- 1Department of Reproductive Center, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, Zhejiang, China
- 2Department of Biomedical Sciences Laboratory, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, Zhejiang, China
- 3Department of Reproductive Health and Infertility, Guangdong Women and Children Hospital, Guangzhou, China
- 4Department of Gynecology, Affiliated Dongyang Hospital of Wenzhou Medical University, Dongyang, Zhejiang, China
Objective: To compare the effects of human menopausal gonadotropin (HMG) combined with letrozole (LE) to HMG only for ovarian stimulation on pregnancy outcome of infertile patients undergoing artificial insemination by husband (AIH) due to unexplained or mild male factors.
Materials and methods: Infertile patients with unexplained or mild male factors treated from July 2015 to December 2021 were selected as subjects. The patients were divided into two groups according to the ovarian stimulation schemes they received, namely HMG combined with LE or HMG only. We analyzed the laboratory examination results before drug treatment (baseline) and during ovarian stimulation and compared the pregnancy outcomes of the two groups using univariable analysis and multivariable logistic regression analysis.
Results: In total, 526 cycles of 372 couples were included. The univariate analysis showed that the clinical pregnancy rate of the HMG combined with LE group was 24.8%, significantly higher than that of the HMG group (14.8%, P = 0.007). The live birth rate (19.9%) of the HMG combined with LE group were also significantly higher than those of the HMG group (11.2%, respectively). In multivariate logistic analysis, the age of males was negatively associated with the clinical pregnancy rate (OR 0.874, 95% CI 0.793~0.963, P=0.006) and live birth (OR0.875, 95% CI 0.783~0.977, P=0.018). Moreover, ovarian stimulation with HMG+LE was the only beneficial factor significantly associated with clinical pregnancy (OR 1.929, 95% CI 1.068~3.485, P=0.029) and live birth (OR 2.255, 95% CI 1.188~4.282, P=0.013).
Conclusion: Ovarian stimulation using HMG combined with LE can increase the clinical outcomes (live birth and clinical pregnancy) among infertile patients undergoing AIH due to explained or mild male factors.
1 Introduction
Infertility is defined as the inability of a couple maintaining a regular sexual life without contraception to conceive within one year (1). Intrauterine insemination (IUI) is one of the most widespread types of assisted conception. It increases the probability of conception in infertile couples. This technique is simple, fast, low-cost and manageable, with a relatively low incidence of complications (2). In many countries, IUI is recommended as the first-line treatment for infertility, including those cases with unexplained or mild male factor (2, 3).
In the process of IUI, ovarian stimulation (OS) is routinely performed in couples with infertility due to unexplained and mild male factors (4–6). Either the single use of letrozole (LE) or the single use of human menopausal gonadotropin (HMG) can be used to induce ovulation. LE is a third-generation aromatase inhibitor, which usually causes mono-ovulation. HMG consists of the follicle stimulating and luteinizing hormones, which play a key role in the development of follicles. However, both drugs have certain limitations. Ovarian stimulation by HMG alone may cause multi-follicular development, leading to excessive estrogen levels and promoting endometrial hyperplasia. In turn, these can result in several complications such as multiple pregnancy, ovarian hyperstimulation syndrome, and endometrial thickening. Furthermore, HMG is usually administered as daily injections in a hospital environment, making it inconvenient and relatively painful. Although LE is an oral drug and thus more advantageous, its efficiency duration does not last long because of LE’s short half-life duration. This phenomenon causes the dosage of LE alone not to be sufficient to promote the development of dominant follicles. Therefore, ovulation stimulation needs to be repeated during the next menstrual cycle with a higher LE dosage.
Due to these issues, finding an OS protocol that can improve the clinical pregnancy rate while also reducing the multiple pregnancy rate has become a goal of many physicians. In recent years, single use of LE has been recommended for anovulatory women and has achieved a high successful rate, thus it is widely used in controlled ovarian stimulation (7). Hence, we propose that HMG combined with LE (HMG + LE) can reduce the multiple pregnancy rate without affecting the clinical pregnancy rate and live birth rate. To explore this hypothesis, a retrospective study was conducted in a tertiary hospital in Southeast China.
2 Materials and methods
2.1 Inclusion and exclusion criteria of subjects
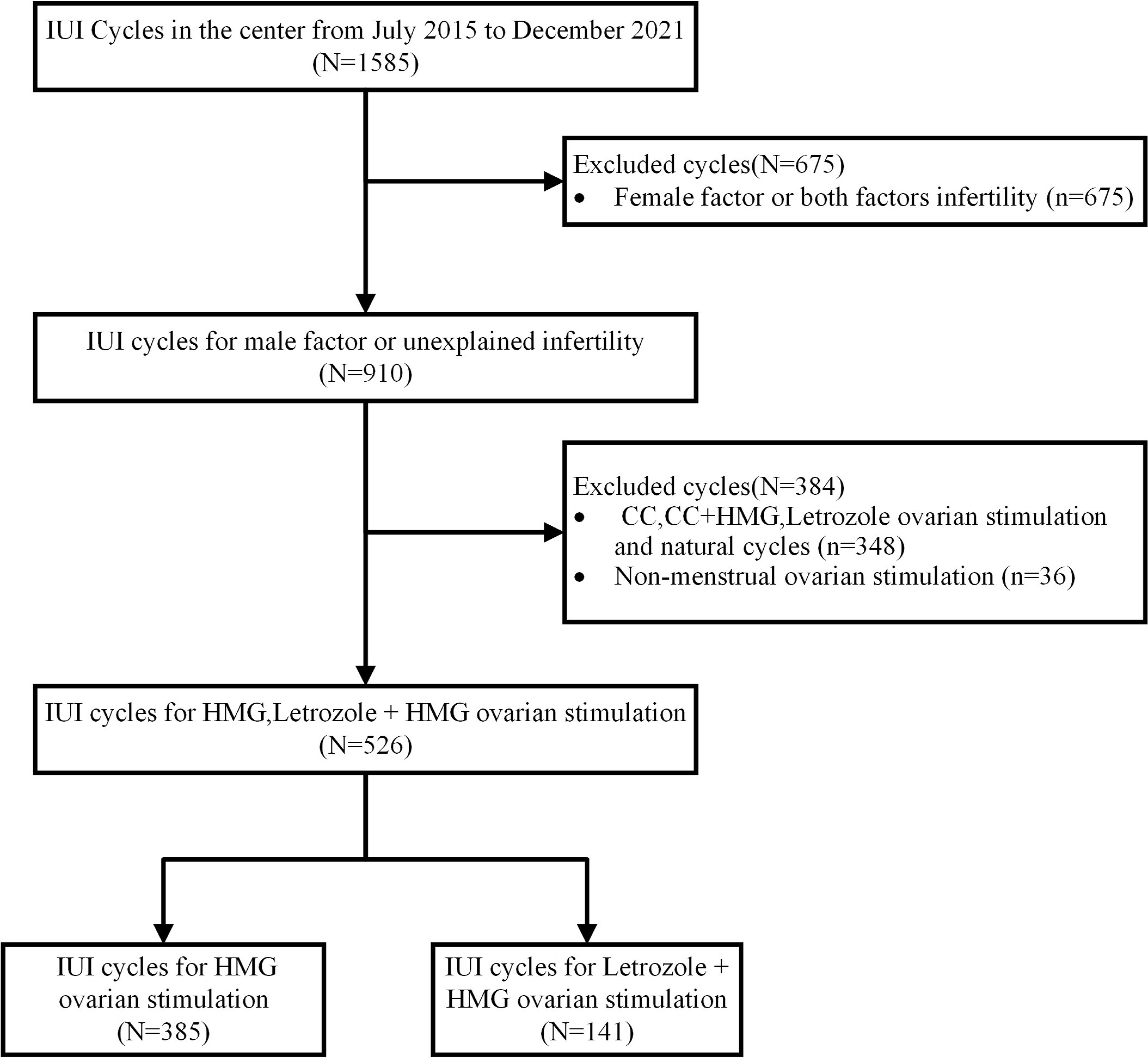
Patients undergoing artificial insemination by husband (AIH) in our hospital from July 2015 to December 2021 were retrospectively collected (Figure 1). These couples received AIH because they were unable conceive after one year or more of regular sexual intercourse without contraception. After excluding cases of abnormal ovulation, endocrine diseases, intrauterine adhesions, and uterine malformations, their conditions were confirmed as unexplained infertility or mild male factor. Informed consent was obtained from the involved patients prior to the study. This study was approved by the Ethics Committee and Institutional Review Board of Dongyang People’s Hospital.
2.2 Intervention of research objects
2.2.1 OS group
Female patients started to use HMG (trade name: Lebaode, Livzon Group Livzon Pharmaceutical Co, Ltd., Zhuhai, China) + LE (trade name: Fu Rui, Hengrui Medicine Co, Ltd., Jiangsu, China) or HMG only to perform OS. The OS regimen was conducted according to the guidelines of ovarian stimulation in assisted reproductive technology in China. Briefly, patients in the HMG only group were administered with HMG on the 2nd-6th day of the menstruation circle (early follicular phase). The initial dose was 75 mg per day but was increased to 112.5 mg if there was no development in follicular diameters and hormones after 5-7 days since the initial stimulation. Patients in the HMG+LE group were administered with 2.5-5.0 mg of LE per day on the 2nd -6th day of the menstruation cycle and the duration would last for 3-5 days. HMG was then administered in the same dosage as in the HMG only group if the follicle did not mature until the mature follicle formed.
2.2.2 Follicle monitoring and laboratory examination
The follicular diameters and hormones, including follicle stimulating hormone, luteinizing hormone, estradiol, and progesterone, were detected on the third day of menstruation as baseline data. In all treatment cycles, follicles were continuously monitored from the 9th day of the menstrual cycle. When the follicle diameter reached ≥ 14 mm, the levels of luteinizing hormone, estradiol and progesterone were monitored. After the peak of estradiol appeared or luteinizing hormone surged, 10000 units of human chorionic gonadotropin (HCG) or 0.2 mg of triptorelin acetate were injected for triggering, and 12h and 36h later, AIH was conducted.
2.2.3 Semen processing and AIH performance
After sexual abstinence for 3–7 days, the husband collected semen through masturbation in a collection cup. The semen parameter (total progressive motile sperm count (TPMSC) was recorded. The semen was processed using density gradient centrifugation followed by AIH in the uterine cavity.
2.2.4 Corpus luteum support
After ovulation, progesterone (200 mg/D) or dydrogesterone (20 mg/D) were administrated continuously for 14 days.
2.3 Determination of pregnancy outcome
Urine HCG (positive) or blood HCG (> 10 mIU/mL) was examined 14 days after IUI to determine whether the patients were biochemically pregnant. A clinical pregnancy was defined as the presence of a gestational sac upon transvaginal ultrasonography after three to four weeks following AIH. An ectopic pregnancy was diagnosed by either laparoscopy or sonographic visualization of an extrauterine gestational sac (8). Early miscarriage was defined as pregnancy loss that occurred spontaneously before 12 weeks of gestation (9). When ultrasonography findings showed two or more gestational sacs in the uterus, a multiple pregnancy was suspected. Live birth was defined as the delivery of a living fetus (or living fetuses) beyond 28 weeks of gestation (10).
2.4 Statistical analysis
SPSS 26.0 (IBM Corporation, USA) was used for statistical analysis of the data. The data conforming to normal distribution in continuous variables were expressed as mean ± SD; otherwise, they were expressed as median and quartile. The differences between groups were analyzed using univariate analysis; t-test for continuous variables with normal distribution, Mann-Whitney U test for continuous variables with abnormal distribution, and chi square test for categorical variables. The significant variables in univariate analysis were included in a multivariate binary logistic regression analysis (ENTER method). The results were expressed as odds ratio (OR) with a 95% confidence interval (CI). A P value < 0.05 was considered statistically significant.
3 Results
3.1 Comparison of patient characteristics between the LE+HMG and HMG group
A total of 372 couples and 526 cycles were included in this study. The average age of the females was 30 years old, with an average duration of infertility of two years. Among all the cycles, 236 cycles corresponded to primary infertility and 290 cycles to secondary infertility. There were 141 cycles in the HMG+LE group and 385 cycles in the HMG group. The basic characteristics of the patients are shown in Table 1. We observed that the ages of females and males were significantly different between the two groups (P<0.05). There were lower baseline levels of FSH and TPMSC and a higher level of LH in HMG+LE group compared to the HMG group. In addition, the percentage of dydrogesterone for luteal support in HMG+LE group was 84.4%, which was significantly higher than that of the HMG group (59.2%; P<0.001). There was no significant difference between the two groups in trigger drugs, female body weight, female body mass index, type of infertility, and duration of infertility (P>0.05).
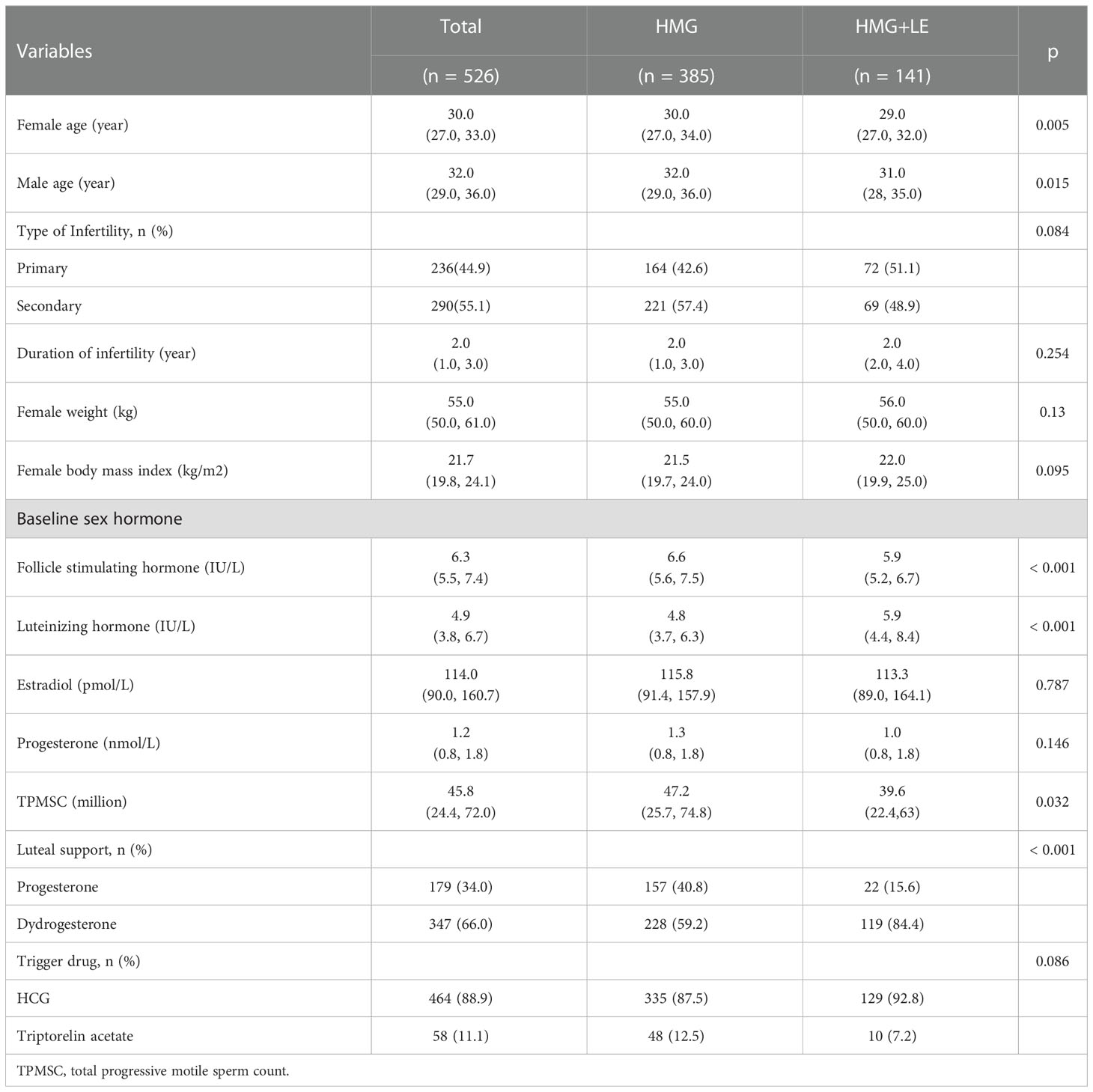
Table 1 The basic characteristics of the patients receiving human menopausal gonadotropin (HMG) with letrozole and HMG alone.
3.2 Hormonal parameters after OS between the LE+HMG and HMG group
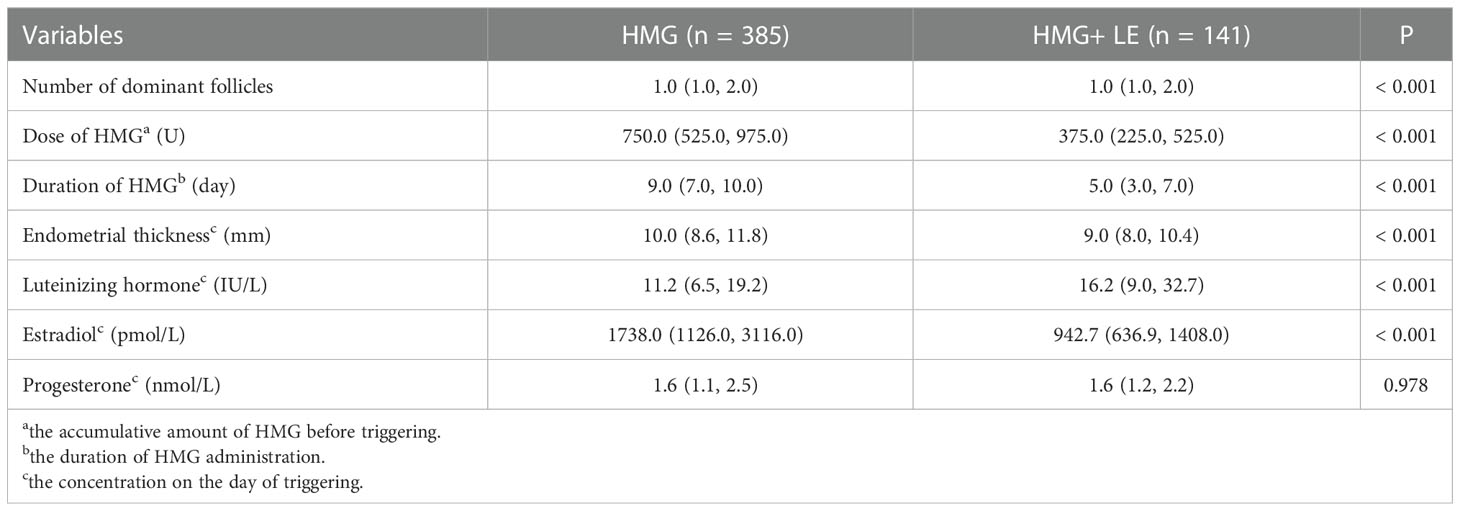
As shown in Table 2, the number of dominant follicles in the HMG group was significantly higher than that in HMG+LE group. In terms of the HMG group, the medication duration, dosage of HMG, and endometrial thickness on the day of triggering were higher than those in HMG + LE group, with statistical significance (P<0.001). Estradiol in the HMG group (1738.0 _pmol/L) on the day of triggering was higher than that in HMG + LE group (942.7pmol/L), with statistical significance (P<0.001). The luteinizing hormone on the day of triggering in the HMG group (11.2 IU/L) was lower than that in HMG + LE group (16.2 IU/L), with statistical significance (P<0.001). Notably, there was no significant difference in progesterone between the two groups on the day of triggering (P = 0.978).
3.3 The pregnancy outcomes between the LE+HMG and HMG group
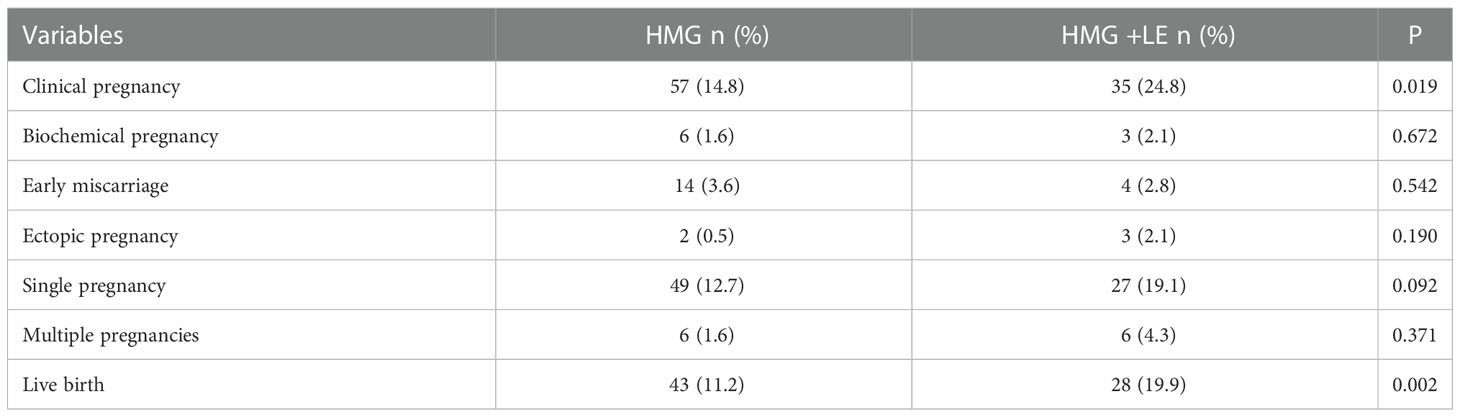
The clinical pregnancy rate and live birth rate of the HMG + LE group were 24.8% and 19.9%, significantly higher than those in the HMG group (14.8% and 11.2%, respectively). However, there were no significant differences in the biochemical pregnancy rate, multiple pregnancy rate, early miscarriage rate, ectopic pregnancy rate, and singleton pregnancy rate between the two groups (Table 3). Notably, there was no case with ovarian hyperstimulation syndrome in this study.
3.4 The factors associated with clinical pregnancy and live birth
Triggering drug was included in multivariate analysis despite the factor not being significant in the univariate analysis because of its importance. Of note, the age of males was negatively significantly associated with clinical pregnancy (OR 0.874, 95% CI 0.793~0.963, P=0.006) and live birth (OR0.875, 95% CI 0.783~0.977, P=0.018) (Table 4). LE+HMG usage increased the clinical pregnancy (OR 1.929, 95% CI 1.068~3.485, P=0.029) and live births (OR 2.255, 95% CI 1.188~4.282, P=0.013). The baseline hormone levels, including FSH and LH, drugs for luteal support and triggering, and semen parameter (TPMSC), were not significantly associated with clinical outcomes (live birth and clinical pregnancy).
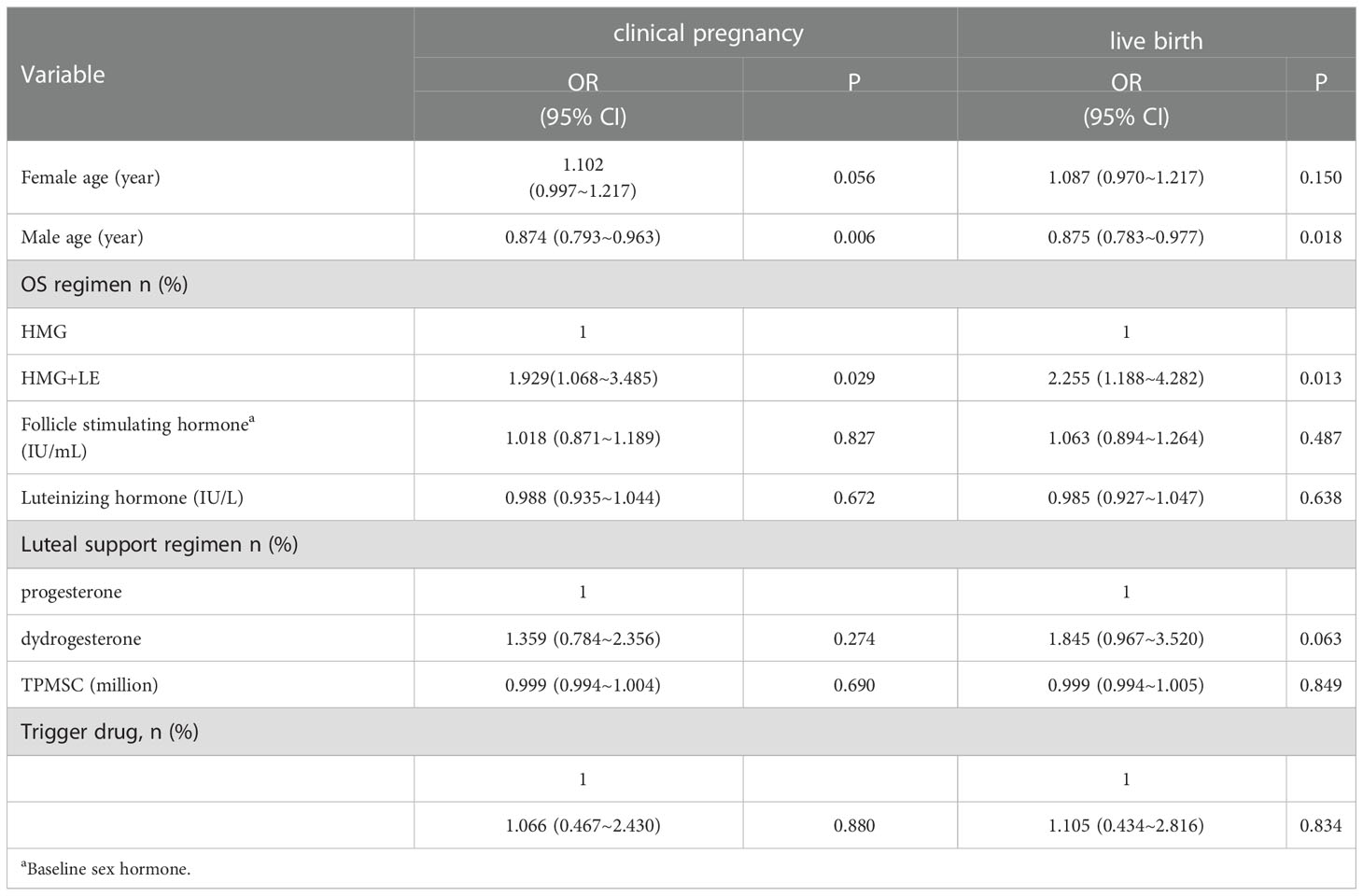
Table 4 Analyses of factors affecting clinical pregnancy rate and live birth rate using multi-variable logistic regression model.
4 Discussion
The effects of HMG or LE alone in artificial insemination have been confirmed in previous studies. However, whether the combination of both would get more benefit remains unknown. In this retrospective observational study, the HMG+LE and HMG OS regimens were adopted to improve the clinical outcomes of infertile patients whose infertility was attributed unexplained or mild male factors. Patients stimulated with HMG+LE had better clinical outcomes than those stimulated with HMG only after adjusting differences in baseline characteristics, semen parameter, trigger drugs, and luteal support.
OS in infertile patients can increase the success probability of artificial insemination and clinical pregnancy rate. The commonly used drugs for OS include clomiphene, LE, HMG, urinary FSH, and GnRH agonists (11–15). A recent meta-analysis compared the effectiveness of clomiphene, LE, HMG, and different natural cycle regimens in the treatment of unexplained infertility (16). The results revealed that OS using HMG ranked the highest in terms of the live births/ongoing pregnancy rate, while natural cycle ranked the lowest. HMG has thus been commonly used for OS by patients receiving IUI (17, 18). Letrozole is a third-generation aromatase inhibitor and is combined with HMG, resulting in effective ovary stimulation with lower cost because it lowers the dosage of HMG (19, 20). There are several mechanisms explaining how LE increases ovary sensitivity to HMG. Letrozole inhibits the conversion of androgen to estrogen in the peripheral ovarian tissues by inhibiting aromatase activity, resulting in a transient accumulation of androgen. Consequently, the increased androgen improves the expression of insulin-like growth factor 1, which increases the response sensitivity of the ovaries to HMG (19). Moreover, low estrogen levels may reduce the ubiquitination of estrogen receptors and lead to faster endometrial proliferation and increased blood flow to the uterus and endometrium, which also has a positive impact on pregnancy outcomes (21). This study found that patients stimulated with HMG+LE had a higher live birth rate than those in the HMG group, confirming the better outcome of HMG + LE as we hypothesized. The higher birth rate in the combination regimen is attributed to an improvement in endometrium condition (22), and should be adopted in patients receiving frozen embryo for endometrial preparation (23). Though clomiphene also has effects on endometrium, its usage in ovary stimulation is associated with lower clinical pregnancy rates than HMG+LE (24).
Previous studies postulate that HMG alone causes significantly higher rates of multiple pregnancy compared to other drug regimens (16). Notably, this issue can potentially be solved by combining it with other ovarian stimulation drugs. Letrozole does not consume estrogen receptors in the brain and retains the reserved normal negative feedback function to control the elevated estrogen levels and growth of dominant follicles (25, 26). Only a single follicle is thus expelled in most cases. The rate of multiple pregnancy can thus be theoretically reduced (22, 23) when HMG is combined with letrozole. However, there was no statistical significance in the rate of multiple pregnancy in this study. This phenomenon was attributed to the limited sample size of multiple pregnancy cases in this retrospective study.
The clinical outcomes following IUI are influenced by many factors, including age, infertility type, sperm quality, mature follicular number, and endometrial thickness among other factors (24–28). Herein, the age of males was negatively associated with the clinical pregnancy and live birth in this study, indicating that it also plays a key role in clinical outcomes in patients with infertility attributed to unexplained or mild male factors (29). Moreover, different ovulatory drugs were found to be the only factor affecting the live birth rate after adjusting the confounding factors through multivariate binary regression analysis, highlighting the main role of ovarian stimulation in determining the final clinical outcome. The clinical outcomes in patients receiving IUI treatment are determined by several parameters, including clinical pregnancy, biochemical pregnancy, and live birth. Live birth might be adopted as the finical outcome because the other two might suffer from miscarriages. In this study, the live birth rate was only associated with ovarian stimulation, with patients receiving HMG combined with LE exhibiting better results compared to those receiving HMG only.
The limitation of the study is that it is a retrospective study conducted in a single center and thus possible bias in determination of different ovarian stimulation by the doctors may exist.
5 Conclusion
HMG+LE is superior to HMG in patients with infertility attributed to unexplained or mild male factors because it induces higher clinical pregnancy and live birth rates. However, the age of males was the negative factor for clinical outcomes. HMG + LE should thus be used for ovarian stimulation in patients receiving intrauterine insemination.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee and Institutional Review Board of Dongyang People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
(I) Conception and design: H-QL, N-JS, J-QC; (II) Administrative support: J-QC, X-PL; (III) Provision of study materials or patients: X-PL, H-QL; (IV) Collection and assembly of data: H-QL, X-LP, X-WC; (V) Data analysis and interpretation: H-QL, X-LP; (VI) Manuscript writing: J-QC, H-QL; (VII). All authors contributed to the article and approved the submitted version.
Funding
This study was supported by Jinhua Health Commission and Jinhua Science and Technology Agency [NO. 2022KY43].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dankert T, Kremer JA, Cohlen BJ, Hamilton CJ, Pasker-de Jong PC, Straatman H, et al. A randomized clinical trial of clomiphene citrate versus low dose recombinant fsh for ovarian hyperstimulation in intrauterine insemination cycles for unexplained and Male subfertility. Hum Reprod (2007) 22(3):792–7. doi: 10.1093/humrep/del441
2. ECW G. Intrauterine insemination. Hum Reprod Update (2009) 15(3):265–77. doi: 10.1093/humupd/dmp003
3. Penzias A, Bendikson K, Falcone T, Hansen K, Hurd W. Evidence-based treatments for couples with unexplained infertility: A guideline. Fertil Steril (2020) 113(2):305–22. doi: 10.1016/j.fertnstert.2019.10.014
4. Safarinejad MR. Infertility among couples in a population-based study in Iran: Prevalence and associated risk factors. Int J Androl (2008) 31(3):303–14. doi: 10.1111/j.1365-2605.2007.00764.x
5. Tomlinson MJ, Amissah-Arthur JB, Thompson KA, Kasraie JL, Bentick B. Prognostic indicators for intrauterine insemination (Iui): Statistical model for iui success. Hum Reprod (1996) 11(9):1892–6. doi: 10.1093/oxfordjournals.humrep.a019513
6. Barroso G, Menocal G, Felix H, Rojas-Ruiz JC, Arslan M, Oehninger S. Comparison of the efficacy of the aromatase inhibitor letrozole and clomiphene citrate as adjuvants to recombinant follicle-stimulating hormone in controlled ovarian hyperstimulation: A prospective, randomized, blinded clinical trial. Fertil Steril (2006) 86(5):1428–31. doi: 10.1016/j.fertnstert.2006.03.044
7. Fouda UM, Sayed AM. Extended high dose letrozole regimen versus short low dose letrozole regimen as an adjuvant to gonadotropin releasing hormone antagonist protocol in poor responders undergoing ivf-et. Gynecol Endocrinol (2011) 27(12):1018–22. doi: 10.3109/09513590.2011.579661
8. Dong L, Lian F, Wu H, Xiang S, Li Y, Wei C, et al. Reproductive outcomes of dual trigger with combination gnrh agonist and hcg versus trigger with hcg alone in women undergoing Ivf/Icsi cycles: A retrospective cohort study with propensity score matching. BMC Pregnancy Childbirth (2022) 22(1):583. doi: 10.1186/s12884-022-04899-2
9. Giakoumelou S, Wheelhouse N, Cuschieri K, Entrican G, Howie SE, Horne AW. The role of infection in miscarriage. Hum Reprod Update (2016) 22(1):116–33. doi: 10.1093/humupd/dmv041
10. Wang H, Gao H, Chi H, Zeng L, Xiao W, Wang Y, et al. Effect of levothyroxine on miscarriage among women with normal thyroid function and thyroid autoimmunity undergoing in vitro fertilization and embryo transfer: A randomized clinical trial. JAMA (2017) 318(22):2190–8. doi: 10.1001/jama.2017.18249
11. Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (Letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev (2018) 5:CD010287. doi: 10.1002/14651858.CD010287.pub3
12. Moro F, Scarinci E, Palla C, Romani F, Familiari A, Tropea A, et al. Highly purified hmg versus recombinant fsh plus recombinant lh in intrauterine insemination cycles in women >/=35 years: A rct. Hum Reprod (2015) 30(1):179–85. doi: 10.1093/humrep/deu302
13. Huang S, Du X, Wang R, Li R, Wang H, Luo L, et al. Ovulation induction and intrauterine insemination in infertile women with polycystic ovary syndrome: A comparison of drugs. Eur J Obstet Gynecol Reprod Biol (2018) 231:117–21. doi: 10.1016/j.ejogrb.2018.08.002
14. Demirol A, Gurgan T. Comparison of different gonadotrophin preparations in intrauterine insemination cycles for the treatment of unexplained infertility: A prospective, randomized study. Hum Reprod (2007) 22(1):97–100. doi: 10.1093/humrep/del335
15. Bernacchioni C, Turano P, Donati C. Targeting sphingosine kinase 1 localization as novel target for ovarian cancer therapy. Trans Cancer Res (2017) 6(S7):S1277–S80. doi: 10.21037/tcr.2017.10.05
16. Danhof NA, Wang R, van Wely M, van der Veen F, Mol BWJ, Mochtar MH. Iui for unexplained infertility-a network meta-analysis. Hum Reprod Update (2020) 26(1):1–15. doi: 10.1093/humupd/dmz035
17. Cantineau AE, Rutten AG, Cohlen BJ. Agents for ovarian stimulation for intrauterine insemination (Iui) in ovulatory women with infertility. Cochrane Database Syst Rev (2021) 11:CD005356. doi: 10.1002/14651858.CD005356.pub3
18. Manganiello PD, Stern JE, Stukel TA, Crow H, Brinck-Johnsen T, Weiss JE. A comparison of clomiphene citrate and human menopausal gonadotropin for use in conjunction with intrauterine insemination. Fertil Steril (1997) 68(3):405–12. doi: 10.1016/s0015-0282(97)00260-4
19. Vendola K, Zhou J, Wang J, Famuyiwa OA, Bievre M, Bondy CA. Androgens promote oocyte insulin-like growth factor I expression and initiation of follicle development in the primate ovary. Biol Reprod (1999) 61(2):353–7. doi: 10.1095/biolreprod61.2.353
20. Mu Z, Song J, Yu Y, Sun Z. Successful live birth in a woman with resistant ovary syndrome treated with letrozole and hmg: A case report. Med (Baltimore) (2020) 99(20):e20199. doi: 10.1097/MD.0000000000020199
21. Hu YJ, Chen Yz Fau - Zhu Y-M, Zhu Ym Fau - Huang H-F, Huang HF. Letrozole stimulation in endometrial preparation for cryopreserved-thawed embryo transfer in women with polycystic ovarian syndrome: A pilot study. Clin Endocrinol (Oxf) (2014) 80(1365-2265(1365-2265 (Electronic):283–9. doi: 10.1111/cen.12280
22. Bansal S, Goyal M, Sharma C, Shekhar S. Letrozole versus clomiphene citrate for ovulation induction in anovulatory women with polycystic ovarian syndrome: A randomized controlled trial. Int J Gynaecol Obstet (2021) 152(3):345–50. doi: 10.1002/ijgo.13375
23. Mitwally MF, Biljan MM, Casper RF. Pregnancy outcome after the use of an aromatase inhibitor for ovarian stimulation. Am J Obstet Gynecol (2005) 192(2):381–6. doi: 10.1016/j.ajog.2004.08.013
24. Hansen KR, He AL, Styer AK, Wild RA, Butts S, Engmann L, et al. Predictors of pregnancy and live-birth in couples with unexplained infertility after ovarian stimulation-intrauterine insemination. Fertil Steril (2016) 105(6):1575–83.e2. doi: 10.1016/j.fertnstert.2016.02.020
25. Vargas-Tominaga L, Alarcon F, Vargas A, Bernal G, Medina A, Polo Z. Associated factors to pregnancy in intrauterine insemination. JBRA Assist Reprod (2020) 24(1):66–9. doi: 10.5935/1518-0557.20190060
26. Sinha P, Pandey K, Srivastava A. Factors determining successful intrauterine insemination. Int J Reprod Contraception Obstetrics Gynecol (2017) 6(9):3887–991. doi: 10.18203/2320-1770.ijrcog20174028
27. Biswas J, Bandhu C, Singh H, Dey M. Relation of endometrial thickness and pregnancy rates in intrauterine insemination following ovulation induction. Int J Reprod Contracept Obstet Gynecol (2016) 5:110–5. doi: 10.18203/2320-1770.ijrcog20151609
28. Elkholi D, Nagy HM. The impact of timing of insemination in relation to ovulation on the cycle pregnancy rate of intrauterine insemination and intrauterine tuboperitoneal insemination in unexplained infertility. Middle East Fertility Soc J (2016) 21(1):4–10. doi: 10.1016/j.mefs.2015.03.001
Keywords: letrozole, human menopausal gonadotropin, ovarian stimulation, intrauterine insemination, artificial insemination by husband
Citation: Li H-q, Pan X-l, Su N-j, Lu X-p, Chen J-q and Chen X-w (2022) Retrospective analysis: The application of human menopausal gonadotropin combined with letrozole for IUI in patients undergoing artificial insemination by husband due to unexplained or mild male factors. Front. Endocrinol. 13:1038433. doi: 10.3389/fendo.2022.1038433
Received: 07 September 2022; Accepted: 05 December 2022;
Published: 20 December 2022.
Edited by:
Bassem Refaat, Umm Al-Qura University, Saudi ArabiaReviewed by:
Leila Warszawski, Instituto Estadual de Diabetes e Endocrinologia Luiz Capriglione, BrazilMing-Jer Chen, Taichung Veterans General Hospital, Taiwan
Copyright © 2022 Li, Pan, Su, Lu, Chen and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xu-wei Chen, MTM2MjU4OTI2ODhAMTYzLmNvbQ==
 Hua-qing Li1
Hua-qing Li1 Xin-ling Pan
Xin-ling Pan Xu-wei Chen
Xu-wei Chen