- 1Section of Endocrinology and Diabetology, Health Promotion, Department of Health Promotion, Mother and Child Care, Internal Medicine and Medical Specialties “G. D’Alessandro”, PROMISE, University of Palermo, Palermo, Italy
- 2Department of Pathology, Diagnostic and Therapeutic Services, IRCCS ISMETT (Istituto di Ricovero e Cura a Carattere Scientifico-Istituto Mediterraneo per i Trapianti e Terapie ad alta specializzazione)-UPMC (University of Pittsburgh Medical Center), Palermo, Italy
- 3Section of “Medicina Nucleare e Terapia Radiometabolica”, La Maddalena, Palermo, Italy
- 4Department of Biomedicine, Neurosciences and Advanced Diagnostics, Institute of Radiology, University of Palermo, Palermo, Italy
- 5Department for the Treatment and Study of Abdominal Diseases and Abdominal Transplantation, IRCCS-ISMETT (Istituto di Ricovero e Cura a Carattere Scientifico-Istituto Mediterraneo per i Trapianti e Terapie ad alta specializzazione), UPMC (University of Pittsburgh Medical Center), Palermo, Italy
- 6Department of General Surgery and Medical-Surgical Specialties, University of Catania, Catania, Italy
An ectopic thyroid is a form of thyroid dysgenesis in which the entire thyroid gland or parts of it may be located in another part of the body than the usual place. The most frequent location is the base of the tongue. Although most cases are asymptomatic, symptoms related to tumor size and its relationship with surrounding tissues, hormonal dysfunction, and seldom malignancy may also occur. Here, we describe the case of an asymptomatic woman who was thyroidectomized 19 years previously for a toxic goiter and treated with conventional L-thyroxine therapy, until we enacted a progressive reduction of dosage of the replacement therapy. Incidentally, because of occasional abdomen discomfort, she was hospitalized in our Division of Endocrinology as there was ultrasound evidence of a large mass in the liver dislocating and imprinting the choledochal duct in the pre-pancreatic site, the gallbladder, and the cystic duct, which could not be dissociated from the contiguous hepatic parenchyma and was in very close proximity to the second duodenal portion and the head of the pancreas. Imaging techniques, such as TC, MR, TC/PET, and 131I scintigraphy, confirmed the large lesion with a diameter on the axial plane of about 8 × 5.5 cm and a cranio-caudal extension of about 6 cm. The impossibility of surgical debulking and/or radiometabolic 131I therapy, in the absence of compression symptoms, led to the multidisciplinary decision of a clinical and instrumental follow-up of this rare lesion.
Introduction
Ectopic thyroid is a rare abnormality of the embryonic development of the thyroid gland. The estimated frequency is 0.17 per 1,000 patients (1–3). The most common locations are along the midline from the foramen cecum to the mediastinum. Ectopic thyroid tissue usually occurs in the base of the tongue but may also develop in the mediastinum or in the subdiaphragmatic organs (1, 4). Lingual thyroid ectopia is the most frequent form (about 90% of all cases of thyroid ectopia), whereas subdiaphragmatic forms are rather rare. Other distant locations in which ectopic thyroid tissue has been reported include the heart, thymus, esophagus, duodenum, gallbladder, and adrenal glands (4). However, there are rare reports regarding ectopic thyroid in the stomach. Ectopic thyroid tissues in distant sites could be due to aberrant migration or heterotopic differentiation of uncommitted endodermal cells (5–9). According to several reports, a consensual hypothesis for the formation of sub-diaphragmatic thyroid ectopia is failure of migration or intemperate descent of the thyroid anlage (5–7).
Ectopic thyroid is generally more frequent in women and tends to be asymptomatic. Hypothyroidism occurs in about 33% of patients with thyroid ectopy, which is the first cause of congenital hypothyroidism in pediatric age (5). A large percentage of patients with ectopic lingual thyroid without a coexisting eutopic thyroid tissue will develop sub-clinical hypothyroidism that becomes clinically manifest during periods of physiological stress (1, 5, 10). Accessory thyroid tissue may therefore be functional but is generally insufficient for normal function if the main thyroid gland is completely removed (5, 10–12).
Case description
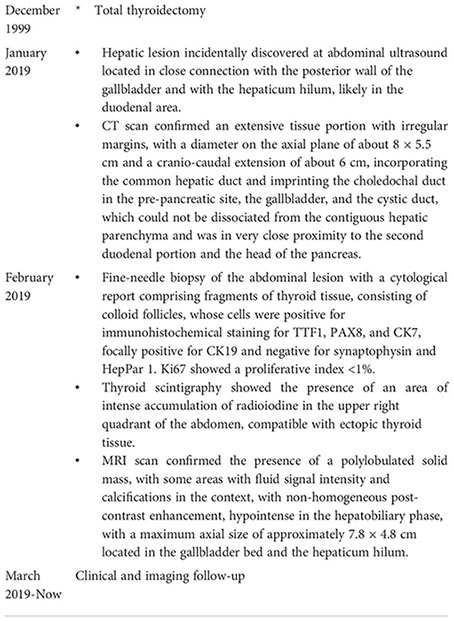
A 65-year-old obese woman was on levothyroxine therapy at the dose of 50 mcg/day (dosage progressively decreased during the last 2 years from 100 mcg/day after surgery). Due to the onset of abdominal pain in the right side and the presence of dark urine, owing to dehydration, the patient had undergone an ultrasound of the abdomen, showing an oval mass of about 7 cm. It had an intensely hyperechoic peripheral portion and a central portion with a dishomogeneous echo structure and was located in close connection with the posterior wall of the gallbladder and with the hepaticum hilum, likely in the duodenal area. Abdomen CT scans with and without contrast (128 slice CT, Somatom Definition AS, Siemens Healthcare, Erlangen, Germany) confirmed the presence in the hepaticum hilum of an extensive tissue portion with irregular margins, unhomogeneously vascularized and characterized by small calcifications in its context, with a diameter on the axial plane of about 8 × 5.5 cm and a cranio-caudal extension of about 6 cm, incorporating the common hepatic duct and imprinting the choledochal duct in the pre-pancreatic site, the gallbladder, and the cystic duct, which could not be dissociated from the contiguous hepatic parenchyma and was in very close proximity to the second duodenal portion and the head of the pancreas (Figure 1).
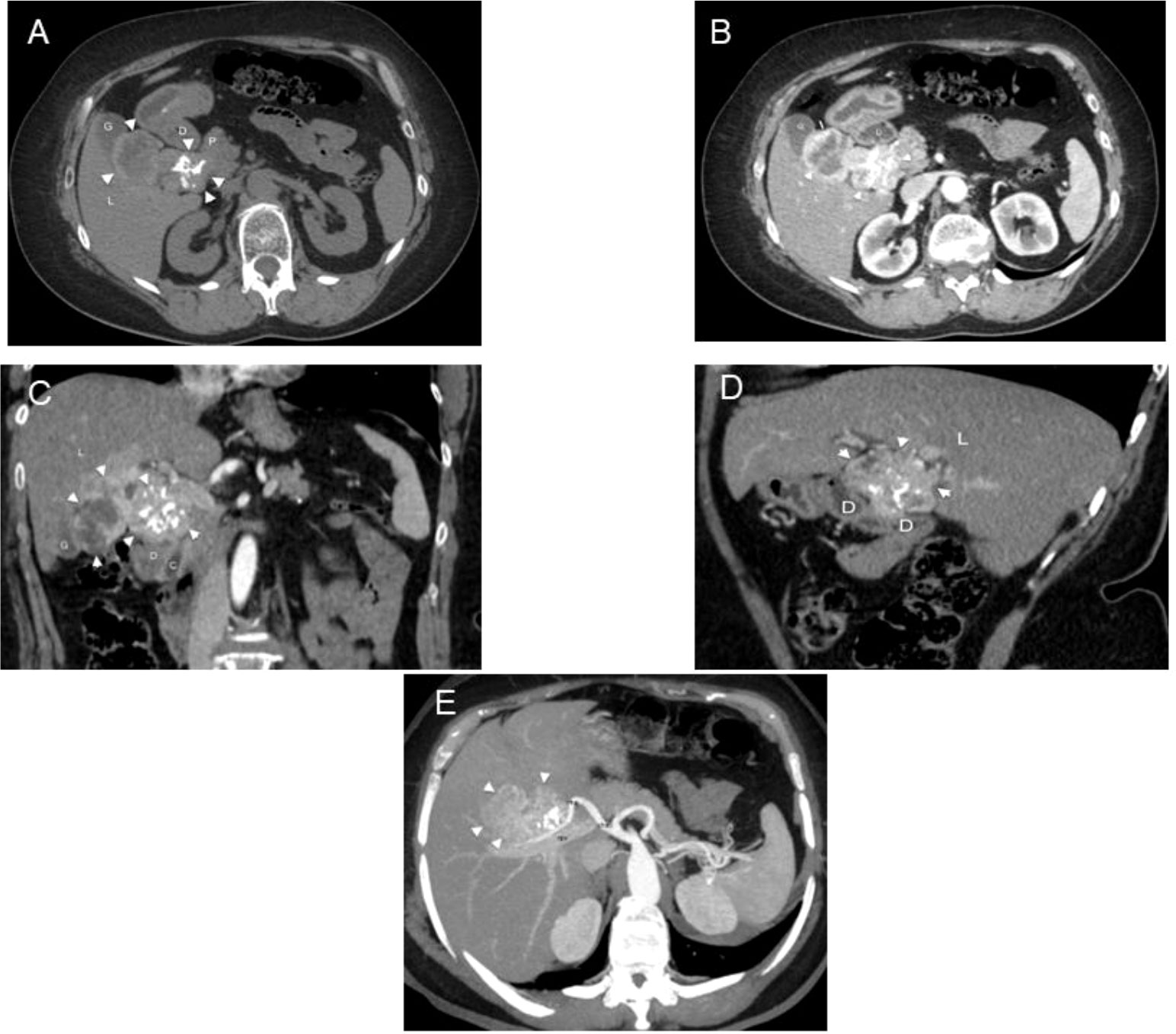
Figure 1 CT in a 65-year-old woman with ectopic thyroid in the hepatic hilum. (A) Unenhanced and (B) enhanced axial CT images. Dishomogeneous density and partially calcified mass (arrowheads) can be observed in the right hypochondrium between the duodenum (D), liver (L), pancreas (P), and gallbladder (G). Dishomogeneous enhancement of the mass is appreciable in the arterial phase. (C) Enhanced coronal CT image. The mass (arrowheads) has a bilobated shape and occupies the hepatic hilum, between the liver (L), gallbladder (G), duodenum (D), pancreas (P), and choledoch (C). (D) Enhanced sagittal CT image. The mass (arrowheads) pushes downward the second portion of the duodenum (D), being interposed between it and the liver (L). (E) Axial 3D MIP. The mass (arrowheads) is supplied by a branch of the right hepatic artery (rha); cha, common hepatic artery; rpv, right portal branch.
In the opinion of the radiologist, as a first hypothesis, the observations pointed to a heteroplastic lesion of likely pertinence of the biliary tract. Because of the patient’s symptoms (abdominal discomfort and dark urine) and the rise in alkaline phosphatase values, ursodeoxycholic acid therapy at the dose of 450 mg per day was started.
To clarify the nature of the lesion, a fine-needle biopsy of the lesion was performed with ultrasound endoscopy, with a cytological report of a hematoxylin and eosin-stained sample comprising fragments of thyroid tissue, consisting of colloid follicles, whose cells were positive for immunohistochemical staining for TTF1 (Figures 2A, B) and PAX8 and CK7 (Figure 2C), focally positive for CK19 and negative for synaptophysin and HepPar 1. Ki67 showed a proliferative index <1% (Figure 2D). The sample also contained cystic content consisting of macrophages (CD68 positive) and rare fragments of gastrointestinal mucosa.
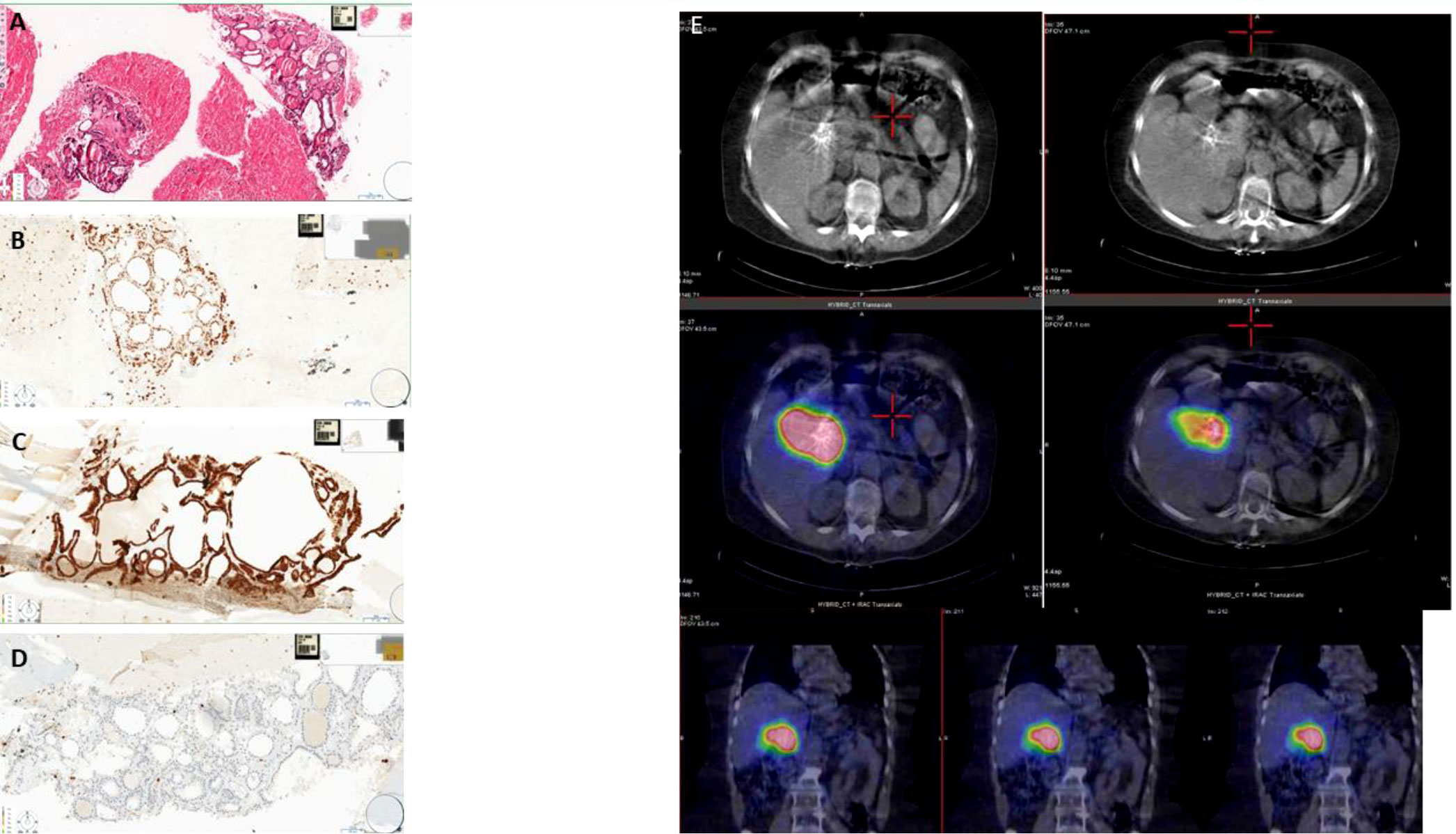
Figure 2 (A) Fine-needle aspiration biopsy (section colored in hematoxylin–eosin showing thyroid follicles mixed with red blood cells). (B) Fine-needle aspiration biopsy (nuclear positivity for TTF1 in the thyroid follicles). (C) Fine-needle aspiration biopsy (membrane positivity for CK7 in the thyroid follicles). (D) Fine-needle aspiration biopsy (rare positive nuclei for Ki67). (E) CT and SPECT/CT fusion images showing intense 131I accumulation in the region of the hepatic hilum site of known ectopic thyroid tissue that contracts with the hepatic parenchyma, its vascular structures, the displaced gallbladder, the duodenum, and the head of the pancreas.
Thyroid scintigraphy with 131I (GE Healthcare Infinia, General Electric Medical Systems, Milwaukee, WI, USA) confirmed the presence of an area of intense accumulation of radioiodine in the upper right quadrant of the abdomen, compatible with ectopic thyroid tissue (Figure 2E). Elevated thyroglobulin levels were consistent with the above diagnosis.
Therefore, excluding the suspicion of a lesion originating from intestinal cells, the presence of thyroid tissue in this site required differential diagnosis distinguishing between ectopic thyroid tissue and metastasis from occult thyroid carcinoma.
In addition, in order to exclude the malignant nature of the lesion, the patient underwent total body PET/CT with 18F-FDG, which did not show tracer distribution abnormalities in the exposed regions (not shown).
MRI of the abdomen with and without contrast (1.5 Tesla, Signa HDxt, GE Medical Systems, Milwaukee, WI, USA) confirmed the presence of a polylobulated solid mass slightly hypointense in the T1-weighted sequences and slightly hyperintense in the T2-weighted sequences, with some areas with fluid signal intensity and calcifications in the context, with non-homogeneous post-contrast enhancement, hypointense in the hepatobiliary phase, with a maximum axial size of approximately 7.8 × 4.8 cm located in the gallbladder bed and the hepaticum hilum (Figure 3). This mass did not present cleavage planes with the liver, biliary branches and vessels to the hepaticum hilum, gallbladder, duodenum, and head of the pancreas. Due to all the abovementioned clinical and imaging findings, we diagnosed a levothyroxine secreting thyroid ectopia.
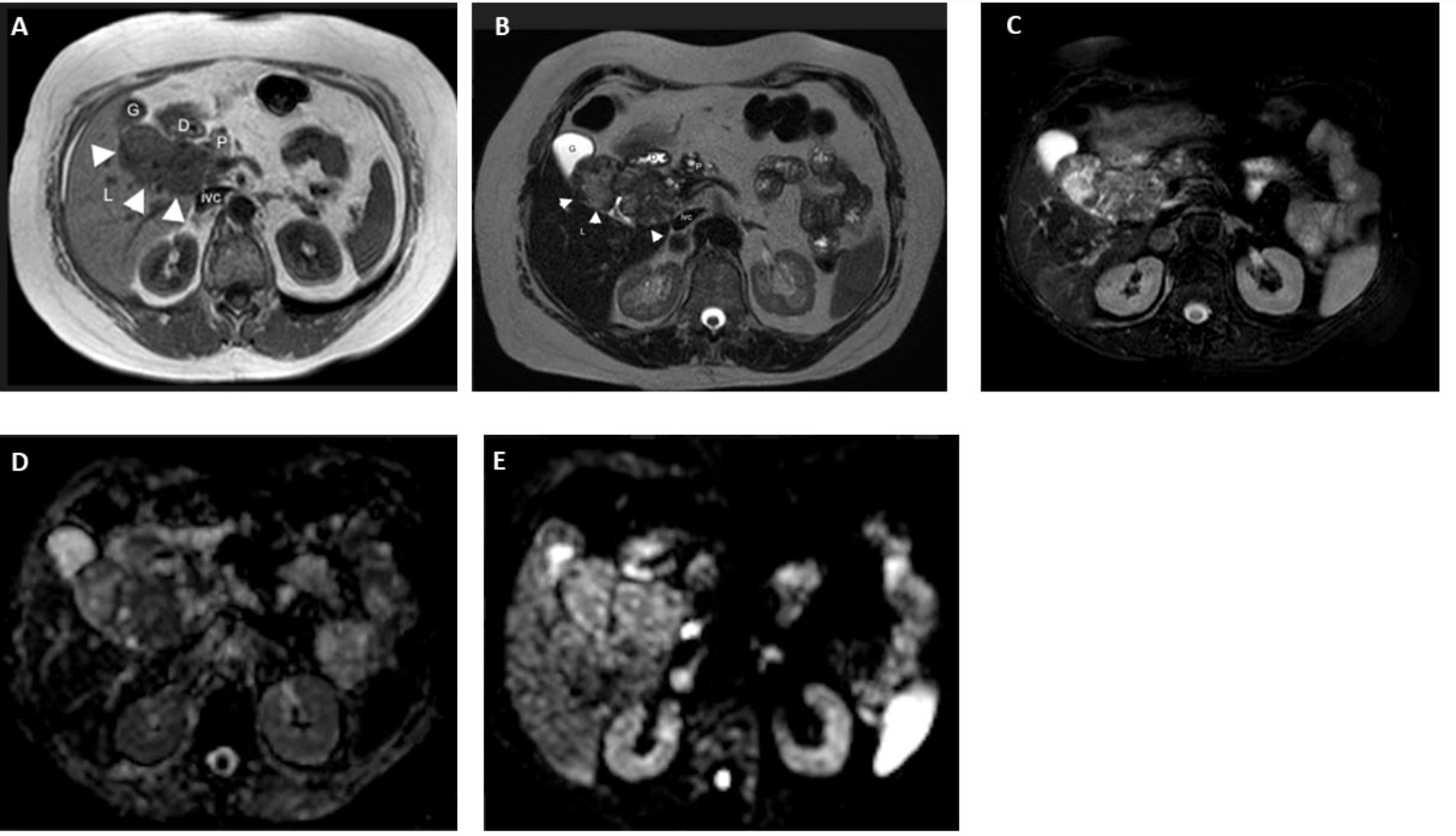
Figure 3 MR in a 65-year-old woman with ectopic thyroid in the hepatic hilum. (A) Axial T1 image. Dishomogeneously hypointense (with respect to the liver) mass in the right hypochondrium between the duodenum (D), liver (L), pancreas (P), gallbladder (G), and inferior vena cava (ivc). Axial (B) T2 and (C) T2 fat-sat images. The mass is dishomogenously hyperintense with respect to the liver. (D) DWI B800 image. The mass shows no meaningful restriction. (E) ADC image. High ADC in the mass. (F) MR-Cholangiography. Common bile duct (cbd) compressed and dislocated by the bilobated mass (m); C, choledocus; G, gallbladder; M (mass); Wirsung (arrowheads).
We excluded the possibility of any medical treatment due to the size of the lesion, and therefore we focused on the possibility of surgical debulking, through a multidisciplinary evaluation with a team of expert surgeons. At present, the size of the lesion and its extension prevent surgical excision due to the absence, in fact, of clear cleavage planes with the contiguous structures.
In our patient, considering the stability of the lesion at instrumental follow-up and the immunohistochemical pattern, not compatible with malignant thyroid tissue, and having established the impossibility of surgical debulking, in the absence of compression symptoms, we decided on a clinical, biochemical, and instrumental follow-up of the lesion (Table 1).
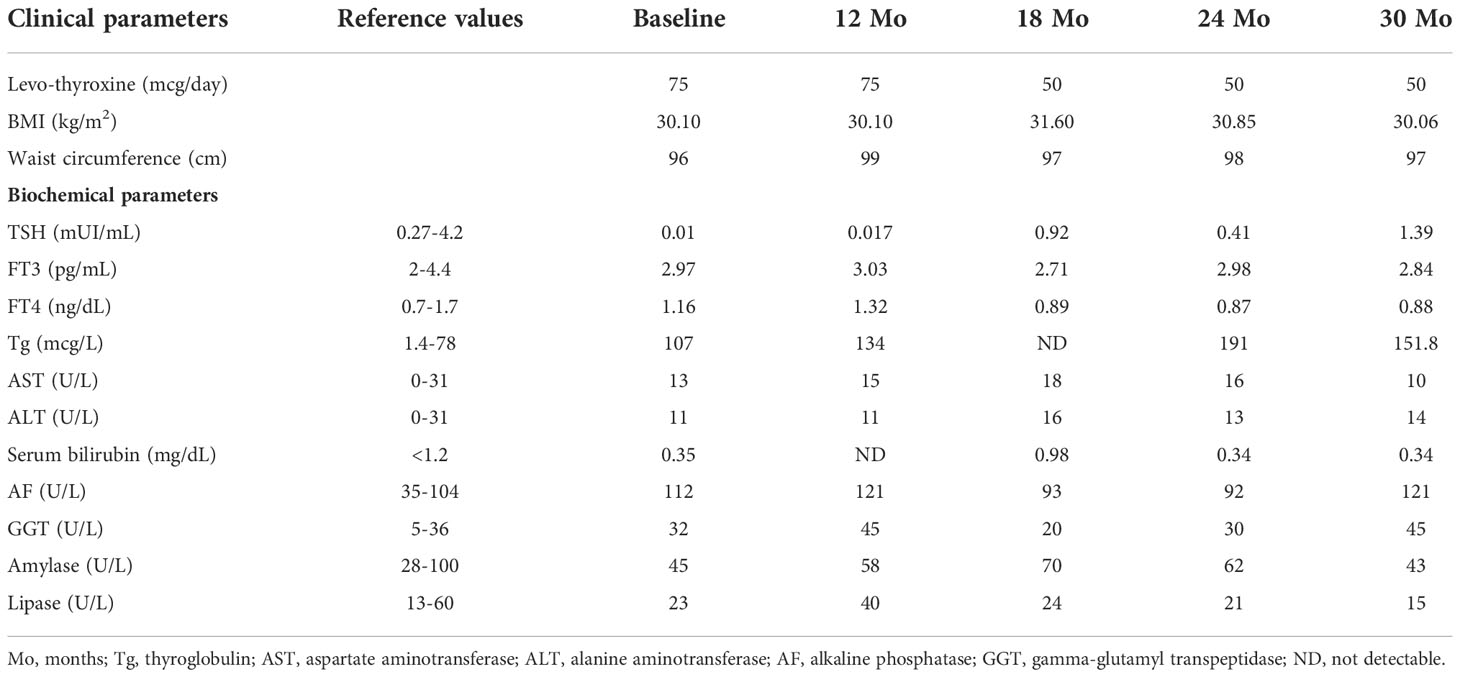
Table 1 Clinical and biochemical parameters of the patient from the first observation and during the follow-up.
Assays
Thyroglobulin, ALT, AST, GGT, alkaline phosphatase, gammaGT, amylase, lipase, and bilirubin were measured in our centralized laboratory with standard methods. TSH, FT4, and FT3 were measured by electrochemiluminescence (ECLIA, Elecsys Insulin, Roche, Milan, Italy).
Timeline
Discussion
The thyroid is the first endocrine gland to form during embryonic development. During the third or fourth week of gestation, an endodermal diverticulum forms in the ventral part of the pharyngeal intestine which descends in the midline, from the caecum (located between the posterior third and anterior two-thirds of the tongue), positioning itself around the seventh week of gestation in the final location of the gland, anterior to the pretrachea and the larynx. During migration, the gland remains in connection with the floor of the branchial intestine through the thyroglossal duct. The latter undergoes atrophy before the definitive formation of the thyroid gland, approximately between the 6th and 10th weeks of gestation (1, 5, 13). A variety of unexpected locations of thyroid tissues have been reported including gallbladder (14–16), lungs (17–19), duodenum (20), porta hepatis (21), pancreas (22), adrenal glands (23, 24), fallopian tube (25–27), and small intestinal mesentery (28). Here, we report a rare case of thyroid tissue located in the gallbladder bed, accompanied by adenoma and a cyst lined with pseudostratified ciliated columnar epithelium in the region of the gallbladder neck.
According to the well-established embryogenetic theory, at the basis of thyroid ectopy there seems to be an abnormal or aberrant descent of the thyroid sketch during intrauterine life; ectopic tissue can lodge in any site of the normal migration path and may or may not coexist with a eutopic thyroid gland (1, 5, 29).
The most common interpretation is that of abnormal differentiation of the endodermal precursors of the anterior intestine (30). Furthermore, ectopic thyroid tissue could also be explained as a teratoma (5). The majority of the causes are multifactorial ones associated with the embryological process, and recently genetic research has demonstrated that the gene transcription factors TITF-1 (Nkx2-1), Foxe1 (TITF-2), and PAX-8 are essential for thyroid maturation and differentiation. Mutation in these genes may share a connection with abnormal migration of the thyroid (1, 31).
The cases of intra-abdominal ectopic thyroid gland reported in the literature are very limited.
In our case, once the thyroid nature of the lesion was ascertained, any metastasis from occult thyroid carcinoma or the neoplastic degeneration of the ectopic tissue itself was excluded, considering that ectopic thyroid tissue can undergo pathological processes that cannot be differentiated, clinically and histologically, from those affecting normal thyroid (5). At a first observation, it was immediately clear that the daily requirement of LT4 was absolutely underestimated compared with the pro-Kg dose required in a patient undergoing total thyroidectomy (body weight post thyroidectomy 72.5 kg: dose per kg 0.68 mg; body weight at our first observation 76 kg, dose per kg 0.65 mg) (32). This circumstance should have promptly led the clinician to suspect the presence of a levothyroxine-secreting mass.
As regards the therapeutic management of the abdominal lesion, the possibility of treating it with radioiodine 131 was immediately excluded. Therapy with 131I (RAI) is indicated for ablative or adjuvant purposes in patients with differentiated thyroid carcinoma undergoing total thyroidectomy aiming at eradicating any residual thyroid and/or microfocus of carcinoma present in the thyroid tissue (33, 34).
Several case histories indicate that hyperthyroidism with large goiter rarely heals after a single administration of 131I, so in these cases the primary indication is surgical (34). The size of the lesion therefore plays a role both in the effectiveness of the treatment and in the dose required and consequently as regards various presumable side effects. RAI is generally well tolerated, whether given for a hyperthyroid disorder or for a compressive goiter. However, adverse effects may occur related to 1) thyroid function; 2) size of the thyroid gland; 3) immunological response; and 4) consequences of extrathyroid irradiation (i.e., transient hyperthyroidism, thyroid inflammation, sialadenitis, immunogenic effects, carcinogenesis) (35). If RAI could have been used in our patient, the site and size of the lesion would significantly have influenced the side effects of its use. Indeed, if we translate the common adverse effects related to the use of RAI to the case in question, it would immediately become clear that there would be catastrophic outcomes related to inflammation, for example, of the surrounding tissues (acute hepatitis) or the thyrotoxic storm that could be unleashed irradiating a lesion of this size. For this reason, although interesting, we dropped the idea of RAI treatment.
The rarity of our case is attributable not only to the site but also to the dimensional and bulk characteristics such as not to present cleavage planes with the liver, biliary branches, vessels to the hepaticum hilum, gallbladder, duodenum, and head of the pancreas and thus compromising the possibility of surgical removal. However, it should be taken into account that further episodes of compression of the biliary duct may occur, and in case of huge enlargement of the mass, surgical resection should be undertaken.
Conclusion
Currently, there are no clear indications on the management of thyroid ectopia. Surgery is generally recommended, notably in the presence of severe obstruction, bleeding, ulceration, refractory hyperthyroidism, severe/local respiratory disorders, and suspected malignancy (1, 5). In other cases, follow-up must be taken into consideration.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Comitato etico di Palermo. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
CS, MB, RP, RM, AC, and SG collected the data. CS and VG drafted the manuscript. CG, PR, and MM edited and revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ibrahim NA, Fadeyibi IO. Ectopic thyroid: Etiology, pathology and management. Hormones (Athens) (2011) 10:261–9. doi: 10.14310/horm.2002.1317
2. Larochelle D, Arcand P, Belzile M, Gagnon NB. Ectopic thyroid tissue: A review of the literature. J Otolaryngol (1979) 8:523–30.
3. Batsakis JG, El-Naggar AK, Luna MA. Thyroid gland ectopia. Ann Otol Rhinol Laryngol (1996) 105:996–1000. doi: 10.1177/000348949610501212
5. Guerra G, Cinelli M, Mesolella M, Tafuri D, Rocca A, Amato B, et al. Morphological, diagnostic and surgical features of ectopic thyroid gland: A review of literature. Int J Surg (2014) 12 Suppl;1:S3–11. doi: 10.1016/j.ijsu.2014.05.076
6. Szinnai G. Genetics of normal and abnormal thyroid development in humans. Best Pract Res Clin Endocrinol Metab (2014) 28:133e150. doi: 10.1016/j.beem.2013.08.005
7. Gillam MP, Kopp P. Genetic regulation of thyroid development. Curr Opin Pediatr (2001) 13:358e363. doi: 10.1097/00008480-200108000-00013
8. Silberschmidt D, Rodriguez-Mallon A, Mithboakar P, Calì G, Amendola E, Sanges R, et al. In vivo role of different domains and of phosphorylation in the transcription factor Nkx2-1. BMC Dev Biol (2011) 11:9. doi: 10.1186/1471-213X-11-9
9. Nitsch R, Di Dato V, di Gennaro A, de Cristofaro T, Abbondante S, De Felice M, et al. Comparative genomics reveals a functional thyroid-specific element in the far upstream region of the PAX8 gene. BMC Genomics (2010) 11:306. doi: 10.1186/1471-2164-11-306
10. Wang F, Zang Y, Li M, Liu W, Wang Y, Yu X, et al. DUOX2 and DUOXA2 variants confer susceptibility to thyroid dysgenesis and gland-in-situ with congenital Hypothyroidism.Front. Endocrinol (Lausanne) (2020) 11:237. doi: 10.3389/fendo.2020.00237
11. Eli SU, Marnane C, Peter R, Winter S. Ectopic, submandibular thyroid causing hyperthyroidism. J Laryngol Otol (2011) 125:1091–3. doi: 10.1017/S0022215111000855
12. Kumar R, Gupta R, Bal CS, Khullar S, Malhotra A. Thyrotoxicosis in a patient with submandibular thyroid. Thyroid (2000) 10:363e365. doi: 10.1089/thy.2000.10.363
13. DeFelice M, Di Lauro ,R. Thyroid development and its disorders: genetic and molecular mechanisms. Endocr Rev (2004) 25:722e746. doi: 10.1210/er.2003-0028
14. Liang K, Liu JF, Wang YH, Tang GC, Teng LH, Li F. Ectopic thyroid pre-senting as a gallbladder mass. Ann R Coll Surg Engl (2010) 92:W4eW6. doi: 10.1308/147870810X12659688852473
15. Cicek Y, Tasci H, Gokdogan C, Ones S, Goksel S. Intra-abdominal ectopic thyroid. Br J Surg (1993) 80:316. doi: 10.1002/bjs.1800800315
16. Evuboglu E, Kapan M, Ipek T, Ersan Y, Oz F. Ectopic thyroid in the abdomen: report of a case. Surg Today (1999) 29:472e474. doi: 10.1007/BF02483044
17. Cattaneo F, Iaccio A, Guerra G, Montagnani S, Ammendola R. NADPH-oxidase-dependent reactive oxygen species mediate EGFR transactivation by FPRL1 in WKYMVm-stimulated human lung cancer cells. Free. Radic Biol Med (2011) 51:1126e1136. doi: 10.1016/j.freeradbiomed.2011.05.040
18. Zhang W, Zhang H, Hou Q, Hou H, Xu D, Liu J, et al. Ectopic thyroid microfollicular adenoma in the lung: A case report. Med (Baltimore) (2019) 98:e16832. doi: 10.1097/MD.0000000000016832
19. Cheng L, Jiang X, Jiang Y. Ectopic thyroid as multiple nodules in bilateral lung lobes: A case report. Gland Surg (2020) 9:806–11. doi: 10.21037/gs-19-499
20. Liu YT, Chen KC, Chen MK, Yang CW. Ectopic thyroid tissue in duodenum-a rare case in a young woman. Am J Gastroenterol (2021) 116:448. doi: 10.14309/ajg.0000000000001040
21. Chooah O, Ding J, Fei JL, Xu FY, Yue T, Pu CL, et al. Radiological insights of ectopic thyroid in the porta hepatis: A case report and review of the literature. World J Clin Cases (2021) 9:3432–41. doi: 10.12998/wjcc.v9.i14.3432
22. Ma A, Liu H. Ectopic thyroid of the pancreas: A case report and literature review. Med (Baltimore) (2017) 96:e8707. doi: 10.1097/MD.0000000000008707
23. Rawitzer J, Kapakoglou A, Walz MK, Schmid KW, Reis H. Ektopes schilddrüsengewebe in der nebenniere: Ein fallbericht in zusammenschau mit der literatur [Ectopic thyroid tissue in the adrenal gland: A case report and review of the literature]. Pathologe (2020) 41:177–80. doi: 10.1007/s00292-019-00724-4
24. Hagiuda J, Kuroda I, Tsukamoto T, Ueno M, Yokota C, Hirose T, et al. Ectopic thyroid in an adrenal mass: A case report. BMC Urol (2006) 6:18. doi: 10.1186/1471-2490-6-18
25. Roth LM, Miller AW, Talerman AM. Typical thyroid-type carcinoma arising in struma ovarii: A report of 4 cases and review of the literature. Int J Gynecol Pathol (2008) 27:496e506. doi: 10.1097/PGP.0b013e31816a74c6
26. Hoda SA, Huvos AG. Struma salpingis associated with struma ovarii. Am J Surg Pathol (1993) 17:1187e1189. doi: 10.1097/00000478-199311000-00013
27. Yilmaz F, Uzunlar AK, Sogutau N. Ectopic thyroid tissue in the uterus. Acta Obstet Gynecol Scand (2005) 84:201e202. doi: 10.1111/j.0001-6349.2005.0255d.x
28. Kutz M, Vila JJ, Urman JM, Garaigorta M, Guinduláin E, Tarifa A, et al. Ectopia tiroidea retroperitoneale [Ectopic retroperitoneal thyroid gland]. Gastroenterol Hepatol (2012) 35:400–3. doi: 10.1016/j.gastrohep.2012.03.013
29. De Falco M, Oliva G, Santangelo G, Del Giudice S, Parmeggiani U. L'ectopia tiroidea: problemi di diagnosi e terapia [Thyroid ectopia: problems in diagnosis and therapy]. G Chir (2010) 31:279–81.
30. Harach HR. Ectopic thyroid tissue adjacent to the gallbladder. Histopathology (1998) 32:90–1. doi: 10.1046/j.1365-2559.1998.0241g.x
31. Abu-Khudir R, Paquette J, Lefort A, Libert F, Chanoine JP, Vassart G, et al. Transcriptome, methylome and genomic variations analysis of ectopic thyroid glands. PloS One (2010) 5:e13420. doi: 10.1371/journal.pone.0013420
32. Jonklaas J, Bianco AC, Bauer AJ, Burman KD, Cappola AR, Celi FS, et al. Guidelines for the treatment of hypothyroidism: prepared by the american thyroid association task force on thyroid hormone replacement. Thyroid (2014) 24:1670–751. doi: 10.1089/thy.2014.0028
33. Salvatori M, Rufini V, Corsello SM, Saletnich I, Rota CA, Barbarino A, et al. Thyrotoxicosis due to ectopic retrotracheal adenoma treated with radioiodine. J Nucl Biol Med (1993) 37:69–72.
34. Ciarallo A, Rivera J. Radioactive iodine therapy in differentiated thyroid cancer: 2020 update. AJR Am J Roentgenol (2020) 215:285–91. doi: 10.2214/AJR.19.22626
Keywords: occult thyroid, hepatic thyroid, hyperthyroidism, 131I scintigraphy, hepatic lesion
Citation: Di Stefano C, Guarnotta V, Barbaccia M, Paratore R, La Monica R, Lo Casto A, Midiri M, Gruttadauria S, Giordano C and Richiusa P (2022) Hepatic incidentaloma: An asymptomatic ectopic thyroid tissue. Front. Endocrinol. 13:1066188. doi: 10.3389/fendo.2022.1066188
Received: 10 October 2022; Accepted: 21 November 2022;
Published: 12 December 2022.
Edited by:
Laura Sterian Ward, State University of Campinas, BrazilReviewed by:
Leonardo Rossi, University of Pisa, ItalyMagdalena Stasiak, Polish Mother’s Memorial Hospital Research Institute, Poland
Copyright © 2022 Di Stefano, Guarnotta, Barbaccia, Paratore, La Monica, Lo Casto, Midiri, Gruttadauria, Giordano and Richiusa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carla Giordano, Y2FybGEuZ2lvcmRhbm9AdW5pcGEuaXQ=; Pierina Richiusa, cGllcmluYS5yaWNoaXVzYUB1bmlwYS5pdA==
†These authors have contributed equally to this work and share first authorship
 Claudia Di Stefano
Claudia Di Stefano Valentina Guarnotta1†
Valentina Guarnotta1† Maria Barbaccia
Maria Barbaccia Rosario Paratore
Rosario Paratore Roberta La Monica
Roberta La Monica Salvatore Gruttadauria
Salvatore Gruttadauria Carla Giordano
Carla Giordano