- 1Department of Reproductive Medicine Center, The third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 2Department of Nutrition, Zhejiang Nutriease Health Technology Company Limited, Hangzhou, Zhejiang, China
- 3State Key Laboratory of Reproductive Medicine (Henan Centre), the Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
- 4Department of Epidemiology, Center for Global Health, School of Public Health, Nanjing Medical University, Nanjing, Jiangsu, China
- 5State Key Laboratory of Reproductive Medicine, Nanjing Medical University, Nanjing, Jiangsu, China
Purpose: The aim of this study was to determine whether a 60-day weight reduction intervention prior to in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) and a higher weight loss ratio effectively improved perinatal outcomes for infertile overweight or obese women.
Methods: This was a retrospective cohort study conducted at a university-affiliated fertility center. Two thousand three hundred and eighty-one overweight or obese infertile women who underwent or did not undergo a 60-day weight reduction intervention prior to IVF/ICSI between February 27, 2017 and November 11, 2020 were included in this study. All of these women achieved clinical pregnancy and delivered a single child after assisted reproductive technology (ART). Primary outcomes included neonatal birth weight and the incidence of pregnancy complications, premature delivery, and low birth weight.
Results: The body mass index (BMI), blood glucose concentration, serum insulin level, and homeostasis model assessment of insulin resistance (HOMA-IR) of the intervention group decreased significantly after the weight reduction intervention. Neonatal birth weight was significantly higher in the intervention group (3519.6 g ±484.8 g) than the control group (3406.8 g ± 554.2 g; P < 0.001). There was no significant difference in the incidence of pregnancy complications between the two groups. Linear regression analysis found that the weight reduction intervention prior to IVF/ICSI and lower HOMA-IR at ovulation induction were associated with increased birth weight. As the weight loss ratio increased, the incidence of hypertensive disorders of pregnancy, premature membrane rupture, premature delivery, stillbirth, and low birth weight showed a downward trend.
Conclusion(s): A 60-day weight reduction intervention prior to IVF/ICSI may increase neonatal birth weight, reduce maternal blood glucose concentration, and improve maternal insulin resistance in infertile overweight or obese women. This results require to be further verified by prospective randomized controlled trials with a larger sample size.
Introduction
Fueled by rapid social development, an increasingly sedentary lifestyle, and a nutritional transition to processed foods and high-calorie diets in recent years, the incidence of overweight and obesity expanded rapidly and the number of cases of infertility caused by overweight and obesity is also increasing (1, 2). For adults, the world health organization (WHO) defined body mass index (BMI) ≥ 25 kg/m2 as overweight and BMI ≥ 30 kg/m2 as obesity (2). Overweight and obesity increases the risk of infertility by causing functional alterations to the hypothalamic–pituitary–ovarian axis (3). Overweight and obesity also impairs ovarian responsiveness to gonadotrophin stimulation, such that higher doses of medication are required. Moreover, compared with women of normal weight, obese women have an increased risk of cycle cancellation and decreased risk of implantation, clinical pregnancy and live birth (4, 5). Overweight and obesity are associated with lower live birth rates in women undergoing assisted reproductive therapy (ART), and previous studies have demonstrated that weight loss prior to ART in overweight and/or obese women increases pregnancy and live birth rates (6–10). Meanwhile, obesity increases the risk of premature delivery, low birth weight, miscarriage, abnormal labor and postpartum complications (11–15). Therefore, a weight reduction intervention prior to in vitro fertilization/intracytoplasmic sperm injection (IVF/ICSI) may be successful at improving ART and perinatal outcomes and may be an appropriate treatment for patients with obesity-related endocrine disorders and irregular menstruation (16). However, there is a lack of researches on whether weight reduction prior to IVF/ICSI affects perinatal outcomes. In addition, there is an urgent need to determine the range of weight loss ratios that is most likely to improve perinatal outcomes. Information gained through studying the effects of weight reduction and the optimal weight loss ratio on perinatal outcomes is required to ensure that overweight and obese infertile patients who accept a weight reduction intervention prior to IVF/ICSI have nicer perinatal outcomes.
Materials and methods
Study design and patients
This was a retrospective cohort study performed at the Assisted Reproduction Center of the Third Affiliated Hospital of Zhengzhou University. Two thousand three hundred and eighty-one overweight or obese infertile women who voluntarily underwent (intervention group, n=495) or rejected/discontinued a 60-day weight reduction intervention (control group, n=1,886) prior to IVF/ICSI from February 27, 2017 to November 11, 2020 and the 2,381 singletons delivered by these patients were included. Patients were randomly selected from the medical record system. The weight reduction intervention was defined as a 60-day weight reduction program that included a low-carbohydrate diet, diet structure adjustment, and an exercise program under the guidance of nutritionists. Nutritionists in the weight management department of the reproductive center instructed patients with a high BMI to purchase appropriate foods and consume a low-carbohydrate diet, to choose foods with a low glycemic index to induce fat breakdown, and to exercise appropriately so that their energy expenditure exceeded their intake. The inclusion criteria of the intervention group included: i) accepting the weight reduction intervention prior to IVF/ICSI, ii) a BMI ≥ 25 kg/m2, iii) undergoing IVF/ICSI after the weight-loss intervention, and iv) delivering a single child. The inclusion criteria for the control group differed in that these patients either interrupted or rejected the weight reduction intervention. Patients were excluded from the study if they had a diagnosis of recurrent spontaneous abortion, repeated implantation failure, abnormal anatomical structure of the uterus, chromosomal abnormalities, or tumors. This study was approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (2021-077-01). Nutritionists supervised and recorded the implementation of weight reduction by providing substitute meals, telephone follow-up, and wechat interaction, guided and adjusted according to the patients’ feedback, and recorded various physical function indexes after weight reduction. All patients included in the study were pregnant after IVF/ICSI and delivered successfully. This study included embryo transfer (ET) and frozen-thawed embryo transfer (FET) cycles.
Controlled ovarian hyperstimulation, endometrial preparation, and embryo transfer
Based on the patients’ condition, a controlled ovarian hyperstimulation protocol (gonadotropin releasing hormone agonist (GnRH-a) long-protocols or GnRH-antagonist protocols) was formulated and follicular growth was monitored by transvaginal ultrasound. The specific methods of ovarian stimulation were described in previous publications by researchers at our center (17). When the diameter of at least one dominant follicle was more than 20 mm, or the diameters of three follicles were more than 18 mm, the ovarian hyperstimulation program was stopped and recombinant human chorionic gonadotropin (Ezer, Merck, Germany) was used to trigger ovulation. Thirty-six hours later, the oocytes were removed under transvaginal ultrasound monitoring. Routine luteal support was given after oocyte retrieval, and IVF or ICSI was performed depending on the patients’ condition. Fresh embryo transfer was performed 3 days after oocyte retrieval or with blastocyst transplantation 5 days after oocyte retrieval. An endometrial preparation protocol for frozen-thawed embryo transfer, including natural cycles, artificial cycles, GnRH-a pre-treatment in hormone replacement therapy (HRT), and induced ovulation cycles, was devised based on the patients’ condition. Natural cycles were applied to patients with regular menstruation, and they usually induces ovulation with or without minimum human chorionic gonadotropin (HCG) that results in the least risk of adverse effects and cost. For convenience, patients with or without normal menstrual cycles all can be treated with artificial cycles which were performed using estrogen and progesterone (18). Artificial cycles can optimize endometrial receptivity with a more consistent interval, duration, and concentration of estrogen and progesterone compared to natural cycles. GnRH-a pre-treatment in HRT cycles were commonly used in patients with polycystic ovary syndrome to downregulate any hormones produced by the ovaries in subsequent therapy when using HRTs (19). Induced ovulation cycles were applied to patients with abnormal ovulation. Either cleavage-stage embryos were transferred on the third day after ovulation or blastocysts were transferred on the fifth day after ovulation. Vaginal ultrasound was used to monitor the presence of a gestational sac, an embryo, and a fetal heart rate on day 30 after transplantation. Basic clinical data and hormone levels were obtained from the case system. Patients were followed up with telephone calls until the end of their pregnancy, and any pregnancy complications and neonatal outcomes were obtained from the medical record system.
Outcome measures
Data related to pregnancy complications and neonatal outcomes were obtained from a review of the patients’ medical charts or through follow-up telephone calls. Hypertensive disorders of pregnancy include the following four categories: chronic hypertension, gestational hypertension, preeclampsia–eclampsia, and chronic hypertension with superimposed preeclampsia (20, 21). Chronic hypertension during pregnancy is defined as the presence of hypertension before conception, the development of elevated blood pressure before a gestational age of 20 weeks, or the persistence of hypertension beyond 12 weeks after delivery. Gestational hypertension, formerly known as pregnancy-induced hypertension, is defined as the development of essential hypertension after 20 weeks of gestation, in the absence of diagnostic criteria for preeclampsia. Preeclampsia is defined as new-onset hypertension that develops after 20 weeks of gestation and is accompanied by proteinuria or evidence of end-organ dysfunction. Eclampsia is defined as seizures in a pregnant woman with preeclampsia with no other identifiable cause. Gestational diabetes, defined as diabetes diagnosed during pregnancy, is a condition in which women without a previous diagnosis of diabetes exhibit abnormal blood glucose concentrations during pregnancy (22, 23). The incidences of placenta praevia (24), premature membrane rupture (25), and stillbirth (26) were also determined. A live birth was defined as an infant born alive after at least 28 weeks of gestation who survived more than 28 days, and a postterm pregnancy was defined as a gestational age > 42 weeks (27, 28). The WHO defines preterm birth as birth before 37 gestational weeks. Birth weights < 2500 g were classified as low birth weight, and birth weights < 1500 g were classified as very low birth weight (27, 29).
Statistical analysis
Continuous variables are presented as means ± standard deviations and categorical variables are presented as frequencies and corresponding percentages. The differences between two groups were assessed using a Student’s t-test for continuous variables and a Pearson’s chi-square test or Fisher’s exact probability method for categorical variables. Logistic and linear regression analyses were used to assess perinatal outcomes. Linear regression analysis was used to identify the factors influencing neonatal birth weight. All statistical analyses were performed using SPSS 22.0 software (IBM, Armonk, NY, USA). Two-tailed P values < 0.05 were considered statistically significant.
Results
Characteristics of the intervention group and the control group
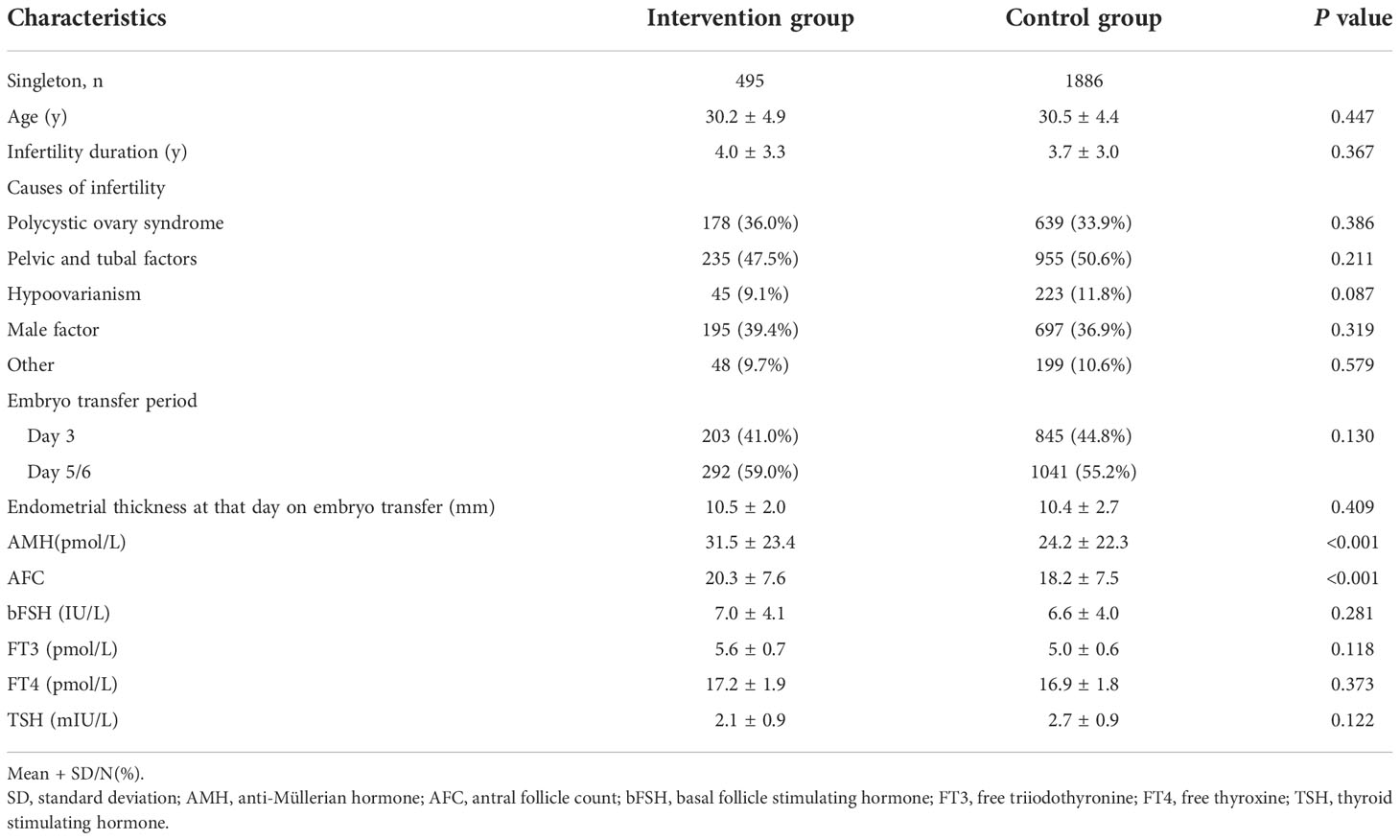
Four hundred and ninety-five singletons from mothers who underwent a weight reduction intervention prior to IVF/ICSI and 1,886 singletons from mothers who directly entered the ovulation induction cycle without a weight reduction intervention were included in this study. The basic characteristics of the intervention group and the control group are presented in Table 1. Anti-Müllerian hormone (AMH) concentration and antral follicle count (AFC) were significantly higher in the intervention group than the control group. There were no significant differences in age; infertility duration; cause of infertility; embryo transfer period; endometrial thickness on the day of embryo transfer; basal follicle-stimulating hormone (bFSH) dose; or free triiodothyronine (FT3), free thyroxine (FT4), and thyroid-stimulating hormone (TSH) concentrations between the two groups.
Comparison of blood glucose and insulin levels between two groups with the change of BMI
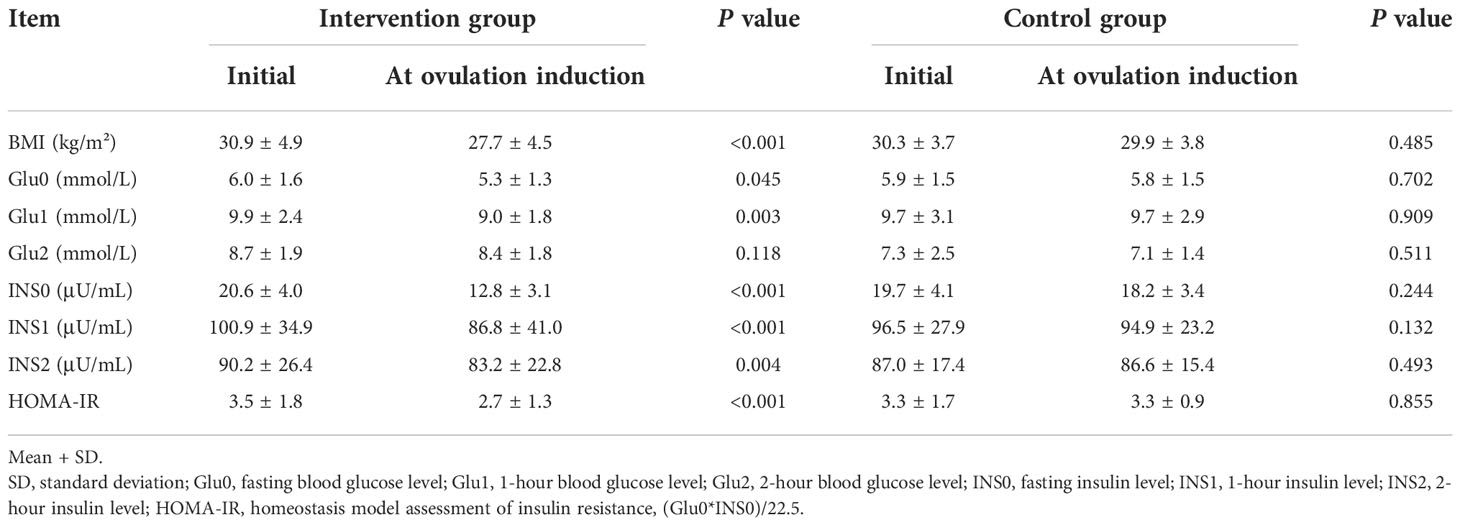
Insulin resistance test and oral glucose tolerance test (OGTT) results were compared after weight loss (Table 2). The BMI of the intervention group decreased significantly after the intervention (P < 0.001), while the BMI of the control group decreased slightly. Fasting glucose, 1-hour blood glucose, fasting insulin, 1-hour insulin, 2-hour insulin concentrations, and HOMA-IR values were lower after the intervention than before the intervention. The OGTT and insulin resistance test results did not change significantly in the control group after the change in BMI.
Comparison of perinatal outcomes of the intervention group and the control group
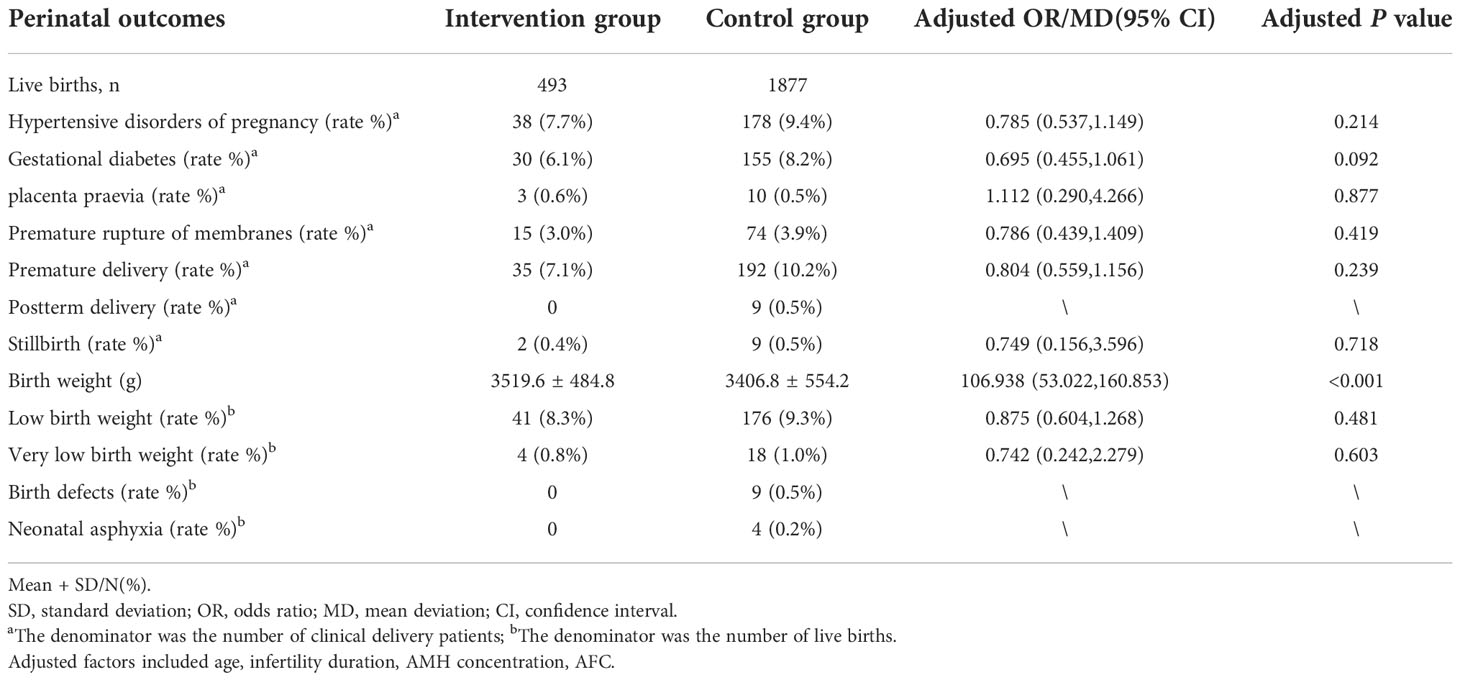
The comparison of perinatal outcomes between the the intervention group and the control group after adjusting for confounding factors (age, infertility duration, AMH concentration, AFC) is shown in Table 3. The incidences of hypertensive disorders of pregnancy, gestational diabetes, placenta praevia, premature membrane rupture, premature delivery, postterm delivery, stillbirth, low birth weight, very low birth weight, birth defect, and neonatal asphyxia were not significantly different between two groups. Birth weight was significantly higher in the intervention group (3519.6 ± 484.8) g than the control group (3406.8 ± 554.2) g after adjusting for confounding factors (P < 0.001).
Linear regression analysis of the influencing factors of birth weight
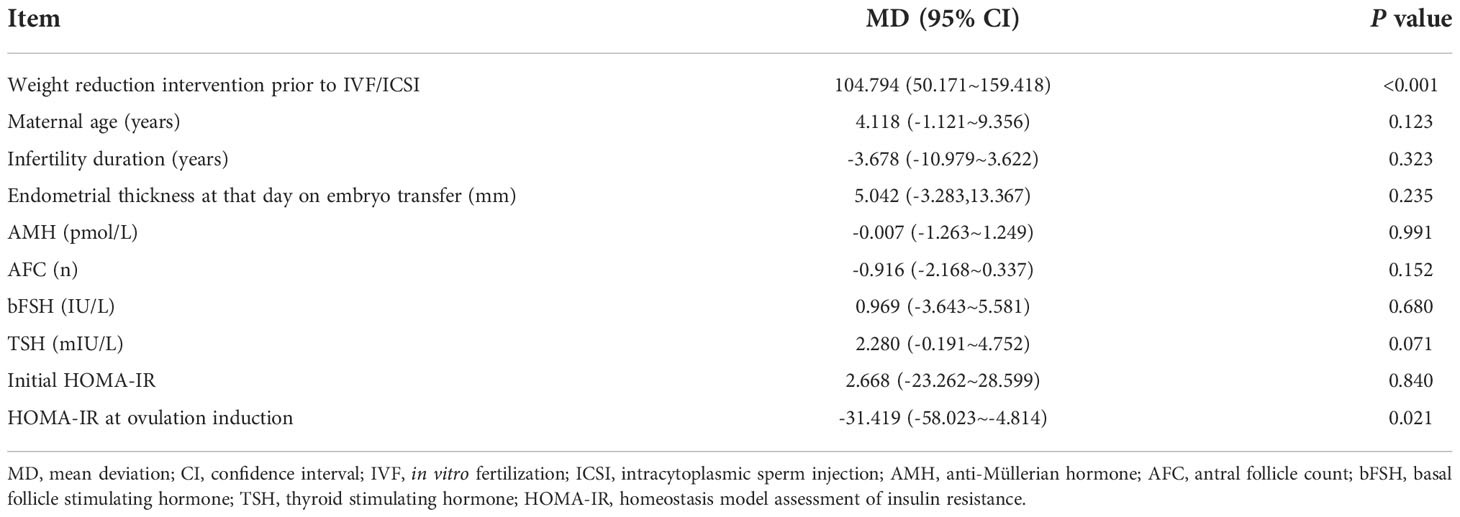
As shown in Table 4, a linear regression analysis found that a weight reduction intervention prior to IVF/ICSI and lower HOMA-IR at ovulation induction were associated with increased neonatal birth weight. Maternal age, infertility duration, endometrial thickness at that day on embryo transfer, AMH concentration, AFC, bFSH dose, TSH concentration and initial HOMA-IR had no effect on birth weight.
Comparison of the incidence of perinatal outcomes with different weight loss ratio
Figure 1 demonstrates the influence of different weight loss ratios on perinatal outcomes. We divided the entire cohort into four weight loss ratio groups (≤0%, 0%-5%, 5%-10%, and ≥10%). With an increase in the weight loss ratio, the incidences of hypertensive disorders of pregnancy, premature membrane rupture, premature delivery, stillbirth and low birth weight gradually decreased. However, the incidences of gestational diabetes and very low birth weight reached their lowest levels at a weight loss ratio of 5%-10%.
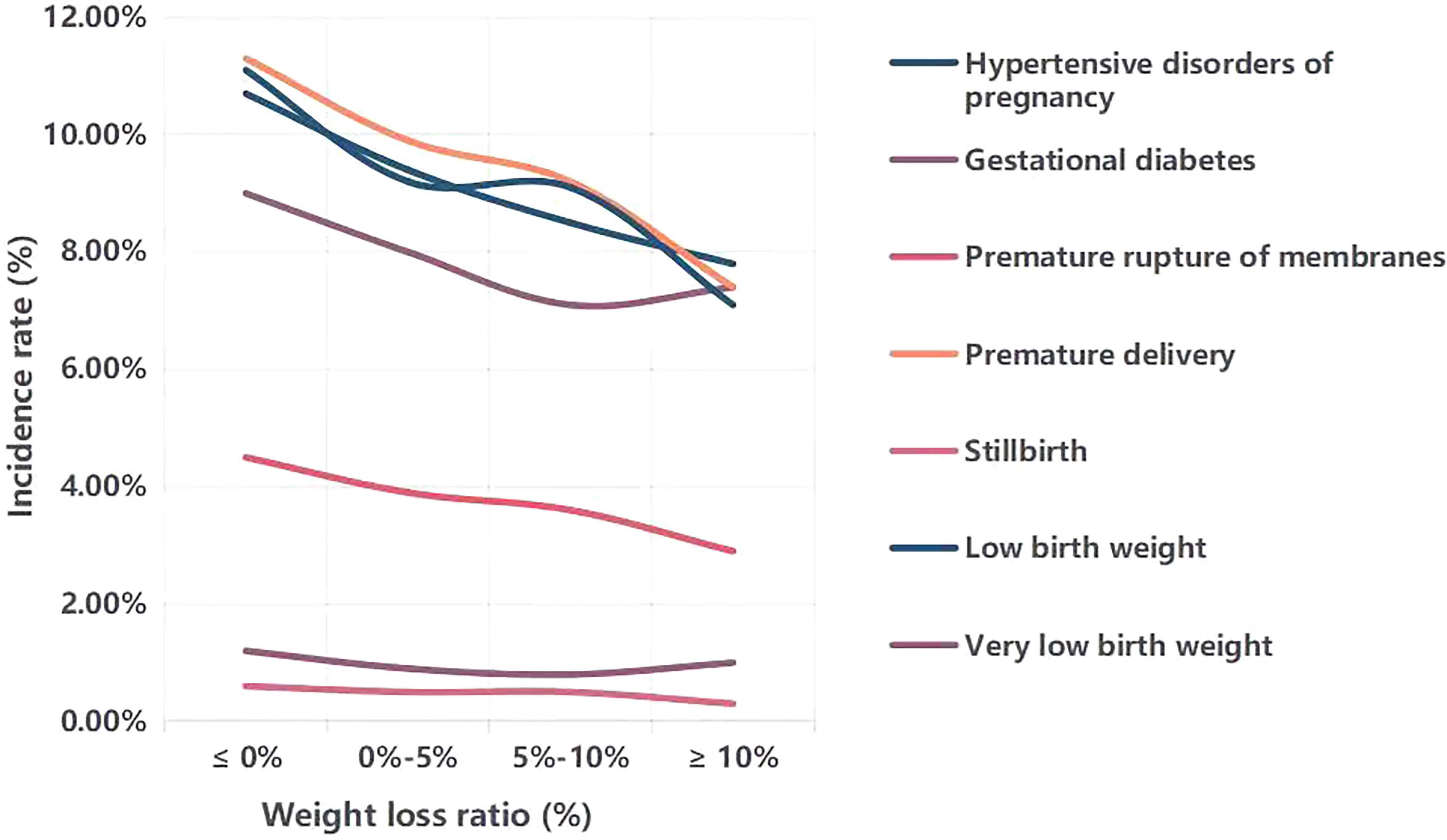
Figure 1 Comparison of the incidence of perinatal outcomes with different weight loss ratio. Weight loss ratio, (initial weight-weight at ovulation induction)/initial weight*100%.
Discussion
Compared with women with a normal BMI, overweight and obese women have a greater risk of adverse pregnancy outcomes, including preeclampsia, gestational diabetes, stillbirth, and cesarean section (30, 31). A meta-analysis confirmed the increased risk of fetal birth defects (neural tube defects, spina bifida, cardiovascular abnormalities, ventricular septal abnormalities, cleft palate, cleft lip and palate, anorectal malformations, hydrocephalus, and limb reduction) in children born to obese mothers (32). This may be related to maternal metabolic disorders (33). Overweight and obesity are also well-described risk factors for infertility (3). Therefore, identifying an appropriate weight loss ratio and determining whether weight loss before ovulation induction is beneficial to perinatal outcomes are topics of widespread concern. To the best of our knowledge, weight reduction in obese women scheduled for IVF/ICSI does not increase the incidence of live births (34, 35) or the cumulative live birth rate (36). However, Liu et al. (37) reported that an appropriate amount of weight loss in overweight or obese women is associated with a significant reduction in the incidence of perinatal complications. Our study focused on the effect of a weight reduction intervention for overweight or obese women prior to IVF/ICSI on perinatal outcomes.
We found that a weight-loss intervention prior to IVF/ICSI increased neonatal birth weight, and this may be related to improvements in placental blood flow by reducing insulin resistance. In a large randomized controlled trial, 290 women were assigned to a 6-month lifestyle-intervention program prior to 18 months of infertility treatment (intervention group) and 290 women were assigned to immediate infertility treatment for 24 months (control group) (38). Birth weight was higher in the intervention group than the control group, which is consistent with the results of our study. In our study, there were no differences in the incidence of pregnancy complications, including hypertensive disorders of pregnancy, gestational diabetes, placenta praevia, and premature membrane rupture, between the intervention and control groups. These are similar to the findings of Mutsaerts and Chavarro (6, 38). In a retrospective cohort study, overweight or obese infertile women who achieved a weight loss ratio > 10% had higher conception rates and live birth rates (39), indicating that perinatal outcomes may be improved with an increased weight reduction ratio. It is worth noting that excessive weight loss over a short time is discouraged, as it has been reported to adversely affect ART outcomes (40) and is associated with an increased risk of adverse pregnancy outcomes, such as low birth weight and miscarriage (41). Overall, weight reduction prior to ART is a reliable treatment method for overweight and obese women preparing for pregnancy, and the inclusion of weight reduction in the pre-pregnancy treatment regime of obese infertile patients is recommended (42).
This study is a retrospective cohort study with limited sample size. As some patients were followed up by telephone, recall bias may have been introduced. This study also enhanced the weight management awareness of patients in the intervention group during assisted reproductive treatment and pregnancy stage, but a handful of patients might give up weight control midway during the whole pregnancy process which affected the results of our study. Nevertheless, compared with previous similar studies, the perinatal outcomes of this study were more comprehensive and the trend of perinatal outcome incidence rate with the change of weight loss ratio was explored for the first time. In addition, there are few studies on the influence of weight reduction in overweight and obese infertile women receiving IVF/ICSI treatment on perinatal outcomes, and there are no guidelines for optimal weight loss rates. Therefore, this study provides significant references to clinicians and overweight or obese patients. Meanwhile, more studies and larger sample sizes are needed to verify the results of this study and to draw more comprehensive and objective conclusions.
Conclusion
In conclusion, weight reduction intervention prior to IVF/ICSI may increase neonatal birth weight, reduce maternal blood glucose concentration, and improve maternal insulin resistance in infertile overweight or obese women. The present study may therefore be helpful for patients with excessive BMI to receive a weight-loss intervention when attending weight counseling before initiating IVF/ICSI cycles.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
This study was approved by the Ethics Committee of the Third Affiliated Hospital of Zhengzhou University (ethics approval number: 2021-077-01).
Author contributions
CY: writing the article, study conception and design, and critical review of the article. SY, WZ, RZ, SR, HW, BR, NL, YK and ML: data processing. JD and YG: supervising the study. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by State Key Laboratory of Reproductive Medicine, Nanjing Medical University (SKLRM-K201902), National Key R&D Program “Fertility Health and Health Security for Women and Children” (2021YFC2700602), Henan Young and Middle-aged Health Science and Technology Innovation Leading Talent Training Project (No. YXKC2021020) and National Health Commission Scientific Research Foundation Henan Medical Science and Technology Research Program Provincial Joint Construction Project (No. SBGJ202102180).
Acknowledgments
Thanks to Professor Guan Yichun and other authors for their support and help.
Conflict of interest
Author NL was employed by company Zhejiang Nutriease Health Technology Company Limited.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Hruby A, Hu FB. The epidemiology of obesity: A big picture. Pharmacoeconomics (2015) 33(7):673–89. doi: 10.1007/s40273-014-0243-x
2. Weir CB, Jan A. BMI classification percentile and cut off points. 2022 jun 27. In: StatPearls. Treasure Island (FL: StatPearls (2022).
3. Broughton DE, Moley KH. Obesity and female infertility: Potential mediators of obesity's impact. Fertil Steril (2017) 107(4):840–7. doi: 10.1016/j.fertnstert.2017.01.017
4. Kasum M, Orešković S, Čehić E, Lila A, Ejubović E, Soldo D. The role of female obesity on in vitro fertilization outcomes. Gynecol Endocrinol (2018) 34(3):184–8. doi: 10.1080/09513590.2017.1391209
5. Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE, et al. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil Steril (2011) 96(4):820–5. doi: 10.1016/j.fertnstert.2011.07.1100
6. Chavarro JE, Ehrlich S, Colaci DS, Wright DL, Toth TL, Petrozza JC, et al. Body mass index and short-term weight change in relation to treatment outcomes in women undergoing assisted reproduction. Fertil Steril (2012) 98(1):109–16. doi: 10.1016/j.fertnstert.2012.04.012
7. Sim KA, Partridge SR, Sainsbury A. Does weight loss in overweight or obese women improve fertility treatment outcomes? A systematic review. Obes Rev (2014) 15(10):839–50. doi: 10.1111/obr.12217
8. Koning AM, Mutsaerts MA, Kuchenbecker WK, Broekmans FJ, Land JA, Mol BW, et al. Complications and outcome of assisted reproduction technologies in overweight and obese women. Hum Reprod (2012) 27(2):457–67. doi: 10.1093/humrep/der416
9. Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female obesity is negatively associated with live birth rate following IVF: A systematic review and meta-analysis. Hum Reprod Update (2019) 25(4):439–51. doi: 10.1093/humupd/dmz011
10. Wang X, Hao J, Zhang F, Li J, Kong H, Guo Y. Effects of female and male body mass indices on the treatment outcomes and neonatal birth weights associated with in vitro fertilization/intracytoplasmic sperm injection treatment in China. Fertil Steril (2016) 106(2):460–6. doi: 10.1016/j.fertnstert.2016.04.021
11. Knight M, Kurinczuk JJ, Spark P, Brocklehurst P, UK Obstetric Surveillance System. Extreme obesity in pregnancy in the united kingdom. Obstet Gynecol (2010) 115(5):989–97. doi: 10.1097/AOG.0b013e3181da8f09
12. Yang X, Zheng B, Wang Y. Effect of pre-pregnancy body mass index on neonatal outcomes in women undergoing autologous frozen-thawed embryo transfer. Fertil Steril (2021) 116(4):1010–9. doi: 10.1016/j.fertnstert.2021.03.044
13. Chen CN, Chen HS, Hsu HC. Maternal prepregnancy body mass index, gestational weight gain, and risk of adverse perinatal outcomes in Taiwan: A population-based birth cohort study. Int J Environ Res Public Health (2020) 17(4):1221. doi: 10.3390/ijerph17041221
14. Sun Y, Shen Z, Zhan Y, Wang Y, Ma S, Zhang S, et al. Effects of pre-pregnancy body mass index and gestational weight gain on maternal and infant complications. BMC Pregnancy Childbirth (2020) 20(1):390. doi: 10.1186/s12884-020-03071-y
15. Dolin CD, Kominiarek MA. Pregnancy in women with obesity. Obstet Gynecol Clin North Am (2018) 45(2):217–32. doi: 10.1016/j.ogc.2018.01.005
16. Hollmann M, Runnebaum B, Gerhard I. Effects of weight loss on the hormonal profile in obese, infertile women. Hum Reprod (1996) 11(9):1884–91. doi: 10.1093/oxfordjournals.humrep.a019512
17. Zhang J, Du M, Li Z, Wang L, Hu J, Zhao B, et al. Fresh versus frozen embryo transfer for full-term singleton birth: a retrospective cohort study. J Ovarian Res (2018) 11(1):59. doi: 10.1186/s13048-018-0432-x
18. Guan Y, Fan H, Styer AK, Xiao Z, Li Z, Zhang J, et al. A modified natural cycle results in higher live birth rate in vitrified-thawed embryo transfer for women with regular menstruation. Syst Biol Reprod Med (2016) 62(5):335–42. doi: 10.1080/19396368.2016.1199064
19. Yu J, Chen P, Luo Y, Lv M, Luo L, Xiao Q, et al. GnRH-agonist pretreatment in hormone replacement therapy improves pregnancy outcomes in women with male-factor infertility. Front Endocrinol (2022) 13:1014558. doi: 10.3389/fendo.2022.1014558
20. Wilkerson RG, Ogunbodede AC. Hypertensive disorders of pregnancy. Emerg Med Clin North Am (2019) 37(2):301–16. doi: 10.1016/j.emc.2019.01.008
21. Hypertension in pregnancy. report of the American college of obstetricians and gynecologists’ task force on hypertension in pregnancy. Obstet Gynecol (2013) 122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88
22. Szmuilowicz ED, Josefson JL, Metzger BE. Gestational diabetes mellitus. Endocrinol Metab Clin North Am (2019) 48(3):479–93. doi: 10.1016/j.ecl.2019.05.001
23. Mack LR, Tomich PG. Gestational diabetes: Diagnosis, classification, and clinical care. Obstet Gynecol Clin North Am (2017) 44(2):207–17. doi: 10.1016/j.ogc.2017.02.002
24. Jauniaux E, Alfirevic Z, Bhide AG, Belfort MA, Burton GJ, Collins SL, et al. Placenta praevia and placenta accreta: Diagnosis and management: Green-top guideline no. 27a. BJOG (2019) 126(1):e1–e48. doi: 10.1111/1471-0528.15306
25. Tchirikov M, Schlabritz-Loutsevitch N, Maher J, Buchmann J, Naberezhnev Y, Winarno AS, et al. Mid-trimester preterm premature rupture of membranes (PPROM): Etiology, diagnosis, classification, international recommendations of treatment options and outcome. J Perinat Med (2018) 46(5):465–88. doi: 10.1515/jpm-2017-0027
26. Kelly K, Meaney S, Leitao S, O'Donoghue K. A review of stillbirth definitions: A rationale for change. Eur J Obstet Gynecol Reprod Biol (2021) 256:235–45. doi: 10.1016/j.ejogrb.2020.11.015
27. Lin J, Guo H, Wang B, Chen Q, Zhu Q. Neonatal outcomes in women with polycystic ovary syndrome after frozen-thawed embryo transfer. Fertil Steril (2021) 115(2):447–54. doi: 10.1016/j.fertnstert.2020.08.1435
28. Practice bulletin no. 146: Management of late-term and postterm pregnancies. Obstet Gynecol (2014) 124(2Pt1):390–6. doi: 10.1097/01.AOG.0000452744.06088.48
29. Patel P, Bhatia J. Total parenteral nutrition for the very low birth weight infant. Semin Fetal Neonatal Med (2017) 22(1):2–7. doi: 10.1016/j.siny.2016.08.002
30. Aune D, Saugstad OD, Henriksen T, Tonstad S. Maternal body mass index and the risk of fetal death, stillbirth, and infant death: A systematic review and meta-analysis. JAMA (2014) 311(15):1536–46. doi: 10.1001/jama.2014.2269
31. Jadresić L, Au H, Woodhouse C, Nitsch D. Pre-pregnancy obesity and risk of congenital abnormalities of the kidney and urinary tract (CAKUT)-systematic review, meta-analysis and ecological study. Pediatr Nephrol (2021) 36(1):119–32. doi: 10.1007/s00467-020-04679-0
32. Stothard KJ, Tennant PW, Bell R, Rankin J. Maternal overweight and obesity and the risk of congenital anomalies: A systematic review and meta-analysis. JAMA (2009) 301(6):636–50. doi: 10.1001/jama.2009.113
33. Luzzo KM, Wang Q, Purcell SH, Chi M, Jimenez PT, Grindler N, et al. High fat diet induced developmental defects in the mouse: oocyte meiotic aneuploidy and fetal growth retardation/brain defects. PloS One (2012) 7(11):e49217. doi: 10.1371/journal.pone.0049217
34. Einarsson S, Bergh C, Friberg B, Pinborg A, Klajnbard A, Karlström PO, et al. Weight reduction intervention for obese infertile women prior to IVF: a randomized controlled trial. Hum Reprod (2017) 32(8):1621–30. doi: 10.1093/humrep/dex235
35. Moran L, Tsagareli V, Norman R, Noakes M. Diet and IVF pilot study: short-term weight loss improves pregnancy rates in overweight/obese women undertaking IVF. Aust N Z J Obstet Gynaecol (2011) 51(5):455–9. doi: 10.1111/j.1479-828X.2011.01343.x
36. Kluge L, Bergh C, Einarsson S, Pinborg A, Mikkelsen Englund AL, Thurin-Kjellberg A. Cumulative live birth rates after weight reduction in obese women scheduled for IVF: follow-up of a randomized controlled trial. Hum Reprod Open (2019) 2019(4):hoz030. doi: 10.1093/hropen/hoz030
37. Liu L, Wang H, Zhang Y, Niu J, Li Z, Tang R. Effect of pregravid obesity on perinatal outcomes in singleton pregnancies following in vitro fertilization and the weight-loss goals to reduce the risks of poor pregnancy outcomes: A retrospective cohort study. PloS One (2020) 15(2):e0227766. doi: 10.1371/journal.pone.0227766
38. Mutsaerts MA, van Oers AM, Groen H, Burggraaff JM, Kuchenbecker WK, Perquin DA, et al. Randomized trial of a lifestyle program in obese infertile women. N Engl J Med (2016) 374(20):1942–53. doi: 10.1056/NEJMoa1505297
39. Kort JD, Winget C, Kim SH, Lathi RB. A retrospective cohort study to evaluate the impact of meaningful weight loss on fertility outcomes in an overweight population with infertility. Fertil Steril (2014) 101(5):1400–3. doi: 10.1016/j.fertnstert.2014.01.036
40. Tsagareli V, Noakes M, Norman RJ. Effect of a very-low-calorie diet on in vitro fertilization outcomes. Fertil Steril (2006) 86(1):227–9. doi: 10.1016/j.fertnstert.2005.12.041
41. Vrebosch L, Bel S, Vansant G, Guelinckx I, Devlieger R. Maternal and neonatal outcome after laparoscopic adjustable gastric banding: a systematic review. Obes Surg (2012) 22(10):1568–79. doi: 10.1007/s11695-012-0740-y
Keywords: weight reduction intervention, obesity, in vitro fertilization, intracytoplasmic sperm injection, perinatal outcomes
Citation: Yang C, Yang S, Zheng W, Zu R, Ran S, Wu H, Ren B, Lv N, Kuang Y, Li M, Du J and Guan Y (2022) Effect of a 60-day weight reduction intervention prior to IVF/ICSI on perinatal outcomes in overweight or obese infertile women. Front. Endocrinol. 13:1062790. doi: 10.3389/fendo.2022.1062790
Received: 06 October 2022; Accepted: 16 November 2022;
Published: 02 December 2022.
Edited by:
Sijia Lu, Yikon Genomics, ChinaReviewed by:
Fang Liu, Affiliated Hospital, Inner Mongolia Medical University, ChinaXiru Liu, First Affiliated Hospital of Chongqing Medical University, China
Copyright © 2022 Yang, Yang, Zheng, Zu, Ran, Wu, Ren, Lv, Kuang, Li, Du and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yichun Guan, bGlzYW1heWd1YW5AMTYzLmNvbQ==
 Chen Yang
Chen Yang Shuheng Yang
Shuheng Yang Wei Zheng1
Wei Zheng1 Ruowen Zu
Ruowen Zu Shiyu Ran
Shiyu Ran Huan Wu
Huan Wu Yichun Guan
Yichun Guan