- 1Department of Respiratory and Critical Care Medicine, Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan, China
- 2Department of Medical Intensive Care Unit, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China
Background: Hyperglycemia is one of the poor prognostic factors in critical ill sepsis patients with diabetes. We aimed to assess the interaction between admission glucose level and clinical endpoints in sepsis patients with diabetes admitted in the intensive care unit (ICU).
Methods: Data from the Medical Information Mart Intensive Care III database were used in this study. The study primary endpoint was 28-day mortality after ICU admission. Multivariate Cox regression models were used to explore the association between admission glucose level and the primary endpoint.
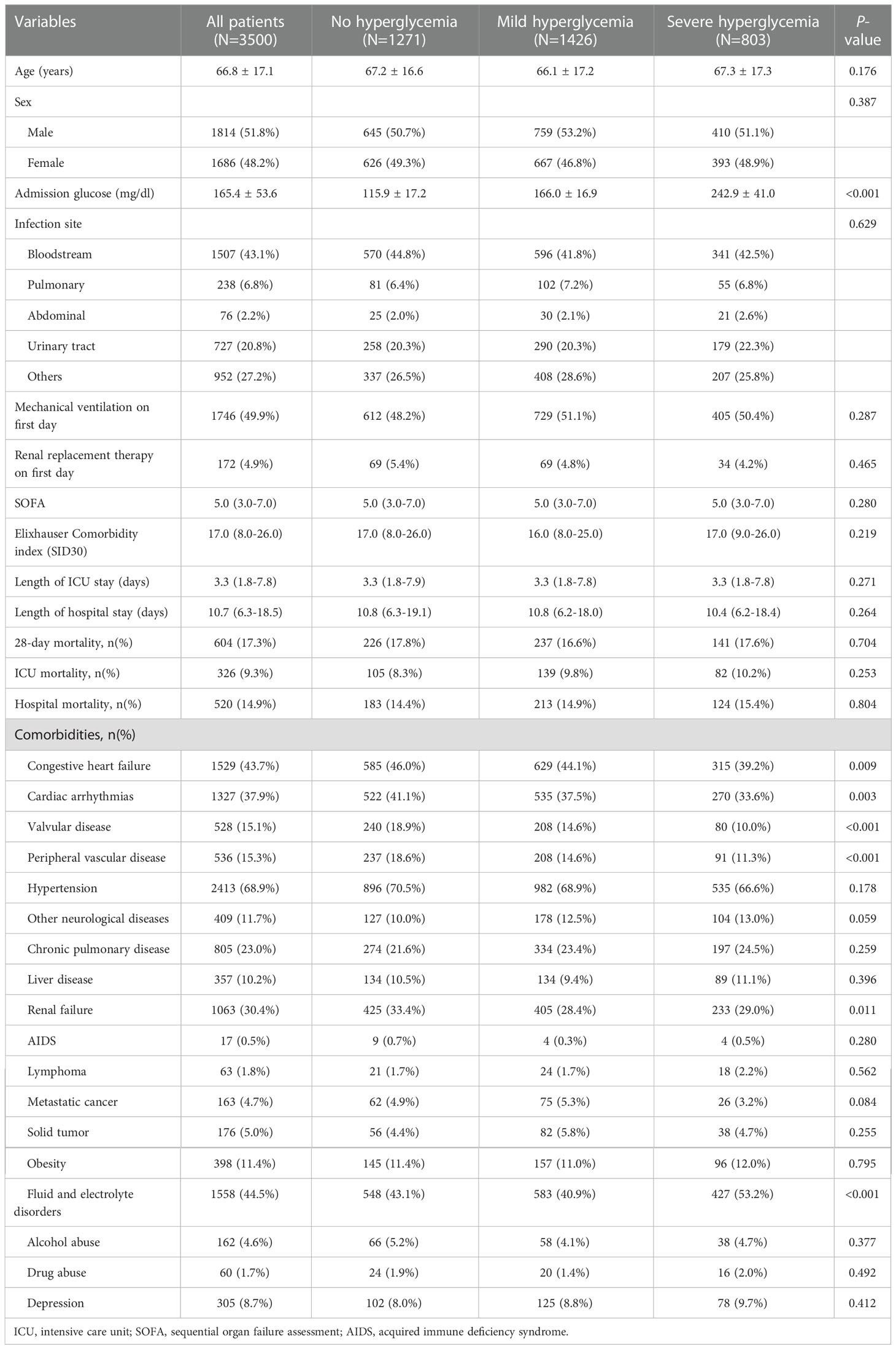
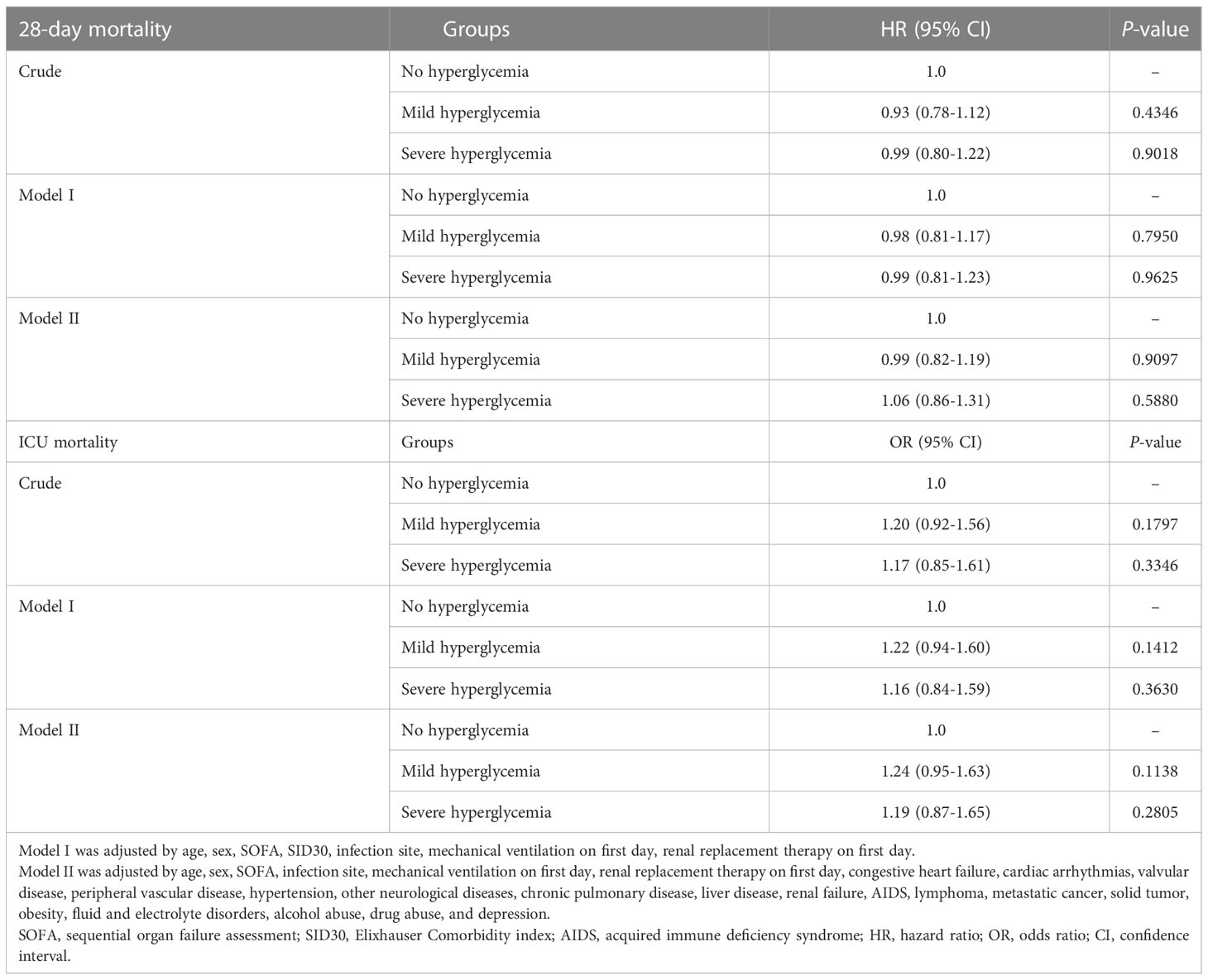
Results: We included 3,500 sepsis patients with diabetes. Of participants with no hyperglycemia, mild hyperglycemia, and severe hyperglycemia, no differences were evident in hospital mortality, ICU mortality, or 28-day mortality (all P >0.05). The multivariable Cox regression analysis demonstrated that severe hyperglycemia did not increase the risk of 28-day mortality (hazard ratio [HR]=1.06, 95% confidence interval [CI]: 0.86–1.31, P=0.5880). Threshold effects analysis identified the inflection points for 28-day mortality as 110 mg/dl and 240 mg/dl. The HRs for 28-day mortality were 0.980 in the <110 mg/dl and 1.008 in the >240 mg/dl. A short-term survival advantage was observed in the 110–240 mg/dl group compared with that in the <110 mg/dl group; meanwhile, no adverse hazard was detected in the >240 mg/dl group. In the stratified analyses, the association effect between the three glucose groups (<110 mg/dl, 110–240 mg/dl, and ≥240 mg/dl) and 28-day mortality was consistent in terms of different sequential organ failure assessment (SOFA) scores and infection sites. The 28-day mortality of the 110–240 mg/dl group with a SOFA score of ≥10 was lower than that of the <110 mg/dl group (HR=0.61, 95% CI: 0.38–0.98).
Conclusion: Admission hyperglycemia was not a risk factor for short-term prognosis in critical ill sepsis patients with diabetes; a lower admission blood glucose level was associated with increased risk of poor prognosis. The potential benefit of higher admission glucose level on 28-day mortality in patients with a more severe condition remains a concern.
Background
Diabetes is a common comorbidity among critically ill sepsis patients and generally causes immune dysfunction and metabolic disorders, including hyperglycemia (1–3). In recent years, diabetes is developing swiftly as a global health epidemic and is one of the top ten causes of adult death (4). Hyperglycemia was closely related to endothelial cell injury, mitochondrial damage, and inflammation activation (5, 6). In terms of clinical research, Vught et al. revealed that severe hypoglycemia contributed to higher 90-day mortality in sepsis patients with diabetes (7). In another study, Vught et al. indicated that severe hyperglycemia was correlated with 30-day mortality in patients with sepsis, regardless of the presence or absence of diabetes (8). Subsequently, multiple studies that examined the glucose levels of this patient group reported different views, and some indicated the adverse effects of glycemic control (9–12). A previous large randomized trial found that a glucose level of 81–108 mg/dl was associated with adverse clinical outcomes of glycemic control compared with a glucose level of ≤180 mg/dl (2).
To our knowledge, evidence on how hyperglycemia affects the clinical outcomes in critical sepsis patients with diabetes remains limited and debatable. Considering that diabetes is consistently correlated with other diseases, the impact of admission glucose level in the outcome of sepsis patients should be explored, potentially determining better individualized glycemic control strategies. Consequently, we aimed to assess the interaction between admission glucose levels and clinical endpoints in sepsis patients with diabetes admitted in the intensive care unit (ICU).
Methods
Patient data
Data from the Medical Information Mart Intensive Care III (MIMIC-III) database were used in this study (13). The institutional review boards of Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology Affiliates approved the access to the database (record identification numbers: 33460949 and 49780033). The requirement for obtaining informed consent was waived due to the use of anonymized data.
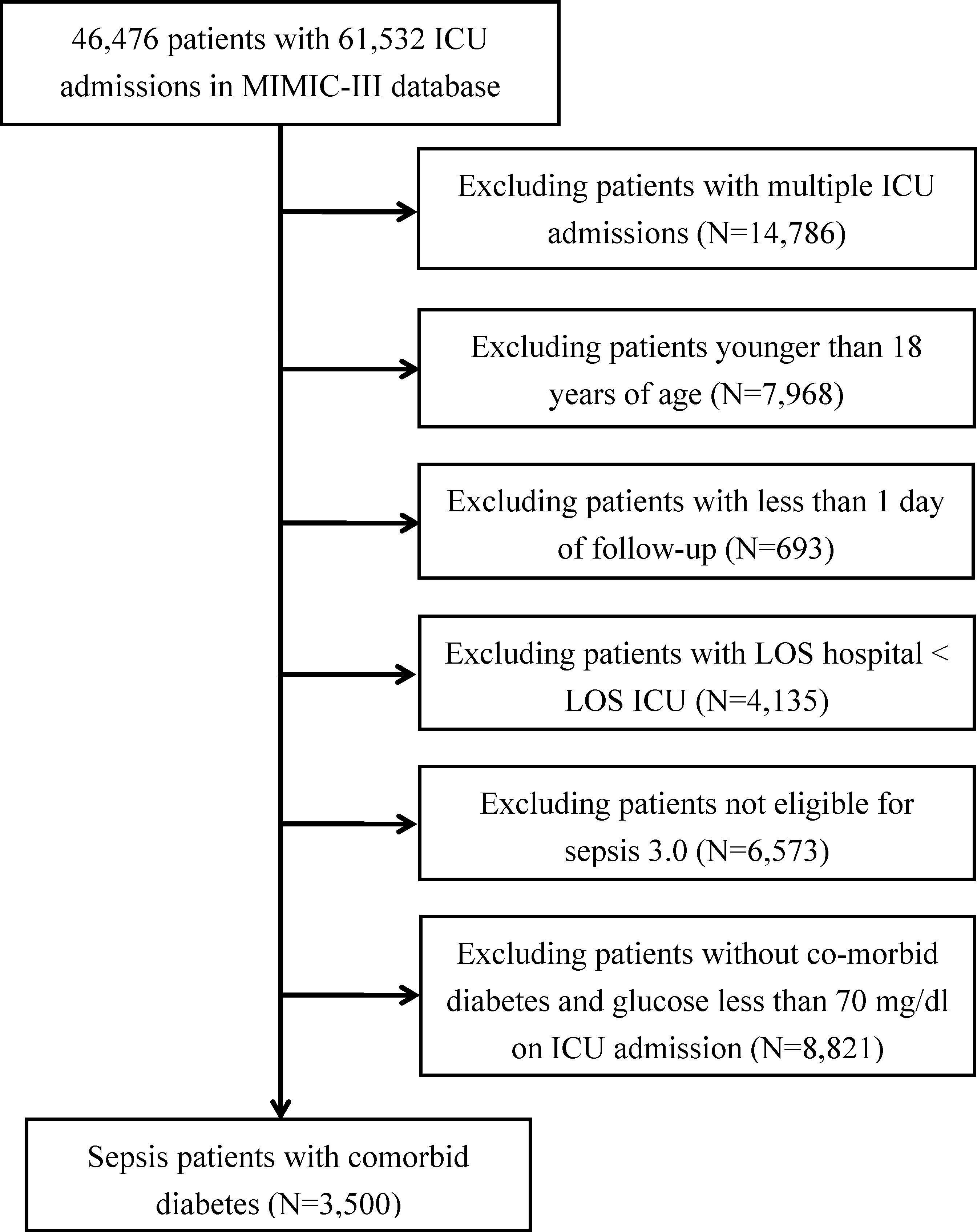
Adult (aged ≥18 years) patients diagnosed with sepsis based on the following criteria were included in the study: suspected infection and a sequential organ failure assessment (SOFA) score of ≥2 (14). We excluded patients with 1) multiple ICU admissions, 2) less than one day of follow-up, 3) hospital length of stay less than the ICU length of stay, 4) no diabetes, and 5) admission blood glucose level of <70 mg/dl. The first plasma glucose measurement obtained in patients admitted in the ICU was used in the study and grouped into the following categories: no hyperglycemia (≤139 mg/dl), mild hyperglycemia (140–199 mg/dl), and severe hyperglycemia (≥200 mg/dl) (7, 8). Along with the patient’s baseline information (e.g., age and sex), therapeutic measures, and clinical endpoints for routine variables, we also extracted the data of patients’ SOFA score, Elixhauser Comorbidity Index (SID30) (15), and specific comorbidities. The code for assisting in the investigation of MIMIC-III is openly available on the website (16).
Outcomes
The primary outcome was 28-day mortality after ICU admission, and the secondary outcome was ICU mortality.
Statistical analysis
The data were expressed as mean ± standard deviation or median (interquartile range) for continuous variables and as numbers and percentages for categorical variables. We compared the characteristics of participants between glucose groups using one-way analysis of variance for continuous variables and chi-square test for categorical variables. Initially, we applied Cox regression models to explore the associations of admission glucose level with the 28-day mortality and logistic regression models to explore the association of admission glucose level with ICU mortality. We presented different adjusted models to assess the effect of admission glucose level on clinical endpoints in sepsis patients with diabetes. In model I, we adjusted for demographic characteristics (age and sex), disease severity (SOFA scores), comorbidity scores (SID30), infection site, and initial treatment (mechanical ventilation and renal replacement therapy on the first); in model II, we substituted the SID30 with the specific diseases (congestive heart failure, cardiac arrhythmias, etc.). Covariate screening was used to include covariates as potential confounders if they changed the estimates of admission glucose level on 28-day mortality by more than 10% or were associated significantly with 28-day mortality.
Subsequently, to explore whether a nonlinear relationship exists between glucose level and 28-day mortality, we performed the smoothed spline method using a Cox model to fit the 28-day mortality (generalized additive model for fitting ICU mortality). If it existed, segmental regression models constructed during the threshold effects analysis were used to detect the inflection points, and the differences were compared by log-likelihood ratio tests (17). Next, the admission glucose level was re-grouped by inflection points, and the different adjustment models described above were used to evaluate the clinical outcome. Finally, stratified analysis and interaction tests were conducted to explore the consistency of the relationship between the inflection point grouping of glucose and 28-day mortality in the patient subgroups based on SOFA scores (<5, 5–10, and ≥10) and infection site. All data were analyzed using EmpowerStats (www.empowerstats.com) and R (http://www.R-project.org). A P-value of <0.05 was considered significant.
Results
Participants’ characteristics
A total of 3,500 sepsis patients with diabetes with a mean age of 66.79 years were enrolled in this study (Figure 1). Majority of the sepsis patients with diabetes were men (51.8% vs. 48.2%). No significant differences were observed between the three groups in terms of SID30, SOFA score, infection site, and need for mechanical ventilation or renal replacement therapy on the first day of ICU admission. Additional detailed results are presented in Table 1.
Clinical outcomes of the participants
With regard to the clinical outcomes, the hospital mortality, ICU mortality, and 28-day mortality in sepsis patients with diabetes in the no hyperglycemia, mild hyperglycemia, and severe hyperglycemia groups were not significant (all P >0.05). No significant difference was found in the length of hospital or ICU stay among the three groups (all P >0.05).
Associations between admission glucose level and clinical outcomes
The Cox regression analysis demonstrated that severe hyperglycemia did not increase the risk of 28-day mortality (crude hazard ratio [HR]=0.99, 95% confidence interval [CI] 0.80-1.22, P =0.9018). After adjusting for confounding factors, hyperglycemia remained a non-risk factor (Table 2). In model II, when compared with the no hyperglycemia group, the 28-day mortality rate in the severe hyperglycemia group did not significantly increase (HR=1.06, 95% CI: 0.86–1.31, P=0.5880). Similar findings were reported in the mild hyperglycemia group (HR=0.99, 95% CI: 0.82–1.19, P=0.9097). With regard to ICU mortality, the results similarly indicated no significant increase in ICU mortality in both the mild hyperglycemia and severe hyperglycemia groups compared with that of the no hyperglycemia group (Table 2).
Smooth splines showed a nonlinear relationship of admission glucose with 28-day and ICU mortality (Figures 2A, B). Threshold effect analysis identified the inflection points for 28-day mortality of 110 mg/dl and 240 mg/dl. For 28-day mortality, the HR was 0.980 for a glucose level of <110 mg/dl and 1.008 for a glucose level of >240 mg/dl (Table 3). Subsequently, the admission glucose level was divided into three categories according to the inflection point: <110 mg/dl, 110–240 mg/dl, ≥240 mg/dl (inflection point grouping of glucose); a Cox regression analysis was performed, and the results revealed a 26% significant reduction of 28-day mortality in the 110–240 mg/dl group compared with the <110 mg/dl group (HR=0.74, 95% CI: 0.59–0.93, P=0.0100); in the >240 mg/dl group, no substantial increase was observed in the risk of 28-day mortality rate (P >0.05) (Table 4). A considerable short-term survival advantage was observed in the 110–240 mg/dl group compared with that in the 110 mg/dl group; meanwhile, no remarkable adverse hazard was detected in the >240 mg/dl group (Figure 3).
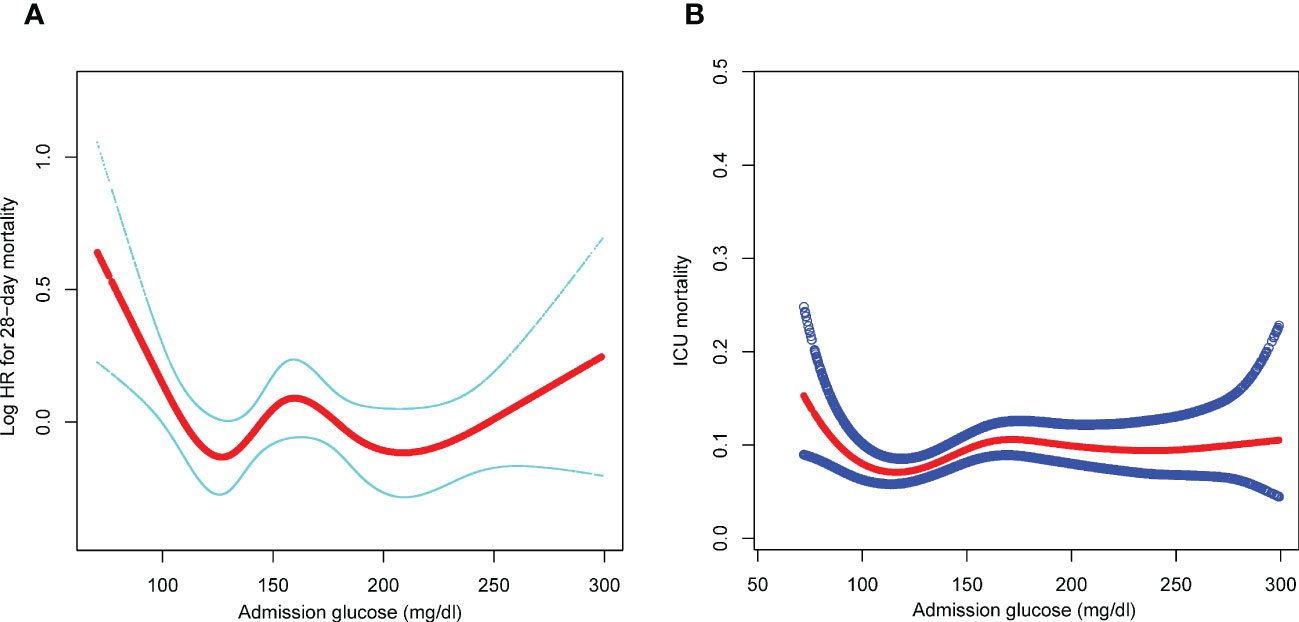
Figure 2 (A) Association of admission glucose level with 28-day mortality. (B) Association of admission glucose level with ICU mortality. adjusted by age, sex, SOFA, infection site, mechanical ventilation on first day, renal replacement therapy on first day, congestive heart failure, cardiac arrhythmias, valvular disease, peripheral vascular disease, hypertension, other neurological diseases, chronic pulmonary disease, liver disease, renal failure, AIDS, lymphoma, metastatic cancer, solid tumor, obesity, fluid and electrolyte disorders, alcohol abuse, drug abuse, and depression. SOFA, sequential organ failure assessment; AIDS, acquired immune deficiency syndrome; ICU, intensive care unit; HR, hazard ratio.
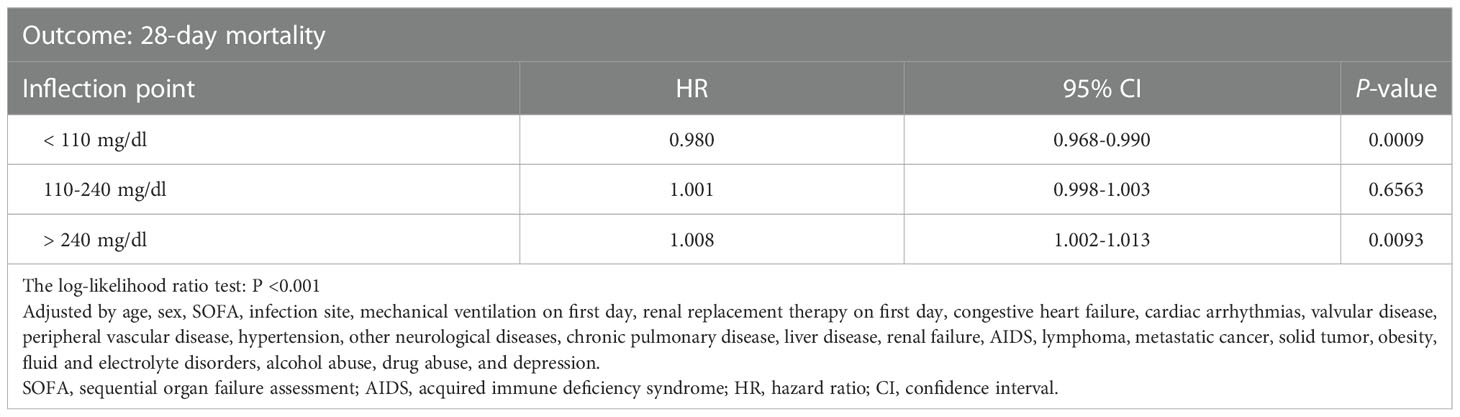
Table 3 Threshold effect analysis of glucose level and 28-day mortality rate using piece-wise linear regression.
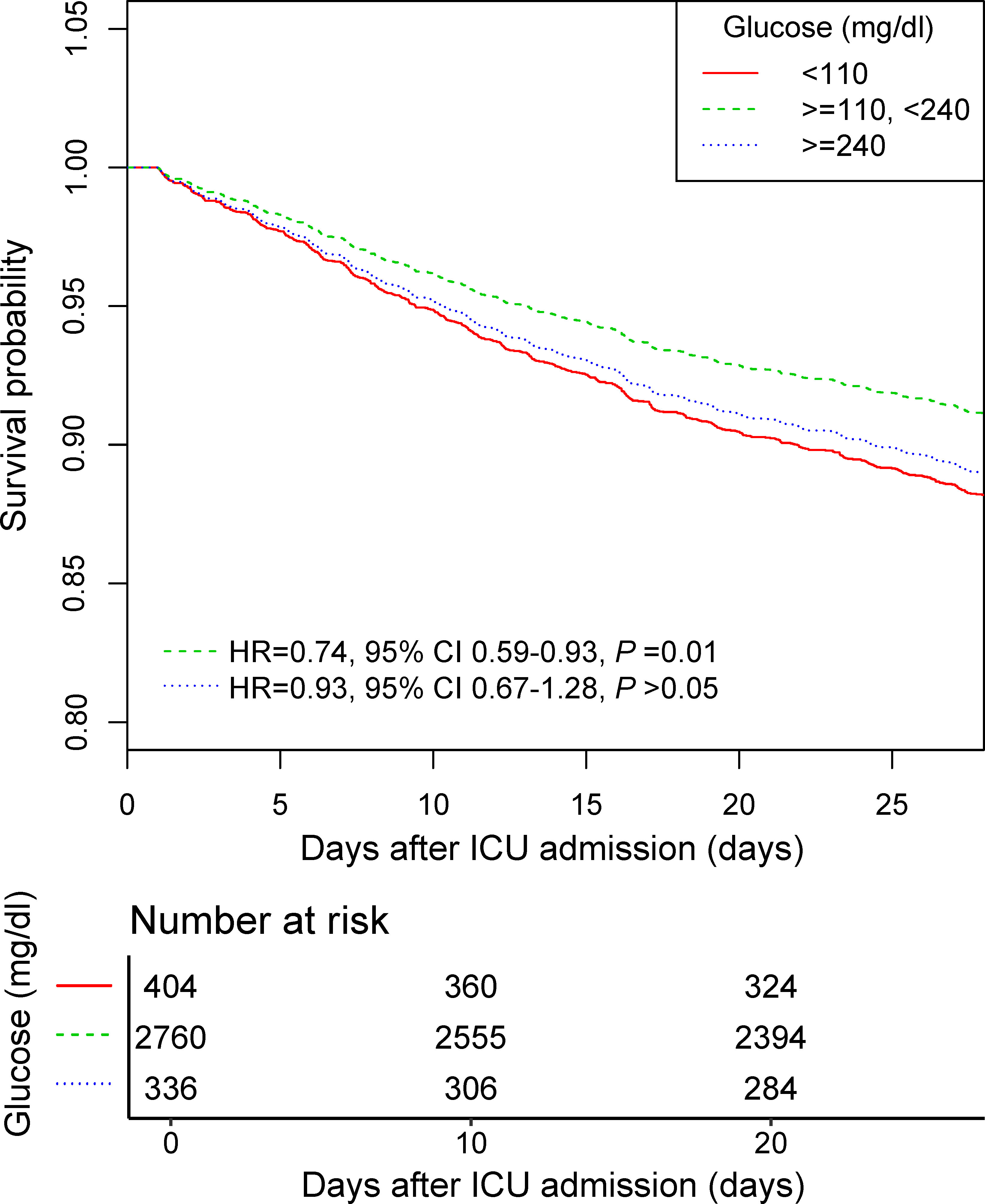
Figure 3 The 28-day survival curve of the Cox regression model for participants with inflection point grouping of glucose. Adjusted by age, sex, SOFA, infection site, mechanical ventilation on first day, renal replacement therapy on first day, congestive heart failure, cardiac arrhythmias, valvular disease, peripheral vascular disease, hypertension, other neurological diseases, chronic pulmonary disease, liver disease, renal failure, AIDS, lymphoma, metastatic cancer, solid tumor, obesity, fluid and electrolyte disorders, alcohol abuse, drug abuse, and depression. SOFA, sequential organ failure assessment; AIDS, acquired immune deficiency syndrome.
In the stratified analysis, the association effect between the new glucose category and the risk of 28-day mortality was generally consistent in the different SOFA scores and infection site (Table 5). Furthermore, the 28-day mortality of the 110–240 mg/dl group with a SOFA score of ≥10 was lower than that of the <110 mg/dl group (HR=0.61, 95% CI: 0.38–0.98). Similarly, patients with bloodstream infection in the 110–240 mg/dl group experienced substantially lower 28-day mortality rate compared with those in the <110 mg/dl group (HR=0.70, 95% CI: 0.49–1.00).
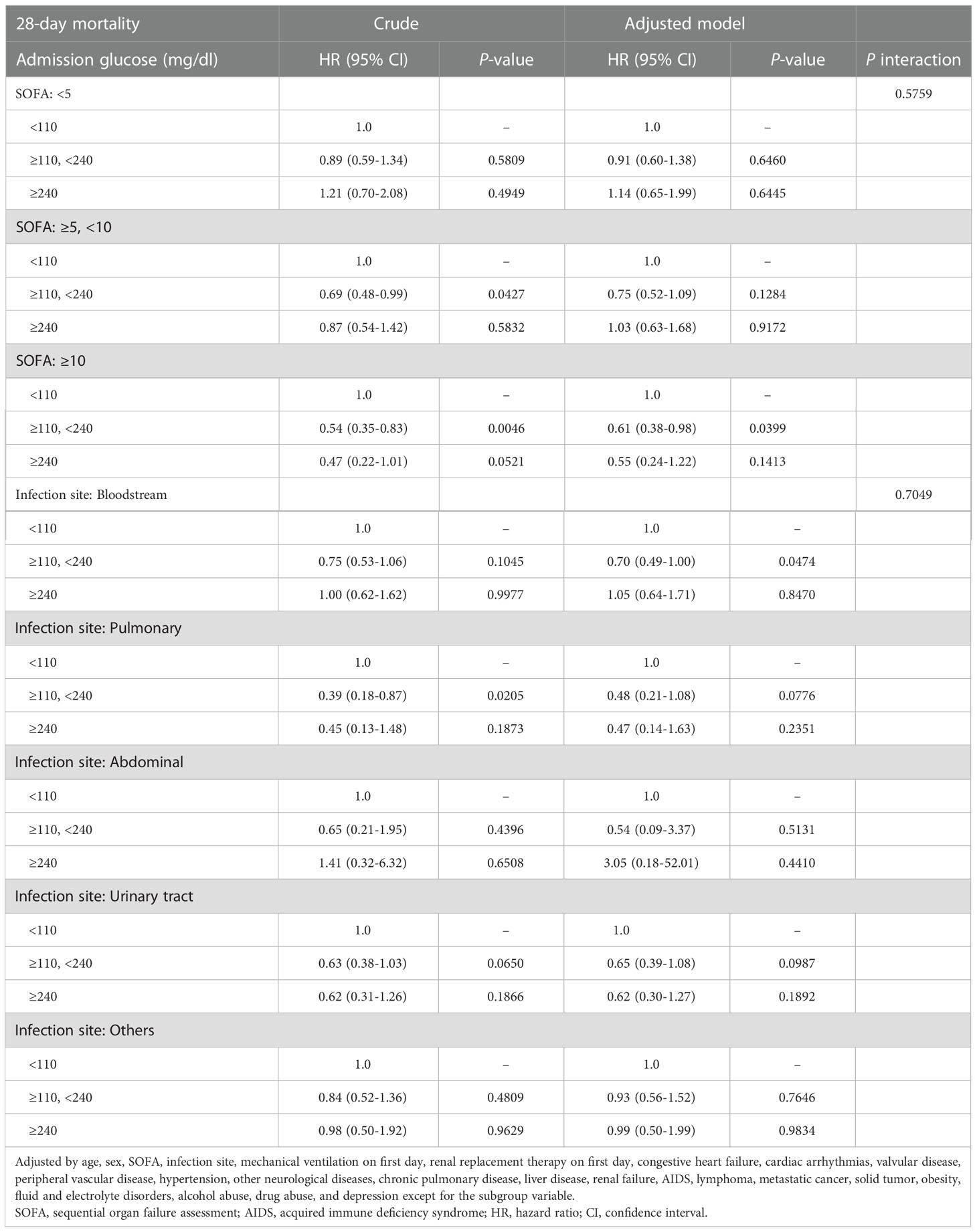
Table 5 Association of inflection point grouping of glucose with 28-day mortality stratified by different scores of SOFA and infection site.
Discussion
The present study explored the association between admission glucose level and clinical outcomes among critical sepsis patients with diabetes and found that the risk of 28-day mortality was not substantially increased in sepsis patients with diabetes who had an admission glucose level of ≥240 mg/dl compared with those who had an admission glucose level of <110 mg/dl; notably, the 28-day mortality rate was markedly reduced in the 110–240 mg/dl group (HR=0.74, 95% CI: 0.59–0.93). Furthermore, an elevated admission glucose level was significantly associated with a reduction in the 28-day mortality rate in the SOFA score ≥10 subgroup, which may imply that patients with serious conditions require a higher energy supply.
Currently, a number of studies have evaluated the glycemic control goals in sepsis patients; the Surviving Sepsis Campaign similarly recommended a glycemic level of 8–10 mmol/L in glycemic management (18). To our knowledge, only a very few studies have investigated the effect of glucose on prognosis in critical sepsis patients with diabetes. A recent study by Zohar et al. included 1,527 patients with community-onset sepsis and found that admission hyperglycemia (>200 mg/dl) correlated with increased in-hospital mortality, 30-day mortality, and 90-day mortality; moreover, this adverse outcomes were more prevalent in patients with diabetes (19). In a study of 1,059 sepsis patients, Vught et al. similarly found that severe hyperglycemia (>200 mg/dl) upon admission did not increase the 30-day mortality rate in patients with sepsis; rather, hyperglycemia was strongly associated with increased 30-, 60-, and 90-day mortality rates in patients with sepsis without diabetes (20). Moreover, Tayek et al. searched the PubMed database for publications related to sepsis, diabetes, glycemia, and prognosis; nine studies were analyzed, which reported that hyperglycemia was not related to poor outcome in sepsis patients with diabetes; the opposite was true in hyperglycemic patients without diabetes, which was an independent hazard factor for ICU and in-hospital mortality (21). Stegenga et al. examined 830 patients with severe sepsis and suggested a measurable increase in 28- and 90-day mortality rates with hyperglycemia (>200 mg/dl) compared with admission glucose at or below 200 mg/dl in sepsis patients without diabetes. Although the authors did not explicitly analyze the admission glucose level in sepsis patients with diabetes, the curve fitting plots in the article indicated that admission hyperglycemia had a relatively slight effect on 28-day mortality in sepsis patients with diabetes (22). In addition, another relatively earlier research conducted by Freire et al. demonstrated that admission hyperglycemia was not appreciably associated with in-hospital mortality (23). All of the abovementioned studies showed results similar to those of our study; that is, in sepsis patients with diabetes, admission hyperglycemia was not an independent hazard factor for poor short-term prognosis. In our study, we further revealed a non-linear relationship between admission glucose level and 28-day mortality using smoothing spline curves, with the lowest 28-day mortality in the admission glucose range of 110–240 mg/dl, which was different from those reported in other studies and was one of the highlights of our study. In the subgroup analysis, we found that higher admission glucose level was significantly associated with lower 28-day mortality rate in the SOFA ≥10 subgroup; whether this means that critically ill patients require higher energy supply deserves further investigation. These results, contrary to our common knowledge of the devastating consequences of diabetes and hyperglycemia, suggest the need for an individualized glycemic control strategy for sepsis patients with diabetes that differs from other critically ill patients since they may be able to benefit from hyperglycemia.
In the light of the available studies, however, it seems that the clinical benefit of hyperglycemia and sepsis with co-existent diabetes remains a topic that cannot be thoroughly elucidated. From the clinical point of view, diabetes can cause immune dysfunction and metabolic disorders, which inevitably induce the organism’s ability to defend against infection, in turn with catastrophic consequences. Physiologically, part of the potential mechanism can be attributed to the metabolic requirements and maintenance of the function of immune cells by glucose, with an equally critical role played by the synthetic action of immunomodulators (24, 25). Furthermore, patients with diabetes have a tolerance to hyperglycemia as a consequence of persistent high blood glucose concentrations, converting the detrimental elevated glucose into an energy reservoir (26). Additionally, the therapies administered to diabetic patients, including sulfonylureas, metformin, thiazolidinediones, and insulin, as well as the effects of diabetes on the immune system, may potentially affect the host’s response to sepsis and clinical endpoints. Therefore, further investigations are imperatively needed to comprehensively address which mechanisms contribute to the overall impact of diabetes on the outcomes of sepsis.
Even with the relatively large sample size included in our study, the limitations should not be overlooked. First, we did not account for the effect of diabetes type and diabetes medications like insulin and metformin; thus, we were unable to assess whether medications and diabetes type have an effect on outcomes at this point. Next, we cannot exclude the possibility of new-onset diabetes since data on HbA1c levels are not available. Moreover, we were not able to obtain information about the duration and severity of diabetes; thus, it was impossible to measure the effect of these factors on the outcome as well. Third, we used the first blood glucose measurement obtained after admission to the ICU for the purpose of eliminating the effect of medical therapies in the ICU, and the results were different from those of studies investigating glycemic control, although our results may help identify appropriate glycemic control strategies to some extent. Finally, we should interpret these results with caution, as the association analysis should not be mistaken for causality. Therefore, further in-depth basic and clinical studies are warranted to enrich the category of findings.
Conclusion
Admission hyperglycemia was not a risk factor for short-term prognosis in critical ill sepsis patients with diabetes; rather, a lower blood glucose level was associated with increased risk of poor prognosis. Notably, an elevated admission glucose level was significantly associated with a reduction in 28-day mortality rate in the SOFA score ≥10 subgroup; whether this implies that patients with severe illness require a higher energy supply deserves further research.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: The raw data itself is from a third-party dataset available from MIMIC-III, a freely accessible critical care database. Reproduction of their data is not permitted according to the Data Use Agreement of the database but access can be requested here: https://mimic.physionet.org/gettingstarted/access.
Ethics statement
The access of the database has been approved by the institutional review boards of both Beth Israel Deaconess Medical Center and Massachusetts Institute of Technology Affiliates (Record ID: 33460949). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
SL and DL designed the study and wrote the draft of this manuscript, WH mainly performed data extraction and statistical analysis, SL revised this manuscript. All authors are involved in data correction. Written consent to publication was obtained.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Van den Berghe G, Wouters P, Weekers F, Verwaest C, Bruyninckx F, Schetz M, et al. Intensive insulin therapy in critically ill patients. N Engl J Med (2001) 345(19):1359–67. doi: 10.1056/NEJMoa011300
2. Nice-Sugar Study Investigators. Intensive versus conventional glucose control in critically ill patients. N Engl J Med (2009) 360(13):1283–97. doi: 10.1056/NEJMoa0810625
3. Benfield T, Jensen JS, Nordestgaard BG. Influence of diabetes and hyperglycaemia on infectious disease hospitalisation and outcome. Diabetologia (2007) 50(3):549–54. doi: 10.1007/s00125-006-0570-3
4. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
5. Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation (2006) 114(6):597–605. doi: 10.1161/CIRCULATIONAHA.106.621854
6. Bonnard C, Durand A, Peyrol S, Chanseaume E, Chauvin MA, Morio B, et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. J Clin Invest (2008) 118(2):789–800. doi: 10.1172/JCI32601
7. van Vught LA, Holman R, de Jonge E, de Keizer NF, van der Poll T. Diabetes is not associated with increased 90-day mortality risk in critically ill patients with sepsis. Crit Care Med (2017) 45(10):e1026–35. doi: 10.1097/CCM.0000000000002590
8. van Vught LA, Wiewel MA, Klein Klouwenberg P, Hoogendijk AJ, Scicluna BP, Ong DS, et al. Admission hyperglycemia in critically ill sepsis patients: association with outcome and host response. Crit Care Med (2016) 44(7):1338–46. doi: 10.1097/CCM.0000000000001650
9. Brunkhorst FM, Engel C, Bloos F, Meier-Hellmann A, Ragaller M, Weiler N, et al. Intensive insulin therapy and pentastarch resuscitation in severe sepsis. N Engl J Med (2008) 358(2):125–39. doi: 10.1056/NEJMoa070716
10. Van den Berghe G, Wilmer A, Hermans G, Meersseman W, Wouters PJ, Milants I, et al. Intensive insulin therapy in the medical ICU. N Engl J Med (2006) 354(5):449–61. doi: 10.1056/NEJMoa052521
11. Preiser JC, Devos P, Ruiz-Santana S, Mélot C, Annane D, Groeneveld J, et al. A prospective randomised multi-centre controlled trial on tight glucose control by intensive insulin therapy in adult intensive care units: the glucontrol study. Intensive Care Med (2009) 35(10):1738. doi: 10.1007/s00134-009-1585-2
12. Kalfon P, Giraudeau B, Ichai C, Guerrini A, Brechot N, Cinotti R, et al. Tight computerized versus conventional glucose control in the ICU: a randomized controlled trial. Intensive Care Med (2014) 40(2):171–81. doi: 10.1007/s00134-013-3189-0
13. Johnson AE, Pollard TJ, Shen L, Lehman LW, Feng M, Ghassemi M, et al. MIMIC-III, a freely accessible critical care database. Sci Data (2016) 3:160035. doi: 10.1038/sdata.2016.35
14. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
15. Steiner C, Elixhauser A, Schnaier J. The healthcare cost and utilization project: an overview. Eff Clin Pract (2002) 5(3):143–51.
16. Johnson A, Stone DJ, Celi LA, Pollard TJ. The MIMIC code repository: Enabling reproducibility in critical care research. J Am Med Inform Assoc (2017) 25(1):32–9. doi: 10.1093/jamia/ocx084
17. Lin L, Chen CZ, Yu XD. Zhonghua liu xing bing xue za zhi. The analysis of threshold effect using Empower Stats software (2013) 34(11):1139–41. doi: 10.3760/cma.j.issn.0254-45.2013.011.021
18. Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, et al. Surviving sepsis campaign: International guidelines for management of sepsis and septic shock 2021. Intensive Care Med (2021) 47(11):1181–247. doi: 10.1007/s00134-021-06506-y
19. Zohar Y, Zilberman Itskovich S, Koren S, Zaidenstein R, Marchaim D, Koren R. The association of diabetes and hyperglycemia with sepsis outcomes: a population-based cohort analysis. Intern Emerg Med (2021) 16(3):719–28. doi: 10.1007/s11739-020-02507-9
20. Vught LAV, Wiewel MA, Klouwenberg PMK, Hoogendijk AJ, Ong DS, Cremer OL, et al. Effect of admission hyperglycemia in sepsis patients with or without a history of diabetes. Crit Care (2015) 19(Suppl 1):P371. doi: 10.1186/cc14451
21. Tayek CJ, Tayek JA. Diabetes patients and non-diabetic patients intensive care unit and hospital mortality risks associated with sepsis. World J Diabetes (2012) 3(2):29–34. doi: 10.4239/wjd.v3.i2.29
22. Stegenga ME, Vincent JL, Vail GM, Xie J, Haney DJ, Williams MD, et al. Diabetes does not alter mortality or hemostatic and inflammatory responses in patients with severe sepsis. Crit Care Med (2010) 38(2):539–45. doi: 10.1097/CCM.0b013e3181c02726
23. Freire AX, Bridges L, Umpierrez GE, Kuhl D, Kitabchi AE. Admission hyperglycemia and other risk factors as predictors of hospital mortality in a medical ICU population. Chest (2005) 128(5):3109–16. doi: 10.1378/chest.128.5.3109
24. Ganeshan K, Chawla A. Metabolic regulation of immune responses. Annu Rev Immunol (2014) 32:609–34. doi: 10.1146/annurev-immunol-032713-120236
25. Pearce EL, Pearce EJ. Metabolic pathways in immune cell activation and quiescence. Immunity (2013) 38(4):633–43. doi: 10.1016/j.immuni.2013.04.005
Keywords: critical care, diabetes, sepsis, glucose, prognosis
Citation: Lin S, Lai D and He W (2023) Association between hyperglycemia and adverse clinical outcomes of sepsis patients with diabetes. Front. Endocrinol. 13:1046736. doi: 10.3389/fendo.2022.1046736
Received: 17 September 2022; Accepted: 22 December 2022;
Published: 09 January 2023.
Edited by:
Xian Wu Cheng, Yanbian University Hospital, ChinaReviewed by:
Xinhua Zou, Nanjing Medical University, ChinaXingji Lian, Guangzhou First People’s Hospital, China
Copyright © 2023 Lin, Lai and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shan Lin, ZHIuc2hhbmxpbkBmb3htYWlsLmNvbQ==
†These authors have contributed equally to this work
 Shan Lin
Shan Lin Dingfeng Lai2†
Dingfeng Lai2†