- Department of Endocrinology, Centre of Postgraduate Medical Education, Warsaw, Poland
Thyroid autoimmunity (TAI) is commonly defined as the presence of thyroperoxidase antibodies (TPOAbs) and/or thyroglobulin antibodies (TgAbs), which predisposes an individual to hypothyroidism. TAI affects nearly 10% of women of reproductive age and evokes great interest from clinicians because of its potentially negative impact on female fertility and pregnancy course. In this mini-review, we review the current literature concerning the influence of TPOAb or TPOAb/TgAb positivity without thyroid dysfunction on reproduction. TAI may negatively affect female fertility; several studies have found an increased prevalence of TAI in infertile women, especially in those with unexplained infertility and polycystic ovary syndrome. According to some observations, TAI might also be connected with premature ovarian insufficiency and endometriosis. The relationship between TAI and an increased risk of pregnancy loss is well documented. The pathophysiological background of these observations remains unclear, and researchers hypothesize on the direct infiltration of reproductive organs by thyroid antibodies, co-existence of TAI with other autoimmune diseases (either organ specific or systemic), immunological dysfunction leading to inhibition of immune tolerance, and relative thyroid hormone deficiency. Interestingly, in the current literature, better outcomes of assisted reproductive technology in women with TAI have been reported compared with those reported in earlier publications. One plausible explanation is the more widespread use of the intracytoplasmic sperm injection method. The results of randomized clinical trials have shown that levothyroxine supplementation is ineffective in preventing adverse pregnancy outcomes in women with TAI, and future research should probably be directed toward immunotherapy.
Introduction
Thyroid autoimmunity (TAI) is commonly characterized by the presence of circulating thyroperoxidase antibodies (TPOAbs) and/or thyroglobulin antibodies (TgAbs) which predisposes to hypothyroidism. In this mini-review, we will focus on the consequences of TAI with elevated concentrations of TPOAb and/or TgAb without thyroid dysfunction for female fertility and maternal obstetric complications.
TAI is the most common autoimmune disease among women of reproductive age (1). Recent publications report the presence of TPOAbs and TgAbs in 5%–14% and 3%–18%, respectively, of pregnant women (2). Moreover, TPOAbs were found in 9.5% of women with previous pregnancy loss or subfertility (3). TPOAb positivity appeared to be connected with iodine deficiency or excess (4), age over 40 years (5), obesity classes II–III (body mass index ≥35 kg/m2), and ethnicity, occurring more frequently in Caucasian women than in Black women (3). TPOAb prevalence in pregnant women might be underestimated because the currently used manufacturer cutoffs for TPOAb may be too high for this population. In a recent study, TPOAb concentrations below the manufacturer cutoffs were associated with higher thyroid-stimulating hormone (TSH) concentrations and a higher risk of premature delivery (6). Due to the fact that TPOAb is recognized as a sensitive marker of TAI, which is associated with the risk of hypothyroidism (7), the majority of publications explore the impact of TPOAb or TPOAb/TgAb, while there are few studies investigating the effect of isolated TgAb. However, isolated TgAb positivity may be as common as isolated TPOAb positivity: 5% vs. 4% (8) and 14.5% vs. 13.5% (5), respectively, and may exert some harmful effects. So far, it has been demonstrated that TgAb can interfere with the thyroidal response to human chorionic gonadotropin (hCG) stimulation similar to TPOAb (9). TgAbs are associated with an increased risk of premature rupture of fetal membranes and low birth weight (10). They might also have a significant impact on TSH concentration (11). In its 2017 guidelines, the American Thyroid Association (ATA) stated that there is a need for further research on the significance of isolated TgAb positivity (12). Increasing evidence confirms the association between TAI and the risk of pregnancy loss and suggests its relationship with reduced fertility. Unfortunately, the pathological pathways through which TAI exerts its adverse effects remain unclear; therefore, treatment options are limited.
Pathophysiology
The association between TAI and procreation can be direct or indirect. A hypothesis on a probable direct harmful effect of thyroid antibodies comes from a small Italian study in which a group of 14 infertile women with TAI undergoing in vitro fertilization (IVF) had TPOAbs and TgAbs in the follicular fluid, where their concentrations correlated positively with those in the blood. Oocyte fertilization, high-quality embryos, and pregnancy rates were lower in TAI women than in 17 negative controls (13). These results were then replicated in a larger study group (14), which suggested that TPO is a common antigen in both thyroid and ovarian tissues (15). As TPOAb has been proven to cause thyrocyte death through antibody-dependent cytotoxicity cells (ADCC) and C3 complement-mediated cytotoxicity and TgAb has been postulated to act via ADCC (16), damage to reproductive organs expressing TPO and Tg might ensue. Additionally, the cross-reactivity of TPOAb with hCG receptors in the zona pellucida has been proposed as another potential mechanism of infertility (17).
As all the components necessary to produce thyroid hormones are present in the endometrium, syncytiotrophoblast, and invasive trophoblast (sodium/iodide symporter, pendrin, TPO, and Tg), one can speculate about the local synthesis of thyroid hormones. In such circumstances, thyroid autoimmunity could lead to thyroid hormone deficiency at the tissue level and disturb the process of embryo implantation and placentation, with consequent infertility or obstetrical complications (1, 18, 19). To support this hypothesis, according to two publications by Italian researchers (20, 21), TAI was linked to increased uterine artery resistance and umbilical artery vasoconstriction, as determined by the Doppler technique. Hemodynamic abnormalities were associated with increased rates of miscarriage, fetal growth restriction, small for gestational age, preeclampsia, and prematurity.
The indirect negative impact of TAI on fertility and pregnancy outcomes might be its co-occurrence with other autoimmune diseases, either organ specific (autoimmune polyglandular syndromes 1–4) with anti-ovarian antibodies or systemic (systemic lupus erythematosus, Sjögren’s syndrome, and rheumatoid arthritis) with antinuclear antibodies, antiphospholipid antibodies, and anti-laminin-1 antibodies (aLN-1), which have documented negative impacts on fertility and pregnancy outcomes (22, 23). It has been reported that 20% of women with TAI have non-organ-specific antibodies that can react with the trophoblast/placenta and induce a prothrombotic state, cytokine imbalance, and complement activation (17).
TAI itself can lead to immunological dysfunction and inhibition of immune tolerance at the systemic and maternal–fetal interface levels. A systemic imbalance of helper lymphocyte Th1/Th2/Th17 and Treg activity, leading to increased secretion of inflammatory cytokines interleukin (Il)-2, IL-17, and interferon-γ (INF-γ)—known factors of implantation failure and pregnancy loss—was observed in women with TAI (24, 25). In addition, excessive activation and cytotoxicity of natural killer (NK) cells in peripheral blood and the uterus, as well as upregulation of NKT-like cells, have been reported (26–28). Interestingly, NK cell numbers and activity can be enhanced not only by Il-2 and INF-γ but also by TSH acting in an endocrine and paracrine manner (17).
Another potentially negative factor in women with TAI might be a relative deficiency of thyroid hormone. Several studies have found that thyroid autoimmunity is associated with higher serum TSH levels within the normal range compared with healthy controls (3, 6, 29). In addition, about 20% of TAI women who were euthyroid in the preconception period developed subclinical hypothyroidism over the course of their pregnancies. In a study by Korevaar et al. (30), TPOAb positivity was associated with an impaired thyroidal response to hCG during pregnancy. TPOAb-positive women with a more-impaired-than-expected thyroidal response to hCG (lower FT4 than expected for hCG) had more than 2.0-fold higher risk of premature delivery. Furthermore, the risk of a negative pregnancy outcome can be modified by TSH concentration, and the combination of TPOAb positivity with high-normal TSH concentration is associated with synergistically higher risks of miscarriage (31) and preterm delivery (6).
Previous publications have pointed out that the older age of women with TAI is an independent factor for decreased fertility and a higher risk of miscarriage (32). However, in a recent publication, infertile women with TAI were not older than women without thyroid autoimmunity (33).
Thyroid autoimmunity and fertility
Publications assessing the prevalence of thyroid autoantibodies in infertile women have reported divergent results, which may be explained by heterogeneous study designs (retrospective, prospective, and cross-sectional), different populations studied (various ages of subjects and causes of infertility), and different assays used to determine thyroid antibodies. It is noteworthy that, in a recent prospective study carried out among 1,054 fertile women with a history of one or two prior pregnancy losses, there was no difference in the pregnancy rates between 154 women with TAI and 900 women without TAI (74% vs. 72.2%, respectively, p = 0.64) (34). However, summarized data have shown an association between TAI and decreased fertility. In a review study of women with various causes of infertility, Poppe et al. demonstrated a significantly higher incidence of TAI [risk ratio (RR): 2.1, p < 0.0001] (35). In a meta-analysis by van den Boogaard et al. (36), the presence of thyroid antibodies was associated with a higher risk of unexplained subfertility [odds ratio (OR): 1.5, 95% confidence interval (95% CI): 1.1–2.0]. An elevated prevalence of TAI might be especially concerning in some particular causes of infertility: polycystic ovary syndrome (PCOS) (26.9%), idiopathic infertility, and endometriosis (25%) (37–40). In a 2018 meta-analysis of 13 cross-sectional and case–control studies evaluating a total of 1,210 women with PCOS and 987 healthy controls, Romitti et al. (41) found a significant association between PCOS and TAI (OR: 3.27, 95% CI: 2.32–4.63). A predisposing factor for the co-occurrence of PCOS and TAI is a polymorphism in the fibrillin gene, which regulates transforming growth factor-β (TGF-β) activity, which, in turn, affects Treg cells. Reduced TGF-β and Treg activity promotes the development of autoimmune diseases. Another predisposing factor is the high estrogen/progesterone ratio found in women with PCOS and vitamin D deficiency (42). TAI leading to (sub)hypothyroidism may negatively affect metabolic performance, higher triglycerides, and free testosterone in women with PCOS (43). Some studies have found TAI in 25%–46% of women with endometriosis attending infertility clinics (29, 40, 44), although this observation has not been confirmed by other observations (41, 45). The pathophysiology of endometriosis is complex and still unclear, but the condition is associated with a variety of inflammatory and immunological phenomena, such as the presence of autoantibodies to endometrial antigens (including aLN-1), complement deposits, apoptosis, a decline in NK cell concentration, and cytotoxic effects on the endometrium (46, 47). Reciprocally, thyroid antibodies can affect the human endometrium, including ectopic endometrium, as all of the transcripts involved in thyroxin synthesis have been found in the endometrium, including TPO and Tg (1). Several studies have pointed out a possible association between TAI and diminished ovarian reserve or premature ovarian insufficiency (POI). From 4% to 30% of POI cases are autoimmune in origin (23); in a recent meta-analysis, the authors confirmed a higher frequency of TPOAb positivity in this group of patients (OR: 2.26, 95% CI: 1.31–3.92, p = 0.004), but not of TgAb positivity (48). After pooling data from 30 studies published between 1997 and 2021, they concluded that women of reproductive age with Hashimoto’s thyroiditis (hypothyroid and euthyroid) have lower concentrations of anti-Müllerian hormone and antral follicle count. Unfortunately, they did not perform a subanalysis in a group of euthyroid women. In a recent publication (49) not included in the abovementioned meta-analysis, retrospective research of 4,302 euthyroid women proved that TAI was associated with POI only in the group with TSH > 2.5 µIU/ml but not in those with TSH ≤ 2.5 µIU/ml. These facts indicate that there may be a role for relative thyroid hormone insufficiency acting together with thyroid autoimmunity in the process of ovarian damage. The European Society of Human Reproduction and Embryology recommends testing for TPOAb in women with POI (50).
Thyroid autoimmunity and the risk of maternal obstetric complications
The association between TAI and miscarriage is well documented. In a 2011 meta-analysis of 31 studies comprising 12,126 women without overt thyroid dysfunction, an elevated risk of miscarriage was demonstrated among women with TPOAb/TgAb positivity (OR: 3.90, 95% CI: 2.48–6.12, p < 0.001) (51). This association was also proven among euthyroid women with thyroid antibody positivity (OR: 1.80, 95% CI: 1.25–2.60, p = 0.002) but was only confirmed in two subgroups: women with recurrent miscarriage and women with infertility. A significant doubling in the odds of preterm birth with the presence of thyroid autoantibodies was also demonstrated (OR: 2.07, 95% CI: 1.17–3.68, p = 0.01). An elevated risk of preterm delivery was documented in another meta-analysis comprising 11 prospective cohort studies and 35,467 participants; the relative risk of preterm delivery was higher for pregnant women with thyroid antibodies compared with controls (RR: 1.41, 95% CI: 1.08–1.84, p = 0.011) and for TPOAb-positive euthyroid women (RR: 1.98, 95% CI: 1.29–3.04, p = 0.002), but not for TgAb positivity (52). The latest meta-analysis of 19 cohorts pooling the data of 47,055 pregnant women also demonstrated that TPOAb-positive euthyroid women had a higher risk of preterm birth vs. TPOAb-negative women (6.8% vs. 4.9%) (OR: 1.36, 95% CI: 1.15–1.60, p < 0.01) (53). Three current studies, two prospective (54, 55) and one retrospective (56), have confirmed the previously established relationship between TAI and the risk of miscarriage, preterm birth, and early-term birth. It is interesting that, in one of the studies, the association between TAI and preterm birth occurred only in cases with female fetuses (55).
The association between TAI and recurrent pregnancy loss was demonstrated in a recent meta-analysis (57). After pooling data from 17 studies that included women with TPOAb positivity or TPOAb/TgAb positivity, the meta-analysis revealed a statistically significant association between recurrent pregnancy loss and thyroid autoimmunity (OR: 1.94, 95% CI: 1.43–2.64).
In a recent study of 454 women with unexplained recurrent pregnancy loss, TPOAb positivity was associated with a lower live birth rate (51.3% vs. 65.2%, p = 0.02) (58). However, the conclusion is hindered because 75% of the TPOAb-positive women and 3.7% of the TPOAb-negative women received L-thyroxine treatment.
Several studies, mostly retrospective, have reported an increased risk of preeclampsia, gestational diabetes mellitus (GDM), anemia, placenta previa, polyhydramnios, placental abruption, and premature rupture of membranes in women with TAI (59). Although these observations are inconsistent and further studies are needed, the last complication has been documented in a cohort of 10,062 women (60, 61). It is interesting that placental abruption was linked to the persistence of TPOAb positivity in the first and second trimesters, and the risk was doubled when TPOAb together with TgAb was increased. It should be noted that the link between TAI and GDM requires further investigation, but it was suggested in the latest meta-analysis (62). The authors found an increased risk of GDM in TAI pregnant women with TSH <4.0 mIU/L, while there was no such risk in controls with TSH <4.0 mIU/L and negative thyroid antibodies (OR: 2.04, 95% CI: 1.32–3.137, p < 0.001). The plausible link between TAI and GDM is an elevated concentration of inflammatory cytokines, which leads to insulin resistance (63).
Thyroid autoimmunity and assisted reproductive technology outcomes
The impact of thyroid autoimmunity on assisted reproductive technology (ART) outcomes has been widely investigated, but the studies have great heterogeneity due to different designs, causes of infertility, various protocols for ovarian stimulation, various definitions of euthyroidism, different fertilization procedures, including IVF, intracytoplasmic sperm injection (ICSI), or intrauterine insemination (IUI), and variously defined outcomes. Previous studies have reported poorer embryo quality (64, 65), lower clinical pregnancy rates, higher risk of miscarriage, and lower live birth rates in women with TAI undergoing ART (66–68). However, in several recent studies (69–71), comprehensive reviews (72, 73), and meta-analyses (74–76), no deleterious effect of TAI on ART outcomes was found. The suggested explanation for this discrepancy might be the increasing usage of the ICSI method of fertilization. As this procedure involves injecting sperm into the center of the egg, it overcomes the potential barrier of thyroid antibodies infiltrating the zona pellucida. However, this hypothesis does not explain the lack of a negative relationship between TAI and IUI outcomes observed at present (77). Although ICSI has mainly been performed in male infertility, this method is suggested by the European Thyroid Association (ETA) to be used in infertile women with thyroid autoimmunity (78).
Special attention should be paid to the risk of hypothyroidism in women with TAI undergoing ART. Diminished thyroid response to hCG, on the one hand, and rapid increase of estradiol and thyroxin-binding globulin concentrations soon after controlled ovarian stimulation, on the other hand, might result in a decrease in free thyroid hormone accessibility (79, 80). Monitoring thyroid function in women with TAI undergoing ART was proposed in the 2021 ETA guidelines (78) and adopted by some endocrine societies (81). It consists of assessing the TSH concentration at the time of the second positive hCG result confirming pregnancy.
An important question exists: “What is the optimal preconceptional value of TSH concentration determining successful ART outcomes in women with TAI?” Unaune et al. did not observe significant differences in cumulative delivery rates after IVF/ICSI between TPOAb-positive and TPOAb-negative women whenever the TSH threshold of 2.5 or 5.0 mIU/L was adopted (69). Similarly, Chai et al. (82) found no difference in IVF outcomes between women with TSH below and above 4.5 mIU/L. Furthermore, in a recent meta-analysis including 18 publications and 14,846 participants, no difference was observed in IVF/ICSI/IUI outcomes when a TSH cutoff value of 2.5 mIU/L was used (83). However, when a broader TSH cutoff value of 3.5–5 mIU/L was used, a higher miscarriage rate was observed (RR: 1.91, 95% CI: 1.09–3.35, p = 0.02). Unfortunately, thyroid autoimmunity status was not taken into account in the studies’ selection criteria.
Treatment options
Levothyroxine
According to the hypothesis postulating that TAI is accompanied by a relative thyroid hormone deficiency in the blood and/or at the tissue level, supplementation with levothyroxine may have beneficial effects on pregnancy outcome. The 2017 ATA guidelines (12) recommended levothyroxine treatment for women with thyroid autoimmunity and TSH above the pregnancy-specific reference range. The ATA also proposed considering levothyroxine for women with thyroid autoimmunity and TSH above 2.5 mIU/L and for euthyroid infertile women with a history of pregnancy loss. After the publication of the ATA guidelines, several important randomized clinical trials (RCTs) were released, which makes it necessary to reconsider this perspective (Table 1) (84–90). Moreover, four meta-analyses including only RCTs could not find any evidence that levothyroxine supplementation in euthyroid women with AITD resulted in an improvement in maternal pregnancy outcomes (94–97). The results of the meta-analyses have also shown that initiating levothyroxine during the preconception period or in the first trimester did not affect the miscarriage risk. Finally, the highly anticipated T4-LIFE trial addressing the impact of levothyroxine treatment in TPOAb-positive euthyroid women with recurrent miscarriage was published, and the levothyroxine intervention failed again to decrease the risk of pregnancy loss (90). Although these observations require further analysis, the only beneficial effect of levothyroxine treatment in TAI women documented so far is prevention of hypothyroidism.
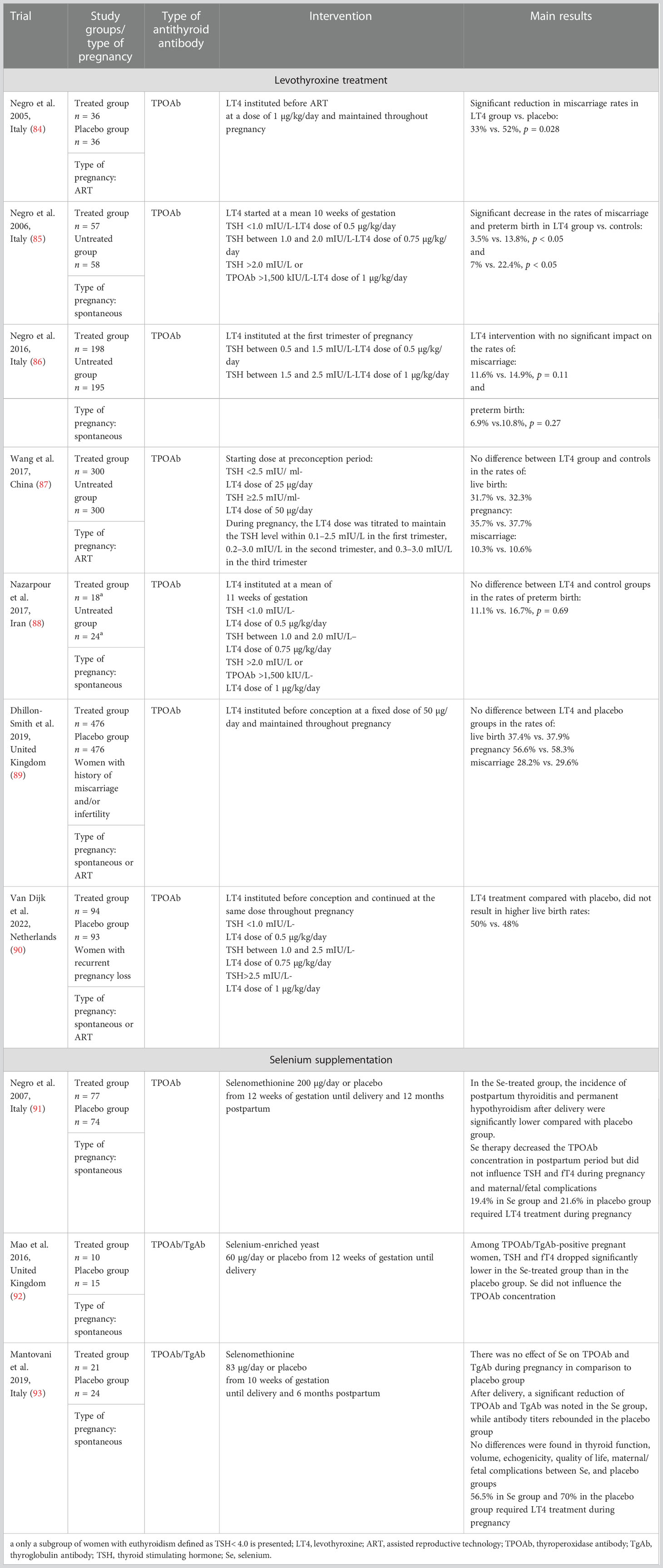
Table 1 Prospective randomized clinical studies of levothyroxine treatment and selenium supplementation in euthyroid pregnant women with thyroid autoimmunity.
Selenium
Selenium (Se) is a micronutrient with immunoregulatory properties and a well-established role in thyroid physiology (98, 99). In many previous studies, Se supplementation reduced the TPOAb and TgAb levels and improved the thyroid echogenicity (100–104). Although Se deficiency has been reported in several European countries, especially among pregnant women (105, 106), the efficacy of Se supplementation among pregnant women with TAI remains controversial (Table 1) (91–93). In the future, many aspects of Se therapy should be taken into account, such as its interaction with other micronutrients, especially iodine, and the postulated inverted U-shape relationship with disease, which means that either Se deficiency or Se excess could lead to adverse outcomes.
Immunotherapy
TAI is regarded as an epiphenomenon of generalized immune dysfunction; therefore, immunotherapy arouses interest, especially since progressively more evidence indicates that levothyroxine has failed to improve fertility and pregnancy outcomes. Unfortunately, randomized studies using oral steroids (small doses of prednisolone) are scarce. The results of two small RCTs (107, 108) showed improved pregnancy rates in TAI women undergoing ART, but the miscarriage rate was still high (up to 75%). The experience of infertility clinics with intravenous immunoglobulin (IVIG) use in the general population of women with recurrent miscarriage is limited, and evidence for the effect of IVIG in recurrent pregnancy loss is weak. Two recent meta-analyses of IVIG use in recurrent pregnancy loss found no evidence of improved live birth rate (109, 110). However, a higher live birth rate was demonstrated in some publications, as well as beneficial changes in immune profile (i.e., a decrease in Th1/Th2 ratio and NK cells) (111, 112). Only a few observational studies concerning IVIG use in TAI women with recurrent miscarriage have shown a successful live birth rate of 80% to 90% (113, 114). Further well-designed RCTs are needed to establish the true efficacy of IVIG. In addition, high costs and possible side effects, including anaphylactic reactions and the risk of thrombosis, must be taken into consideration.
In Table 2, the current recommendations of endocrine societies (12, 78, 115) for the clinical care of women with TAI who are pregnant or who are planning pregnancy are presented.
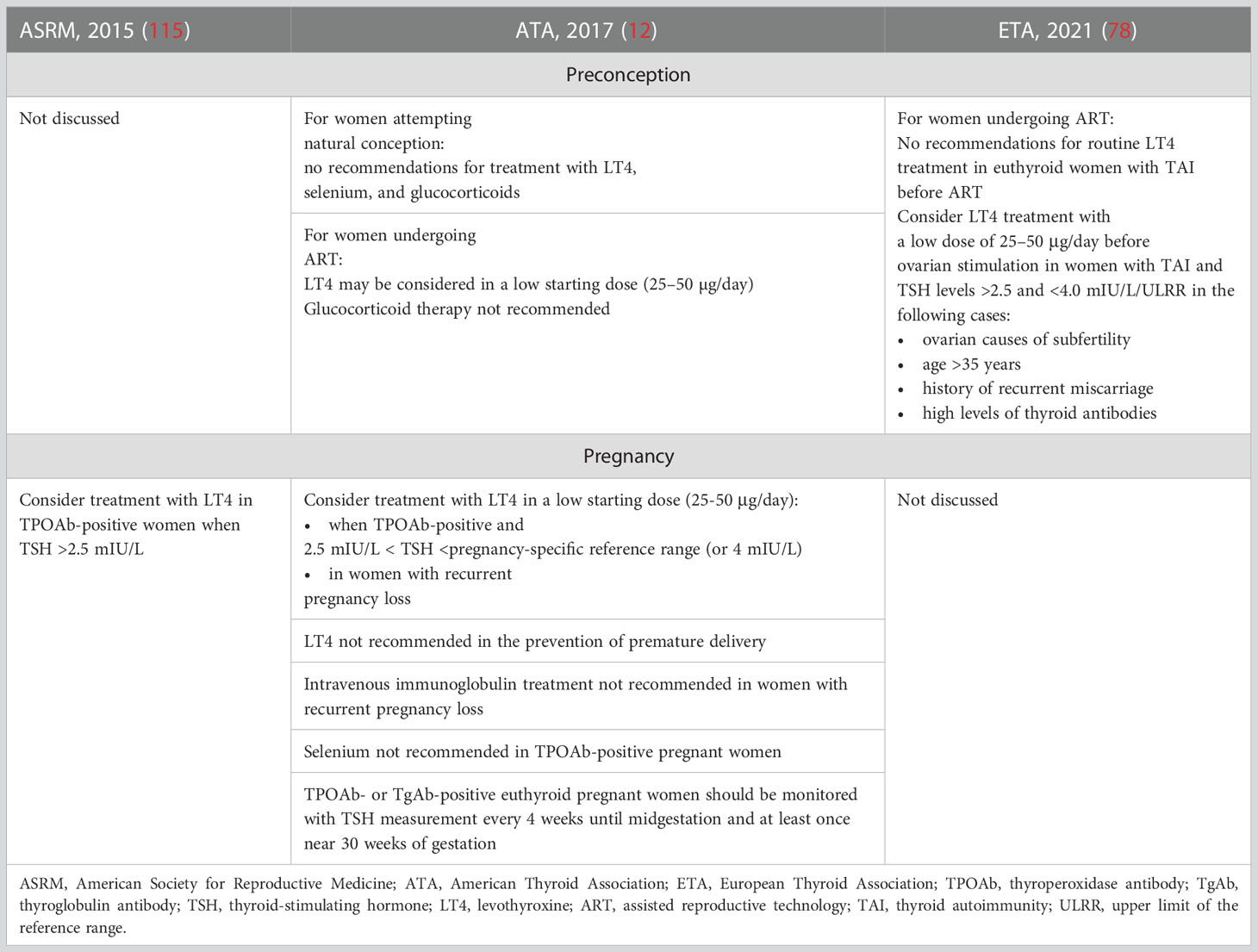
Table 2 Recommendations regarding the treatment for thyroid autoimmunity during preconception period and pregnancy.
Conclusions
Thyroid autoimmunity is not only connected with a possible thyroid hormone deficiency but often represents a broader spectrum of immune disturbances that lead to decreased fertility and an increased risk of pregnancy loss. A better understanding of the pathophysiological pathways of TAI is the cornerstone for successful therapies in the future. Levothyroxine supplementation appeared to be ineffective in preventing adverse pregnancy outcomes, so future research should probably be directed toward repairing the immune imbalance.
Author contributions
KT: conception and study design, literature review, and preparing the paper. MG-C: conception, literature review, and preparing the manuscript. PG and JK: preparing and critical revision of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vissenberg R, Manders VD, Mastenbroek S, Fliers E, Afink GB, Ris-Stalpers C, et al. Pathophysiological aspects of thyroid hormone disorders/thyroid peroxidase autoantibodies and reproduction. Hum Reprod Update (2015) 21(3):378–87. doi: 10.1093/humupd/dmv004
2. De Leo S, Pearce EN. Autoimmune thyroid disease during pregnancy. Lancet Diabetes Endocrinol (2018) 6(7):575–86. doi: 10.1016/S2213-8587(17)30402-3
3. Dhillon-Smith RK, Tobias A, Smith PP, Middleton LJ, Sunner KK, Baker K, et al. The prevalence of thyroid dysfunction and autoimmunity in women with history of miscarriage or subfertility. J Clin Endocrinol Metab (2020) 105(8):2667–77. doi: 10.1210/clinem/dgaa302
4. Teng D, Yang W, Shi X, Li Y, Ba J, Chen B, et al. An inverse relationship between iodine intake and thyroid antibodies: A national cross-sectional survey in mainland China. Thyroid. (2020) 30(11):1656–65. doi: 10.1089/thy.2020.0037
5. Li Y, Shan Z, Teng W, Thyroid Disorders IdSaDESG. The iodine status and prevalence of thyroid disorders among women of childbearing age in China: National cross-sectional study. Endocr Pract (2021) 27(10):1028–33. doi: 10.1016/j.eprac.2021.03.017
6. Korevaar TIM, Pop VJ, Chaker L, Goddijn M, de Rijke YB, Bisschop PH, et al. Dose dependency and a functional cutoff for TPO-antibody positivity during pregnancy. J Clin Endocrinol Metab (2018) 103(2):778–89. doi: 10.1210/jc.2017-01560
7. Hollowell JG, Staehling NW, Flanders WD, Hannon WH, Gunter EW, Spencer CA, et al. T(4), and thyroid antibodies in the united states population (1988 to 1994): National health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab (2002) 87(2):489–99. doi: 10.1210/jcem.87.2.8182
8. Unuane D, Velkeniers B, Anckaert E, Schiettecatte J, Tournaye H, Haentjens P, et al. Thyroglobulin autoantibodies: Is there any added value in the detection of thyroid autoimmunity in women consulting for fertility treatment? Thyroid (2013) 23(8):1022–8. doi: 10.1089/thy.2012.0562
9. Hou Y, Liu A, Li J, Wang H, Yang Y, Li Y, et al. Different thyroidal responses to human chorionic gonadotropin under different thyroid peroxidase antibody and/or thyroglobulin antibody positivity conditions during the first half of pregnancy. Thyroid. (2019) 29(4):577–85. doi: 10.1089/thy.2018.0097
10. Chen LM, Zhang Q, Si GX, Chen QS, Ye EL, Yu LC, et al. Associations between thyroid autoantibody status and abnormal pregnancy outcomes in euthyroid women. Endocrine (2015) 48(3):924–8. doi: 10.1007/s12020-014-0420-x
11. Bliddal S, Derakhshan A, Xiao Y, Chen LM, Männistö T, Ashoor G, et al. Association of thyroid peroxidase antibodies and thyroglobulin antibodies with thyroid function in pregnancy: An individual participant data meta-analysis. Thyroid (2022) 32(7):828–40. doi: 10.1089/thy.2022.0083
12. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid (2017) 27(3):315–89. doi: 10.1089/thy.2016.0457
13. Monteleone P, Parrini D, Faviana P, Carletti E, Casarosa E, Uccelli A, et al. Female infertility related to thyroid autoimmunity: The ovarian follicle hypothesis. Am J Reprod Immunol (2011) 66(2):108–14. doi: 10.1111/j.1600-0897.2010.00961.x
14. Medenica S, Garalejic E, Arsic B, Medjo B, Bojovic Jovic D, Abazovic D, et al. Follicular fluid thyroid autoantibodies, thyrotropin, free thyroxine levels and assisted reproductive technology outcome. PLos One (2018) 13(10):e0206652. doi: 10.1371/journal.pone.0206652
15. Monteleone P, Faviana P, Artini PG. Thyroid peroxidase identified in human granulosa cells: Another piece to the thyroid-ovary puzzle? Gynecol Endocrinol (2017) 33(7):574–6. doi: 10.1080/09513590.2017.1296424
16. Bogusławska J, Godlewska M, Gajda E, Piekiełko-Witkowska A. Cellular and molecular basis of thyroid autoimmunity. Eur Thyroid J (2022) 11(1):e210024. doi: 10.1530/ETJ-21-0024
17. Twig G, Shina A, Amital H, Shoenfeld Y. Pathogenesis of infertility and recurrent pregnancy loss in thyroid autoimmunity. J Autoimmun (2012) 38(2-3):J275–81. doi: 10.1016/j.jaut.2011.11.014
18. Colicchia M, Campagnolo L, Baldini E, Ulisse S, Valensise H, Moretti C. Molecular basis of thyrotropin and thyroid hormone action during implantation and early development. Hum Reprod Update (2014) 20(6):884–904. doi: 10.1093/humupd/dmu028
19. Rahnama R, Mahmoudi AR, Kazemnejad S, Salehi M, Ghahiri A, Soltanghoraee H, et al. Thyroid peroxidase in human endometrium and placenta: A potential target for anti-TPO antibodies. Clin Exp Med (2021) 21(1):79–88. doi: 10.1007/s10238-020-00663-y
20. Spinillo A, De Maggio I, Ruspini B, Bellingeri C, Cavagnoli C, Giannico S, et al. Placental pathologic features in thyroid autoimmunity. Placenta (2021) 112:66–72. doi: 10.1016/j.placenta.2021.07.287
21. Beneventi F, De Maggio I, Bellingeri C, Cavagnoli C, Spada C, Boschetti A, et al. Thyroid autoimmunity and adverse pregnancy outcomes: A prospective cohort study. Endocrine (2022) 76(1):198–207. doi: 10.1007/s12020-021-02958-w
22. Tektonidou MG, Anapliotou M, Vlachoyiannopoulos P, Moutsopoulos HM. Presence of systemic autoimmune disorders in patients with autoimmune thyroid diseases. Ann Rheum Dis (2004) 63(9):1159–61. doi: 10.1136/ard.2004.022624
23. Szeliga A, Calik-Ksepka A, Maciejewska-Jeske M, Grymowicz M, Smolarczyk K, Kostrzak A, et al. Autoimmune diseases in patients with premature ovarian insufficiency-our current state of knowledge. Int J Mol Sci (2021) 22(5):2594. doi: 10.3390/ijms22052594
24. Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol (2010) 63(6):601–10. doi: 10.1111/j.1600-0897.2010.00852.x
25. Lu H, Huang Y, Xin H, Hao C, Cui Y. The expression of cytokines IFN-γ, IL-4, IL-17A, and TGF-β1 in peripheral blood and follicular fluid of patients testing positive for anti-thyroid autoantibodies and its influence on in vitro fertilization and embryo transfer pregnancy outcomes. Gynecol Endocrinol (2018) 34(11):933–9. doi: 10.1080/09513590.2018.1459546
26. Konova E. The role of NK cells in the autoimmune thyroid disease-associated pregnancy loss. Clin Rev Allergy Immunol (2010) 39(3):176–84. doi: 10.1007/s12016-010-8201-7
27. Miko E, Meggyes M, Doba K, Farkas N, Bogar B, Barakonyi A, et al. Characteristics of peripheral blood NK and NKT-like cells in euthyroid and subclinical hypothyroid women with thyroid autoimmunity experiencing reproductive failure. J Reprod Immunol (2017) 124:62–70. doi: 10.1016/j.jri.2017.09.008
28. Bucci I, Giuliani C, Di Dalmazi G, Formoso G, Napolitano G. Thyroid autoimmunity in female infertility and assisted reproductive technology outcome. Front Endocrinol (Lausanne) (2022) 13:768363. doi: 10.3389/fendo.2022.768363
29. Poppe K, Glinoer D, Van Steirteghem A, Tournaye H, Devroey P, Schiettecatte J, et al. Thyroid dysfunction and autoimmunity in infertile women. Thyroid. (2002) 12(11):997–1001. doi: 10.1089/105072502320908330
30. Korevaar TIM, Steegers EA, Pop VJ, Broeren MA, Chaker L, de Rijke YB, et al. Thyroid autoimmunity impairs the thyroidal response to human chorionic gonadotropin: Two population-based prospective cohort studies. J Clin Endocrinol Metab (2017) 102(1):69–77. doi: 10.1210/jc.2016-2942
31. Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: A prospective cohort study. Thyroid (2014) 24(11):1642–9. doi: 10.1089/thy.2014.0029
32. Prummel MF, Wiersinga WM. Thyroid autoimmunity and miscarriage. Eur J Endocrinol (2004) 150(6):751–5. doi: 10.1530/eje.0.1500751
33. Poppe K, Autin C, Veltri F, Sitoris G, Kleynen P, Praet JP, et al. Thyroid disorders and in vitro outcomes of assisted reproductive technology: An unfortunate combination? Thyroid (2020) 30(8):1177–85. doi: 10.1089/thy.2019.0567
34. Plowden TC, Schisterman EF, Sjaarda LA, Zarek SM, Perkins NJ, Silver R, et al. Subclinical hypothyroidism and thyroid autoimmunity are not associated with fecundity, pregnancy loss, or live birth. J Clin Endocrinol Metab (2016) 101(6):2358–65. doi: 10.1210/jc.2016-1049
35. Poppe K, Velkeniers B, Glinoer D. Thyroid disease and female reproduction. Clin Endocrinol (Oxf) (2007) 66(3):309–21. doi: 10.1111/j.1365-2265.2007.02752.x
36. Van den Boogaard E, Vissenberg R, Land JA, Van Wely M, van der Post JA, Goddijn M, et al. Significance of (sub)clinical thyroid dysfunction and thyroid autoimmunity before conception and in early pregnancy: A systematic review. Hum Reprod Update (2011) 17(5):605–19. doi: 10.1093/humupd/dmr024
37. Janssen OE, Mehlmauer N, Hahn S, Offner AH, Gärtner R. High prevalence of autoimmune thyroiditis in patients with polycystic ovary syndrome. Eur J Endocrinol (2004) 150(3):363–9. doi: 10.1530/eje.0.1500363
38. Poppe K, Velkeniers B, Glinoer D. The role of thyroid autoimmunity in fertility and pregnancy. Nat Clin Pract Endocrinol Metab (2008) 4(7):394–405. doi: 10.1038/ncpendmet0846
39. Abalovich M, Mitelberg L, Allami C, Gutierrez S, Alcaraz G, Otero P, et al. Subclinical hypothyroidism and thyroid autoimmunity in women with infertility. Gynecol Endocrinol (2007) 23(5):279–83. doi: 10.1080/09513590701259542
40. Petta CA, Arruda MS, Zantut-Wittmann DE, Benetti-Pinto CL. Thyroid autoimmunity and thyroid dysfunction in women with endometriosis. Hum Reprod (2007) 22(10):2693–7. doi: 10.1093/humrep/dem267
41. Romitti M, Fabris VC, Ziegelmann PK, Maia AL, Spritzer PM. Association between PCOS and autoimmune thyroid disease: A systematic review and meta-analysis. Endocr Connect (2018) 7(11):1158–67. doi: 10.1530/EC-18-0309
42. Gaberšček S, Zaletel K, Schwetz V, Pieber T, Obermayer-Pietsch B, Lerchbaum E. Mechanisms in endocrinology: Thyroid and polycystic ovary syndrome. Eur J Endocrinol (2015) 172(1):R9–21. doi: 10.1530/EJE-14-0295
43. Glintborg D, Rubin KH, Nybo M, Abrahamsen B, Andersen M. Increased risk of thyroid disease in Danish women with polycystic ovary syndrome: A cohort study. Endocr Connect (2019) 8(10):1405–15. doi: 10.1530/EC-19-0377
44. Gerhard I, Becker T, Eggert-Kruse W, Klinga K, Runnebaum B. Thyroid and ovarian function in infertile women. Hum Reprod (1991) 6(3):338–45. doi: 10.1093/oxfordjournals.humrep.a137335
45. Shigesi N, Kvaskoff M, Kirtley S, Feng Q, Fang H, Knight JC, et al. The association between endometriosis and autoimmune diseases: A systematic review and meta-analysis. Hum Reprod Update (2019) 25(4):486–503. doi: 10.1093/humupd/dmz014
46. Eisenberg VH, Zolti M, Soriano D. Is there an association between autoimmunity and endometriosis? Autoimmun Rev (2012) 11(11):806–14. doi: 10.1016/j.autrev.2012.01.005
47. Inagaki J, Sugiura-Ogasawara M, Nomizu M, Nakatsuka M, Ikuta K, Suzuki N, et al. An association of IgG anti-laminin-1 autoantibodies with endometriosis in infertile patients. Hum Reprod (2003) 18(3):544–9. doi: 10.1093/humrep/deg148
48. Li F, Lu H, Huang Y, Wang X, Zhang Q, Li X, et al. A systematic review and meta-analysis of the association between hashimoto's thyroiditis and ovarian reserve. Int Immunopharmacol (2022) 108:108670. doi: 10.1016/j.intimp.2022.108670
49. Li Z, Xu S, Luo W, Hu J, Zhang T, Jiao X, et al. Association between thyroid autoimmunity and the decline of ovarian reserve in euthyroid women. Reprod BioMed Online (2022) 45(3):615–22. doi: 10.1016/j.rbmo.2022.05.015
50. Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. European Society of human reproduction and embryology (ESHRE) guideline: Management of women with premature ovarian insufficiency. Hum Reprod (2016) 31(5):926–37. doi: 10.1093/humrep/dew027
51. Thangaratinam S, Tan A, Knox E, Kilby MD, Franklyn J, Coomarasamy A. Association between thyroid autoantibodies and miscarriage and preterm birth: Meta-analysis of evidence. BMJ (2011) 342:d2616. doi: 10.1136/bmj.d2616
52. He X, Wang P, Wang Z, Xu D, Wang B. Thyroid antibodies and risk of preterm delivery: A meta-analysis of prospective cohort studies. Eur J Endocrinol (2012) 167(4):455–64. doi: 10.1530/EJE-12-0379
53. Korevaar TIM, Derakhshan A, Taylor PN, Meima M, Chen L, Bliddal S, et al. Association of thyroid function test abnormalities and thyroid autoimmunity with preterm birth: A systematic review and meta-analysis. JAMA. (2019) 322(7):632–41. doi: 10.1001/jama.2019.10931
54. Han Y, Mao LJ, Ge X, Huang K, Yan SQ, Ren LL, et al. Impact of maternal thyroid autoantibodies positivity on the risk of early term birth: Ma'anshan birth cohort study. Endocrine. (2018) 60(2):329–38. doi: 10.1007/s12020-018-1576-6
55. Yuan N, Sun J, Li Z, Chai S, Zhang X, Ji L. Relationship between anti-thyroid peroxidase antibody positivity and pregnancy-related and fetal outcomes in euthyroid women: A single-center cohort study. BMC Pregnancy Childbirth (2020) 20(1):491. doi: 10.1186/s12884-020-03176-4
56. Kaplan S. The relationship between thyroid autoantibody positivity and abnormal pregnancy outcomes and miscarriage in euthyroid patients. J Obstet Gynecol Investig (2020) 3(1):17–22. doi: 10.5114/jogi.2020.100977
57. Dong AC, Morgan J, Kane M, Stagnaro-Green A, Stephenson MD. Subclinical hypothyroidism and thyroid autoimmunity in recurrent pregnancy loss: A systematic review and meta-analysis. Fertil Steril (2020) 113(3):587–600.e1. doi: 10.1016/j.fertnstert.2019.11.003
58. Bliddal S, Feldt-Rasmussen U, Rasmussen Å, Kolte AM, Hilsted LM, Christiansen OB, et al. Thyroid peroxidase antibodies and prospective live birth rate: A cohort study of women with recurrent pregnancy loss. Thyroid. (2019) 29(10):1465–74. doi: 10.1089/thy.2019.0077
59. Dhillon-Smith RK, Coomarasamy A. TPO antibody positivity and adverse pregnancy outcomes. Best Pract Res Clin Endocrinol Metab (2020) 34(4):101433. doi: 10.1016/j.beem.2020.101433
60. Haddow JE, McClain MR, Palomaki GE, Neveux LM, Lambert-Messerlian G, Canick JA, et al. Thyroperoxidase and thyroglobulin antibodies in early pregnancy and placental abruption. Obstet Gynecol (2011) 117(2 Pt 1):287–92. doi: 10.1097/AOG.0b013e31820513d9
61. Haddow JE, Cleary-Goldman J, McClain MR, Palomaki GE, Neveux LM, Lambert-Messerlian G, et al. Thyroperoxidase and thyroglobulin antibodies in early pregnancy and preterm delivery. Obstet Gynecol (2010) 116(1):58–62. doi: 10.1097/AOG.0b013e3181e10b30
62. Kent NL, Young SL, Akison LK, Cuffe JSM. Is the link between elevated TSH and gestational diabetes mellitus dependant on diagnostic criteria and thyroid antibody status: A systematic review and meta-analysis. Endocrine. (2021) 74(1):38–49. doi: 10.1007/s12020-021-02733-x
63. Kyrilli A, Unuane D, Poppe KG. Thyroid autoimmunity and pregnancy in euthyroid women. Best Pract Res Clin Endocrinol Metab (2022) 101632. doi: 10.1016/j.beem.2022.101632
64. Andrisani A, Sabbadin C, Marin L, Ragazzi E, Dessole F, Armanini D, et al. The influence of thyroid autoimmunity on embryo quality in women undergoing assisted reproductive technology. Gynecol Endocrinol (2018) 34(9):752–5. doi: 10.1080/09513590.2018.1442427
65. Weghofer A, Himaya E, Kushnir VA, Barad DH, Gleicher N. The impact of thyroid function and thyroid autoimmunity on embryo quality in women with low functional ovarian reserve: A case-control study. Reprod Biol Endocrinol (2015) 13:43. doi: 10.1186/s12958-015-0041-0
66. Poppe K, Glinoer D, Tournaye H, Devroey P, van Steirteghem A, Kaufman L, et al. Assisted reproduction and thyroid autoimmunity: An unfortunate combination? J Clin Endocrinol Metab (2003) 88(9):4149–52. doi: 10.1210/jc.2003-030268
67. Busnelli A, Paffoni A, Fedele L, Somigliana E. The impact of thyroid autoimmunity on IVF/ICSI outcome: A systematic review and meta-analysis. Hum Reprod Update (2016) 22(6):775–90. doi: 10.1093/humupd/dmw019
68. Seungdamrong A, Steiner AZ, Gracia CR, Legro RS, Diamond MP, Coutifaris C, et al. Preconceptional antithyroid peroxidase antibodies, but not thyroid-stimulating hormone, are associated with decreased live birth rates in infertile women. Fertil Steril (2017) S0015-0282(17):31748–X. doi: 10.1016/j.fertnstert.2017.08.026
69. Unuane D, Velkeniers B, Deridder S, Bravenboer B, Tournaye H, De Brucker M. Impact of thyroid autoimmunity on cumulative delivery rates in in vitro fertilization/intracytoplasmic sperm injection patients. Fertil Steril (2016) 106(1):144–50. doi: 10.1016/j.fertnstert.2016.03.011
70. Huang N, Chen L, Lian Y, Wang H, Li R, Qiao J, et al. Impact of thyroid autoimmunity on in vitro Fertilization/Intracytoplasmic sperm injection outcomes and fetal weight. Front Endocrinol (Lausanne) (2021) 12:698579. doi: 10.3389/fendo.2021.698579
71. Inagaki Y, Takeshima K, Nishi M, Ariyasu H, Doi A, Kurimoto C, et al. The influence of thyroid autoimmunity on pregnancy outcome in infertile women: A prospective study. Endocr J (2020) 67(8):859–68. doi: 10.1507/endocrj.EJ19-0604
72. Unuane D, Velkeniers B. Impact of thyroid disease on fertility and assisted conception. Best Pract Res Clin Endocrinol Metab (2020) 34(4):101378. doi: 10.1016/j.beem.2020.101378
73. Grigoriadis S, Maziotis E, Simopoulou M, Sfakianoudis K, Giannelou P, Rapani A, et al. The impact of thyroid autoantibodies positivity on in vitro fertilization outcome: A comprehensive review. Int Arch Clin Physiol (2019) 1:002. doi: 10.23937/iacph-2017/1710002
74. Leiva P, Schwarze JE, Vasquez P, Ortega C, Villa S, Crosby J, et al. There is no association between the presence of anti-thyroid antibodies and increased reproductive loss in pregnant women after ART: A systematic review and meta-analysis. JBRA Assist Reprod (2017) 21(4):361–5. doi: 10.5935/1518-0557.20170057
75. Poppe K, Autin C, Veltri F, Kleynen P, Grabczan L, Rozenberg S, et al. Thyroid autoimmunity and intracytoplasmic sperm injection outcome: A systematic review and meta-analysis. J Clin Endocrinol Metab (2018) 103(5):1755–66. doi: 10.1210/jc.2017-02633
76. Venables A, Wong W, Way M, Homer HA. Thyroid autoimmunity and IVF/ICSI outcomes in euthyroid women: A systematic review and meta-analysis. Reprod Biol Endocrinol (2020) 18(1):120. doi: 10.1186/s12958-020-00671-3
77. Unuane D, Velkeniers B, Bravenboer B, Drakopoulos P, Tournaye H, Parra J, et al. Impact of thyroid autoimmunity in euthyroid women on live birth rate after IUI. Hum Reprod (2017) 32(4):915–22. doi: 10.1093/humrep/dex033
78. Poppe K, Bisschop P, Fugazzola L, Minziori G, Unuane D, Weghofer A. 2021 European Thyroid association guideline on thyroid disorders prior to and during assisted reproduction. Eur Thyroid J (2021) 9(6):281–95. doi: 10.1159/000512790
79. Poppe K, Glinoer D, Tournaye H, Schiettecatte J, Devroey P, van Steirteghem A, et al. Impact of ovarian hyperstimulation on thyroid function in women with and without thyroid autoimmunity. J Clin Endocrinol Metab (2004) 89(10):5273. doi: 10.1210/jc.2004-0105
80. Busnelli A, Somigliana E, Benaglia L, Sarais V, Ragni G, Fedele L. Thyroid axis dysregulation during in vitro fertilization in hypothyroid-treated patients. Thyroid. (2014) 24(11):1650–5. doi: 10.1089/thy.2014.0088
81. Hubalewska-Dydejczyk A, Gietka-Czernel M, Trofimiuk-Müldner M, Zgliczyński W, Ruchała M, Lewiński A, et al. Thyroid diseases and fertility disorders - guidelines of the polish society of endocrinology [Choroby tarczycy a zaburzenia płodności - rekomendacje polskiego towarzystwa endokrynologicznego]. Endokrynol Pol (2022) 73(4):645–79. doi: 10.5603/EP.a2022.0069
82. Chai J, Yeung WYT, Lee CYV, Li HWR, Ho PC, Ng HYE. Live birth rates following in vitro fertilization in women with thyroid autoimmunity and/or subclinical hypothyroidism. Clin Endocrinol (Oxf). (2014) 80(1):122–7. doi: 10.1111/cen.12220
83. Zhao T, Chen BM, Zhao XM, Shan ZY. Meta-analysis of ART outcomes in women with different preconception TSH levels. Reprod Biol Endocrinol (2018) 16(1):111. doi: 10.1186/s12958-018-0424-0
84. Negro R, Mangieri T, Coppola L, Presicce G, Casavola EC, Gismondi R, et al. Levothyroxine treatment in thyroid peroxidase antibody-positive women undergoing assisted reproduction technologies: A prospective study. Hum Reprod (2005) 20(6):1529–33. doi: 10.1093/humrep/deh843
85. Negro R, Formoso G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. Levothyroxine treatment in euthyroid pregnant women with autoimmune thyroid disease: Effects on obstetrical complications. J Clin Endocrinol Metab (2006) 91(7):2587–9. doi: 10.1210/jc.2005-1603
86. Negro R, Schwartz A, Stagnaro-Green A. Impact of levothyroxine in miscarriage and preterm delivery rates in first trimester thyroid antibody-positive women with TSH less than 2.5 mIU/L. J Clin Endocrinol Metab (2016) 101(10):3685–90. doi: 10.1210/jc.2016-1803
87. Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing In vitro fertilization and embryo transfer: A randomized clinical trial. JAMA. (2017) 318(22):2190–8. doi: 10.1001/jama.2017.18249
88. Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Alavi Majd H, Azizi F. Effects of levothyroxine treatment on pregnancy outcomes in pregnant women with autoimmune thyroid disease. Eur J Endocrinol (2017) 176(2):253–65. doi: 10.1530/EJE-16-0548
89. Dhillon-Smith RK, Middleton LJ, Sunner KK, Cheed V, Baker K, Farrell-Carver S, et al. Levothyroxine in women with thyroid peroxidase antibodies before conception. N Engl J Med (2019) 380(14):1316–25. doi: 10.1056/NEJMoa1812537
90. Van Dijk MM, Vissenberg R, Fliers E, van der Post JAM, van der Hoorn MP, de Weerd S, et al. Levothyroxine in euthyroid thyroid peroxidase antibody positive women with recurrent pregnancy loss (T4LIFE trial): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol (2022) 10(5):322–29. doi: 10.1016/S2213
91. Negro R, Greco G, Mangieri T, Pezzarossa A, Dazzi D, Hassan H. The influence of selenium supplementation on postpartum thyroid status in pregnant women with thyroid peroxidase autoantibodies. J Clin Endocrinol Metab (2007) 92(4):1263–68. doi: 10.1210/jc.2006-1821
92. Mao J, Pop VJ, Bath SC, Vader HL, Redman CW, Rayman MP. Effect of low-dose selenium on thyroid autoimmunity and thyroid function in UK pregnant women with mild-to-moderate iodine deficiency. Eur J Nutr (2016) 55(1):55–61. doi: 10.1007/s00394-014-0822-9
93. Mantovani G, Isidori AM, Moretti C, Di Dato C, Greco E, Ciolli, et al. Selenium supplementation in the management of thyroid autoimmunity during pregnancy: Results of the “SERENA study”, a randomized, double-blind, placebo-controlled trial. Endocrine (2019) 66:542–50. doi: 10.1007/s12020-019-01958-.1
94. Sun X, Hou N, Wang H, Ma L, Sun J, Liu Y. A meta-analysis of pregnancy outcomes with levothyroxine treatment in euthyroid women with thyroid autoimmunity. J Clin Endocrinol Metab (2020) 105(4):1009–19. doi: 10.1210/clinem/dgz217
95. Wang X, Zhang Y, Tan H, Bai Y, Zhou L, Fang F, et al. Effect of levothyroxine on pregnancy outcomes in women with thyroid autoimmunity: A systematic review with meta-analysis of randomized controlled trials. Fertil Steril (2020) 114(6):1306–14. doi: 10.1016/j.fertnstert.2020.06.034
96. Lau L, Benham JL, Lemieux P, Yamamoto J, Donovan LE. Impact of levothyroxine in women with positive thyroid antibodies on pregnancy outcomes: A systematic review and meta-analysis of randomised controlled trials. BMJ Open (2021) 11(2):e043751. doi: 10.1136/bmjopen-2020-043751
97. Di Girolamo R, Liberati M, Silvi C, D'Antonio F. Levothyroxine supplementation in euthyroid pregnant women with positive autoantibodies: A systematic review and meta-analysis. Front Endocrinol (Lausanne) (2022) 13:759064. doi: 10.3389/fendo.2022.759064
98. Rayman MP. Selenium and human health. Lancet (2012) 379(9822):1256–68. doi: 10.1016/S0140-6736(11)61452-9
99. Schomburg L. Selenium, selenoproteins and the thyroid gland: Interactions in health and disease. Nat Rev Endocrinol (2011) 8(3):160–71. doi: 10.1038/nrendo.2011.174
100. Gärtner R, Gasnier BC, Dietrich JW, Krebs B, Angstwurm MW. Selenium supplementation in patients with autoimmune thyroiditis decreases thyroid peroxidase antibodies concentrations. J Clin Endocrinol Metab (2002) 87(4):1687–91. doi: 10.1210/jcem.87.4.8421
101. Duntas LH, Mantzou E, Koutras DA. Effects of a six month treatment with selenomethionine in patients with autoimmune thyroiditis. Eur J Endocrinol (2003) 148(4):389–93. doi: 10.1530/eje.0.1480389
102. Turker O, Kumanlioglu K, Karapolat I, Dogan I. Selenium treatment in autoimmune thyroiditis: 9-month follow-up with variable doses. J Endocrinol (2006) 190(1):151–56. doi: 10.1677/joe.1.06661
103. Gärtner R, Gasnier BC. Selenium in the treatment of autoimmune thyroiditis. Biofactors (2003) 19(3-4):165–70. doi: 10.1002/biof.5520190309
104. Nacamulli D, Mian C, Petricca D, Lazzarotto F, Barollo S, Pozza D, et al. Influence of physiological dietary selenium supplementation on the natural course of autoimmune thyroiditis. Clin Endocrinol (Oxf). (2010) 73(4):535–39. doi: 10.1111/j.1365-2265.2009.03758.x
105. Ambroziak U, Hybsier S, Shahnazaryan U, Krasnodębska-Kiljańska M, Rijntjes E, Bartoszewicz Z, et al. Severe selenium deficits in pregnant women irrespective of autoimmune thyroid disease in an area with marginal selenium intake. J Trace Elem Med Biol (2017) 44:186–91. doi: 10.1016/j.jtemb.2017.08.005
106. Zachara BA. Selenium in complicated pregnancy. A Review Adv Clin Chem (2018) 86:157–78. doi: 10.1016/bs.acc.2018.05.004
107. Litwicka K, Arrivi C, Varricchio MT, Mencacci C, Greco E. In women with thyroid autoimmunity, does low-dose prednisolone administration, compared with no adjuvant therapy, improve in vitro fertilization clinical results? J Obstet Gynaecol Res (2015) 41(5):722–8. doi: 10.1111/jog.12615
108. Turi A, Giannubilo SR, Zanconi S, Mascetti A, Tranquilli AL. Preconception steroid treatment in infertile women with antithyroid autoimmunity undergoing ovarian stimulation and intrauterine insemination: A double-blind, randomized, prospective cohort study. Clin Ther (2010) 32(14):2415–21. doi: 10.1016/j.clinthera.2011.01.010
109. Egerup P, Lindschou J, Gluud C, Christiansen OB, Group IIS. The effects of intravenous immunoglobulins in women with recurrent miscarriages: A systematic review of randomised trials with meta-analyses and trial sequential analyses including individual patient data. PLos One (2015) 10(10):e0141588. doi: 10.1371/journal.pone.0141588
110. Rasmark Roepke E, Hellgren M, Hjertberg R, Blomqvist L, Matthiesen L, Henic E, et al. Treatment efficacy for idiopathic recurrent pregnancy loss - a systematic review and meta-analyses. Acta Obstet Gynecol Scand (2018) 97(8):921–41. doi: 10.1111/aogs.13352
111. Ahmadi M, Abdolmohammadi-Vahid S, Ghaebi M, Aghebati-Maleki L, Afkham A, Danaii S, et al. Effect of intravenous immunoglobulin on Th1 and Th2 lymphocytes and improvement of pregnancy outcome in recurrent pregnancy loss (RPL). BioMed Pharmacother (2017) 92:1095–102. doi: 10.1016/j.biopha.2017.06.001
112. Lee SK, Kim JY, Han AR, Hur SE, Kim CJ, Kim TH, et al. Intravenous immunoglobulin G improves pregnancy outcome in women with recurrent pregnancy losses with cellular immune abnormalities. Am J Reprod Immunol (2016) 75(1):59–68. doi: 10.1111/aji.12442
113. Stricker RB, Steinleitner A, Bookoff CN, Weckstein LN, Winger EE. Successful treatment of immunologic abortion with low-dose intravenous immunoglobulin. Fertil Steril (2000) 73(3):536–40. doi: 10.1016/s0015-0282(99)00572-5
114. Kiprov DD, Nachtigall RD, Weaver RC, Jacobson A, Main EK, Garovoy MR. The use of intravenous immunoglobulin in recurrent pregnancy loss associated with combined alloimmune and autoimmune abnormalities. Am J Reprod Immunol (1996) 36(4):228–34. doi: 10.1111/j.1600-0897.1996.tb00168.x
Keywords: thyroid autoimmunity, female fertility, pregnancy outcomes, assisted reproductive technology outcomes, levothyroxine treatment, immunotherapy
Citation: Tańska K, Gietka-Czernel M, Glinicki P and Kozakowski J (2023) Thyroid autoimmunity and its negative impact on female fertility and maternal pregnancy outcomes. Front. Endocrinol. 13:1049665. doi: 10.3389/fendo.2022.1049665
Received: 20 September 2022; Accepted: 12 December 2022;
Published: 11 January 2023.
Edited by:
Tuija Männistö, NordLab Oulu, FinlandReviewed by:
Luigi Carbone, University of Naples Federico II, ItalyCopyright © 2023 Tańska, Gietka-Czernel, Glinicki and Kozakowski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kamila Tańska, a3RhbnNrYUBjbWtwLmVkdS5wbA==; Małgorzata Gietka-Czernel, bWdpZXRrYUBjbWtwLmVkdS5wbA==
 Kamila Tańska
Kamila Tańska Małgorzata Gietka-Czernel
Małgorzata Gietka-Czernel Piotr Glinicki
Piotr Glinicki Jarosław Kozakowski
Jarosław Kozakowski