- 1Division of Pediatrics, Department of Health Science University of Piemonte Orientale, Ospedale Maggiore della Carità, Novara, Italy
- 2Department of Endocrinology and Metabolic Diseases, Istituto Auxologico Italiano IRCCS, Milan, Italy
- 3Department of Paediatric Endocrinology, St. George’s University Hospital NHS Foundation Trust, London, United Kingdom
- 4Department of Medical Biotechnology and Translational Medicine, University of Milan, Milan, Italy
Despite decades of experience, the diagnosis of growth hormone deficiency (GHD) remains challenging, especially in peripubertal children. Failure to respond to GH stimulation tests (GHSTs) is needed to confirm GHD, but long-standing controversies regarding the number of tests needed and the interpretation of GH peaks are still a matter of debate worldwide. Diagnostic workup is even more problematic in short children with slow growth and delayed sexual development: they often exhibit low GH peaks under GHST, which often normalize as puberty progresses. Consequently, this transient suboptimal response to GHST may result in GH overtreatment, carrying both health and economic concerns. Considering the complex and bound link between GH axis and sex steroids, the use of sex steroid priming prior to GHST might be helpful in peripubertal setting. However, its use is still controversial. There is no consensus regarding patient selection, timing, dose, and preparation of sex steroids. In this review, we aim to overview the use of sex steroid priming in clinical practice, highlighting the need to develop appropriate guidelines in order to overcome diagnostic pitfalls in peripubertal age.
Introduction
Despite decades of experience, the diagnosis of growth hormone deficiency (GHD) remains a challenge for the paediatric endocrinologist. It should result from “auxologic, anatomical and laboratory data”, as stated in the recent document from Growth Hormone Research Society Workshop (1), and therefore appropriate selection of patients eligible for growth hormone (GH) investigation is crucial. Family and previous medical history should be taken into account, as well as accurate physical examination to rule out body disproportions and syndromic features and to evaluate pubertal status. Radiological findings such as brain MRI for hypothalamus-pituitary study and hand-wrist X-ray for bone age assessment do also contribute to the diagnostic evaluation. Finally, serum IGF-I and IGFBP 3 values are supportive biochemical findings. Since measurement of random serum GH concentrations are useless, except for neonates (2), failure to respond to GH stimulation tests (GHSTs) is needed to confirm GHD, when an alternative aetiology for short stature is not evident.
Long-standing controversies continue to generate debate, regarding how to perform and interpret GHSTs (3–7). Arbitrary and not universally adopted cut-off levels, reliability and reproducibility of these tests are the main issues. In a study by Marin et al. investigating GH response to provocative tests in prepubertal children with normal stature, 61% of them failed three different tests with a cut-off fixed at 7 mcg/l (8). Difficulties in distinguishing partial GHD from idiopathic short stature (ISS) or constitutional delay of growth and puberty (CDGP) have already been extensively highlighted (9–12), showing normalization of GH peaks at early retesting (10). Both GH and sex steroids are required for the pubertal growth spurt and there is strong evidence, at least in boys, that sex steroids are a potent stimulus facilitating GH release (13). Diagnostic workup is challenging in short children with slow growth velocity and delayed sexual development: they often exhibit low GH peaks under GHST, which reverts to normal levels as puberty progresses. As a consequence, this transient suboptimal response to GHST may result in GH overtreatment, with both health and economic concerns (4).
According to the 2019 GH Research Society guidelines, the use of sex steroid priming prior to GHST might be helpful in the peripubertal setting. It was first introduced in 1968 to reduce the percentage of false positive results to GHST, since availability of GH treatment was limited at that time (14). However, its use is still controversial. There is no consensus regarding patient selection, timing, dosage, preparation, and administration of sex steroids.
In this review, we aim to overview the use of sex steroid priming in clinical practice, highlighting the need to develop appropriate guidelines in order to overcome diagnostic pitfalls in peripubertal age.
Rationale for use of priming
After minipuberty occurs, during childhood the hypothalamus-pituitary-gonadal system becomes quiescent. A significant change and maturation in the hypothalamic “gonadostat” occur in girls at approximately 10.5 years and in boys at 11.5 years. GnRH neurons amplify their signal to increase amplitude and frequency of FSH and LH pulsatile release by the pituitary gonadotropic cells with a prominent nocturnal rhythm (15). This in turn triggers sex steroid production by gonads with feedback regulation of gonadotropin secretion by both testosterone and oestrogen (16). In girls growth acceleration starts with the onset of breast development (Tanner Stage B2), whereas in boys the rate of growth increases significantly only after achieving Tanner stage III-IV (with approximately 10 ml of testicular volume) (17, 18). The different timing of puberty onset between sexes may be related to an increased sensitivity of the gonadotrophs to GnRH in girls or to a greater bioactivity of oestrogen in prepubertal females compared to prepubertal males (19).
Historical data have demonstrated a complex and close link between GH axis and sex steroids both in animals models and humans (20–24). The hypothalamic regulation of GH secretion results primarily from a stimulating control by GH-releasing hormone (GHRH) and by an inhibiting control by somatostatin. On one hand, sex steroids are known to potentiate GH responsiveness to GHRH in somatotroph cells in the anterior pituitary gland; on the other hand, GH modulates pubertal development by stimulating local production of insulin-like growth factors in gonads and by enhancing gonadal response to gonadotrophin secretion and these axes constitute a regulated network whose feedback relationships manifest important changes at the time of puberty.
The use of sex steroid priming in the diagnosis of GHD is based on three considerations:
A) GH levels increase physiologically during puberty.
Rose et al. (25) analysed circadian GH secretion of 132 normal children and adolescents (every 20 minutes for 24 hours) and found that spontaneous GH secretion increases during puberty, with a peak during early-mid puberty in girls (sometimes before the earliest clinical signs of puberty) and during mid-late puberty in boys, corresponding to their peak of growth velocity. If correlated with bone maturation, mean GH levels and pulse amplitude increased in girls beyond a bone age of 8 years, whereas a decrease in growth velocity was observed in boys till bone age of 11 years. This means that the interpretation of GH levels according to chronological age may be misleading and generate a high amount of false GHD diagnosis.
Similarly, Mauras et al. (26) confirmed that prepubertal boys showed lower GH concentrations compared to sexually mature boys of same age and these findings were secondary to variations in amplitude rather than in the pulse frequency of GH secretions. A study from a cross-sectional group of healthy North American males showed that mean 24-hour GH concentration of young adult is similar to that in the prepubertal state, suggesting that the relative impact of sex steroids on GH concentrations is limited to the last stages of puberty (27).
The role of IGF-I as a modulator of pubertal timing is increasingly recognized (28, 29). High GH secretion is most certainly responsible for the increased IGF-I levels during puberty; nevertheless, previous studies have found a suboptimal growth response to GH stimulation test in girls with central precocious puberty (30, 31). Negative feedback of IGF-I levels on pituitary may be reduced in puberty, emphasizing their synergic anabolic role during growth spurt. IGF-I levels peak 2 years after growth spurt and might play a role in gonadal and secondary sexual characteristics maturation (32).
B) Sex steroids regulate GH secretion and actions, both directly or via modulators, through paracrine or endocrine signalling (33).
The evidence of high levels of oestrogen receptors in hypothalamus and pituitary demonstrates that oestrogens act as regulator of GH secretion by reducing somatostatin receptor expression, increasing the number of GHRH-binding sites and increasing ghrelin-induced GH production (34). Moreover, 80% of the somatotropes in human pituitary co-express aromatase, and in patients with aromatase deficiency the GH response to stimulation is substantially blunted (35). Similarly, late pubertal boys receiving oestrogen receptor blocker (Tamoxifen) to evaluate the role of endogenous oestrogens in the control of GH secretion showed a significant decrease in GH production rates, in mean GH pulse amplitudes, and in serum IGF-I levels (36). These data support the paracrine effect of oestrogens derived from aromatization of androgens in men. Peripherally, oestrogen exerts tissue-specific effect: for example, in bones it potentiates GH signalling via SOCS-2 pathway promoting osteoblast proliferation and bone growth (37). Testosterone also acts peripherally, amplifying GH-mediated secretion of IGF-I, sodium retention, substrate metabolism and protein anabolism, while exhibiting similar but independent actions of its own and interacts directly with GH in the liver to regulate protein metabolism by enhancing GH receptor expression (38). Contrary to androgens, oestrogens do not influence whole body protein anabolism, and this may explain sex differences in muscle bulk. Sex steroids modulate GH secretion during lifespan. Evidence of a regulatory role of sex steroids on GH comes from association studies in children and adults. Physiologically, in children, a positive correlation between sex steroid and GH status has been proved from the evidence of a threefold increase in GH secretion along with an increase in gonadal steroid concentrations during puberty (39).
C) Exogenous sex steroids stimulate GH synthesis, release, and action.
Sex steroids administration exerts a stimulatory effect on GH secretory episodes. In the above mentioned study by Marin et al. 40 mcg/m2 oral ethynil oestradiol given two days before GHST increased GH peak in normal prepubertal children from 1.9-20 mcg/L to 7.2-40.5 mcg/L, reaching similar levels of pubertal children (8-63.2 mcg/L) (8). Low doses of ethinyloestradiol (0.1 mcg/kg/day) could rise GH concentrations after 1 to 5 weeks and improve height gain in patients with Turner Syndrome, without significantly advancing bone maturation (40). The effect of oestrogen on GH secretion is dependent on the route of administration. When administered orally, oestrogen reduces hepatic IGF-I production as a result of first-pass effect. The fall in IGF-I after oral oestrogen therapy reduces negative feedback on GH secretion, as seen in postmenopausal women (41). In men with hypogonadism, testosterone replacement stimulates GH/IGF-I system peripherally and enhances tissue responsiveness to GH. Importantly, non-aromatizable androgens do not stimulate GH secretion (42).
Concerns and benefits
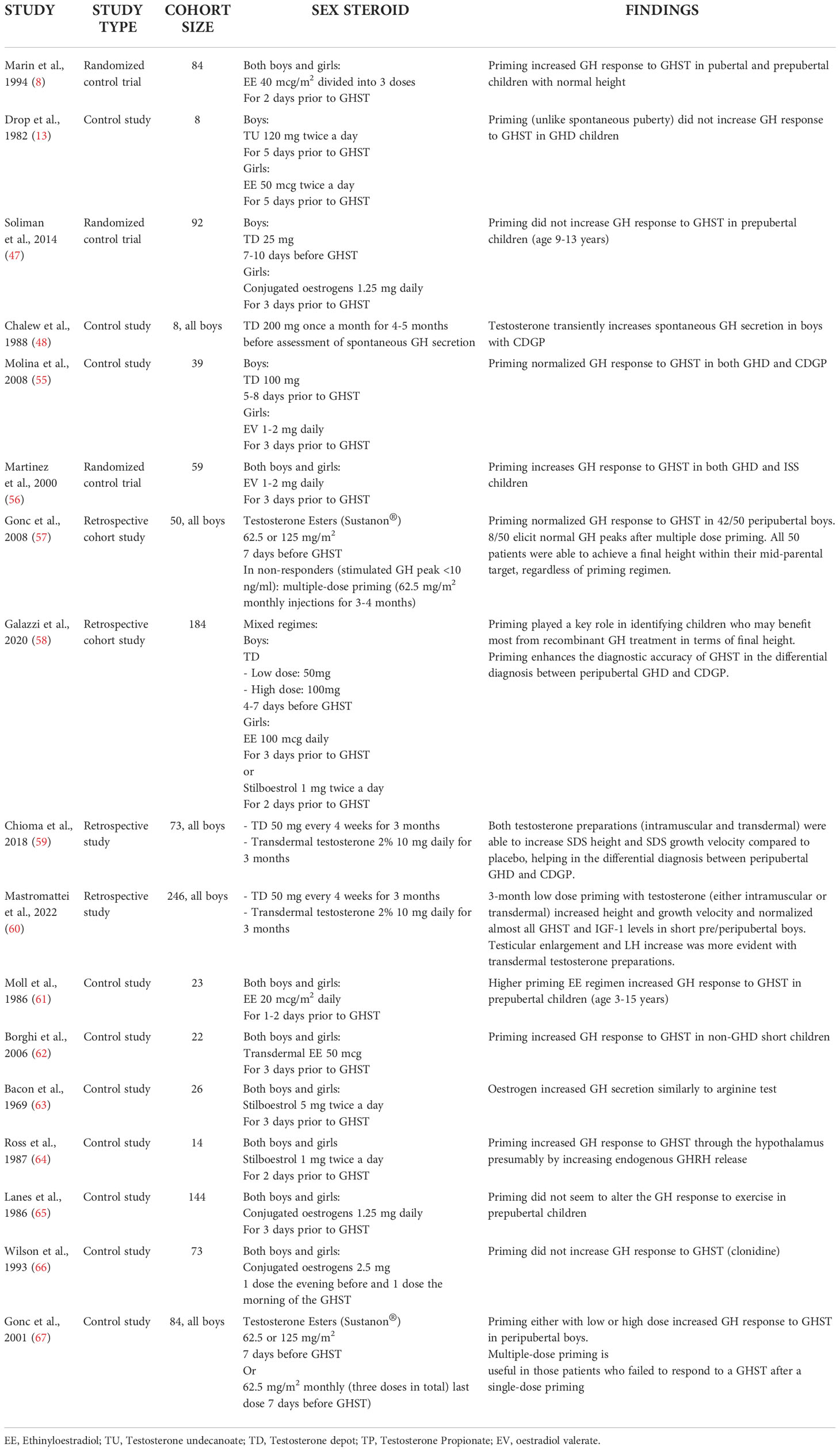
A recent audit among nine American and European expert paediatric endocrinologists showed that priming is recommended in 5 out of 9 countries (the UK, the Netherlands, Denmark, Spain, and Germany), but protocols differ significantly (43). The prevalence was higher (up to 85%) among tertiary endocrine centres in UK (44). In contrast, different data result from a French population-based registry (45): in 2,165 patients with idiopathic GHD sex steroid priming was used in only 2% of patients before GHST. Pubertal development has been reported not to increase GH reserve when evaluated by GHST (3, 46). It should be noted, however, that these studies were performed decades ago with different GH assays and sometimes with obsolete and unreliable diagnostic test (i.e. treadmill exercise). Soliman et al. found that the mean GH response to provocative testing did not differ between primed and not primed-children, although testosterone intramuscular injections were administered at a lower dose compared to other reports; this study however included younger children (starting from 9 years old) compared to other papers and this could had influenced the results (47). Another concern against routine use of priming is that primed GH peak may be unphysiological and transient, therefore many peripubertal GH deficient patients may be not identified, preventing them from receiving appropriate and potentially beneficial treatment (13, 48, 49). The existence of “transient GHD” in adolescents with delayed puberty is still debated, as the underlying pathology is more often consistent with sex hormones deficiency rather than GHD. The majority of the patients with idiopathic GHD show normal GH secretion when retested after achieving of final height, whereas the likelihood of permanent GHD is higher in adults with congenital panhypopituitarism and acquired pituitary lesions (50, 51). For this reason some authors suggested the need to retest patients with idiopathic GHD after one year of therapy: the Belgian Study Group for Paediatric Endocrinology reported normal GH peak in 44% of cases (52). 28 out of 33 GHD patients of an Italian cohort with normal pituitary morphology at brain MRI normalized GH secretion even before commencement of GH treatment (10). Recently published data suggested patients with isolated GHD without a hypothalamic-pituitary abnormality on MR scanning (including small anterior pituitary) can also be considered for early retesting of the GH axis once they are established in puberty (Tanner stages B2/3 in girls & 6-12 ml testes in boys) (53). In addition, GH treatment seems to have little effect on final height in adolescents with transient GHD (54).
On the contrary, many other studies reported that sex steroid priming could improve diagnostic efficacy of GHSTs in peripubertal patients. Molina et al. demonstrated that 53.8% of short children who underwent clonidine stimulation test normalized GH secretion after priming (55); these data were confirmed also among children affected by ISS compared to GHD when micronized oestradiol was administered before GHST (56). A prospective study including 50 boys with poor growth who failed to respond to unprimed GHST showed that some of them normalized GH response to GHST after testosterone priming with different protocols (57): 31/50 boys after single low dose testosterone (62.5 mg/m2), 11/50 after single conventional dose (125 mg/m2) and 8/50 boys after multiple-dose testosterone (62.5 mg/m2 weekly for 4 weeks). Mean peak GH increased from 4.9 ± 3.0 to 19.3 ± 5.9 mcg/L in the low dose group, from 5.4 ± 2.1 to 17.0 ± 5.9 mcg/L in the conventional dose group, and from 5.1 ± 2.1 to 15.4 ± 5.1 mcg/L in the multiple-dose group. There was no statistical difference among mean peak GH level of the three groups before and after priming. Most relevantly, those subjects were able to reach a final height well within their genetic target (mean final height -1.27 +/-0.72 SDS versus mean mid-parental height -1.38 +/-0.72 SDS) without any rhGH treatment. More recently, a retrospective study among ENDO-ERN centres confirmed that sex steroid priming enhanced the specificity of GHST in differential diagnosis between GHD and CDGP in a cohort of 184 peripubertal children (74 females), selecting children who may benefit the most from priming. In fact, those children diagnosed as GHD upon a primed GHST reached a greater final height compared to untreated CDGP (primed CDGP vs GHD FH: -1.5 vs -0.81; p = 0.023) and closer to their midparental target (primed CDGP vs GHD ΔSDS FH-TH: -0.74 vs 0.12, p = 0.025), whereas those diagnosed upon an unprimed GHST, final height was similar between GHD children treated with rhGH and untreated CDGP children (unprimed CDGP vs GHD FH: -0.9 vs -0.93, p =n.s.)
(58). Lastly, two recent retrospective Italian studies on short pre/peripubertal boys primed with a prolonged low-dose testosterone protocols (either with intramuscular or transdermal preparation) showed an increase in height and growth velocity SDS and a normalization of GHST peaks compared to untreated boys (59, 60).
A summary of the clinical research studies included in this review is reported in Table 1.
Use of priming in clinical practice
The actual limitation for use of priming is the current absence of standardized protocol for sex steroids administration with reference to patients’ age, type, dose, and timing. The 2019 international audit (43) revealed that priming may be used in boys between the ages of 10 to 13 years and in girls between the ages of 8 to 12 years. According to 2016 Pediatric Endocrine Society Guidelines priming should be considered in prepubertal boys older than 11 and in prepubertal girls older than 10 years with final height prognosis > –2 SD of the reference population (68). Another adopted strategy is to prime all prepubertal children (boys >9 years, girls >8 years, either on chronological age or bone age) (4). Lazar and Phillip (49) advocated the use of priming only in selected cases, i.e. girls aged > 11.5–12 years and boys aged > 13–13.5 years with any or only initial signs of puberty. Similarly, the recent update from GH Research Society advised to limit its use to adolescents with delayed puberty only, but did not provide any age cut-off due to lack of consensus (1).
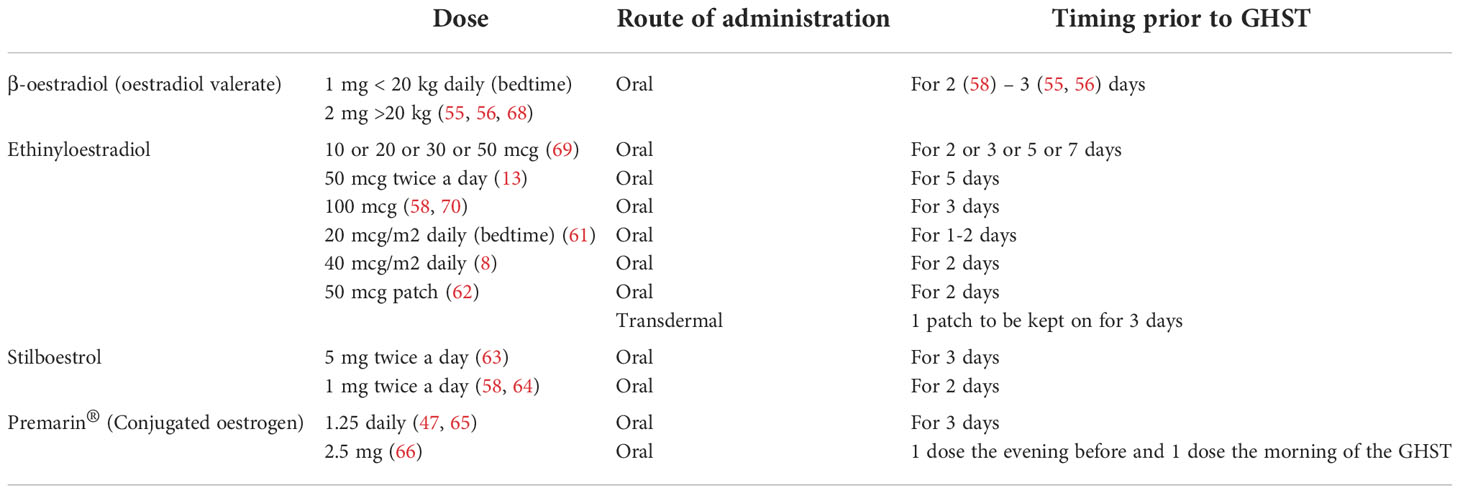
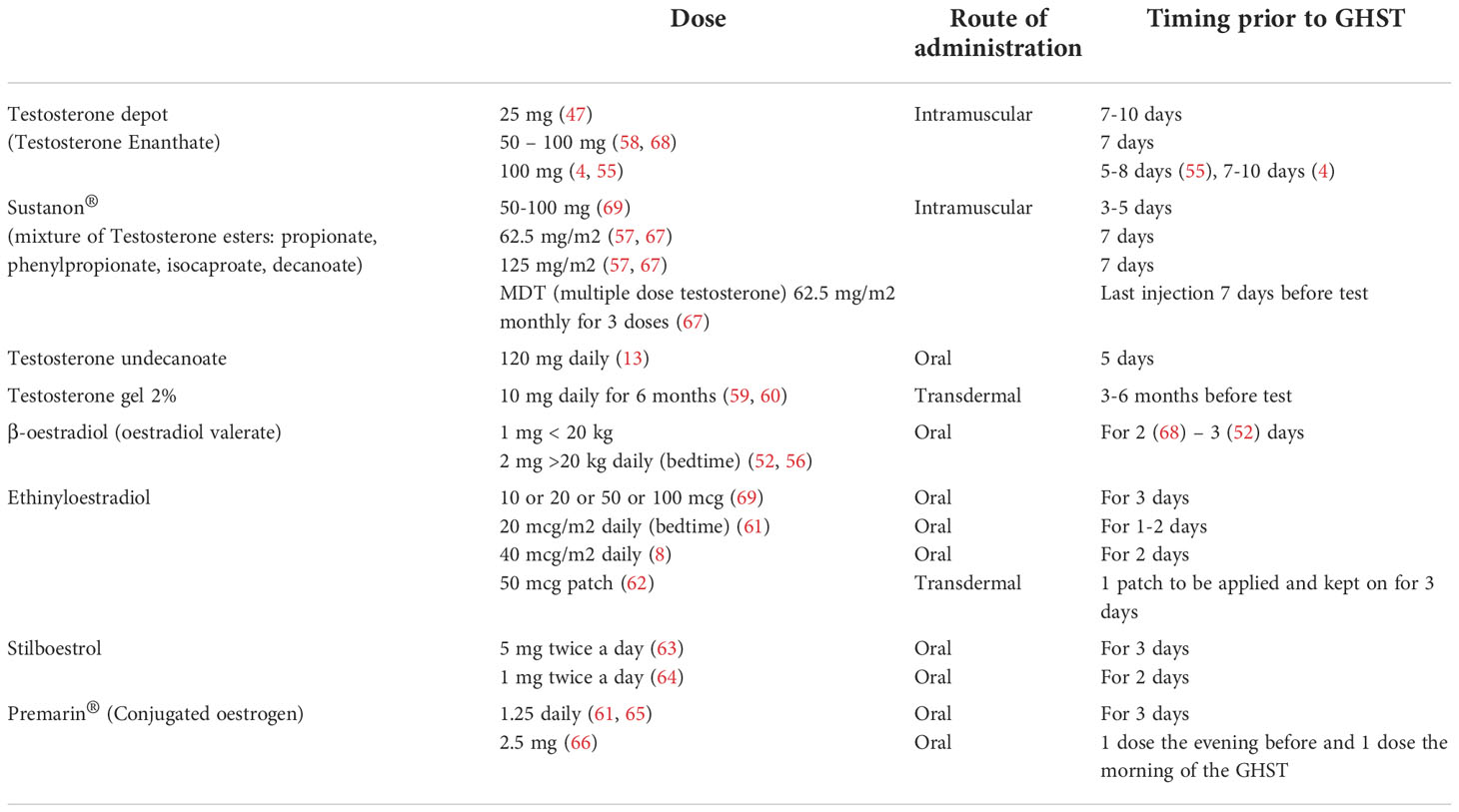
Published data revealed a large heterogeneity in current practice about dose and type of sex steroid preparations across centres and countries, as listed in Tables 2, 3.
A reasonable, easy to use and commonly used approach in both boys and girls would be 2 mg (1 mg for body weight <20 kg) of β-oestradiol orally on each of the 3 evenings preceding the test, as indicated in the BSPED UK Consensus National Guidelines for Sex Hormone Priming (71). Alternatively, for instance in case of lack of supply of β-oestradiol, children of both sexes can be primed with oral Stilboestrol (1 mg twice a day for 2 days before the test). Promising results have been proven for boys primed with transdermal testosterone 2% (59, 60).
As previously mentioned, clinical and experimental data strongly suggest that oestrogens control the feedback amplification of GH levels during puberty even in males and that the modulation of GH production by androgens is mainly secondary to their aromatization. Moreover, the use of oral or transdermal preparations is suitable for needle-phobic patients and could possibly increase patient compliance to treatment. Several oestrogen and testosterone products are available and their effects and pharmacokinetics may vary according to different route and strength, so that a comparison is not always possible (72, 73). For example, oral ethynyloestradiol elicits a sharp response on IGF-I and achieves its peak plasma concentration within 0.5-1.5 hours with a half-life of approximately 12-14 hours, whilst transdermal formulations result in lower oestrogen metabolite concentrations. In a pilot study by Borghi et al. oestrogen patches are considered safe and viable since they deliver a continuous release of oestradiol, guaranteeing stable plasma levels for 72 hours (70). In addition, this route of administration avoids the first passage hepatic effect and does not directly affect IGF-I synthesis. Testosterone transdermal gel are commonly accepted for puberty induction in boys with CDGP and hypopituitarism (74) and their use can be theorized for priming prior to GHST as suggested in the study by Mastromattei et al. 2022 (60).
For research purposes, Radetti et al. reported that priming GHST with Pegvisomant, a GH receptor antagonist, would enhance the accuracy of the test, although this approach has not been extensively confirmed in clinical practice (75).
Side effects
Data are lacking on potential side effects of sex steroid priming. Albrecht et al. analysed the consequences of priming with testosterone enanthate i.m. (50 mg, 125 mg, 250 mg) given 7 days before GHST on 188 prepubertal boys. Overall, only 5 subjects displayed side effects (2.7%), irrespective of testosterone plasma levels: 2/188 developed severe priapism requiring cavernosal aspiration (after testosterone 125 mg single dose), 1/188 mild self-limiting priapism, 2/188 complained testicular pain (after testosterone 50 mg single dose (76). Other common adverse effects related to intramuscular administration are local inflammation and pain at injection site. It is worth mentioning that, as cottonseed or sesame/peanut oil are the formulation vehicle, testosterone vials are contraindicated in case of known hypersensitivity/allergy to nuts or soy (74). Side effects such as severe nausea and vomiting are frequently observed following priming with Stilboestrol. Transient breast tenderness has been reported as well (49).
The need for a structured approach
Since GH production and release are significantly and physiologically influenced by androgen and oestrogen milieu during puberty, sex steroid priming has been proposed and proved to improve diagnostic performance of GH provocation tests. We therefore recommend including priming to all protocols for diagnostic workup of short patients. However, there is no consensus on who, when and how to use it. Nowadays, the availability of biosynthetic GH has eased limitations for GH prescription and has led to the conclusion that sex hormone priming is not necessary in the routine evaluation of every prepubertal child. It should be considered only in a subgroup of adolescents with delayed puberty (e.g. Tanner stages 1 and 2 in girls older than 12 years and boys older than 13 years) in order to prevent unnecessary GH treatment of children with CDGP. Assessment of bone age is warranted to evaluate pubertal delay and to select candidates for sex steroid priming. Several protocols have been suggested for priming and no one demonstrated evident superiority over others. Nevertheless, among different preparations and dosages, oral oestrogen seems preferable in both girls and boys as oestrogen plays a pivotal role in the regulation of GH secretion also in males.
Conclusion
Although sex hormone priming prior to GHST is not mandatory to diagnose GHD, several evidence recommend its use in peripubertal children in order to select those who may benefit the most from rhGH treatment, avoiding redundant treatment in CDGP, who can either achieve normalization of their auxological parameters with a low dose short course of sex steroids. Large prospective studies following patients until final height are still needed to clarify the optimal priming regimen and the correct timing of these preparations during their growth, especially in girls.
Author contributions
CP drafted the manuscript. AA and EG critically revised the manuscript. LP supervised the whole process. All authors contributed to the article and approved the submitted version.
Funding
This work was partially funded by the Italian Ministry of Health, Rome (grant code:05C202_2012).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Collett-Solberg PF, Ambler G, Backeljauw PF, Bidlingmaier M, Biller BMK, Boguszewski MCS, et al. Diagnosis, genetics, and therapy of short stature in children: A growth hormone research society international perspective. Horm Res Paediatr (2019) 92:1–14. doi: 10.1159/000502231
2. Rose SR, Ross JL, Uriarte M, Barnes KM, Cassorla FG, Cutler GB. The advantage of measuring stimulated as compared with spontaneous growth hormone levels in the diagnosis of growth hormone deficiency. N Engl J Med (1988) 319(4):201–7. doi: 10.1056/NEJM198807283190403
3. Ghigo E, Bellone J, Aimaretti G, Bellone S, Loche S, Cappa M, et al. Reliability of provocative tests to assess growth hormone secretory status. study in 472 normally growing children. J Clin Endocrinol Metab (1996) 81(9):3323–7. doi: 10.1210/jcem.81.9.8784091
4. Murray PG, Dattani MT, Clayton PE. Controversies in the diagnosis and management of growth hormone deficiency in childhood and adolescence. Arch Dis Child (2016) 101:96–100. doi: 10.1136/archdischild-2014-307228
5. Shen Y, Zhang J, Zhao Y, Yan Y, Liu Y, Cai J. Diagnostic value of serum IGF-1 and IGFBP-3 in growth hormone deficiency: A systematic review with meta-analysis. Eur J Pediatr (2015) 174(4):419–27. doi: 10.1007/s00431-014-2406-3
6. Mazzola A, Meazza C, Travaglino P, Pagani S, Frattini D, Bozzola E, et al. Unreliability of classic provocative tests for the diagnosis of growth hormone deficiency. J Endocrinol Invest (2008) 31(2):159–62. doi: 10.1007/BF03345583
7. Bidlingmaier M, Freda PU. Measurement of human growth hormone by immunoassays: Current status, unsolved problems and clinical consequences. Growth Horm IGF Res (2010) 20(1):19–25. doi: 10.1016/j.ghir.2009.09.005
8. Marin G, Domené HM, Barnes KM, Blackwell BJ, Cassorla FG, Cutler GB. The effects of estrogen priming and puberty on the growth hormone response to standardized treadmill exercise and arginine-insulin in normal girls and boys. J Clin Endocrinol Metab (1994) 79(2):537–41. doi: 10.1210/jcem.79.2.8045974
9. Bollino A, Cangiano B, Goggi G, Federici S, Duminuco P, Giovanelli P, et al. Pubertal delay: The challenge of a timely differential diagnosis between congenital hypogonadotropic hypogonadism and constitutional delay of growth and puberty. Minerva Pediatr (2020) 72(4):278–87. doi: 10.23736/S0026-4946.20.05860-0
10. Loche S, Bizzarri C, Maghnie M, Faedda A, Tzialla C, Autelli M, et al. Results of early reevaluation of growth hormone secretion in short children with apparent growth hormone deficiency. J Pediatr (2002) 140(4):445–9. doi: 10.1067/mpd.2002.122729
11. Wei C, Crowne EC. Recent advances in the understanding and management of delayed puberty. Arch Dis Child (2016) 101(5):481–8. doi: 10.1136/archdischild-2014-307963
12. Galazzi E, Persani LG. Differential diagnosis between constitutional delay of growth and puberty, idiopathic growth hormone deficiency and congenital hypogonadotropic hypogonadism: A clinical challenge for the pediatric endocrinologist. Minerva Endocrinol (2020) 45(4):354–75. doi: 10.23736/S0391-1977.20.03228-9
13. Drop SLS, Sabbe-Claus L, Visser HKA. The effect of puberty and short-term oral administration of testosterone undecanoate on gh tests and sex-steroid related plasma compounds in GH deficient patients. Clin Endocrinol (Oxf) (1982) 16:375–81. doi: 10.1111/j.1365-2265.1982.tb00730.x
14. Martin LG, Clark JW, Connor TB. Growth hormone secretion enhanced by androgens. J Clin Endocrinol Metab (1968) 28:425–8. doi: 10.1210/jcem-28-3-425
15. Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med (1972) 287(12):582–6. doi: 10.1056/NEJM197209212871203
16. Jakacki RI, Kelch RP, Sauder SE, Lloyd JS, Hopwood NJ, Marshall JC. Pulsatile secretion of luteinizing hormone in children. J Clin Endocrinol Metab (1982) 55:453–8. doi: 10.1210/jcem-55-3-453
17. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child (1969) 44(235):291–303. doi: 10.1136/adc.44.235.291
18. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child (1970) 45(239):13–23. doi: 10.1136/adc.45.239.13
19. Klein KO, Baron J, Colli MJ, McDonnell DP, Cutler GB. Estrogen levels in childhood determined by an ultrasensitive recombinant cell bioassay. J Clin Invest (1994) 94(6):2475–80. doi: 10.1172/JCI117616
20. Homburg R, Eshel A, Hi A, Jacobs HS. : Growth hormone facilitates ovulation induction by gonadotrophins. Clin Endocrinol (Oxf) (1988) 29(1):113–7. doi: 10.1111/j.1365-2265.1988.tb00252.x
21. Daniel WA, Aynsley-Green A, Zachmann M, Prader A. Interrelation of the therapeutic effects of growth hormone and testosterone on growth in hypopituitarism. J Pediatr (1976) 89(6):992–9. doi: 10.1016/S0022-3476(76)80619-1
22. van der Werff ten Bosch JJ, Bot A. Does hGH treatment promote adult height of hypopituitary children? Neth J Med (1988) 32(5-6):217–25.
23. Zachmann M, Prader A. Anabolic and androgenic effect of testosterone in sexually immature boys and its dependency on growth hormone. J Clin Endocrinol Metab (1970) 30(1):85–95. doi: 10.1210/jcem-30-1-85
24. Albanese A, Stanhope R. Growth hormone deficiency throughout puberty. J Endocrinol Invest. (1992) 15(10):777–81. doi: 10.1007/BF03347651
25. Rose SR, Municchi G, Barnes KM, Kamp GA, Uriarte MM, Ross JL, et al. Spontaneous growth hormone secretion increases during puberty in normal girls and boys. Pediatr Nephrol (1992) 6:318. doi: 10.1007/BF00878392
26. Mauras N, Blizzard RM, Link K, Johnson ML, Rogol AD, Veldhuis JD. Augmentation of growth hormone secretion during puberty: Evidence for a pulse amplitude-modulated phenomenon. J Clin Endocrinol Metab (1987) 64(3):596–601. doi: 10.1210/jcem-64-3-596
27. Martha PM, Rogol AD, Veldhuis JD, Kerrigan JR, Goodman DW, Blizzard RM. Alterations in the pulsatile properties of circulating growth hormone concentrations during puberty in boys. J Clin Endocrinol Metab (1989) 69(3):563–70. doi: 10.1210/jcem-69-3-563
28. Mauras N, Rogol AD, Haymond MW, Veldhuis JD. Sex steroids, growth hormone, insulin-like growth factor-1: Neuroendocrine and metabolic regulation in puberty. Horm Res (1996) 45(1-2):74–80. doi: 10.1159/000184763
29. Sørensen K, Aksglaede L, Petersen JH, Andersson AM, Juul A. Serum IGF1 and insulin levels in girls with normal and precocious puberty. Eur J Endocrinol (2012) 166(5):903–10. doi: 10.1530/EJE-12-0106
30. Kamp GA, Manasco PK, Barnes KM, Jones J, Rose SR, Hill SC, et al. Low growth hormone levels are related to increased body mass index and do not reflect impaired growth in luteinizing hormone-releasing hormone agonist-treated children with precocious puberty. J Clin Endocrinol Metab (1991) 72(2):301–7. doi: 10.1210/jcem-72-2-301
31. Cara JF, Kreiter ML, Rosenfield RL. Height prognosis of children with true precocious puberty and growth hormone deficiency: Effect of combination therapy with gonadotropin releasing hormone agonist and growth hormone. J Pediatr (1992) 120(5):709–15. doi: 10.1016/S0022-3476(05)80232-X
32. Juul A, Skakkebæk NE. Why do normal children have acromegalic levels of IGF-I during puberty? J Clin Endocrinol Metab (2019) 104(7):2770–6. doi: 10.1210/jc.2018-02099
33. Birzniece V, Ho KKY. Sex steroids and the GH axis: Implications for the management of hypopituitarism. Best Pract Res Clin Endocrinol Metab (2017) 31:59–69. doi: 10.1016/j.beem.2017.03.003
34. Simard J, Hubert JF, Hosseinzadeh T, Labrie F. Stimulation of growth hormone release and synthesis by estrogens in rat anterior pituitary cells in culture. Endocrinology (1986) 119:2004–11. doi: 10.1210/endo-119-5-2004
35. Rochira V, Zirilli L, Maffei L, Premrou V, Aranda C, Baldi M, et al. Tall stature without growth hormone: Four male patients with aromatase deficiency. J Clin Endocrinol Metab (2010) 95(4):1626–33. doi: 10.1210/jc.2009-1743
36. Metzger DL, Kerrigant JR. Estrogen receptor blockade with tamoxifen diminishes growth hormone secretion in boys: Evidence for a stimulatory role of endogenous estrogens during male adolescence. J Clin Endocrinol Metab (1994) 79(2):513–8. doi: 10.1210/jcem.79.2.8045971
37. Bolamperti S, Mrak E, Moro GL, Sirtori P, Fraschini G, Guidobono F, et al. 17β-estradiol positively modulates growth hormone signaling through the reduction of SOCS2 negative feedback in human osteoblasts. Bone (2013) 55(1):84–92. doi: 10.1016/j.bone.2013.03.016
38. Birzniece V, Meinhardt UJ, Umpleby MA, Handelsman DJ, Ho KKY. Interaction between testosterone and growth hormone on whole-body protein anabolism occurs in the liver. J Clin Endocrinol Metab (2011) 96(4):1060–7. doi: 10.1210/jc.2010-2521
39. KERRIGAN JR, ROGOL AD. The impact of gonadal steroid hormone action on growth hormone secretion during childhood and adolescence*. Endocr. Rev (1992) 13:281–98. doi: 10.1210/edrv-13-2-281
40. Mauras N, Rogol AD, Veldhuis JD. Specific, time-dependent actions of low-dose ethinyl estradiol administration on the episodic release of growth hormone, follicle-stimulating hormone, and luteinizing hormone in prepubertal girls with turner’s syndrome. J Clin Endocrinol Metab (1989) 69(5):1053–8. doi: 10.1210/jcem-69-5-1053
41. Weissberger AJ, Ho KKY, Lazarus L. Contrasting effects of oral and transdermal routes of estrogen replacement therapy on 24-hour growth hormone (GH) secretion, insulin-like growth factor I, and gh-binding protein in postmenopausal women. J Clin Endocrinol Metab (1991) 72(2):374–81. doi: 10.1210/jcem-72-2-374
42. Veldhuis JD, Metzger DL, Martha PM, Mauras N, Kerrigan JR, Keenan B, et al. Estrogen and testosterone, but not a nonaromatizable androgen, direct network integration of the hypothalamo-somatotrope (growth homone)-insulin- like growth factor I axis in the human: Evidence from pubertal pathophysiology and sex-steroid hormone replac. J Clin Endocrinol Metab (1997) 82:3414–20. doi: 10.1210/jcem.82.10.4317
43. Binder G, Reinehr T, Ibáñez L, Thiele S, Linglart A, Woeful J, et al. GHD diagnostics in Europe and the US: An audit of national guidelines and practice. Horm Res Paediatr (2019) 92(3):150–6. doi: 10.1159/000503783
44. Frerichs C, Raymond L, Senniappan S. Variations in sex steroid priming for growth hormone stimulation testing in UK. Arch Dis Child (2017) 102(3):294. doi: 10.1136/archdischild-2016-311186
45. Carel JC, Ecosse E, Nicolino M, Tauber M, Leger J, Cabrol S, et al. Adult height after long term treatment with recombinant growth hormone for idiopathic isolated growth hormone deficiency: Observational follow up study of the French population based registry. Br Med J (2002) 325(7355):70. doi: 10.1136/bmj.325.7355.70
46. Cavallo L, Acquafredda A, Liuzzi S, Russo R, Zecchino C, Leuzzi R, et al. Growth hormone release during insulin tolerance, clonidine, arginine and growth hormone releasing hormone tests in short normal children and adolescents. J Endocrinol Investig Off J Ital Scociety Endocrinol (1992) 15(2):131–5. doi: 10.1007/BF03348678
47. Soliman AT, Adel A, Sabt A, Elbukhari E, Ahmed H, De Sanctis V. Does priming with sex steroids improve the diagnosis of normal growth hormone secretion in short children? Indian J Endocrinol Metab (2014) 18(Suppl 1):S80–3. doi: 10.4103/2230-8210.145078
48. Chalew SA, Udoff LC, Hanukoglu A, Bistritzer T, Armour KM, Kowarski AA. The effect of testosterone therapy on spontaneous growth hormone secretion in boys with constitutional delay. Am J Dis Child. (1988) 142(12):1345–8. doi: 10.1001/archpedi.1988.02150120099049
49. Lazar L, Phillip M. Is sex hormone priming in peripubertal children prior to growth hormone stimulation tests still appropriate? Horm Res Paediatr (2010) 73:299–302. doi: 10.1159/000284396
50. Ho KKY. Consensus guidelines for the diagnosis and treatment of adults with GH deficiency II: A statement of the GH research society in association with the European society for pediatric endocrinology, Lawson Wilkins society, European society of endocrinology. J Eur J Endocrinol (2007) 157(6):695–700. doi: 10.1530/EJE-07-0631
51. Loche S, Di Iorgi N, Patti G, Noli S, Giaccardi M, Olivieri I, et al. Growth hormone deficiency in the transition age. Endocr Dev (2018) 33:46–56. doi: 10.1159/000487525
52. Thomas M, Massa G, Maes M, Beckers D, Craen M, François I, et al. Growth hormone (GH) secretion in patients with childhood-onset GH deficiency: Retesting after one year of therapy and at final height. Horm Res (2003) 59(1):7–15. doi: 10.1159/000067936
53. Laurer E, Sirovina A, Blaschitz A, Tischlinger K, Montero-Lopez R, Hörtenhuber T, et al. The landscape of retesting in childhood-onset idiopathic growth hormone deficiency and its reversibility: A systematic review and meta-analysis. Eur J Endocrinol (2022) 187(2):265–78. doi: 10.1530/EJE-21-1179
54. Zucchini S, Pirazzoli P, Baronio F, Gennari M, Bal MO, Balsamo A, et al. Effect on adult height of pubertal growth hormone retesting and withdrawal of therapy in patients with previously diagnosed growth hormone deficiency. J Clin Endocrinol Metab (2006) 91(11):4271–6. doi: 10.1210/jc.2006-0383
55. Molina S, Paoli M, Camacho N, Arata-Bellabarba G, Lanes R. Is testosterone and estrogen priming prior to clonidine useful in the evaluation of the growth hormone status of short peripubertal children? J Pediatr Endocrinol Metab (2008) 21(3):257–66. doi: 10.1515/JPEM.2008.21.3.257
56. Martínez AS, Domené HM, Ropelato MG, Jasper HG, Pennisi PA, Escobar ME, et al. Estrogen priming effect on growth hormone (GH) provocative test: A useful tool for the diagnosis of GH deficiency. J Clin Endocrinol Metab (2000) 85(11):4168–72. doi: 10.1210/jc.85.11.4168
57. Gonc EN, Kandemir N, Ozon A, Alikasifoglu A. Final heights of boys with normal growth hormone responses to provocative tests following priming. J Pediatr Endocrinol Metab (2008) 21:963–71. doi: 10.1515/jpem.2008.21.10.963
58. Galazzi E, Improda N, Cerbone M, Soranna D, Moro M, Fatti LM, et al. Clinical benefits of sex steroids given as a priming prior to GH provocative test or as a growth-promoting therapy in peripubertal growth delays: Results of a retrospective study among ENDO-ERN centres. Clin Endocrinol (Oxf). (2021) 94(2):219–28. doi: 10.1111/cen.14337
59. Chioma L, Papucci G, Fintini D, Cappa M. Use of testosterone gel compared to intramuscular formulation for puberty induction in males with constitutional delay of growth and puberty: A preliminary study. J Endocrinol Invest. (2018) 41(2):259–63. doi: 10.1007/s40618-017-0726-7
60. Mastromattei S, Todisco T, Chioma L, Ubertini G, Pattumelli MG, Fintini D, et al. Efficacy of short-term induction therapy with low-dose testosterone as a diagnostic tool in the workup of delayed growth and puberty in boys. J Endocrinol Invest. (2022) 45(12):2377–84. doi: 10.1007/s40618-022-01879-3
61. Moll GW, Rosenfield RL, Fang VS. Administration of low-dose estrogen rapidly and directly stimulates growth hormone production. Am J Dis Child (1986) 140(2):124–7. doi: 10.1001/archpedi.1986.02140160042027
62. Borghi MMS, Longui CA, Calliari LE, Faria CDC, Kochi C, Monte O. Transdermal estradiol priming during clonidine stimulation test in non-growth hormone deficient children with short stature: A pilot study. J Pediatr Endocrinol Metab (2006) 19(3):223–7. doi: 10.1515/JPEM.2006.19.3.223
63. Bacon GE, Lowrey GH, Knoller M. Comparison of arginine infusion and diethylstilbestrol as a means of provoking growth hormone secretion. J Pediatr (1969) 75(3):385–90. doi: 10.1016/S0022-3476(69)80263-5
64. Ross RJ, Grossman A, Davies PS, Savage MO, Besser GM. Stilboestrol pretreatment of children with short stature does not affect the growth hormone response to growth hormone-releasing hormone. Clin Endocrinol (Oxf). (1987) 27(2):155–61. doi: 10.1111/j.1365-2265.1987.tb01140.x
65. Lanes R, Lifshitz F, Sekaran C, Fort P, Recker B. Premarin priming does not alter growth hormone release following exercise. J Endocrinol Invest (1986) 9(6):443–6. doi: 10.1007/BF03346963
66. Wilson DM, Dotson RJN, Neely EK, Cohen P, Hintz RL, Rosenfeld RG. Effects of estrogen on growth hormone following clonidine stimulation. Am J Dis Child (1993) 147(1):63–5. doi: 10.1001/archpedi.1993.02160250065019
67. Gönç EN, Yordam N, Kandemir N, Alikasifoglu A. Comparison of stimulated growth hormone levels in primed versus unprimed provocative tests: Effect of various testosterone doses on growth hormone levels. Horm Res (2001) 56(1–2):32–7. doi: 10.1159/000048087
68. Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, et al. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: Growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr (2017) 86:361–97. doi: 10.1159/000452150
69. Chesover AD, Dattani MT. Evaluation of growth hormone stimulation testing in children. Clin Endocrinol (Oxf) (2016) 84(5):708–14. doi: 10.1111/cen.13035
70. Joint Formulary Committee. British national formulary 83. (2019). BMJ Publishing and the Royal Pharmaceutical Society.
71. BSPED UK consensus national guidelines for sex hormone priming (2021). Available at: https://www.bsped.org.uk/media/1875/bsped-priming-guidelines-v3-16521.pdf.
72. Shoham Z, Kopernik G. Tools for making correct decisions regarding hormone therapy. part I: Background and drugs. Fertil Steril (2004) 81(6):1447–57. doi: 10.1016/j.fertnstert.2003.10.052
73. Shoskes JJ, Wilson MK, Spinner ML. Pharmacology of testosterone replacement therapy preparations. Transl Androl Urol (2016) 5(6):834–43. doi: 10.21037/tau.2016.07.10
74. Stancampiano MR, Lucas-Herald AK, Russo G, Rogol AD, Ahmed SF. Testosterone therapy in adolescent boys: The need for a structured approach. Horm Res Paediatr (2020) 92:215–28. doi: 10.1159/000504670
75. Radetti G, Elsedfy HH, Khalaf R, Meazza C, Pagani S, El Kholy M, et al. Pegvisomant-primed growth hormone (GH) stimulation test is useful in identifying true GH deficient children. Hormones (2017) 16(3):291–6. doi: 10.14310/horm.2002.1748
Keywords: pubertal delay, sex steroid priming, GH deficiency (GHD), short stature, peripubertal age, growth hormone stimulation test (GHST)
Citation: Partenope C, Galazzi E, Albanese A, Bellone S, Rabbone I and Persani L (2022) Sex steroid priming in short stature children unresponsive to GH stimulation tests: Why, who, when and how. Front. Endocrinol. 13:1072271. doi: 10.3389/fendo.2022.1072271
Received: 17 October 2022; Accepted: 14 November 2022;
Published: 29 November 2022.
Edited by:
Sandro Loche, Ospedale Microcitemico, ItalyReviewed by:
Nicola Improda, University of Naples Federico II, ItalyKatharina Schilbach, Medizinische Klinik und Poliklinik IV, LMU Klinikum, Germany
Copyright © 2022 Partenope, Galazzi, Albanese, Bellone, Rabbone and Persani. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cristina Partenope, cGFydGVub3BlLmNyaXN0aW5hQGdtYWlsLmNvbQ==
 Cristina Partenope
Cristina Partenope Elena Galazzi2
Elena Galazzi2 Simonetta Bellone
Simonetta Bellone Ivana Rabbone
Ivana Rabbone Luca Persani
Luca Persani