
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 06 January 2023
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1046381
Introduction: The reference intervals (RIs) are of great importance for physicans to determine whether or not an individual is healthy. However, many clinical laboratories in China still adopted the default RI provided by the manufacturers; and these “uncalibrated” RIs might lead to the misdiagnosis of diseases. In the present study, we enroll reference people with the purpose of determining the RIs of serum triiodothyronine (T3), thyroxine (T4), free triiodothyronine (FT3), free thyroxine (FT4) and thyroid-stimulating hormone (TSH) in Chinese population, and explore the possible roles of age and sex on the levels of biomarkers.
Methods: Serum samples from 66,609 individuals who met the inclusion criteria were analyzed using an Roche Cobas E 601 hormone analyzer. The dynamic trends of biomarker were visually assessed by their concentrations over age and sex. Specific partitions were determined by the method of Harris and Boyd. RIs, corresponding to the 2.5th and 97.5th percentiles, as well as the 0.5th, 25th, 50th, 75th and 99.5th percentiles were calculated for each reference partition using a non-parametric rank approach.
Results: The serum level of T3, T4, FT4 or TSH showed a right-skewed distribution in both males and females while FT3 presented an approximate normal distribution. Females had a higher mode value of serum T3 or T4, but a lower mode value of serum TSH, FT3 or FT4. All five biomarkers did not need age partitioning according to the approach of harris and boyd, while T3 and FT3 need sex partitioning.
Conclusions: The present study not only determined the age- and sex-specific trends of the five thyroid hormones, but provided sex-stratified RIs for T3 and FT3, valuably contributing to the current literature and timely evaluation of thyroid health and disease.
In China, approximately 40% of adults (≥18 years of age) suffer from thyroid-related diseases, and subclinical hypothyroidism accounts for most of these cases (1). As the symptoms of patients with subclinical thyroid disease are not apparent, biomarkers such as serum triiodothyronine (T3), thyroxine (T4), free triiodothyronine (FT3), free thyroxine (FT4) and thyroid-stimulating hormone (TSH) are used to assist in the diagnosis of thyroid diseases.
Clinically, reference intervals (RIs) are used to determine whether the level of a biomarker is within the normal range, and the use of inappropriate RIs as judgment criteria may lead to serious consequences. For example, Droke et al. suggested that the use of inadequate serum ferritin reference intervals caused an underdiagnosis (>15%) of iron deficiency in low-income children (2). In the Netherlands, using the TSH reference interval recommended by the American Thyroid Association for pregnant women caused 8.6% and 4.9% of pregnant women with normal TSH levels to be misdiagnosed in the first and second trimesters, respectively (3, 4).
Currently, most clinical laboratories in China adopt the default RIs from manufacturers. These RIs, however, were developed on limited samples and do not reflect the actual values of biomarkers in specific populations. To improve screening performance, the American Thyroid Association (ATA) and the National Academy of Clinical Biochemistry (NACB) published guidelines in 2017 suggesting that clinical laboratories should establish their own RIs based on the screened populations (5, 6). Considering this, we launched a cross-sectional study in 2016. The goal of such an investigation was to determine the baseline levels of serum TSH, T3, FT3, T4 and FT4 in a population of healthy Chinese individuals and explore the possible roles of age and sex on the levels of biomarkers. In this pilot study, we reported the dynamic trends of TSH, T3, FT3, T4 and FT4 according to age and sex and developed age- and/or sex-stratified RIs.
Individuals aged 22 to 70 years who had a physical exam at West China Fourth Hospital (WCFH) between August 2016 and September 2020 were enrolled in this study. The physical conditions of the study participants were assessed by licensed medical practitioners based on their questionnaire responses and physical exam reports. Subjects with visible or palpable thyroid tumors were excluded, as were breastfeeding or pregnant women, individuals with pituitary disorders, hypothyroidism, hyperthyroidism, a family history of thyroid disease, and those taking medications that affect thyroid function (Supplementary Table S1).
The biochemical laboratory of the West China Fourth Hospital (BLWCFH) was the participating laboratory. BLWCFH passed multiple rounds of external quality assessments (EQA) organized by the National Center for Clinical Laboratories (NCCL) since 2012. The detail of the NCCL EQA procedure included the following: the NCCL distributed two batches of quality control products containing low, median and high concentration of certain target analytes to each participating laboratory every year; participating laboratories were required to test all quality control products and return the results to the NCCL. The allowable deviation for any test analyte in the NCCL was ±25% from the median value of the peer group laboratories, while the deviation for TSH, T3, T4, FT3 or FT4 in BLWCFH was less than 8%. The EQA results of BLWCFH from 2016 to 2020 are listed in Supplementary Table S2.
Except for the EQA, BLWCFH also performed strict internal quality control (IQC) as follows (1): control products containing high and low levels of target analytes were measured every day for 20 consecutive days; and (2) all test values were between the mean ± 2 SD, while the coefficient of variation (CV) was limited to <8%. Over the 4-year period during which samples were tested, 5 batches of control products were used to monitor the performance of the testing equipment. The overall imprecision for each analyte is listed in Supplementary Table S3.
For all study participants, 8 hours of fasting were required before blood sampling. In brief, 3 ml of blood was collected from each subject using a vacutainer blood collection tube (Sanfeng Medical Co., Ltd., Chongqing, China). After incubation at room temperature for 10 min, the serum was isolated by centrifugation at 3,000 rpm for 10 minutes. The levels of T3, T4, FT3, FT4 and TSH were determined using a Cobas E 601 automatic electro-chemiluminescence immunoassay analyzer (Roche Diagnostics, Shanghai, China) with the corresponding Elecsys® detection Kit. The time interval between sampling and testing was <60 min. The limits of detection (LOD)/quantification (LOQ) are listed in Supplementary Table S4.
Data were analyzed in accordance with CLSI C28-A3 guidelines (7). Statistical analysis was implemented using R studio Ver. 4.0.5 or EXCEL Ver. 16.65. In brief, the frequency histograms of each biomarker were used to visually inspect the data. Outliers were identified using Tukey’s tests and removed. The normality of the data was determined by the Shapiro−Wilk test. The RIs of each biomarker were partitioned by the Harris & Boyd approach (7); if the results did not suggest partitioning, the data were combined and re-estimated. The nonparametric rank method was used to calculate the 0.5th, 2.5th, 25th, 50th, 75th, 97.5th and 99.5th percentiles of each reference partition. The central 95% interval, corresponding to the range between the 2.5th and 97.5th percentiles, was defined as the RI. For each RI, the 90% confidence intervals were calculated for the end points.
A total of 66,609 adults (22 to 70 years old) who met the inclusion criteria were included in the study analysis. As the study subjects were allowed to choose their health check packages and different packages included varying numbers of tested thyroid hormones, it is not surprising that the data size for serum levels of TSH, T3, T4, FT3 and FT4 are different. Specifically, the TSH level was determined for all subjects. While the serum level of T3 and T4 were measured for approximately 54,000 subjects. In comparison, only 34,000 subjects choose to take all five thyroid hormones. The sample size of each analyte and the number of outliers removed are listed in Table 1.
As indicated in Figure 1, the serum level of T3 in females and the serum level of TSH in both males and females showed a right-skewed distribution, while T4, FT3 and FT4 showed an approximately normal distribution. Females had a higher mode value of TSH (2.15 mIU/L), whereas males had a higher mode values of T3 (1.87 nM), T4 (107.0 nM), FT3 (5.14 pM), and FT4 (17.90 pM) (Table 2). The skewness value for each biomarker by sex is listed in Table 2. The distribution of biomarker values according to various age groups is listed in Supplementary Figure S6–S10.
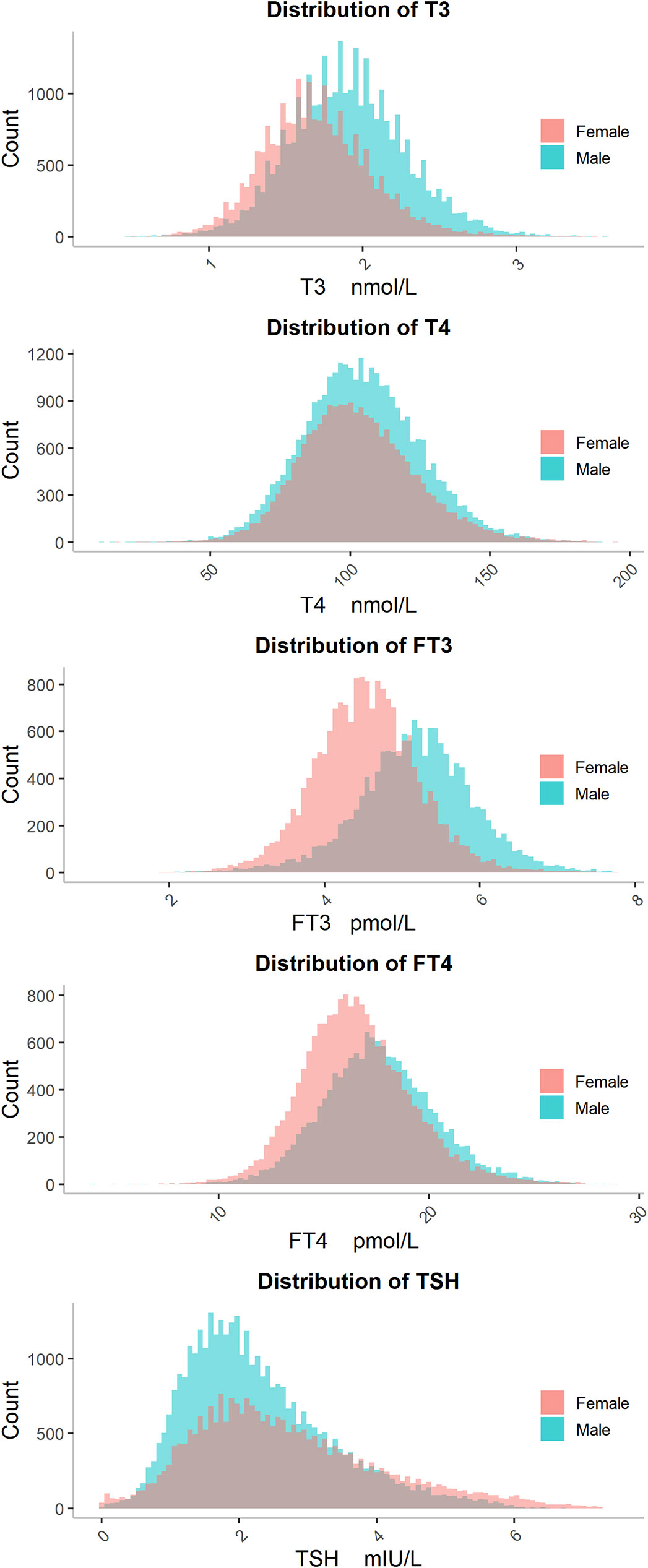
Figure 1 The distributions of T3, T4, FT3, FT4 and TSH. The distributions of five thyroid hormones are shown separately for males (blue bars) and females (red bars). Abbreviations are listed in the legend of Table 1.
As shown in Figure 2, the median of T3 decreased in males and remained at a steady state in females according to age. In contrast, the median of FT3 in all male partitions was higher than that in female partitions, except for the age group of 70 years old. The dynamic trend of serum FT4 was similar to that of serum FT3, except that the median of FT4 in males aged 68 years was lower than that of females of the same age. Although the level of serum T4 did not change with age or sex, the median and interquartile range (IQR) of TSH increased with age in females.
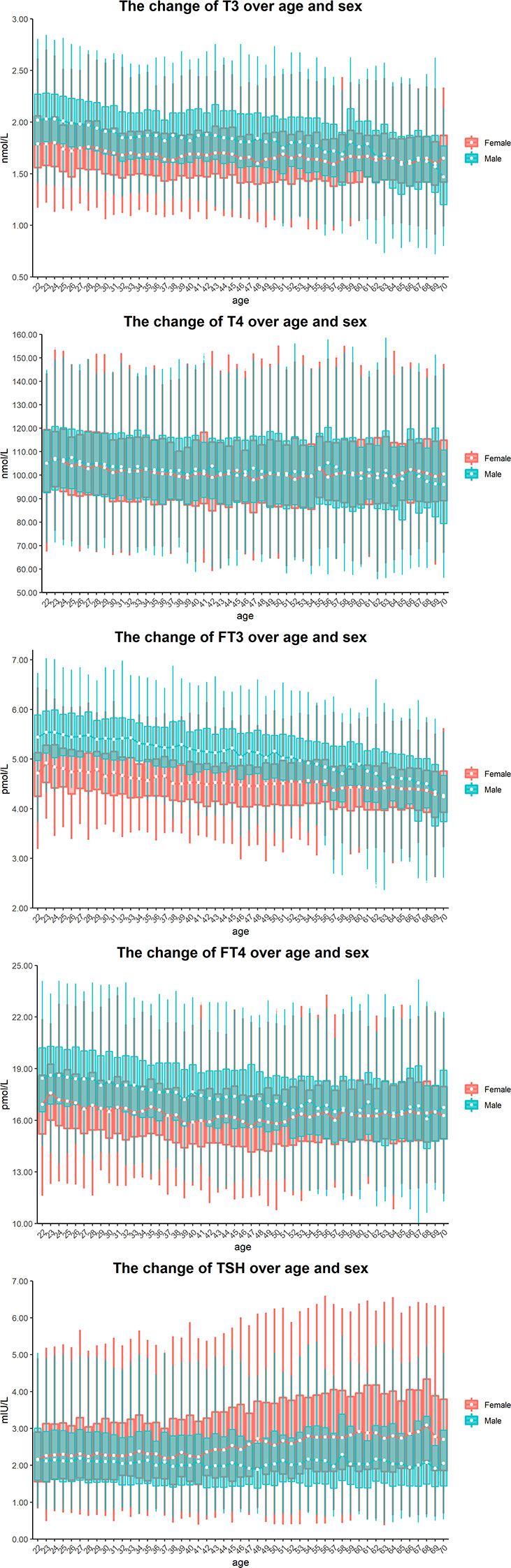
Figure 2 Sex and age-related dynamic changes of T3, T4, FT3, FT4 and TSH. Data of male and female partitions are shown in blue and red boxes with whiskers, respectively. The boxes extend from the 25th to the 75th percentile, with whiskers extending to the 2.5th or 97.5th percentile. Medians are shown as white circles in the body of boxes, and are linked with blue (male) or red (female) line to shown the dynamics. Abbreviations are listed in the legend of Table 1.
As the Harris & Boyd method did not suggest any age and/or sex partitioning for T4, FT4 and TSH but suggested sex partitioning for both T3 and FT3, the estimated RIs for T4, FT4 and TSH were [65.90-147.5] nM, [12.11-22.82] pM and [0.683-5.45] mIU/L, respectively (Table 3). The RIs of T3 and FT3 for males were [1.20-2.70] nM and [3.54-6.63] pM, respectively (Table 3), while the RIs of T3 and FT3 for females were [1.06-2.49] nM and [3.24-5.93] pM, respectively (Table 3). For each RI, the 90% confidence intervals for the end points calculated by the bootstrap resampling method are shown in Table 4. The details of the other percentiles for each biomarker are listed in Supplementary Table S5.
Estimating RIs has never been an easy job, but determining the reference partition is even tougher. Although several reports published age- and/or sex-stratified RIs for thyroid hormones, they did not interpret how they partitioned the reference groups. For example, Vadiveloo et al. divided the reference population into 8 age groups and established age-specific RIs for serum TSH. In their study, less information regarding the rationale for group partitioning is available (8). In another case, Yoshihara and colleagues established age- and sex-stratified RIs for serum TSH and FT4. However, again, they did not explain how they confirmed the reference partitions (9). In the present study, the reference partitions were determined by the Harris & Boyd method. The merit of using such a statistical method is that many cofactors, e.g., sample size and value distribution, could be taken into consideration when determining the reference groups to avoid any subjective preference (10, 11).
The actual distribution of TSH, T3, T4, FT3 and FT4 in serum samples was assessed by estimating the 0.5th, 2.5th, 25th, 50th, 75th, 97.5th and 99.5th percentiles in each reference partition. As shown in Figure 2, the median TSH level was lower in males than in females. This result is consistent with the findings reported previously (12, 13). Unlike TSH, the median level of FT4 was higher in males than in females. This may be attributed to the differential expression of DIO1, LHX3 and FOXE1 between men and women. Such genes were demonstrated to affect the level of FT4 via the hypothalamic-pituitary-thyroid axis (14). As the activity of 5’-deiodinase deiodinase found in young males was strong (15), it is not surprising that the levels of T3 and FT3 were higher in adolescent males (20–24 years old) since 5’-deiodinase is able to convert T4 to T3 (16). Regarding the level of T4, previous studies reported a higher level of T4 in males, which is in line with our study (17, 18). The TSH level barely changed over age in males but increased steadily in females from the age of 40 to 68 years (Figure 2). One report attributed this TSH increase either to the decreased activity of TSH in middle-aged women or to a decreased sensitivity of TSH to the negative feedback of thyroid hormones (19), while another report attributed this to the increased uptake of iodine or the accelerated organ failure of thyroid tissues with age in females (20).
Although elderly individuals are more likely to suffer from thyroid diseases that affect their life expectancy and increase the burden on society as a whole (1, 21), few studies have investigated the baseline levels and dynamic trends of thyroid hormones in elderly individuals. Our study closed this gap by including elderly individuals aged 60 to 70 years. As people age, the levels of T3 and FT3 tend to decrease, while T4 and FT4 levels remain relatively stable (Figure 2). In a previous study, the levels of FT3 and T3 were found to be higher in people aged 18-50 years compared to those aged 51-90 years. Specifically, FT3 levels were about 6%~7% higher in the younger group, while T3 levels were about 8%~12% higher (22). Additionally, the study found no correlation between age and the level of FT4 and T4 (22), which is consistent with the data of the present study.
The most noteworthy finding in this study is that we captured the dynamic trends of TSH, T3, FT3, T4 and FT4 over age and sex. Comparing to the RIs provided by the manufacturers, the RIs that we proposed had a lower endpoint for both T3 and T4, which aligns with several studies (23, 24). Furthermore, a large sample size allowed us to determine a more precise reference interval (RI) for FT3 than the previous studies (25, 26). In a recent investigation, Wang and colleges showed that the reference range of TSH in healthy Chinese adults was (0.67-7.87) mIU/L (27). In contrast, our RIs presented a lower upper limit. Differential results could be due to the sample size, age and ethnicity of the study population.
It is important to show the limitations of our study. As the inclusion and exclusion criteria for the healthy population were not rigorous, there may have been a mix of false-positive and false negative cases, which might impair the accuracy of thyroid hormone RIs. However, since tens of thousands of samples were included in this study, such an error can be largely ignored. Although our study showed statistically significant sex differences in T3 and FT3 levels, some differences may be due to the large sample sizes and are not clinically significant. Other limitations are that we did not address whether additional cofactors such as iodine intake, ethnicity, climate, and season changed. That is because the submitted data from the participating laboratory did not include more detailed information. In addition, the concentration of thyroid hormones outside of the RIs does not necessarily indicate the presence of disease.
In conclusion, this study established age- and sex-stratified reference intervals for five thyroid function indicators based on CLSI EP28-A3c. Most importantly, our data provided strong evidence regarding the age- and sex-specific trends in thyroid hormones based on a large number of observations. Thus, these data made a valuable contribution to the current literature and could be important for the early assessment of disease.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the Medical Ethics Committee of West China Fourth Hospital of Sichuan University. The patients/participants provided their written informed consent to participate in this study.
CW, TT, MS, and TX contributed to the conception and designed of the project. Recruitment of subjects, sample collection and measurements were conducted by TX, MS, JF, and XP. The initial draft of the manuscript was written by TX and MS. CW and TT reviewed the draft manuscript and made comments and suggestions. TX and MS made revisions to the manuscript. All authors contributed to the article and approved the submitted version.
This study is supported by the Key Program of Sichuan Science and Technology Department (No. 2021YFS0179 & No. 2021YFQ0060).
We sincerely appreciate all participants in this project, medical workers for the data collection, and any individuals who helped us to finish this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1046381/full#supplementary-material
1. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and safety of long-term universal salt iodization on thyroid disorders: Epidemiological evidence from 31 provinces of mainland China. Thyroid (2020) 30(4):568–79. doi: 10.1089/thy.2019.0067
2. Droke EA, Kennedy TS, Hubbs-Tait L. Potential for misclassification of micronutrient status in children participating in a head start program. J Am Diet Assoc (2006) 106(3):376–82. doi: 10.1016/j.jada.2005.12.011
3. Medici M, de Rijke YB, Peeters RP, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VV, et al. Maternal early pregnancy and newborn thyroid hormone parameters: The generation r study. J Clin Endocrinol Metab (2012) 97(2):646–52. doi: 10.1210/jc.2011-2398
4. Stagnaro-Green A, Abalovich M, Alexander E, Azizi F, Mestman J, Negro R, et al. Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid (2011) 21(10):1081–125. doi: 10.1089/thy.2011.0087
5. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid (2017) 27(3):315–89. doi: 10.1089/thy.2016.0457
6. Kratzsch J, Fiedler GM, Leichtle A, Brügel M, Buchbinder S, Otto L, et al. New reference intervals for thyrotropin and thyroid hormones based on national academy of clinical biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem (2005) 51(8):1480–6. doi: 10.1373/clinchem.2004.047399
7. Horowitz GL, Altaie S, Boyd JC, Ceriotti F, Garg U, Horn P, et al. Defining, establishing, and verifying reference intervals in the clinical laboratory; approved guideline. 3rd ed. USA: CLSI and IFCC (2010) p. 12–20.
8. Vadiveloo T, Donnan PT, Murphy MJ, Leese GP. Age- and gender-specific tsh reference intervals in people with no obvious thyroid disease in Tayside, Scotland: The thyroid epidemiology, audit, and research study (Tears). J Clin Endocrinol Metab (2013) 98(3):1147–53. doi: 10.1210/jc.2012-3191
9. Yoshihara A, Noh JY, Ohye H, Sato S, Sekiya K, Kosuga Y, et al. Reference limits for serum thyrotropin in a Japanese population. Endocr J (2011) 58(7):585–8. doi: 10.1507/endocrj.k11e-082
10. Harris EK, Boyd JC. On dividing reference data into subgroups to produce separate reference ranges. Clin Chem (1990) 36(2):265–70. doi: 10.1093/clinchem/36.2.265
11. Harris EK, Wong ET, Shaw ST Jr. Statistical criteria for separate reference intervals: Race and gender groups in creatine kinase. Clin Chem (1991) 37(9):1580–2. doi: 10.1093/clinchem/37.9.1580
12. Ehrenkranz J, Bach PR, Snow GL, Schneider A, Lee JL, Ilstrup S, et al. Circadian and circannual rhythms in thyroid hormones: Determining the tsh and free T4 reference intervals based upon time of day, age, and sex. Thyroid (2015) 25(8):954–61. doi: 10.1089/thy.2014.0589
14. Porcu E, Medici M, Pistis G, Volpato CB, Wilson SG, Cappola AR, et al. A meta-analysis of thyroid-related traits reveals novel loci and gender-specific differences in the regulation of thyroid function. PloS Genet (2013) 9(2):e1003266. doi: 10.1371/journal.pgen.1003266
15. Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev (2002) 23(1):38–89. doi: 10.1210/edrv.23.1.0455
16. Bianco AC, Dumitrescu A, Gereben B, Ribeiro MO, Fonseca TL, Fernandes GW, et al. Paradigms of dynamic control of thyroid hormone signaling. Endocr Rev (2019) 40(4):1000–47. doi: 10.1210/er.2018-00275
17. Li ZZ, Yu BZ, Wang JL, Yang Q, Ming J, Tang YR. Reference intervals for thyroid-stimulating hormone and thyroid hormones using the access tsh 3rd is method in China. J Clin Lab Anal (2020) 34(5):e23197. doi: 10.1002/jcla.23197
18. Wang D, Yu S, Cheng X, Cao L, Zhang H, Liu L, et al. Nationwide Chinese study for establishing reference intervals for thyroid hormones and related tests. Clin Chim Acta (2019) 496:62–7. doi: 10.1016/j.cca.2019.06.011
19. Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, et al. Age-related changes in thyroid function: A longitudinal study of a community-based cohort. J Clin Endocrinol Metab (2012) 97(5):1554–62. doi: 10.1210/jc.2011-3020
20. Cho NH, Choi HS, Kim KW, Kim HL, Lee SY, Choi SH, et al. Interaction between cigarette smoking and iodine intake and their impact on thyroid function. Clin Endocrinol (Oxf) (2010) 73(2):264–70. doi: 10.1111/j.1365-2265.2010.03790.x
21. Valeix P, Dos Santos C, Castetbon K, Bertrais S, Cousty C, Hercberg S. [Thyroid hormone levels and thyroid dysfunction of French adults participating in the su. Vi.Max Study]. Ann Endocrinol (Paris) (2004) 65(6):477–86. doi: 10.1016/s0003-4266(04)95956-2
22. Hubl W, Schmieder J, Gladrow E, Demant T. Reference intervals for thyroid hormones on the architect analyser. Clin Chem Lab Med (2002) 40(2):165–6. doi: 10.1515/cclm.2002.028
23. Mirjanic-Azaric B, Avram S, Stojakovic-Jelisavac T, Stojanovic D, Petkovic M, Bogavac-Stanojevic N, et al. Direct estimation of reference intervals for thyroid parameters in the republic of srpska. J Med Biochem (2017) 36(2):137–44. doi: 10.1515/jomb-2017-0008
24. Zou Y, Wang D, Cheng X, Ma C, Lin S, Hu Y, et al. Reference intervals for thyroid-associated hormones and the prevalence of thyroid diseases in the Chinese population. Ann Lab Med (2021) 41(1):77–85. doi: 10.3343/alm.2021.41.1.77
25. Wang Y, Zhang YX, Zhou YL, Xia J. Establishment of reference intervals for serum thyroid-stimulating hormone, free and total thyroxine, and free and total triiodothyronine for the beckman coulter dxi-800 analyzers by indirect method using data obtained from Chinese population in zhejiang province, China. J Clin Lab Anal (2017) 31(4):e22069. doi: 10.1002/jcla.22069
26. Marwaha RK, Tandon N, Ganie MA, Mehan N, Sastry A, Garg MK, et al. Reference range of thyroid function (Ft3, Ft4 and tsh) among Indian adults. Clin Biochem (2013) 46(4-5):341–5. doi: 10.1016/j.clinbiochem.2012.09.021
Keywords: thyroid hormones, reference intervals, age, sex, Chinese population
Citation: Xie T, Su M, Feng J, Pan X, Wang C and Tang T (2023) The reference intervals for thyroid hormones: A four year investigation in Chinese population. Front. Endocrinol. 13:1046381. doi: 10.3389/fendo.2022.1046381
Received: 16 September 2022; Accepted: 19 December 2022;
Published: 06 January 2023.
Edited by:
Loredana Pagano, University of Turin, ItalyReviewed by:
Marina Caputo, Università degli Studi del Piemonte Orientale, ItalyCopyright © 2023 Xie, Su, Feng, Pan, Wang and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tian Tang, dGFuZ3RpYW4xMjM0NUBhbGl5dW4uY29t; dGFuZ3RpYW4xMjM0NUBzY3UuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.