
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Endocrinol. , 09 January 2023
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1043595
This article is part of the Research Topic Approaches to the Management of Weight Regain After Bariatric Surgery View all 5 articles
While bariatric surgery restults in significant long-term weight loss for most patients with obesity, post-surgical weight gain affects a considerable percentage of patients to varying degrees of severity. Furthermore, a small but significant percentage of patients experience inadequate post-surgical weight loss. Although many studies have examined the role of anti-obesity medications to address post-operative weight regain, an evidence-based consensus has not yet been achieved because of the heterogeneity of populations studied and the studies themselves. Observational studies in the post-bariatric surgery population consistently demonstrate the benefit of medical weight management after bariatric surgery, with most evidence highlighting liraglutide, topiramate, and phentermine/topiramate. New anti-obesity medications are anticipated to be helpful for post-surgical weight optimization given their efficacy in the non-surgical population.
Bariatric surgery has been demonstrated to achieve significant weight loss in patients with obesity. The sleeve gastrectomy (SG) and Roux-en-Y gastric bypass (RYGB) have historically been the most popular bariatric surgical procedures in the US and remained so in 2019 at 61.4% and 17.0% respectively, with bariatric revision of a prior procedure ranking 3rd most common at 16.7% (1). Multiple definitions of what constitutes significant post-bariatric weight regain and inadequate post-bariatric procedure weight loss have been used in the literature, which can make comparisons difficult. Nevertheless, the heterogeneous studies can inform an evidence-based approach for the evaluated groups of patients.
Various studies have reported on the magnitude, timing, and durability of weight loss following bariatric surgeries. The ongoing Longitudinal Assessment of Bariatric Surgery (LABS) prospective observational study examined 1406 individuals after RYGB who were followed up for 5 years or longer and found that weight regain (WR) occurred as early as within one year of RYGB after the nadir weight was achieved, with patients regaining a median of 26.8% of maximum weight loss at 5 years after surgery, or a median weight regain of 9.7% relative to their presurgical weight at 5 years after surgery (2). In the landmark prospective observational Swedish Obese Subjects (SOS) study, 2010 individuals who had undergone bariatric surgery were compared to contemporaneously matched control subjects with obesity (3, 4). The average maximal weight loss following gastric banding, vertical banded gastroplasty (VGB), and RYGB was 21-38% and occurred around 1-2 years post-procedure. Patients experienced gradual weight regain totaling 8-13% before reaching a weight loss plateau (WLP) at 8-10 years after the procedure. The largest observational study of bariatric surgery patients was conducted by Baig et al. who reviewed 9617 patient charts from 26 weight centers in India to characterize clinically significant post-bariatric WR at 5 years following SG, RYGB, and one-anastomosis gastric bypass (OAGB). Mean WR ranged from 6-22% at 5 years depending on type of bariatric surgery (5). The percentage of patients who experienced WR at 5 years following bariatric surgery ranged from 15.9% to 35.1% for SG, 5% to 14.6% for RYGB, and 1% to 3% for OAGB, with percentages varying depending on the definition used for WR in the study.
The LABS, SOS and Baig et al. studies highlight that WR following bariatric surgery is not uncommon and affects patients to varying degrees of severity depending on the type of bariatric surgery performed. WR can be a distressing experience for patients, and excess weight regain is associated with many diseases, such as diabetes, hypertension, hyperlipidemia, asthma, arthritis, depression, coronary heart disease, and various malignancies (6). Therefore, it is incumbent upon weight management providers to offer interventions to prevent post-bariatric surgery weight regain and optimize the weight status of such patients. Anti-obesity medications (AOMs) have been investigated in a variety of contexts related to type of bariatric surgery, the specific AOM or combination of AOM used, and the timing of AOM initiation and are evidence-based options for post-bariatric surgery weight optimization.
Medical management of obesity is recommended for individuals with a body mass index (BMI) of 30 kg/m2 or greater and for individuals with a BMI of 27 kg/m2 plus comorbidities (7). Pharmacologic agents can facilitate weight loss by opposing various physiologic mechanisms that contribute to obesity (8). Currently, five medications are FDA-approved for the long-term treatment of obesity: orlistat, phentermine/topiramate, liraglutide 3.0mg, naltrexone/bupropion, and semaglutide 2.4mg. Several medications are used off-label for long-term weight management: phentermine (>3 months), topiramate, liraglutide 1.8mg, naltrexone, bupropion, semaglutide 2.0mg, and tirzepatide. Their effectiveness in treating post-bariatric weight regain remains an area of active investigation.
Table 1 summarizes studies that evaluated the weight loss effect of AOMs in patients who underwent bariatric surgery. All results in Table 1 achieved statistical significance within referenced study unless otherwise specified.
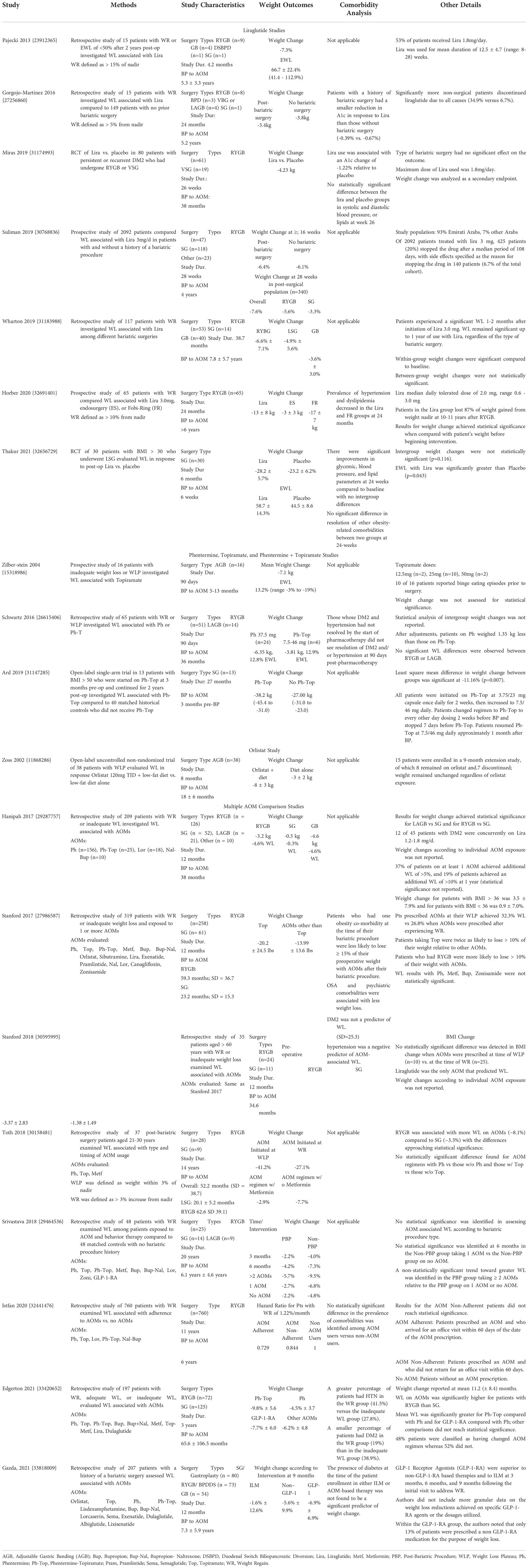
Table 1 Summary of Studies on Pharmacotherapies Aimed at Addressing Weight Gain After Bariatric Surgery.
Orlistat is an intestinal lipase inhibitor that reduces fat absorption by approximately 30% (9) FDA-approved in 1999 for the treatment of obesity, it is available as an over-the-counter medication at doses up to 60 mg three times per day and as a prescription at doses up to 120 mg three times per day. Orlistat was associated with weight loss in a non-randomized intervention study of 38 patients experiencing a weight loss plateau (WLP) after adjustable gastric banding, with a weight change of -8 ± 3 kg at 8 months compared to -3 ± 2 kg with dietary intervention alone (10). Although not assessed for statistical significance, patients on orlistat in this study reported improvement of constipation symptoms.
Phentermine is a sympathomimetic that suppresses appetite via central neural pathways (11). Topiramate is an anti-epileptic that also has central anorexigenic effects with benefits demonstrated in binge eating disorder (12, 13). The combination phentermine/topiramate was FDA-approved for the treatment of obesity in 2012. Phentermine, topiramate, and phentermine/topiramate benefit from a large body of evidence supporting their use in patients with a history of bariatric surgery (14–18). In the largest retrospective study of 319 patients, topiramate was associated with the greatest weight change (-20.2lbs) after RYGB or SG as compared to 15 other AOMs (-13.99 lbs. for AOMs other than topiramate) (17). However, this signal for topiramate was not observed in a subgroup of patients ages 21-30 years (19). A similar study of 197 post-surgical patients reported that phentermine/topiramate was associated with the greatest odds of achieving 5, 10, and 15% weight loss compared to other AOMs (20). Adherence to topiramate, phentermine, or combination phentermine/topiramate has been consistently associated with greater weight loss (18). Among 16 patients with binge eating disorder and a history of bariatric surgery, topiramate 12.5-50mg per day was associated with an additional excess weight loss of 13.7% (14). In a retrospective study of 30 patients, when phentermine 37.5mg was compared with phentermine-topiramate 7.5-46mg, both produced significant weight losses of 6.3 kg and 3.8 kg, respectively, over 90 days, and phentermine 37.5mg was statistically superior (15). Finally, in patients with a BMI > 50 who underwent laparoscopic sleeve gastrectomy, initiation of phentermine-topiramate 7.5-46 mg at 3 months pre-operatively was found to facilitate superior weight loss results relative to historically matched controls who did not receive phentermine-topiramate 7.5-46 mg (16).
Liraglutide is a glucagon-like-peptide-1 receptor agonist (GLP-1-RA) and is FDA-approved for type 2 diabetes as liraglutide 1.8mg and for obesity as liraglutide 3.0mg. Benefit of liraglutide in the post-bariatric surgery setting is supported by a randomized controlled trial (21) and multiple observational studies (22–27). The randomized double-blind placebo-controlled trial, which was performed to investigate the effect of liraglutide 1.8mg for the management of patients with a history of bariatric surgery and persistent or recurrent type 2 diabetes, reported as a secondary endpoint a statistically significant mean weight change of -4.23 kg with liraglutide vs. placebo at 26 weeks (21). Of the observational studies, the largest included 787 patients treated with liraglutide 3.0 mg for ≥ 16 weeks and demonstrated a weight change of -6.4% for patients with a history of bariatric surgery versus -6.1% for patients without a history of bariatric surgery (24). Use of liraglutide in patients with a history of bariatric surgery has also been associated with improvements in blood pressure (25) and hemoglobin A1c (21, 26). In comparing the effect of liraglutide 3.0 mg in patients with different bariatric surgeries, those who had a history of RYGB lost significantly more weight (-5.6%) than patients who had a history of SG (-3.3%) (24); similar results have been replicated (-6.6% with RYGB vs. -3.6% with SG) but were not found to be statistically significant (25). Furthermore, there were significantly fewer discontinuations of liraglutide in patients with a history of bariatric surgery compared to those without a history of bariatric surgery (26).
Naltrexone is an opioid receptor antagonist that inhibits the auto-inhibition of anorexigenic neurons in the hypothalamus (28). Bupropion is a dopamine and norepinephrine reuptake inhibitor that can suppress appetite (28). The combination naltrexone/bupropion was FDA-approved for the treatment of obesity in 2014. There are no studies evaluating the individual efficacies of naltrexone, bupropion, or naltrexone/bupropion specifically in the post-bariatric surgery population. A few studies have investigated the effectiveness of obesity pharmacotherapy in general, in which monotherapies naltrexone or bupropion and combination naltrexone/bupropion were minimally represented (17, 29, 30). The strongest body of evidence for weight loss efficacy stems from phase 3 randomized controlled trials of combination naltrexone/bupropion, from which individuals with a history of bariatric surgery were excluded, but demonstrated about 5% placebo-subtracted weight loss at one year (31, 32).
Semaglutide is a GLP-1-RA that is FDA-approved for type 2 diabetes as semaglutide 2.0mg and for obesity as semaglutide 2.4mg. There are no studies evaluating semaglutide specifically in the post-bariatric surgery population. Several phase 3 randomized controlled trials, which excluded individuals with a history of bariatric surgery, proved superior weight loss with semaglutide 2.4mg compared to placebo in individuals with obesity or metabolically complicated overweight: 12.4% in general (33), 11.1% in East Asian populations (34), 6.2% in individuals with type 2 diabetes (35), and 13.3% in the setting of intensive lifestyle modification (36). A double-blinded, randomized, placebo-controlled trial of semaglutide 2.4mg in patients with inadequate weight loss following bariatric surgery is underway (BARI-STEP) (37).
Tirzepatide is a dual GLP-1-RA and gastric inhibitory peptide receptor agonist (GIP-RA). It is FDA-approved for the treatment of type 2 diabetes. There are no studies evaluating tirzepatide specifically in the post-bariatric surgery population. However, a recent phase 3 trial, which excluded individuals with a history of bariatric surgery, demonstrated weight loss efficacy approaching that of some bariatric surgeries. In a double-blind, randomized, controlled trial of 2539 adults with obesity (BMI ≥ 30) or medically complicated overweight (BMI ≥ 27), tirzepatide 15mg weekly resulted in 20.9% weight loss over 72 weeks compared to 3.1% weight loss with placebo (38). More than half of participants on tirzepatide 15 mg achieved weight loss of ≥ 20%: 57% vs. 3% with placebo. Reduction in body weight of ≥ 25% was observed in 36% of participants on tirzepatide 15 mg vs. 1.5% on placebo.
Retrospective studies have investigated weight loss associated with obesity pharmacotherapy in general among patients with a history of bariatric surgery without identifying specific AOM monotherapies or AOM combination therapies. A trend toward greater weight loss has been observed with two or more AOMs compared to patients taking zero or one AOM (29). GLP-1-RAs were found to produce significantly greater weight loss than regimens with a non-GLP-1-RA or intensive lifestyle modification alone (30). Among 37 patients ages 21-30 years, AOM regimens sans metformin were found to be more effective than AOM regimens with metformin (19).
Other studies identified factors associated with greater weight loss in the population of patients with medically managed obesity after bariatric surgery. Greater weight loss results were observed with use of AOMs for patients with a history of laparoscopic adjustable gastric band vs. SG (39) and RYGB vs. SG (20, 39, 40), for patients with pre-operative BMI > 36 vs. those with BMI < 36 (39), and for initiation of AOM at the weight loss plateau rather than waiting until weight regain (17, 19, 20, 40).
There are no official guidelines on perioperative weight optimization in patients undergoing bariatric surgery, and there is no consensus on the pharmacologic management of weight regain, inadequate weight loss, or weight loss plateau after bariatric surgery. Whether initiation of AOM preoperatively has long-term benefits to mitigate these post-operative weight issues is unknown. When initiated post-operatively, the choice of which AOM to use for weight optimization is an important component in the long-term care of patients with obesity.
There is a paucity of data available to guide pharmacotherapeutic weight management decisions before bariatric surgery and whether this impacts the sustainability of clinically significant weight loss post-operatively. Weight loss in the pre-operative period has proven benefits, including a reduction in the likelihood of post-operative complications after RYGB (41, 42) but not SG (43). However, there was no effect seen in long-term weight loss outcomes. Notably, these studies did not include patients with pre-surgical weight loss using AOMs. Only one study, conducted in patients with BMI > 50, investigated this question of pre-operative weight optimization and demonstrated significantly greater post-operative weight loss at 24 months with pre-operative phentermine/topiramate 7.5-46mg daily (16). Although post-operative weight loss outcomes associated with using other AOMs before bariatric surgery have not been assessed, other AOMs should still be considered if phentermine/topiramate is contraindicated or if an obesity-associated co-morbidity can be concomitantly addressed (e.g., GLP-1-RA in a patient with obesity and diabetes). It is reasonable for providers to prescribe AOMs in the pre-operative period given the potential to improve long-term weight loss outcomes following bariatric surgery.
Weight regain after bariatric surgery most often begins 1-2 years post-operatively (3). Patients should monitor their weight changes and follow up with their weight management providers at regular intervals after bariatric surgery, as the timing of weight regain or WLP will vary. When a patient reaches a WLP, providers should assess for any factors responsible for preventing additional weight loss, such as dietary changes, initiation of an obesogenic medication, or a post-operative anatomic etiology (e.g., gastro-gastric fistula). The period when patients reach a weight loss plateau represents an opportunity to intervene, with or without an AOM, to address obesogenic factors. Several studies have suggested that greater weight loss was achieved when AOMs were initiated at the weight loss plateau rather than waiting for weight regain (17, 19, 40), with one study demonstrating statistical significance (20). If no clear explanation for the WLP is discovered that can be managed by other means (e.g., endoscopic or surgical revision of a gastro-gastric fistula), AOM initiation should be considered at the time of WLP to promote further weight loss and prevent weight regain.
Of all AOMs, liraglutide, topiramate, and phentermine/topiramate are the pharmacologic agents best supported with current observational studies. Use of GLP-1-RAs for weight optimization after bariatric surgery will likely gain popularity given evidence in the post-surgical population (30) and the proven weight loss efficacy of newer agents in the non-surgical population with obesity. Semaglutide and tirzepatide are the newest additions to the family of GLP-1-RAs, with the latter being a dual GLP-1 and GIP receptor agonist. However, there are no publications to date describing the efficacy of semaglutide or tirzepatide in patients who have undergone bariatric surgery. Pharmacotherapies with gut peptide modulators are emerging as the most effective AOMs (44, 45), with the most recent addition, tirzepatide 15 mg, demonstrating about 20% average weight loss, greater than lap band but less than sleeve gastrectomy and gastric bypass (38). Despite the absence of data detailing the use of these newer agents after bariatric surgery, the existing GLP-1-RA data in this setting and the substantial weight loss seen in non-surgical patients with obesity using semaglutide 2.4mg and tirzepatide suggest that these agents will likely provide more post-operative weight loss than prior GLP-1-RAs.
Medical co-morbidities may influence the choice of on- or off-label AOM used for weight optimization after bariatric surgery. In addition to significant weight loss, semaglutide has proven systemic benefits in the non-surgical population (46–50), whereas tirzepatide trials are still ongoing. Semaglutide would be an optimal AOM for patients with post-surgical weight regain, WLP, or inadequate weight loss who also have a history of a stroke, coronary artery disease, non-alcoholic fatty liver disease, or type 2 diabetes. Tirzepatide is FDA-approved for individuals with type 2 diabetes (51) and may be cardioprotective (52), pending official results of its cardiovascular outcome trial (53). As another example, patients who also experience migraine headaches may benefit from topiramate given its FDA-approval as a migraine prophylaxis.
Contraindications should also inform the choice of AOM used for weight optimization after bariatric surgery. Topiramate or phentermine/topiramate are options in patients who have a contraindication to GLP-1-RAs (e.g., personal or family history of medullary thyroid carcinoma) or who have poorly controlled gastroesophageal reflux disease, which is a common concern after sleeve gastrectomy. For patients who are not candidates for GLP-1-RAs and have a contraindication to topiramate (e.g., nephrolithiasis) and/or phentermine (e.g., uncontrolled hypertension), monotherapy bupropion or bupropion/naltrexone might be considered, though little data exists regarding its efficacy after bariatric surgery.
The use of an AOM may not be appropriate or effective for all patients with weight regain, inadequate weight loss, or WLP following bariatric surgery. If a patient’s unexpected post-operative weight trajectory is due to an anatomic etiology, surgical or endoscopic revision may be indicated. After SG and RYGB, revisional bariatric surgery has become the third most common type of bariatric surgery performed in the Unites States (54), with weight regain as the most common indication for revision (55). Revisional surgeries include conversion to a different bariatric operation (e.g., gastric band to SG), re-sleeve after SG, or prolongation of the biliopancreatic limb after RYGB. Endoscopic options for revision include a transoral outlet reduction (TORe) and revisional endoscopic sleeve gastroplasty (ESG), which result in approximately 10-15% additional weight loss at 1 year after revision (56, 57). A TORe aims to decrease the diameter of a dilated gastro-jejunal anastomosis using an endoscopic suture device. Endoscopic suturing is also used during the revisional ESG to reduce the gastric volume in patients with a dilated gastric pouch after SG.
In patients with a history of bariatric surgery, the presentations of weight regain, weight loss plateau, or inadequate weight loss are opportunities for weight optimization. Observational studies have consistently demonstrated the effectiveness of AOMs in treating these post-operative weight issues, with liraglutide, topiramate, and phentermine/topiramate having the best supporting evidence among all AOMs. The future of perioperative weight optimization in patients undergoing bariatric surgery is an increasingly empowered field due to the availability of on- and off-label AOMs, evolution of gut peptide modulators as pharmacotherapy, and the development of endoscopic interventions.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
EL: Created first draft and performed edits according to feedback from senior authors OS: Contributed to second draft, primarily within Expect Opinion and Conclusion sections BT: Provided feedback and guidance on all drafts LA: Provided feedback and guidance on all drafts. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. American Society for Metabolic and Bariatric Surgery. Estimate of bariatric surgery numbers, 2011-2020. Available at: https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers.
2. King WC, Hinerman AS, Belle SH, Wahed AS, Courcoulas AP. Comparison of the performance of common measures of weight regain after bariatric surgery for association with clinical outcomes. JAMA (2018) 320(15):1560–9. doi: 10.1001/jama.2018.14433
3. Sjöström L. Review of the key results from the Swedish obese subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med (2013) 273(3):219–34. doi: 10.1111/joim.12012
4. Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med (2004) 351(26):2683–93. doi: 10.1056/NEJMoa035622
5. Baig SJ, Priya P, Mahawar KK, Shah S. Indian Bariatric surgery outcome reporting (IBSOR) group. weight regain after bariatric surgery-a multicentre study of 9617 patients from Indian bariatric surgery outcome reporting group. Obes Surg (2019) 29(5):1583–92. doi: 10.1007/s11695-019-03734-6
6. Apovian CM. Obesity: Definition, comorbidities, causes, and burden. Am J Manag Care (2016) 22(7 Suppl):s176–85.
7. Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: A report of the American college of Cardiology/American heart association task force on practice guidelines and the obesity society. J Am Coll Cardiol (2014) 63(25 Pt B):2985–3023. doi: 10.1016/j.jacc.2013.11.004
8. Schwartz MW, Seeley RJ, Zeltser LM, Drewnowski A, Ravussin E, Redman LM, et al. Obesity pathogenesis: An endocrine society scientific statement. Endocr Rev (2017) 38(4):267–96. doi: 10.1210/er.2017-00111
9. Roche Pharmaceuticals. Xenical package insert (2009). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020766s026lbl.pdf.
10. Zoss I, Piec G, Horber FF. Impact of orlistat therapy on weight reduction in morbidly obese patients after implantation of the Swedish adjustable gastric band. Obes Surg (2002) 12(1):113–7. doi: 10.1381/096089202321144685
11. Teva Pharmaceuticals USA. ADIPEX-p package insert (2012). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/085128s065lbl.pdf.
12. Verrotti A, Scaparrotta A, Agostinelli S, Di Pillo S, Chiarelli F, Grosso S. Topiramate-induced weight loss: A review. Epilepsy Res (2011) 95(3):189–99. doi: 10.1016/j.eplepsyres.2011.05.014
13. McElroy SL, Hudson JI, Capece JA, Beyers K, Fisher AC, Rosenthal NR, et al. Topiramate for the treatment of binge eating disorder associated with obesity: A placebo-controlled study. Biol Psychiatry (2007) 61(9):1039–48. doi: 10.1016/j.biopsych.2006.08.008
14. Zilberstein B, Pajecki D, Garcia de Brito AC, Gallafrio ST, Eshkenazy R, Andrade CG. Topiramate after adjustable gastric banding in patients with binge eating and difficulty losing weight. Obes Surg (2004) 14(6):802–5. doi: 10.1381/0960892041590926
15. Schwartz J, Chaudhry UI, Suzo A, Durkin N, Wehr AM, Foreman KS, et al. Pharmacotherapy in conjunction with a diet and exercise program for the treatment of weight recidivism or weight loss plateau post-bariatric surgery: A retrospective review. Obes Surg (2016) 26(2):452–8. doi: 10.1007/s11695-015-1979-x
16. Ard JD, Beavers DP, Hale E, Miller G, McNatt S, Fernandez A. Use of phentermine-topiramate extended release in combination with sleeve gastrectomy in patients with BMI 50 kg/m(2) or more. Surg Obes Relat Dis (2019) 15(7):1039–43. doi: 10.1016/j.soard.2019.04.017
17. Stanford FC, Alfaris N, Gomez G, Ricks ET, Shukla AP, Corey KE, et al. The utility of weight loss medications after bariatric surgery for weight regain or inadequate weight loss: A multi-center study. Surg Obes Relat Dis (2017) 13(3):491–500. doi: 10.1016/j.soard.2016.10.018
18. Istfan NW, Anderson WA, Hess DT, Yu L, Carmine B, Apovian CM. The mitigating effect of phentermine and topiramate on weight regain after roux-en-Y gastric bypass surgery. Obes (Silver Spring) (2020) 28(6):1023–30. doi: 10.1002/oby.22786
19. Toth AT, Gomez G, Shukla AP, Pratt JS, Cena H, Biino G, et al. Weight loss medications in young adults after bariatric surgery for weight regain or inadequate weight loss: A multi-center study. Children (Basel) (2018) 5(9):116. doi: 10.3390/children5090116
20. Edgerton C, Mehta M, Mou D, Dey T, Khaodhiar L, Tavakkoli A. Patterns of weight loss medication utilization and outcomes following bariatric surgery. J Gastrointest Surg (2021) 25(2):369–77. doi: 10.1007/s11605-020-04880-4
21. Miras AD, Pérez-Pevida B, Aldhwayan M, Kamocka A, McGlone ER, Al-Najim W, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol (2019) 7(7):549–59. doi: 10.1016/S2213-8587(19)30157-3
22. Horber FF, Steffen R. Reversal of long-term weight regain after roux-en-Y gastric bypass using liraglutide or surgical revision. a prospective study. Obes Surg (2021) 31(1):93–100. doi: 10.1007/s11695-020-04856-y
23. Pajecki D, Halpern A, Cercato C, Mancini M, de Cleva R, Santo MA. Short-term use of liraglutide in the management of patients with weight regain after bariatric surgery. Rev Col Bras Cir (2013) 40(3):191–5. doi: 10.1590/S0100-69912013000300005
24. Suliman M, Buckley A, Al Tikriti A, Tan T, le Roux CW, Lessan N, et al. Routine clinical use of liraglutide 3 mg for the treatment of obesity: Outcomes in non-surgical and bariatric surgery patients. Diabetes Obes Metab (2019) 21(6):1498–501. doi: 10.1111/dom.13672
25. Wharton S, Kuk JL, Luszczynski M, Kamran E, Christensen RAG. Liraglutide 3.0 mg for the management of insufficient weight loss or excessive weight regain post-bariatric surgery. Clin Obes (2019) 9(4):e12323. doi: 10.1111/cob.12323
26. Gorgojo-Martínez JJ, Feo-Ortega G, Serrano-Moreno C. Effectiveness and tolerability of liraglutide in patients with type 2 diabetes mellitus and obesity after bariatric surgery. Surg Obes Relat Dis (2016) 12(10):1856–63. doi: 10.1016/j.soard.2016.02.013
27. Thakur U, Bhansali A, Gupta R, Rastogi A. Liraglutide augments weight loss after laparoscopic sleeve gastrectomy: A randomised, double-blind, placebo-control study. Obes Surg (2021) 31(1):84–92. doi: 10.1007/s11695-020-04850-4
28. Takeda Pharmaceuticals America. Contrave package insert (2014). Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/200063s000lbl.pdf.
29. Srivastava G, Buffington C. A specialized medical management program to address post-operative weight regain in bariatric patients. Obes Surg (2018) 28(8):2241–6. doi: 10.1007/s11695-018-3141-z
30. Gazda CL, Clark JD, Lingvay I, Almandoz JP. Pharmacotherapies for post-bariatric weight regain: Real-world comparative outcomes. Obes (Silver Spring) (2021) 29(5):829–36. doi: 10.1002/oby.23146
31. Greenway FL, Fujioka K, Plodkowski RA, Mudaliar S, Guttadauria M, Erickson J, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-i): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2010) 376(9741):595–605. doi: 10.1016/S0140-6736(10)60888-4
32. Apovian CM, Aronne L, Rubino D, Still C, Wyatt H, Burns C, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obes (Silver Spring) (2013) 21(5):935–43. doi: 10.1002/oby.20309
33. Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med (2021) 384(11):989–1002. doi: 10.1056/NEJMoa2032183
34. Kadowaki T, Isendahl J, Khalid U, SY L, Nishida T, Ogawa W, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): A randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. Lancet Diabetes Endocrinol (2022) 10(3):193–206. doi: 10.1016/S2213-8587(22)00008-0
35. Davies M, Færch L, Jeppesen OK, Pakseresht A, Pedersen SD, Perreault L, et al. Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): A randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet (2021) 397(10278):971–84. doi: 10.1016/S0140-6736(21)00213-0
36. Wadden TA, Bailey TS, Billings LK, Davies M, Frias JP, Koroleva A, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: The STEP 3 randomized clinical trial. JAMA (2021) 325(14):1403–13. doi: 10.1001/jama.2021.1831
37. ClinicalTrials.gov. Semaglutide 2.4 mg in patients with poor weight-loss (BARI-STEP). Betheseda (MD: National Library of Medicine (US. Available at: https://clinicaltrials.gov/ct2/show/NCT05073835 (Accessed Aug 23, 2022). NCT05073835.
38. Jastreboff AM, Aronne LJ, Ahmad NN, Wharton S, Connery L, Alves B, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med (2022) 387(3):205–16. doi: 10.1056/NEJMoa2206038
39. Nor Hanipah Z, Nasr EC, Bucak E, Schauer PR, Aminian A, Brethauer SA, et al. Efficacy of adjuvant weight loss medication after bariatric surgery. Surg Obes Relat Dis (2018) 14(1):93–8. doi: 10.1016/j.soard.2017.10.002
40. Stanford FC, Toth AT, Shukla AP, Pratt JS, Cena H, Biino G, et al. Weight loss medications in older adults after bariatric surgery for weight regain or inadequate weight loss: A multicenter study. Bariatr Surg Pract Patient Care (2018) 13(4):171–8. doi: 10.1089/bari.2018.0037
41. Benotti PN, Still CD, Wood GC, Akmal Y, King H, El Arousy H, et al. Preoperative weight loss before bariatric surgery. Arch Surg (2009) 144(12):1150–5. doi: 10.1001/archsurg.2009.209
42. Anderin C, Gustafsson UO, Heijbel N, Thorell A. Weight loss before bariatric surgery and postoperative complications: data from the Scandinavian obesity registry (SOReg). Ann Surg (2015) 261(5):909–13. doi: 10.1097/SLA.0000000000000839
43. Samaan JS, Zhao J, Qian E, Hernandez A, Toubat O, Alicuben ET, et al. Preoperative weight loss as a predictor of bariatric surgery postoperative weight loss and complications. J Gastrointest Surg (2022) 26(1):86–93. doi: 10.1007/s11605-021-05055-5
44. Rubino DM, Greenway FL, Khalid U, O'Neil PM, Rosenstock J, Sørrig R, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: The STEP 8 randomized clinical trial. JAMA (2022) 327(2):138–50. doi: 10.1001/jama.2021.23619
45. Khera R, Murad MH, Chandar AK, Dulai PS, Wang Z, Prokop LJ, et al. Association of pharmacological treatments for obesity with weight loss and adverse events: A systematic review and meta-analysis. JAMA (2016) 315(22):2424–34. doi: 10.1001/jama.2016.7602
46. Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med (2016) 375(4):311–22. doi: 10.1056/NEJMoa1603827
47. Mann JFE, Hansen T, Idorn T, Leiter LA, Marso SP, Rossing P, et al. Effects of once-weekly subcutaneous semaglutide on kidney function and safety in patients with type 2 diabetes: a post-hoc analysis of the SUSTAIN 1-7 randomised controlled trials. Lancet Diabetes Endocrinol (2020) 8(11):880–93. doi: 10.1016/S2213-8587(20)30313-2
48. Flint A, Andersen G, Hockings P, Johansson L, Morsing A, Sundby Palle M, et al. Randomised clinical trial: Semaglutide versus placebo reduced liver steatosis but not liver stiffness in subjects with non-alcoholic fatty liver disease assessed by magnetic resonance imaging. Aliment Pharmacol Ther (2021) 54(9):1150–61. doi: 10.1111/apt.16608
49. Frías JP, Auerbach P, Bajaj HS, Fukushima Y, Lingvay I, Macura S, et al. Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. Lancet Diabetes Endocrinol (2021) 9(9):563–74. doi: 10.1016/S2213-8587(21)00174-1
50. Newsome PN, Buchholtz K, Cusi K, Linder M, Okanoue T, Ratziu V, et al. A placebo-controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med (2021) 384(12):1113–24. doi: 10.1056/NEJMoa2028395
51. Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet (2021) 398(10295):143–55. doi: 10.1016/S0140-6736(21)01324-6
52. Sattar N, McGuire DK, Pavo I, Weerakkody GJ, Nishiyama H, Wiese RJ, et al. Tirzepatide cardiovascular event risk assessment: A pre-specified meta-analysis. Nat Med (2022) 28(3):591–8. doi: 10.1038/s41591-022-01707-4
53. ClinicalTrials.gov. A study of tirzepatide (LY3298176) compared with dulaglutide on major cardiovascular events in participants with type 2 diabetes (SURPASS-CVOT) . Betheseda (MD: National Library of Medicine (US. Available at: https://www.clinicaltrials.gov/ct2/show/NCT04255433 (Accessed Aug 24, 2022). Identifier NCT04255433.
54. Clapp B, Harper B, Dodoo C, Klingsporn W, Barrientes A, Cutshall M, et al. Trends in revisional bariatric surgery using the MBSAQIP database 2015-2017. Surg Obes Relat Dis (2020) 16(7):908–15. doi: 10.1016/j.soard.2020.03.002
55. Mahawar KK, Nimeri A, Adamo M, Borg CM, Singhal R, Khan O, et al. Practices concerning revisional bariatric surgery: a survey of 460 surgeons. Obes Surg (2018) 28(9):2650–60. doi: 10.1007/s11695-018-3226-8
56. Jaruvongvanich V, Vantanasiri K, Laoveeravat P, Matar RH, Vargas EJ, Maselli DB, et al. Endoscopic full-thickness suturing plus argon plasma mucosal coagulation versus argon plasma mucosal coagulation alone for weight regain after gastric bypass: A systematic review and meta-analysis. Gastrointest Endosc (2020) 92(6):1164–1175.e6. doi: 10.1016/j.gie.2020.07.013
Keywords: bariatric (weight loss) surgery, anti-obesity medications, obesity, weight regain after bariatric surgery, obesity pharmacotherapy
Citation: Lucas E, Simmons O, Tchang B and Aronne L (2023) Pharmacologic management of weight regain following bariatric surgery. Front. Endocrinol. 13:1043595. doi: 10.3389/fendo.2022.1043595
Received: 13 September 2022; Accepted: 28 October 2022;
Published: 09 January 2023.
Edited by:
Meera Shah, Mayo Clinic, United StatesReviewed by:
Carolina Nicoletti, University of São Paulo, BrazilCopyright © 2023 Lucas, Simmons, Tchang and Aronne. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Eugene Lucas, ZWpsOTAwOUBtZWQuY29ybmVsbC5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.