- 1The First Clinical College of China Medical University, Shenyang, China
- 2Department of Endocrinology and Metabolism, The Fourth People’s Hospital of Shenyang, Shenyang, China
- 3Department of Endocrinology and Metabolism, The First Affiliated Hospital of Soochow University, Suzhou, China
- 4Department of Cardiology, The Second Affiliated Hospital of Dalian Medical University, Dalian, China
Background: Polycystic ovary syndrome (PCOS) is one of the most common gynecological endocrine disorders. Apelin and chemerin are newly identified adipokines, which are higher in obesity and diabetes. Studies have found that the serum apelin and chemerin levels in patients with PCOS are significantly increased. However, other studies showed the opposite results. Therefore, the relationship between those two adipokines and PCOS is still controversial.
Aim: This meta-analysis was conducted to statistically evaluate the apelin and chemerin levels of patients with PCOS.
Methods: We searched the Web of Science, Embase, PubMed, and Google Scholar databases for potential studies. “Polycystic ovary syndrome” or “PCOS” in combination with the terms “apelin” or “chemerin” were used as keywords search titles or abstracts. The publication period examined was between 1990 and 2021. Standardized mean differences (SMD) with corresponding 95% confidence intervals (CIs) were determined as the results of the meta-analysis.
Results: A total of 148 articles were initially retrieved, and 18 qualified articles were finally obtained through preliminary screening and quality evaluation. The publications together contain 1,265 cases and 894 controls. The results of the meta-analysis showed that the circulating chemerin levels in patients with PCOS were significantly higher than those in the controls (SMD: 0.79, 95% CI [0.36, 1.23]), and there was no significant difference in circulating apelin between patients with PCOS and controls (SMD: 0.57, 95% CI [-0.21, 1.35]).
Conclusions: This meta-analysis is the first to evaluate circulating apelin and chemerin levels in patients with PCOS. Our findings suggest that circulating chemerin levels of patients with PCOS are significantly higher than those of healthy controls.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=218316, identifier CRD42020218316.
Introduction
Polycystic ovary syndrome (PCOS) is one of the most common gynecological endocrine disorders with a complex pathogenesis (1). It is characterized by dysmenorrhea, amenorrhea, infertility, hairiness, and obesity, all of which seriously affect the physical and mental health of women of childbearing age. PCOS also increases the risk of endometrial cancer, gestational diabetes mellitus, and hyperlipidemia. Approximately 5%–10% of women of childbearing age are affected by PCOS. Patients with PCOS account for 15%–20% of infertility cases (2, 3). Studies have shown that abnormal follicular development in patients with PCOS is not only regulated by sex hormones but is also closely related to disorders in the follicular development microenvironment caused by ovarian autocrine/paracrine dysfunction. Recent studies suggested that insulin resistance may initiate PCOS development (4).
Apelin and chemerin are newly identified adipokines. Apelin is an APJ receptor ligand, is widely expressed in different organs, and plays an important role in glucose and lipid metabolism (5). Recent studies found that serum apelin levels are significantly correlated with type 2 diabetes mellitus and obesity (6, 7). Chemerin, also called TIG2 or RARRES2, is secreted as an 18 kDa precursor protein (chem163s), which can be transformed into a 16 kDa active molecule only after C-terminal cleavage (8). The precursor protein has multiple restriction sites, which can produce a variety of subtypes upon treatment with different proteases. Chemerin also contributes to adipogenesis, glucose homeostasis, food intake, and body weight and is associated with elevated levels of obesity, diabetes, and cancer (9–11).
Studies have found that serum apelin and chemerin levels in patients with PCOS are significantly increased (12–15), and are potential targets for the treatment of PCOS (16, 17). However, other studies showed that the serum apelin and chemerin levels of PCOS patients are lower than those of healthy individuals (18, 19). Therefore, the relationship between these adipokines and PCOS remains controversial. This meta-analysis aimed to statistically evaluate apelin and chemerin levels in patients with PCOS.
Methods
Search design
We searched the Web of Science, Embase, PubMed, and Google Scholar databases for potential studies. “Polycystic ovary syndrome” or “PCOS” in combination with the terms “apelin” or “chemerin” were used as keywords search titles or abstracts. The full electronic search strategy is provided in the Supplementary Data Sheet 1. The publication period examined was between 1990 and 2021. Concurrently, manual retrieval of relevant literature was performed and the references included in clinical trials were consulted to uncover relevant studies that might have been omitted. This review and meta-analysis were conducted according to the recommendations of the Cochrane Collaboration and following the PRISMA statement. The PRISMA list is provided in the Supplementary Data Sheet 2 and the PROSPERO registration number is CRD42020218316.
Inclusion criteria
The studies included in this meta-analysis met the following criteria: (1) case-controlled or prospective design; (2) detailed data on circulating apelin or chemerin levels in patients with PCOS and healthy controls; and (3) written in English. All the patients with PCOS included in the studies had no medical history or evidence of diabetes, hypertension, hyperprolactinemia, thyroid disease, Cushing’s syndrome, and congenital adrenal hyperplasia. Patients taking drugs such as insulin-sensitizing drugs, oral contraceptives, corticosteroids, anti-androgens, and gonadotropin-releasing hormone agonists or antagonists within 3 months were also excluded from the study.
Data extraction and risk of bias
Two independent evaluators screened the studies according to the inclusion and exclusion criteria. First, the evaluators read the topic and abstract and eliminated duplicate studies and those who did not meet the inclusion criteria. Next, they read the full text of the documents marked for inclusion and cross-checked the results. Finally, the two reviewers discussed and came to consensus on any publications with objections. If they still could not reach an agreement, a third researcher was invited for further evaluation. For documents with questions or missing data, we contacted the author or corresponding author to obtain as much confirmation or supplemental data as possible. The extracted content of the original publication data included the first author, publication year, study period, region, study design, and details of cases and controls.
The Newcastle Ottawa Scale (NOS) was used as the standard to evaluate the quality of the included literature. The NOS is applicable to the evaluation of cohort and case-controlled studies. It consists of three parts: selection of exposure and control populations, comparability, and evaluation of exposure or outcome. It has eight entries. NOS uses the semi-quantitative principle of a star scale to evaluate the quality of literature with a maximum of nine stars (20, 21).
Statistical analysis
Standardized mean differences (SMD) with corresponding 95% confidence intervals (CIs) were determined as the results of the meta-analysis. Cochran’s Q test and I2 statistics were used to test the heterogeneity among the studies. When I2 ≤ 50%, there was no heterogeneity and a fixed effects model was used for combined analysis. When I2 > 50%, there was significant heterogeneity and the random effects model was used for combined analysis. The stability of the meta-analysis results was evaluated by a sensitivity analysis. Low-quality literature was excluded and the impact of a single study on the overall research results was excluded for each study. Begg’s test was used to analyze publication bias. The significance level was set at P < 0.05. Stata 12.0 (College Station, TX, USA) was used for analysis.
Results
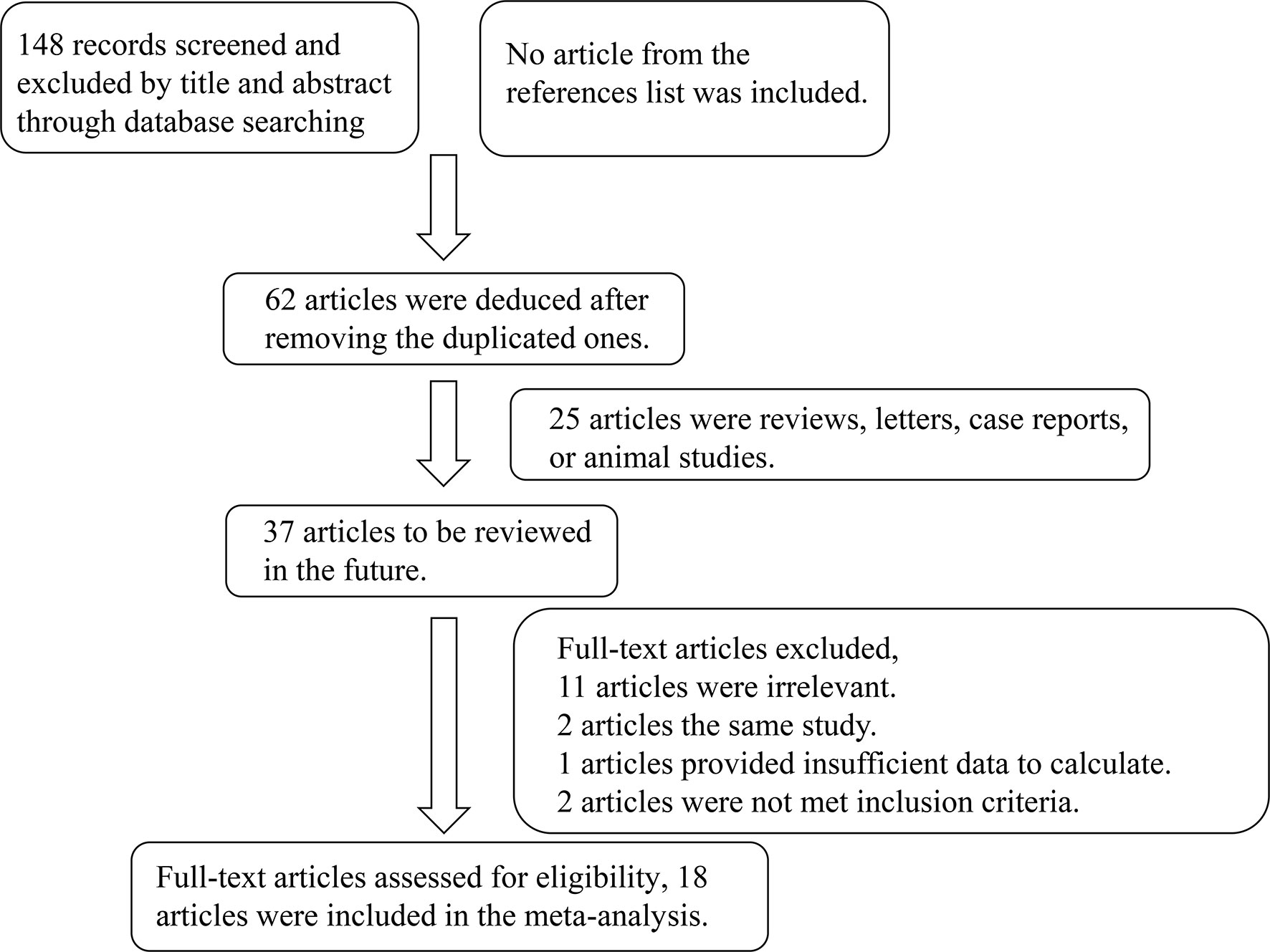
A total of 148 articles were initially retrieved, and 18 qualified articles were finally obtained through preliminary screening and quality evaluation (5–25). The publications together contain 1,265 cases and 894 controls (12–15, 18, 19, 22–33). The literature retrieval process is shown in Figure 1 and the baseline data and quality evaluation of the included case-controlled studies are shown in Table 1.
Results of the meta-analysis
The results of the meta-analysis showed that there was no significant difference in circulating apelin between patients with PCOS and controls (SMD: 0.57, 95% CI [-0.21, 1.35]; I2 = 96.6%). Forest plots of circulating apelin levels in patients with PCOS compared with controls are shown in Figure 2. The circulating chemerin levels in patients with PCOS were significantly higher than those in the controls (SMD: 0.79, 95% CI [0.36, 1.23]; I2 = 91.7%). Forest plots of circulating chemerin levels are shown in Figure 3. The funnel plots of circulating apelin and chemerin were presented in Supplementary Figures 1, 2.
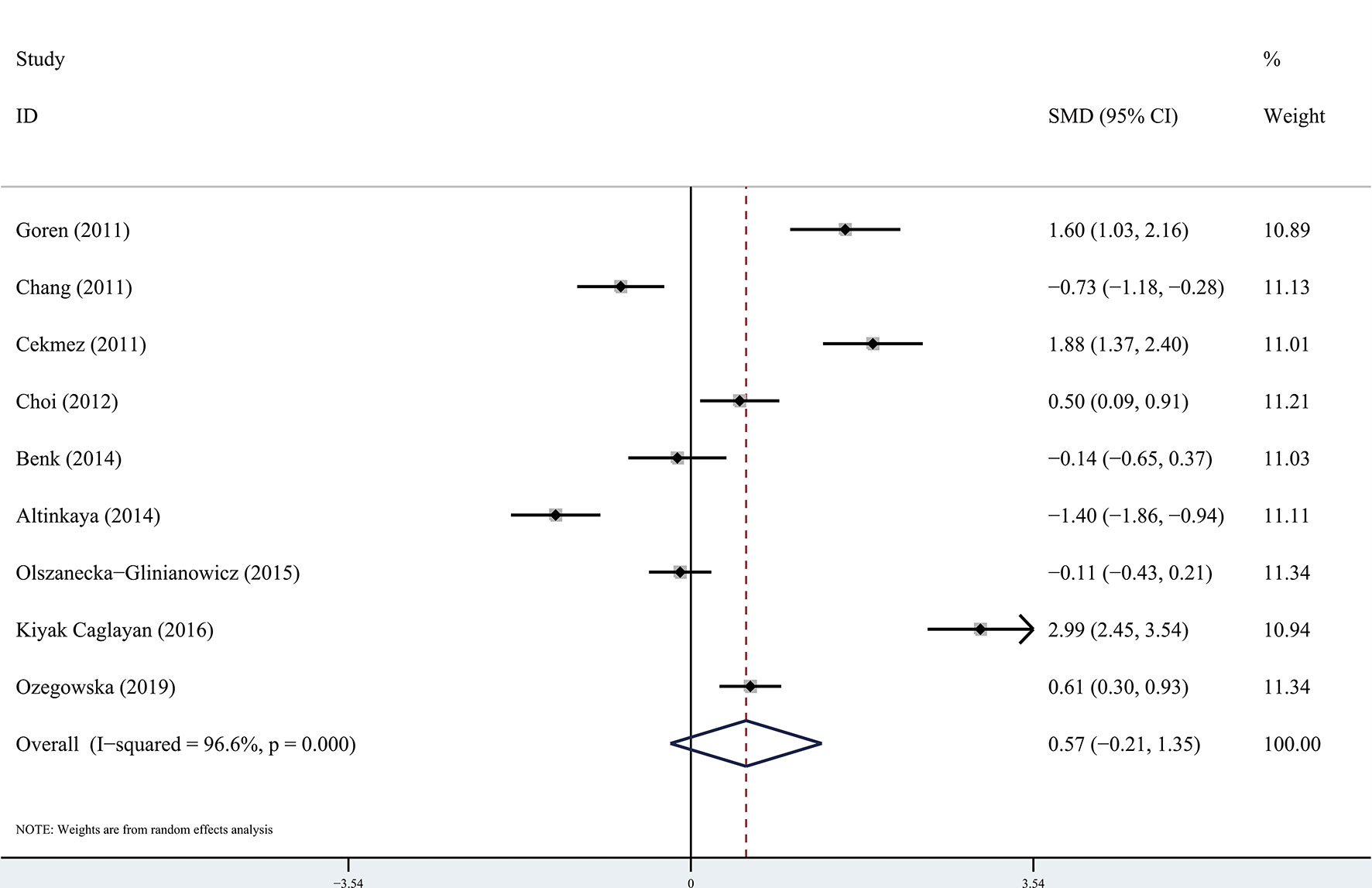
Figure 2 Forest plots of circulating apelin levels in patients with polycystic ovary syndrome compared with controls. Diamond represents the pooled odds ratio at 95% confidence interval.
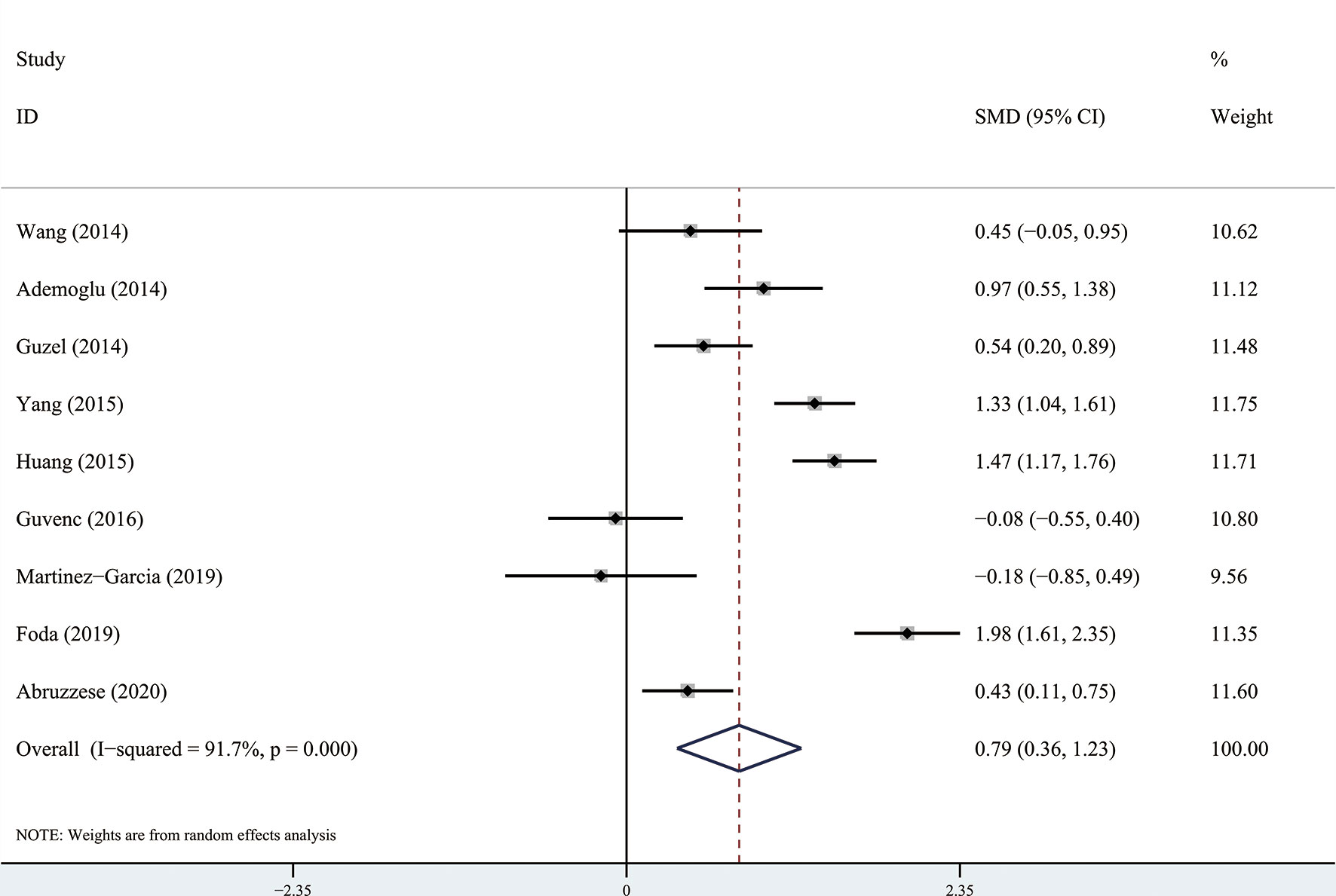
Figure 3 Forest plots of circulating chemerin levels in patients with polycystic ovary syndrome compared with controls. Diamond represents the pooled odds ratio at 95% confidence interval.
Sensitivity analysis and publication bias
Using the sensitivity analysis by excluding individual studies one by one, the results showed little difference, suggesting that the results of this study were relatively credible (Figures 4, 5). A comprehensive search of articles obtained from the database was performed. Begg’s test was also performed to determine whether there was a potential publication bias in the reviewed literature. The results (P > 0.05) suggest that there was no publication bias.

Figure 4 The sensitivity analysis results of circulating apelin levels in patients with polycystic ovary syndrome compared with controls.
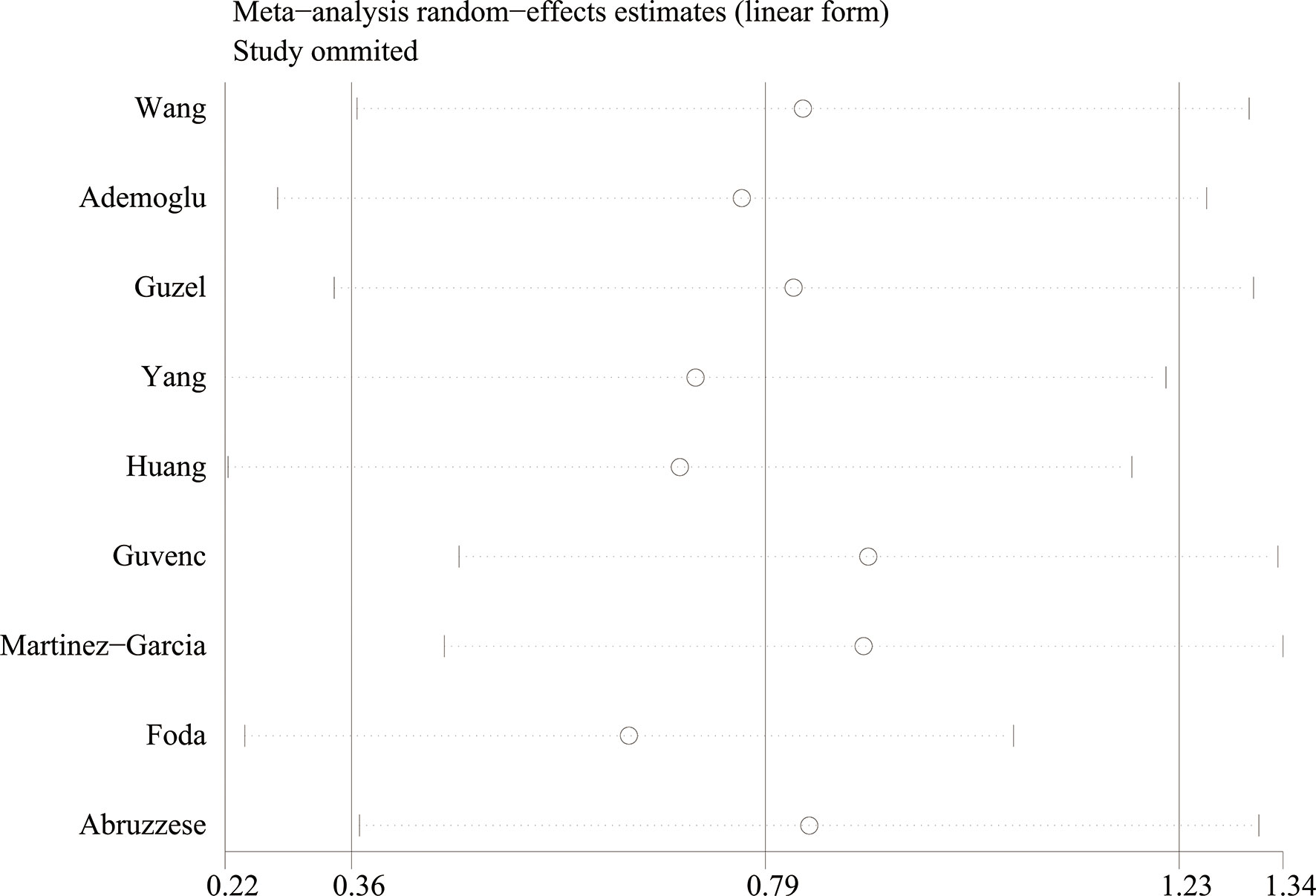
Figure 5 The sensitivity analysis results of circulating chemerin levels in patients with polycystic ovary syndrome compared with controls.
Discussion
This systematic review is the first to evaluate circulating apelin and chemerin levels in patients with PCOS. Although most studies have shown that circulating apelin and chemerin levels in patients with PCOS are higher than those in healthy controls, some found that they are lower. In this meta-analysis, 18 independent studies were included and analyzed. We concluded that the circulating chemerin levels in patients with PCOS were significantly higher than those in the healthy controls (SMD: 0.79, 95% CI [0.36, 1.23]), whereas there was no significant association between circulating apelin and PCOS (SMD: 0.57, 95% CI [-0.21, 1.35]).
A 5-dihydrotestosterone (DHT)-induced rat model was used to simulate the reproductive and metabolic phenotypes of PCOS. These animal experiments showed that recombinant chemerin inhibits basal estradiol secretion in DHT-induced rat granulosa cells. In vitro, chemerin suppressed follicle-stimulating hormone-induced progesterone and estradiol secretion in cultured preantral follicles and granulosa cells (34). Chemokine-like receptor-1 (CMKLR1), an orphan G-protein-coupled receptor, is specifically expressed by monocyte-derived dendritic cells, macrophages, and circulating plasmacytoid dendritic cells. Chemerin is a chemoattractant ligand for CMKLR1. CMKLR1 gene deletion attenuates the effects of chronic DHT treatment on ovarian function in mouse models of DHT-induced PCOS, likely via BMP4 signaling (35). Chemerin also reduces IGF-1-induced steroidogenesis and cell proliferation by decreasing the activation of the IGF-1R signaling pathway in primary human granulosa cells (36). Additionally, metformin treatment has been shown to significantly reduce serum chemerin levels in PCOS patients (32, 37). Chemerin treatment in vitro stimulates the process of angiogenesis (38). Therefore, Anusha et al. hypothesized that the increased expression of ovarian chemerin protein in PCOS subjects may cause derangements in ovarian steroidogenesis or angiogenesis that may trigger the development and progression of metabolism related reproductive disorder (39). In addition, murine model of polycystic ovaries when treated with pioglitazone and metformin showed improved insulin resistance and abnormal steroid production by attenuating the ovarian chemerin gene expression (40). These findings suggest that chemerin is a novel negative regulator that may contribute to PCOS pathogenesis. However, further investigation is necessary to understand the effects of chemerin on PCOS.
Although circulating apelin levels are significantly associated with diabetes, in our meta-analysis, there was no significant association between circulating apelin levels and PCOS. Apelin is expressed in granulosa cells, follicles, and follicular fluid and participates in the normal development of follicles, selection of dominant follicles, and proliferation and apoptosis of granulosa cells (41, 42). However, the influence of apelin on PCOS pathogenesis seems to be more complicated, as indicated by controversial data regarding the association between apelin levels, HOMA-IR, and body mass index (BMI) (12, 18, 22, 27). More high-quality studies are needed to better support the association between apelin and PCOS.
This meta-analysis aimed to statistically evaluate circulating apelin and chemerin levels in PCOS patients. However, this study had some limitations. Due to the lack of large sample case-controlled studies, most of the studies included in this meta-analysis were small. Additionally, some studies did not use BMI-matched healthy controls. Different detection methods for apelin and chemerin were used in these studies. All these factors may have affected the results; therefore, the results of this meta-analysis should be interpreted cautiously, as further research is needed.
Conclusion
This meta-analysis is the first to evaluate circulating apelin and chemerin levels in PCOS patients. Our findings suggest that circulating chemerin levels in PCOS patients are significantly higher than those in healthy controls. More high-quality studies are needed to better support the association between serum apelin levels and PCOS.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
XS designed the study. YG and XS searched databases and collected the data. HF and HW assessed the quality of the study. XS performed the analysis. HF and XS wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1076951/full#supplementary-material
Supplementary Data Sheet 1 | The full electronic search strategy.
Supplementary Data Sheet 2 | Preferred reporting items for systematic review and meta-analyses (PRISMA) checklist
Supplementary Figure 1 | The funnel plots of circulating apelin levels in patients with polycystic ovary syndrome compared with controls.
Supplementary Figure 2 | The funnel plots of circulating chemerin levels in patients with polycystic ovary syndrome compared with controls.
References
1. Carmina E, Lobo RA. Polycystic ovary syndrome (PCOS) arguably the most common endocrinopathy is associated with significant morbidity in women. J Clin Endocrinol Metab (1999) 84:1897–9. doi: 10.1210/jcem.84.6.5803
2. The Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod (2004) 19:41–7. doi: 10.1093/humrep/deh098
3. Wild RA, Carmina E, Diamanti-Kandarakis E, Dokras A, Escobar-Morreale HF, Futterweit W, et al. Assessment of cardiovascular risk and prevention of cardiovascular disease in women with the polycystic ovary syndrome a consensus statement by the androgen excess and polycystic ovary syndrome (AE-PCOS) society. J Clin Endocrinol Metab (2010) 95:2038–49. doi: 10.1210/jc.2009-2724
4. Amato MC, Vesco R, Vigneri E, Ciresi A, Giordano C. Hyperinsulinism and polycystic ovary syndrome (PCOS) role of insulin clearance. J Endocrinol Invest (2015) 38:1319–26. doi: 10.1007/s40618-015-0372-x
5. Antushevich H, Wojcik M. Review apelin in disease. Clin Chim Acta (2018) 483:241–8. doi: 10.1016/j.cca.2018.05.012
6. Castan-Laurell I, Dray C, Attane C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine (2011) 40:1–9. doi: 10.1007/s12020-011-9507-9
7. Noori-Zadeh A, Bakhtiyari S, Khanjari S, Haghani K, Darabi S. Elevated blood apelin levels in type 2 diabetes mellitus a systematic review and meta-analysis. Diabetes Res Clin Pract (2019) 148:43–53. doi: 10.1016/j.diabres.2018.12.012
8. Kennedy AJ, Davenport AP. International union of basic and clinical pharmacology CIII chemerin receptors CMKLR1 (Chemerin1) and GPR1 (Chemerin2) nomenclature, pharmacology, and function. Pharmacol Rev (2018) 70:174–96. doi: 10.1124/pr.116.013177
9. Helfer G, Wu QF. Chemerin a multifaceted adipokine involved in metabolic disorders. J Endocrinol (2018) 238:R79–94. doi: 10.1530/JOE-18-0174
10. Li Y, Shi B, Li S. Association between serum chemerin concentrations and clinical indices in obesity or metabolic syndrome a meta-analysis. PloS One (2014) 9:e113915. doi: 10.1371/journal.pone.0113915
11. Qi X, Fan J, Zhu J, Ling Y, Mi S, Chen H, et al. Circulating chemerin level and risk of cancer a systematic review and meta-analysis. biomark Med (2020) 14:919–28. doi: 10.2217/bmm-2019-0500
12. Cekmez F, Cekmez Y, Pirgon O, Canpolat FE, Aydinöz S, Metin Ipcioglu O, et al. Evaluation of new adipocytokines and insulin resistance in adolescents with polycystic ovary syndrome. Eur Cytokine Netw (2011) 22:32. doi: 10.1684/ecn.2011.0279
13. Goren K, Sagsoz N, Noyan V, Yucel A, Caglayan O, Bostancİ MS. Plasma apelin levels in patients with polycystic ovary syndrome. J Turkish-German Gynecol Assoc (2012) 13:27–31. doi: 10.5152/jtgga.2011.74
14. Wang L, Zhong Y, Ding Y, Shi X, Huang J, Zhu F. Elevated serum chemerin in Chinese women with hyperandrogenic PCOS. Gynecol Endocrinol (2014) 30:746–50. doi: 10.3109/09513590.2014.928687
15. Ademoglu E, Berberoglu Z, Carlioglu A, Dellal F, Gorar S, Alphan Z, et al. Higher levels of circulating chemerin in both lean and obese patients with polycystic ovary syndrome. Minerva Ginecol (2014) 66:535–42.
16. Liu Q, Jiang J, Shi Y, Mo Z, Li M. Apelin/Apelin receptor a new therapeutic target in polycystic ovary syndrome. Life Sci (2020) 260:118310. doi: 10.1016/j.lfs.2020.118310
17. Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest (2017) 40:1–8. doi: 10.1007/s40618-016-0523-8
18. Chang C, Tsai Y, Lee C, Chan T, Wang S, Su J. Lower serum apelin levels in women with polycystic ovary syndrome. Fertil Steril (2011) 95:2520–3. doi: 10.1016/j.fertnstert.2011.04.044
19. Guvenc Y, Var A, Goker A, Kuscu NK. Assessment of serum chemerin, vaspin and omentin-1 levels in patients with polycystic ovary syndrome. J Int Med Res (2016) 44:796–805. doi: 10.1177/0300060516645421
20. Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses (2014) (Accessed 2014 Aug 5).
21. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 (2014) (Accessed 2014 Aug).
22. Choi YS, Yang HI, Cho S, Jung JA, Jeon YE, Kim HY, et al. Serum asymmetric dimethylarginine, apelin, and tumor necrosis factor-α levels in non-obese women with polycystic ovary syndrome. Steroids (2012) 77:1352–8. doi: 10.1016/j.steroids.2012.08.005
23. Altinkaya SO, Nergiz S, Kucuk M, Yuksel H. Apelin levels in relation with hormonal and metabolic profile in patients with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol (2014) 176:168–72. doi: 10.1016/j.ejogrb.2014.02.022
24. Benk SD, Gokce C, Keskin KR, Yilmaz AN, Ozturk OH, Turhan E, et al. Does polycystic ovary syndrome itself have additional effect on apelin levels? Obstet Gynecol Int (2014) 2014:536896. doi: 10.1155/2014/536896
25. Guzel EC, Celik C, Abali R, Kucukyalcin V, Celik E, Guzel M, et al. Omentin and chemerin and their association with obesity in women with polycystic ovary syndrome. Gynecol Endocrinol (2014) 30:419–22. doi: 10.3109/09513590.2014.888412
26. Huang R, Yue J, Sun Y, Zheng J, Tao T, Li S, et al. Increased serum chemerin concentrations in patients with polycystic ovary syndrome relationship between insulin resistance and ovarian volume. Clin Chim Acta (2015) 450:366–9. doi: 10.1016/j.cca.2015.09.015
27. Olszanecka-Glinianowicz M, Madej P, Owczarek A, Chudek J, Skalba P. Circulating anti-mullerian hormone levels in relation to nutritional status and selected adipokines levels in polycystic ovary syndrome. Clin Endocrinol (OXF) (2015) 83:98–104. doi: 10.1111/cen.12687
28. Kiyak Caglayan E, Engin-Üstun Y, Sari N, Göçmen AY, Seckin L, Kara M, et al. Is there association between vitamin d levels, apelin 36, and visfatin in PCOS? Gynecol Endocrinol (2016) 32:386–9. doi: 10.3109/09513590.2015.1124260
29. Yang S, Wang Q, Huang W, Song Y, Feng G, Zhou L, et al. Are serum chemerin levels different between obese and non-obese polycystic ovary syndrome women? Gynecol Endocrinol (2016) 32:38–41. doi: 10.3109/09513590.2015.1075501
30. Ożegowska K, Bartkowiak-Wieczorek J, Bogacz A, Seremak-Mrozikiewicz A, Duleba AJ, Pawelczyk L. Relationship between adipocytokines and angiotensin converting enzyme gene insertion/deletion polymorphism in lean women with and without polycystic ovary syndrome. Gynecol Endocrinol (2019) 36:1–5. doi: 10.1080/09513590.2019.1695248
31. Martinez-Garcia MA, Moncayo S, Insenser M, Alvarez-Blasco F, Luque-Ramirez M, Escobar-Morreale HF. Metabolic cytokines at fasting and during macronutrient challenges influence of obesity, female androgen excess and sex. Nutrients (2019) 11:2566. doi: 10.3390/nu11112566
32. Foda AA, Foda EA, El-Negeri MA, El-Said ZH. Serum chemerin levels in polycystic ovary syndrome after metformin therapy. Diabetes Metab Syndr (2019) 13:1309–15. doi: 10.1016/j.dsx.2019.01.050
33. Abruzzese GA, Gamez J, Belli SH, Levalle OA, Mormandi E, Otero P, et al. Increased chemerin serum levels in hyperandrogenic and normoandrogenic women from Argentina with polycystic ovary syndrome. Gynecol Endocrinol (2020) 36:1057–61. doi: 10.1080/09513590.2020.1769061
34. Wang Q, Kim JY, Xue K, Liu JY, Leader A, Tsang BK. Chemerin, a novel regulator of follicular steroidogenesis and its potential involvement in polycystic ovarian syndrome. Endocrinology (2012) 153:5600–11. doi: 10.1210/en.2012-1424
35. Tang M, Huang C, Wang YF, Ren PG, Chen L, Xiao TX, et al. CMKLR1 deficiency maintains ovarian steroid production in mice treated chronically with dihydrotestosterone. Sci Rep (2016) 6:21328. doi: 10.1038/srep21328
36. Reverchon M, Cornuau M, Rame C, Guerif F, Royere D, Dupont J. Chemerin inhibits IGF-1-induced progesterone and estradiol secretion in human granulosa cells. Hum Reprod (2012) 27:1790–800. doi: 10.1093/humrep/des089
37. Sun X, Wu X, Zhou Y, Yu X, Zhang W. Evaluation of apelin and insulin resistance in patients with PCOS and therapeutic effect of drospirenone-ethinylestradiol plus metformin. Med Sci Monit (2015) 21:2547–52. doi: 10.12659/MSM.894926
38. Tal S, Seifer DB, Arici A. The emerging role of angiogenic factor dysregulation in the pathogenesis of polycystic ovarian syndrome. semin. Reprod Med (2015) 33:195–207. doi: 10.1055/s-0035-1552582
39. Anusha S, Mayank C, Puran B, Amitabh K. Adiponectin and chemerin contrary adipokines in regulating reproduction and metabolic disorders. Reprod Sci (2018) 25:1462–73. doi: 10.1177/1933719118770547
40. Kabiri N, Tabandeh M, Tabatabaie SR. Beneficial effects of pioglitazone and metformin in murine model of polycystic ovaries via improvement of chemerin gene up-regulation. DARU (2014) 22:39. doi: 10.1186/2008-2231-22-39
41. Shimizu T, Kosaka N, Murayama C, Tetsuka M, Miyamoto A. Apelin and APJ receptor expression in granulosa and theca cells during different stages of follicular development in the bovine ovary involvement of apoptosis and hormonal regulation. Anim Reprod Sci (2009) 116:28–37. doi: 10.1016/j.anireprosci.2009.01.009
Keywords: apelin, chemerin, polycystic ovary syndrome, PCOS, meta- analysis
Citation: Gao Y, Xin C, Fan H, Sun X and Wang H (2023) Circulating apelin and chemerin levels in patients with polycystic ovary syndrome: A meta-analysis. Front. Endocrinol. 13:1076951. doi: 10.3389/fendo.2022.1076951
Received: 22 October 2022; Accepted: 22 December 2022;
Published: 11 January 2023.
Edited by:
Didier Dewailly, Université de Lille, FranceReviewed by:
Mayank Choubey, New York University, United StatesChuan Lv, The People’s Hospital of Liaoning Province, China
Xiaowen Zhang, Nanjing Drum Tower Hospital, China
Copyright © 2023 Gao, Xin, Fan, Sun and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xin Sun, c3VueGluNzdAMTI2LmNvbQ==; Hongli Wang, aG9uZ2xpd2FuZ19kb2N0b3JAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Yiming Gao
Yiming Gao Caihong Xin2†
Caihong Xin2† Huaying Fan
Huaying Fan Xin Sun
Xin Sun