- 1Reproductive Medicine Center, Tongji Hospital, Tongji Medicine College, Huazhong University of Science and Technology, Wuhan, China
- 2Center for Reproductive Medicine, Department of Obstetrics and Gynecology, Peking University Third Hospital, Beijing, China
Introduction: To investigate whether rescue in vitro maturation (R-IVM) improves the reproductive outcomes among women undergoing intracytoplasmic sperm injection (ICSI) after one oocyte retrieved cycle.
Methods: Between January 2019 and December 2020, 2602 women who underwent ICSI in the Reproductive Medicine Center of Tongji Hospital, Wuhan, China, were included in our retrospective cohort study. There were 2112 women undergoing only ICSI and 490 women with R-IVM followed by ICSI. The intermediate reproductive outcomes and pregnancy outcomes were assessed, including the number of normally fertilized embryos, number of cleaved embryos, number of good-quality embryos, number of day-3 available embryos, number of embryos cultured past day-3, number of blastocysts, number of available blastocysts, biochemical pregnancy, miscarriage, clinical pregnancy and live birth. The perinatal outcomes were also assessed, including preterm birth and birth weight. The abovementioned outcomes were also calculated for in vivo matured and R-IVM oocytes separately in women undergoing ICSI with R-IVM group.
Result(s): Compared with the women who underwent only ICSI, those who underwent ICSI with R-IVM had higher numbers of MII oocytes, normally fertilized embryos, cleaved embryos, day-3 available embryos, embryos cultured past day-3, and higher oocyte maturation rate, available embryo rate than women undergoing only ICSI. Additionally, we found that women undergoing ICSI with R-IVM had an increased chance of clinical pregnancy (adjusted OR=1.50, 95% CI: 1.17–1.93) and cumulative live birth (adjusted OR=1.35, 95% CI: 1.07–1.71). After propensity score matching (PSM), the cumulative live birth rate was 60.1% for women undergoing ICSI with R-IVM versus 54.9% for women undergoing only ICSI (OR=1.24, 95% CI: 0.94–1.63). The reproductive outcomes were also significantly different when calculated for in vivo matured and R-IVM oocytes separately in women undergoing ICSI with R-IVM group. All live births from R-IVM embryos were healthy and without malformations or complications.
Conclusion: R-IVM may improve the reproductive outcomes of women undergoing ICSI. It may also provide a reference for the safety of R-IVM. This study maybe support a routine application of R-IVM among patients who intend to undergo ICSI.
Introduction
Assisted reproductive technology (ART) is an effective and the final treatment for infertile couples. According to International Committee for Monitoring Assisted Reproductive Technologies (ICMART) annual world report, in 2014, the number of ART cycles reached 2.92 million globally and 1.15 million in China, 62.3% used intracytoplasmic sperm injection (ICSI) as the fertilization method (1, 2). An important aspect of the ICSI procedure was a precise determination of nuclear maturation status and oocyte morphology. About 15% of retrieved oocytes after controlled ovarian hyperstimulation (COH) are still immature and usually discarded (3). Women with a high proportion of immature oocytes after COH have a reduced chance of pregnancy or even cancel the cycle (4). Therefore, it is important to find ways to improve the utilization of immature oocytes.
In vitro maturation (IVM) is an alternative ART in which immature oocytes are retrieved at germinal vesicle(GV)/metaphase I(MI) stage and then matured in vitro to reach the metaphase II (MII) stage (5, 6). Generally, the classical IVM involves the immature oocytes from unstimulated or minimally stimulated cycles and applies to patients with polycystic ovary syndrome (PCOS) or patients with premature ovarian failure (POF) (7–9). According to the American Society for Reproductive Medicine(ASRM) committee opinion on IVM, a type of IVM known as rescue IVM (R-IVM) has been developed to benefit more infertile couples, which is the in vitro maturation of immature oocytes from conventional COH (10). Additionally, the zona pellucida of immature oocytes may harden and be completely exposed during IVM, so ICSI has been advocated as the preferred fertilization method for IVM oocytes (11).
Previous studies suggested that IVM is safe for infertile women, and for children born from the technique (12, 13). However, studies generally suggest that the development capacity of embryos derived from IVM oocytes is not comparable to sibling embryos derived from in vivo matured oocytes, which can still provide extra blastocysts for transfer (14–16). In addition, a previous study implied that women transferred with embryos from R-IVM oocytes can achieve live births, especially for those with poor prognoses (17). Some studies found that women with a high antral follicle count (AFC) after IVM treatment could achieve acceptable live birth rates and have no increased risk of adverse perinatal outcomes (18–20). There is no consistent opinion about the clinical application of R-IVM. It is still unclear whether ICSI with R-IVM increases reproductive outcomes after one oocyte retrieved cycle and supports routine utilization among all patients. Thus, we compared the reproductive outcomes of women undergoing conventional ICSI and ICSI with R-IVM to determine whether women undergoing ICSI with R-IVM increase reproductive outcomes after one oocyte retrieved cycle.
Materials and methods
Patient cohort
We performed a retrospective cohort study at the Reproductive Medicine Center of Tongji Hospital, Wuhan, China. Women aged 20-45 years who underwent ICSI cycles (with at least one mature oocyte and one immature oocyte) between January 2019 and December 2020 were included, and only one oocyte retrieved cycle per woman. Women chose between only ICSI or combined with R-IVM after consulting the ART specialist about the benefits and risks of each strategy. We excluded women with chromosomal abnormality (n=5), who underwent oocyte cryopreservation-thaw cycles (n=16), oocyte donation cycles (n=3), or preimplantation genetic testing (PGT) cycles (n=63). Finally, a total of 2602 women were included and divided into two groups according to the treatment strategy adopted (i.e., only ICSI or ICSI with R-IVM), with follow-up until July 2022. This study was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology.
ICSI procedures and in vitro maturation
Details on COH and ICSI procedures were described in our previous publications (21, 22). Briefly, COH protocols included gonadotropin-releasing hormone (GnRH) agonist, GnRH antagonist, luteal phase stimulation, and mild-stimulation protocols. During COH, we monitored serum hormone levels, follicle size, follicle count, and endometrial thickness. When at least two leading follicles reached ≥18 mm in diameter, recombinant HCG (Ovidrel; Merck-Serono) was then applied to trigger ovulation. The cumulus-oocyte complexes (COCs) were retrieved 36-38 hours after the HCG trigger. For the only ICSI group, cumulus cells of oocytes were removed 2 hours after retrieval with 80 IU hyaluronidase(Vitrolife, Sweden). And then nuclear maturation status of all denuded oocytes was assessed under an inverted microscope and classified as GV, MI, and MII stage. Only those oocytes at the MII stage were selected for ICSI while GV and MI oocytes were typically discarded. However, for the ICSI with R-IVM group, in addition to MII oocytes undergoing ICSI, those denuded immature oocytes, including GV and MI oocytes retrieved from COH cycles, were also used for further culture and utilization in vitro. They were subjected to rescue IVM treatment, which was continued to individually culture in G1-plus medium (Vitrolife, Sweden) at 37°C in a humidified atmosphere of 5% O2, 6% CO2, and 89% nitrogen for 24 hours. Moreover, it was necessary to monitor the maturity of immature oocytes periodically at 6 hours intervals until 24 hours. When immature oocytes that completed nuclear maturation during the preincubation period were selected for subsequent fertilization with the partner’s semen by ICSI.
Fresh embryo transfer (ET) was performed on day 3 after oocyte retrieval. The surplus embryos that were considered good or fair quality would be cryopreserved or extensively cultured to day 5 or 6 to reach the blastocyst stage for later transfer in subsequent frozen ET cycles. All the additional embryos were cryopreserved by vitrification using the Cryotop system. For a maximum of two day 3 embryos or one or two day 5-6 blastocysts were transferred according to the Code of Practice for Assisted Reproductive Technology developed by the Ministry of Health of the People’s Republic of China. Embryo transfers were performed by specified gynecologists with the same standard ET protocol.
Study variables
Data on maternal age, body mass index (BMI), previous conventional IVF attempts, duration of infertility (years), cause of infertility (ovulation disorder, diminished ovarian reserve, tubal or pelvic factor, endometriosis, male factor, uterine factor, unexplained), type of infertility (primary or secondary), baseline follicle-stimulating hormone (FSH), AFC, anti-Müllerian hormone (AMH), stimulation protocol (agonist, antagonist or others), gonadotropin duration (days), gonadotropin dosage, E2 and P level on the day of hCG injection, endometrial thickness, number of oocytes retrieved, number of embryos transferred, stage of embryo transferred (cleavage embryo or blastocyst), and type of first transfer (fresh or frozen ET) were extracted from the medical records. BMI (kg/m2) was calculated as the weight in kilograms divided by the square of the height in meters. Ovulation disorder included PCOS and abnormal uterine bleeding (AUB). Male factors included semen abnormalities, coital infertility (i.e. erectile dysfunction and ejaculatory dysfunction). Infertility without a clear known cause was classified as unexplained infertility.
Outcome assessments
The intermediate reproductive and pregnancy outcomes of ICSI treatment, including the number of normally fertilized embryos, number of cleaved embryos, number of good-quality embryos, number of day-3 available embryos, number of embryos cultured past day-3, number of blastocysts, number of available blastocysts, biochemical pregnancy, miscarriage, clinical pregnancy and live birth were abstracted from the medical records. The perinatal outcomes, including delivery mode (natural labor or cesarean delivery), fetal sex (male or female), gestational age, and birth weight were obtained from telephone interviews after delivery. The abovementioned outcomes were also calculated for in vivo matured and R-IVM oocytes separately in women undergoing ICSI with R-IVM group.
Normal fertilization was defined as the presence of two pronuclei (2PN). The normal fertilization rate was defined as the number of 2PN oocytes divided by the number of MII oocytes. The cleavage rate was defined as the number of cleaved embryos developed from 2PN oocytes divided by the number of 2PN oocytes. Cleavage embryonic development was evaluated using Veeck systems. Good-quality embryos were defined as normally fertilized embryos with 7-9 cells, fragmentation less than 10%, and without multinucleation on day-3. The good-quality embryo rate was defined as the number of good-quality embryos divided by the number of cleaved embryos. The available embryo rate was defined as the number of day-3 available embryos divided by the number of oocytes retrieved. The blastocysts were evaluated using the Gardner system. On Day 5 or 6, blastocysts with ≥3BC grade were considered to be available for cryopreservation. The blastocyst formation rate was defined as the blastocysts divided by the number of embryos cultured past day-3. The available blastocyst rate was defined as the number of blastocysts for cryopreservation divided by the number of embryos cultured past day-3. Biochemical pregnancy was defined as a positive result of HCG measurement without ultrasonographic visualization of clinical pregnancy. Clinical pregnancy was defined as the presence of a gestational sac with fetal heart activity by ultrasound 28 days after embryo transfer. Miscarriage was defined as pregnancy loss before gestational week 28. Live birth (LB) was defined as the delivery of a live newborn after gestational week 28. Cumulative LBR was defined as the chance of having LB after fresh and frozen transfers of embryos derived from one ICSI cycle. Preterm birth was defined as delivery at <37 weeks of gestation. Low birth weight was defined as child birth weight <2500g. Macrosomia was defined as child birth weight >4000g.
Statistical analysis
Differences between groups were evaluated using Student’s t-tests, Mann-Whitney U test, Wilcoxon signed-rank test, chi-squared tests, or Fisher’s exact tests based on variable distribution and property. Results were reported as the mean ± SD or n (%).
Propensity score matching (PSM) was adapted to avoid confounding bias and excessive numerical difference. Confounders related to the reproductive outcomes were chosen based on literature review, including age, BMI, AFC, ovarian stimulation protocol, cause of infertility, previous conventional IVF attempts, number of oocytes retrieved, and oocyte maturation rate at retrieval. The propensity scores (PS) were estimated from a logistic regression model that considered the aforementioned confounders. Each woman who underwent only ICSI was matched (a 1:1 match) to a corresponding woman who underwent R-IVM followed by ICSI using an optimal matching algorithm by randomly selecting each pair with the closest PS. The caliper was set to 0.02.
Univariate generalized linear models were applied for the matched cohorts to evaluate the associations between treatment and reproductive outcomes. Multivariate generalized linear models were applied for the unmatched cohorts, and regression coefficients and 95% confidence intervals (CIs) adjusting for confounders were calculated. For intermediate reproductive outcomes, the obtained regression coefficients were converted into percentage changes using the following formula: [exp(β) -1] ×100%. For pregnancy outcomes, odds ratio (OR) and 95% CI were reported. The Kaplan-Meier method was used to estimate the cumulative LBR, and comparisons of cumulative LBR were made using the log-rank test. We performed a subgroup analysis based on women with or without diminished ovarian reserve (DOR). We also investigated the modification effects by type of infertility (primary and secondary). To test the robustness of our results, we conducted several sensitivity analyses. First, we reanalyzed the data by excluding women with PCOS. Second, we reanalyzed the data after excluding cycles from couples diagnosed with male infertility only. Statistical analysis was conducted using SPSS 22.0 software and R 4.2.1 software. Statistical significance was defined as a P-value <0.05.
Results
Demographics
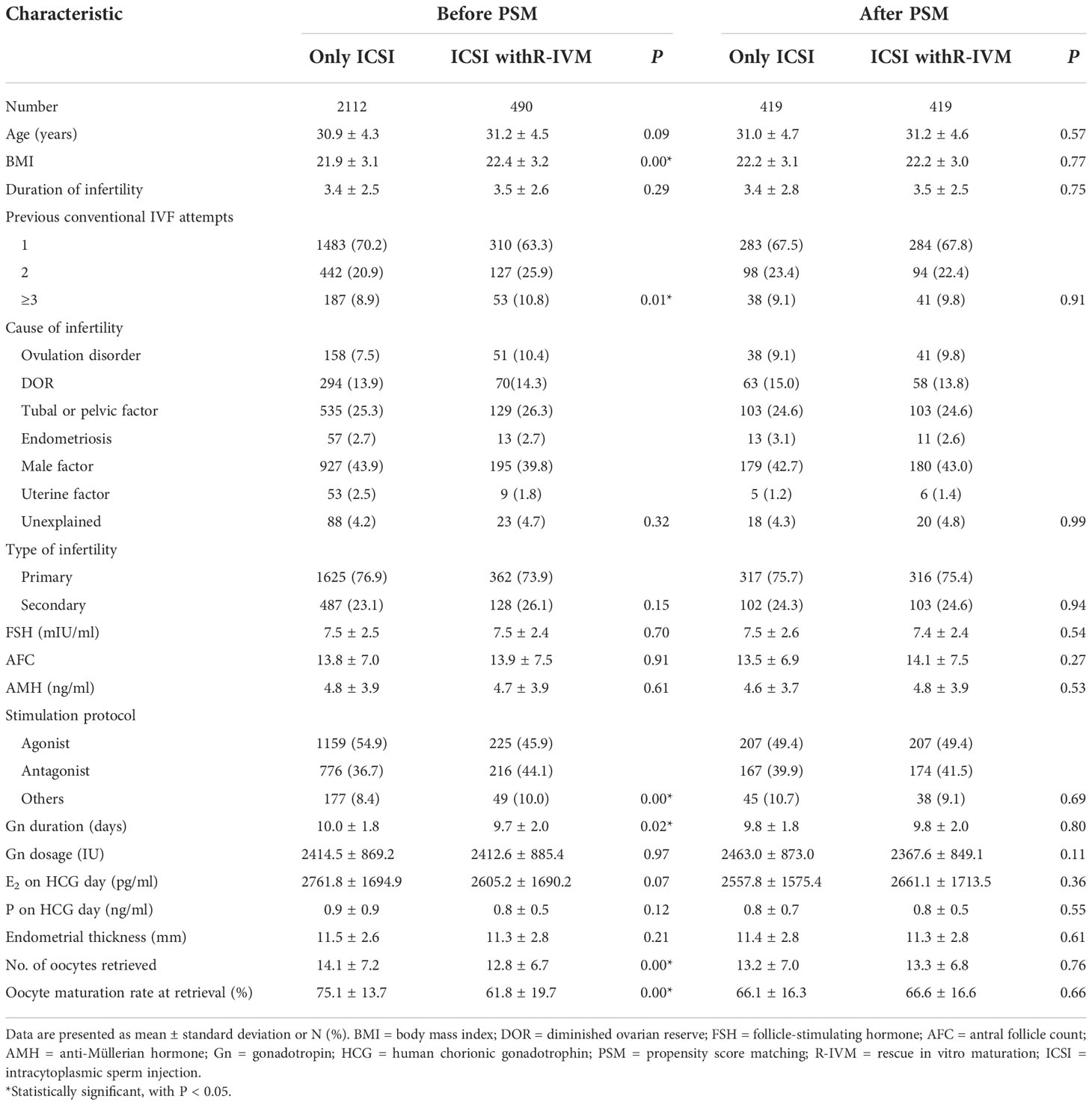
Table 1 shows the demographic characteristics. Between January 2019 and December 2020, 2602 women undergoing ICSI in our center were included in this analysis (Figure S1). There were 2112 women undergoing only ICSI and 490 women with R-IVM followed by ICSI. There were significant differences between only ICSI and ICSI with R-IVM in terms of BMI (21.9 ± 3.1 versus 22.4 ± 3.2, P<0.01), previous conventional IVF attempts, stimulation protocol, gonadotrophin duration, number of retrieved oocytes, and oocyte maturation rate at retrieval. After PSM, 838 women (419 women in each group) were included in the analysis, and none of the demographic characteristics demonstrated a significant difference between groups. And distributions of PS and standard differences indicated a balance between the compared cohorts (Figure S2).
Intermediate reproductive outcomes
The intermediate reproductive outcomes are shown in Table 2 and Table S1. Before matching, compared with the women who underwent only ICSI, the women who underwent ICSI with R-IVM had higher numbers of MII oocytes (16.7%; 95%CI: 12.9% to 20.5%), normally fertilized embryos (14.3%; 95%CI: 9.8% to 18.9%), cleaved embryos (12.7%; 95%CI: 8.2% to 17.3%), good-quality embryos (6.7%; 95%CI: 0.4% to 13.3%), day-3 available embryos (12.8%; 95%CI: 8.3% to 17.5%), embryos cultured past day-3 (16.2%; 95%CI:11.0% to 21.6%), blastocysts (12.5%; 95%CI:6.2% to 19.1%), available blastocysts (9.2%; 95%CI:1.9% to 16.9%), oocyte maturation rate (113.9%; 95%CI: 98.2% to 131.0%), available embryo rate (27.8%; 95%CI: 20.4% to 35.6%), after adjustment for confounders. The good-quality embryo rate was lower (–9.5%; 95%CI: –16.6% to –1.9%) among women undergoing ICSI with R-IVM. After PSM, compared with the women who underwent only ICSI, the women who underwent ICSI with R-IVM had higher numbers of MII oocytes (18.2%; 95%CI: 13.2% to 23.5%), normally fertilized embryos (15.5%; 95%CI: 9.6% to 21.8%), cleaved embryos (9.9%; 95%CI: 4.2% to 15.9%), day-3 available embryos (14.8%; 95%CI: 8.7% to 21.2%), embryos cultured past day-3 (15.2%; 95%CI:8.4% to 22.5%), oocyte maturation rate (82.8%; 95%CI: 67.7% to 99.2%), available embryo rate (27.8%; 95%CI: 18.6% to 37.7%).
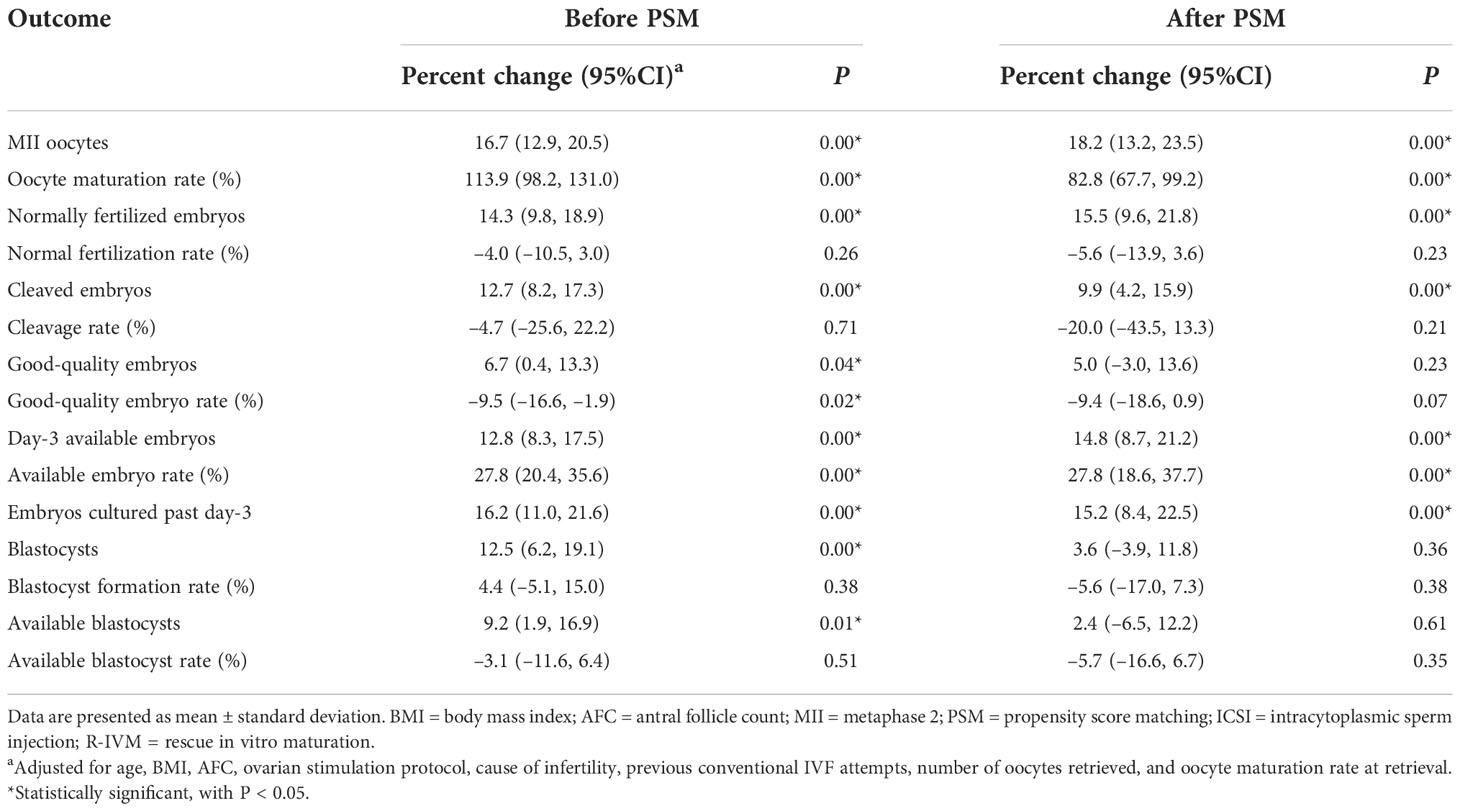
Table 2 Intermediate reproductive outcomes of women undergoing only ICSI versus women undergoing ICSI with R-IVM.
The intermediate reproductive outcomes were also calculated for in vivo matured and R-IVM oocytes separately in women undergoing ICSI with R-IVM group (Table S3). Compared with in vivo matured oocytes, combined R-IVM and in vivo matured oocytes group had significant higher numbers of MII oocytes, normally fertilized embryos, cleaved embryos, good-quality embryos, day-3 available embryos, embryos cultured past day-3, blastocysts, available blastocysts, had lower normal fertilization rate.
Pregnancy and perinatal outcome
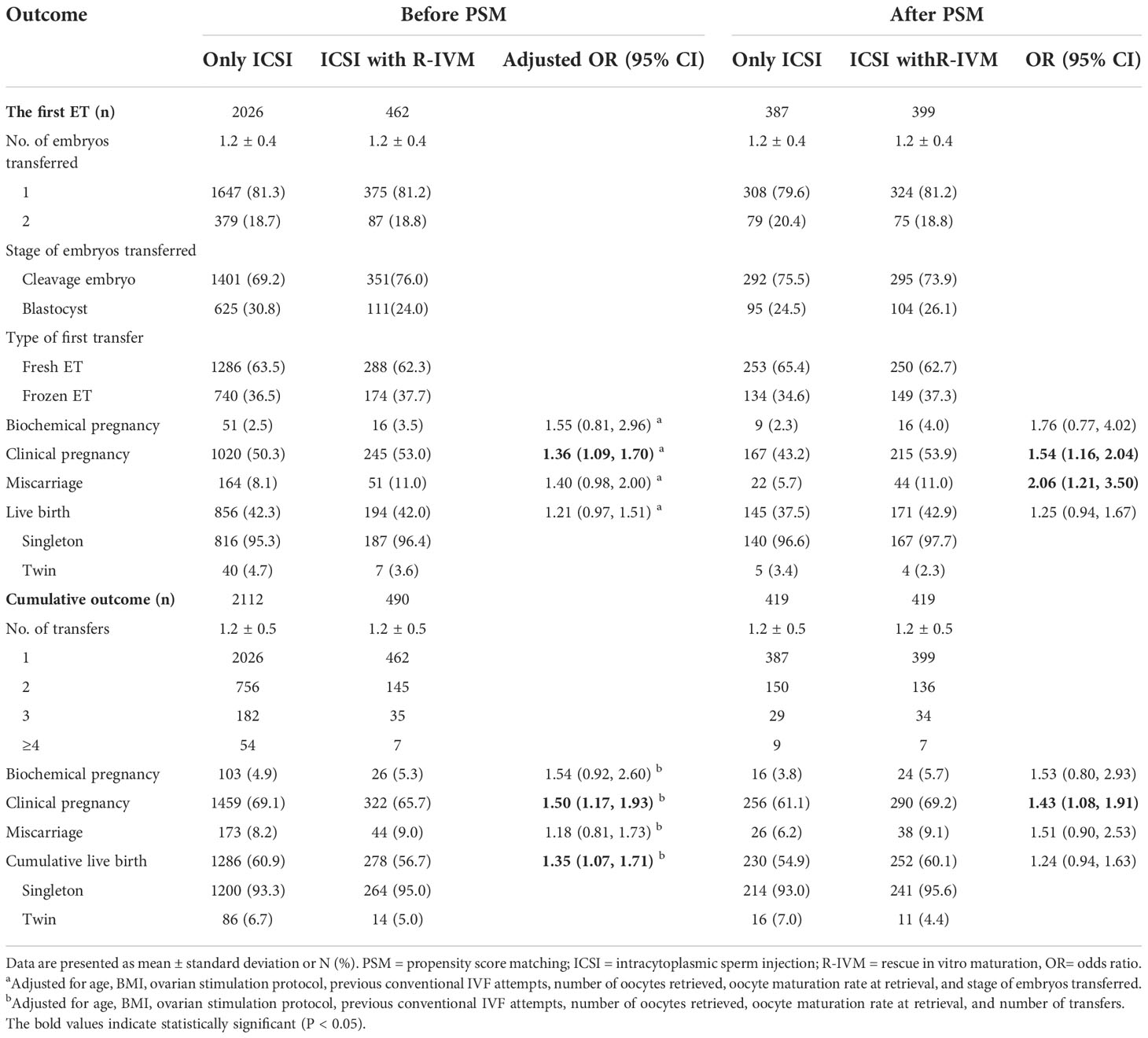
The pregnancy outcomes are shown in Table 3 and Table S2. Before matching, compared with the women who underwent only ICSI, those who underwent ICSI with R-IVM had an increased chance of clinical pregnancy after the fresh ET (adjusted OR=1.32, 95% CI: 1.00–1.73), after the first ET (adjusted OR=1.36, 95% CI: 1.09–1.70). The live birth rate after the first ET was 42.0% for women undergoing ICSI with R-IVM versus 42.3% for women undergoing only ICSI (adjusted OR=1.21, 95% CI: 0.97–1.51). Women undergoing ICSI with R-IVM had an increased chance of cumulative clinical pregnancy (adjusted OR=1.50, 95% CI: 1.17–1.93), and cumulative live birth (adjusted OR=1.35, 95% CI: 1.07–1.71).
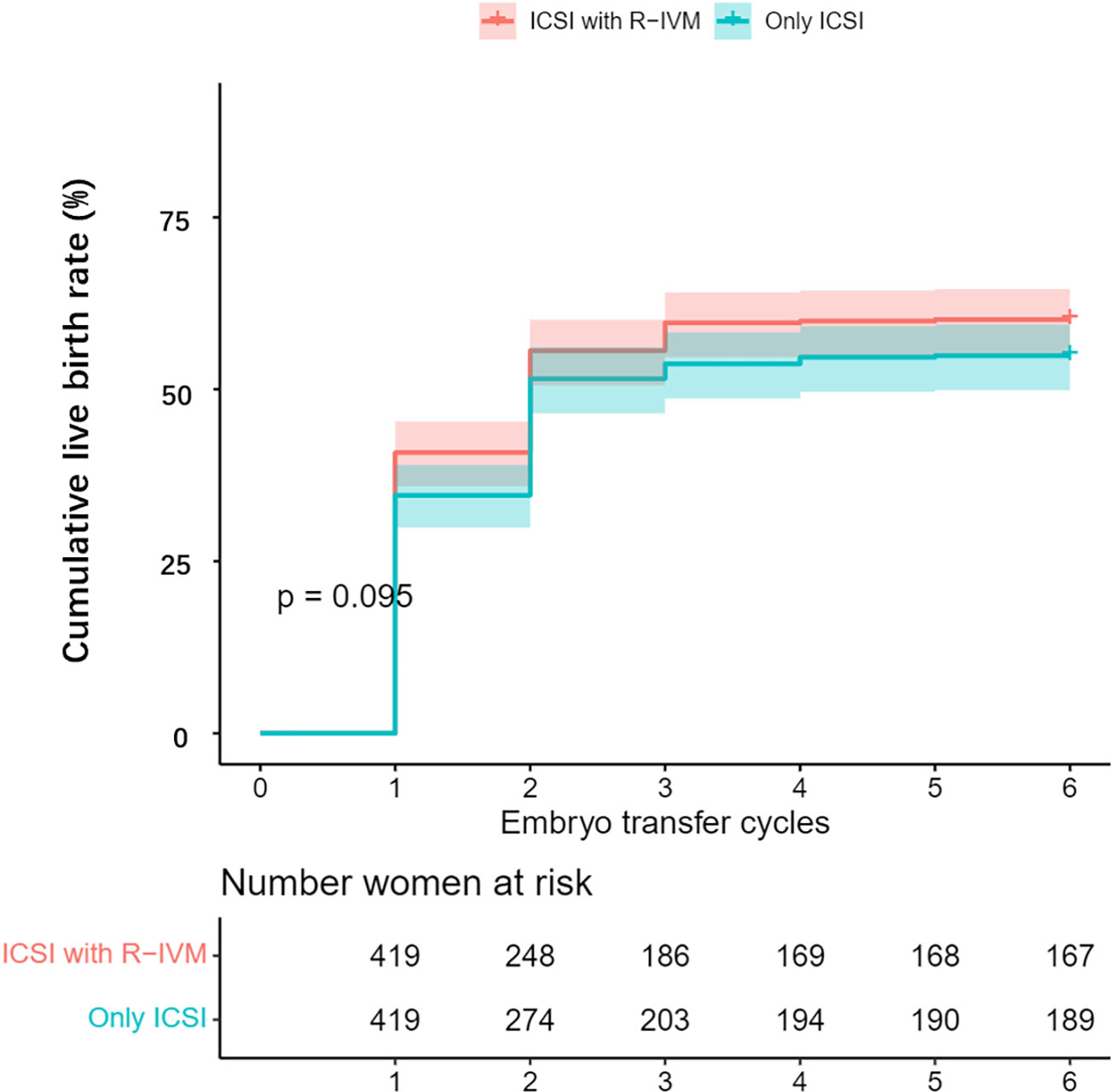
After PSM, compared with the women who underwent only ICSI, those who underwent ICSI with R-IVM had an increased chance of clinical pregnancy after the fresh ET cycle (OR=1.43, 95% CI: 1.01–2.03), after the first ET (OR=1.54, 95% CI: 1.16–2.04), and miscarriage (OR=2.06, 95% CI: 1.21–3.50) after the first ET. The live birth rate after the first ET was 42.9% for women undergoing ICSI with R-IVM versus 37.5% for women undergoing only ICSI (OR=1.25, 95% CI: 0.94–1.67). Women undergoing ICSI with R-IVM had an increased chance of cumulative clinical pregnancy (OR=1.43, 95% CI: 1.08–1.91; Figure 1). The cumulative live birth rate was 60.1% for women undergoing ICSI with R-IVM versus 54.9% for women undergoing only ICSI (OR=1.24, 95% CI: 0.94–1.63; Figure 2).

Figure 1 Kaplan-Meier curve for the cumulative clinical pregnancy rate after propensity score matching.
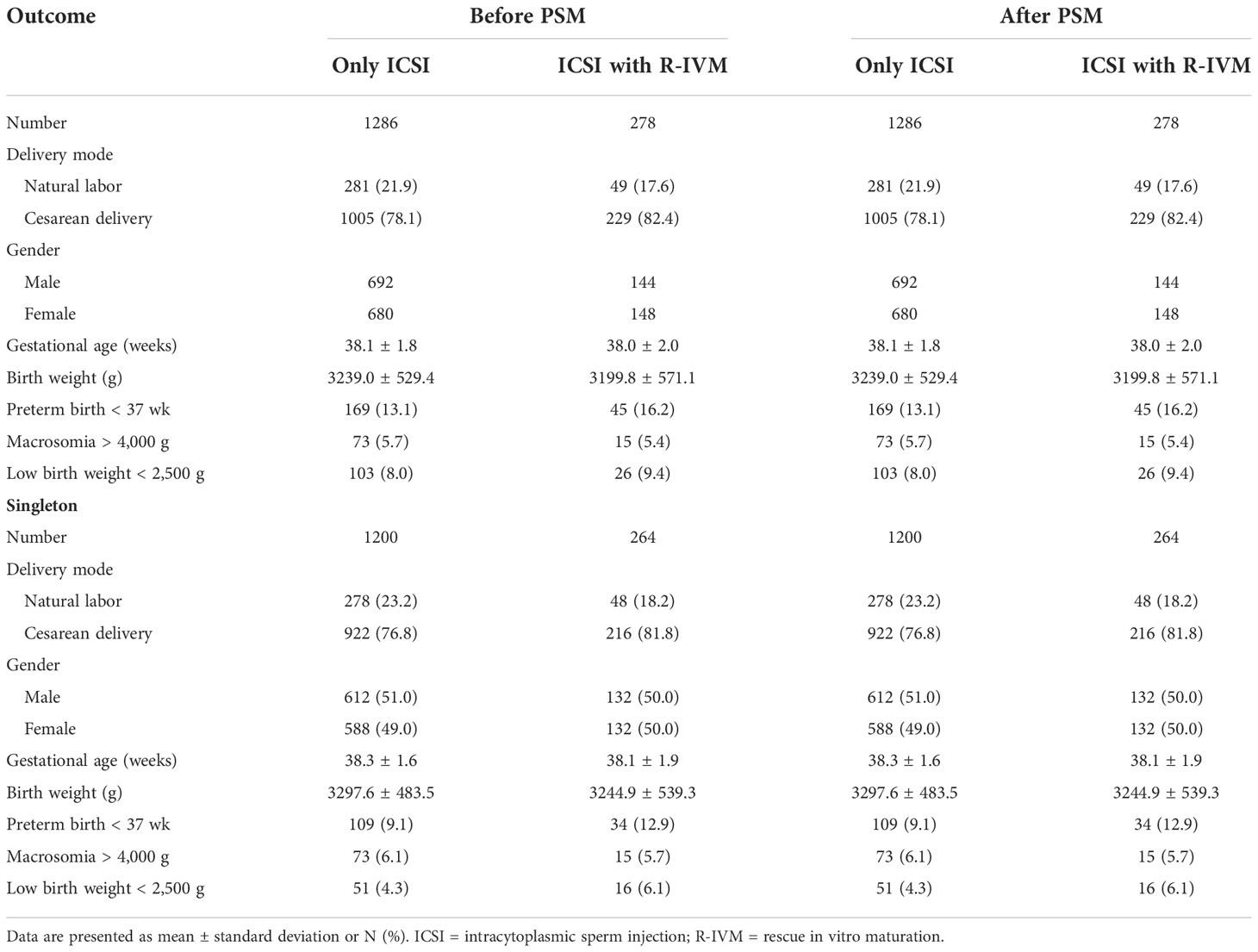
The perinatal outcomes of all live birth in the two group are shown in Table 4. There were no significant differences between the two groups in terms of delivery mode, gestational age, birth weight, preterm birth rate, macrosomia, and low birth weight.
The pregnancy and perinatal outcome were also calculated for in vivo matured and R-IVM oocytes separately in women undergoing ICSI with R-IVM group (Table S4). Compared with in vivo matured oocytes, combined R-IVM and in vivo matured oocytes group had higher cumulative clinical pregnancy rate and cumulative live birth rate. Sixty-seven cases transferred embryos originated from R-IVM oocytes, 33 cases had pregnancy, 27 cases had live birth. In addition, only 17 cases transferred embryos originated from mixed in vivo matured and R-IVM oocytes, 9 cases had pregnancy, 8 cases had live birth, and one case had twin delivery. All live births from R-IVM embryos were healthy and without malformations or complications.
Sensitivity analysis
The abovementioned results were largely unchanged when we restricted the analysis to the women without DOR (Table S5), to women with primary infertility (Table S6), to women without PCOS (Table S7), or when the cycles from the couples with male infertility only were excluded (Table S8). However, when we restricted our analysis to women with DOR, significant differences can be only observed in MII oocytes and oocyte maturation rate, and 2 live births were achieved from 7 cycles that transfer embryos from R-IVM; when we restricted our analysis to women with secondary infertility, significant differences can be only observed in oocyte maturation rate and available embryo rate.
Discussion
In this study, we observed that women undergoing ICSI with R-IVM had higher numbers of MII oocytes, normally fertilized embryos, cleaved embryos, day-3 available embryos, embryos cultured past day-3, and had higher oocyte maturation rate, available embryo rate than women undergoing only ICSI. Additionally, we found that women undergoing ICSI with R-IVM had an increased chance of clinical pregnancy and cumulative live birth. The reproductive outcomes were also significantly different when calculated for in vivo matured and R-IVM oocytes separately in women undergoing ICSI with R-IVM group. All live births from R-IVM embryos were healthy and without malformations or complications.
Consistent with our results, previous studies also found that the fertilization rate and good-quality embryo rate were significantly lower in the R-IVM followed by ICSI group (23–25). These results suggest that post-IVM nuclear maturation is morphologically complete, but cytoplasmic maturation is insufficient or incomplete. Insufficient ooplasmic maturation inhibits the release of cortical granules into the perivitelline space, resulting in zona hardening with consequent interference with fertilization and blastocyst development (26–30). Additionally, the oocytes cultured in the medium for an extended period may lead to oocyte aging and poorer embryonic development quality (31–33). There were different results in reproductive outcomes between groups when we restricted the analyses to women with secondary infertility or with DOR, which also can be explained by their older age (secondary infertility: 33.6 ± 4.9 years, DOR: 34.6 ± 5.4 years). However, other studies reported that the numbers of cleaved embryos, good-quality embryos, day-3 available embryos, and the available embryo rate were similar between groups (34–36). This inconsistency with our results may be related to the differences in various IVM protocols, with oocyte aspiration performed in unstimulated cycles or stimulated cycles, and with or without an HCG trigger.
Our study showed that women undergoing ICSI with R-IVM had an increased chance of clinical pregnancy and cumulative live birth. Inconsistent with our results, a few observational studies found that cumulative rates of clinical pregnancy and live birth were significantly lower in the IVM group compared with the standard ICSI group (19, 20, 37). The inconsistency may be related to the differences in the study population. In addition, the proportion of MII oocytes can be affected by ovarian stimulation protocols (38). The IVM in our study involved the oocytes from conventional COH cycles, but the IVM in other studies involved the oocytes from unstimulated or minimally stimulated cycles. And we cultured immature oocytes in a standard G1-plus medium, but they cultured immature oocytes in a pre-maturation medium supplemented with recombinant FSH. Regarding the recently traditional IVM research, the application of a pre-maturation step, a biphasic IVM culturing system, and adjustments to cumulus cell removal or culture media/conditions have a marked effect on the clinical outcomes of IVM oocytes (39–42). The higher success rate of women undergoing ICSI with R-IVM in our study could be also explained by the higher numbers of cleaved embryos, good-quality embryos, and day-3 available embryos. The clinical pregnancy and live birth rate after the first ET were higher among women undergoing ICSI with R-IVM, which may be an additive effect of the higher cumulative live birth rate. The oocyte maturation rate at retrieval was associated with the live birth rate (4, 43). Accordingly, we adjusted for the oocyte maturation rate at retrieval in our current analysis to indicate the clinical application of ICSI with R-IVM. This study may be of great significance for patients with a high proportion of immature oocytes. Besides, two live births were achieved from 7 cycles that transfer embryos from R-IVM oocytes in women with DOR. Because of the small number of embryos for DOR patients, every additional embryo is of considerable potential clinical significance for them. Thus, these retrospective data suggest that ICSI with R-IVM is more successful, but larger retrospective and well-designed prospective studies are needed to confirm and reinforce the findings.
With respect to birth outcomes, all live births from R-IVM embryos were healthy and without malformations or complications. Studies have reported that the obstetric, perinatal, and neonatal outcomes and development of children conceived from IVM cycles seem similar to spontaneous conceptions or IVF treatment (12, 44, 45). Additionally, some studies have proved that an embryo from IVM oocytes could obtain a normal karyotype (46, 47). We should consider that data on IVM children are limited in our study, both in numbers and duration of follow-up. Further research about a more comprehensive appraisal of the health status of IVM children is still needed.
The strengths of this study include the relatively large sample size and the use of PSM and multivariable regression to minimize bias. However, some limitations should be noted. First, it was a retrospective cohort study conducted in a single-center, which may limit the external validity of our conclusions, as the heterogeneous practices between centers. The retrospective cohort study unlike randomized controlled trials (RCT), cannot control for unmeasured variables such as sperm concentration, sperm motility, and maternal comorbidities. Nevertheless, PSM presents a range of advantages over conventional regression models and some methodological similarities with RCTs. Second, data on pregnancy-related complications, congenital anomalies, and children’s development were not available. Third, small numbers of embryos from R-IVM oocytes were transferred; a future larger study is needed to compare the pregnancy outcomes between the two groups. Fourth, cycles with all mature oocytes were excluded, which comprised nearly one-third of the cycles at our center. Nevertheless, few studies have investigated differences in reproductive outcomes between only ICSI and ICSI with R-IVM. Therefore, our study results may provide useful insights for both clinicians and patients. Our study may provide immediate and long-term advantages by reducing the cancellation rate and providing extra blastocysts or transfers per attempt, and has the potential to give rise to pregnancies and live births, it may also provide a reference for future large-scale studies.
In conclusion, our study indicates that ICSI with R-IVM may increase pregnancy outcomes compared with only ICSI. It may also provide a reference for the safety of R-IVM. Adapting R-IVM as a routine may benefit the women undergoing ICSI. Cohort includes multiple centers worldwide and prospective design are needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
This study was approved by the ethics committee of Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology. The patients/participants provided their written informed consent to participate in this study.
Author contributions
D-YQ: Conceptualization, Methodology, Investigation, Formal analysis, Writing-original draft, Writing-review and editing; H-HJ: Investigation, Methodology, Formal analysis, Supervision, Writing-review and editing; Q-YY: Investigation, Methodology, Writing-review and editing; WY: Investigation, Writing-review and editing; X-QY: Investigation, Writing-review and editing; YW: Investigation, Data Curation; T-RD: Investigation, Writing-review and editing; Y-YD: Investigation, Methodology; X-LR: Investigation, Writing-review and editing; NG: Supervision, Investigation, Formal analysis, Methodology, and Writing-review and editing; Y-FL: Project administration, Conceptualization, Supervision, Writing-review and editing, Funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81771654).
Acknowledgments
The authors sincerely thank all the doctors, nurses, and embryologists in the Reproductive Medicine Center of Tongji Hospital and all study participants.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1047571/full#supplementary-material
References
1. Chambers GM, Dyer S, Zegers-Hochschild F, de Mouzon J, Ishihara O, Banker M, et al. International committee for monitoring assisted reproductive technologies world report: assisted reproductive technology, 2014dagger. Hum Reprod (2021) 36(11):2921–34. doi: 10.1093/humrep/deab198
2. Qiao J, Wang Y, Li X, Jiang F, Zhang Y, Ma J, et al. A lancet commission on 70 years of women’s reproductive, maternal, newborn, child, and adolescent health in China. Lancet (2021) 397(10293):2497–536. doi: 10.1016/S0140-6736(20)32708-2
3. Telfer EE, Andersen CY. In vitro growth and maturation of primordial follicles and immature oocytes. Fertil Steril (2021) 115(5):1116–25. doi: 10.1016/j.fertnstert.2021.03.004
4. Parrella A, Irani M, Keating D, Chow S, Rosenwaks Z, Palermo GD. High proportion of immature oocytes in a cohort reduces fertilization, embryo development, pregnancy and live birth rates following ICSI. Reprod Biomed Online (2019) 39(4):580–7. doi: 10.1016/j.rbmo.2019.06.005
5. Escrich L, Pellicer A, Meseguer M. Let’s rescue oocytes: in vitro maturation 2.0 is coming. Fertil Steril (2018) 110(4):638–9. doi: 10.1016/j.fertnstert.2018.05.019
6. De Vos M, Smitz J, Thompson JG, Gilchrist RB. The definition of IVM is clear-variations need defining. Hum Reprod (2016) 31(11):2411–5. doi: 10.1093/humrep/dew208
7. Walls ML, Hart RJ. In vitro maturation. Best Pract Res Clin Obstet Gynaecol (2018) 53:60–72. doi: 10.1016/j.bpobgyn.2018.06.004
8. Gong X, Li H, Zhao Y. The improvement and clinical application of human oocyte In vitro maturation (IVM). Reprod Sci (2021) 29(8):2127–35. doi: 10.1007/s43032-021-00613-3
9. Yang ZY, Chian RC. Development of in vitro maturation techniques for clinical applications. Fertil Steril (2017) 108(4):577–84. doi: 10.1016/j.fertnstert.2017.08.020
10. Practice Committees of the American Society for Reproductive Medicine, the Society of Reproductive Biologists and Technologists, and the Society for Assisted Reproductive Technology Electronic address. In vitro maturation: a committee opinion. Fertil Steril (2021) 115(2):298–304. doi: 10.1016/j.fertnstert.2020.11.018
11. Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Intracytoplasmic sperm injection (ICSI) for non-male factor indications: a committee opinion. Fertil Steril (2020) 114(2):239–45. doi: 10.1016/j.fertnstert.2020.05.032
12. Yu EJ, Yoon TK, Lee WS, Park EA, Heo JY, Ko YK, et al. Obstetrical, neonatal, and long-term outcomes of children conceived from in vitro matured oocytes. Fertil Steril (2019) 112(4):691–9. doi: 10.1016/j.fertnstert.2019.05.034
13. Roesner S, von Wolff M, Elsaesser M, Roesner K, Reuner G, Pietz J, et al. Two-year development of children conceived by IVM: a prospective controlled single-blinded study. Hum Reprod (2017) 32(6):1341–50. doi: 10.1093/humrep/dex068
14. Margalit T, Ben-Haroush A, Garor R, Kotler N, Shefer D, Krasilnikov N, et al. Morphokinetic characteristics of embryos derived from in-vitro-matured oocytes and their in-vivo-matured siblings after ovarian stimulation. Reprod Biomed Online (2019) 38(1):7–11. doi: 10.1016/j.rbmo.2018.10.002
15. Ozturk S. Molecular determinants of the meiotic arrests in mammalian oocytes at different stages of maturation. Cell Cycle (2022) 21(6):547–71. doi: 10.1080/15384101.2022.2026704
16. Krisher RL. Present state and future outlook for the application of in vitro oocyte maturation in human infertility treatment. Biol Reprod (2022) 106(2):235–42. doi: 10.1093/biolre/ioac010
17. Holubcova Z, Kyjovska D, Martonova M, Paralova D, Klenkova T, Otevrel P, et al. Egg maturity assessment prior to ICSI prevents premature fertilization of late-maturing oocytes. J Assist Reprod Genet (2019) 36(3):445–52. doi: 10.1007/s10815-018-1393-0
18. Rubino P, Vigano P, Luddi A, Piomboni P. The ICSI procedure from past to future: a systematic review of the more controversial aspects. Hum Reprod Update (2016) 22(2):194–227. doi: 10.1093/humupd/dmv050
19. Ho V, Braam SC, Pham TD, Mol BW, Vuong LN. The effectiveness and safety of in vitro maturation of oocytes versus in vitro fertilization in women with a high antral follicle count. Hum Reprod (2019) 34(6):1055–64. doi: 10.1093/humrep/dez060
20. Vuong LN, Ho V, Ho TM, Dang VQ, Phung TH, Giang NH, et al. In-vitro maturation of oocytes versus conventional IVF in women with infertility and a high antral follicle count: a randomized non-inferiority controlled trial. Hum Reprod (2020) 35(11):2537–47. doi: 10.1093/humrep/deaa240
21. Zhu L, Xi Q, Zhang H, Li Y, Ai J, Jin L. Blastocyst culture and cryopreservation to optimize clinical outcomes of warming cycles. Reprod Biomed Online (2013) 27(2):154–60. doi: 10.1016/j.rbmo.2013.04.006
22. Xu B, Chen Y, Geerts D, Yue J, Li Z, Zhu G, et al. Cumulative live birth rates in more than 3,000 patients with poor ovarian response: a 15-year survey of final in vitro fertilization outcome. Fertil Steril (2018) 109(6):1051–9. doi: 10.1016/j.fertnstert.2018.02.001
23. Zhao HC, Ding T, Ren Y, Li TJ, Li R, Fan Y, et al. Role of Sirt3 in mitochondrial biogenesis and developmental competence of human in vitro matured oocytes. Hum Reprod (2016) 31(3):607–22. doi: 10.1093/humrep/dev345
24. Vandenberghe L, Santos-Ribeiro S, De Munck N, Desmet B, Meul W, De Vos A, et al. Expanding the time interval between ovulation triggering and oocyte injection: does it affect the embryological and clinical outcome? Hum Reprod (2021) 36(3):614–23. doi: 10.1093/humrep/deaa338
25. Karavani G, Wasserzug-Pash P, Mordechai-Daniel T, Bauman D, Klutstein M, Imbar T. Age-dependent in vitro maturation efficacy of human oocytes - is there an optimal age? Front Cell Dev Biol (2021) 9:667682. doi: 10.3389/fcell.2021.667682
26. Richani D, Dunning KR, Thompson JG, Gilchrist RB. Metabolic co-dependence of the oocyte and cumulus cells: essential role in determining oocyte developmental competence. Hum Reprod Update (2021) 27(1):27–47. doi: 10.1093/humupd/dmaa043
27. Ferrer-Vaquer A, Barragan M, Rodriguez A, Vassena R. Altered cytoplasmic maturation in rescued in vitro matured oocytes. Hum Reprod (2019) 34(6):1095–105. doi: 10.1093/humrep/dez052
28. Lan Y, Zhang S, Gong F, Lu C, Lin G, Hu L. The mitochondrial DNA copy number of cumulus granulosa cells may be related to the maturity of oocyte cytoplasm. Hum Reprod (2020) 35(5):1120–9. doi: 10.1093/humrep/deaa085
29. Straczynska P, Papis K, Morawiec E, Czerwinski M, Gajewski Z, Olejek A, et al. Signaling mechanisms and their regulation during in vivo or in vitro maturation of mammalian oocytes. Reprod Biol Endocrinol (2022) 20(1):37. doi: 10.1186/s12958-022-00906-5
30. Trebichalska Z, Kyjovska D, Kloudova S, Otevrel P, Hampl A, Holubcova Z. Cytoplasmic maturation in human oocytes: an ultrastructural study dagger. Biol Reprod (2021) 104(1):106–16. doi: 10.1093/biolre/ioaa174
31. Carvalho M, Leal F, Mota S, Aguiar A, Sousa S, Nunes J, et al. The effect of denudation and injection timing in the reproductive outcomes of ICSI cycles: new insights into the risk of in vitro oocyte ageing. Hum Reprod (2020) 35(10):2226–36. doi: 10.1093/humrep/deaa211
32. Maggiulli R, Cimadomo D, Fabozzi G, Papini L, Dovere L, Ubaldi FM, et al. The effect of ICSI-related procedural timings and operators on the outcome. Hum Reprod (2020) 35(1):32–43. doi: 10.1093/humrep/dez234
33. Ruth KS, Day FR, Hussain J, Martinez-Marchal A, Aiken CE, Azad A, et al. Genetic insights into biological mechanisms governing human ovarian ageing. Nature (2021) 596(7872):393–7. doi: 10.1038/s41586-021-03779-7
34. Escrich L, Galiana Y, Grau N, Insua F, Soler N, Pellicer A, et al. Do immature and mature sibling oocytes recovered from stimulated cycles have the same reproductive potential? Reprod Biomed Online (2018) 37(6):667–76. doi: 10.1016/j.rbmo.2018.08.023
35. Wang X, Xiao Y, Sun Z, Zhen J, Yu Q. Effect of the time interval between oocyte retrieval and ICSI on embryo development and reproductive outcomes: a systematic review. Reprod Biol Endocrinol (2021) 19(1):34. doi: 10.1186/s12958-021-00717-0
36. Sanchez F, Lolicato F, Romero S, De Vos M, Van Ranst H, Verheyen G, et al. An improved IVM method for cumulus-oocyte complexes from small follicles in polycystic ovary syndrome patients enhances oocyte competence and embryo yield. Hum Reprod (2017) 32(10):2056–68. doi: 10.1093/humrep/dex262
37. Zheng X, Guo W, Zeng L, Zheng D, Yang S, Xu Y, et al. In vitro maturation without gonadotropins versus in vitro fertilization with hyperstimulation in women with polycystic ovary syndrome: a non-inferiority randomized controlled trial. Hum Reprod (2022) 37(2):242–53. doi: 10.1093/humrep/deab243
38. Lonergan P, Fair T. Maturation of oocytes in vitro. Annu Rev Anim Biosci (2016) 4:255–68. doi: 10.1146/annurev-animal-022114-110822
39. Richani D, Gilchrist RB. Approaches to oocyte meiotic arrest in vitro and impact on oocyte developmental competence. Biol Reprod (2022) 106(2):243–52. doi: 10.1093/biolre/ioab176
40. Yang H, Kolben T, Meister S, Paul C, van Dorp J, Eren S, et al. Factors influencing the In vitro maturation (IVM) of human oocyte. Biomedicines (2021) 9(12):1904. doi: 10.3390/biomedicines9121904
41. Rodriguez-Varela C, Labarta E. Does coenzyme Q10 supplementation improve human oocyte quality? int. J Mol Sci (2021) 22(17):9541. doi: 10.3390/ijms22179541
42. Saenz-de-Juano MD, Ivanova E, Romero S, Lolicato F, Sanchez F, Van Ranst H, et al. DNA Methylation and mRNA expression of imprinted genes in blastocysts derived from an improved in vitro maturation method for oocytes from small antral follicles in polycystic ovary syndrome patients. Hum Reprod (2019) 34(9):1640–9. doi: 10.1093/humrep/dez121
43. Dozortsev DI, Diamond MP. Luteinizing hormone-independent rise of progesterone as the physiological trigger of the ovulatory gonadotropins surge in the human. Fertil Steril (2020) 114(2):191–9. doi: 10.1016/j.fertnstert.2020.06.016
44. Strowitzki T, Bruckner T, Roesner S. Maternal and neonatal outcome and children’s development after medically assisted reproduction with in-vitro matured oocytes-a systematic review and meta-analysis. Hum Reprod Update (2021) 27(3):460–73. doi: 10.1093/humupd/dmaa056
45. Belva F, Roelants M, Vermaning S, Desmyttere S, De Schepper J, Bonduelle M, et al. Growth and other health outcomes of 2-year-old singletons born after IVM versus controlled ovarian stimulation in mothers with polycystic ovary syndrome. Hum Reprod Open (2020) 2020(1):hoz043. doi: 10.1093/hropen/hoz043
46. Kornilov NV, Pavlova MN, Yakovlev PP. The live birth in a woman with resistant ovary syndrome after in vitro oocyte maturation and preimplantation genetic testing for aneuploidy. J Assist Reprod Genet (2021) 38(6):1303–9. doi: 10.1007/s10815-021-02085-5
Keywords: rescue in vitro maturation, intracytoplasmic sperm injection, reproductive outcomes, live birth rate, cumulative live birth rate, propensity score matching
Citation: Qin D-Y, Jiang H-H, Yao Q-Y, Yao W, Yuan X-Q, Wang Y, Deng T-R, Du Y-Y, Ren X-L, Guo N and Li Y-F (2022) Rescue in vitro maturation may increase the pregnancy outcomes among women undergoing intracytoplasmic sperm injection. Front. Endocrinol. 13:1047571. doi: 10.3389/fendo.2022.1047571
Received: 18 September 2022; Accepted: 28 November 2022;
Published: 12 December 2022.
Edited by:
Eleonora Porcu, University of Bologna, ItalyReviewed by:
Safak Hatirnaz, Medicana Hospital, TurkeyWeibing Qin, National Health and Family Planning Commission, China
Copyright © 2022 Qin, Jiang, Yao, Yao, Yuan, Wang, Deng, Du, Ren, Guo and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Guo, dHNndW9uYUAxMjYuY29t; Yu-Feng Li, dGpseWY2NkAxMjYuY29t
†These authors have contributed equally to this work
 Dan-Yu Qin1
Dan-Yu Qin1 Hua-Hua Jiang
Hua-Hua Jiang Yu-Feng Li
Yu-Feng Li