-x
25. Lei Y, Gao S, Xiang X, Li X, Yu X, Li S. Physicochemical, structural and adhesion properties of walnut protein isolate-xanthan gum composite adhesives using walnut protein modified by ethanol. Int J Biol Macromol (2021) 192:644–53. doi: 10.1016/j.ijbiomac.2021.10.022
26. Zhao X, Liu H, Zhang X, Zhu H. Comparison of structures of walnut protein fractions obtained through reverse micelles and alkaline extraction with isoelectric precipitation. Int J Biol Macromol (2019) 125:1214–20. doi: 10.1016/j.ijbiomac.2018.09.095
27. Zhao J, Zhang R, Ma YWang X, Feng B, Zhang Y. Optimization of Protein Extraction from Walnut Dregs. Food Sci (2014) 35(18):40–6. doi: 10.7506/spkx1002-6630-201418008
28. Yu Y, Bao Y. Extraction and SDS-PAGE Analysis of Alkali-Soluble Proteins from Juglans mandshurica Maxim Kernels. Food Science (2012) 33(18):10–3. doi: 1002-6630(2012)18-0010-04
29. Lv S, Taha A, Hu H, Lu Q, Pan S. Effects of ultrasonic-assisted extraction on the physicochemical properties of different walnut proteins. Molecules (2019) 24(23):4260. doi: 10.3390/molecules24234260
30. Li X, Guo M, Chi J, Ma J. Bioactive peptides from walnut residue protein. Molecules (2020) 25(6):1285. doi: 10.3390/molecules25061285
31. Wang J, Liu J, John A, Jiang Y, Zhu H, Yang B, et al. Structure identification of walnut peptides and evaluation of cellular antioxidant activity. Food Chem (2022) 388:132943. doi: 10.1016/j.foodchem.2022.132943
32. Chen N, Yang H, Sun Y, Niu J, Liu S. Purification and identification of antioxidant peptides from walnut (Juglans regia l.) protein hydrolysates. Peptides (2012) 38(2):344–9. doi: 10.1016/j.peptides.2012.09.017
33. Li X, Du MX, FuL W, MY S, JH X, Xie MY. Research progress in the preparation and separation and purification of bioactive peptides. Sci Technol Food Ind (2017) 38(20):336–40+46. doi: 10.13386/j.issn1002-0306.2017.20.061
34. Li J, Ye L, Rong R. Research Advances on Enzymatic Preparation and Separation Identification of Bioactive Peptides. Food Research and Development (2012) 33(02):195–9. doi: 10.3969/j.issn.1005-6521.2012.02.058
35. Wu WX, Chen CY, Zhao SL, Ge F, Liu DQ, Liu BQ, et al. Study on the preparation of active peptides and their antioxidative activity from solid-state fermented walnut meal. Sci Technol Food Ind (2013) 34(16):266–71. doi: 10.13386/j.issn1002-0306.2013.16.044
36. Liu D, Guo Y, Ma H. Production, bioactivities and bioavailability of bioactive peptides derived from walnut origin by-products: a review. Crit Rev Food Sci Nutr (2022) 1:1–16. doi: 10.1080/10408398.2022.2054933
37. Li T, Wu C, Liao J, Jiang T, Xu H, Lei H. Application of protein hydrolysates from defatted walnut meal in high-gravity brewing to improve fermentation performance of lager yeast. Appl Biochem Biotechnol (2020) 190(2):360–72. doi: 10.1007/s12010-019-03109-8
38. Wang X, Yu H, Xing R, Li P. Characterization, preparation, and purification of marine bioactive peptides. BioMed Res Int (2017) 2017:9746720. doi: 10.1155/2017/9746720
39. Lv M, Shi X, Zhang L, Meng J, Wang N, Wang F, et al. Functional properties of walnut peptides and progress of preparation process. China Oils Fats (2013) 38(05):34–8. doi: 10.3969/j.issn.1003-7969.2013.05.009
40. Hao J, Zhang W, Yang H, Zhao J, Zhang R, Zhang Y, et al. Study on the preparation process of microcapsule instant walnut powder by walnut meal. Journal of Shaanxi Normal University (Natural Science Edition) (2019) 425(5):103–8. doi: 10.15983/j.cnki.jsnu.2014.05.044
41. Uğurlu S, Okumuş E, Bakkalbaşı E. Reduction of bitterness in green walnuts by conventional and ultrasound-assisted maceration. Ultrason Sonochem (2020) 66:105094. doi: 10.1016/j.ultsonch.2020.105094
42. Liu MC, SJ Y, Hong D, JP Y, Liu M, Lin Y, et al. A simple and convenient method for the preparation of antioxidant peptides from walnut (Juglans regia l.) protein hydrolysates. Chem Cent J (2016) 10:39. doi: 10.1186/s13065-016-0184-x
43. Miao FJ, Ning DL. Research progress on biological activity of walnut peptides. China Oils Fats (2021) 46(03):48–51. doi: 10.19902/j.cnki.zgyz.1003-7969.2021.03.010
44. Wang D, Zhou H, Wang X, Qiu S, Tian Y. Optimization of enzymatic extraction of walnut peptides by response surface methodology. Food Res Dev (2015) 36(15):19–22. doi: 10.13386/j.issn1002-0306.2017.16.027
45. Lu X, Hua Y, Chen Y, Zhang C, Kong X. Effect of processing conditions on the dissolution rate of walnut protein. J Anhui Agric. Sci (2018) 46(13):155–9. doi: 10.13989/j.cnki.0517-6611.2018.13.047
46. Chen S, Li L, Shi Y, Hu J, Xu X, Li J, et al. Optimization of enzymatic preparation of walnut peptides by response surface methodology. Sci Technol Food Ind (2017) 38(16):142–9+58. doi: 10.13386/j.issn1002-0306.2017.16.027
47. Idowu AT, Benjakul S. Bitterness of fish protein hydrolysate and its debittering prospects. J Food Biochem (2019) 43(9):e12978. doi: 10.1111/jfbc.12978
48. Behera SS, Ray RC. Solid state fermentation for production of microbial cellulases: Recent advances and improvement strategies. Int J Biol Macromol (2016) 86:656–69. doi: 10.1016/j.ijbiomac.2015.10.090
49. Sun S, Song JM, Zhang CS. Current status of research and application of solid-state fermentation technology. China Food Addit (2007) 04):54–8. doi: 10.19902/j.cnki.zgyz.1003-7969.2021.03.010
50. Li L, Wang L, Fan W, Jiang Y, Zhang C, Li J, et al. The application of fermentation technology in traditional Chinese medicine: A review. Am J Chin Med (2020) 48(4):899–921. doi: 10.1142/S0192415X20500433
51. He Y, Chen J. Peptides prepared by lactic acid bacteria from walnut meal and their in vitro antioxidant activity. preparation of peptides from fermented walnut meal by lactic acid bacteria and their in vitro antioxidant activity. Food Res Dev (2022) 43(10):117–23+46. doi: 10.12161/j.issn.1005-6521.2022.10.016
52. Liu X, Guo L, Ma H, Wang K, Wu P, Hong C, et al. Optimization of process conditions for the preparation of walnut peptides by solid-state fermentation of bacillus subtilis and aspergillus niger. Mod Food Sci Technol (2018) 34(08):130–7. doi: 10.13982/j.mfst.1673-9078.2018.8.020
53. Wu W, Chen Z, Zhao S, Ge F, Liu D, Liu B, et al. Study on the preparation of active peptides from solid fermented walnut meal and their antioxidant activity. Sci Technol Food Ind (2013) 34(16):266–71. doi: 10.13386/j.issn1002-0306.2013.16.044
54. Liang X, Zhao S, Cao G, Cai X, Cheng X, He X, et al. Preliminary study of liquid fermented walnut cake meal. China Brew (2013) 32(12):66–9. doi: 10.3969/j.issn.0245-5071.2013.12.016
55. Xu Dian. Preparation and biological activity of walnut peptides by aspergillus niger fermentation. (Beijing: Beijing Forestry University) (2014).
56. Jahanbani R, Ghaffari SM, Salami M, Vahdati K, Sepehri H, Sarvestani NN, et al. Antioxidant and anticancer activities of walnut (Juglans regia l.) protein hydrolysates using different proteases. Plant Foods Hum Nutr (2016) 71(4):402–9. doi: 10.1007/s11130-016-0576-z
57. Tang X, He Z, Dai Y, Xiong YL, Xie M, Chen J. Peptide fractionation and free radical scavenging activity of zein hydrolysate. J Agric Food Chem (2010) 58(1):587–93. doi: 10.1021/jf9028656
58. Xie B, Fu H, Yang F. Research progress on preparation, purification, identification and structure-activity relationship of bioactive peptides. Sci Technol Food Ind (2021) 42(05):383–91. doi: 10.13386/j.issn1002-0306.2020050012
59. Cheng J, Chen D, Qu M, Yu L, Mo Z. Optimization of the preparation process of walnut peptide and its memory improvement function. Sci Technol Food Ind (2021) 42(11):135–41. doi: 10.13386/j.issn1002-0306.2021040288
60. Wang W, Li Y, Jiang Y, Shen M. Optimization of enzymatic digestion and decolorization process of walnut protein. Sci Technol Cereals Oils Foods (2022) 30(03):105–12. doi: 10.16210/j.cnki.1007-7561.2022.03.012
61. Bi D, Zhao Y, Jiang R, Wang Y, Tian Y, Chen X, et al. Phytochemistry, bioactivity and potential impact on health of juglans: the original plant of walnut. Nat Prod Commun (2016) 11(6):869–80. doi: 10.1177/1934578X1601100643
62. Zhi T, Hong D, Zhang Z, Li S, Xia J, Wang C, et al. Anti-inflammatory and gut microbiota regulatory effects of walnut protein derived peptide LPF in vivo. Food Res Int (2022) 152:110875. doi: 10.1016/j.foodres.2021.110875
63. Wang M, Amakye WK, Guo L, Gong C, Zhao Y, Yao M, et al. Walnut-derived peptide PW5 ameliorates cognitive impairments and alters gut microbiota in APP/PS1 transgenic mice. Mol Nutr Food Res (2019) 63(18):e1900326. doi: 10.1002/mnfr.201900326
64. Wu L, Liu R, Du Q, Chen Q, Ren J, Fan R, et al. Anti-fatigue effects of walnut peptides in mice. Food Nutr China (2018) 24(12):50–4. doi: 10.3969/j.issn.1006-9577.2018.12.012
65. Wang J, Wu T, Fang L, Liu C, Liu X, Li H, et al. Peptides from walnut (Juglans mandshurica maxim.) protect hepatic HepG2 cells from high glucose-induced insulin resistance and oxidative stress. Food Funct (2020) 11(9):8112–21. doi: 10.1039/d0fo01753a
66. Zhao F, Liu C, Fang L, Lu H, Wang J, Gao Y, et al. Walnut-derived peptide activates PINK1 via the NRF2/KEAP1/HO-1 pathway, promotes mitophagy, and alleviates learning and memory impairments in a mice model. J Agric Food Chem (2021) 69(9):2758–72. doi: 10.1021/acs.jafc.0c07546
67. Zou J, Cai PS, Xiong CM, Ruan JL. Neuroprotective effect of peptides extracted from walnut (Juglans sigilata dode) proteins on Aβ25-35-induced memory impairment in mice. J Huazhong Univ Sci Technol Med Sci (2016) 36(1):21–30. doi: 10.1007/s11596-016-1536-4
68. Liu R, Wu L, Du Q, Ren JW, Chen QH, Li D, et al. Small molecule oligopeptides isolated from walnut (Juglans regia l.) and their anti-fatigue effects in mice. Molecules (2018) 24(1):45. doi: 10.3390/molecules24010045
69. Kim DI, Kim KS. Walnut extract exhibits anti-fatigue action via improvement of exercise tolerance in mice. Lab Anim Res (2013) 29(4):190–5. doi: 10.5625/lar.2013.29.4.190
70. Jahanbani R, Bahramnejad E, Rahimi N, Shafaroodi H, Sheibani N, Moosavi-Movahedi AA, et al. Anti-seizure effects of walnut peptides in mouse models of induced seizure: The involvement of GABA and nitric oxide pathways. Epilepsy Res (2021) 176:106727. doi: 10.1016/j.eplepsyres.2021.106727
71. Li X, Peng X, Guo K, Tan Z. Bacterial diversity in intestinal mucosa of mice fed with dendrobium officinale and high-fat diet. 3 Biotech (2021) 11(1):22. doi: 10.1007/s13205-020-02558-x
72. Guo K, Xu S, Zhang Q, Peng M, Yang Z, Li W, et al. Bacterial diversity in the intestinal mucosa of mice fed with asparagus extract under high-fat diet condition. 3 Biotech (2020) 10(5):228. doi: 10.1007/s13205-020-02225-1
73. Yang XY, Zhong DY, Wang GL, Zhang RG, Zhang YL. Effect of walnut meal peptides on hyperlipidemia and hepatic lipid metabolism in rats fed a high-fat diet. Nutrients (2021) 13(5):1410. doi: 10.3390/nu13051410
74. Ma S, Huang D, Zhai M, Yang L, Peng S, Chen C, et al. Isolation of a novel bio-peptide from walnut residual protein inducing apoptosis and autophagy on cancer cells. BMC Complement Altern Med (2015) 15:413. doi: 10.1186/s12906-015-0940-9
75. Zhang T, Zhu N, Liu R, Du Q, Wulan, Wang T, et al. Functional effects of walnut oligopeptides on laxative function. Chin J Public Health (2019) 35(09):1225–8. doi: 10.11847/zgggws1119521
76. He L, Liu Y, Guo Y, Shen K, Hui H, Tan Z. Diversity of intestinal bacterial lactase gene in antibiotics-induced diarrhea mice treated with Chinese herbs compound qi wei bai Zhu San. 3 Biotech (2018) 8(1):4. doi: 10.1007/s13205-017-1024-y
77. Hui H, Wu Y, Zheng T, Zhou S, Tan Z. Bacterial characteristics in intestinal contents of antibiotic-associated diarrhea mice treated with qiweibaizhu powder. Med Sci Monit (2020) 26:e921771. doi: 10.12659/MSM.921771
Keywords: walnut plant base, walnut protein, walnut peptide, bioactivity, gut microbiota
Citation: Zhou X, Peng X, Pei H, Chen Y, Meng H, Yuan J, Xing H and Wu Y (2022) An overview of walnuts application as a plant-based. Front. Endocrinol. 13:1083707. doi: 10.3389/fendo.2022.1083707
Received: 29 October 2022; Accepted: 24 November 2022;
Published: 15 December 2022.
Edited by:
Guang Chen, Huazhong University of Science and Technology, ChinaReviewed by:
Yi Wu, Hunan University of Chinese Medicine, ChinaCopyright © 2022 Zhou, Peng, Pei, Chen, Meng, Yuan, Xing and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haijing Xing, eGgtamluZ0AxNjMuY29t; Yueying Wu, bWlzc3d5eUBzaW5hLmNu
†These authors have contributed equally to this work and share the first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.