
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 17 January 2023
Sec. Thyroid Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1044655
 Magdalena Zgliczynska1*
Magdalena Zgliczynska1* Magdalena Ostrowska2
Magdalena Ostrowska2 Iwona Szymusik1
Iwona Szymusik1 Michal Ciebiera3
Michal Ciebiera3 Katarzyna Kosinska-Kaczynska1
Katarzyna Kosinska-Kaczynska1Background: The function of the thyroid gland during pregnancy undergoes physiological changes to ensure the proper amount of thyroid hormones for both the pregnant woman and the fetus. Multiple pregnancies (MP) are characterized by specific differences compared to single pregnancies, e.g., higher concentrations of human chorionic gonadotropin, which also affect thyroid function. The aim was to collect available knowledge on maternal thyroid function in MP.
Methods: We have systematically searched three databases: the PubMed/MEDLINE, Scopus and the Cochrane Library. The last search was run on the 4th of August 2022. We included full-text original observational and experimental studies written in English. Case reports, editorials, letters, conference abstracts, reviews and meta-analyses were excluded. No time criterion was established. Studies were considered eligible if at least one maternal thyroid function test was performed and reported. Studies on MP with a co-existing mole were excluded. The risk of bias was assessed with the use of the AXIS tool. The qualitative synthesis of evidence was applied.
Results: The search strategy resulted in the identification of 821 manuscripts. After removing duplicates, we screened the titles and abstracts of 552 articles, out of which 57 were selected for full-text analysis. Finally, 12 articles were included in the review. They were conducted in 6 different countries and published between the years 1997 and 2022. The number of examined women with MP ranged from 9 to 1 626.
Discussion and conclusions: Thyroid function differs between women with MP and SP. Scarce data are available on the topic, but MPs are most likely characterized by higher HCG levels, which influences thyroid-stimulating hormone and free thyroid hormone levels. These differences are mainly expressed in the 1st trimester of pregnancy. Separate population-based reference ranges are needed to correctly diagnose thyroid diseases in MP and to avoid unnecessary treatment. Further research is needed to fill the knowledge gaps.
The thyroid is an endocrine gland whose main function is the secretion of thyroid hormones, mainly triiodothyronine (T3) and thyroxine (T4). The above-mentioned thyroid hormones (TH) exert a wide range of effects in the human body, primarily acting as metabolism and growth regulators in almost all tissues. Their secretion is regulated by the thyroid-stimulating hormone (TSH), which is secreted by the anterior pituitary gland, whereas TSH secretion is further controlled by thyrotropin-releasing hormone (TRH) produced by the hypothalamus and by TH via negative feedback (1).
The function of the thyroid gland during pregnancy undergoes significant physiological changes to ensure the proper amount of TH for both the pregnant woman and the developing fetus (2). Importantly, TH takes part in the development of the fetal brain by regulating the migration, proliferation, differentiation and myelination of neuronal cells and the synapse formation (3). An extreme state of TH deficit caused by severe iodine deficiency during the prenatal period leads to cretinism, a symptom complex which is primarily manifested as severe intellectual disability (4). Preserving maternal euthyroidism is especially important in the first half of pregnancy (5). The fetal thyroid becomes fully functional around the 20th week of gestation, so the fetus remains completely dependent on maternal supply until that time (5, 6). Under normal conditions, several mechanisms contribute to the proper distribution of TH. Primarily, human chorionic gonadotropin (HCG) produced by the placental trophoblast, structurally similar to TSH by containing an identical alpha subunit (α-HCG) and a specific beta subunit (ß-HCG), acts as a weak agonist on the TSH receptor (TSHR) and becomes the main thyrotropic factor during the first trimester (7). It results in the so-called mirror image – a gradual decrease in TSH level in response to an increase in HCG concentrations, especially expressed in the 1st trimester (8). Conversely, high levels of estrogens contribute to an increase in thyroxine-binding globulin (TBG) levels, which results in a 50% rise in the total fraction of TH (9). Another regulatory mechanism is related to the activity of placental deiodinases, especially placental deiodinase type 3. This enzyme catalyzes the conversion of T4 and T3 to inactive metabolites: reverse triiodothyronine (rT3) and diiodothyronine (T2), which facilitates the regulation of fetal exposure to maternal TH (10). The above-described adjustments, along with an increased demand for iodine, might exacerbate maternal thyroid dysfunctions making them latent until that moment.
Various data are available on the incidence of intragestational thyroid diseases, especially as regards subclinical hypothyroidism, which is partially related to the adoption of different reference ranges (11). Notably, nowadays the topic seems so debatable that essential differences in care are pronounced not only between scientific societies but also between different clinical centers and individual healthcare providers (12). Consequently, it is increasingly postulated to use population-specific reference ranges for TSH and TH levels in pregnancy. It seems particularly important, since unnecessary treatment, especially thyrostatic, exposes the mother and the fetus to potential side effects of the therapy (13). Numerous factors may impact the reference values in different populations, primarily: body mass index, iodine supply, ethnicity, the presence of anti-thyroid antibodies. Numerous other factors may play a role, e.g., the presence of focal lesions in the thyroid gland (14, 15). Therefore, specific reference values are currently being developed by various scientific societies but they are still insufficiently widespread (14, 16, 17). Nevertheless, such studies most often excluded the population of patients with multiple pregnancies (MP) from further calculations since they were characterized by specific differences (18). In the context of thyroid function, these mostly include higher concentrations of HCG and estrogens (19). Moreover, given the increased metabolic requirements, women with MP may constitute a risk group as regards various nutritional deficiencies (20). It was also demonstrated that they were more likely to suffer from nausea and vomiting of pregnancy (NVP). However, the relationship between NVP and the deficiency of iodine, the most important micronutrient in the context of proper thyroid function, has not been fully elucidated yet (21–24). Therefore, it should be considered that disturbances in thyroid function in this particular group of pregnant women may occur more frequently which was demonstrated by Chen et al. on a large group of patients with twin pregnancies (TP). The study showed that TPs were associated with a higher risk of overt hyperthyroidism and isolated hypothyroxinemia in early pregnancy, but the risk of subclinical hypothyroidism was lower than in singleton pregnancy (SP). Later in pregnancy, the authors associated TP with a higher risk of subclinical hypothyroidism, isolated hypothyroxinemia and subclinical hyperthyroidism (25).
Considering the above, the aim of this systematic review is to gather currently available knowledge on maternal thyroid function in MP.
This systematic review was developed in correspondence with the updated Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement (PRISMA) dated from 2020 (26, 27). We have systematically searched three databases: the PubMed/MEDLINE, Scopus and the Cochrane Library. Articles found in the databases were considered regardless of the publication date. The last search was initiated on the 4th of August 2022. The detailed search design is presented in Table 1.
The search retrieved 821 manuscripts. We used the automatic duplicate identifier function built in EndNote X9 (Clarivate Analytics, London, UK). As a consequence, 222 duplicates were removed, while another 47 items were manually deleted. The remaining articles (599) were independently screened by two study authors. The next step included eligibility assessment that was performed by two other authors. We included full-text original observational and experimental studies written in English, and excluded case reports, editorials, letters to the editor, conference abstracts, as well as reviews and meta-analyses. Studies were considered eligible if at least one maternal thyroid function test was performed (for example TSH, FT3, FT4) and reported. MPs with a co-existing mole were excluded. Any disagreement which occurred between study authors was resolved through discussion in the presence of all other authors.
The entire selection process is presented in Figure 1 using the PRISMA 2020 flow diagram.
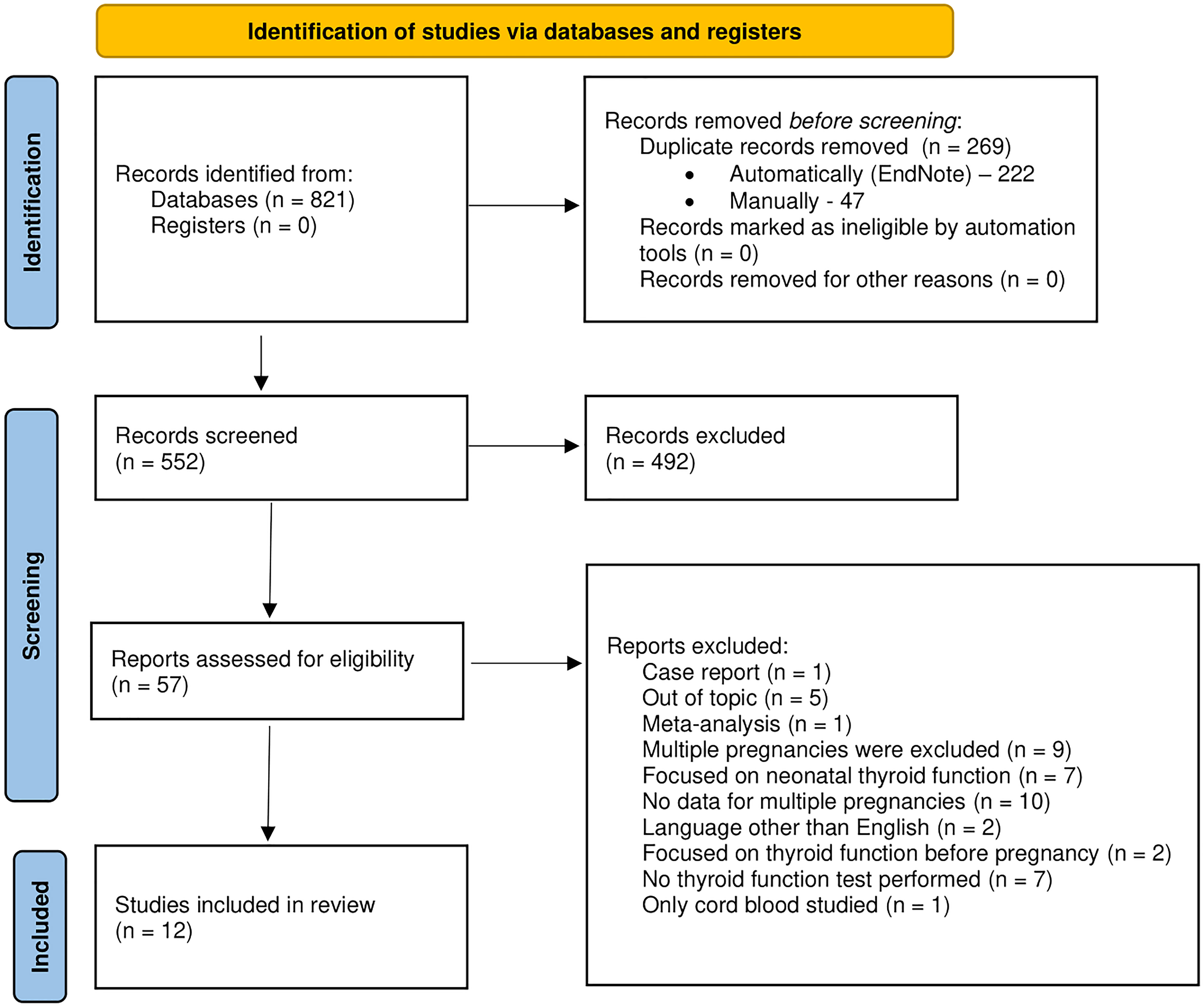
Figure 1 The PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only.
Using a custom-built data extraction form we sought the following information: the authors, year and country of origin, type of study, the main objective, population characteristics, obstetrical data, diagnostic tools and methods used, study timepoints and the results. Two study authors extracted the above-mentioned data from the selected full-text articles, while the third author double-checked their accuracy. No quantitative analyses were performed due to the significant diversity of the surveyed populations and research methodologies.
We attempted to contact the authors of several studies in order to obtain detailed information on their projects: Huang et al., Zhang et al., Liu et al., Rosner et al. and Chen et al. (25, 28–31). We have received answers from: Zhang et al. and Rosner et al. (29, 31).
The risk of bias was assessed with the use of the AXIS tool (32).
The implemented search strategy facilitated the identification of 12 eligible articles (25, 30, 31, 33–41). They were published between 1997 and 2022. At least 4330 women with TP and 48 women with MP were studied. As regards the selected studies: 3 were conducted in China (25, 30, 40), 2 in the United Kingdom (35, 37), 2 in the United States of America (31, 36), 2 in the Czech Republic (39, 41), 2 in Japan (34, 38) and 1 in Belgium (33). The number of examined women with MP ranged from 9 to 1 626 in individual studies. All the studies included TSH measurement results (25, 30, 31, 33–41), 11 out of 12 – FT4 results (25, 30, 31, 33–35, 37–41), and 2 out of 12 – FT3 results (34, 38). Half of the studies concurrently examined HCG and/or its subunits (25, 33–35, 37, 38). Only 2 studies included patients pregnant with more than two fetuses, with the maximum of 8 fetuses (34, 35). 4 studies aimed at establishing reference ranges for TP (36, 37, 39, 40).
The basic characteristics of the retrieved studies are presented in Table 2. A report on the methodology of laboratory examinations implemented in each study can be found in the Supplementary Materials (Supplementary Material 1).
Table 3 presents data on the levels on TSH and TH in MP.
TSH examination was performed in 11 out of the studies (25, 30, 31, 33–40). The comparison of TSH levels between SP and TP/MP in the 1st trimester of pregnancy was available in 8 studies. In 5 cases, a lower TSH level in the 1st trimester was found in SP compared to TP (25, 36, 37, 39, 40). Such a phenomenon was not confirmed by Grün et al., Sakaguchi et al. and Ogueh et al., but their studies were characterized by the lowest number of participants among the included papers (33–35). Interestingly, Ogueh et al. showed significantly lower TSH in pregnancies with more than two fetuses compared to SP, but not to TP (35). Regarding the remaining two trimesters, such data were available in articles authored by Grün et al., Sakaguchi et al., Ogueh et al., Dashe et al., and Chen et al. (25, 33–36). In this aspect, Chen et al. reported higher TSH in TP than in SP in the 3rd trimester of pregnancy, while the remaining authors failed to demonstrate statistically significant differences (25). As for other included studies, Hanaoka et al. did not report detailed results for TSH They only mentioned that twin-to-twin transfusion syndrome (TTTS) associated with elevated HCG levels might be the cause of the increase in TH concentrations along with the suppression of TSH (38). At this point, it should be mentioned that Ashoor et al. showed no difference in TSH between TTTS TP and normal TP (37). Rosner et al. and Liu et al. only reported the mean or median values of TSH. However, in our viewpoint, they should not be directly compared due to the variability of the populations and methods used, both laboratory and statistical (30, 31).
The results of at least one TH were reported in 10 out of 12 studies, while the comparison of TP/MP and SP was available in 7 studies (25, 30, 31, 33–35, 37, 38, 40, 41). In the 1st trimester, FT4 concentrations were statistically higher in TP than in SP according to Grün et al. (pooled, between 8-15 weeks), Jiang et al. (only between 7-12 GWs, not 4-6 GWs) and Chen et al. (25, 33, 40). Higher levels of TH in TP were not confirmed by Sakaguchi et al. (also for FT3), Ogueh et al., Ashoor et al. and Salek et al. (34, 35, 37, 41). Conversely, Ogueh et al. found higher FT4 concentrations in MP than in SP and TP (35). As for non-comparative studies, Hanaoka et al. reported a gradual decline in TH results after successful TTTS laser treatment, whereas Rosner et al. and Liu et al. provided only direct values as in case of TSH – see Table 3 (30, 31, 38).
Rosner et al., who studied patients with TP at the beginning of providing health care, reported the prevalence of overt hypothyroidism of 14% while assuming the upper limit of TSH at the level of 2.5 IU/mL and FT4 values proposed by the laboratory as reference (31). Chen et al., who adopted population-adjusted norms established in healthy SP (1st trimester: TSH 0.03-3.6 mU/l, FT4 11.7-19.8 pmol/L), confirmed overt hypothyroidism in 1.8% of cases, while subclinical hypothyroidism in 0.7% of cases. This group was the only one to examine women with TP in this aspect in the 3rd trimester, reporting the prevalence of overt hypothyroidism at the level of 1.3% and subclinical hypothyroidism at 8.2%. Interestingly, the occurrence of subclinical hypothyroidism was lower in TP than in SP in the 1st trimester, while in the 3rd trimester it was higher (25). Šálek et al. reported no cases of overt hypothyroidism in the studied group of 1st- trimester TP. However, they claimed a 6.4% prevalence of subclinical hypothyroidism in TP when adopting ranges based on TP population (TSH 0.02-2.95 mU/l, FT4 12.2-23.2 pmol/L). They demonstrated no significant difference between SP and TP. However, all cases of hypothyroidism in TP were subclinical, while 7.6% of SP ones were overt (41).
Chen et al. were the only authors who reported the incidence of isolated hypothyroxinemia and hyperthyroidism in TP. Thus, the percentage of isolated hypothyroxinemia was 4.7% in early TP and 3.3% in late TP, which in both cases it was significantly higher than in SP. Regarding hyperthyroidism, the subclinical form was present in 7.2% in early TP and 3.9% in late TP (more frequently than in SP), and the overt one occurred in 8.5% of early TP, which was more common than in SP. However, in late TP, it was diagnosed in only 0.5% and there was no significant difference compared to SP (25).
Table 4 presents a summary of 5 studies reporting thyroid autoimmunity in MP (25, 30, 37, 40, 41). The prevalence of autoimmunity reported in TP varied from 10.4% in the study by Liu et al. up to 15.7% in the study by Ashoor et al. (30, 37). Šálek et al. reported TPOAb (+) positivity in 71% of cases of hypothyroid TP compared to 52% in hypothyroid SP with no statistical significance of the difference (41). Ashoor et al. compared DC TP and MC TP regarding the ATA status. However, they found the difference to be in significant (37). This group of authors also reported higher 1st trimester TSH in ATA- positive TP, but FT4 and β-HCG were not different compared to ATA- negative subgroup (37).
Basing on the data review, the first conclusion that emerges is that there is still too little research-based knowledge concerning the problem. Fortunately, when analyzing the years when the papers were published, it may be concluded that the interest in the field has been growing recently with the increasing occurrence of MP. It is mainly due to the spread of assisted reproductive techniques (ART) and increasing maternal age. According to a study by Monden et al. who based on the data from 2010-2015 obtained from 165 countries, approximately 1.6 million pairs of twins were born annually, about 1 in 40 babies were born as a twin and the direct number of twin deliveries was higher than ever before (44, 45). MPs were found to develop more adverse outcomes than singletons, so it is especially important to act on any modifiable risk factor which could also include the function of the thyroid gland (46, 47). Based on the presented results, it may be concluded that multiple pregnancy may be associated with a decrease in TSH in the 1st trimester and a simultaneous increase in TH concentrations compared to SP, although some studies have failed to prove such differences. Based on a single, yet a large study by Chen et al., it may be concluded that thyroid function disorders are probably more common in women with TP/MP. However, none of the studies showed differences in the frequency of thyroid autoimmunity between SP and TP/MP. The obtained results and postulated mechanisms are summarized in Figure 2.
The analyzed studies were carried out mainly during the 1st trimester of pregnancy, which is probably related to the pathophysiology of MP. Namely, the pattern of HCG secretion is one of the main differences between SP and MP. Half of the studies included in the review focused on this aspect. The results of studies concerning the fluctuations of HCG and its subunit concentrations during pregnancy and its influence on the thyroid axis in MP are presented in Table 5.Grün et al., Ogueh et al. and Chen et al. reported a higher 1st trimester peak of HCG in TP/MP compared to SP (25, 33, 35). Conversely, Sakaguchi et al. did not report such a phenomenon. However, similarly to Ogueh et al., they observed that HCG remained higher in the 2nd and 3rd trimester compared to SP as well (34). Grün et al., who performed the measurements at the highest number of timepoints, noted that the increase in the concentration of dimeric HCG was not only higher, but also occurred earlier and lasted longer than in SP (33). Nevertheless, their study did not reveal statistically significant differences between SP and TP as regards HCG levels after the 20th week of gestation (33). Sakaguchi et al. and Ogueh et al. included pregnancies of a greater number of fetuses than twins and reported no differences in HCG levels between TP and multifetal pregnancies (34, 35). Studies in which both HCG and the β-HCG subunits were measured showed that they acted similarly throughout the pregnancy (34, 35). Ashoor et al. reported higher β-HCG in the 1st trimester of TP (37). α-HCG was found to act differently and increase gradually during pregnancy. However, Grün et al. only observed its higher concentration in MP at week 11 (33), whereas Sakaguchi et al. noted it in all trimesters (34).
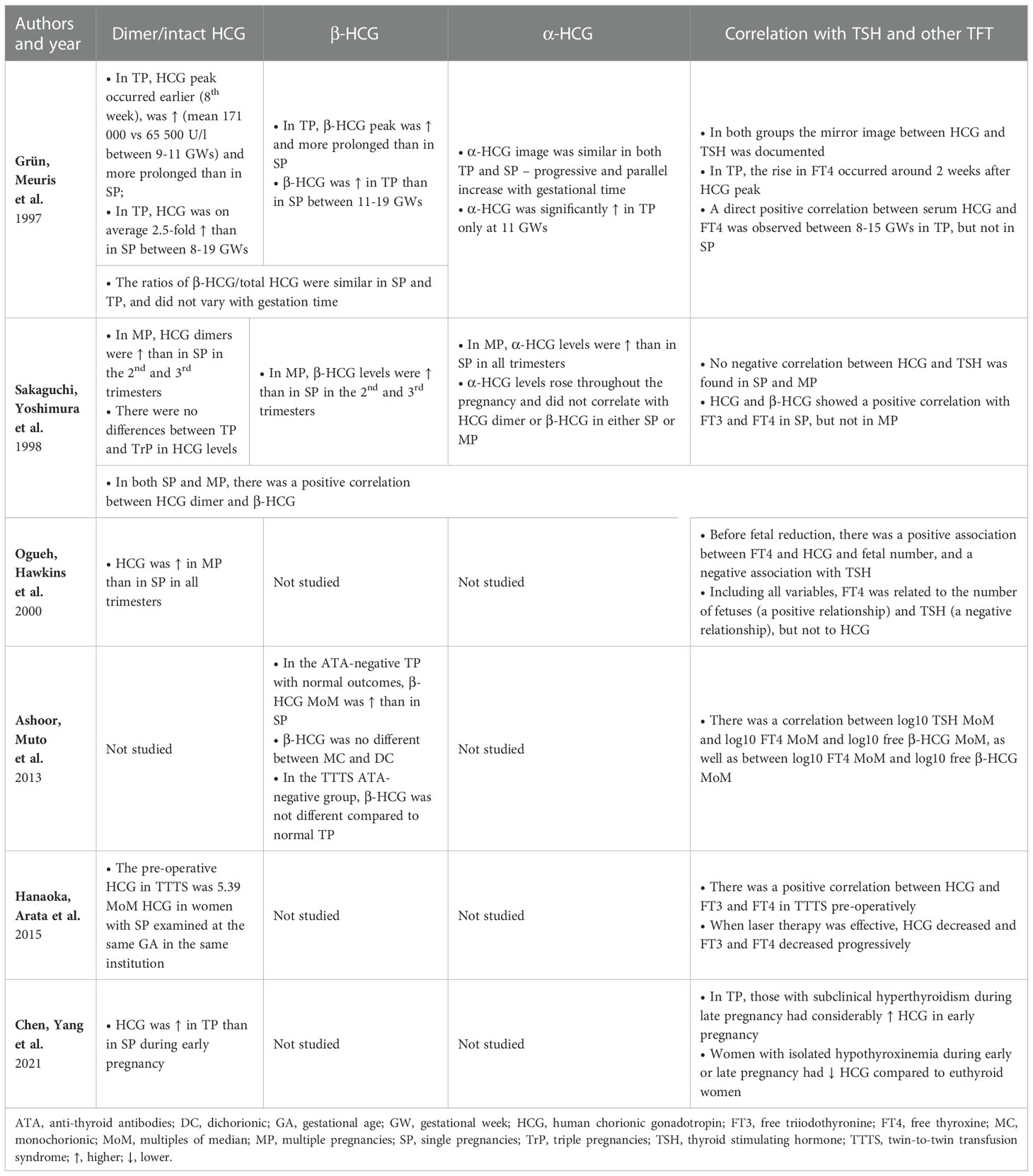
Table 5 Human chorionic gonadotropin, its subunits and its influence on the thyroid function in multiple pregnancies.
It is worth noting the actual HCG impact on hormones in the pituitary-thyroid axis. Lockwood et al. found TSH below ≤0.2 mIU/L in 67% of cases of women with HCG >200,000 IU/L and in all cases with HCG >400,000 IU/L. Elevated FT4 was noted in 32% of specimens with HCG concentrations >200,000 IU/L and in 80% with HCG >400,000 IU/L. However, only 6% of women with >200,000 IU/L had the symptoms of hyperthyroidism (48). In this study, 10% of women with HCG >200,000 IU/L were pregnant with twins, and the result of one of them exceeded 400,000 IU/L, but no separate results were reported for this group (48). As regards studies included in the review, Grün et al. discussed a case of transient hyperthyroidism in TP with HCG >200,000 IU/L which confirmed the hypothesis (33). Molar pregnancies with a co-existing live fetus are a remarkable example of the thyrotropic action of extremely high concentrations of HCG in MP. Molar pregnancies were not included in this review due to their different pathophysiology. Such patients face a considerable risk of developing severe hyperthyroidism constituting one of the main challenges for caregivers (49, 50).
According to the majority of studies included in the review, HCG/β-HCG concentrations were characterized by a negative relationship with the level of TSH and a positive relationship with the levels of free TH (33, 35, 37, 38). Sakaguchi et al. were the only ones who found no such correlation (34). Ogueh et al. also studied pregnancies with a higher fetal number than TP. However, after considering all factors, they suggested that the number of fetuses might be a more important factor regulating FT4 or TSH than the concentration of HCG itself, which was also supported by the delayed response of FT4 levels to HCG decrease (35). Grün et al. also demonstrated an approximately 2-week long delay in FT4 response to HCG, while Hanaoka et al. reported a 4-week long delay in TSH response to HCG (33, 38).
Notably, the role of ATA is another aspect in the thyroid response to the stimulating effect of HCG. Korevaar et al. conducted a study in a large group and found that HCG was positively associated with FT4 and negatively associated with TSH in TPOAb-negative women. However, as regards TPOAb-positive women, HCG was not associated with FT4 or TSH (51). Ashoor et al. demonstrated that TSH was higher in ATA-positive group, but FT4 and β-HCG were not different compared to ATA-negative group (37). It is possible that many more factors influence thyroid response to HCG. Based on the results of a study conducted by Koreevar et al. in a group of women with single pregnancies, higher maternal BMI, male fetal sex and maternal parity over 2 were found to be associated with lower thyroid response to HCG stimulation (52).
The authors of available studies indicated that MC TP might differ from DC in terms of HCG/β-HCG concentrations, but data regarding the direction of the differences are inconsistent (53–55). In this context, the aspect raised by Hanaoka et al., i.e., differences in HCG concentrations in TTTS, seems particularly interesting (38). The authors found HCG concentrations in TTTS patients to be higher than previously reported in TP (56, 57). Moreover, after successful laser treatment, HCG, FT3 and FT4 concentrations decreased. The postulated HCG increase mechanism was mostly due to placental hypoxia secondary to impaired blood flow triggered by polyhydramnios in the recipient (54, 58). Lamine et al. described a case of a 30-year-old woman pregnant with MC complicated by TTTS which resulted in severe hyperemesis gravidarum and hyperthyroidism that resolved after successful fetoscopic laser coagulation. In this case, the levels of HCG were markedly increased before and decreased after the procedure with the simultaneous fluctuations of the levels of free TH (58). Conversely, a study by Ashoor et al. did not confirm the association between TTTS and higher levels of free β-HCG or any differences related to chorionicity (37). The above data show that further exploration of differences in HCG secretion depending on chorionicity and TTTS or twin anemia-polycythemia syndrome (TAPS) could reveal interesting data.
The risk of overtreatment has risen in recent years due to the widespread use of levothyroxine in pregnant women. Sufficient and irrefutable evidence is available to conclude that it is advisable to treat overt hypothyroidism in pregnancy, since it is associated with abnormal neurodevelopment in the offspring, as well as an increased risk of fetal loss, preterm labor and many other pregnancy complications. Standard therapy was confirmed to improve the outcomes (16, 59). As regards the treatment of subclinical hypothyroidism, the matter remains controversial. Firstly, the fact that authors use different definitions of subclinical hypothyroidism in pregnancy constitutes a major problem with the interpretation of individual study results. Moreover, while some studies confirmed a positive impact of treatment on reducing the risk of pregnancy complications such as preterm delivery, a large randomized trial published in 2017 showed that treatment for subclinical hypothyroidism or hypothyroxinemia introduced between 8 and 20 weeks of gestation did not result in significantly better cognitive outcomes in 5- year -old children compared to no treatment (60–62). Similar results were provided by the CATS (Controlled Antenatal Thyroid Screening) and CATS II studies (63–65). However, it was postulated that treatment may have been initiated too late to prevent potential damage in such a case (59). At the same time the actual risk of overtreatment was confirmed by studies showing the so-called U-shaped maternal FT4 relationship with the child’s Intelligence Quotient and child’s gray matter and cortex volume as well as studies associating excessive FT4 with a higher prevalence of attention deficit hyperactivity disorder and behavioral difficulties in offspring (63, 66). Thus, the treatment of subclinical conditions is again becoming more controversial. It is even more difficult as they are only recognized on the basis of laboratory test results. Therefore, in order to diagnose them correctly, reference values must be developed in a reliable and individualized manner (67). Factors to be considered when developing reference ranges for specific populations are described in the American Thyroid Association Guidelines (59). They recommend the inclusion of women without thyroid diseases, with optimal iodine intake and a negative TPOAb status in the determination of reference intervals (59). However, it was also suggested that other factors may also significantly affect TFT, including body mass index, thyroid nodules, or ethnicity. Based on the evidence gathered, the authors of the present review suggest that the number of fetuses should also be taken into account. It is debatable whether different reference values should be used for women with MC than for women with DC TP. Previously, a hypothesis was proposed that women who became spontaneously pregnant with DC might have a different pituitary function from those with current or past singleton pregnancies (68). They were characterized by higher levels of gonadotropins, FSH in particular. They were also statistically taller, with earlier menarche and earlier menopause, which might also be related to a globally differently functioning pituitary (69–72). At present, these populations certainly differ in the frequency of conception through ART, although it was postulated that ART might have an impact on MC twinning, especially blastocyst transfer (73). Little reference was made to chorionicity in the papers included in the review. Ashoor et al. found no difference in the values of β-HCG, TSH and FT4 and ATA prevalence between DC and MC, but the population did not differ in terms of the frequency of assisted conception (51.3% DC and 48.3% MC) in their study (37). Rosner et al., who studied patients starting maternity care, reported no difference in overt hypothyroidism rates between MC and DC (31).
Our review showed that four groups of authors attempted at creating TP-specific reference ranges (36, 37, 39–41). The direct comparison of values is of limited importance here as the authors used different determination methodologies (Supplementary Material 1). However, it is worth mentioning that the lower limit of TSH near the end of the 1st trimester of TP was accounted for almost zero in all those cases, i.e., on the verge of the sensitivity of the method (36, 37, 39–41). According to Ashoor et al., the lowest upper limit of TSH was 2.35 mIU/L for Caucasian women with TP and 1.82 mIU/L for African women with TP, both in groups with BMIs <25 during the 11th week of TP (37). Interestingly, Jiang et al. who examined patients twice in the first 12 weeks of gestation noted significantly lower TSH values at 7-12 weeks compared to 4-6 weeks and suggested the potential need for separate references at that crucial time (40). Ashoor et al. provided separate reference ranges depending on the age, BMI and ethnicity of TP patients, which resulted in noticeable differences in values (37). Similar observations on the population of women with single pregnancies were also published by Laurberg et al. (74).
The problem has not been fully elucidated so far. Firstly, subsequent studies should be conducted to establish population-based reference ranges for each trimester for women with MP, including TP in the first place as the most common type. In our opinion, since individual countries differ in basic demographic parameters, ethnicity, iodine supply, fortification of food, thyroid disease profile and the frequency of autoimmune diseases, the reference ranges should at least be created at the national level. Nevertheless, such studies should also focus on the influence of thyroid function in MP on the final pregnancy outcomes, for example by assessing the frequency of maternal and fetal complications or children’s intellectual achievements. The difficulty in establishing such reference ranges is due to the fact that multiple pregnancy is by definition a pathological state. Normally, physiological mechanisms, such as the stimulation of the thyroid gland by HCG, may take the form of a pathology, given the excessive levels of this thyrotropic hormone. It would also be especially valuable to note the type of conception (ART or spontaneous) and chorionicity in studies assessing thyroid function in TP. Research should include information on the supply of iodine in a given population in the studied years and on the applied population-based measures to prevent deficiencies, e.g., salt fortification. Data should also be provided on autoimmunity, i.e., TPOAb, TgAb and TSHR antibodies. It would also be helpful to investigate whether the sera of women with twin pregnancies were more likely to produce laboratory errors, for example by cross-reacting with HCG.
Moreover, it would be valuable to establish reference parameters for fetal thyroid dimensions in TP as in case of SP, as well as to compare the results of children born from MP. According to some authors, congenital hypothyroidism was relatively common in children born from multiple pregnancies (75, 76). It would be interesting to explore potential general hormonal differences between women who conceive twins spontaneously and others in the context of the hypothalamic-pituitary-thyroid axis.
The advantage of the review is the novelty of the undertaken subject. To our knowledge, no similar systematic review has been conducted so far. The study discusses the theory, the results of available research and indicates future directions. However, it is not free from limitations. One of the main limitations is associated with the lack of the statistical synthesis of research results or meta-analysis. Nevertheless, in the opinion of the present authors, performing it in a reliable manner would be impossible. Firstly, the studies included in the review were carried out in different populations. Secondly, various laboratory methods were used, which is of great importance for the results obtained. Another limitation is related to the fact that the included studies lacked potentially relevant data. The authors rarely reported maternal age, BMI, parity or chorionicity. Moreover, the vast majority of studies only considered TP, so the results could not be extrapolated to other types of MP. None of the authors reported if antibodies against TSH receptors were tested, although they are the main factor differentiating gestational thyrotoxicosis from Graves’ disease. However, we do not expect that it is associated with high bias due to the rarity of pregnancy-onset disease (77). Therefore, the quality of the included studies is another limitation (see details in Supplementary Material 2). When evaluated with the AXIS tool, the lowest score achieved was 10 out of 20, while the highest was 16 out of 20, with the average of 14.5 ± 1.8. Noticeably more points were scored in newer papers, one reason for that being the possible gradual implementation of good clinical practice and appropriate reporting guidelines over time. The biggest problem was associated with the failure to report the phenomenon of non-respondents and the lack of the analysis of the group size as well as the strength of statistical tests. Regrettably, considering the prevalence of the phenomenon in the general population, one may have concerns about the sufficient power of the tests performed in studies involving few or over a dozen so patients. Furthermore, a substantial number of authors did not discuss the limitations of their own manuscripts. Furthermore, some of them focused on even more selected groups than MP, e.g., only MP conceived as a result of ART or only TP suffering from TTTS, which also certainly limits their representativeness for all MPs.
Women with MP seem to have a different thyroid function than women with SP. Currently, little research-based knowledge is available on the topic, but it seems that MPs are most likely characterized by higher HCG levels, which has an impact on TSH and free TH levels. Such differences are mainly expressed in the 1st trimester of pregnancy. Separate population-based reference ranges are needed to diagnose individual thyroid diseases in MP correctly and to avoid unnecessary treatment. More research is needed to fulfill the underlying knowledge gaps.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Conceptualization: MZ, KK-K. Methodology: MZ, MC. Investigation: MZ, MO, KK-K, IS. Resources: MZ, MO, IS. Abstracts screening: MZ, MC. Full-text screening: IS, KK-K. Data collection: MZ, MO. Data curation: MZ, MO, MC, KK-K. Risk of bias assessment: MZ. Writing—original draft preparation: MZ, KK-K, MO, MC. Writing—review and editing: KK-K, IS, MC. Supervision: KK-K, IS, MC. Project administration: MZ. Funding acquisition, KK-K, MC. All authors contributed to the article and approved the submitted version.
Center of Postgraduate Medical Education, Warsaw, Poland – grant number 501-999999-22.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1044655/full#supplementary-material
1. Beynon ME, Pinneri K. An overview of the thyroid gland and thyroid-related deaths for the forensic pathologist. Acad Forensic Pathol (2016) 6(2):217–36. doi: 10.23907/2016.024
2. Korevaar TIM, Medici M, Visser TJ, Peeters RP. Thyroid disease in pregnancy: new insights in diagnosis and clinical management. Nat Rev Endocrinol (2017) 13(10):610–22. doi: 10.1038/nrendo.2017.93
3. Drover SSM, Villanger GD, Aase H, Skogheim TS, Longnecker MP, Zoeller RT, et al. Maternal thyroid function during pregnancy or neonatal thyroid function and attention deficit hyperactivity disorder: A systematic review. Epidemiology. (2019) 30(1):130–44. doi: 10.1097/EDE.0000000000000937
4. Chen ZP, Hetzel BS. Cretinism revisited. Best Pract Res Clin Endocrinol Metab (2010) 24(1):39–50. doi: 10.1016/j.beem.2009.08.014
5. Lee SY, Cabral HJ, Aschengrau A, Pearce EN. Associations between maternal thyroid function in pregnancy and obstetric and perinatal outcomes. J Clin Endocrinol Metab (2020) 105(5):e2015–23. doi: 10.1210/clinem/dgz275
6. Jansen TA, Korevaar TIM, Mulder TA, White T, Muetzel RL, Peeters RP, et al. Maternal thyroid function during pregnancy and child brain morphology: a time window-specific analysis of a prospective cohort. Lancet Diabetes Endocrinol (2019) 7(8):629–37. doi: 10.1016/S2213-8587(19)30153-6
7. Mawet M, Nisolle M, Geenen V. Human chorionic gonadotropin and early embryogenesis: Review. Int J Mol Sci (2022) 23(3):1380. doi: 10.3390/ijms23031380
8. Hershman JM. The role of human chorionic gonadotropin as a thyroid stimulator in normal pregnancy. J Clin Endocrinol Metab (2008) 93(9):3305–6. doi: 10.1210/jc.2008-1461
9. Visser WE, Peeters RP. Interpretation of thyroid function tests during pregnancy. Best Pract Res Clin Endocrinol Metab (2020) 34(4):101431. doi: 10.1016/j.beem.2020.101431
10. Adu-Gyamfi EA, Wang YX, Ding YB. The interplay between thyroid hormones and the placenta: a comprehensive reviewdagger. Biol Reprod (2020) 102(1):8–17. doi: 10.1093/biolre/ioz182
11. Dong AC, Stagnaro-Green A. Differences in diagnostic criteria mask the true prevalence of thyroid disease in pregnancy: A systematic review and meta-analysis. Thyroid. (2019) 29(2):278–89. doi: 10.1089/thy.2018.0475
12. Maraka S, Mwangi R, Yao X, Sangaralingham LR, Singh Ospina NM, O'Keeffe DT, et al. Variation in treatment practices for subclinical hypothyroidism in pregnancy: US national assessment. J Clin Endocrinol Metab (2019) 104(9):3893–901. doi: 10.1210/jc.2019-00057
13. Lee SY, Pearce EN. Testing, monitoring, and treatment of thyroid dysfunction in pregnancy. J Clin Endocrinol Metab (2021) 106(3):883–92. doi: 10.1210/clinem/dgaa945
14. Ollero MD, Toni M, Pineda JJ, Martinez JP, Espada M, Anda E. Thyroid function reference values in healthy iodine-sufficient pregnant women and influence of thyroid nodules on thyrotropin and free thyroxine values. Thyroid. (2019) 29(3):421–9. doi: 10.1089/thy.2018.0324
15. Gietka-Czernel M, Glinicki P. Subclinical hypothyroidism in pregnancy: controversies on diagnosis and treatment. Pol Arch Intern Med (2021) 131(3):266–75. doi: 10.20452/pamw.15626
16. Hubalewska-Dydejczyk A, Trofimiuk-Muldner M, Ruchala M, Lewinski A, Bednarczuk T, Zgliczynski W, et al. Thyroid diseases in pregnancy: guidelines of the polish society of endocrinology [Choroby tarczycy w ciazy: zalecenia postepowania polskiego towarzystwa endokrynologicznego]. Endokrynol Pol (2021) 72(5):425–88. doi: 10.5603/EP.a2021.0089
17. Kostecka-Matyja M, Fedorowicz A, Bar-Andziak E, Bednarczuk T, Buziak-Bereza M, Dumnicka P, et al. Reference values for TSH and free thyroid hormones in healthy pregnant women in Poland: A prospective, multicenter study. Eur Thyroid J (2017) 6(2):82–8. doi: 10.1159/000453061
18. Korevaar TI, Steegers EA, Chaker L, Medici M, Jaddoe VW, Visser TJ, et al. The risk of preeclampsia according to high thyroid function in pregnancy differs by hCG concentration. J Clin Endocrinol Metab (2016) 101(12):5037–43. doi: 10.1210/jc.2016-2397
19. Povoa A, Xavier P, Matias A, Blickstein I. First trimester beta-hCG and estradiol levels in singleton and twin pregnancies after assisted reproduction. J Perinat Med (2018) 46(8):853–6. doi: 10.1515/jpm-2017-0132
20. Zgliczynska M, Kosinska-Kaczynska K. Micronutrients in multiple pregnancies-the knowns and unknowns: A systematic review. Nutrients. (2021) 13(2):386. doi: 10.3390/nu13020386
21. Mitsuda N, Eitoku M, Maeda N, Fujieda M, Suganuma N. Severity of nausea and vomiting in singleton and twin pregnancies in relation to fetal sex: The Japan environment and children's study (JECS). J Epidemiol. (2019) 29(9):340–6. doi: 10.2188/jea.JE20180059
22. Adalsteinsdottir S, Tryggvadottir EA, Hrolfsdottir L, Halldorsson TI, Birgisdottir BE, Hreidarsdottir IT, et al. Insufficient iodine status in pregnant women as a consequence of dietary changes. Food Nutr Res (2020) 6:64. doi: 10.29219/fnr.v64.3653
23. McMullan P, Hamill L, Doolan K, Hunter A, McCance D, Patterson C, et al. Iodine deficiency among pregnant women living in northern Ireland. Clin Endocrinol (Oxf). (2019) 91(5):639–45. doi: 10.1111/cen.14065
24. Medici M, Ghassabian A, Visser W, de Muinck Keizer-Schrama SM, Jaddoe VW, Visser WE, et al. Women with high early pregnancy urinary iodine levels have an increased risk of hyperthyroid newborns: the population-based generation r study. Clin Endocrinol (Oxf). (2014) 80(4):598–606. doi: 10.1111/cen.12321
25. Chen Z, Yang X, Zhang C, Ding Z, Zhang Y, Korevaar TIM, et al. Thyroid function test abnormalities in twin pregnancies. Thyroid. (2021) 31(4):572–9. doi: 10.1089/thy.2020.0348
26. Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (2021) 372:n160. doi: 10.1136/bmj.n160
27. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. (2021) 134:178–89. doi: 10.1016/j.jclinepi.2021.03.001
28. Huang N, Chen L, Lian Y, Wang H, Li R, Qiao J, et al. Impact of thyroid autoimmunity on In vitro Fertilization/Intracytoplasmic sperm injection outcomes and fetal weight. Front Endocrinol (Lausanne). (2021) 12:698579. doi: 10.3389/fendo.2021.698579
29. Zhang L, Yang X, Sun X, Xu Y, Xue Q, Shang J, et al. Influence of hypothyroidism on in vitro fertilization outcomes. Int J Clin Exp Med (2016) 9(2):4551–6.
30. Liu XS, Su XJ, Li GH, Huang SJ, Liu Y, Sun HX, et al. Maternal thyroid function and birth weight in twins. Endocrinology (2022) 163(8). doi: 10.1210/endocr/bqac082
31. Rosner JY, Fox NS, Saltzman D, Rebarber A, Gupta S. The effect of treated overt hypothyroidism on outcomes in twin pregnancies. Am J Perinatology. (2017) 34(14):1447–50. doi: 10.1055/s-0037-1604289
32. Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open (2016) 6(12):e011458. doi: 10.1136/bmjopen-2016-011458
33. Grün JP, Meuris S, De Nayer P, Glinoer D. The thyrotrophic role of human chorionic gonadotrophin (hCG) in the early stages of twin (versus single) pregnancies. Clin Endocrinol (Oxf). (1997) 46(6):719–25. doi: 10.1046/j.1365-2265.1997.2011011.x
34. Sakaguchi N, Yoshimura M, Nishikawa M, Yoshikawa N, Toyoda N, Yonemoto T, et al. Maternal thyroid function in multiple pregnancy: the variable thyrotropic activity of human chorionic gonadotropin. Horm Metab Res (1998) 30(11):689–93. doi: 10.1055/s-2007-978959
35. Ogueh O, Hawkins AP, Abbas A, Carter GD, Nicolaides KH, Johnson MR. Maternal thyroid function in multifetal pregnancies before and after fetal reduction. J Endocrinol (2000) 164(1):7–11. doi: 10.1677/joe.0.1640007
36. Dashe JS, Casey BM, Wells CE, McIntire DD, Byrd EW, Leveno KJ, et al. Thyroid-stimulating hormone in singleton and twin pregnancy: importance of gestational age-specific reference ranges. Obstet Gynecol. (2005) 106(4):753–7. doi: 10.1097/01.AOG.0000175836.41390.73
37. Ashoor G, Muto O, Poon LC, Muhaisen M, Nicolaides KH. Maternal thyroid function at gestational weeks 11-13 in twin pregnancies. Thyroid. (2013) 23(9):1165–71. doi: 10.1089/thy.2012.0537
38. Hanaoka M, Arata N, Sago H. Change of maternal thyroid function in twin-twin transfusion syndrome. Endocr J (2015) 62(10):949–52. doi: 10.1507/endocrj.EJ14-0509
39. Šálek T, Dhaifalah I, Langova D, Havalová J. Maternal thyroid-stimulating hormone reference ranges for first trimester screening from 11 to 14 weeks of gestation. J Clin Lab Anal (2018) 32(6):e22405. doi: 10.1002/jcla.22405
40. Jiang YX, Sun WJ, Zhang Y, Huang Y, Huang YY, Lu GZ, et al. Thyroid function of twin-pregnant women in early pregnancy. Chin Med J (Engl) (2019) 132(17):2033–8. doi: 10.1097/CM9.0000000000000381
41. Salek T, Dhaifalah I, Langova D, Havalova J. The prevalence of maternal hypothyroidism in first trimester screening from 11 to 14 weeks of gestation. BioMed Pap Med Fac Univ Palacky Olomouc Czech Repub. (2019) 163(3):265–8. doi: 10.5507/bp.2018.063
42. Zhang Y, Liu F, Sun W, Huang Y, Zhang W, Wang B, et al. [Establishment of reference ranges for thyroid-related indicators in normal pregnant women]. Zhonghua Yi Xue Za Zhi. (2016) 96(5):339–43. doi: 10.3760/cma.j.issn.0376-2491.2016.05.005
43. Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine status and prevalence of thyroid disorders after introduction of mandatory universal salt iodization for 16 years in China: A cross-sectional study in 10 cities. Thyroid. (2016) 26(8):1125–30. doi: 10.1089/thy.2015.0613
44. Monden C, Pison G, Smits J. Twin peaks: more twinning in humans than ever before. Hum Reprod (2021) 36(6):1666–73. doi: 10.1093/humrep/deab029
45. Human Multiple Births Database. French Institute for demographic studies - INED (distributor). Available at: https://www.twinbirths.org.
46. Rissanen AS, Jernman RM, Gissler M, Nupponen I, Nuutila ME. Maternal complications in twin pregnancies in Finland during 1987-2014: a retrospective study. BMC Pregnancy Childbirth. (2019) 19(1):337. doi: 10.1186/s12884-019-2498-x
47. Cheong-See F, Schuit E, Arroyo-Manzano D, Khalil A, Barrett J, Joseph KS, et al. Prospective risk of stillbirth and neonatal complications in twin pregnancies: systematic review and meta-analysis. BMJ (2016) 354:i4353. doi: 10.1136/bmj.i4353
48. Lockwood CM, Grenache DG, Gronowski AM. Serum human chorionic gonadotropin concentrations greater than 400,000 IU/L are invariably associated with suppressed serum thyrotropin concentrations. Thyroid. (2009) 19(8):863–8. doi: 10.1089/thy.2009.0079
49. Raj R, Uy EM, Hager M, Asadipooya K. Delivery of euthyroid baby following hyperthyroidism in twin gestation with coexisting complete hydatidiform mole. Case Rep Endocrinol (2019) 2019:2941501. doi: 10.1155/2019/2941501
50. Polonsky A, Olteanu I, Ben-David M, Mamet J, Agranat A, Fridman E. Viable triplet pregnancy coexisting with a complete molar pregnancy. Clin Case Rep (2016) 4(3):247–9. doi: 10.1002/ccr3.417
51. Korevaar TI, Steegers EA, Pop VJ, Broeren MA, Chaker L, de Rijke YB, et al. Thyroid autoimmunity impairs the thyroidal response to human chorionic gonadotropin: Two population-based prospective cohort studies. J Clin Endocrinol Metab (2017) 102(1):69–77. doi: 10.1210/jc.2016-2942
52. Korevaar TI, de Rijke YB, Chaker L, Medici M, Jaddoe VW, Steegers EA, et al. Stimulation of thyroid function by human chorionic gonadotropin during pregnancy: A risk factor for thyroid disease and a mechanism for known risk factors. Thyroid. (2017) 27(3):440–50. doi: 10.1089/thy.2016.0527
53. Prats P, Rodriguez I, Nicolau J, Comas C. Early first-trimester free-beta-hCG and PAPP-a serum distributions in monochorionic and dichorionic twins. Prenat Diagn. (2012) 32(1):64–9. doi: 10.1002/pd.2902
54. Fox CE, Pretlove SJ, Chan BC, Mahony RT, Holder R, Kilby MD. Maternal serum markers of placental damage in uncomplicated dichorionic and monochorionic pregnancies in comparison with monochorionic pregnancies complicated by severe twin-to-twin transfusion syndrome and the response to fetoscopic laser ablation. Eur J Obstet Gynecol Reprod Biol (2009) 144(2):124–9. doi: 10.1016/j.ejogrb.2009.02.049
55. Muller F, Dreux S, Dupoizat H, Uzan S, Dubin MF, Oury JF, et al. Second-trimester down syndrome maternal serum screening in twin pregnancies: impact of chorionicity. Prenat Diagn. (2003) 23(4):331–5. doi: 10.1002/pd.594
56. Drugan A, O'Brien JE, Dvorin E, Krivchenia EL, Johnson MP, Sokol RJ, et al. Multiple marker screening in multifetal gestations: failure to predict adverse pregnancy outcomes. Fetal Diagn Ther (1996) 11(1):16–9. doi: 10.1159/000264273
57. Neveux LM, Palomaki GE, Knight GJ, Haddow JE. Multiple marker screening for down syndrome in twin pregnancies. Prenat Diagn. (1996) 16(1):29–34. doi: 10.1002/(SICI)1097-0223(199601)16:1<29::AID-PD801>3.0.CO;2-K
58. Lamine F, Camponovo C, Baud D, Werner D, Marino L, Sykiotis GP. Relapse of human chorionic gonadotropin-induced hyperthyroidism and severe hyperemesis gravidarum secondary to twin-twin transfusion syndrome, with rapid recovery following fetoscopic laser coagulation: Case report. Front Endocrinol (Lausanne). (2021) 12:705567. doi: 10.3389/fendo.2021.705567
59. Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American thyroid association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid (2017) 27(3):315–89. doi: 10.1089/thy.2016.0457
60. Casey BM, Thom EA, Peaceman AM, Varner MW, Sorokin Y, Hirtz DG, et al. Treatment of subclinical hypothyroidism or hypothyroxinemia in pregnancy. N Engl J Med (2017) 376(9):815–25. doi: 10.1056/NEJMoa1606205
61. Nazarpour S, Ramezani Tehrani F, Simbar M, Tohidi M, Minooee S, Rahmati M, et al. Effects of levothyroxine on pregnant women with subclinical hypothyroidism, negative for thyroid peroxidase antibodies. J Clin Endocrinol Metab (2018) 103(3):926–35. doi: 10.1210/jc.2017-01850
62. Nazarpour S, Ramezani Tehrani F, Amiri M, Bidhendi Yarandi R, Azizi F. Levothyroxine treatment and pregnancy outcomes in women with subclinical hypothyroidism: a systematic review and meta-analysis. Arch Gynecol Obstet. (2019) 300(4):805–19. doi: 10.1007/s00404-019-05245-2
63. Hales C, Taylor PN, Channon S, McEwan K, Thapar A, Langley K, et al. Controlled antenatal thyroid screening II: Effect of treating maternal suboptimal thyroid function on child behavior. J Clin Endocrinol Metab (2020) 105(3). doi: 10.1210/clinem/dgz098
64. Hales C, Taylor PN, Channon S, Paradice R, McEwan K, Zhang L, et al. Controlled antenatal thyroid screening II: Effect of treating maternal suboptimal thyroid function on child cognition. J Clin Endocrinol Metab (2018) 103(4):1583–91. doi: 10.1210/jc.2017-02378
65. Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, et al. Antenatal thyroid screening and childhood cognitive function. N Engl J Med (2012) 366(6):493–501. doi: 10.1056/NEJMoa1106104
66. Korevaar TI, Muetzel R, Medici M, Chaker L, Jaddoe VW, de Rijke YB, et al. Association of maternal thyroid function during early pregnancy with offspring IQ and brain morphology in childhood: a population-based prospective cohort study. Lancet Diabetes Endocrinol (2016) 4(1):35–43. doi: 10.1016/S2213-8587(15)00327-7
67. Yang X, Meng Y, Zhang Y, Zhang C, Guo F, Yang S, et al. Thyroid function reference ranges during pregnancy in a large Chinese population and comparison with current guidelines. Chin Med J (Engl). (2019) 132(5):505–11. doi: 10.1097/CM9.0000000000000051
68. Milham S Jr. Pituitary gonadotrophin and dizygotic twinning. Lancet. (1964) 2(7359):566. doi: 10.1016/S0140-6736(64)90626-9
69. Corney G, Seedburgh D, Thompson B, Campbell DM, MacGillivray I, Timlin D. Multiple and singleton pregnancy: differences between mothers as well as offspring. Prog Clin Biol Res (1981) 69a:107–14.
70. Wyshak G. Reproductive and menstrual characteristics of mothers of multiple births and mothers of singletons only: a discriminant analysis. Prog Clin Biol Res (1981) 69a:95–105.
71. Wyshak G, Honeyman MS. Increased deaths due to endocrine system diseases and allergies among mothers of dizygotic twins. Metabolism. (1984) 33(4):375–8. doi: 10.1016/0026-0495(84)90202-6
72. Wyshak G. Menopause in mothers of multiple births and mothers of singletons only. Soc Biol (1978) 25(1):52–61. doi: 10.1080/19485565.1978.9988318
73. Mateizel I, Santos-Ribeiro S, Done E, Van Landuyt L, Van de Velde H, Tournaye H, et al. Do ARTs affect the incidence of monozygotic twinning? Hum Reprod (2016) 31(11):2435–41. doi: 10.1093/humrep/dew216
74. Laurberg P, Andersen SL, Hindersson P, Nohr EA, Olsen J. Dynamics and predictors of serum TSH and fT4 reference limits in early pregnancy: A study within the Danish national birth cohort. J Clin Endocrinol Metab (2016) 101(6):2484–92. doi: 10.1210/jc.2016-1387
75. Jiang X, Huang YL, Feng Y, Tang F, Jia XF, Chen QY, et al. Same-sex twins have a high incidence of congenital hypothyroidism and a high probability to be missed at newborn screening. Clin Chim Acta (2020) 502:111–5. doi: 10.1016/j.cca.2019.12.018
76. Gietka-Czernel M, Debska M, Kretowicz P, Debski R, Zgliczynski W. Fetal thyroid in two-dimensional ultrasonography: nomograms according to gestational age and biparietal diameter. Eur J Obstet Gynecol Reprod Biol (2012) 162(2):131–8. doi: 10.1016/j.ejogrb.2012.02.013
Keywords: thyroid, pregnancy, twin pregnancy, multiple pregnancy, human chorionic gonadotropin
Citation: Zgliczynska M, Ostrowska M, Szymusik I, Ciebiera M and Kosinska-Kaczynska K (2023) Maternal thyroid function in multiple pregnancies – a systematic review. Front. Endocrinol. 13:1044655. doi: 10.3389/fendo.2022.1044655
Received: 14 September 2022; Accepted: 28 December 2022;
Published: 17 January 2023.
Edited by:
George Paltoglou, National and Kapodistrian University of Athens, GreeceReviewed by:
Chen Zhang, Fudan University, ChinaCopyright © 2023 Zgliczynska, Ostrowska, Szymusik, Ciebiera and Kosinska-Kaczynska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Magdalena Zgliczynska, emdsaWN6eW5za2EubWFnZGFAZ21haWwuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.