- 1The Diabetes, Endocrine and Metabolism Pediatric Unit, Pediatric Department (DEMPU), Cairo University, Cairo, Egypt
- 2Department of Clinical and Chemical Pathology, Cairo University, Cairo, Egypt
Background: Differences of sex development (DSD) are congenital conditions linked to atypical development of chromosomal, gonadal, or anatomical sex.
Objective: The aim of this study was to demonstrate our experiences at the Diabetes Endocrine and Metabolism Pediatric Unit (DEMPU), Faculty of Medicine, Cairo University in the field of DSD by focusing on the clinical presentation, laboratory profile, classification, and etiological diagnosis of these conditions. In addition, the present study intended to delineate the importance of serum anti-Müllerian hormone (AMH) and inhibin B in detecting the presence of functioning testicular tissue.
Methods: This cohort study included 451 infants and children with various clinical presentations of DSD. The study performed a retrospective analysis on medical records of established DSD cases to evaluate the clinical importance of AMH and inhibin B. In addition, newly diagnosed patients were prospectively analyzed.
Results: Three hundred thirty-six (74.5%) patients were 46,XY DSD, 98 (21.7%) were 46,XX DSD, 14 patients had other karyotypes and 3 had missing karyotypes. Among the 46XY DSD patients, the most common cause was partial androgen insensitivity. In contrast, congenital adrenal hyperplasia constituted the most common diagnosis in 46,XX DSD cases. The cut off value of serum AMH was 14.5 ng/ml with 100% sensitivity and 55.1% specificity.
Conclusion: Partial androgen insensitivity was the most important cause of 46,XY DSD in Egyptian children, and congenital adrenal hyperplasia was the most common cause of 46,XX DSD. AMH was valuable in detecting functioning testicular tissue.
1 Introduction
Differences of sex development (DSD) area group of congenital conditions linked to atypical development of chromosomal, gonadal, or anatomical sex (1). DSD is associated with many clinical presentations, the most common of which is atypical genitalia (2). The global incidence of DSD is nearly 1 in 4500 to 5500 (3), while its incidence in Egypt has been estimated to be 1 in 3,000 live births (4). The most common cause of atypical genitalia is congenital adrenal hyperplasia (2), followed by androgen insensitivity (5).
Meticulous history, detailed physical examination, and biochemical investigations, including karyotyping, radiological, and genetic testing, are important diagnostic processes to determine the etiology of DSD, subsequently allowing the implementation of appropriate management strategies, such as hormonal replacement and gonadal malignancy risk stratification (6, 7).
In children with 46,XY DSD, serum anti-Müllerian hormone (AMH) and/or inhibin B, as well as basal or post-human chorionic gonadotropin (HCG)stimulation test androgens including, androstenedione, testosterone, and dihydrotestosterone levels (DHT), are necessary in order to assess androgen synthesis and mechanism of action (8). In patients with impalpable gonads, serum AMH and inhibin B can also detect the presence of functioning testicular tissue secreted by sertoli cells (9). The aim of the present study was to demonstrate our experiences at the Diabetes Endocrine and Metabolism Pediatric Unit (DEMPU), Faculty of Medicine, Cairo University in the field of DSD in light of the increased number of referrals of children presenting with atypical genitalia to our tertiary care hospital from across Egypt. In addition, we focused on determining the etiological distribution and clinical and laboratory profile among DSD patients, and evaluate the importance of AMH and inhibin B in detecting functioning testicular tissue.
2 Materials and methods
This was a longitudinal cohort study, which included 451 infants and children with DSD, followed at the DEMPU of Cairo University Children's Hospital between November2019 and January 2022.The minimum age was seven days, and the maximum age was 14 years. The present study was approved by the Cairo University Hospitals Research Committee on 12-10-2019 (MD-92-2019). Records of infants and children who had already been diagnosed with DSD were retrospectively analyzed, while new cases presented to our clinical settings during the timeline of the study were prospectively analyzed.
All patients were subjected to history taking, including atypical genitalia, salt-losing crisis, consanguinity, and family history of infertility.
Physical examination: A comprehensive physical examination was performed in a private setting after explaining the examination steps and obtaining informed consent from the children’s parents or caregivers.
General examination: Participants’ blood pressure and other vitals that would indicate signs of dehydration were measured. Furthermore, we evaluated potential dysmorphic features or any other associated anomalies, and we used Tanner staging for pubertal assessement (10).
Genital examination: Different degrees of virilization in female participants were assessed with the Prader scale (11). In addition to calculating the external masculinization score (EMS), the Quigley scale was used to assess the development of external genitalia in 46,XY children (12, 13).
The following investigations were also performed:
1. Abdomino-pelvic scrotal ultrasonography (U/S) was performed as the first-line screening imaging modality.
2. Laboratory investigations: All blood samples were collected under complete aseptic conditions; karyotype analysis (peripheral blood): 3–5 mL in sodium/ lithium heparin vacutainer tube. Hormonal evaluation: 17-Hydroxyprogesterone was used to screen for CAH secondary to 21-hydroxylase deficiency; Dehydroepiandrosterone (DHEA), progesterone, and 11-deoxycortisol were used to diagnose less common forms of CAH; Testosterone, its precursors and metabolites (DHT and Δ4 androstendione), follicle-stimulating hormone, and luteinizing hormone were evaluated in patients with 46,XY DSD. Basal cortisol and adrenocorticotropic hormone (ACTH) were measured to determine the diagnosis of panhypopituitarism and enzymatic disorders affecting adrenal steroidogenesis. Human chorionic gonadotrophin (HCG) stimulation tests, including measurements of basal testosterone, DHT, and Δ4 androstenedionelevels, were performed, and then 1000–1500 IU (<1 year old, 500 units; 1–10 years, 1000 units; >10 years, 1500 units) of HCG were given intramuscularly (IM) daily for three consecutive days. Consequently, potential increase in testosterone, DHT, and Δ-4 androstenedione levels were assessed 24 hours after the last HCG injection. In cases where testosterone response to the standard 3-day HCG stimulation test was poor, long HCG stimulation was subsequently indicated. Administration of 1500 IU HCG twice weekly for the following 2 weeks was then performed, with a total of seven injections in three weeks and samples were withdrawn on day 22 to assess the testosterone function (14, 15). Response to HCG was considered normal when the absolute testosterone concentrations reached a higher value than the upper limit of the normal prepubertal range or increased by more than twofold the baseline value (16). Increased testosterone levels after HCG testing is suggestive of 5-alpha-reductase deficiency or androgen insensitivity. Stimulated T: DHT ratio>30 is likely to be 5-alpha-reductase-2 deficiency (17, 18). The different etiologies of DSD were diagnosed according to the guidelines of the British Society for Endocrinology (18). Serum AMH levels and previous hormonal profiles were collected from patients’ files.
In this study, we assessed the serum inhibin B levels of patients with 46,XY DSD using enzyme-linked immunoassay (ELISA).The normal reference range for AMH and inhibin B were as follows (19) :Inhibin B (ng/dl): <one month (133–196); one month < one year (195–256); 1–10 years (91–163); and 10–20 years (169–216). AMH (ng/ml): <14 days (35–140); 15 days– six months (55–210); six months– two years (85–320); 2–9 years (55–250); 9–18 years: Tanner 1(35–200), Tanner 2(10–140), Tanner 3 (4–55), Tanner 4 (4–22), and Tanner 4 (4–21).
3. Interventional investigations: a) In fluoroscopy-Genitography, genitograms were performed in preparation for surgery in 46,XX virilized females.b)Laparoscopic procedures were performed in 46,XX DSD patients to assess the urogenital anatomy. Contrary to patients with 46,XY DSD, endoscopic examination was performed for the identification of any Mullerian remnants and gonads in all patients with impalpable testes.
2.1 Statistical analysis
Data were statistically described as mean ± standard deviation (SD), median and range or frequencies (number of cases), and percentages when appropriate. The assumption of normality for numerical data was tested using the Kolmogorov Smirnov test. Comparisons between the study groups were performed using Student t test for independent samples when comparing two groups of normally distributed data and/or large enough samples, and using the Mann–Whitney U test for independent samples when comparing not-normal data. Also, we used the Kruskal–Wallis test to compare normally distributed numerical variables among groups, and the Chi-square (χ2) test was used to compare categorical data. However, the exact test was employed when the expected frequency was less than five. Spearman rank correlation equation was used to demonstrate correlations among various variables. Two-sided p values less than 0.05 were considered statistically significant. The IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, USA) release 22 for Microsoft Windows was used for all statistical analyses. The cut off value of serum AMH was detected using ROC curve.
3 Results
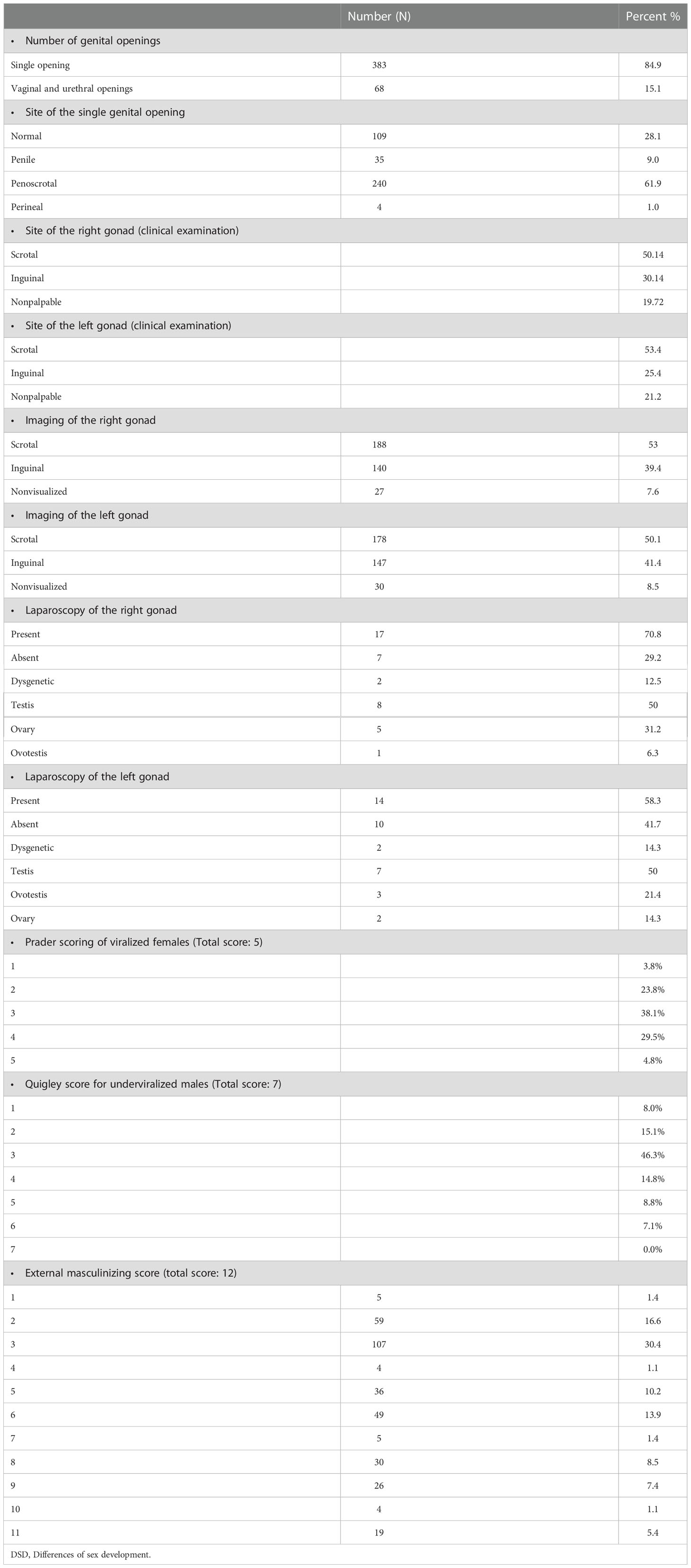
This study included 451 infants and children with DSD of which 336 patients (74.5%) had 46,XY DSD, 98 patients (21.7%) had 46XX DSD, and only 14 patients (3.1%) had another karyotype, including 10 patients with mixed gonadal dysgenesis, one with Klinefelter syndrome (47 XXY), one with trisomy 21/ Klinefelter syndrome (47XXY/ 48 XXY), one patient with trisomy 18, and one with trisomy 13. There were three patients for which karyotype results could not be obtained. Figure 1 summarizes the spectrum of different etiologies of DSD among the patients included in this study. PAIS was the most prevalent diagnosis, representing 42.8 % of all patients, while CAH represented 24.2 % of the population study, with 21 hydroxylase deficiency being the most common form of CAH.
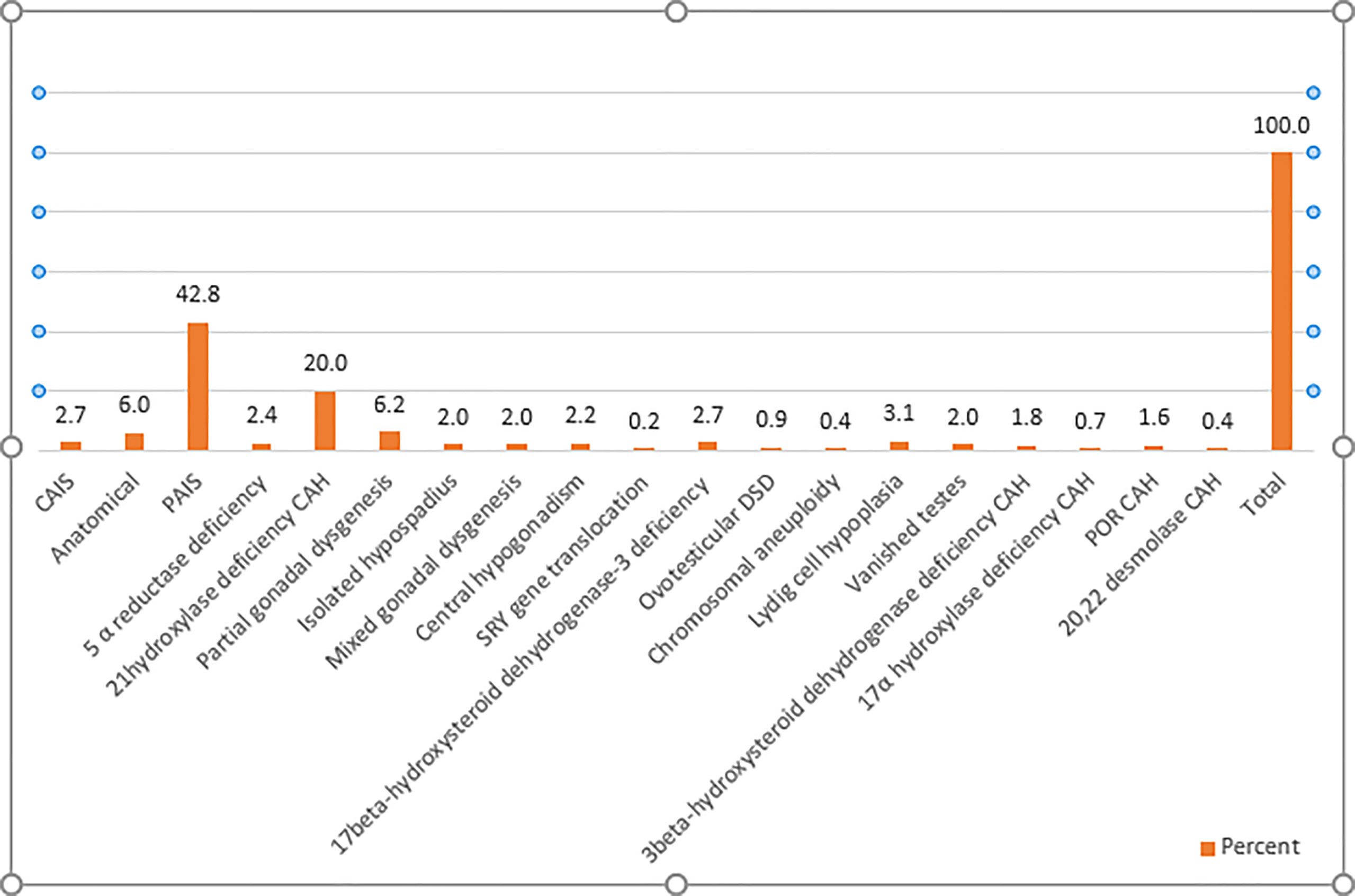
Figure 1 Etiological classification of cases of DSD. CAH, congenital adrenal hyperplasia; CAIS, complete androgen insensitivity; DSD, differences of sex development; PAIS, partial androgen insensitivity; POR CAH, P450 oxidoreductase deficiency.
In addition to one patient with Robinow syndrome and two patients with cloacal dystrophy and associated renal anomalies, respectively, the associated anomalies primarily included the characteristic features of trisomy 21, 13, and 18. Other disorders included congenital heart defects, limb anomalies, and dysmorphic features. Moreover, one patient had a double uterus, double vagina, and imperforate anus.
The mean age of presentation among non salt-losing individuals was 3.45 years (± SD 3.865). Table 1 outlines the clinical, imaging, and laparoscopic findings of the gonads of patients with DSD.
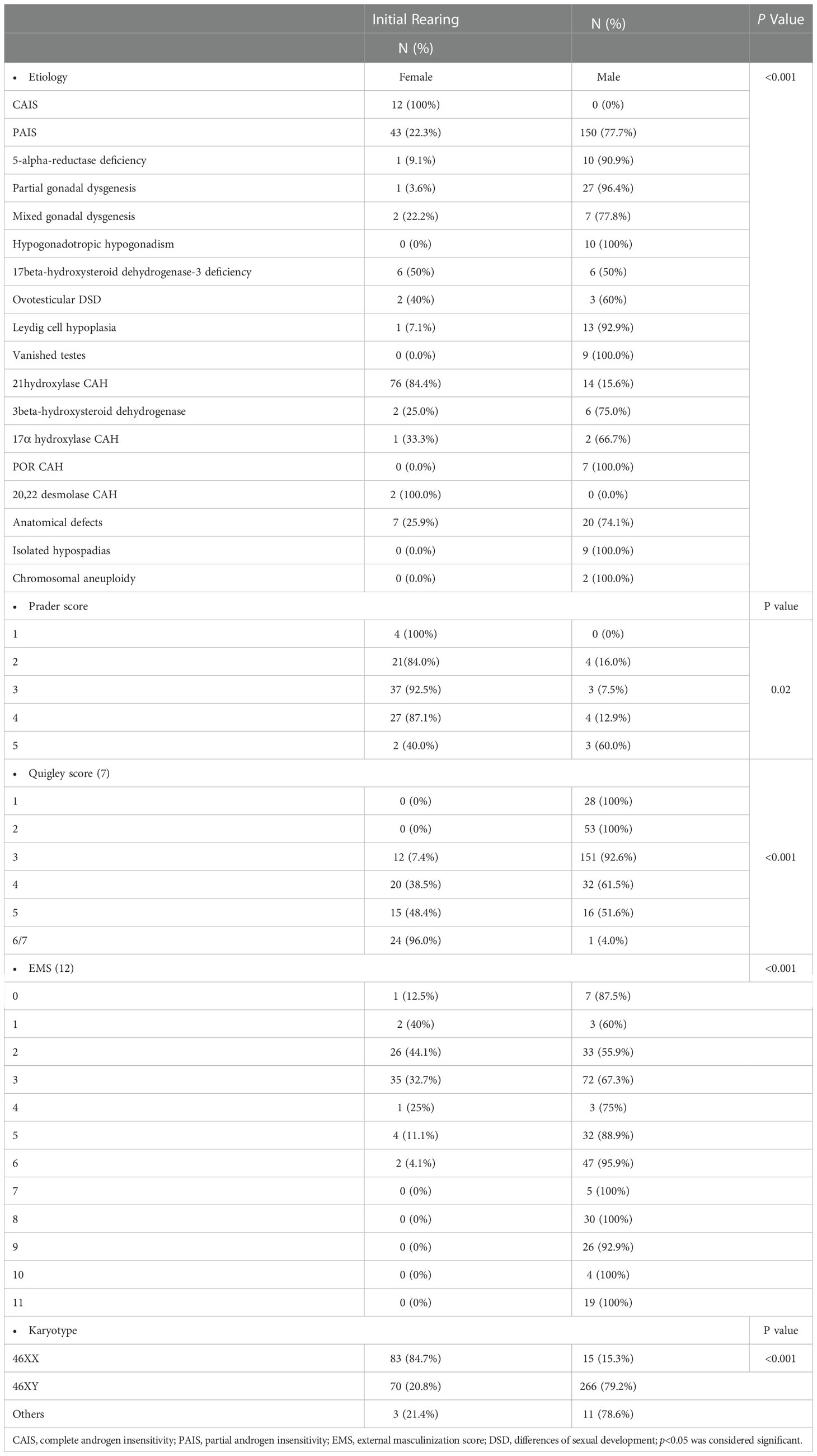
Our results showed a significant correlation between the Quigley score and initial sex of rearing (p<0.001). Compared to 51.6% of 46,XY DSD patients with a Quigley score of 5, all 46,XY DSD patients with a Quigley score of 1–2 were initially reared as males. Among virilized females, all patients with a Prader score of 1 were initially reared as females. In contrast, 60% of patients with a Prader score of 5 were initially reared as males (Table 2). Table 2 also demonstrates the initial sex of rearing for patients with different etiological diagnoses. In patients with 46,XY DSD, Comparison between hormonal profile of patients with poor HCG response and those with normal HCG response was outlined in Table 3.The minimum of post HCG stimulation serum total testosterone level in patients with good response in this study was 0.2 ng/ml, which was near the normal prepubertal level and > twofold the basal testosterone level for this patient (0.02ng/ml).
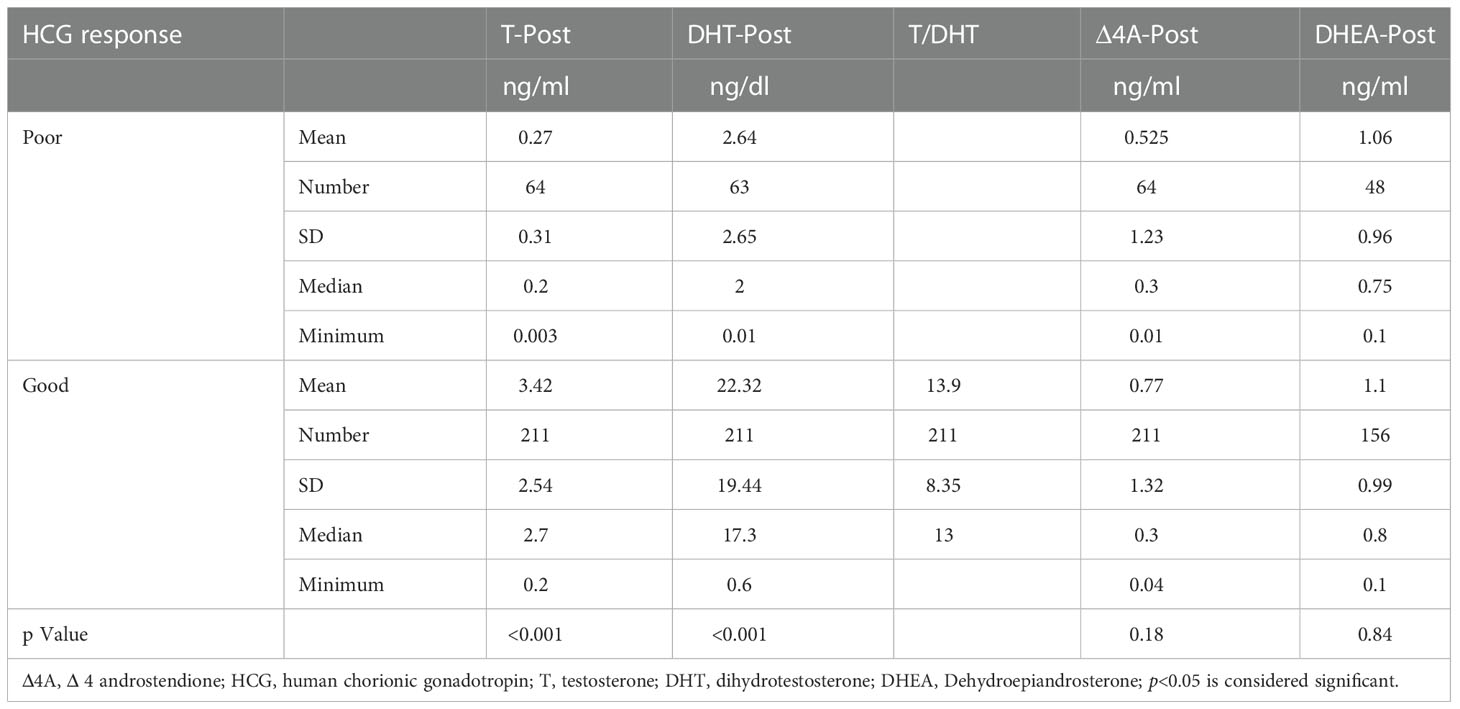
Table 3 Comparison between patients with sufficient and inadequate HCG response regarding post stimulation hormonal levels.
The mean ±SD of inhibin B level among 46,XY DSD patients was 174.38 pg/dl ±122.58. Fifty patients (38.8%) showed low levels of inhibin B to the age-appropriate reference ranges. The mean serum inhibin B level of patients with undescended testes (n = 58) was 204.74 pg/dl (range: 7–679) with a mean serum AMH level of 51.21 ng/ml (range: 0.01–490).
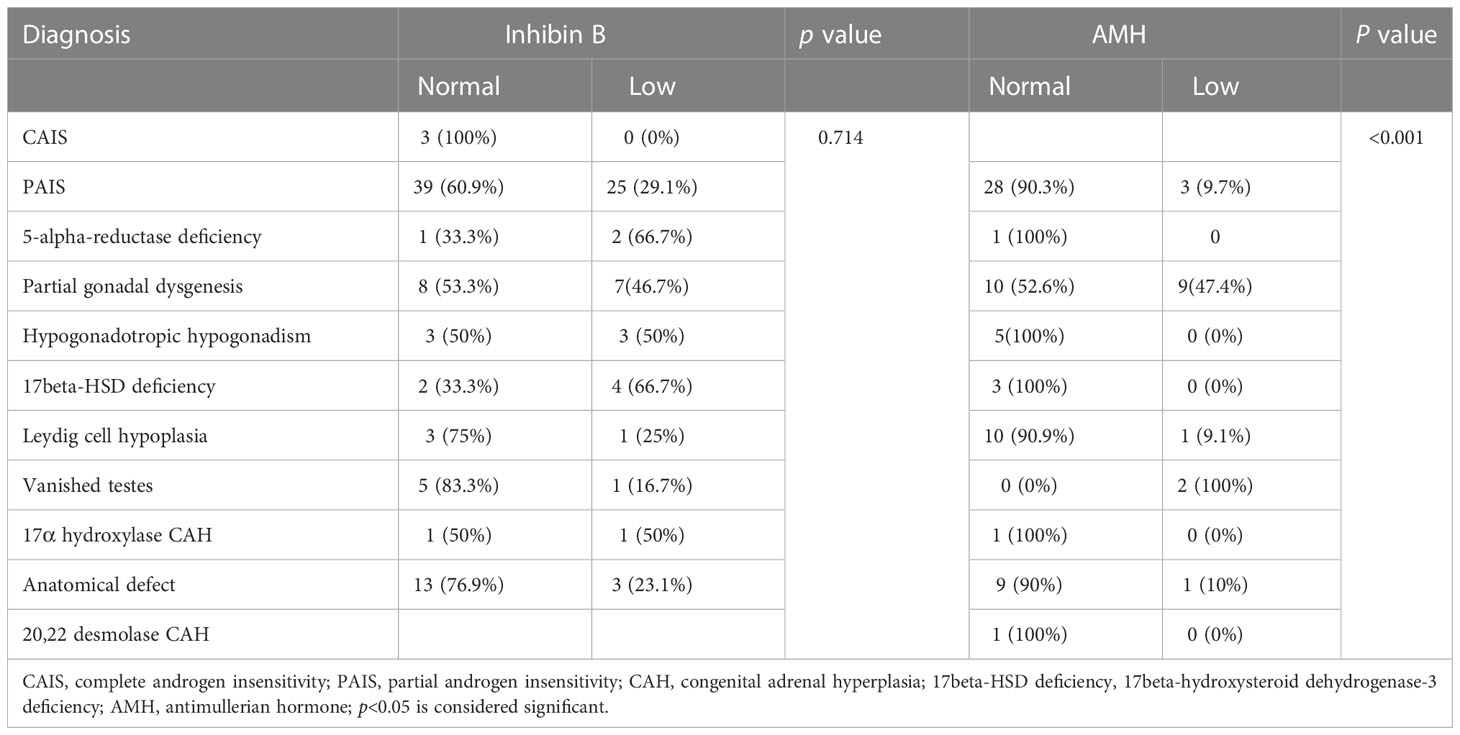
The median AMH in patients with poor HCG response was statistically significantly lower compared to the median AMH in patients with normal HCG response (p<0.001). However, there was no significant difference in serum inhibin B among patients with poor HCG response (p= 0.23) (Table 4). Table 5 outlines the AMH and inhibin B state of patients diagnosed with different etiologies of 46,XY DSD.
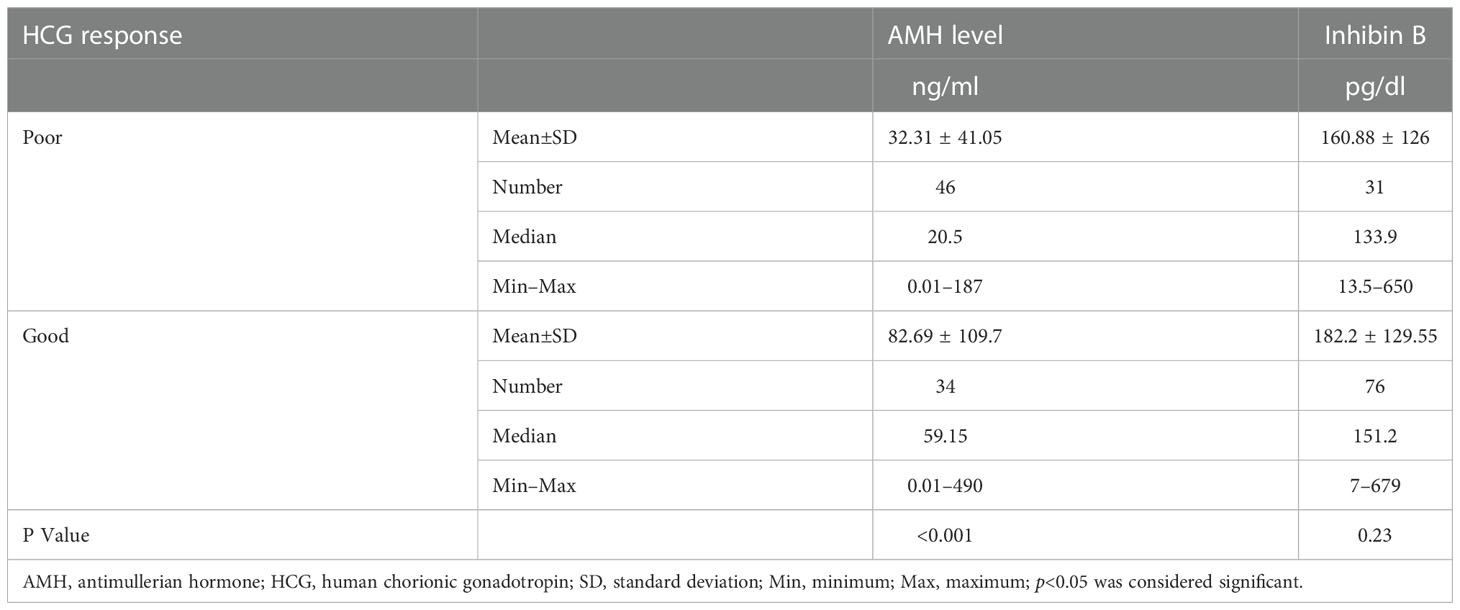
Table 4 Comparison between patients with sufficient and inadequate HCG response with respect to AMH and Inhibin B levels.
The cut off value of AMH, which indicates the presence of functioning testicular tissue, was 14.5 ng/ml (100% sensitivity and 55.1% specificity), and the Positive Predictive Value and Negative Predictive Value were 60.71% and 100.00%, respectively, using the ROC curve (Figure 2).
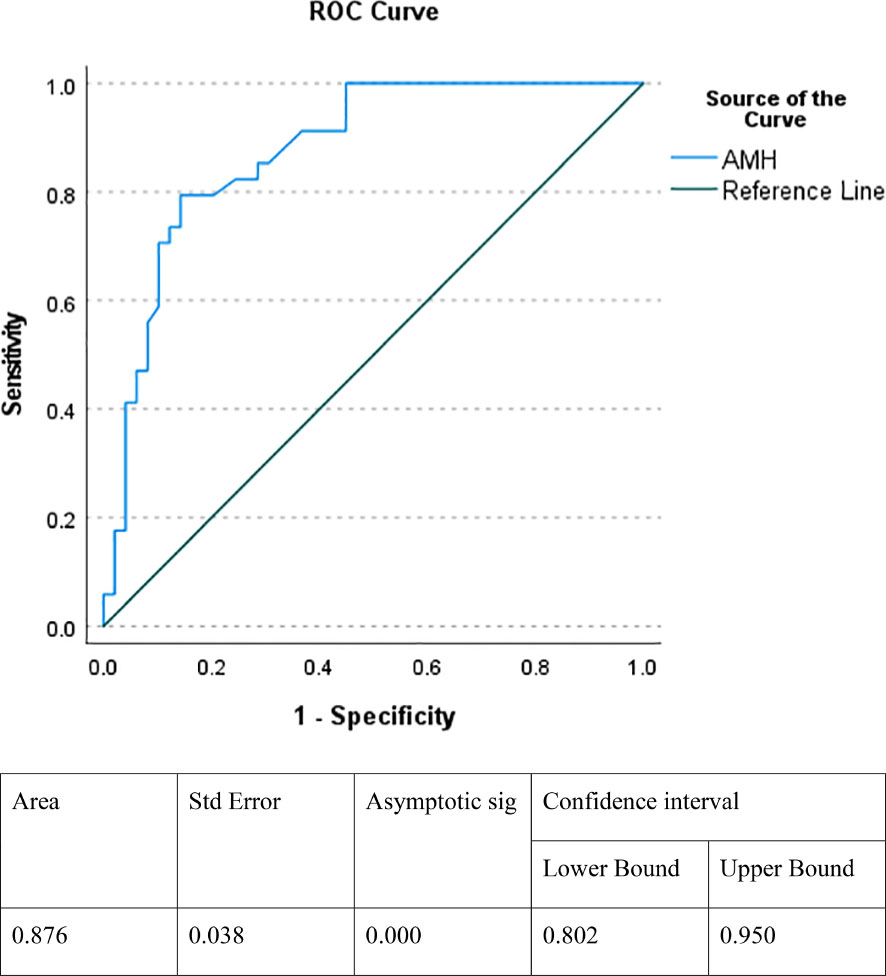
Figure 2 ROC curve indicating the specificity and sensitivity of AMH in detecting functioning testicular tissue. AMH, antimullerian hormone.
Our results showed the AMH level was positively correlated with the post-HCG stimulation testosterone level (p<0.001, r= 0.42) and DHT level (p<0.001, r = 0.40) (Figure 3). In contrast, serum inhibin B was not significantly correlated with stimulated testosterone and DHT levels (p = 0.595 and p = 0.454, respectively).
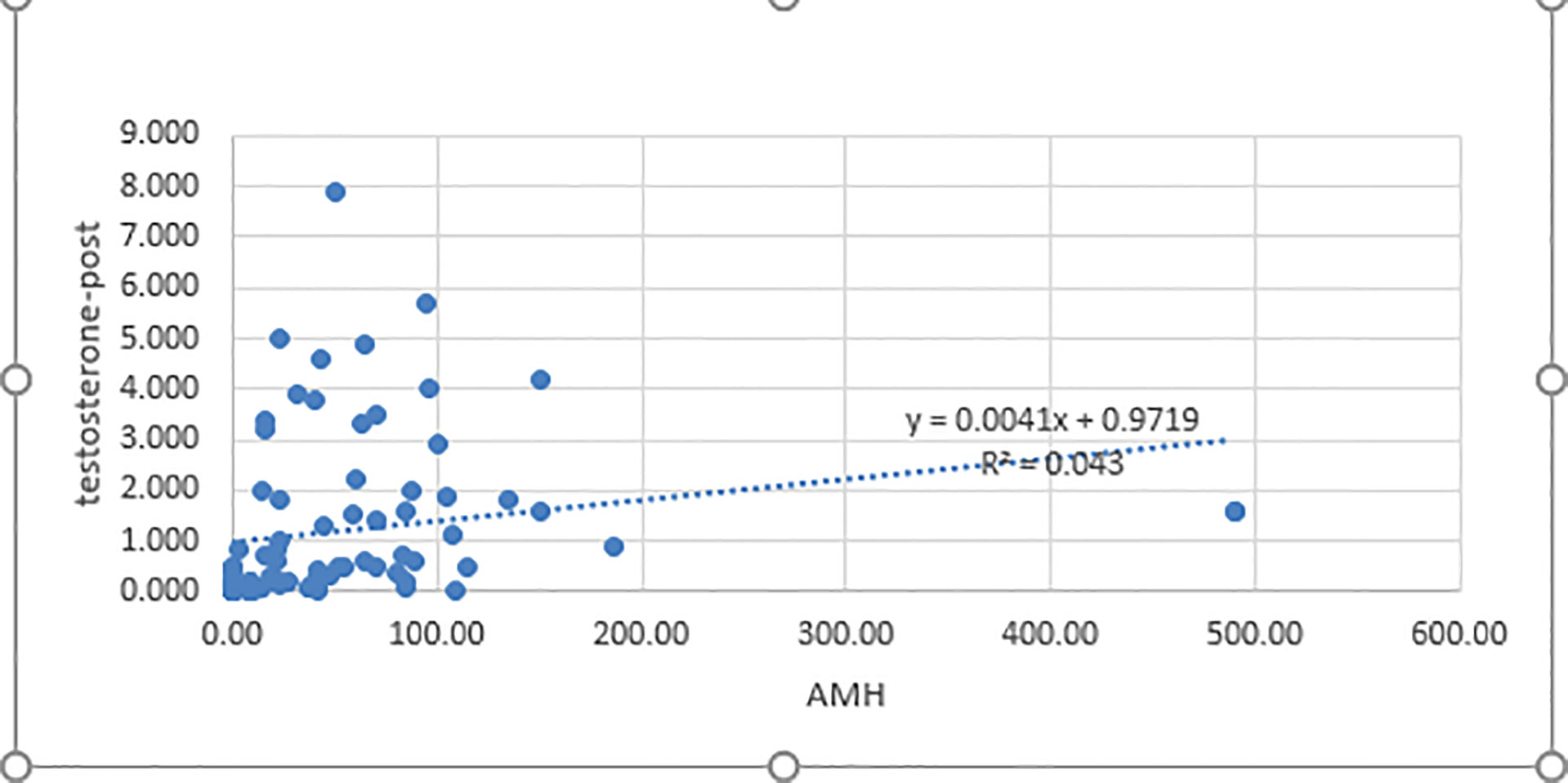
Figure 3 Correlation between AMH and post-HCG testosterone levels. AMH, antimullerian hormone; testosterone post: post-human chorionic gonadotropin test stimulation; p< 0.05 was considered significant.
4 Discussion
This study included 451 infants and children followed at the DEMPU, 76.7% of which presented with atypical genitalia, including isolated micropenis, isolated hypospadias, and isolated undescended testes. This finding was consistent with previous studies conducted in Pakistan and Turkey (20, 21). In addition to atypical genitalia, salt-losing manifestations were present in 21.3% of our patients. Patients who were diagnosed with complete androgen insensitivity (CAIS) presented either accidental palpable testes in labial folds or were accidently discovered during inguinal hernia, in accordance with the findings of Konar et al. (22). The mean age of presentation among patients without salt-losing manifestations was 3.5 years. This comes in contrast with studies performed in developed countries reporting neonatal presentation and early management, including surgical treatment, before the age of six months (23). A possible explanation for this delayed presentation in cases with atypical genitalia, which has also been reported by Nasir et al., may be that health care providers failed to perform a thorough examination of the genitalia (24). Also, contrary to the developed countries, ignorance of our conservative society with regard to DSD is a significant cause of delayed presentations of cases with atypical genitalia (25). However, patients with salt-losing manifestations, who represented 87.5% of all CAH patients, had earlier presentation during infancy and the neonatal period.
Patients with 46,XY DSD represented 74.5% of our patients while 21.7% had a 46,XX karyotype, which was consistent with the results of previous studies (4, 26). However, Al-Jurayyan et al., Dar et al. and Nasir et al. reported that 46,XX DSD were more prevalent than 46,XY DSD (24, 27, 28) All cases with the 46 XX karyotype were diagnosed as CAH except one patient who had SRY gene translocation. We included the CAH population in this study, but most women with CAH do not identify as having a DSD; they identify as having an adrenal steroid disorder (29).
PAIS was the most prevalent etiology of 46,XY DSD, representing 42.8% of all patients, a result that is consistent with the findings of Hafez et al. (9), as PAIS or 5-alpha-reductase deficiency represented 44.19% of their studied patients. The frequency of bilateral undescended testes was found to be 40.4% in our study, including patients with inguinal or nonpalpable testes. In contrast, the prevalence of this condition was 1.52% in a study conducted in Bulgaria on children aged <1 years up to 19 years old (30) and 20% in Pakistan (31).
There was a predilection for the male sex of rearing among under virilized males despite the high degrees of ambiguity; 51.6% and 61.5% of patients with Quigley scores 5 and 4, respectively, were assigned as males. Mazen et al. (4) reported similar findings in the Egyptian society.
Sertoli cells continue to release high levels of AMH during infancy and childhood (32). AMH has been considered a good biomarker for testicular function due to the little clinical use of basal testosterone and gonadotropin levels following minipuberty (33). Similarly, inhibin B is secreted by the sertoli cells in children and adults and can be used as a marker for testicular function in these populations (34).
Our results showed a positive correlation between AMH level and post-HCG stimulation DHT and testosterone levels. However, there was no significant correlation between the inhibin B level and stimulated DHT and testosterone levels. Hafez et al. found similar results regarding AMH; however, the authors found significant correlations between inhibin B and post-HCG stimulated testosterone and dihydrotestosterone (9). In addition, Kunini et al. found a significant correlation between inhibin B and post-HCG stimulated testosterone (35).
In our study, 90 % patients with PAIS, 100% of patients with 5-alpha-reductase deficiency and 90 % of those with anatomical defects had normal AMH levels, which was consistent with the results of Szarras-Czapnik et al. (36) Also Karaoglan reported normal AMH in patients with AIS and those with anatomical defects; however, the authors also found relatively low AMH levels in patients with 5-alpha-reductase deficiency (37). Our results demonstrated low AMH levels in more than half of patients with partial gonadal dysgenesis, which agreed with the results of Karaoglan (37).
5 Conclusions
In the present study, the most prevalent DSD category was patients with 46,XY DSD. However, CAH, predominantly classic salt-wasting CAH, was a major etiology of DSD. In patients with XY DSD, AMH was a reliable marker for detecting the presence and function of testes in infants and prepubertal children. Furthermore, AMH levels were a valuable tool for making differential diagnosis of DSD, with low AMH levels indicating global testicular disorders and normal or elevated AMH levels indicating disorders of androgen biosynthesis or action.
Our study has several limitations. First, this was a longitudinal study but there was no long-term follow-up. Second, some patients had missing data. Third, molecular analysis for specific disorders was not performed. However, our study has significant strengths as well. To our knowledge, this was the first study that evaluated the etiological diagnoses of DSD in the DEMPU, Faculty of medicine Cairo University. In addition, the large sample size included in our study allowed for a wide variety of etiological diagnoses, including rare conditions with detailed clinical profiles, with biochemical and histopathological confirmation whenever possible.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the scientific ethics committee of pediatric department, Faculty of Medicine, Cairo University on 12/10/2019 with code n: MD-92-2019. An informed consent was obtained from each child or his parents before enrollment. The consent was verbal, as our research was part of MD thesis of a candidate in our institute so it was performed under Cairo University regulations at the time of performance of the study. Written informed consent from the participants' legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SH conceived of the study, participated in its design, supervised data collections from the patients and helped to draft the manuscript. MM participated in study design and data interpretation coordination and helped to draft the manuscript. SA participated in the study design, data interpretation and coordination and helped to draft the manuscript. NR participated in the study design, supervised collecting data and laboratory work and helped to draft the manuscript. EA participated in patient selection and data collection. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to acknowledge our patients and their caregivers, the medical staff and nursing team of the Diabetes, Endocrine, and Metabolism Pediatric Unit (DEMPU) at the Abo El Rish Children’s Hospital, Faculty of Medicine, Cairo University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Lee PA, Nordenstr¨om A, Houk CP, Ahmed SF, Auchus R, Baratz A, et al. Global DSD update consortium. global disorders of sex development update since 2006: perceptions, approach, and care. Horm Res Paediatr (2016) 85:158–80. doi: 10.1159/000442975
2. Manzoor J, Aftab S, Yaqoob M. Ambiguous genitalia: an overview of 7 years experience at the children’s hospital & institute of child health, Lahore, Pakistan. Pak J Med Sci (2019) 35:151–5. doi: 10.12669/pjms.35.1.289
3. Bashamboo A, Mcelreavey K. Consanguinity and disorders of sex development. Hum Hered (2014) 77:108–17. doi: 10.1159/000360763
4. Mazen I, Hiort O, Bassiouny R, El Gammal M. Differential diagnosis of disorders of sex development in Egypt. Horm Res (2008) 70:118–23. doi: 10.1159/000137657
5. Mehmood KT, Rentea RM. Ambiguous genitalia and disorders of sexual differentiation. In: StatPearls. Treasure Island (FL: StatPearls Publishing (2021).
6. Krishnan S, Meyer J, Khattab A. Ambiguous genitalia in the newborn. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Grossman A, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Stratakis CA, Trence DL, Wilson DP, editors. Endotext. South Dartmouth (MA).MDText.com, Inc (2019). p. p2000.
7. Hughes IA, Nihoul-Fékété C, Thomas B, Cohen-Kettenis PT. Consequences of the ESPE/LWPES guidelines for diagnosis and treatment of disorders of sex development. Best Pract Res Clin Endocrinol Metab (2007) 21:351–65. doi: 10.1016/j.beem.2007.06.003
8. Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, et al. Society for endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (Revised 2015). Clin Endocrinol (Oxf) (2016) 84:771–88. doi: 10.1111/cen.12857
9. Hafez M, El Dayem SM, El Mougy F, Atef A, Kandil M, Galal A, et al. The role of anti-mullerian and inhibin b hormones in the evaluation of 46 XY disorders of sex development. J Pediatr Endocrinol Metab (2014) 27:891–9. doi: 10.1515/jpem-2013-0355
10. Tanner JM, Davies PS. Clinical longitudinal standards for height and height velocity for north American children. J Pediatr (1985) 107:317–29. doi: 10.1016/s0022-3476(85)80501-1
11. Prader A. Genital findings in the female pseudo-hermaphroditism of the congenital adrenogenital syndrome; morphology, frequency, development, and heredity of the different genital forms. Helv Paediatr Acta (1954) 9:231–48.
12. Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocr Rev (1995) 16:271–321. doi: 10.1210/edrv-16-3-271
13. Ahmed SF, Cheng A, Dovey L, Hawkins JR, Martin H, Rowland J, et al. Phenotypic features, androgen receptor binding, and mutational analysis in 278 clinical cases reported as androgen insensitivity syndrome. J Clin Endocrinol Metab (2000) 85:658–65. doi: 10.1210/jcem.85.2.6337
14. Ahmed SF, Chenge A, Huges IA. Biochemical evaluation of the gonadotropin-gonadotrophin-gonadal axis in androgen insenstivity syndrome. Arch Dis Child (1999) 80:324–29. doi: 10.1136/adc.80.4.324
15. Dixon JR, Wallace AM O, Toole S, Ahmed SF. Prolonged human chorionic gonadotrophin ( hCG) stimulation as a tool for investigating and managing undescended testes. Clin Endocrinol (2007) 67:816–21. doi: 10.1111/j.1365-2265.2007.02968.x
16. Segal TY, Mehta A, Anazodo A, Hindmarsh PC, Dattani MT. Role of gonadotrophin-releasing hormone and human chorionic gonadotrphin stimulation tests in differentiating patients with hypogonadotropic hypogonadism from those with constitutional delay of growth and puberty. J Clin Endocrinol Metab (2009) 94:780–85. doi: 10.1210/jc.2008-0302
17. Guerrero-Fernández J, Azcona San Julián C, Barreiro Conde J, Bermúdez de la Vega JA, Carcavilla Urquí A, Castaño González LA. Management guidelines for disorders / different sex development (DSD). Ann Pediatr (Engl ed) (2018) 89:315.e1–315.e19. doi: 10.1016/j.anpedi.2018.06.009
18. Ahmed SF, Achermann J, Alderson J, Crouch NS, Elford S, Hughes IA, et al. Society for endocrinology UK guidance onthe initial evaluation ofa suspected difference or disorder of sex development (Revised 2021). Clin Endocrinol (Oxf) (2021) 95:818–40. doi: 10.1111/cen.14528
19. Edelsztein NY, Grinspon RP, Schteingart HF, Rey RA. Anti-müllerian hormone as a marker of steroid and gonadotropin action in the testis of children and adolescents with disorders of the gonadal axis. Int J Pediatr Endocrinol (2016) 2016:20. doi: 10.1186/s13633-016-0038-2
20. Rehman UL, Ahsan T, Jabeen R, Zehra F. Clinical spectrum of disorders of sexual differentiation. J Coll Phys Surg Pak (2016) 26:199–203.
21. Erdoğan S, Kara C, Uçaktürk A, Aydın M. Etiological classification and clinical assessment of children and adolescents with disorders of sex development. J Clin Res Pediatr Endocrinol (2011) 3:77–83. doi: 10.4274/jcrpe.v3i2.16
22. Konar S, Dasgupta D, Patra DK, De A, Mallick B. Chromosomal study is must for prepubertal girl with inguinal hernia: opportunity to diagnose complete androgen insensitivity syndrome. J Clin Diagn Res (2015) 9:GD01–3. doi: 10.7860/JCDR/2015/11411.5750
23. Al-Agha AE, Thomsett MJ, Batch JA. The child of uncertain sex: 17 years of experience. J Paediatr Child Health (2001) 37:348–51. doi: 10.1046/j.1440-1754.2001.00669.x
24. Nasir AA, Abdur-Rahman LO, Adesiyun OO, Bamigbola KT, Adegboye MB, Raji HO, et al. Analysis of presentations and outcomes of care of children with disorders of sexual development in a Nigerian hospital. J Pediatr Adolesc Gynecol (2019) 32:21–6. doi: 10.1016/j.jpag.2018.08.005
25. Özbey H, Etker S. Disorders of sexual development in a cultural context. Arab J Urol (2013) 11:33–9. doi: 10.1016/j.aju.2012.12.003
26. Joshi RR, Rao S, Desai M. Etiology, and clinical profile of ambiguous genitalia. overview 10 years experience. Indian Pediatr (2006) 43:974–9.
27. Al Jurayyan NA, Al-Jurayyan RN, Mohamed SH, Babiker AM, Al Otaibi HM. Radiological imaging of disorders of sex development (DSD). Sudan J Paediatr (2013) 13:10–6.
28. Dar SA, Nazir M, Lone R, Sameen D, Ahmad I, Wani WA, et al. Clinical spectrum of disorders of sex development: a cross-sectional observational study. Indian J Endocrinol Metab (2018) 22:774–9. doi: 10.4103/ijem.IJEM_159_18
29. Lin-Su K, Lekarev O, Poppas DP, Vogiatzi MG. Congenital adrenal hyperplasia patient perception of 'disorders of sex development' nomenclature. Int J Pediatr Endocrinol (2015) 2015(1):9. doi: 10.1186/s13633-015-0004-4
30. Kumanov P, Tomova A, Robeva R, Hubaveshki S. Prevalence of cryptorchidism among Bulgarian boys. J Clin Res Pediatr Endocrinol (2008) 1:72–9. doi: 10.4008/jcrpe.v1i2.42
31. Khan S, Tafweez R, Haider A, Yaqoob M. Spectrum of external genital anomalies in disorders of sex development at children hospital & institute of child health, Lahore, Pakistan. Pak J Med Sci (2021) 37:244–49. doi: 10.12669/pjms.37.1.2991
32. Kanakatti Shankar R, Dowlut-McElroy T, Dauber A, Gomez-Lobo V. Clinical utility of anti-mullerian hormone in pediatrics. J Clin Endocrinol Metab (2022) 107:309–23. doi: 10.1210/clinem/dgab687
33. Josso N, Rey RA. What does AMH tell us in pediatric disorders of sex development? Front Endocrinol (Lausanne) (2020) 11:619. doi: 10.3389/fendo.2020.00619
34. Freire AV, Grinspon RP, Rey RA. Importance of serum testicular protein hormone measurement in the assessment of disorders of sex development. Sex Dev (2018) 12:30–40. doi: 10.1159/000479572
35. Kubini K, Zachmann M, Albers N, Hiort O, Bettendorf M, Wölfle J, et al. Basal inhibin b and the testosterone response to human chorionic gonadotropin correlate in prepubertal boys. J Clin Endocrinol Metab (2000) 85:134–8. doi: 10.1210/jcem.85.1.6285
36. Szarras-Czapnik M, Gajewska M, Ksiazyk J, Janas R, Ginalska-Malinowska M. Anti-müllerian hormone (AMH) measurements in the assessment of testicular function in prepubertal boys and in sexual differentiation disorders. Endokrynol Diabetol Chor Przemiany Materii Wieku Rozw (2006) 12:195–9.
Keywords: anti-mullerian hormone, inhibin B, congenital adrenal hyperplasia, androgen insensitivity syndromes, atypical genitalia, differences of sex development
Citation: Abdelghaffar S, AbdelMoneam EN, Hassanein SA, Radwan NA and Mira MF (2023) Categorization of differences of sex development among Egyptian children and the role of antimullerian hormone and inhibin B. Front. Endocrinol. 13:1072399. doi: 10.3389/fendo.2022.1072399
Received: 17 October 2022; Accepted: 14 December 2022;
Published: 06 January 2023.
Edited by:
Ahmed Khattab, The State University of New Jersey, United StatesReviewed by:
Karen Su, NewYork-Presbyterian, United StatesOksana Lekarev, NewYork-Presbyterian, United States
Copyright © 2023 Abdelghaffar, AbdelMoneam, Hassanein, Radwan and Mira. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marwa Farouk Mira, bWFyd2EubWlyYUBrYXNyYWxhaW55LmVkdS5lZw==
 Shereen Abdelghaffar1
Shereen Abdelghaffar1 Engy Nasr AbdelMoneam
Engy Nasr AbdelMoneam Samah A. Hassanein
Samah A. Hassanein Noha Abdelhalim Radwan
Noha Abdelhalim Radwan Marwa Farouk Mira
Marwa Farouk Mira