
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 10 January 2023
Sec. Adrenal Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1048663
This article is part of the Research Topic Recent advances in diagnosis and treatment of congenital adrenal hyperplasia due to 21-hydroxylase deficiency View all 12 articles
 Afif Nakhleh1,2*†‡
Afif Nakhleh1,2*†‡ Leonard Saiegh3,4†
Leonard Saiegh3,4† Naim Shehadeh1,2,3
Naim Shehadeh1,2,3 Naomi Weintrob5,6
Naomi Weintrob5,6 Mohammad Sheikh-Ahmad4
Mohammad Sheikh-Ahmad4 Lia Supino-Rosin7
Lia Supino-Rosin7 Sandra Alboim7
Sandra Alboim7 Raya Gendelman8
Raya Gendelman8 Moshe Zloczower1
Moshe Zloczower1Context: The 250µg-cosyntropin stimulation test (CST) is used to diagnose non-classic congenital adrenal hyperplasia (NCCAH). The current recommendation is to perform CST when follicular 17-hydroxyprogesterone (17OHP) is 6-30 nmol/L, a cutoff derived from radioimmunoassay (RIA). Recently, enzyme-linked immunosorbent assay (ELISA) has replaced RIA.
Objectives: We aimed to (1) determine the RIA and ELISA-based 17OHP cutoffs at which CST should be performed, (2) identify predictors of NCCAH.
Methods: A retrospective study at an Israeli Health Maintenance Organization. Data were retrieved from women with suspected NCCAH, referred for CST during 2001–2020. NCCAH was defined as a stimulated 17OHP >30 nmol/L. Serum 17OHP levels were assayed by RIA from 1/2000-3/2015, and by ELISA from 4/2015-12/2020. ROC curves were generated and optimal 17OHP thresholds were determined. Multivariate analysis was performed.
Results: CST was performed in 2409 women (1564 in RIA, 845 in ELISA). NCCAH was diagnosed in 4.7% of the RIA group and 7.5% of the ELISA group. The optimal basal 17OHP cutoff values predicting NCCAH were 6.1 nmol/L in RIA (sensitivity=93.2%, specificity=91.7%) and 8.2 nmol/L in ELISA (sensitivity=93.7%, specificity=92.3%). In multivariate analysis, higher basal 17OHP, lower LH: FSH ratio, and oligomenorrhea were predictors of NCCAH in RIA. Higher basal 17OHP, androstenedione, and total testosterone were predictors of NCCAH in ELISA. A lower LH: FSH ratio showed similar trend in ELISA.
Conclusions: Optimal RIA-based basal 17OHP cutoff was comparable with that recommended in guidelines. The results suggest adopting a higher 17OHP cutoff when using ELISA. LH : FSH ratio improves the negative predictive value of basal 17OHP.
The 250 µg cosyntropin stimulation test (CST) is used to diagnose non-classic congenital adrenal hyperplasia (NCCAH) due to 21-hydroxylase deficiency. The current recommendation is to perform CST when basal serum 17-hydroxyprogesterone (17OHP) levels (performed in the early follicular phase in females) are 6-30 nmol/L, and the test is considered positive for NCCAH diagnosis when the 60-minutes post-CST 17OHP level is > 30 nmol/L (1). These 17OHP cutoffs were mainly derived from radioimmunoassay (RIA) data (2). The Endocrine Society recommends screening with an early morning (before 8 AM) basal serum 17OHP measurement by liquid chromatography with tandem mass spectrometry (LC-MS/MS) (1). However, because of the limited availability of LC-MS/MS, immunoassays remain the assays most frequently used.
There are several disadvantages to using RIA including reagent instability, radioactive waste management, and the need for manual handling. Recently, a validated enzyme-linked immunosorbent assay (ELISA) has widely replaced RIA in the measurement of serum 17OHP (3). ELISA is simple, easy to perform, and uses commercially available reagents (4). A validation study by an Australian laboratory showed that IBL’s 17OHP ELISA assay provides an acceptable alternative for the Siemens Healthcare Diagnostics 17OHP RIA (3, 5).
The 17OHP cutoff of 6 nmol/L for NCCAH screening has been questioned by several studies utilizing RIA assays that suggested lower cutoffs (6–9). Several studies showed that between 2% and 11% of adult patients with NCCAH might be missed using this approach (10). Implementation of a new assay justifies re-evaluation of the basal 17OHP threshold level for performing CST, especially in the era of cost-effective medicine.
Since most of the patients diagnosed with NCCAH are females (11, 12), we studied only females aged >16 years with clinical suspicion of NCCAH. The current study aimed to determine the best cutoff for performing CST using ELISA compared to RIA. In addition, we aimed to identify clinical and laboratory factors that could predict the diagnosis of NCCAH.
This non-interventional, retrospective, cohort study was conducted using the electronic medical database of Maccabi Healthcare Services (MHS), a large health maintenance organization (HMO) in Israel serving over 2 million patients. All medical data were obtained from the MHS automated database. The retrieval of patients’ records was performed using MDClone, a query tool that provides comprehensive patient-level data for a wide range of variables in a defined time frame around an index event (13, 14). This platform was used to elicit information on demographic, clinical, and biochemical data, and dispensed community prescriptions. Approval was obtained from MHS institutional review board (IRB) and ethics committee to access and analyze data. Individual patient informed consent was not required because of the anonymized nature of patient records.
We retrieved data on consecutive women over 16 years of age with suspected NCCAH, referred for 250 µg CST from January 2001 – December 2020. As it is a real-life study, all CSTs for suspected NCCAH were included regardless of pretest 17OHP levels. The time of the CST was set as the index date. Subjects meeting inclusion criteria had clinical data available for at least 12 months before the index date.
Subjects using estrogen-containing oral contraceptives (OC), systemic or topical hormone replacement therapy (HRT), or systemic glucocorticoids (GC) were excluded (n=141). Subjects were considered as users of OC (n=109), HRT (n=3), or GC (n=29) if their prescription was filled within three months before the index date.
Clinical presentations that led to 17OHP measurements were hirsutism, oligomenorrhea, amenorrhea, acne, alopecia, or infertility that were identified within 12 months before the index date, based on the International Classification of Diseases-9 (ICD-9) or MHS internal diagnostic codes—called “Y” codes.
Demographic variables included age and last body mass index (BMI) at the index date. Laboratory values were defined as the last available value during the six months before or at the index date. Laboratory values included pre- and post-CST (basal and stimulated) serum 17OHP and cortisol, serum TSH, prolactin, total testosterone, dehydroepiandrosterone sulfate (DHEAS), androstenedione, LH, and FSH; LH to FSH ratio was also calculated.
NCCAH was defined as a 60-minute post-CST 17OHP serum level >30 nmol/L.
Serum cortisol, TSH, prolactin, total testosterone, LH, and FSH levels were measured by chemiluminescent immunoassays (CLIAs) (Advia Centaur or Centaur XP, Siemens). Serum DHEAS levels were measured by CLIA (Immulite 2000, Siemens) from January 2000 through May 2014, and by CLIA (Centaur XP, Siemens) from June 2014 to December 2020. Serum androstenedione levels were measured using RIA (DSL) from January 2000 through December 2011, and by CLIA (Immulite 2000, Siemens) from January 2012 through December 2020.
From January 2000 through March 2015, serum 17OHP levels were assayed by direct RIA using Wizard gamma counter, Perkin-Elmer (OHP-CT: Cis Bio, Gif-sur-Yvette, France), and from April 2015 to December 2020 by ELISA (MG12181: Tecan IBL GmbH, Hamburg, Germany). The intra-assay coefficient of variation was 12.3-8.3% (range 0.345-13.21 nmol/L) for RIA, and 2.8-4.9% (range 7.39-34.57 nmol/L) for ELISA. The inter-assay coefficient of variation was 12-12.8% (range 2.94-22.81 nmol/L) for RIA, and 5.8-9.2% (range 0.78-17.39 nmol/L) for ELISA. The limit of detection was 0.09 nmol/L in both methods. MHS central laboratory performed validation for 17OHP assays, comparing 48 samples from their daily routine. Passing-Bablok regression revealed a good correlation between ELISA and RIA assays (r=0.97; range 0-25 nmol/L). The absolute 17OHP values measured by ELISA were slightly (34% on average) higher than by RIA (ELISA = 1.3462 RIA - 0.04); however, they were still within the allowable limits in accordance with the manufacturer’s package insert reference range.
Categorical variables were described as numbers and frequencies. Continuous variables were described as means and standard deviations (SD), or medians and ranges. The Chi-square test or Fisher’s exact test was used to compare categorical variables, and the student’s t-test was used to compare continuous variables, respectively. We allocated the individuals into two groups according to the 17OHP assay method used (RIA vs ELISA). For each group, a receiver-operating characteristic (ROC) curve was generated and an optimal basal 17OHP threshold with the highest sensitivity and specificity was determined. Positive (PPV) and negative (NPV) predictive values were calculated. We compared the sensitivity and specificity between the RIA and the ELISA groups when using the optimal RIA-based basal 17OHP cutoff. For each 17OHP assay group, univariate and multivariate logistic regression analyses were performed to identify variables that predict NCCAH, defined as a post-CST 17OHP serum level of >30 nmol/L. Variables with a p-value <0.1 on the univariate analysis were included in the multivariate analysis, which aimed to determine any independent predictors of NCCAH.
A two-sided P-value < 0.05 was considered statistically significant. All analyses were conducted with the statistical software SPSS, version 25 (IBM Corporation, Armonk, NY, USA).
CST was performed in 2409 women who satisfied the inclusion criteria (1564 in the RIA group and 845 in the ELISA group). The mean (± SD) age was 24.1 ± 7 years. Symptoms that prompted cosyntropin testing were hirsutism in 45% of subjects, oligomenorrhea in 40%, amenorrhea in 25%, acne in 39.4%, infertility in 13.5%, and alopecia in 12.5%. The two groups were comparable in terms of the indication for testing, except for hirsutism which was more common in the RIA group (p<0.001) (Table 1).
The mean basal and stimulated 17OHP levels were lower in the RIA group as compared to the ELISA group (4.1 ± 6.4 vs. 5.9 ± 8.9 and 9.9 ± 15.3 vs. 12.3 ± 17.3, respectively, p<0.001 for both comparisons). Basal serum cortisol, total testosterone, and LH : FSH ratio were higher in the RIA group. Serum androstenedione levels were higher in the ELISA group (Table 1). The same pattern of differences was also observed between the two groups when comparing subjects without NCCAH (Table 2).
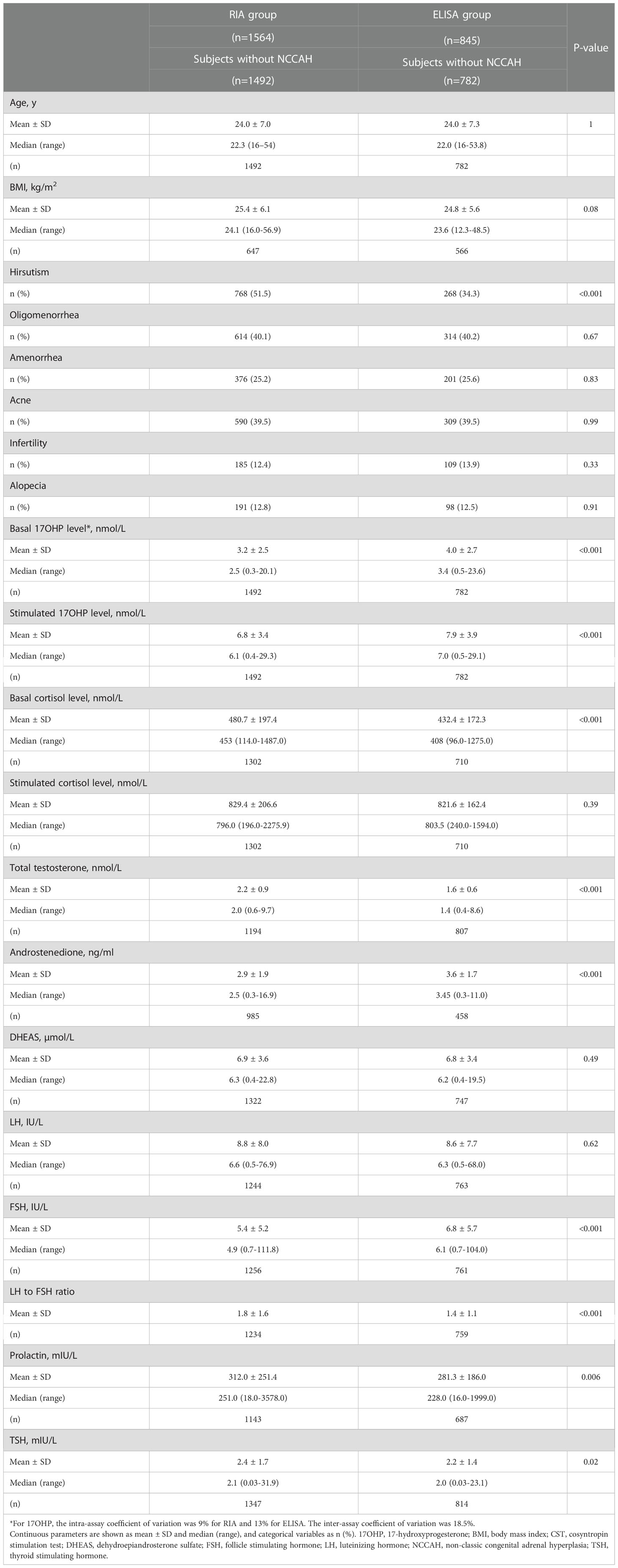
Table 2 Comparison of clinical and biochemical characteristics among women without NCCAH from the RIA and the ELISA groups.
NCCAH was diagnosed in 74 (4.7%) subjects in the RIA group and 63 (7.5%) in the ELISA group (p=0.008) (Table 1). NCCAH subjects in the two groups had comparable parameters, except for higher total testosterone levels in the RIA group and higher androstenedione levels in the ELISA group (Table 3).
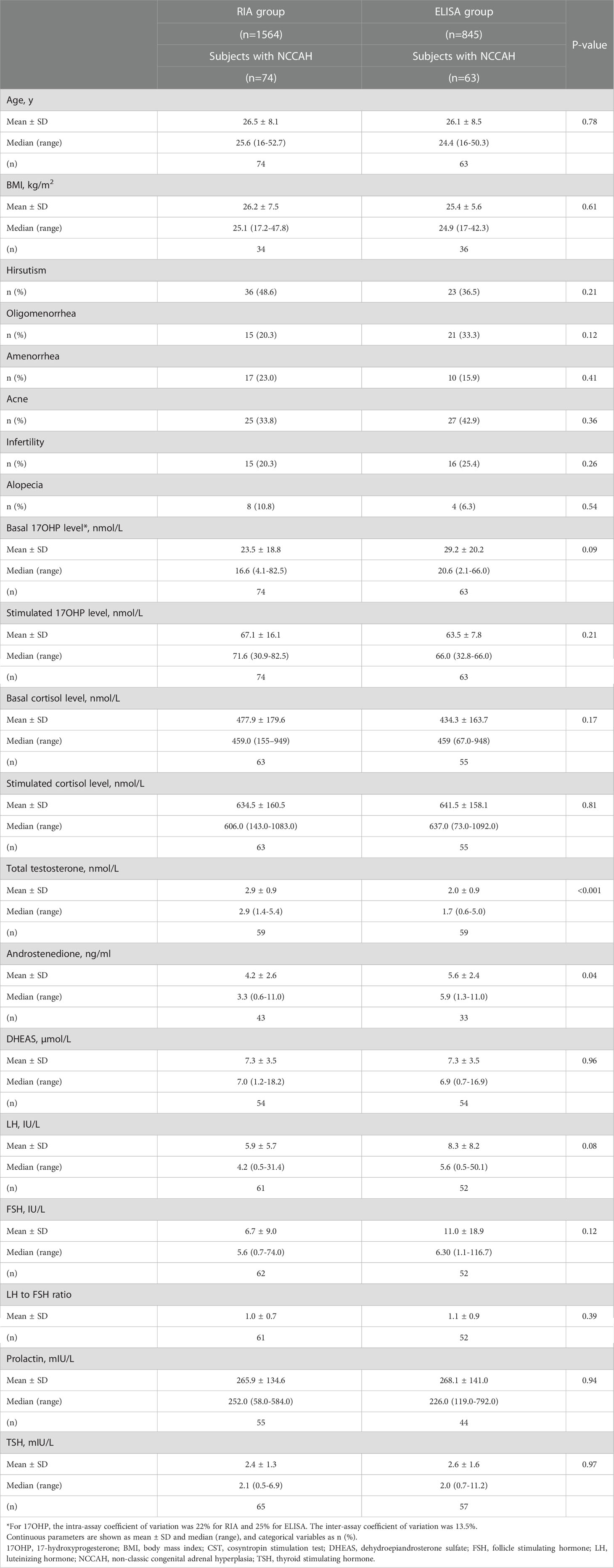
Table 3 Comparison of clinical and biochemical characteristics among women with NCCAH from the RIA and the ELISA groups.
Using ROC analysis, the optimal basal 17OHP cutoff values predicting NCCAH were 6.1 nmol/L in the RIA group (sensitivity 93.2%, specificity 91.7%, NPV 99.6%, and PPV 35.8%) (Figure 1) and 8.2 nmol/L in the ELISA group (sensitivity 93.7%, specificity 92.3%, NPV 99.5%, PPV 49.6%) (Figure 2). When basal 17OHP cutoff value of 6.1 nmol/L was used in the ELISA group, sensitivity was 93.7% (p=1 for comparison with RIA), but specificity decreased to 84.5% (p<0.001 for comparison with RIA).
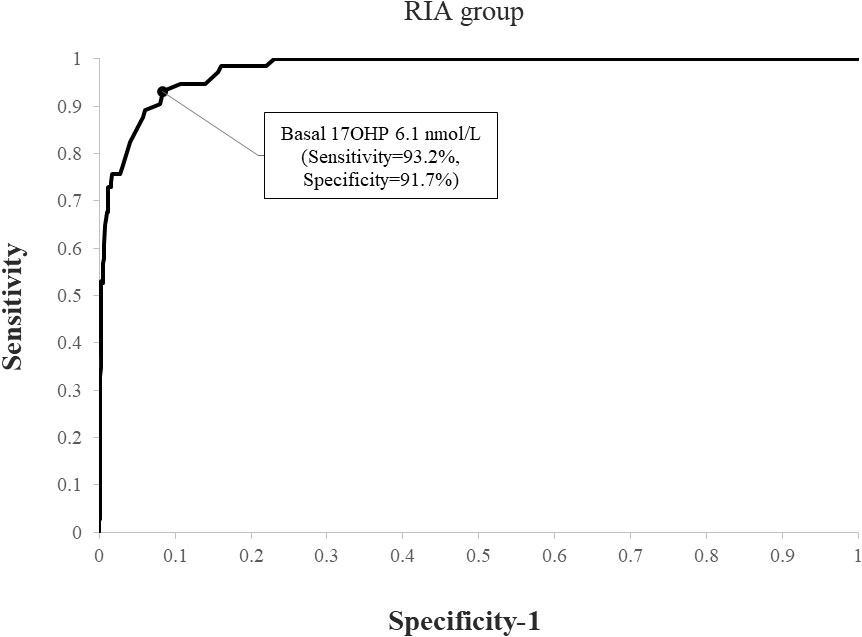
Figure 1 ROC curve analysis for determination of optimal basal 17OHP threshold in RIA (using a post-CST 17OHP cutoff > 30 nmol/L for the diagnosis of NCCAH).
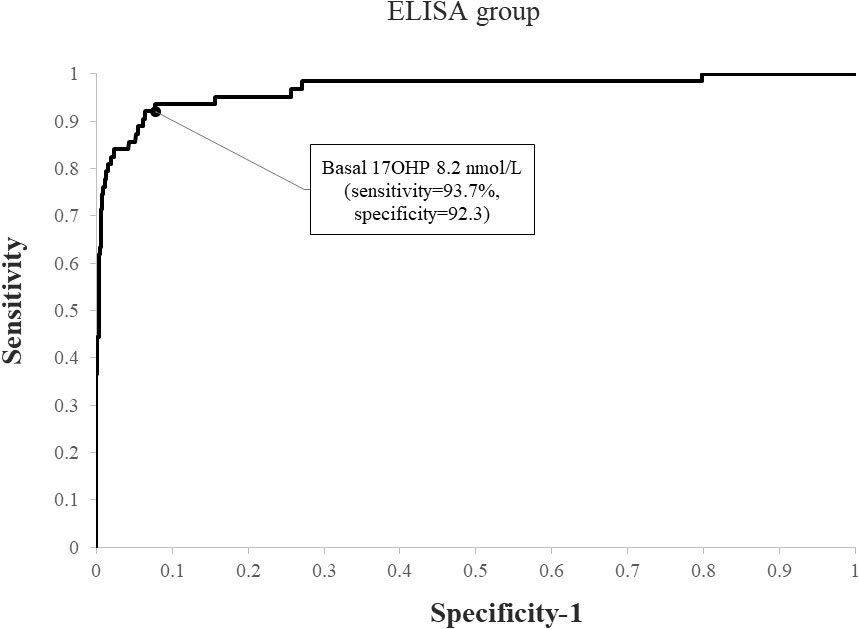
Figure 2 ROC curve analysis for determination of optimal basal 17OHP threshold in ELISA (using a post-CST 17OHP cutoff > 30 nmol/L for the diagnosis of NCCAH).
When applying a post-CST 17OHP diagnostic cutoff of > 40 nmol/L, NCCAH was diagnosed in 69 (4.4%) subjects in the RIA group and 60 (7.1%) in the ELISA group (p=0.007). Using ROC analysis, the optimal basal 17OHP cutoff values predicting NCCAH were 6.1 nmol/L in the RIA group (sensitivity 92.8%, specificity 91.4%, NPV 99.6%, PPV 33.2%) (Figure 3) and 8.2 nmol/L in the ELISA group (sensitivity 95%, specificity 92%, NPV 99.6%, PPV 47.5%) (Figure 4).
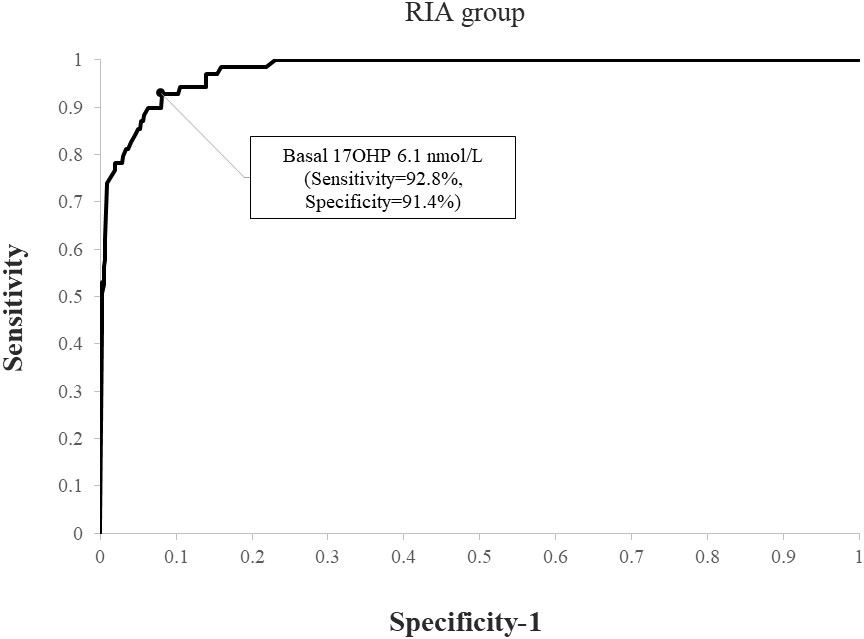
Figure 3 ROC curve analysis for determination of optimal basal 17OHP threshold in RIA (using a post-CST 17OHP cutoff > 40 nmol/L for the diagnosis of NCCAH).
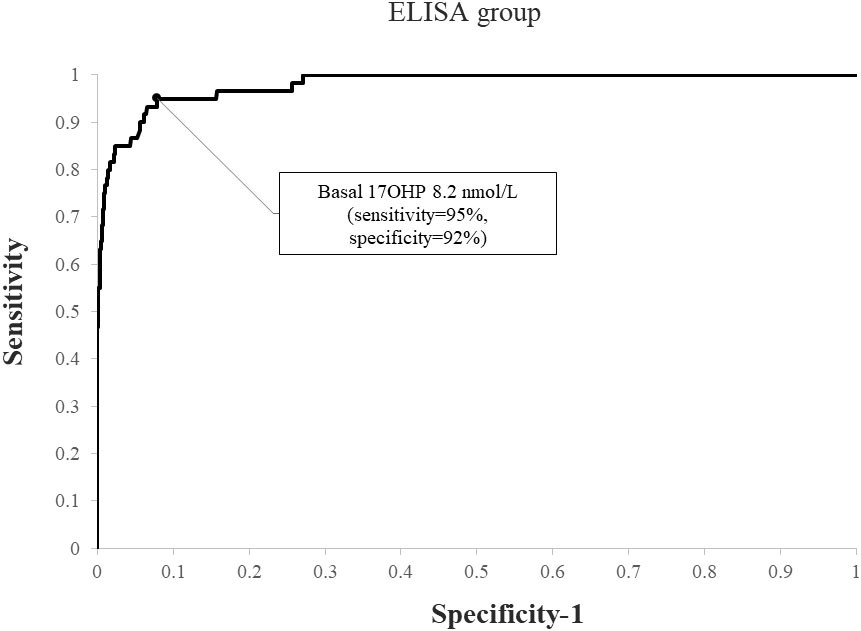
Figure 4 ROC curve analysis for determination of optimal basal 17OHP threshold in ELISA (using a post-CST 17OHP cutoff > 40 nmol/L for the diagnosis of NCCAH).
Table 4 compares the clinical and biochemical characteristics of women with and without NCCAH (defined as a post-CST 17OHP serum level of >30 nmol/L) in the RIA and the ELISA groups. In both groups, the following variables met the criteria for inclusion in the multivariate logistic regression analysis: age, basal serum 17OHP, total testosterone, androstenedione, and LH : FSH ratio. A history of oligomenorrhea and a history of infertility were included in the analyses of the RIA and the ELISA groups, respectively.
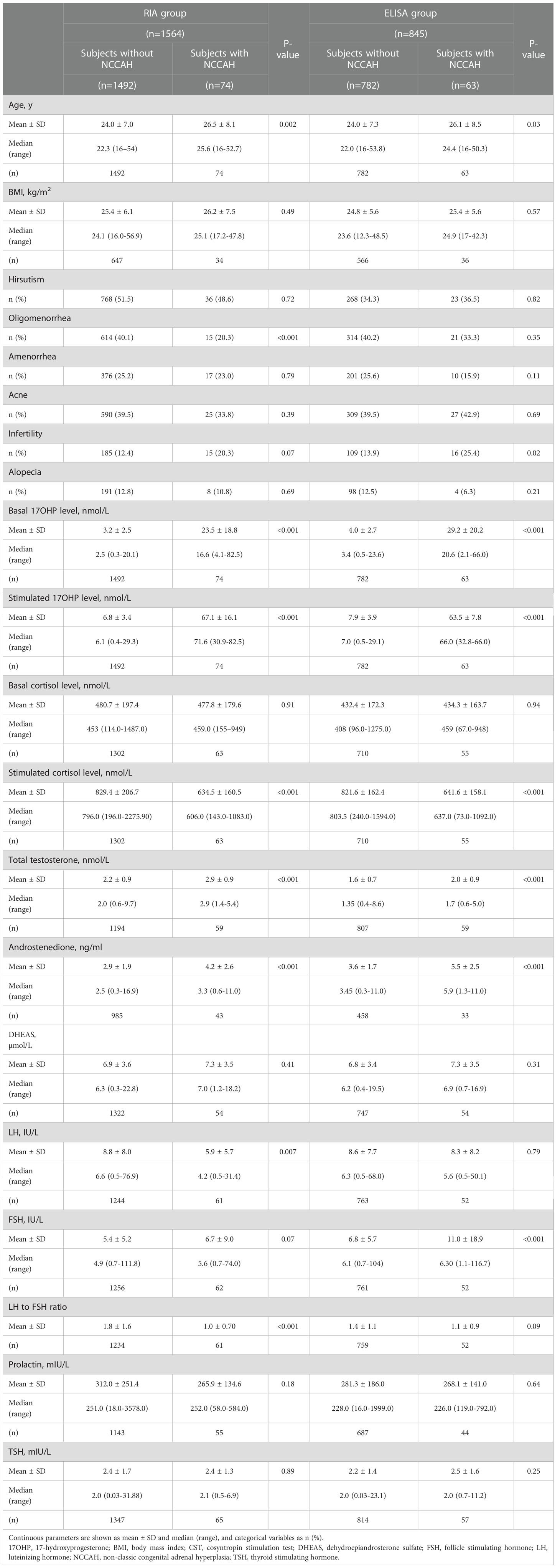
Table 4 Comparison of clinical and biochemical characteristics between women with and without NCCAH in the RIA and the ELISA groups.
In the multivariate analysis, higher basal 17OHP level (p<0.001), lower LH: FSH ratio (p=0.005), and oligomenorrhea (p=0.021) emerged as independent predictors of NCCAH in the RIA group. Higher basal 17OHP (p<0.001), androstenedione (p=0.004), and total testosterone (p=0.012) levels were identified as independent predictors of NCCAH in the ELISA group. Of note, a lower LH : FSH ratio showed a similar trend in ELISA but did not reach significance (p=0.054). The results of the multivariate analyses are shown in Tables 5 and 6.
Using ROC analysis, the optimal LH : FSH ratio cutoff values for predicting NCCAH were 1.4 in the RIA group (sensitivity 85.3%, specificity 46.8%, NPV 98.5%, PPV 7.3%) and 1.2 in the ELISA group (sensitivity 71.2%, specificity 43.4%, NPV 95.6%, PPV 7.9%).
In the RIA group, the combination of a serum basal 17OHP value below 6.1 and an LH : FSH ratio above 1.4 increased the NPV to 100%. In the ELISA group, the combination of a serum basal 17OHP value below 8.2 and LH : FSH ratio above 1.2 increased the NPV to 99.7%. Combining basal serum 17OHP values below the optimal cutoffs with an LH : FSH ratio > 2, yielded an increase in the NPV to 100% in both groups.
This study showed that the optimal RIA-based basal 17OHP cutoff at which to refer a woman suspected of NCCAH to CST was 6.1 nmol/L, comparable with that recommended in the guidelines (6 nmol/L) (1). When using ELISA, we observed an upward shift in the basal 17OHP cutoff to 8.2 nmol/L. This result is in accordance with a recent report by Domagala et al. (15) who examined the validity of the currently accepted 17OHP threshold at which CST should be performed in 343 polish individuals with suspected NCCAH. They showed that the basal ELISA-based 17OHP value that best qualified patients for testing was 2.8 ng/mL (8.4 nmol/L), with a sensitivity and a specificity of 77.2% and 91.3%, respectively.
While LC-MS/MS-based 17OHP measurement has been recommended by the Endocrine Society for the screening of NCCAH due to 21-hydroxylase deficiency (1), the use of LC-MS/MS is expensive, labor consuming and therefore not widespread, especially in adult outpatient clinics. In addition, standard cutoffs for this technique are still evolving (10).
Immunoassays are still the assays most frequently used in clinical settings and present good performance in NCCAH diagnosis. The classical basal 17OHP cutoff value of 6 nmol/L for performing CST was established based on immunoassays, mainly RIA (1). This cutoff has been called into question by several studies, most of which used RIA assays (2). Maffazoli et al. showed that 6.2% of women with NCCAH could have been missed using the classical threshold and Bidet et al. reported that 8% of NCCAH women had basal 17OHP levels < 6 nmol/L (6, 7). Escobar-Morreale et al. suggested a lower basal 17OHP level (5.1 nmol/L) to improve the screening sensitivity for NCCAH diagnosis in a cohort of women with hyperandrogenism (9).
Nonetheless, in light of the widespread use of immunoassays and the replacement of RIA by the ELISA 17OHP assay, it is pertinent to check whether the guideline-recommended basal 17OHP cutoff applies. This is the first sizable study gleaning data from a large cohort of women with hyperandrogenism and comparing data between the old RIA and the new ELISA method.
In the present study, 137/2409 (5.7%) women were diagnosed with NCCAH, defined as a stimulated 17OHP level >30 nmol/L. This figure is similar to previous studies of hyperandrogenic females (16, 17). This implies a significant number of unnecessary and costly CSTs in a real-world setting. Thus, adopting a higher 17OHP cutoff in ELISA assays might reduce the number of unnecessary tests.
Noteworthy, NCCAH was diagnosed more often in the ELISA (7.5%) than the RIA (4.7%) group. This difference could be explained by the upward shift in 17OHP levels in the ELISA group (Tables 1, 2) which might have led to more false-positive tests, although among subjects diagnosed with NCCAH the basal 17OHP levels did not differ significantly between assays (Table 3). This hypothesis is supported by the Ambroziak et al. study that used an ELISA-based 17OHP assay for the diagnosis of NCCAH among women with hyperandrogenism and observed a considerable probability of false-positive tests when based only on a stimulated 17OHP cutoff value of ≥30 nmol/l (18). They showed that among 21 women with pre- or post-CST 17OHP ≥30 nmol/l, NCCAH was confirmed by genetic testing only in five women, of whom four subjects were heterozygous carriers. They also showed that post-CST ELISA-based 17OHP <30 nmol/l excludes NCCAH with a high degree of confidence (18). In addition, Azziz et al. showed that when post-CST 17OHP values were within the range of 30–45 nmol/l, most patients were heterozygous carriers (19). In the absence of genetic data, we cannot rule out the possibility of more heterozygous carriers (of CYP21A2 monoallelic mutations) in the ELISA group.
The validation study, performed by MHS central laboratory, established that when using ELISA, the absolute 17OHP values are higher (34% on average) than that achieved if the same samples were analyzed using RIA. This raises a question of what effect would employing a 34% higher diagnostic 17OHP cutoff (~ 40 nmol/L) have on our results. Interestingly, applying a higher post-CST 17OHP cutoff of > 40 nmol/L did not affect the observed discordance in the diagnosis rates of NCCAH nor the optimal basal 17OHP thresholds in the RIA and the ELISA groups.
NCCAH diagnosis might be truly more prevalent in the ELISA group. This might be explained by the introduction of guidelines in recent years that led to more precise referrals and testing. The higher androstenedione levels in the ELISA group may remark a higher degree of clinical suspicion that led to more positive CSTs. However, further studies that incorporate genetic data are needed.
We observed that androstenedione levels were higher in the ELISA than the RIA group, while testosterone levels were higher in the RIA. We do not have a clear explanation for these differences; however, as the androstenedione assay was changed in 2012, assays were not identical in the two groups. Consequently, the difference in assays could explain these discrepancies, and the fact that we still have these differences between RIA and ELISA groups in both women with and without NCCAH (Tables 2, 3), supports this assumption. Basal cortisol, TSH and prolactin levels were higher in the RIA than in the ELISA group. However, they did not differ in NCCAH subjects between the two groups (Table 3) and did not differ within each group of RIA and ELISA, between women with and without NCCAH (Table 4). We do not have a decent explanation for that. However, as it is a real-life and not a randomized controlled trial, we cannot exclude the possibility that other unknown factors may have played a role in these differences.
Remarkably, in the RIA group, lower LH: FSH ratio and oligomenorrhea emerged as independent predictors of NCCAH. In the ELISA group, higher androstenedione and total testosterone levels were independent predictors, while lower LH: FSH ratio showed a marginal trend of significance.
Polycystic ovary syndrome (PCOS) is an important differential diagnosis of NCCAH in women and is much more frequent (20). NCCAH and PCOS share several clinical and biochemical features. Basal 17OHP, androstenedione, and testosterone levels might be increased in both NCCAH and PCOS female patients (6). Thus, it is imperative to investigate new biochemical markers rather than 17OHP alone, to better differentiate between these entities. Although elevated LH : FSH ratio (usually >2) is much more prevalent in polycystic ovary syndrome (PCOS) than in NCCAH (21), its discriminatory utility has not been thoroughly addressed.
In our study, a low LH: FSH ratio emerged as an independent predictor of NCCAH in the RIA group and showed a similar trend in the ELISA group. We found that combining basal serum 17OHP below the suggested cutoff with an LH: FSH ratio > 2 increases the NPV for the diagnosis of NCCAH to 100% in both RIA and ELISA groups. To our knowledge, this is the first study to show that the LH : FSH ratio improves the NPV for the diagnosis of NCCAH.
Our study’s main strength is the large number of subjects in each group, which enabled several analyses and allowed drawing important implications for clinical practice. We studied females older than 16 years to avoid 17OHP level differences derived from different pubertal development (22).
This study has some limitations which stem from its retrospective design. An unknown confounding may have influenced our results. The results of this study may not be generalizable to users of other assays. We cannot rule out the inclusion of peri- or post-menopausal women in the study population that may have affected the results, although the median age was 22 years. The lack of genetic profiling is another key limitation, as it may have helped better define the NCCAH populations and draw further distinctions between the 17OHP-assay groups. Further studies that incorporate genetic data are mandatory.
In conclusion, our results suggest adopting a higher ELISA-based 17OHP cutoff (8.2 nmol/L) at which patients suspected of NCCAH should be referred for further evaluation. This may help eliminate the number of unnecessary tests. When using RIA, the classical 17OHP cutoff level of 6 nmol/L seems to apply. Utilization of LH : FSH ratio can improve the negative predictive value of the basal 17OHP levels.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Maccabi Healthcare Services (MHS) institutional review board (IRB) and ethics committee. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
AN, LS, and MZ were responsible for the design of the study and data acquisition. AN, LS, and MZ contributed to the analysis and the interpretation of data, drafted the manuscript and revised it critically for important intellectual content. NS, NW, and MS-A contributed to the analysis and the interpretation of data and revised the manuscript critically for important intellectual content. LS-R, SA, and RG contributed to the interpretation of data. All authors read and approved the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Speiser PW, Arlt W, Auchus RJ, Baskin LS, Conway GS, Merke DP, et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2018) 103(11):4043–88. doi: 10.1210/jc.2018-01865
2. Azziz R, Zacur HA. 21-hydroxylase deficiency in female hyperandrogenism: Screening and diagnosis. J Clin Endocrinol Metab (1989) 69(3):577–84. doi: 10.1210/jcem-69-3-577
3. 17-OH-Progesterone ELISA . Available at: https://www.ibl-international.com/en/17-hydroxy-progesterone-17-ohp-elisa (Accessed 2022 Apr 21).
4. Engvall E, The ELISA. Enzyme-linked immunosorbent assay. Clin Chem (2010) 56(2):319–20. doi: 10.1373/clinchem.2009.127803
5. Escape from ria! comparing the ibl elisa 17 oh progesterone assay with the siemens coat-a-count manual ria assay . Available at: https://www.aacb.asn.au/documents/item/947.
6. Maffazioli GDN, Bachega TASS, Hayashida SAY, Gomes LG, Valassi HPL, Marcondes JAM, et al. Steroid screening tools differentiating nonclassical congenital adrenal hyperplasia and polycystic ovary syndrome. J Clin Endocrinol Metab (2020) 105(8):e2895–902. doi: 10.1210/clinem/dgaa369
7. Bidet M, Bellanné-Chantelot C, Galand-Portier MB, Tardy V, Billaud L, Laborde K, et al. Clinical and molecular characterization of a cohort of 161 unrelated women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency and 330 family members. J Clin Endocrinol Metab (2009) 94(5):1570–8. doi: 10.1210/jc.2008-1582
8. Moran C, Azziz R, Carmina E, Dewailly D, Fruzzetti F, Ibañez L, et al. 21-hydroxylase-deficient nonclassic adrenal hyperplasia is a progressive disorder: a multicenter study. Am J Obstet Gynecol (2000) 183(6):1468–74. doi: 10.1067/mob.2000.108020
9. Escobar-Morreale HF, Sanchón R, San Millán JL. A prospective study of the prevalence of nonclassical congenital adrenal hyperplasia among women presenting with hyperandrogenic symptoms and signs. J Clin Endocrinol Metab (2008) 93(2):527–33. doi: 10.1210/jc.2007-2053
10. Nordenström A, Falhammar H. MANAGEMENT OF ENDOCRINE DISEASE: Diagnosis and management of the patient with non-classic CAH due to 21-hydroxylase deficiency. Eur J Endocrinol (2019) 180(3):R127–45. doi: 10.1530/EJE-18-0712
11. Weintrob N, Brautbar C, Pertzelan A, Josefsberg Z, Dickerman Z, Kauschansky A, et al. Genotype-phenotype associations in non-classical steroid 21-hydroxylase deficiency. Eur J Endocrinol (2000) 143(3):397–403. doi: 10.1530/eje.0.1430397
12. Eyal O, Tenenbaum-Rakover Y, Shalitin S, Israel S, Weintrob N. Adult height of subjects with nonclassical 21-hydroxylase deficiency. Acta Paediatr (2013) 102(4):419–23. doi: 10.1111/apa.12147
13. Benaim AR, Almog R, Gorelik Y, Hochberg I, Nassar L, Mashiach T, et al. Analyzing medical research results based on synthetic data and their relation to real data results: Systematic comparison from five observational studies. JMIR Med Inf (2020) 8(2):e16492. doi: 10.2196/16492
14. Hochberg I. Insulin detemir use is associated with higher occurrence of hypoglycemia in hospitalized patients with hypoalbuminemia. Diabetes Care (2018) 41(4):e44–6. doi: 10.2337/dc17-1957
15. Domagala B, Trofimiuk-Muldner M, Krawczyk A, Topor-Kolkowska J, Skalniak A, Przybylik-Mazurek E, et al. What cut-off value of 17-hydroxyprogesterone should be an indication to perform a 250 µg cosyntropin stimulation test when NCCAH is suspected? - a retrospective study. J Endocr Soc (2021) 5(Suppl 1):A102. doi: 10.1210/jendso/bvab048.204
16. Eldar-Geva T, Hurwitz A, Vecsei P, Palti Z, Milwidsky A, Rösler A. Secondary biosynthetic defects in women with late-onset congenital adrenal hyperplasia. N Engl J Med (2010) 323(13):855–63. doi: 10.1056/NEJM199009273231302
17. Carmina E, Dewailly D, Escobar-Morreale HF, Kelestimur F, Moran C, Oberfield S, et al. Non-classic congenital adrenal hyperplasia due to 21-hydroxylase deficiency revisited: an update with a special focus on adolescent and adult women. Hum Reprod Update (2017) 23(5):580–99. doi: 10.1093/humupd/dmx014
18. Ambroziak U, Kępczyńska-Nyk A, Kuryłowicz A, Małunowicz EM, Wõjcicka A, Mis̈kiewicz P, et al. The diagnosis of nonclassic congenital adrenal hyperplasia due to 21-hydroxylase deficiency, based on serum basal or post-ACTH stimulation 17-hydroxyprogesterone, can lead to false-positive diagnosis. Clin Endocrinol (Oxf). (2016) 84(1):23–9. doi: 10.1111/cen.12935
19. Azziz R, Dewailly D, Owerbach D. Clinical review 56: Nonclassic adrenal hyperplasia: current concepts. J Clin Endocrinol Metab (1994) 78(4):810–5. doi: 10.1210/jcem.78.4.8157702
20. Moran C, Azziz R. 21-hydroxylase-deficient nonclassic adrenal hyperplasia: the great pretender. Semin Reprod Med (2003) 21(3):295–300. doi: 10.1055/s-2003-43307
21. Pignatelli D. Non-classic adrenal hyperplasia due to the deficiency of 21-hydroxylase and its relation to polycystic ovarian syndrome. Front Horm Res (2013) 40:158–70. doi: 10.1159/000342179
Keywords: non-classic congenital adrenal hyperplasia, radioimmunoassay, enzyme-linked immunosorbent assay, 17-hydroxyprogesterone, cosyntropin stimulation test, LH : FSH ratio
Citation: Nakhleh A, Saiegh L, Shehadeh N, Weintrob N, Sheikh-Ahmad M, Supino-Rosin L, Alboim S, Gendelman R and Zloczower M (2023) Screening for non-classic congenital adrenal hyperplasia in women: New insights using different immunoassays. Front. Endocrinol. 13:1048663. doi: 10.3389/fendo.2022.1048663
Received: 19 September 2022; Accepted: 19 December 2022;
Published: 10 January 2023.
Edited by:
Semra Çaglar Çetinkaya, University of Health Sciences, TurkeyReviewed by:
Dominika Janus, Jagiellonian University Medical College, PolandCopyright © 2023 Nakhleh, Saiegh, Shehadeh, Weintrob, Sheikh-Ahmad, Supino-Rosin, Alboim, Gendelman and Zloczower. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Afif Nakhleh, YW5ha2hsZWhAZ21haWwuY29t
†These authors have contributed equally to this work
‡ORCID: Afif Nakhleh, orcid.org/0000-0003-1206-1516
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.