- 1Department of Obstetrics and Gynaecology, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 2Department of Obstetrics and Gynaecology, The Second Affiliated Hospital, The Chinese University of Hong Kong, Shenzhen, China
- 3Reproduction and Development, Li Ka Shing Institute of Health Sciences, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 4School of Biomedical Sciences, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 5Chinese University of Hong Kong-Sichuan University Joint Laboratory in Reproductive Medicine, The Chinese University of Hong Kong, Hong Kong, Hong Kong SAR, China
Endometriosis is a common reproductive disorder characterized by the presence of endometrial implants outside of the uterus. It affects ~1 in 10 women of reproductive age. Endometriosis in the ovary, also known as endometrioma (OMA), is the most frequent implantation site and the leading cause of reproductive failure in affected women. Ovarian aging is one of the characteristic features of OMA, however its underlying mechanism yet to be determined. Accumulated evidence has shown that pelvic and local microenvironments in women with OMA are manifested, causing detrimental effects on ovarian development and functions. Whilst clinical associations of OMA with poor ovarian reserve, premature ovarian insufficiency, and early menopause have been reported. Moreover, surgical ablation, fenestration, and cystectomy of OMA can further damage the normal ovarian reservoir, and trigger hyperactivation of primordial follicles, subsequently resulting in the undesired deterioration of ovarian functions. Nevertheless, there is no effective treatment to delay or restore ovarian aging. This review comprehensively summarised the pathogenesis and study hypothesis of ovarian aging caused by OMA in order to propose potential therapeutic targets and interventions for future studies.
1 Introduction
Endometriosis is a chronic inflammatory disease characterized by the presence of ectopic implants, including endometrium and granules, outside of the uterus. Its prevalence reaches 15% of reproductive-aged women and caused disturbances in their life quality due to severe pain and infertility (1). Endometrioma (OMA), the most common subtype of endometriosis, affects up to 44% of women with endometriosis worldwide (2). A strong correlation between OMA and infertility has been indicated in lots of prior studies to support the hypothesis that OMA per se and its treatments may imply quantitative and qualitative disturbance of ovarian reserve (3). OMA has been related to a lower ovarian reserve among infertile women, which is associated with ovarian aging and early menopause (4–7). Although the association is nonlinear and the underlying mechanism is obscured, molecular studies recently emerged to point out the potential mechanisms including iron accumulation, fibrosis, oxidative stress, DNA damage, genetics, and folliculogenesis interruption create a detrimental environment to impair follicle development and ovarian function, may subsequently lead to ovarian aging and early menopause (8–10).
The fertility capacity has long been known to diminish along with chronological age increase. In addition to natural aging, premature ovarian failure (POF)/primary ovarian insufficiency (POI) is defined as primary hypogonadism in women before the age of 40, characterized by interrupted folliculogenesis, reduced follicles, and interfered hormone production. POF/POI can also lead to premature ovarian aging manifested as early menopause and infertility (11–14). To date, many clinical studies demonstrated POF/POI could be induced by OMA and its treatments, crossing the bridge between OMA and ovarian aging. It is reported that patients with OMA who underwent surgical interventions have an increased risk of POF/POI [(15)]. It has been validated in animal models (16). Besides, it is addressed that hyperactivation of dormant primordial follicles, the onset of POF/POI, is induced by iron accumulation, fibrosis, and oxidative stress from OMA lesions (16). Despite the cause-effect relationship being under exploration, the investigations of the association and underlying mechanisms of OMA and ovarian aging are crucial for the development of potential therapies.
In this review, we thoroughly evaluated the clinical relationship between OMA and ovarian aging, summarized their potential mechanisms based on in vitro, in vivo, and clinical studies, as well as point out therapeutic targets, which may benefit the fecundity of infertile women with ovarian aging induced by OMA.
2 Clinical relevance of oma and ovarian reserve
Ovarian reserve is defined as the quality and quantity of the ovarian dormant primordial follicles. It determines the ovarian potential to provide functional eggs that are competent to fertilize (17). Only a limited number of primordial follicles are recruited to develop into growing follicles which either be selected for ovulation or go through atresia. Since most growing follicles are destined toward apoptosis and degeneration, only the left primordial follicles, remaining dormant in the cortex, reflect the ovarian reserve (18, 19). Ovarian reserve determines fecundity and fertility treatment success, its decline dictates the onset of ovarian aging (18, 20–22). Therefore, its assessment is pivotal for monitoring women’s ovarian function during ovarian aging and reproductive treatment. Due to the small size and lack of hormone secretion of primordial follicles, a clinical tool to directly assess primordial follicles does not exist (23). Currently available assessment tools for ovarian reserve, i.e., serum follicle-stimulating hormone (FSH), serum anti-müllerian hormone (AMH), and antral follicle count (AFC), only assess a small fraction of all follicles. Both FSH and AMH are predominantly produced by the developing follicles rather than dormant primordial follicles (24). Therefore the clinical value of serum FSH is limited for predicting ovarian reserve from a meta-analysis (25). The inaccuracy of serum AMH in resembling the number of primordial follicles was also confirmed when compared with ovarian cortical biopsies, which is a gold standard for ovarian reserve assessment (26). In addition, ultrasound detects AFC by identifying follicles with fluid-filled antrum, while it is limited to identify follicles in earlier stages and has difficulties in distinguishing healthy antral follicles from the ones undergoing atresia (27). The biopsy is a traumatic procedure that may decrease ovarian reserve and lead to other complications if not done properly (28). In summary, there is a lack of proper methods for assessing ovarian reserve accurately and safely. In this review, we analyze the effect of OMA on ovarian reserve according to the results from existing tools, but we also provoke for more suitable tools to be explored for an accurate assessment of ovarian reserve with less detrimental effects.
A meta-analysis presented that the ovaries with OMA had a lower AFC before and after surgical removal of lesions compared to the contralateral healthy ovaries, indicating both OMA itself and its surgical intervention can affect the number of growing follicles (29). Besides, a reduction in the serum level of AMH has been reported in women with OMA before surgical treatment (30). As a previous study has shown, there is a significantly lower serum level of AMH in women with bilateral OMA than the ones with unilateral or without OMA. Moreover, the extent of reduction is positively associated with the size of endometriotic lesions, indicating the OMA itself appears to be related to impaired ovarian reserve, and the effect depends on the size and bilaterality (31). Declined ovarian reserve after surgical removal of endometriotic lesions in patients with OMA has been widely reported in observational studies. A prospective cohort study evaluated the consequence of laparoscopic cystectomy of OMA on ovarian reserve and found a reduction in serum level of AMH postpone surgery (32). A decrease in ovarian reserve assessed by basal FSH and ovarian response during assisted reproductive technology (ART) treatment was observed in patients after surgical removal of bilateral OMA, and there is no correlation between the decline extend and the patient’s age (33). With the undisputable destroy effect of surgical interventions on the ovarian reserve of patients suffering from OMA and the advancement in modern surgical technology, some modifications were applied to the surgical process, including the choice of cystectomy or drainage, and the use of homeostatic agent during the surgical process. They were evidenced to improve fertility preservation to some extent but still cannot avoid the injurious impact of surgery (34–37). Recently, it is revealed that diminished ovarian reserve might be a consequence of premature primordial follicle activation, which is regulated by the phosphoinositide 3-kinase (PI3K)/Protein kinase B (Akt)/mammalian target of rapamycin (mTOR) and PI3K/Phosphatase and tensin homolog (PTEN)/Akt/Forkhead box protein O3 (FOXO3) signaling pathway (18, 38). The regulatory pathways are also proven to participate in the pathophysiology of endometriosis which thereafter leads to an increased rate of primordial follicle activation in ovaries with OMA (39–41). It can be verified among patients with unilateral OMA, whose density of primordial follicles in the ovarian cortex is lower in ovaries with OMA than the contralateral ones (7). Besides, the impact of surgical treatment on the activation of primordial follicles through the PI3K/PTEN/Akt/FOXO3 and mTOR signaling pathways has been addressed in both clinical and animal studies (42, 43).
There is limited evidence to show a direct relationship between ovarian aging and OMA, however, the hyperactivation of the primordial follicle in ovaries with OMA leads to ovarian reserve exhaustion, which therefore accelerates ovarian aging has been addressed in several studies (44). Since ovarian reserve in females decreased with chronological age in the natural aging process (45). It is reasonable to foresee that the prematurely primordial activation leading to loss of ovarian reserve may result in POF and subsequent ovarian aging. A research article demonstrated the PI3K/Akt/FOXO3 signaling pathway, which plays a main role in the primordial follicles hyperactivation in ovaries with OMA, is also important in females suffering from POF and the suppression of related pathways could improve the pregnancy rate in patients (46). POF represents the final stage of continuous loss of ovarian function and the absence of menstruation is one of the diagnoses of POF. Menopause represents the end of ovarian aging. However, the transitional process from a normal to absolute regression of ovarian function during the ovarian aging process caused by OMA has yet to be clarified (47–49). There is a commence of processes that took part in the pathogenesis of both OMA and ovarian aging, for example, oxidative stress, cytokines, DNA damage and repair, etc. On account of the limited studies to directly elucidate the association and underlying mechanisms between OMA and ovarian aging, extensive and longitudinal human studies are eagerly needed.
3 The mechanisms of OMA per se. leading to ovarian aging
3.1 Hyperactivation of primordial follicles and diminished ovarian reserve
The pool of dormant primordial follicles located in the cortical region of ovaries reflects ovarian function, which includes secreting ovarian steroids for homeostasis and producing qualified oocytes for fertilization (50). Folliculogenesis is a well-organized process that starts from primordial follicle activation to ovulation (51). Its disturbance during both physiological processes i.e., aging, or pathological diseases may result in a diminished ovarian reserve and impaired quality of oocytes (38, 51). Genome-wide microarray analysis of mouse ovary reported that adverse influence on folliculogenesis may contribute to the aging-dependent diminished ovarian function (52). Ovaries surrounding OMA were found with interrupted folliculogenesis, manifesting a decreased density of primordial follicles while a higher distribution of growing follicles compared with the contralateral healthy ovaries, which indicates a potential activation of primordial follicles in ovaries with OMA (53, 54). The initial activation of the primordial follicle is mainly under the regulation of the PI3K/Akt/mTOR, PI3K/PTEN/Akt/FOXO3 signaling pathways (18, 38). In addition, the Hippo/Yes-association protein (YAP) pathway is pertained to the process of primordial follicles by promoting oocyte development and granulosa cell proliferation (38). The involvement of PI3K/Akt/mTOR, PI3K/PTEN/Akt/FOXO3, and Hippo/YAP pathways were reported in both the pathophysiology of endometriosis and the physiological ovarian aging, uncovering its role in bridging OMA and ovarian aging (39–41, 55–57).
3.2 Fibrosis
In lots of tissues, especially the lung and liver, fibrosis is recognized in related diseases and induces organ failure. Characterized by excessive extracellular matrix (ECM) deposition and connective tissue elongation, fibroblasts developed and expand in response to constant tissue injury and inflammation, as well as physiological processes such as aging (58–60). With increased proinflammatory chemokines, immune cells, mostly M1 macrophages, are recruited to the damage site and trigger anti-inflammation which induces their differentiation to M2 macrophages. The M2 macrophages subsequently stimulate adjacent fibroblasts to produce collagen for scar formation and wound healing (61).
Increased collagen deposition has been demonstrated in the ovaries of women post-menopause as well as in the animal model of reproductive aging (62–67). A recent study documented that ovarian fibrosis originates from cellular stress-induced mitochondrial damage, which then leads to declined bioenergetics, oxidative stress, inflammatory mediators, and collagen accumulation, with reproductive aging. The fibrosis within the ovarian stroma results in anovulation, thereby causing fertility loss. The removal of fibrotic collagen from ovaries was demonstrated to prolong the female reproductive lifespan in mice (68).
The presence of dense fibrosis in the ovarian cyst’s pseudocapsule is widely known, which is also an important characteristic of ovarian OMA (69). One study revealed a higher fibrotic content in the ovarian endometriotic lesions compared to other subtypes of endometriosis (70). The expression of α-smooth muscle isoform of actin (α-SMA), which is pivotal for microfibroblast activation, was detected in ovarian cysts through immunostaining, suggesting myofibroblast proliferation (71, 72). Additionally, fibrosis was significantly extensive in ovaries with OMA compared to the contralateral healthy ovaries (53). Therefore, it is hypothesized that ovarian OMA affects the microenvironment and leads to fibrosis of the surrounding ovarian tissue. The presence of fibrosis in the ovarian cortex adjacent to OMA might furtherly result in a declined follicular density and decreased ovarian reserve, eventually acting as a causative factor for ovarian aging (70). The transforming growth factor-β (TGF-β)/Smad signaling pathway was found essential to the epithelial-to-mesenchymal transition of endometriotic cells derived from OMA, which is involved in the pathophysiology of OMA. Briefly, fibrosis formation can be accelerated by fibroblast-to-myofibroblast transdifferentiation and subsequent surge in collagen production and cell contractility. This process was proved to be reversed by TGF-β blockade (73).
3.3 Stiff and stretch
Tissue stiffening is one of the hallmarks of fibrosis. Increased stiffness accelerates the microfibroblast to produce collagen and then further promotes matrix stiffness, leading to a fibrotic microenvironment of surrounding tissues over time (63, 74, 75). Increased stiffness could activate EMT through myriad transcription factors predominantly TGF-β1. TGF-β1 also underlies the pathophysiology of endometriosis (76–78). Continuous stiffening of surrounding ovarian tissues by OMA lesions has been assumed to reduce ovarian reserve (79).
Stretch from OMA lesions may activate Yes-associated protein (YAP) and transcriptional co-activator with PDZ- binding motif (TAZ), the effectors in downstream of the Hippo signaling pathway, in the adjacent ovarian cortex (80). A meta-analysis compared the serum level of AMH in patients with OMA or benign ovarian cysts. Although the persistent stretch also presents in benign cysts, only the ovaries with OMA presented a significantly reduced serum level of AMH (30). While the reduction of AMH expression is independent of the size of OMA before surgical incision (81). Therefore, instead of stretching alone, there might be other factors that incorporate the mechanical stimuli to facilitate follicle loss in ovaries with OMA. An in vitro study demonstrated stretch and stiffness share a similar mechanotransduction mechanism, and there is an interconnected effect of stretch and stiffness levels on cell phenotypes (82). It is conceivable that stretch and stiffness co-ordinately induce mechanotransduction to activate YAP/TAZ.
The hippo signaling pathway acts as a downstream effector of Akt signaling and inhibits FOXOs and TSC1/TSC2 to activate mTORC1 (83–85). However, sole activation of Akt is insufficient to activate YAP/TAZ, mechanical stimulus such as stretch is also needed (85). The hippo signaling pathway regulates the activation of primordial follicles has been reported in mice (83). During in vitro activation of human follicles, a reduced expression of TSC1 and LATS1, inhibitors of Hippo and PI3K/Akt/mTOR signaling pathways, was demonstrated (43). This indicated that stiffness and stretch activate the related signaling pathways of primordial follicle activation and subsequently defect ovarian function.
3.4 Oxidative stress and cytokines
OMA cyst is proven to contain numerous toxic contents which could also trigger hyperactivation of primordial follicles and diminish the follicle density, thereby accelerating ovarian aging in patients with OMA. According to the molecular milieu of endometriotic cysts, a higher level of free iron was reported in both cyst wall and cyst fluid for a long time (86). Unlike combined iron which plays an essential role in several physiological activities, free iron mediates the generation of reactive oxygen species (ROS) and produces oxygen-free radicals through the Fenton reaction (87). The surrounding ovarian cortex is affected by excessive oxidative stress and presented a significantly increased expression level of 8-OHdG, a DNA damage marker, compared with benign ovarian cysts (88). Oxidative stress triggers hyperactivation of primordial follicles through the PI3K/Akt/mTOR signaling pathway in which PTEN is inhibited and oncogenes like Akt are activated (89). Meanwhile, myofibroblasts proliferation and collagen production are stimulated by excessive oxygen stress, therefore leading to tissue stiffening of the ovarian cortex similarly to the phenotype of senescence (90). Since the guanine bases, composing unit of telomeres, are vulnerable to oxidative damage and the oxidized lesions are inefficient to be repaired, oxidative stress is regarded as the main cause of telomere shortening, leading to reproductive senescence (91).
Numerous chemokines, cytokines, and growth factors arising from the OMA regulate PI3K/Akt/mTOR pathways activation and therefore have a potentially detrimental effect on follicle growth in the adjacent ovaries. Among factors involved in the pathophysiology of OMA, some molecules like vascular endothelial growth factor (VEGF) and interleukin (IL)-8 also participate in the PI3K/Akt/mTOR pathways (92, 93). The expression levels of proinflammatory cytokines such as tumor necrosis factor-alpha (TGF-α), IL-1, IL-6, and IL-8 are significantly increased in endometriotic lesions and fluids from patients with OMA (86, 94–96). The pivotal role of IL-1 in regulating folliculogenesis and the positive effect of IL-1β on the activation of primordial follicles were verified in in vitro culture system of the bovine ovary (97). The IL-16 promotes primordial follicle activation and development during in vitro culturing of the rat ovarian tissue (98). Recently, in an animal model of ovarian aging, the cytokines including IL-6, IL-8, and TNF-α were proved to contribute to the depletion of ovarian reserve (99). It adds plausibility to the concept that chemicals derived from OMA could result in ovarian aging although there is no direct evidence from both human studies and animal models.
3.5 DNA damage and repair
Various DNA damage agents can be continuously exposed to human beings which then impact health situations and modulate disease states (100). However, intricate and complicated systems in cells, involving DNA repair, damage tolerance, cell death pathways, and cell cycle checkpoints, faithfully protect DNA from deleterious consequences (100). DNA repair pathways are intrigued by the robust DNA damage response (DDR) followed by DNA damage. Major DNA repair pathways such as homologous recombination (HR), mismatch repair (MMR), nucleotide excision repair (NER), non-homologous end joining (NHEJ), and base excision repair (BER) are active along the cell cycle, permitting the DNA damage in cells (100).
Preserving genomic sequence information is pivotal for life perturbation. While the well-toned system of DNA damage/repair has been disrupted or deregulated along natural aging and many diseases, consequently, leading to declining fertility as the earliest phenotype of human aging (101, 102). With the advent of next-generation sequencing (NGS), alteration of proteins charging DNA recombination and repair have been screened in patients with POF (103–114). Minichromosome maintenance (MCM) 8 and MCM9 belong to the Mini Chromosome Maintenance family of proteins involved in HR (103). The lack of MCM8 leads to sterility and degenerated ovarian function in animal models (104). Consistent with the observation, a missense variant in MCM8 was found in the patients with POF and primary amenorrhea (105). Since HR initiates DNA double-strand breaks and is important for meiosis, the fibroblasts that come from the patient with POF were more sensitive to chromosomal breaks and the recruitment and activity of MCM8 at the site of DNA breaks were revealed to be impaired (105). Likewise, the absent expression of MCM9 in mice impairs meiotic recombination and oocyte generation (104). Pathogenic variants on MCM9 were also found to be responsible for POF in several studies (109–111). In addition, BRCA genes are involved in ataxia-telangiectasia-mutated (ATM)-mediated DNA double-strand break repairing (115). Mutations of BRCA1 and BRCA2 genes were found to boost reproductive aging and premature infertility (116).
ROS, the pivotal mechanism of OMA-related infertility, was reported to cause DNA damage via attaching DNA bases and compromising the DNA backbone (100, 117). A decreased expression level of copious genes implicated in DNA double-strand break repair, including BRCA1, was found in patients with endometriosis, and its level is positively correlated with ovarian reserves (118). Mammalian oocytes respond to extensive DNA damage at the prophase stage of meiosis through the activity of the DDR and Spindle Assembly Checkpoint (SAC) pathways (119, 120). It is verified by in vitro maturation of mouse oocytes with follicular fluid (FF) from women with endometriosis, in which the FF from patients with endometriosis upraised ROS levels in oocytes and switch on the DDR and SAC pathways. The sensitivity of oocytes to DNA damage checkpoints is increased and prevents oocyte maturation in women with endometriosis (121). A modulated DNA damage response was also observed in the eutopic endometrium of endometriosis, indicating the disturbance of DDR and DNA repair genes and their implication for impaired infertility in patients with endometriosis (122).
These studies highlight the role of pathogenic variants of essential regulators during DNA damage/repair in maintaining fertility in patients with ovarian aging and OMA. The therapeutic targets of related genes may have the potential to alleviate DNA damage and restore fertility potential for these patients.
3.6 Dysregulation of ovarian angiogenesis
Angiogenesis is a highly programmed process of growing new blood vessels from existing vascular structures (123). Active angiogenesis can be found in both physiological and pathological conditions in the reproductive organs of adult females, such as ovaries (124). Highly regulated angiogenesis is crucial for reproduction to support folliculogenesis and endometrial development (125). Dysregulation in angiogenesis, which generates extensive blood vessels, may contribute to the onset and development of many diseases (126).
Dysregulated angiogenesis plays a critical role in the pathogenesis of endometriosis as it enables the engraftment of endometriotic implants and their subsequent progression (127). Elevated expression of proangiogenic factors, such as vascular endothelial growth factor (VEGF)-A and hypoxia-inducible factor (HIF) -1/2α were found positively correlated to OMA, aiding in the growth of endometriotic lesions (128). Bevacizumab, an inhibitor of VEGF, showed no detrimental effect on ovarian reserve while suppressing the progression of endometriotic implants in a rat model of endometriosis (129).
Nevertheless, robust angiogenesis in ovarian follicles and corpus luteum (CL) was recently uncovered with an imaging system, confirming that the generation of new blood vessels was pivotal to guaranteeing a sufficient supply of nutrients and hormones during follicle maturation and development (130). It was reported that the administration of axitinib, the blocker of angiogenesis, decreased ovarian follicle consumption, postponed ovarian aging, and extended female reproductive longevity by suppressing follicle recruiting and development (130). It implied a potential clinical approach to pause lesion progression and delay ovarian aging in patients with OMA.
3.7 Genetics
Early menopause, the result of ovarian aging, can happen in women with a disrupted ovarian function which stop producing sexual hormones, especially estrogen. Age at menopause is estimated to be attributed 50% attributed to genetic factors (131). The presence of myriad genetic aberrations has been identified in POF genomics (132). Promising candidate genes like Forkhead Box L2 (FOXL2), growth differentiation factor 9 (GDF9), and bone morphogenetic protein 15 (BMP15) are also contribute to the pathophysiology of OMA (133–136). the overlapped genetic aberrations are hardly surprising since both OMA and POI potentially affect the formation of the ovarian reserve from the primordial follicle pool, disrupt oogenesis and meiosis, and lead to follicle dysfunction by interrupting folliculogenesis (11, 132). The insights revealed the essence of the genetic analysis point to potential new drug targets for improving fertility in women with OMA-related-ovarian aging. Figure 1 presented the potential mechanisms of OMA itself which leads to ovarian aging.

Figure 1 The potential mechanisms of ovarian aging in OMA per se and their potential target therapies. PI3K, phosphoinositide 3-kinase; PTEN, phosphatase and tensin homolog; Akt, Protein kinase B; FOXO3, Forkhead box protein O3; mTOR, mammalian target of rapamycin; YAP, Yes-associated protein; TGF, Transforming growth factor; FoxL2, Forkhead Box L2; GDF9, Growth Differentiation Factor 9; BMP15, Bone morphogenetic protein 15; VEGF, Vascular endothelial growth factor; IL, interleukin; POF, premature ovarian failure; POI, premature ovarian insufficiency.
4 The mechanisms of OMA treatment leading to ovarian aging
4.1 Tissue damages
Because of the ineffectiveness of medical therapies, there is a general consensus that OMA requires surgical treatment (137). There are various surgical methods including ablation, fenestration, cystectomy, etc. Thereinto, cystectomy by the stripping method is the most common method applied to patients with OMA, because of the lower recurrence rate and more favorable reproductive outcomes compared with others (138). However, OMA cysts are difficult to be removed without damaging surrounding follicular tissue (139). Cystectomy may also cause adhesion and injury to the surrounding blood vessel, which further impeded the development of growing follicles since they are extensively surrounded by blood vessels (140, 141). Moreover, the combination of bipolar diathermy will speed up the damage to the follicles since it literately burns out the follicles using thermal energy. In a word, it is hypothesized that the damage to surrounding tissue and blood vessels may result in impaired ovarian function and thereby speeds up ovarian aging (142).
The hypothesis is supported by numerous clinical studies. It was observed that after ovarian OMA excision, women’s responsiveness to hyperstimulation was reduced and the menopausal transition occurred earlier (15, 143). A retrospective crossover study examined the ovulation rate in 28 infertile patients with unilateral OMA to evaluate the result of ovarian cystectomy. It showed that the ovulation rate significantly declined in the affected ovary after laparoscopic cystectomy as compared with before surgery (144). A cohort study presented that compared with the control group, women with OMA had significantly lower AMH concentrations at baseline and exhibited a further reduction at 6 months postoperatively (145). A prospective randomized study evaluated women who underwent ovarian surgery to remove OMA underwent substantially longer stimulations and required substantially higher dosages of recombinant FSH compared with those who proceeded directly with IVF-ICSI. Additionally, these patients with OMA surgery had a substantially lower oocytes retrieval rate. However, for the fertilization and pregnancy rates, there was no observed difference (146). Surgery for OMA greater than 5 cm in diameter and bilateral OMA resulted in more extensive damage to ovarian reserve (33, 147). Overall the surgical incision of OMA potentially implies a detrimental effect on the surrounding ovarian tissue which subsequently boosts ovarian aging.
4.2 Hyperactivation of primordial follicles
The impact of surgical injury on primordial follicle activation has been determined in several studies. An in vitro study demonstrated that surgical injury to the surrounding ovary could activate dormant primordial follicles near the surgical incision through the mTOR signaling pathway (42). mTOR plays an important role in ovarian aging. It allows different types of cells to escape from the normal biochemical system and regulates the balance between apoptosis and survival (148). Furthermore, surgery could induce local inflammation. The triggered cytokines could affect primordial follicles and/or ovarian reserve in ovaries with resected OMA. for instance, IL-1α may play a pivotal role in the age-related exhaustion of the ovarian reserve in mice by promoting apoptotic pathways and enhancing the expression of pro-inflammatory cytokines IL-1β, IL-6, and TNF-α (149). In addition, a mouse study presented that lipopolysaccharide (LPS) accelerated primordial follicle activation through the PI3K/PTEN/Akt/FOXO3 signaling pathway (150). The activation of this pathway may lead to a compromised DNA damage response, then impacting the growth of primordial follicles and ovarian aging (55). An in vitro experiment illustrated that human ovarian fragmentation culturing resulted in immediate translocation of the Hippo/YAP into the nucleus of granulosa cells (43). In specific regards to the development of ovary tissue, and ovarian follicles, actin polymerization-enhancing drugs promote ovarian follicle growth mediated by YAP (151).
Taking all information together, when deciding whether a patient needs to go through surgery to remove an OMA, every clinician should not only consider symptom relief and recurrence of disease but also ovarian responsiveness, chances of conception during IVF cycles, ovarian reserve, and the possible tendency of ovarian aging. The possible pathogenesis of OMA interventions leading to ovarian senescence is illustrated in Figure 2.
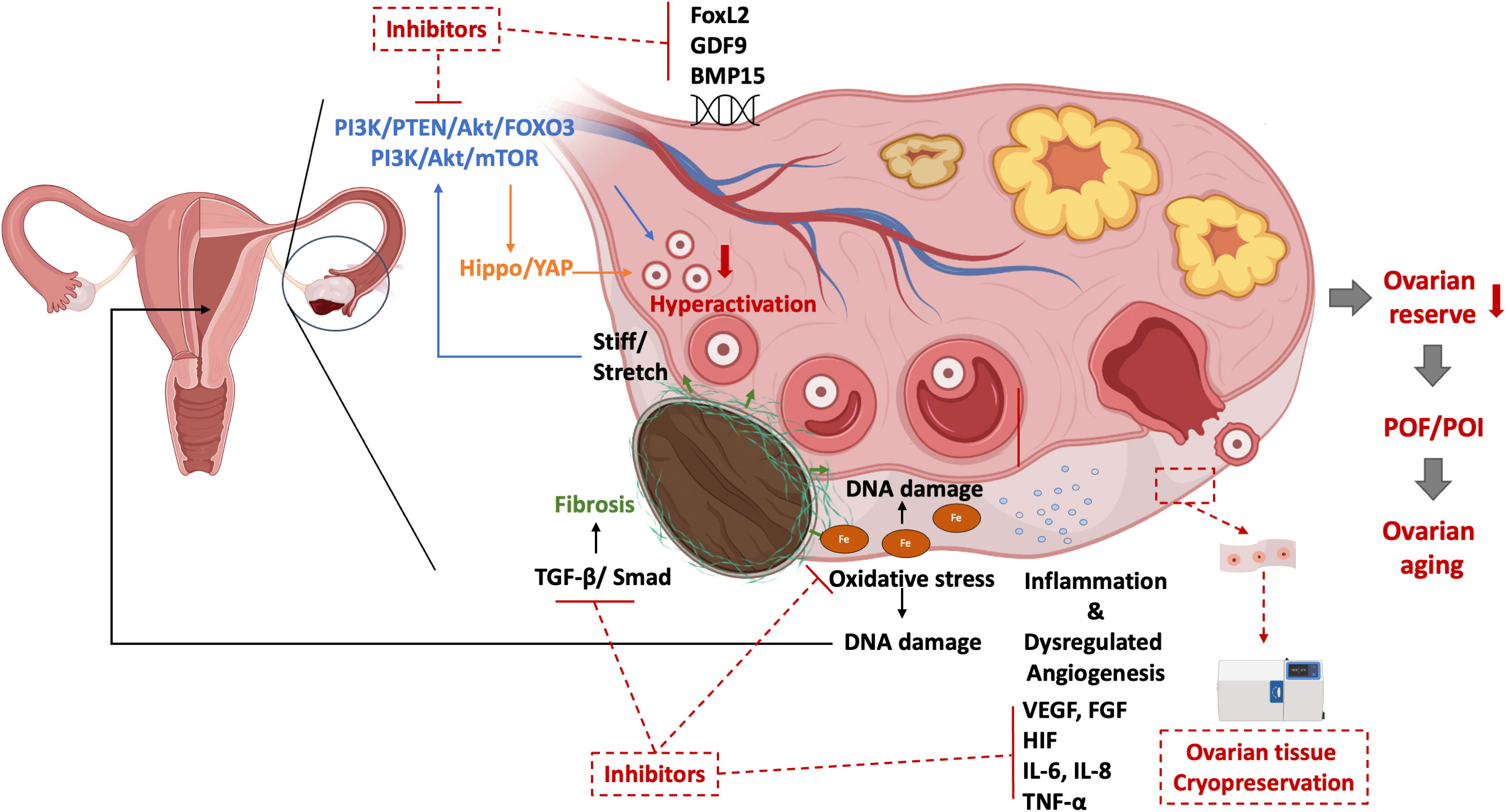
Figure 2 The possible pathogenesis of ovarian senescence due to OMA interventions and their treatments.
5 New treatment and target therapy
5.1 Therapeutic targets
Therapeutic targets aim at reversing ovarian aging and thereby restoring fertility in the aspect of ovarian function are essential for the investigation and development of novel drugs. Based on the mechanisms of OMA leading to ovarian aging described in parts 3&4, 15 drugs targeting the related pathogenesis and signaling pathways were screened in the Therapeutic Target Database (Table 1). Some of them are already put on the market for other conditions, such as myeloma, pulmonary fibrosis, and diabetes, but still with potential applications in the area of OMA-induced ovarian aging from related studies. For instance, both in-vitro and in-vivo studies demonstrated that Sirolimus, which was approved for myeloma, induced regression of endometriotic lesions through inhibiting angiogenesis and proliferation (152–154). Anti-fibrotic agent Pirfenifone was proven to reduce postoperative adhesions for women with endometriosis (155). Siltuximab, an antiviral agent targeting IL-6, is one of the drug candidates for endometriosis-related infertility from the computational drug discovery (156). In addition, Menotropins stimulation may attenuate infertility caused by endometriosis and benefit the IVF-ET outcome (157). And Bevacizumab, an angiogenesis inhibitor, dramatically reduced the size of endometriotic lesions with no impairment to ovarian reserve (129). In account of the utilization in reproductive medicine, the pregnancy risk of the potential drugs is necessary to be considered. According to the guidelines proposed by the United States Food and Drug Administration, five-letter risk categories (A, B, C, D, and X) indicate the potential of a drug to induce birth defects if used during pregnancy (159). It is noted that Menotropins are classified as category X, and thus need to be applied under cautions and contraindicated if conception has occurred. Furthermore, some preclinical therapeutic agents are observed to reverse ovarian function in in-vitro and in-vivo models of OMA and aging. Quercetin, an antioxidant, delayed oocyte aging and improved the developmental potential of aged oocytes during in vitro culturing system (160). Application of ammonium trichloro (dioxoethylene-o,o’) tellurate (AS101), a modulator of the PI3K-PTEN-Akt pathway, was proved to preserve ovarian reserve in mice ovaries with OMA by inhibiting the hyperactivation of primordial follicles (41). It was first reported that ovarian fibrosis in reproductive-aged mice could be reversed with antifibrosis drugs (pirfenidone and BGP-15) and thereby improved female fertility (68). The insights revealed in these therapeutic agents point to a prospective application in treating women with OMA accompanied by ovarian aging. More preclinical and clinical trials should be launched for their further development.
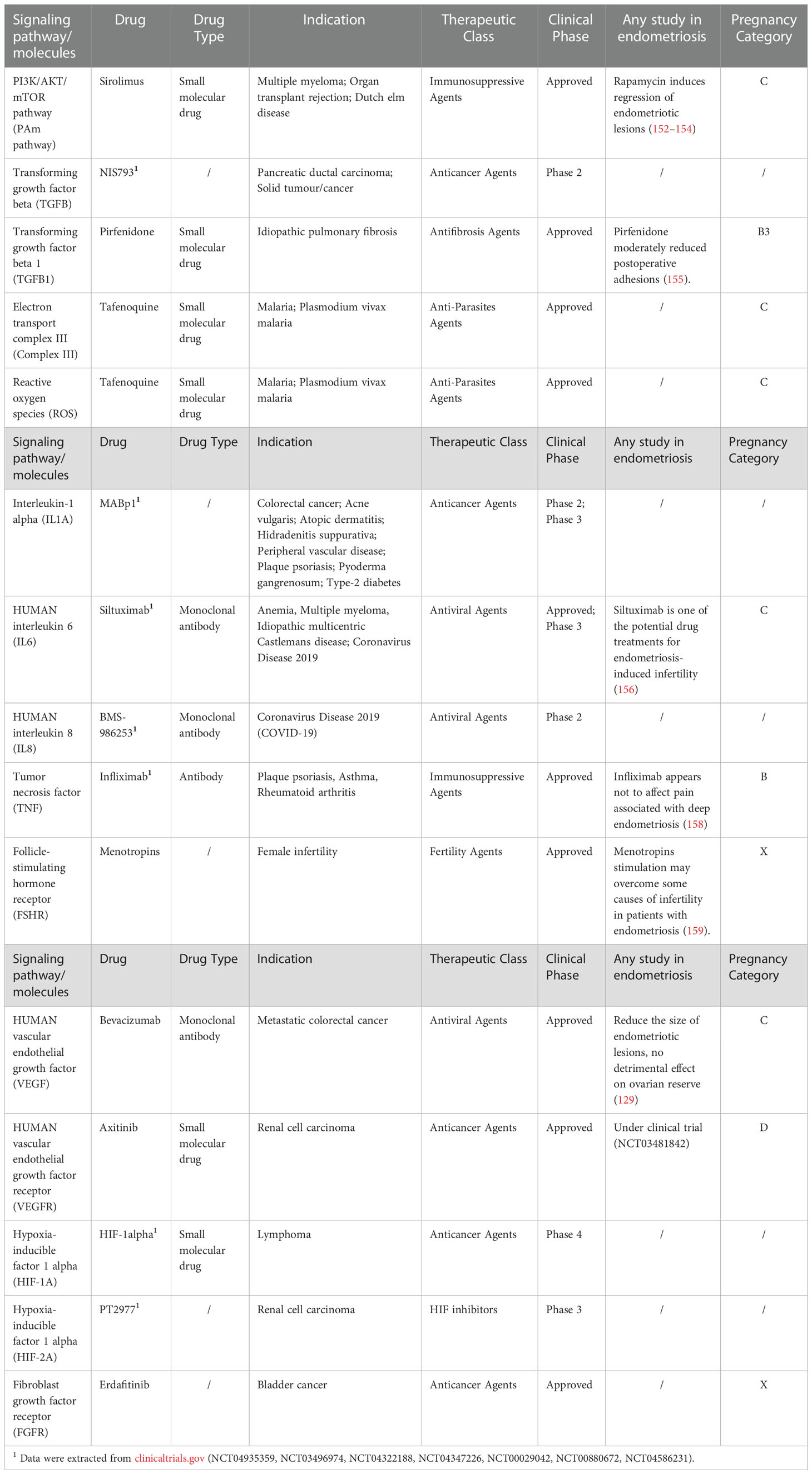
Table 1 Potential drugs targeting the signaling pathways and molecules of OMA related to ovarian aging.
5.2 Peri-surgical interventions
As mentioned above, the possible mechanisms of surgery-related damage to ovarian reserve cannot be ignored. Accordingly, some measures have been taken to optimize the procedure to minimize the deleterious effect on ovarian reserve. A recent RCT randomized 200 women with unilateral OMA (≥5 cm) to receive bipolar coagulation or oxidized regenerated cellulose (ORC) during drainage or cystectomy for hemostasis (34). The trial showed that the use of ORC (drainage + ORC group and cystectomy + ORC group) significantly reduced recurrence rates, with minimal affection for the ovarian reserve in the drainage + ORC group. The use of ORC was generally safe, while encapsulation of fluid and foreign body granulomatous reaction had been reported (161). Some other RCTs also found that hemostatic sealant was non-inferior to bipolar coagulation for hemostasis during laparoscopic cystectomy for OMA patients and might be beneficial to preserve ovarian reserve (36, 162–164).
Similarly, to minimize the use of electrocoagulation and preserve ovarian reserve, some studies used vasopressin injection or epinephrine compress technique to reduce intraoperative bleeding, whereas there is a controversy as to whether this approach could preserve ovarian reserve. Alborzi et al. conducted an RCT to compare ovarian cystectomy after vasopressin injection in the mesovarium space (n=60) and direct cystectomy (n=60) for patients with unilateral OMA (3-6 cm) (165). The results showed that the control group had significantly higher hemostasis points and bleeding compared with the vasopressin group, but there was no difference between the two groups in postoperative serum levels of AMH and pregnancy outcomes. However, another retrospective cohort study indicated that for patients with bilateral OMA (>5cm), vasopressin injection could preserve ovarian reserve (166). An additional RCT revealed that the epinephrine compression method for ovarian stripping had the benefit of the preservation of the ovarian reserve, especially for those with OMA, which might be attributed to epinephrine ameliorated fibrotic changes and necrotic findings in the injured lesion (167). Importantly, no matter which surgical strategy is applied, the assessment of the ovarian reserve is crucial for counselling before the operation. And the patient should be fully aware of the effect of ovarian damage before proceeding to operation.
In addition, there are some studies discussing the impact of hormone therapy on ovarian reserve after cystectomy for OMA. A small single-center RCT compared two groups of women with OMA who received perioperative GnRHa treatment (n=22) or dienogest treatment (n=27) to study the effect on ovarian reserve (168). They found that dienogest was effective for preserving ovarian reserve by reducing the inflammatory response.
However, clinicians should aware that the different methods of peri-surgical interventions could be effective to reduce damage to the ovary, but the trauma still could not be fully reduced.
5.3 Assisted reproductive technology
Around 25-50% of infertile patients are diagnosed with endometriosis and up to 50% of women with endometriosis are referred to IVF centers for ART intervention (169). Although the mechanism of OMA-related infertility is unclear, previous studies suggested that the adhesion of the fallopian tube and ovarian (170), the oxidative damage on oocytes (171), and inflammation (172) might be responsible for it. In a systematic review and meta-analysis, Hamdam et al. investigated the impact of OMA on IVF/ICSI outcomes (173). The study showed that although the mean number of oocytes retrieved per cycle (MNOR) was lower and the cycle cancellation rate (CCR) was higher in women with OMA compared with those without OMA, the live birth rate (LBR) and the clinical pregnancy rate (CPR) were similar between the two groups. In subgroup analysis, women with OMA who received surgical treatment before IVF/ICSI had a similar CPR, LBR, and MNOR compared with those without surgical treatment. The results suggested that surgical treatment of OMA did not affect the IVF/ICSI treatment outcomes. Considering the surgical treatment of OMA might reduce ovarian reserve, physicians should weigh the pros and cons before stripping ovarian OMA prior to IVF/ICSI. HJ Park concluded that surgery prior to IVF was necessary when patients were suffering from severe dysmenorrhea or suspected of cancer. And when the size of OMA was very large, laparoscopic ovarian cystectomy could be considered before IVF (174).
Several studies compared GnRH agonist and GnRH antagonist ovarian stimulation protocols in women with endometriosis. An RCT found that the implantation rate and clinical pregnancy rate were similar in a GnRH antagonist cycle and a GnRH agonist protocol for women with stage I/II endometriosis and OMA (175). Drakopoulos et al. conducted a retrospective cohort study to compare long GnRH agonist with GnRH antagonist ART protocols for women with endometriosis (176). In patients with stage I-II endometriosis, the β-hCG positive, clinical pregnancy, and live birth rates were higher in the GnRH agonist group, but the difference was not statistically significant (P=0.07). No differences in pregnancy outcomes was observed between the two ovarian stimulation protocols in stage III/IV endometriosis group. Overall, there is no sufficient evidence to recommend the best ovarian stimulation protocol for OMA patients. More relevant clinical studies are required.
5.4 Fertility preservation
Fertility preservation (FP) has addressed massive attention since the development of reproductive technologies. FP is legislatively available in most European countries for patients with oncological, and benign diseases, as well as in transgender men (177). Cryopreservation of oocyte, embryo, and ovarian tissue can be applied together with potential medical and surgical interventions to preserve fertility. Oocyte and embryo cryopreservation requires ovarian stimulation while ovarian tissue cryopreservation (OTC) does not. Up to now, oocyte and embryo cryopreservation are preferable for women with age-related fertility loss, due to the advanced development of oocyte and embryo vitrification. Oocyte cryopreservation is usually for single women and embryo preservation is widely applied as a part of ART for married couples as the joint legal ownership with the male partner is necessary. OTC is an essential choice for patients who either have no sufficient time for ovarian stimulation or have adjacent tissue resected in a prior surgery. A combination of different approaches should be considered according to the individual’s situation (178).
As discussed above, both OMA itself and its surgical removal lead to reduced ovarian reserve with impaired yield and quality of oocytes. The preservation of fertility in patients diagnosed with OMA is especially important (179). However, there are limited data describing the effect of FP before surgical interventions in women with OMA so far (180). The first case of oocyte preservation in women with endometriosis was reported in 2009, which proposed the indication of FP in young women with severe endometriosis (181). Another publication reported the success of primordial follicle survival after ovarian tissue cryopreservation and transplantation in patients with severe endometriosis (182). However, these two studies had limitations to be presented as case reports. One observational study showed that FP for patients with a surgical history of OMA was related to poorer responsiveness of ovarian stimulation compared with OMA per se. The authors highlighted the importance of FP counseling before surgical resection in young women with severe endometriosis, however, there was no results of the FP results in healthy controls (183). The effect of OMA on controlled ovarian stimulation and the cumulative effect of stimulation on oocyte yield had been demonstrated in a research group in South Korea (184). Simultaneously, their study verified the efficacy of pre-operative FP in patients with OMA to prolong their fertility age (184).
There are several options for improving fecundities in patients with OMA, but FP should be with great potential for those with severe and repeated OMA. As OMA is still a novel topic in fertility preservation. It is conflicted about the timing, necessaries, approaches, and the patient’s willingness for the application of FP in those patients. The scenario is multifaceted, and both patients and physicians may be overwhelmed by the proper decision (185). Therefore, Marie-Madeleine Dolmans proposed an algorithm for fertility preservation in patients with endometriosis based on the strict indications, in which low level of AMH, age beyond 30 years, bilateral OMA, a high recurrence rate after surgery, OMA growing fast, and OMA at a young age should be taken into account (186).
6 Conclusion and perspectives
OMA is a prevalent disease in infertile women with a decreased ovarian reserve and impaired ovarian function. Surgery, the most common treatment of OMA, is disputable on the potential to destruct surrounding ovarian tissues. The interaction between OMA and ovarian aging can be found in numerous clinical cases, but there is no review to clarify the relevance and underlying mechanisms. Here we comprehensively summarised the clinical relevance and possible pathogenesis and mechanisms in commence of OMA and ovarian aging for the first time. Thereinto, fibrosis, inflammation, dysregulated angiogenesis, and oxidative stress may lead to the imbalance of DNA damage/repair and hyperactivation of primordial follicles, further resulting in a decreased ovarian reserve, which is not only an important characteristic of ovaries with OMA but also characterise the beginning of ovarian aging. The surgical removal of OMA also implies a detrimental effect on the surrounding ovaries, resecting the healthy ovarian cortex, hyperactivating primordial follicles, and thereby diminishing the ovarian function. Therapeutic targets on etiology pathways and molecules, i.e. PI3K/PTEN/Akt/FOXO3, TGF-β, IL-1β, IL-6, and TNF-α, are with the possibility to delay ovarian aging and restore fertility. Besides, modifications of surgical interventions like hemostasis methods are demonstrated to improve fertility loss to some extent but cannot absolutely avoid the detrimental effect of surgery. Fertility preservation is a recent-developed reproductive technology with great potential in maintaining fecundity in women with OMA. Due to the complexity and sophistication of this technique, more details, especially the different approaches to cryopreservation, the timing of tissue collection, ethical issues, and availability are needed to be discussed thoroughly before implying the application to patients.
Increased attention has been raised to seek an understanding of the pathophysiology and mechanisms of OMA leading to ovarian aging, which assists to propose new treatments and target therapy. However, many of these are still incompletely understood. We aimed to raise awareness of the missing pieces of puzzles and advocate more related studies. While the limited access to human samples, large-scale experiments on animals which share similar anatomy to human beings are also important. Novel animal models of OMA have been proposed recently, but they failed to manifest OMA exclusively. On account of the disparities of different subtypes of endometriosis, a proper animal model specific to OMA is the top priority, so to launch more related research for better management of OMA and its associated ovarian aging.
Author contributions
ZT and CC participated in the research design. ZT participated in data evaluation, extraction, and interpretation. ZT, XG, and YL participated in the data validation and in the drafting of the manuscript. ZT, XG, YL, SH, JC, and CC critically revised the manuscript. All authors approved the final version of the manuscript.
Funding
This study is funded by the Theme-based Research Scheme granted by the Research Grants Council of the Hong Kong Government of Special Administrative Region (T13-602/21-N).
Acknowledgments
We express our gratitude to Basecare Medical Device Co. for the setting up of a fertility preservation centre in the department for research.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Smolarz B, Szyłło K, Romanowicz H. Endometriosis: Epidemiology, classification, pathogenesis, treatment and genetics (Review of literature). Int J Mol Sci (2021) 22(19). doi: 10.3390/ijms221910554
2. Puckett. Y. Endometrioma Vol. 12. StatPearls (2022). p. 2022. Available at https://www.ncbi.nlm.nih.gov/books/NBK559230/
3. Yılmaz Hanege B, Güler Çekıç S, Ata B. Endometrioma and ovarian reserve: effects of endometriomata per se and its surgical treatment on the ovarian reserve. Facts Views Vis Obgyn (2019) 11(2):151–7.
4. Seyhan A, Ata B, Uncu G. The impact of endometriosis and its treatment on ovarian reserve. Semin Reprod Med (2015) 33(6):422–8. doi: 10.1055/s-0035-1567820
5. Romanski PA, Brady PC, Farland LV, Thomas AM, Hornstein MD. The effect of endometriosis on the antimüllerian hormone level in the infertile population. J Assisted Reprod Genet (2019) 36(6):1179–84. doi: 10.1007/s10815-019-01450-9
6. Prescott J, Farland LV, Tobias DK, Gaskins AJ, Spiegelman D, Chavarro JE, et al. A prospective cohort study of endometriosis and subsequent risk of infertility. Hum Reprod (2016) 31(7):1475–82. doi: 10.1093/humrep/dew085
7. Kitajima M, Defrère S, Dolmans M-M, Colette S, Squifflet J, Van Langendonckt A, et al. Endometriomas as a possible cause of reduced ovarian reserve in women with endometriosis. Fertility Sterility (2011) 96(3):685–91. doi: 10.1016/j.fertnstert.2011.06.064
8. Mier-Cabrera J, Jiménez-Zamudio L, García-Latorre E, Cruz-Orozco O, Hernández-Guerrero C. Quantitative and qualitative peritoneal immune profiles, T-cell apoptosis and oxidative stress-associated characteristics in women with minimal and mild endometriosis. Bjog (2011) 118(1):6–16. doi: 10.1111/j.1471-0528.2010.02777.x
9. Podgaec S, Abrao MS, Dias JA, Rizzo LV, de Oliveira RM, Baracat EC. Endometriosis: an inflammatory disease with a Th2 immune response component. Hum Reprod (2007) 22(5):1373–9. doi: 10.1093/humrep/del516
10. Bertone-Johnson ER, Manson JE, Purdue-Smithe AC, Steiner AZ, Eliassen AH, Hankinson SE, et al. Anti-müllerian hormone levels and incidence of early natural menopause in a prospective study. Hum Reproduction (2018) 33(6):1175–82. doi: 10.1093/humrep/dey077
11. Chon SJ, Umair Z, Yoon MS. Premature ovarian insufficiency: Past, present, and future. Front Cell Dev Biol (2021) 9:672890. doi: 10.3389/fcell.2021.672890
12. Karlberg S, Tiitinen A, Alfthan H, Lipsanen-Nyman M. Premature ovarian insufficiency and early depletion of the ovarian reserve in the monogenic mulibrey nanism disorder. Hum Reprod (2018) 33(7):1254–61. doi: 10.1093/humrep/dey103
13. Torrealday S, Kodaman P, Pal L. Premature ovarian insufficiency - an update on recent advances in understanding and management. F1000Res (2017) 6:2069. doi: 10.12688/f1000research.11948.1
14. Sükür YE, Kıvançlı IB, Ozmen B. Ovarian aging and premature ovarian failure. J Turk Ger Gynecol Assoc (2014) 15(3):190–6. doi: 10.5152/jtgga.2014.0022
15. Coccia ME, Rizzello F, Mariani G, Bulletti C, Palagiano A, Scarselli G. Ovarian surgery for bilateral endometriomas influences age at menopause. Hum Reprod (2011) 26(11):3000–7. doi: 10.1093/humrep/der286
16. Matsuzaki S, Pankhurst MW. Hyperactivation of dormant primordial follicles in ovarian endometrioma patients. Reproduction (2020) 160(6):R145–r53. doi: 10.1530/REP-20-0265
17. Jirge PR. Poor ovarian reserve. J Hum Reprod Sci (2016) 9(2):63–9. doi: 10.4103/0974-1208.183514
18. Adhikari D, Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocrine Rev (2009) 30(5):438–64. doi: 10.1210/er.2008-0048
19. Lew R. Natural history of ovarian function including assessment of ovarian reserve and premature ovarian failure. Best Pract Res Clin Obstet Gynaecol (2019) 55:2–13. doi: 10.1016/j.bpobgyn.2018.05.005
20. Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: mechanisms and clinical consequences. Endocr Rev (2009) 30(5):465–93. doi: 10.1210/er.2009-0006
21. Sills ES, Alper MM, Walsh AP. Ovarian reserve screening in infertility: practical applications and theoretical directions for research. Eur J Obstet Gynecol Reprod Biol (2009) 146(1):30–6. doi: 10.1016/j.ejogrb.2009.05.008
22. Coccia ME, Rizzello F. Ovarian reserve. Ann N Y Acad Sci (2008) 1127:27–30. doi: 10.1196/annals.1434.011
23. Nagamatsu G. Regulation of primordial follicle formation, dormancy, and activation in mice. J Reprod Dev (2021) 67(3):189–95. doi: 10.1262/jrd.2021-040
24. Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol (2011) 9(1):23. doi: 10.1186/1477-7827-9-23
25. Bancsi LF, Broekmans FJ, Mol BW, Habbema JD, te Velde ER. Performance of basal follicle-stimulating hormone in the prediction of poor ovarian response and failure to become pregnant after in vitro fertilization: A meta-analysis. Fertil Steril (2003) 79(5):1091–100. doi: 10.1016/S0015-0282(03)00078-5
26. von Wolff M, Roumet M, Stute P, Liebenthron J. Serum anti-mullerian hormone (AMH) concentration has limited prognostic value for density of primordial and primary follicles, questioning it as an accurate parameter for the ovarian reserve. Maturitas (2020) 134:34–40. doi: 10.1016/j.maturitas.2020.02.001
27. Weenen C, Laven JS, Von Bergh AR, Cranfield M, Groome NP, Visser JA, et al. Anti-müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod (2004) 10(2):77–83. doi: 10.1093/molehr/gah015
28. Łuczak J, Bagłaj M, Ciaputa R, Szymerowski A, Nowak M. Does open ovarian biopsy in prepubertal age affect ovarian reserve in a rat model? J Pediatr Surg (2021) 56(2):360–7. doi: 10.1016/j.jpedsurg.2020.05.046
29. Muzii L, Di Tucci C, Di Feliciantonio M, Marchetti C, Perniola G, Panici PB. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: a systematic review and meta-analysis. Hum Reprod (2014) 29(10):2190–8. doi: 10.1093/humrep/deu199
30. Muzii L, Di Tucci C, Di Feliciantonio M, Galati G, Di Donato V, Musella A, et al. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: a systematic review and meta-analysis. Fertil Steril (2018) 110(5):932–40.e1. doi: 10.1016/j.fertnstert.2018.06.025
31. Karadağ C, Yoldemir T, Demircan Karadağ S, Turgut A. The effects of endometrioma size and bilaterality on ovarian reserve. J Obstet Gynaecol (2020) 40(4):531–6. doi: 10.1080/01443615.2019.1633518
32. Rasoul NS, Al Allak MM. A prospective cohort study on laparoscopic cystectomy of endometrioma and its effects on ovarian reserve. J Pak Med Assoc (2021) 71(Suppl 9):S8–s11.
33. Coccia ME, Rizzello F, Capezzuoli T, Evangelisti P, Cozzi C, Petraglia F. Bilateral endometrioma excision: Surgery-related damage to ovarian reserve. Reprod Sci (2019) 26(4):543–50. doi: 10.1177/1933719118777640
34. Shaltout MF, Elsheikhah A, Maged AM, Elsherbini MM, Zaki SS, Dahab S, et al. A randomized controlled trial of a new technique for laparoscopic management of ovarian endometriosis preventing recurrence and keeping ovarian reserve. J Ovarian Res (2019) 12(1):66. doi: 10.1186/s13048-019-0542-0
35. Martinez-Garcia JM, Candas B, Suarez-Salvador E, Gomez M, Merino E, Castellarnau M, et al. Comparing the effects of alcohol sclerotherapy with those of surgery on anti-müllerian hormone and ovarian reserve after endometrioma treatment. a prospective multicenter pilot cohort study. Eur J Obstet Gynecol Reprod Biol (2021) 259:60–6. doi: 10.1016/j.ejogrb.2021.01.027
36. Park SJ, Seol A, Lee N, Lee S, Kim HS. A randomized controlled trial of ovarian reserve preservation and hemostasis during ovarian cystectomy. Sci Rep (2021) 11(1):8495. doi: 10.1038/s41598-021-87965-7
37. Kostrzewa M, Wilczyński JR, Głowacka E, Żyła M, Szyłło K, Stachowiak G. One-year follow-up of ovarian reserve by three methods in women after laparoscopic cystectomy for endometrioma and benign ovarian cysts. Int J Gynaecol Obstet (2019) 146(3):350–6. doi: 10.1002/ijgo.12884
38. Hsueh AJW, Kawamura K, Cheng Y, Fauser BCJM. Intraovarian control of early folliculogenesis. Endocrine Rev (2015) 36(1):1–24. doi: 10.1210/er.2014-1020
39. Matsuzaki S, Darcha C. Co-Operation between the AKT and ERK signaling pathways may support growth of deep endometriosis in a fibrotic microenvironment in vitro†. Hum Reprod (2015) 30(7):1606–16. doi: 10.1093/humrep/dev108
40. Song Y, Fu J, Zhou M, Xiao L, Feng X, Chen H, et al. Activated Hippo/Yes-associated protein pathway promotes cell proliferation and anti-apoptosis in endometrial stromal cells of endometriosis. J Clin Endocrinol Metab (2016) 101(4):1552–61. doi: 10.1210/jc.2016-1120
41. Takeuchi A, Koga K, Satake E, Makabe T, Taguchi A, Miyashita M, et al. Endometriosis triggers excessive activation of primordial follicles via PI3K-PTEN-Akt-Foxo3 pathway. J Clin Endocrinol Metab (2019) 104(11):5547–54. doi: 10.1210/jc.2019-00281
42. He Y, Peng X, Wu T, Yang W, Liu W, Zhang J, et al. Restricting the induction of NGF in ovarian stroma engenders selective follicular activation through the mTOR signaling pathway. Cell Death Dis (2017) 8(5):e2817–e. doi: 10.1038/cddis.2017.168
43. Grosbois J, Demeestere I. Dynamics of PI3K and hippo signaling pathways during in vitro human follicle activation. Hum Reprod (2018) 33(9):1705–14. doi: 10.1093/humrep/dey250
44. Monniaux D, Clément F, Dalbiès-Tran R, Estienne A, Fabre S, Mansanet C, et al. The ovarian reserve of primordial follicles and the dynamic reserve of antral growing follicles: What is the link? 1. Biol Reprod (2014) 90(4):85. doi: 10.1095/biolreprod.113.117077
45. Amanvermez R, Tosun M. An update on ovarian aging and ovarian reserve tests. Int J Fertil Steril (2016) 9(4):411–5. doi: 10.22074/ijfs.2015.4591
46. Kawamura K, Kawamura N, Hsueh AJW. Activation of dormant follicles: a new treatment for premature ovarian failure? Curr Opin Obstetrics Gyneco (2016) 28(3):217-22. doi: 10.1097/GCO.0000000000000268
47. Coulam CB, Adamson SC, Annegers JF. Incidence of premature ovarian failure. Obstet Gynecol (1986) 67(4):604–6.
48. Knauff EA, Eijkemans MJ, Lambalk CB, ten Kate-Booij MJ, Hoek A, Beerendonk CC, et al. Anti-mullerian hormone, inhibin b, and antral follicle count in young women with ovarian failure. J Clin Endocrinol Metab (2009) 94(3):786–92. doi: 10.1210/jc.2008-1818
49. Welt CK. Primary ovarian insufficiency: a more accurate term for premature ovarian failure. Clin Endocrinol (Oxf) (2008) 68(4):499–509. doi: 10.1111/j.1365-2265.2007.03073.x
50. Fauser BC, Van Heusden AM. Manipulation of human ovarian function: physiological concepts and clinical consequences. Endocr Rev (1997) 18(1):71–106. doi: 10.1210/edrv.18.1.0290
51. Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, et al. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Sci Transl Med (2013) 5(172):172ra21. doi: 10.1126/scitranslmed.3004925
52. Ye W, Shen W, Yan W, Zhou S, Cheng J, Pan G, et al. What changed on the folliculogenesis in the process of mouse ovarian aging? BioMed Res Int (2019) 2019:3842312. doi: 10.1155/2019/3842312
53. Kitajima M, Dolmans MM, Donnez O, Masuzaki H, Soares M, Donnez J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil Steril (2014) 101(4):1031–7. doi: 10.1016/j.fertnstert.2013.12.049
54. Schubert B, Canis M, Darcha C, Artonne C, Pouly J-L, Déchelotte P, et al. Human ovarian tissue from cortex surrounding benign cysts: a model to study ovarian tissue cryopreservation. Hum Reprod (2005) 20(7):1786–92. doi: 10.1093/humrep/dei002
55. Maidarti M, Anderson RA, Telfer EE. Crosstalk between PTEN/PI3K/Akt signalling and DNA damage in the oocyte: Implications for primordial follicle activation, oocyte quality and ageing. Cells (2020) 9(1):200. doi: 10.3390/cells9010200
56. Ye H, Li X, Zheng T, Hu C, Pan Z, Huang J, et al. The hippo signaling pathway regulates ovarian function via the proliferation of ovarian germline stem cells. Cell Physiol Biochem (2017) 41(3):1051–62. doi: 10.1159/000464113
57. Li J, Zhou F, Zheng T, Pan Z, Liang X, Huang J, et al. Ovarian germline stem cells (OGSCs) and the hippo signaling pathway association with physiological and pathological ovarian aging in mice. Cell Physiol Biochem (2015) 36(5):1712–24. doi: 10.1159/000430144
58. Henderson NC, Rieder F, Wynn TA. Fibrosis: from mechanisms to medicines. Nature (2020) 587(7835):555–66. doi: 10.1038/s41586-020-2938-9
59. Marcelin G, Silveira ALM, Martins LB, Ferreira AV, Clément K. Deciphering the cellular interplays underlying obesity-induced adipose tissue fibrosis. J Clin Invest (2019) 129(10):4032–40. doi: 10.1172/JCI129192
60. Heukels P, Moor CC, von der Thüsen JH, Wijsenbeek MS, Kool M. Inflammation and immunity in IPF pathogenesis and treatment. Respir Med (2019) 147:79–91. doi: 10.1016/j.rmed.2018.12.015
61. Vasse GF, Nizamoglu M, Heijink IH, Schlepütz M, van Rijn P, Thomas MJ, et al. Macrophage-stroma interactions in fibrosis: biochemical, biophysical, and cellular perspectives. J Pathol (2021) 254(4):344–57. doi: 10.1002/path.5632
62. McCloskey CW, Cook DP, Kelly BS, Azzi F, Allen CH, Forsyth A, et al. Metformin abrogates age-associated ovarian fibrosis. Clin Cancer Res (2020) 26(3):632–42. doi: 10.1158/1078-0432.CCR-19-0603
63. Amargant F, Manuel SL, Tu Q, Parkes WS, Rivas F, Zhou LT, et al. Ovarian stiffness increases with age in the mammalian ovary and depends on collagen and hyaluronan matrices. Aging Cell (2020) 19(11):e13259. doi: 10.1111/acel.13259
64. Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, et al. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reprod (2016) 152(3):245–60. doi: 10.1530/REP-16-0129
65. Mara JN, Zhou LT, Larmore M, Johnson B, Ayiku R, Amargant F, et al. Ovulation and ovarian wound healing are impaired with advanced reproductive age. Aging (Albany NY) (2020) 12(10):9686–713. doi: 10.18632/aging.103237
66. Umehara T, Kawai T, Kawashima I, Tanaka K, Okuda S, Kitasaka H, et al. The acceleration of reproductive aging in Nrg1(flox/flox) ;Cyp19-cre female mice. Aging Cell (2017) 16(6):1288–99. doi: 10.1111/acel.12662
67. Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, et al. Single-cell transcriptomic atlas of primate ovarian aging. Cell (2020) 180(3):585–600.e19. doi: 10.1016/j.cell.2020.01.009
68. Umehara T, Winstanley YE, Andreas E, Morimoto A, Williams EJ, Smith KM, et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci Adv (2022) 8(24):eabn4564. doi: 10.1126/sciadv.abn4564
69. Matsuzaki S, Canis M, Darcha C, Dechelotte P, Pouly JL, Bruhat MA. Fibrogenesis in peritoneal endometriosis. a semi-quantitative analysis of type-I collagen. Gynecol Obstet Invest (1999) 47(3):197–9. doi: 10.1159/000010094
70. Liu X, Zhang Q, Guo S-W. Histological and immunohistochemical characterization of the similarity and difference between ovarian endometriomas and deep infiltrating endometriosis. Reprod Sci (2018) 25(3):329–40. doi: 10.1177/1933719117718275
71. Khare VK, Martin DC, Eltorky M. A comparative study of ovarian and pelvic wall-infiltrating endometriosis. J Am Assoc Gynecol Laparosc (1996) 3(2):235–9. doi: 10.1016/S1074-3804(96)80006-5
72. Anaf V, Simon P, Fayt I, Noel J. Smooth muscles are frequent components of endometriotic lesions. Hum Reprod (2000) 15(4):767–71. doi: 10.1093/humrep/15.4.767
73. Zhang Q, Duan J, Liu X, Guo SW. Platelets drive smooth muscle metaplasia and fibrogenesis in endometriosis through epithelial-mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation. Mol Cell Endocrinol (2016) 428:1–16. doi: 10.1016/j.mce.2016.03.015
74. Matsuzaki S, Darcha C, Pouly JL, Canis M. Effects of matrix stiffness on epithelial to mesenchymal transition-like processes of endometrial epithelial cells: Implications for the pathogenesis of endometriosis. Sci Rep (2017) 7:44616. doi: 10.1038/srep44616
75. Wells RG. Tissue mechanics and fibrosis. Biochimica et Biophysica Acta (BBA) - molecular basis of disease. Biochimica et Biophysica Acta (2013) 1832(7):884–90. doi: 10.1016/j.bbadis.2013.02.007
76. Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest (2009) 119(6):1420–8. doi: 10.1172/JCI39104
77. Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol (2014) 15(3):178–96. doi: 10.1038/nrm3758
78. Omwandho CO, Konrad L, Halis G, Oehmke F, Tinneberg HR. Role of TGF-betas in normal human endometrium and endometriosis. Hum Reprod (2010) 25(1):101–9. doi: 10.1093/humrep/dep382
79. Somigliana E, Berlanda N, Benaglia L, Viganò P, Vercellini P, Fedele L. Surgical excision of endometriomas and ovarian reserve: a systematic review on serum antimüllerian hormone level modifications. Fertil Steril (2012) 98(6):1531–8. doi: 10.1016/j.fertnstert.2012.08.009
80. Yu FX, Zhao B, Guan KL. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell (2015) 163(4):811–28. doi: 10.1016/j.cell.2015.10.044
81. Mohamed AA, Al-Hussaini TK, Fathalla MM, El Shamy TT, Abdelaal II, Amer SA. The impact of excision of benign nonendometriotic ovarian cysts on ovarian reserve: A systematic review. Am J Obstet Gynecol (2016) 215(2):169–76. doi: 10.1016/j.ajog.2016.03.045
82. Throm Quinlan AM, Sierad LN, Capulli AK, Firstenberg LE, Billiar KL. Combining dynamic stretch and tunable stiffness to probe cell mechanobiology in vitro. PLoS One (2011) 6(8):e23272. doi: 10.1371/journal.pone.0023272
83. Hu LL, Su T, Luo RC, Zheng YH, Huang J, Zhong ZS, et al. Hippo pathway functions as a downstream effector of AKT signaling to regulate the activation of primordial follicles in mice. J Cell Physiol (2019) 234(2):1578–87. doi: 10.1002/jcp.27024
84. Chen CC, Jeon SM, Bhaskar PT, Nogueira V, Sundararajan D, Tonic I, et al. FoxOs inhibit mTORC1 and activate akt by inducing the expression of Sestrin3 and rictor. Dev Cell (2010) 18(4):592–604. doi: 10.1016/j.devcel.2010.03.008
85. Borreguero-Muñoz N, Fletcher GC, Aguilar-Aragon M, Elbediwy A, Vincent-Mistiaen ZI, Thompson BJ. The hippo pathway integrates PI3K-akt signals with mechanical and polarity cues to control tissue growth. PLoS Biol (2019) 17(10):e3000509. doi: 10.1371/journal.pbio.3000509
86. Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: from pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update (2013) 20(2):217–30. doi: 10.1093/humupd/dmt053
87. Entezari S, Haghi SM, Norouzkhani N, Sahebnazar B, Vosoughian F, Akbarzadeh D, et al. Iron chelators in treatment of iron overload. J Toxicol (2022) 2022:4911205. doi: 10.1155/2022/4911205
88. Matsuzaki S, Schubert B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil Steril (2010) 93(7):2431–2. doi: 10.1016/j.fertnstert.2009.08.068
89. Koundouros N, Poulogiannis G. Phosphoinositide 3-Kinase/Akt signaling and redox metabolism in cancer. Front Oncol (2018) 8:160. doi: 10.3389/fonc.2018.00160
90. Otoupalova E, Smith S, Cheng G, Thannickal VJ. Oxidative stress in pulmonary fibrosis. Compr Physiol (2020) 10(2):509–47. doi: 10.1002/cphy.c190017
91. Yang L, Chen Y, Liu Y, Xing Y, Miao C, Zhao Y, et al. The role of oxidative stress and natural antioxidants in ovarian aging. Front Pharmacol (2020) 11:617843. doi: 10.3389/fphar.2020.617843
92. Wang L, Tang C, Cao H, Li K, Pang X, Zhong L, et al. Activation of IL-8 via PI3K/Akt-dependent pathway is involved in leptin-mediated epithelial-mesenchymal transition in human breast cancer cells. Cancer Biol Ther (2015) 16(8):1220–30. doi: 10.1080/15384047.2015.1056409
93. Fasciani A, D'Ambrogio G, Bocci G, Monti M, Genazzani AR, Artini PG. High concentrations of the vascular endothelial growth factor and interleukin-8 in ovarian endometriomata. Mol Hum Reprod (2000) 6(1):50–4. doi: 10.1093/molehr/6.1.50
94. Shi LB, Zhou F, Zhu HY, Huang D, Jin XY, Li C, et al. Transforming growth factor beta1 from endometriomas promotes fibrosis in surrounding ovarian tissues via Smad2/3 signaling†. Biol Reprod (2017) 97(6):873–82. doi: 10.1093/biolre/iox140
95. Carmona F, Chapron C, Martínez-Zamora M-Á, Santulli P, Rabanal A, Martínez-Florensa M, et al. Ovarian endometrioma but not deep infiltrating endometriosis is associated with increased serum levels of interleukin-8 and interleukin-6. J Reprod Immunol (2012) 95(1):80–6. doi: 10.1016/j.jri.2012.06.001
96. Mikhaleva LM, Davydov AI, Patsap OI, Mikhaylenko EV, Nikolenko VN, Neganova ME, et al. Malignant transformation and associated biomarkers of ovarian endometriosis: A narrative review. Adv Ther (2020) 37(6):2580–603. doi: 10.1007/s12325-020-01363-5
97. Passos JRS, Costa JJN, da Cunha EV, Silva AWB, Ribeiro RP, de Souza GB, et al. Protein and messenger RNA expression of interleukin 1 system members in bovine ovarian follicles and effects of interleukin 1β on primordial follicle activation and survival in vitro. Domest Anim Endocrino (2016) 54:48–59. doi: 10.1016/j.domaniend.2015.09.002
98. Feeney A, Nilsson E, Skinner MK. Cytokine (IL16) and tyrphostin actions on ovarian primordial follicle development. Reprod (2014) 148(3):321–31. doi: 10.1530/REP-14-0246
99. Lliberos C, Liew SH, Mansell A, Hutt KJ. The inflammasome contributes to depletion of the ovarian reserve during aging in mice. Front Cell Dev Biol (2020) 8:628473. doi: 10.3389/fcell.2020.628473
100. Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen (2017) 58(5):235–63. doi: 10.1002/em.22087
101. Ward LD, Parker MM, Deaton AM, Tu HC, Flynn-Carroll AO, Hinkle G, et al. Rare coding variants in DNA damage repair genes associated with timing of natural menopause. HGG Adv (2022) 3(2):100079. doi: 10.1016/j.xhgg.2021.100079
102. Katari S, Aarabi M, Kintigh A, Mann S, Yatsenko SA, Sanfilippo JS, et al. Chromosomal instability in women with primary ovarian insufficiency. Hum Reprod (2018) 33(3):531–8. doi: 10.1093/humrep/dey012
103. Zhai Y, Tye BK. Structure of the MCM2-7 double hexamer and its implications for the mechanistic functions of the Mcm2-7 complex. Adv Exp Med Biol (2017) 1042:189–205. doi: 10.1007/978-981-10-6955-0_9
104. Lutzmann M, Grey C, Traver S, Ganier O, Maya-Mendoza A, Ranisavljevic N, et al. MCM8- and MCM9-deficient mice reveal gametogenesis defects and genome instability due to impaired homologous recombination. Mol Cell (2012) 47(4):523–34. doi: 10.1016/j.molcel.2012.05.048
105. AlAsiri S, Basit S, Wood-Trageser MA, Yatsenko SA, Jeffries EP, Surti U, et al. Exome sequencing reveals MCM8 mutation underlies ovarian failure and chromosomal instability. J Clin Invest (2015) 125(1):258–62. doi: 10.1172/JCI78473
106. Dou X, Guo T, Li G, Zhou L, Qin Y, Chen ZJ. Minichromosome maintenance complex component 8 mutations cause primary ovarian insufficiency. Fertil Steril (2016) 106(6):1485–9.e2. doi: 10.1016/j.fertnstert.2016.08.018
107. Bouali N, Francou B, Bouligand J, Imanci D, Dimassi S, Tosca L, et al. New MCM8 mutation associated with premature ovarian insufficiency and chromosomal instability in a highly consanguineous Tunisian family. Fertil Steril (2017) 108(4):694–702. doi: 10.1016/j.fertnstert.2017.07.015
108. Tenenbaum-Rakover Y, Weinberg-Shukron A, Renbaum P, Lobel O, Eideh H, Gulsuner S, et al. Minichromosome maintenance complex component 8 (MCM8) gene mutations result in primary gonadal failure. J Med Genet (2015) 52(6):391–9. doi: 10.1136/jmedgenet-2014-102921
109. Wood-Trageser MA, Gurbuz F, Yatsenko SA, Jeffries EP, Kotan LD, Surti U, et al. MCM9 mutations are associated with ovarian failure, short stature, and chromosomal instability. Am J Hum Genet (2014) 95(6):754–62. doi: 10.1016/j.ajhg.2014.11.002
110. Fauchereau F, Shalev S, Chervinsky E, Beck-Fruchter R, Legois B, Fellous M, et al. A non-sense MCM9 mutation in a familial case of primary ovarian insufficiency. Clin Genet (2016) 89(5):603–7. doi: 10.1111/cge.12736
111. Goldberg Y, Halpern N, Hubert A, Adler SN, Cohen S, Plesser-Duvdevani M, et al. Mutated MCM9 is associated with predisposition to hereditary mixed polyposis and colorectal cancer in addition to primary ovarian failure. Cancer Genet (2015) 208(12):621–4. doi: 10.1016/j.cancergen.2015.10.001
112. Wang J, Zhang W, Jiang H, Wu BL. Mutations in HFM1 in recessive primary ovarian insufficiency. N Engl J Med (2014) 370(10):972–4. doi: 10.1056/NEJMc1310150
113. Pu D, Wang C, Cao J, Shen Y, Jiang H, Liu J, et al. Association analysis between HFM1 variation and primary ovarian insufficiency in Chinese women. Clin Genet (2016) 89(5):597–602. doi: 10.1111/cge.12718
114. Zhang W, Song X, Ni F, Cheng J, Wu B-L, Jiang H. Association analysis between HFM1 variations and idiopathic azoospermia or severe oligozoospermia in Chinese men. Sci China Life Sci (2017) 60(3):315–8. doi: 10.1007/s11427-016-0274-9
115. Panier S, Boulton SJ. Double-strand break repair: 53BP1 comes into focus. Nat Rev Mol Cell Biol (2014) 15(1):7–18. doi: 10.1038/nrm3719
116. Oktay K, Turan V, Titus S, Stobezki R, Liu L. BRCA mutations, DNA Repair Deficiency, and ovarian aging. Biol Reprod (2015) 93(3):67. doi: 10.1095/biolreprod.115.132290
117. Henner WD, Rodriguez LO, Hecht SM, Haseltine WA. Gamma ray induced deoxyribonucleic acid strand breaks. 3' glycolate termini. J Biol Chem (1983) 258(2):711–3. doi: 10.1016/S0021-9258(18)33104-1
118. Choi YS, Park JH, Lee JH, Yoon JK, Yun BH, Park JH, et al. Association between impairment of DNA double strand break repair and decreased ovarian reserve in patients with endometriosis. Front Endocrinol (Lausanne) (2018) 9:772. doi: 10.3389/fendo.2018.00772
119. Collins JK, Lane SIR, Merriman JA, Jones KT. DNA Damage induces a meiotic arrest in mouse oocytes mediated by the spindle assembly checkpoint. Nat Commun (2015) 6(1):8553. doi: 10.1038/ncomms9553
120. Pailas A, Niaka K, Zorzompokou C, Marangos P. The DNA damage response in fully grown mammalian oocytes. Cells (2022) 11(5):798. doi: 10.3390/cells11050798
121. Hamdan M, Jones KT, Cheong Y, Lane SI. The sensitivity of the DNA damage checkpoint prevents oocyte maturation in endometriosis. Sci Rep (2016) 6:36994. doi: 10.1038/srep36994
122. Bane K, Desouza J, Shetty D, Choudhary P, Kadam S, Katkam RR, et al. Endometrial DNA damage response is modulated in endometriosis. Hum Reprod (2021) 36(1):160–74. doi: 10.1093/humrep/deaa255
124. Shimizu T, Hoshino Y, Miyazaki H, Sato E. Angiogenesis and microvasculature in the female reproductive organs: physiological and pathological implications. Curr Pharm Des (2012) 18(3):303–9. doi: 10.2174/138161212799040367
125. Chung MS, Han SJ. Endometriosis-associated angiogenesis and anti-angiogenic therapy for endometriosis. Front Glob Womens Health (2022) 3:856316. doi: 10.3389/fgwh.2022.856316
126. Goel S, Duda DG, Xu L, Munn LL, Boucher Y, Fukumura D, et al. Normalization of the vasculature for treatment of cancer and other diseases. Physiol Rev (2011) 91(3):1071–121. doi: 10.1152/physrev.00038.2010
127. Laschke MW, Menger MD. Basic mechanisms of vascularization in endometriosis and their clinical implications. Hum Reprod Update (2018) 24(2):207–24. doi: 10.1093/humupd/dmy001
128. Filippi I, Carrarelli P, Luisi S, Batteux F, Chapron C, Naldini A, et al. Different expression of hypoxic and angiogenic factors in human endometriotic lesions. Reprod Sci (2016) 23(4):492–7. doi: 10.1177/1933719115607978
129. Ozer H, Boztosun A, Açmaz G, Atilgan R, Akkar OB, Kosar MI. The efficacy of bevacizumab, sorafenib, and retinoic acid on rat endometriosis model. Reprod Sci (2013) 20(1):26–32. doi: 10.1177/1933719112452941
130. Xu X, Mu L, Li L, Liang J, Zhang S, Jia L, et al. Imaging and tracing the pattern of adult ovarian angiogenesis implies a strategy against female reproductive aging. Sci Adv (2022) 8(2):eabi8683. doi: 10.1126/sciadv.abi8683
131. Stolk L, Zhai G, van Meurs JBJ, Verbiest MMPJ, Visser JA, Estrada K, et al. Loci at chromosomes 13, 19 and 20 influence age at natural menopause. Nat Genet (2009) 41(6):645–7. doi: 10.1038/ng.387
132. Cloke B, Rymer J. Premature ovarian insufficiency - the need for a genomic map. Climacteric (2021) 24(5):444–52. doi: 10.1080/13697137.2021.1945025
133. Governini L, Carrarelli P, Rocha AL, Leo VD, Luddi A, Arcuri F, et al. FOXL2 in human endometrium: hyperexpressed in endometriosis. Reprod Sci (2014) 21(10):1249–55. doi: 10.1177/1933719114522549
134. De Conto E, Matte Ú, Bilibio JP, Genro VK, Souza CA, Leão DP, et al. Endometriosis-associated infertility: GDF-9, AMH, and AMHR2 genes polymorphisms. J Assist Reprod Genet (2017) 34(12):1667–72. doi: 10.1007/s10815-017-1026-z
135. Shi L, Wei X, Wu B, Yuan C, Li C, Dai Y, et al. Molecular signatures correlated with poor IVF outcomes: Insights from the mRNA and lncRNA expression of endometriotic granulosa cells. Front Endocrinol (Lausanne) (2022) 13:825934. doi: 10.3389/fendo.2022.825934
136. Wiweko B, Iffanolida PA, Rectifa Z, Muna N, Mutia K, Febri RR, et al. BMP15 mRNA profile in granulosa cells from endometriosis patients undergoing in vitro fertilization. J Physics: Conf Ser (2018) 1073:032052. doi: 10.1088/1742-6596/1073/3/032052
137. Leone Roberti Maggiore U, Scala C, Venturini PL, Remorgida V, Ferrero S. Endometriotic ovarian cysts do not negatively affect the rate of spontaneous ovulation. Hum Reprod (2015) 30(2):299–307. doi: 10.1093/humrep/deu308
138. Hart R, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomata: a cochrane review. Hum Reprod (2005) 20(11):3000–7. doi: 10.1093/humrep/dei207
139. Rolla E. Endometriosis: advances and controversies in classification, pathogenesis, diagnosis, and treatment. F1000Res (2019) 8:529. doi: 10.12688/f1000research.14817.1
140. Komatsu K, Masubuchi S. Increased supply from blood vessels promotes the activation of dormant primordial follicles in mouse ovaries. J Reprod Dev (2020) 66(2):105–13. doi: 10.1262/jrd.2019-091
141. Kim TN, Chung MK, Nam JK, Lee JZ, Chung JH, Lee SW. Effectiveness of hyaluronic acid/carboxymethylcellulose in preventing adhesive bowel obstruction after laparoscopic radical cystectomy. Asian J Surg (2019) 42(1):394–400. doi: 10.1016/j.asjsur.2018.08.007
142. Hendriks ML, van der Valk P, Lambalk CB, Broeckaert MA, Homburg R, Hompes PG. Extensive tissue damage of bovine ovaries after bipolar ovarian drilling compared to monopolar electrocoagulation or carbon dioxide laser. Fertil Steril (2010) 93(3):969–75. doi: 10.1016/j.fertnstert.2008.10.046
143. Somigliana E, Benaglia L, Vigano P, Candiani M, Vercellini P, Fedele L. Surgical measures for endometriosis-related infertility: A plea for research. Placenta (2011) 32 Suppl 3:S238–42. doi: 10.1016/j.placenta.2011.06.011
144. Horikawa T, Nakagawa K, Ohgi S, Kojima R, Nakashima A, Ito M, et al. The frequency of ovulation from the affected ovary decreases following laparoscopic cystectomy in infertile women with unilateral endometrioma during a natural cycle. J Assist Reprod Genet (2008) 25(6):239–44. doi: 10.1007/s10815-008-9229-y
145. Uncu G, Kasapoglu I, Ozerkan K, Seyhan A, Oral Yilmaztepe A, Ata B. Prospective assessment of the impact of endometriomas and their removal on ovarian reserve and determinants of the rate of decline in ovarian reserve. Hum Reprod (2013) 28(8):2140–5. doi: 10.1093/humrep/det123
146. Demirol A, Guven S, Baykal C, Gurgan T. Effect of endometrioma cystectomy on IVF outcome: a prospective randomized study. Reprod BioMed Online (2006) 12(5):639–43. doi: 10.1016/S1472-6483(10)61192-3
147. Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: a systematic review and meta-analysis. J Clin Endocrinol Metab (2012) 97(9):3146–54. doi: 10.1210/jc.2012-1558
148. Liu J, Wu DC, Qu LH, Liao HQ, Li MX. The role of mTOR in ovarian neoplasms, polycystic ovary syndrome, and ovarian aging. Clin Anat (2018) 31(6):891–8. doi: 10.1002/ca.23211
149. Uri-Belapolsky S, Shaish A, Eliyahu E, Grossman H, Levi M, Chuderland D, et al. Interleukin-1 deficiency prolongs ovarian lifespan in mice. Proc Natl Acad Sci USA (2014) 111(34):12492–7. doi: 10.1073/pnas.1323955111
150. Bromfield JJ, Sheldon IM. Lipopolysaccharide reduces the primordial follicle pool in the bovine ovarian cortex ex vivo and in the murine ovary In Vivo1. Biol Reprod (2013) 88(4):98. doi: 10.1095/biolreprod.112.106914
151. Cheng Y, Feng Y, Jansson L, Sato Y, Deguchi M, Kawamura K, et al. Actin polymerization-enhancing drugs promote ovarian follicle growth mediated by the hippo signaling effector YAP. FASEB J (2015) 29(6):2423–30. doi: 10.1096/fj.14-267856
152. Laschke MW, Elitzsch A, Scheuer C, Holstein JH, Vollmar B, Menger MD. Rapamycin induces regression of endometriotic lesions by inhibiting neovascularization and cell proliferation. Br J Pharmacol (2006) 149(2):137–44. doi: 10.1038/sj.bjp.0706857
153. Venkata PP, Chen Y, Alejo S, He Y, Palacios BE, Loeffel I, et al. KDM1A inhibition augments the efficacy of rapamycin for the treatment of endometrial cancer. Cancer Letters (2022) 524:219–31. doi: 10.1016/j.canlet.2021.10.019
154. Ren XU, Wang Y, Xu G, Dai L. Effect of rapamycin on endometriosis in mice. Exp Ther Med (2016) 12(1):101–6. doi: 10.3892/etm.2016.3280
155. El-Halwagy A, Elgergawi A, Dawood A, Dawood A. Reduction of postoperative adhesions after laparoscopic surgery for endometriosis by using a novel anti-fibrotic drug pirfenidone: A randomized double blind study. Gynecology Obstetrics (sunnyvale) (2017) 07:422. doi: 10.4172/2161-0932.1000422
156. Lin Y, Chen Z, Huang J, Fang J, Qi Y, Xie L, et al. Computational drug discovery in patients with endometriosis-induced infertility via text mining and biomedical databases. Clin Exp Obstet Gynecol (2022) 49(8):168. doi: 10.31083/j.ceog4908168
157. Nakamura K, Oosawa M, Kondou I, Inagaki S, Shibata H, Narita O, et al. Menotropin stimulation after prolonged gonadotropin releasing hormone agonist pretreatment for in vitro fertilization in patients with endometriosis. J Assist Reprod Genet (1992) 9(2):113–7. doi: 10.1007/BF01203749
158. Koninckx PR, Craessaerts M, Timmerman D, Cornillie F, Kennedy S. Anti-TNF-alpha treatment for deep endometriosis-associated pain: a randomized placebo-controlled trial. Hum Reprod (2008) 23(9):2017–23. doi: 10.1093/humrep/den177
159. Arif. JCLH. Pregnancy medications StatPearls. Treasure Island (FL: StatPearls (2022). Available at: https://www.ncbi.nlm.nih.gov/books/NBK507858/.
160. Wang H, Jo YJ, Oh JS, Kim NH. Quercetin delays postovulatory aging of mouse oocytes by regulating SIRT expression and MPF activity. Oncotarget (2017) 8(24):38631–41. doi: 10.18632/oncotarget.16219
161. Keshavarzi S, MacDougall M, Lulic D, Kasasbeh A, Levy M. Clinical experience with the surgicel family of absorbable hemostats (oxidized regenerated cellulose) in neurosurgical applications: a review. Wounds (2013) 25(6):160–7.
162. Song T, Lee SH, Kim WY. Additional benefit of hemostatic sealant in preservation of ovarian reserve during laparoscopic ovarian cystectomy: a multi-center, randomized controlled trial. Hum Reprod (2014) 29(8):1659–65. doi: 10.1093/humrep/deu125
163. Chung J, Law T, Chung C, Mak J, Sahota DS, Li TC. Impact of haemostatic sealant versus electrocoagulation on ovarian reserve after laparoscopic ovarian cystectomy of ovarian endometriomas: a randomised controlled trial. Bjog (2019) 126(10):1267–75. doi: 10.1111/1471-0528.15807
164. Chung JPW, Law TSM, Mak JSM, Sahota DS, Li TC. Ovarian reserve and recurrence 1 year post-operatively after using haemostatic sealant and bipolar diathermy for haemostasis during laparoscopic ovarian cystectomy. Reprod BioMed Online (2021) 43(2):310–8. doi: 10.1016/j.rbmo.2021.05.003
165. Alborzi S, Poordast T, Askary E, Chamanara K, Zahiri Sorouri Z, Hosseini Najar Kellaii E, et al. The effect of vasopressin injection on ovarian reserve in patients with ovarian endometrioma: a randomized controlled trial. Reprod BioMed Online (2022) 44(4):651–8. doi: 10.1016/j.rbmo.2021.11.024
166. Xin L, Ye M, Yang L, Chen L, Wang L, Hou Q. The effects of vasopressin injection technique on ovarian reserve in laparoscopic cystectomy of bilateral ovarian endometrioma: a retrospective cohort study. Am J Transl Res (2022) 14(4):2343–9.
167. Park EY, Hwang KH, Kim JH, Lee SH, Park KS, Choi SJ, et al. Epinephrine minimizes the use of bipolar coagulation and preserves ovarian reserve in laparoscopic ovarian cystectomy: a randomized controlled trial. Sci Rep (2020) 10(1):20911. doi: 10.1038/s41598-020-77781-w
168. Muraoka A, Osuka S, Yabuki A, Bayasula, Yoshihara M, Tanaka H, et al. Impact of perioperative use of GnRH agonist or dienogest on ovarian reserve after cystectomy for endometriomas: a randomized controlled trial. Reprod Biol Endocrinol (2021) 19(1):179. doi: 10.1186/s12958-021-00866-2
169. Holoch KJ, Lessey BA. Endometriosis and infertility. Clin Obstet Gynecol (2010) 53(2):429–38. doi: 10.1097/GRF.0b013e3181db7d71
170. Young VJ, Brown JK, Saunders PT, Horne AW. The role of the peritoneum in the pathogenesis of endometriosis. Hum Reprod Update (2013) 19(5):558–69. doi: 10.1093/humupd/dmt024
171. Gupta S, Agarwal A, Agarwal R, Loret de Mola JR. Impact of ovarian endometrioma on assisted reproduction outcomes. Reprod BioMed Online (2006) 13(3):349–60. doi: 10.1016/S1472-6483(10)61439-3
172. Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction (2002) 123(2):217–26. doi: 10.1530/rep.0.1230217
173. Hamdan M, Dunselman G, Li TC, Cheong Y. The impact of endometrioma on IVF/ICSI outcomes: a systematic review and meta-analysis. Hum Reprod Update (2015) 21(6):809–25. doi: 10.1093/humupd/dmv035
174. Park HJ, Kim H, Lee GH, Yoon TK, Lee WS. Could surgical management improve the IVF outcomes in infertile women with endometrioma?: a review. Obstet Gynecol Sci (2019) 62(1):1–10. doi: 10.5468/ogs.2019.62.1.1
175. Pabuccu R, Onalan G, Kaya C. GnRH agonist and antagonist protocols for stage I-II endometriosis and endometrioma in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril (2007) 88(4):832–9. doi: 10.1016/j.fertnstert.2006.12.046
176. Drakopoulos P, Rosetti J, Pluchino N, Blockeel C, Santos-Ribeiro S, de Brucker M, et al. Does the type of GnRH analogue used, affect live birth rates in women with endometriosis undergoing IVF/ICSI treatment, according to the rAFS stage? Gynecol Endocrinol (2018) 34(10):884–9. doi: 10.1080/09513590.2018.1460346
177. Nawroth F, von Wolff M. Networks for fertility preservation. In: von Wolff M, Nawroth F, editors. Fertility preservation in oncological and non-oncological diseases: A practical guide. Cham: Springer International Publishing (2020). p. 7–9.
178. Anderson RA, Amant F, Braat D, D'Angelo A, Chuva de Sousa Lopes SM, Demeestere I, et al. ESHRE guideline: female fertility preservation. Hum Reprod Open (2020) 2020(4):hoaa052. doi: 10.1093/hropen/hoaa052
179. Cobo A, Giles J, Paolelli S, Pellicer A, Remohí J, García-Velasco JA. Oocyte vitrification for fertility preservation in women with endometriosis: an observational study. Fertil Steril (2020) 113(4):836–44. doi: 10.1016/j.fertnstert.2019.11.017
180. De Vos M, Smitz J, Woodruff TK. Fertility preservation in women with cancer. Lancet (2014) 384(9950):1302–10. doi: 10.1016/S0140-6736(14)60834-5
181. Elizur SE, Chian RC, Holzer HE, Gidoni Y, Tulandi T, Tan SL. Cryopreservation of oocytes in a young woman with severe and symptomatic endometriosis: a new indication for fertility preservation. Fertil Steril (2009) 91(1):293.e1–3. doi: 10.1016/j.fertnstert.2007.06.040
182. Donnez J, Squifflet J, Dolmans MM, Martinez-Madrid B, Jadoul P, Van Langendonckt A. Orthotopic transplantation of fresh ovarian cortex: a report of two cases. Fertil Steril (2005) 84(4):1018. doi: 10.1016/j.fertnstert.2005.06.011
183. Raad J, Sonigo C, Tran C, Sifer C, Durnerin IC, Grynberg M. Oocyte vitrification for preserving fertility in patients with endometriosis: first observational cohort study… and many unresolved questions. letter to the Editor. Eur J Obstet Gynecol Reprod Biol (2018) 220:140–1. doi: 10.1016/j.ejogrb.2017.12.001
184. Hong YH, Lee HK, Kim SK, Lee JR, Suh CS. The significance of planned fertility preservation for women with endometrioma before an expected ovarian cystectomy. Front Endocrinol (Lausanne) (2021) 12:794117. doi: 10.3389/fendo.2021.794117
185. Lessey BA, Gordts S, Donnez O, Somigliana E, Chapron C, Garcia-Velasco JA, et al. Ovarian endometriosis and infertility: in vitro fertilization (IVF) or surgery as the first approach? Fertil Steril (2018) 110(7):1218–26. doi: 10.1016/j.fertnstert.2018.10.003
Keywords: endometriosis, ovarian preservation, premature ovarian insufficiency, treatment, target therapy
Citation: Tan Z, Gong X, Li Y, Hung SW, Huang J, Wang CC and Chung JPW (2023) Impacts of endometrioma on ovarian aging from basic science to clinical management. Front. Endocrinol. 13:1073261. doi: 10.3389/fendo.2022.1073261
Received: 18 October 2022; Accepted: 08 December 2022;
Published: 04 January 2023.
Edited by:
Xiangrong Cui, Affiliated of Shanxi Medical University, ChinaReviewed by:
Ruining Liang, Jiangxi University of Traditional Chinese Medicine, ChinaTsung-Hsuan Lai, Fu Jen Catholic University, Taiwan
Copyright © 2023 Tan, Gong, Li, Hung, Huang, Wang and Chung. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi Chiu Wang, Y2N3YW5nQGN1aGsuZWR1Lmhr; Jacqueline Pui Wah Chung, amFjcXVlbGluZWNodW5nQGN1aGsuZWR1Lmhr
 Zhouyurong Tan
Zhouyurong Tan Xue Gong1
Xue Gong1 Yiran Li
Yiran Li Sze Wan Hung
Sze Wan Hung Jin Huang
Jin Huang Chi Chiu Wang
Chi Chiu Wang Jacqueline Pui Wah Chung
Jacqueline Pui Wah Chung