
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 16 January 2023
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1058651
This article is part of the Research Topic Novel Insights into Sperm Function and Selection: from Basic Research to Clinical Application View all 14 articles
 Yanwei Sha1,2,3†
Yanwei Sha1,2,3† Wensheng Liu4†
Wensheng Liu4† Hua Nie4†
Hua Nie4† Lu Han4
Lu Han4 Chunjie Ma4
Chunjie Ma4 Xiaoya Zhang5
Xiaoya Zhang5 Ziyi Xiao6
Ziyi Xiao6 Weibing Qin4*
Weibing Qin4* Xiaoming Jiang7*
Xiaoming Jiang7* Xiaoli Wei6*‡
Xiaoli Wei6*‡Asthenozoospermia is the most common cause of male infertility. Dynein protein arms play a crucial role in the motility of sperm flagella and defects in these proteins generally impair the axoneme structure and affect sperm flagella function. In this study, we performed whole exome sequencing for a cohort of 126 infertile patients with asthenozoospermia and identified homozygous DNALI1 mutation in one patient from a consanguineous family. This identified homozygous mutation was verified by Sanger sequencing. In silico analysis showed that this homozygous mutation is very rare, highly pathogenic, and very conserved. Sperm routine analysis confirmed that the motility of the spermatozoa from the patient significantly decreased. Further sperm morphology analysis showed that the spermatozoa from the patient exhibited multiple flagella morphological defects and a specific loss in the inner dynein arms. Fortunately, the patient was able to have his child via intracytoplasmic sperm injection treatment. Our study is the first to demonstrate that homozygous DNALI1 mutation may impair the integration of axoneme structure, affect sperm motility and cause asthenoteratozoospermia in human beings.
More than 80% of male infertility cases exhibit asthenozoospermia, which is caused by the dysfunction of sperm motility, such as reduced or completely absent sperm motility in the ejaculated (1, 2). Many factors, such as infection, varicocele, and pollution exposure, may predispose to asthenozoospermia. However, the genetic factors underlying asthenozoospermia cannot be ignored (3, 4).
The sperm flagellum plays an essential role in sperm motility through its conserved axonemal structure (5). Sperm axonemes consist of highly ordered “9+2” microtubules characterized by a central pair of microtubules surrounded by nine peripheral microtubule doublets (MTD) (6). There are various protein complexes, such as radial spokes, nexin-dynein regulatory complex, central complex, and dynein arms, as major components of the axoneme (7). Among these important complexes, dynein arms consisting of an inner and an outer dynein arm (IDA and ODA, respectively) are attached to each of the nine MTDs, which are essential for generating the beating forces of sperm flagellum (8). Strikingly, each dynein arm possesses a similar molecular composition: several light-chain proteins, at least two heavy-chain proteins, and at least two intermediate-chain proteins (9–11).
Dynein axonemal light intermediate chain 1 (DNALI1), also called P28, encodes a flagellar protein that is essential for the assembly of the inner dynein arm (12). Previous studies have shown that p28 mutation disrupted dynein heavy chain composition in Tetrahymena thermophila, leading to defects in beat frequency and waveform patterns of cilia (13). Furthermore, DNALI1 is strongly expressed in spermatocytes, spermatids, and flagella of mature sperm in the murine testis, indicating its potential function in male reproduction (12). Based on structural analysis, DNALI1 was found to be linked to the C-terminus of DNAH1, and infertile patients with DNAH1 mutations also presented DNALI1 defect in human beings (12, 14). Unfortunately, the role of DNALI1 in male reproduction has not been reported.
In the present study, we conducted whole-exome sequencing on 126 patients with asthenozoospermia and identified a homozygous mutation in DNALI1 from an infertile patient. The spermatozoa of this patient showed motility and morphological defects, as well as a significant loss of the internal dynein arms. Our findings proposed that mutation in DNALI1 is novel genetic pathogenesis of asthenoteratozoospermia and this infertile defect can be overcome by ICSI for the first time. These results demonstrate that DNALI1 plays an important role in the motility of sperm flagellum, which may extend the spectrum of etiological genes and provide new insight into the diagnosis and treatment of patients with asthenoteratozoospermia.
We recruited 126 patients who were infertile due to asthenozoospermia for genetic analysis and 60 fertile healthy men as control subjects. The parents of the DNALI1 mutated patient had consanguineous marriage. All infertile patients included in the study were excluded for abnormal karyotype, translocations, Y chromosome microdeletions, etc. We performed a routine analysis of semen for the participants.
This study was approved by the Ethics Committee of Women and Children’s Hospital of Xiamen University. All subjects participating in the study signed a written informed consent form.
Whole-exome sequencing (WES) was performed on these asthenozoospermia patients as previously described (15). Briefly, genomic DNA for each patient was isolated from the peripheral blood sample and processed for exome enrichment using the TruSeq Exome Enrichment kit according to the manufacturer’s protocol. DNA sequencing was performed on an Illumina Hiseq 2000 sequencer and sequence reads were aligned to the human genome reference (hg19) using Burrows-Wheeler Aligner and sorted by Picard software. Candidate variants were annotated by using ANNOVAR and other bioinformatics databases. Further Sanger sequencing was performed to validate the selected mutation site in the DNALI1 mutated patient. We were unable to validate this mutation site in his parents because both of his parents passed away.
Transmission electron microscopy (TEM) was performed at the core facility of biomedical sciences of Xiamen University as described elsewhere (16). Briefly, the fresh spermatozoa were first fixed by incubation in 2.5% glutaraldehyde. The samples were washed twice with 0.1M phosphate buffer and resuspended in 0.2 M sodium cacodylate buffer. After embedding with Epon 812, the ultrathin sections were stained with uranyl acetate and lead citrate and observed by TEM (JEM-1400, Jeol, Japan).
Intracytoplasmic sperm injection (ICSI) treatment for assisted fertilization was performed as described previously (17). The percentage of fertilization was evaluated by the presence of two polar bodies and two pronuclei. Then the embryos were individually cultured in Vitrolife G-SERIES culture media. Serum HCG levels were measured 14 days after embryo transfer and clinical pregnancy was confirmed by ultrasound performed 28 days after embryo transfer.
We performed WES and bioinformatic analyses to reveal the genetic etiology of 126 patients with asthenozoospermia and identified the homozygous DNALI1 mutation NM_003462.5:c.691_693del in a patient from a consanguineous family (Figure 1A). This homozygous DNALI1 mutation in this patient was further confirmed by Sanger sequencing (Figure 1B). Among all homozygous or compound heterozygous mutated genes in this patient, no genes that have been reported to be associated with asthenozoospermia, teratozoospermia, or structural components of spermatozoa were identified. Among the testis-specific or highly expressed genes, also only the DNALI1 gene was mutated and has been associated with primary ciliary dyskinesia. The ultrastructural defects of the inner dynein arms could be found. In Chlamydomonas, mutations in this gene exhibits similar defects. Moreover, by TEM, we also observed a defect in the inner dynein arms of axonemes in this patient. Based on these evidences, we hypothesize that DNALI1 mutation is likely responsible for asthenoteratozoospermia in this patient.
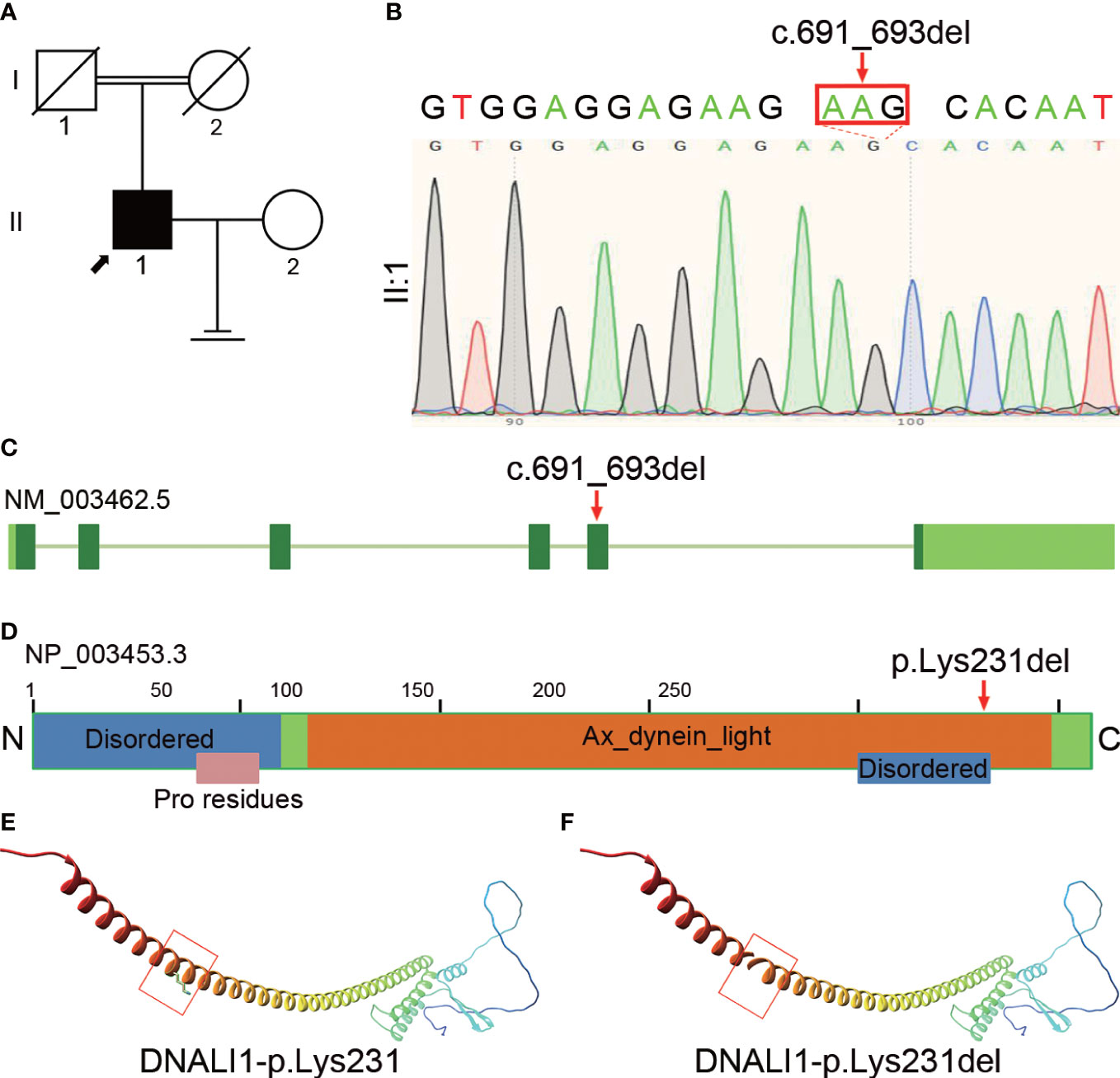
Figure 1 Identification of homozygous DNALI1 mutation in an infertile man with asthenoteratozoospermia. (A) Pedigree chart of the patient with asthenoteratozoospermia. The black square and the black arrow represent the proband. (B) Sanger sequencing verified the variant in the patient. The mutated bases are indicated by the red arrow and red rectangle. (C) The location of the mutated bases on the genome of DNALI1. (D) The position of the amino acid substitution on the domain map of DNALI1. The blue rectangle represents the “Disordered” domain, the orange rectangle represents the “Ax_dynein_light” domain, and the pink rectangle represents the “Pro residues” domain. (E) The position of the affected amino acid on the three-dimensional structure of the wild-type DNALI1. The red rectangle shows p.Lys231 of DNALI1. (F) Effect of the deletion mutation on DNALI1 three-dimensional structure. The red rectangle shows the site of the deleted amino acid.
This patient had some symptoms of suspected PCD such as cough, chronic sinusitis, and recurrent upper respiratory tract infections. Based on this, we hypothesized that this patient is a suspected PCD patient. This patient had a history of infertility for 5 years after marriage, but his parents had normal fertility with only one child. Although further verification was not available due to the passing of his parents, we hypothesized that the patient’s parents were heterozygous carriers of this mutation and that the patient’s homozygous mutation locus was likely inherited from his parents who had a consanguineous marriage.
The homozygous DNALI1 mutation was further evaluated using in silico analysis. This DNALI1 mutation NM_003462.5:c.691_693del (p.Lys231del) is very rare in the EXAC, 1000 genome, ESP6500, and gnomAD databases of the human population. This variant is classified as of uncertain significance with minor pathogenic evidence according to the ACMG Classification.
This homozygous NM_003462.5:c.691_693del (p.Lys231del) mutation is located at the fifth exon of the DNALI1 genome (Figure 1C) and results in the deletion of the 231th amino acid in the Ax_dynein_light and the Disordered domains (Figure 1D). We then aligned the amino acid sequences of DNALI1 from Homo sapiens to that of Drosophila melanogaster and found that the amino acid affected by the NM_003462.5:c.691_693del (p.Lys231del) mutation is highly conserved among these species from human beings to fruit fly (Supplementary Figure 1). In addition, we constructed the mutated protein structure using SWISS-MODEL and found that this mutation affected the three-dimensional structure of DNALI1. Compared to the original DNALI1 three-dimensional structure (Figure 1E), the NM_003462.5:c.691_693del (p.Lys231del) mutation resulted in the deletion of Lysine at the 231th (Figure 1F). Lysine is a positively charged basic hydrophilic essential amino acid of the basic amino acid class. This change could significantly affect the nearby steric hindrance and the three-dimensional structure of DNALI1, possibly affecting its stability and function.
Clinical examination showed that the DNALI1-mutated patient had normal physical development. No significant abnormalities were found in the development of organs or accessory glands of the reproductive system. Serum hormone levels were within the normal range, with only a slight increase in PRL values (Table 1). Routine semen analysis was performed for the patient, and the results showed that the patient had normal sperm concentration. However, the percentages of progressive motility and non-progressive motility were significantly decreased, and the percentage of normal morphology sperm was also lower than the reference value (Table 2). We performed CASA on the patient’s spermatozoa, and the results showed that the patients had significantly lower sperm parameters (Table 3).
Compared with the normal morphology of the spermatozoa from the control subject, the results of Papanicolaou staining showed that the patient’s spermatozoa exhibited multiple flagellar defects, including absent or coiled flagella (Figure 2A). The results of field emission scanning electron microscopy further confirmed the morphological defects of the patient’s spermatozoa (Figure 2B). In addition, TEM analysis was performed to explore ultrastructural defects, and it was found that the cross-section of the control sample showed a typical “9+2” axoneme structure, but the cross-sections of the patient’s spermatozoa exhibited specific inner dynein arm defects (Figure 2C).

Figure 2 Morphological and ultrastructural analysis of the spermatozoa from the DNALI1-mutated patient. (A) Morphological analysis of the spermatozoa from a control subject and the patient with homozygous DNALI1 mutation. Scale bar: 10 μm. (B) Morphological analysis of the patient’s spermatozoa by field emission scanning electron microscopy. Scale bar: 3 μm. (C) Ultrastructure analysis of the spermatozoa from the patient at the midpiece. The green “*” indicates the loss of IDA. Scale bar: 200 nm. CP, central pair of microtubules; ODF, outer dense fiber; MS, mitochondrial sheath; ODA, outer dynein arm; IDA, inner dynein arm.
This couple underwent two cycles of ICSI treatment. In the first cycle, we retrieved 18 oocytes, 14 of which were in the MII stage. All these 14 oocytes at the MII stage were injected with the proband’s sperm and nine of them were fertilized. After embryo culture, only one blastocyst was formed on day 3. This embryo was transferred, but his wife failed to be conceived. In the second cycle, 14 oocytes were retrieved, nine of which were in the MII stage. All nine of these MII oocytes were injected with the proband’s sperm and all of them were fertilized. After embryo culture, two blastocysts were formed and transferred. The embryos were successfully implanted and his wife had a clinically successful pregnancy.
In this study, we recruited 126 infertile patients due to asthenozoospermia and detected a homozygous DNALI1 mutation from one patient with asthenoteratozoospermia. This homozygous mutation in DNALI1 resulted in the deletion of the inner dynein arms, which severely impairs sperm motility. These data suggest that DNALI1 is a novel gene associated with sperm flagellar function and defects in this gene may contribute to asthenoteratozoospermia in humans.
The formation and function of sperm flagellum are essential for sperm motility (18). Typically, the sperm flagellum has a highly organized axoneme portion consisting of nine outer doublet microtubules and a central pair microtubule, called the “9+2” structure (19). Axonemal dyneins, including ODA and IDA, are observed on outer doublet microtubules, which play a central role in the beating and motility of sperm flagellum (20). Indeed, mutations in genes associated with the formation of the sperm tail are responsible for sperm motility and fertility defect (18). For example, mutations in IDA and ODA genes, such as DNAH1, DNAH2, DNAH8, and DNAH10, cause male infertility due to asthenozoospermia with multiple morphological abnormalities of the sperm flagella (21). However, mutations in DNAH5, DNAH11, and DNAI1 also lead to male infertility due to isolated non-syndromic asthenozoospermia (22, 23). In this study, we identified for the first time homozygous DNALI1 mutation as potential pathogenesis for asthenoteratozoospermia.
DNALI1 is a kind of axonemal IDA protein and is mainly expressed in the human ciliated tissues, including the testis, ovary, and lung (12). DNALI1 mutation altered dynein heavy chain composition, which further resulted in defects in beat frequency and waveform patterns of cilia in Tetrahymena thermophila (13). In the present study, spermatozoa from the DNALI1-mutated patient exhibited specific inner dynein arm loss, resulting in a significant decrease in progressive and non-progressive motility. Moreover, CASA results further demonstrated that the motility parameters of the patient’s sperm were significantly reduced. In addition to the motility of spermatozoa, the flagella morphology of the patient’s sperm also exhibited various abnormalities characterized by absent or coiled flagella. These findings suggest that defects in DNALI1 may affect IDA assembly during flagellar axoneme formation, leading to IDA deficiency, sperm flagellar morphology anomalous, and asthenozoospermia.
ICSI is the preferred clinical treatment for patients with asthenoteratozoospermia (24). However, for some patients with idiopathic asthenoteratozoospermia, multiple attempts are often required and some do not end up with satisfactory results (25). Therefore, reports on assisted reproduction are of clinical importance when studying infertility due to genetic defects. In this study, the patient underwent two cycles of ICSI treatment and obtained a clinical pregnancy, which could provide a reference for other infertility due to DNALI1 deficiency.
In summary, our work demonstrates that genetic defects of DNALI1 severely impair sperm motility and contribute to the human azoospermia phenotype, for which ICSI treatment is an effective remedy for the first time. Our results prove the importance of DNALI1 in the structure and function of the sperm axoneme, which provides novel evidence for a comprehensive understanding of the axonemal assembly and function of sperm flagellum.
The datasets presented in this article are not readily available because the CNGB regulations. Requests to access the datasets should be directed to Y-WS, c2hheWFud2VpOTI4QDEyNi5jb20=.
The studies involving human participants were reviewed and approved by the Ethics Committee of Women and Children’s Hospital of Xiamen University. The patients/participants provided their written informed consent to participate in this study.
XW and XJ designed this study. XW and WL drafted the manuscript. YS, WQ, and HN performed bioinformatic analysis. XZ and ZX performed molecular genetics experiments. YS and XJ conducted clinical phenotyping. LH and CM interpreted the data. All authors approved the final manuscript.
This work was supported by the following grants: the National Natural Science Foundation of China (82071697, and 81871200), the Medical Innovation Project of Fujian Province (2020-CXB-051), the open project of NHC Key Laboratory of Male Reproduction and Genetics in Guangzhou (KF202004).
We express our deepest gratitude to the participants for their cooperation.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1058651/full#supplementary-material
DNALI1, dynein axonemal light intermediate chain 1; WES, whole exome sequencing; MTD, microtubule doublets; TEM, transmission electron microscopy; ICSI, intracytoplasmic sperm injection; IDA, inner dynein arm; ODA, outer dynein arm; CASA, computer-assisted sperm analysis; EXAC, exome aggregation consortium; ACMG, American college of medical genetics and genomics.
1. Curi SM, Ariagno JI, Chenlo PH, Mendeluk GR, Pugliese MN, Sardi Segovia LM, et al. Asthenozoospermia: analysis of a large population. Arch Androl (2003) 49:343–9. doi: 10.1080/01485010390219656
2. Sha Y, Wei X, Ding L, Mei L, Huang X, Lin S, et al. DNAH17 is associated with asthenozoospermia and multiple morphological abnormalities of sperm flagella. Ann Hum Genet (2020) 84:271–9. doi: 10.1111/ahg.12369
3. Tu C, Wang W, Hu T, Lu G, Lin G, Tan YQ. Genetic underpinnings of asthenozoospermia. Best Pract Res Clin Endocrinol Metab (2020) 34:101472. doi: 10.1016/j.beem.2020.101472
4. Shahrokhi SZ, Salehi P, Alyasin A, Taghiyar S, Deemeh MR. Asthenozoospermia: Cellular and molecular contributing factors and treatment strategies. Andrologia (2020) 52:e13463. doi: 10.1111/and.13463
5. Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod (2011) 17:524–38. doi: 10.1093/molehr/gar034
6. Linck RW, Chemes H, Albertini DF. The axoneme: the propulsive engine of spermatozoa and cilia and associated ciliopathies leading to infertility. J Assist Reprod Genet (2016) 33:141–56. doi: 10.1007/s10815-016-0652-1
7. Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol (2009) 187:921–33. doi: 10.1083/jcb.200908067
8. Sui H, Downing KH. Molecular architecture of axonemal microtubule doublets revealed by cryo-electron tomography. Nature (2006) 442:475–8. doi: 10.1038/nature04816
9. Mische S, He Y, Ma L, Li M, Serr M, Hays TS. Dynein light intermediate chain: an essential subunit that contributes to spindle checkpoint inactivation. Mol Biol Cell (2008) 19:4918–29. doi: 10.1091/mbc.e08-05-0483
10. Asai DJ, Wilkes DE. The dynein heavy chain family. J Eukaryot Microbiol (2004) 51:23–9. doi: 10.1111/j.1550-7408.2004.tb00157.x
11. Ide T, Owa M, King SM, Kamiya R, Wakabayashi K. Protein-protein interactions between intermediate chains and the docking complex of chlamydomonas flagellar outer arm dynein. FEBS Lett (2013) 587:2143–9. doi: 10.1016/j.febslet.2013.05.058
12. Rashid S, Breckle R, Hupe M, Geisler S, Doerwald N, Neesen J. The murine Dnali1 gene encodes a flagellar protein that interacts with the cytoplasmic dynein heavy chain 1. Mol Reprod Dev (2006) 73:784–94. doi: 10.1002/mrd.20475
13. Subramanian A, Kabi A, Gray SF, Pennock D. p28 dynein light chains and ciliary motility in tetrahymena thermophila. Cytoskeleton (Hoboken) (2016) 73:197–208. doi: 10.1002/cm.21295
14. Ben Khelifa M, Coutton C, Zouari R, Karaouzene T, Rendu J, Bidart M, et al. Mutations in DNAH1, which encodes an inner arm heavy chain dynein, lead to male infertility from multiple morphological abnormalities of the sperm flagella. Am J Hum Genet (2014) 94:95–104. doi: 10.1016/j.ajhg.2013.11.017
15. Sha Y, Liu W, Huang X, Li Y, Ji Z, Mei L, et al. EIF4G1 is a novel candidate gene associated with severe asthenozoospermia. Mol Genet Genomic Med (2019) 7:e807. doi: 10.1002/mgg3.807
16. Liu W, Sha Y, Li Y, Mei L, Lin S, Huang X, et al. Loss-of-function mutations in SPEF2 cause multiple morphological abnormalities of the sperm flagella (MMAF). J Med Genet (2019) 56:678–84. doi: 10.1136/jmedgenet-2018-105952
17. Sha YW, Zhang Q, Ding L, Li P. First successful pregnancy outcome after intracytoplasmic sperm injection with short-tailed sperm from an infertile han Chinese man. Asian J Androl (2017) 19:613–4. doi: 10.4103/1008-682X.182395
18. Lehti MS, Sironen A. Formation and function of sperm tail structures in association with sperm motility defects. Biol Reprod (2017) 97:522–36. doi: 10.1093/biolre/iox096
19. King SM. Axonemal dyneins winch the cilium. Nat Struct Mol Biol (2010) 17:673–4. doi: 10.1038/nsmb0610-673
20. Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol (2007) 69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236
21. Tu C, Cong J, Zhang Q, He X, Zheng R, Yang X, et al. Et al: Bi-allelic mutations of DNAH10 cause primary male infertility with asthenoteratozoospermia in humans and mice. Am J Hum Genet (2021) 108:1466–77. doi: 10.1016/j.ajhg.2021.06.010
22. He J, Li L, Yu Y, Hu X, Zhang H, Liu R, et al. Two mutations in the axonemal dynein heavy chain gene 5 in a Chinese asthenozoospermia patient: A case report. Med (Baltimore) (2020) 99:e20813. doi: 10.1097/MD.0000000000020813
23. Zuccarello D, Ferlin A, Cazzadore C, Pepe A, Garolla A, Moretti A, et al. Mutations in dynein genes in patients affected by isolated non-syndromic asthenozoospermia. Hum Reprod (2008) 23:1957–62. doi: 10.1093/humrep/den193
24. Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, et al. European Academy of andrology guideline management of oligo-astheno-teratozoospermia. Andrology (2018) 6:513–24. doi: 10.1111/andr.12502
Keywords: asthenoteratozoospermia, whole-exome sequencing, DNALI1, inner dynein arms, intracytoplasmic sperm injection
Citation: Sha Y, Liu W, Nie H, Han L, Ma C, Zhang X, Xiao Z, Qin W, Jiang X and Wei X (2023) Homozygous mutation in DNALI1 leads to asthenoteratozoospermia by affecting the inner dynein arms. Front. Endocrinol. 13:1058651. doi: 10.3389/fendo.2022.1058651
Received: 30 September 2022; Accepted: 25 October 2022;
Published: 16 January 2023.
Edited by:
Tao Luo, Nanchang University, ChinaReviewed by:
Yang Xiaoyu, Nanjing Medical University, ChinaCopyright © 2023 Sha, Liu, Nie, Han, Ma, Zhang, Xiao, Qin, Jiang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoli Wei, eGlhb2xpQHludS5lZHUuY24=; Xiaoming Jiang, a2VhaXNobWlseUAxMjYuY29t; Weibing Qin, Z3VhcmRxaW5AMTYzLmNvbQ==
†These authors have contributed equally to this work
‡ORCID: Xiaoli Wei, orcid.org/0000-0002-3459-7357
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.