- 1Department of Ophthalmology, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan
- 2School of Medicine, College of Medicine, Chang Gung University, Taoyuan, Taiwan
- 3Department of Pharmacy, Keelung Chang Gung Memorial Hospital, Keelung, Taiwan
- 4Department of Ophthalmology, Linkou Chang Gung Memorial Hospital, Taoyuan, Taiwan
Purpose: To examine tear function in patients with diabetes mellitus (DM).
Design: Systematic review and meta-analysis.
Method: We searched Embase and PubMed from database inception to March 16, 2022. We included observational studies that compared tear function between patients with and without DM. Tear function was measured using invasive tear breakup time (ITBUT) and Schirmer’s 1 test. Pooled results are presented as standard mean difference (SMD) with 95% confidence interval (CI) based on random-effects models.
Results: We included 59 studies (7,234 eyes) comparing the tear function between patients with and without DM. This meta-analysis indicated that patients with DM had worse tear function than those without DM (ITBUT: SMD: −0.98, 95% CI: −1.27 to −0.69; Schirmer’s 1 test: SMD: −0.45, 95% CI: −0.64 to −0.26), and the results remained consistent in patients with different types of DM (e.g., type 1 DM and type 2 DM) and from different ethnic backgrounds (e.g., Asian vs. non-Asian). Patients with DM under poor glycemic control had worse tear function than those of the non-DM group (ITBUT: SMD: −1.26, 95% CI: −1.86 to −0.66; Schirmer’s 1 test: SMD: −0.25, 95% CI: −0.48 to −0.02), whereas there were no significant differences in tear function between patients with DM under optimal glycemic control and non-DM groups.
Conclusions: We found that patients with type 1 or type 2 DM had significantly reduced tear function. The level of tear function could be determined by glycemic control, and therefore, our findings suggest that glycemic control in patients with DM is critical for maintaining tear function.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero, identifier CRD42021250498.
Introduction
Diabetes mellitus (DM), a leading public health issue, affects more than 240 million people worldwide, and this number is expected to reach 370 million by 2030 (1). In addition to vascular complications, ocular complications of DM, such as dry eye disease (DED), diabetic retinopathy, glaucoma, and cataracts, negatively affect quality of life and may impose a huge economic burden (2). Among these ocular complications, DED occurs most frequently in patients with DM (3). For example, Seifart et al. reported that 52.8% of patients with DM suffered from DED compared with 9.3% in healthy controls (4).
Patients with DED often complain of a burning sensation, photopsia, foreign body sensation, soreness, itchiness, redness, and blurred vision. The corneal complications of DED include superficial punctate keratitis, neurotrophic keratopathy, and epithelial defects. In fact, both DM and DED are risk factors for corneal infection, scarring, perforation, and irreversible tissue damage (1). DM increases the risk of developing diabetic keratopathy, which presents as dry eye or recurrent erosions in the early or mild stage and neurotrophic ulcers with secondary infection in the advanced stage (5). In patients with DM, decreased lacrimal tear production results from being neurotrophic with loss of corneal sensation because of injury to the corneal receptors, which may further develop into a dry eye vicious cycle (6, 7).
A previous systematic review and meta-analysis by Lv et al. indicated that tear function is worse in patients with DM than in individuals without the disease, but we recommend that more detailed subgroup analyses should be considered to deal with the impacts of the clinical heterogeneity within the included studies, especially as regard different types of DM (8). For example, Kan et al. recently found no negative effects on tear function in patients with gestational DM (GDM) (9). This implies that different types of DM may cause varying pathophysiologies of DM-related DED. Furthermore, previous studies have reported that the corneal conditions in patients with DM may be determined by glycemic control, age, and ethnicity (10–12), but there is insufficient evidence to explore these factors in patients with DM-related DED.
In this study, we aimed to systematically examine the evidence on tear function in patients with DM; specifically, we evaluated tear function in these patients by conducting different subgroup analyses, including type of DM, age, ethnicity, and glycemic control status.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Supplemental Table S2) (13). The study protocol has been registered on PROSPERO (CRD42021250498) (14).
Search strategy and study selection
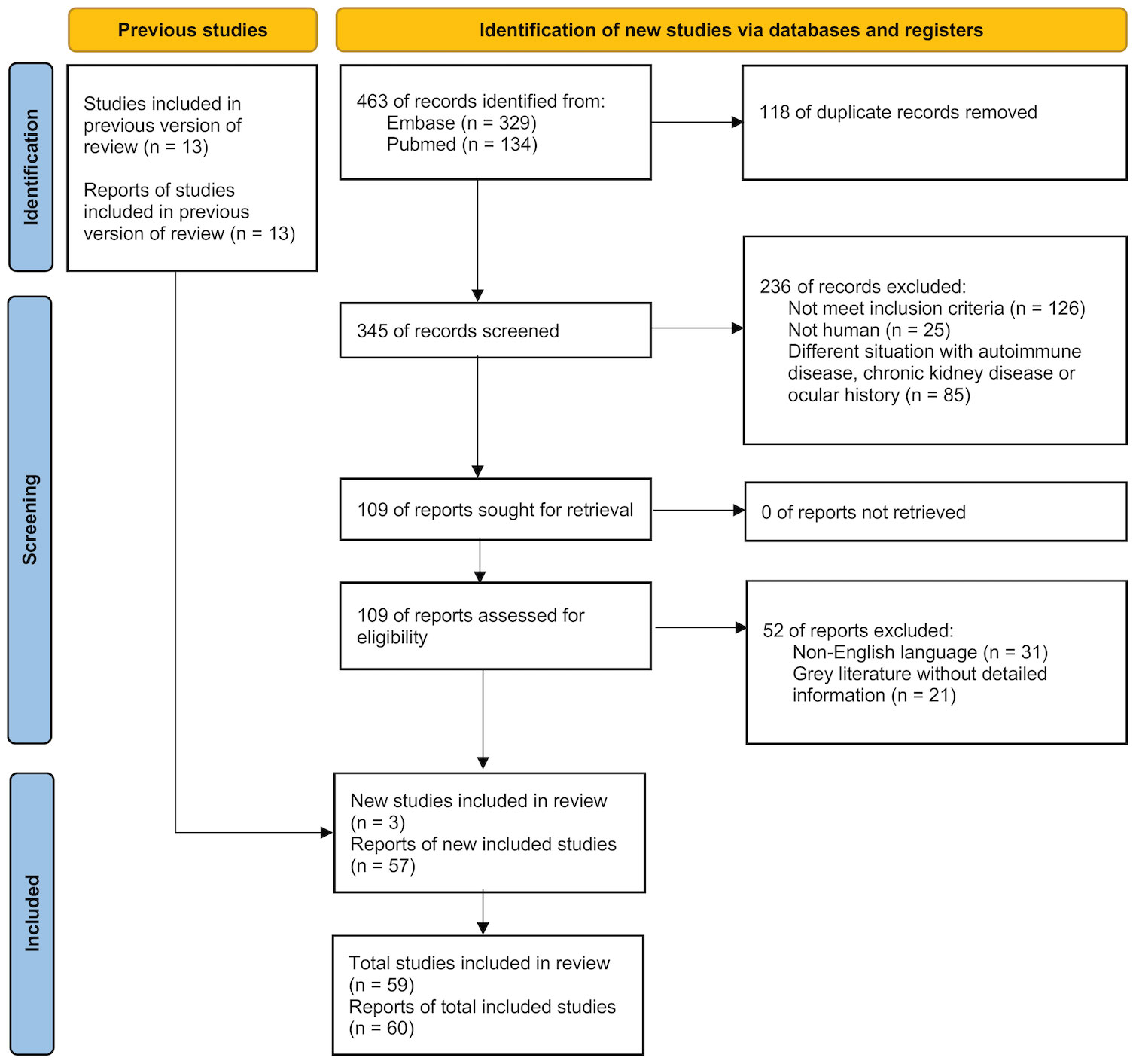
We searched Embase and PubMed for relevant records from the inception of these databases to March 16, 2022. The search strategy is presented in Supplemental Table S3. We also examined reference lists from previously published material and included studies from the lists to obtain further eligible studies. After potential records were identified from the abovementioned databases, two investigators (YKK and ETL) independently screened the study titles and abstracts. The same investigators selected studies by reviewing the full text based on our inclusion and exclusion criteria. Any disagreement about the study selection was resolved through full discussions with the third investigator (CCS).
Eligibility criteria for study selection
Inclusion criteria for studies were as follows: (a) study groups included participants with DM, including type 1 DM, type 2 DM, GDM, and unclassified DM, and the control groups included participants without DM; (b) study outcome assessments used common tests to assess DED severity (15); and (c) study designs were cohort, case–control, or cross-sectional. We excluded studies in which (a) study or control groups focused on non-human participants; (b) study participants had Graves’ disease, connective tissue disorders, chronic kidney disease, or other autoimmune diseases (as autoimmune diseases disturb lacrimal secretion and dialysis alters tear quality) (16, 17); (c) study participants had a medical history of corneal disease, glaucoma, contact lens wearing, current use of ocular medication, or previous intraocular surgery (as structural damage to the cornea and eye drops interrupt tear secretion); (d) the literature was gray (e.g., conference abstracts) without detailed information on participants’ baseline characteristics, risk of bias evaluation, or results extraction; (e) data reports were duplicated (from the same source population); and (f) the language of publication was not English.
Study outcomes
We included two tests for DED severity as study outcomes (18). First, Schirmer’s test is typically used to detect the amount of secretion of the aqueous layer of the tear film, and Schirmer’s 1 test measures total tear secretion function without topical anesthesia. Second, tear breakup time is used to determine the stability of the tear film, whereas invasive tear breakup time (ITBUT) is performed using strips soaked in fluorescein.
To evaluate differences in tear function within different subgroups of DM types, age, ethnicity, and DM control status, we further compared (1) tear function in the pertinent DM group with type 1 DM versus type 2 DM versus GDM versus unclassified DM; (2) mean participants’ age as those <65 versus >65 years old; (3) participants’ ethnicity as Asian versus non-Asian; and (4) mean glycosylated hemoglobin (HbA1C) levels as <7% versus >7%.
Data extraction and quality assessment
Two investigators (YKK and ETL) independently extracted data regarding the study region, inclusion period, trial design, subgroups, sample size, mean age, sex ratio (male/female), DM duration, and HbA1C levels from the included studies. The outcome data for the meta-analyses included Schirmer’s 1 test and ITBUT. Because the outcomes of study interest were continuous data, we first extracted the mean and standard deviation (SD) from the included studies for the meta-analyses. If the included studies only reported the standard error (SE) or interquartile range (IQR), we calculated the SD using the formula SE = SD/√N and IQR/1.35, respectively (19, 20). If the included studies only reported the maximum and minimum values, we calculated the SD using the formula reported by Hozo et al. (21). If the studies only presented the subgroup data, we pooled them together into one group for the final meta-analysis.
Two investigators (YKK and ETL) independently assessed the study quality using an adapted form of the Newcastle–Ottawa Quality Assessment Scale for observational studies (22). This scale includes three major domains (selection, comparability, and outcome), with a total of 10 points. We defined studies with 7–10, 5–6, and 0–4 points as good, moderate, and low study quality, respectively (22). Any disagreement about the study quality assessments was resolved through full discussion with the third investigator (CCS).
Statistical analysis
We conducted quantitative syntheses using meta-analysis to present the mean difference with a 95% confidence interval (CI) based on the random-effects model. We used Review Manager 5.4 software provided by the Cochrane Collaboration Network for the meta-analysis (23). We calculated the standard mean difference (SMD) to adjust for various measurement units from different measurement tools used among the included studies. We calculated I2 values to measure statistical heterogeneity among the studies. Furthermore, we performed subgroup analyses to evaluate the differences in tear function in patients with different types of DM, age, ethnicity, and HbA1C levels. We considered absolute SMDs of <0.2, 0.2–0.5, and >0.8 as small, medium, and large differences in DED severity, respectively, between the DM and control groups (24). Results with two-sided p < 0.05 were considered to be statistically significant.
Results
We initially identified 466 records from Embase (n = 329), PubMed (n = 134), and three additional studies from the reference lists of previous literature. After applying our inclusion and exclusion criteria, we included 60 reports from 59 studies in this systematic review and meta-analysis (Figure 1). Specifically, the Zou et al. study presented two separate reports on adults (type 2 DM) and on children (either type 1 or 2 DM) (25). Hence, the meta-analysis included 60 reports from 59 studies.
Characteristics and quality of included studies
The 59 studies contributed 7,234 eyes of participants with and without DM from China (20 studies, 2,780 eyes) (25–44), Turkey (8 studies, 891 eyes) (45–52), the United States (4 studies, 272 eyes) (9, 53–55), Japan (5 studies, 722 eyes) (56–60), Brazil (4 studies, 373 eyes) (61–64), the United Kingdom (2 studies, 117 eyes) (65, 66), India (3 studies, 569 eyes) (67–69), Korea (2 studies, 330 eyes) (70, 71), and other countries (11 studies, 1,180 eyes) (71–81). There were 1,210 (16.7%) eyes in the type 1 DM group, 4,345 (60.0%) in the type 2 DM group, 1,597 (22.1%) in the unclassified DM group, and 82 (1.1%) in the GDM group. Participants’ ages ranged from 10.1 ± 2.5 to 73.7 ± 5.7 years. The other study characteristics are listed in Supplemental Table S1.
Methodological quality of included studies
Details of the risk of bias assessment are presented in Supplemental Table S4. All studies were assessed as having a low risk of bias, except for the domains of comparability. In general, the quality of the included studies was good.
Main outcome
In this meta-analysis, 59 studies of DM evaluated severity of DED (Figures 2 and 3) (9, 25–82). Compared with the control group, we found that participants with DM had a lower ITBUT (41 studies, SMD: −0.98, 95% CI: −1.27 to −0.69, I2: 95%) and Schirmer’s 1 test result (41 studies, SMD: −0.45, 95% CI: −0.64 to −0.26, I2: 90%).
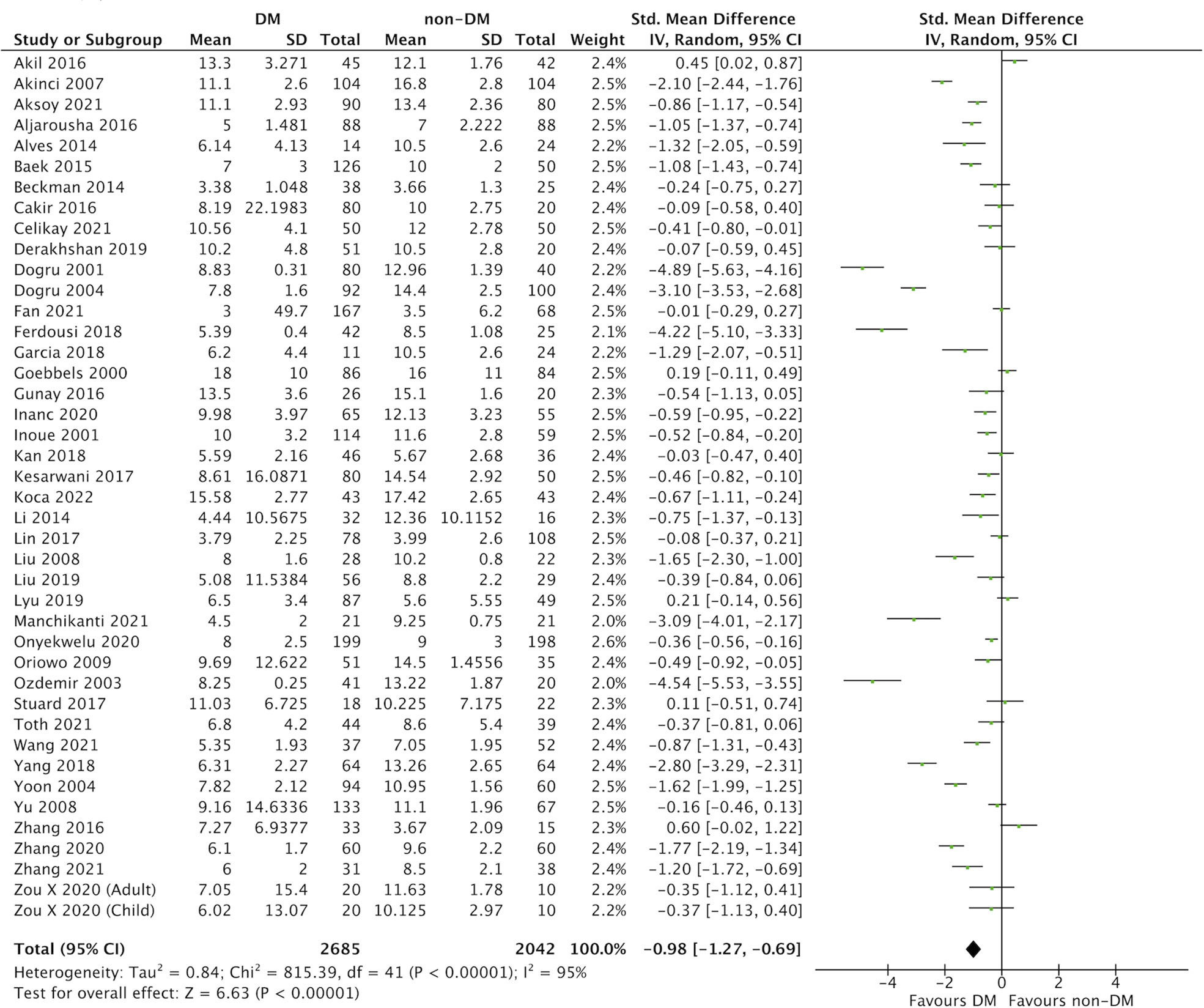
Figure 2 Comparison of the severity of dry eye disease (DED) between diabetes mellitus (DM) and non-DM based on invasive tear breakup time (ITBUT).
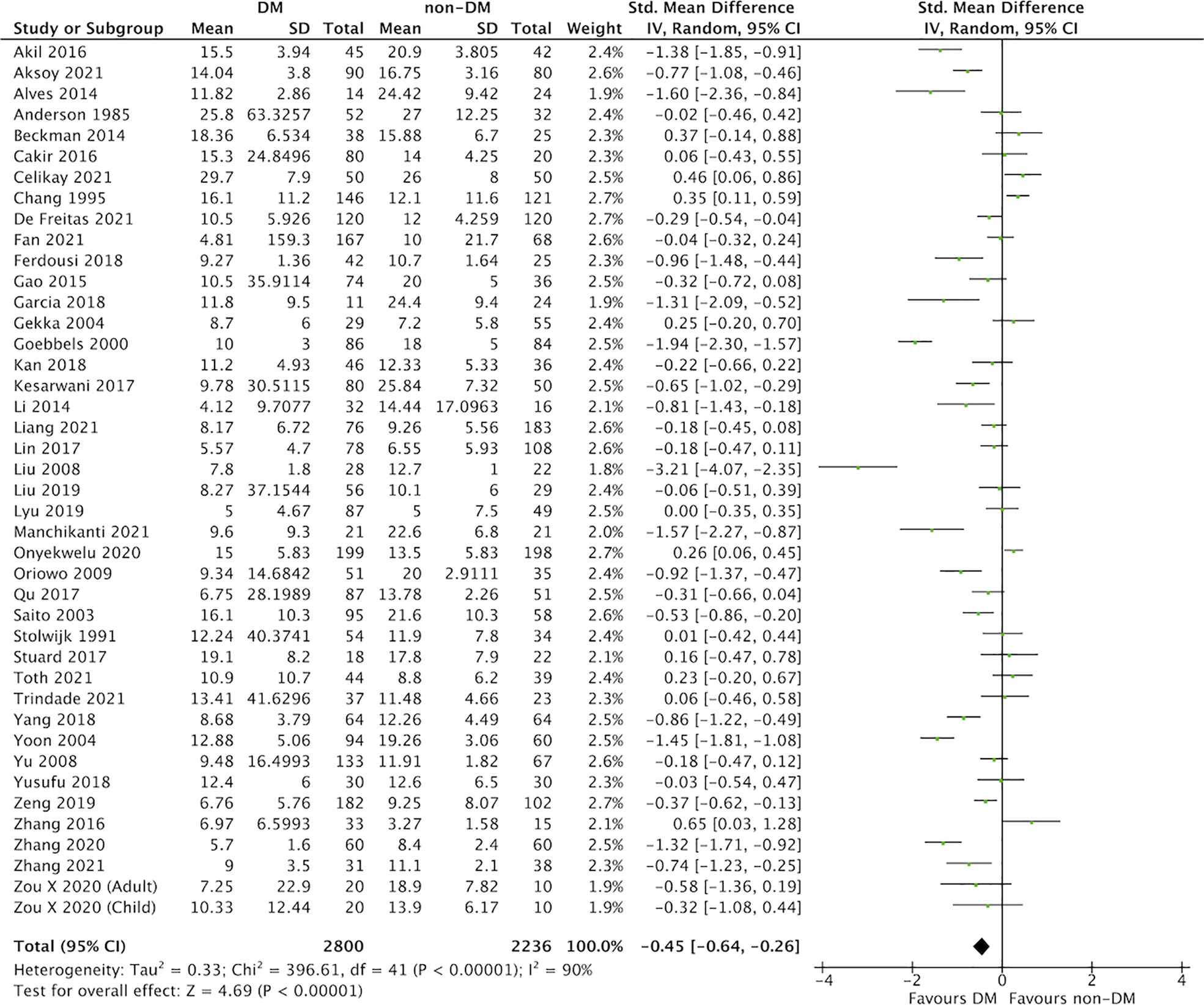
Figure 3 Comparison of the severity of dry eye disease (DED) between diabetes mellitus (DM) and non-DM based on Schirmer’s 1 test.
Subgroup analysis stratified by different types of DM
This meta-analysis included 41 studies that evaluated ITBUT in relation to DM type (Supplemental Figure S1A) (9, 25, 28–32, 34, 35, 39–43, 45–57, 60, 62, 64, 65, 67–71, 73, 74, 79, 80, 82). Compared with the control group, we found lower ITBUTs in participants with type 1 DM (8 studies, SMD: −0.98, 95% CI: −1.70 to −0.26, I2: 96%), type 2 DM (21 studies, SMD: −1.26, 95% CI: −1.76 to −0.76, I2: 96%), and unclassified DM (12 studies, SMD: −0.59, 95% CI: −0.86 to −0.32, I2: 77%). No statistical differences in ITBUT were found in participants with GDM (one study, SMD: −0.03, 95% CI: −0.47 to 0.40, I2: not applicable).
There were 41 studies of DM where Schirmer’s 1 test results were evaluated (Supplemental Figure S1B) (9, 25, 26, 28–35, 37–39, 41–44, 47, 48, 51, 53–55, 58, 59, 61–65, 67–69, 71–75, 77, 82). Compared with the control group, we found lower Schirmer’s 1 test results in participants with type 1 DM (7 studies, SMD: −0.86, 95% CI: −1.39 to −0.33, I2: 91%) and type 2 DM (25 studies, SMD: −0.41, 95% CI: −0.63 to −0.18, I2: 88%). However, no statistical differences were found in participants with unclassified DM (nine studies, SMD: −0.24, 95% CI: −0.63 to 0.14, I2: 86%) or GDM (one study, SMD: −0.22, 95% CI: −0.66 to 0.22, I2: not applicable).
Subgroup analysis stratified by age
Supplemental Figure S2A presents the 35 studies where ITBUT was evaluated in relation to age (9, 25, 28–32, 34, 39–43, 45–54, 56, 57, 60, 65, 67–71, 79, 80, 82). Compared with the control group, we found younger participants had a lower ITBUT (30 studies, SMD: −1.19, 95% CI: −1.55 to −0.83, I2: 96%), whereas no statistical differences in ITBUT were found among elderly participants (five studies, SMD: −0.27, 95% CI: −0.83 to 0.28, I2: 89%).
The 36 studies of DM where Schirmer’s 1 test results were evaluated in relation to age are shown in Supplemental Figure S2B (9, 25, 26, 28–34, 38, 39, 41–44, 47, 48, 51, 53, 54, 58, 59, 61, 63, 65, 67–69, 71–75, 77, 82). Compared with the control group, we found lower Schirmer’s 1 test results in younger participants (29 studies, SMD: −0.51, 95% CI: −0.76 to −0.26, I2: 92%) and elderly participants (7 studies, SMD: −0.23, 95% CI: −0.46 to −0.01, I2: 67%).
Subgroup analysis stratified by ethnicity
There were 41 studies of DM that evaluated ITBUT together with ethnicity (Supplemental Figure S3A) (9, 25, 28–32, 34, 35, 39–43, 45, 47–57, 60, 62, 64, 65, 67–71, 73, 74, 79, 80, 82). Compared with the control group, we found a lower ITBUT in Asian (26 studies, SMD: −1.01, 95% CI: −1.35 to −0.66, I2: 95%) and non-Asian patients with DM (15 studies, SMD: −0.94, 95% CI: −1.49 to −0.39, I2: 95%).
With regard to Schirmer’s 1 test results, 41 studies included ethnicity (Supplemental Figure S3B) (9, 25, 26, 28–35, 37–39, 41–44, 47, 48, 51, 53–55, 58, 59, 61–65, 67–69, 71–75, 77, 82). Compared with the control group, we found lower Schirmer’s 1 test results in Asian (26 studies, SMD: −0.47, 95% CI: −0.69 to −0.26, I2: 89%) and non-Asian patients with DM (15 studies, SMD: −0.41, 95% CI: −0.80 to −0.02, I2: 91%).
Subgroup analysis stratified by HbA1C levels
Twenty-one studies of DM investigated ITBUT in relation to HbA1C levels (Supplemental Figure S4A) (9, 30, 32, 34, 42, 45–51, 53, 54, 56, 60, 65, 69, 70, 80, 82). Compared with the control group, we found a lower ITBUT in participants with both poor control of DM (15 studies, SMD: −1.26, 95% CI: −1.86 to −0.66, I2: 97%) and good control of DM (6 studies, SMD: −0.47, 95% CI: −0.87 to −0.07, I2: 84%).
Twenty studies evaluated Schirmer’s 1 test results with HbA1C levels (Supplemental Figure S4B) (9, 30, 32–34, 38, 42, 47, 48, 51, 53, 54, 58, 59, 61, 63, 65, 69, 75, 82). Compared with the control group, we found lower Schirmer’s 1 test results in participants with poor control of DM (15 studies, SMD: −0.25, 95% CI: −0.48 to −0.02, I2: 83%), but no statistical differences were found in participants with good control of DM (5 studies, SMD: −0.25, 95% CI: −0.72 to 0.22, I2: 86%).
Discussion
Based on the meta-analyses of ITBUT and Schirmer’s 1 tests, this study indicated that patients with DM presented with worse tear function than those without DM. More importantly, our findings could be the first summarized evidence on tear function within different DM subgroups. For example, unlike types 1 and 2 DM, we found patients with GDM had similar tear function to control groups. Moreover, patients with DM with good glycemic control had similar tear function to those without DM. However, tear function was similar in Asian and non-Asian patients with DM.
The influence of chronic hyperglycemia, such as in type 1 and type 2 DM, on DED has been elucidated by several mechanisms, including microvascular changes of the lacrimal gland, a reduced lipid layer in tear film composition, a high grade of conjunctival squamous metaplasia, an increased inflammatory process, and a low goblet cell density (83, 84). However, our subgroup analysis showed no significant difference between the tear function of patients with GDM and healthy pregnant women. A possible explanation could be the short duration of DM with a low degree of hyperglycemia in patients with GDM (9), so the clinical impacts from GDM on tear function may be relatively minor. Our finding may provide the fundamental evidence for further studies to confirm this proposed hypothesis.
Previous evidence regarding the role of ethnicity in tear function suggested that Asian populations were associated with higher risk of DED (12, 85, 86). However, in this systematic review and meta-analysis, we observed similar tear function in Asian and non-Asian patients with DM. Our findings may support the previous study from Butovich et al. indicating that minimal differences in meibogenesis and the process of lipid secretion from meibomian glands among different ethnicities were unlikely to differentially affect tear function between Asians and Caucasians (87).
Early studies reported age as a significant risk factor for decline of tear function, because it is associated with lacrimal gland atrophy with lymphocyte infiltration, eyelid laxity, and meibomian gland dysfunction (88–93). In this presented meta-analysis, we found elderly patients with DM may have better tear function than younger patients, contrary to previous reports. However, the impact of glycemic control on tear function in this subgroup analysis could not be ignored, because elderly patients usually have better glycemic control compared with younger patients (32, 42). Among five and seven included studies with elderly patients reporting ITBUT and Schirmer’s 1 test in our meta-analysis, respectively, only two studies reported the mean baseline HbA1C levels. We found both included studies had a mean HbA1C of less than 7%, whereas there were no differences in ITBUT (two studies, SMD: −0.48, 95% CI: −1.87 to 0.90, I2: 95%; Supplemental Figure S5A) and Schirmer’s 1 test results between this population and the controls (two studies, SMD: −0.35, 95% CI: −1.07 to 0.37, I2: 83%; Supplemental Figure S5B). Taking together all our results, we suggest that, under the optimal glycemic controls in elderly patients with DM could maintain the tear function as the control group. In addition, more studies on the tear function form elderly patients with inadequate glycemic controls should be determined.
Compared with previous systematic review with meta-analysis (8), this presented work included 46 more recent studies from China, Turkey, the United States, and other countries, which makes our findings more generalizable to clinical practice. However, some limitations should be noted before the interpretations of our study findings. First, we conducted various subgroup analyses (e.g., types of DM, age, ethnicity, and glycemic controls) with random-effects analyses to address the substantial clinical heterogeneity among the included studies. For example, some included studies were not based on well-matched designs to compare tear function between DM- and non-DM groups, so potential impacts from possible confounders could not be totally excluded. Second, result inconsistency among the studies were found, even after the subgroup stratifications with the random-effects analyses. Third, not every included study reported the mean with SD data for our meta-analysis; however, using different published approaches, we were able to convert SE, IQR, or maximum and minimum values. Finally, because this study mainly focused on type 1 or type 2 DM, our findings may not apply to prediabetic patients whose tear function may be substantially different from type 1 or type 2 DM patients (94). Regularly updated meta-analyses with future studies are required to replicate our findings.
In conclusion, this systematic review and meta-analysis found that patients with type 1 or type 2 DM had worse tear function compared with the non-DM groups. The level of tear function could be determined by glycemic control. Our findings suggest that glycemic control in patients with DM is critical for maintaining tear function.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Y-KK contributed to study planning, performed the systematic review search, and wrote the manuscript. S-CS performed study conception and meta-analysis and reviewed/edited the manuscript. E-TL contributed to study planning and implementation of supplemental analyses and reviewed/edited the manuscript. L-YP contributed to study planning and reviewed/edited the manuscript. LY contributed to study planning, wrote the methods, and reviewed/edited the manuscript. C-CS contributed to study conception and planning and reviewed/edited the manuscript. C-CS is the study guarantor. All authors contributed to the article and approved the submitted version.
Funding
This research received the research grant MW#70986493 from Alcon Services AG, Taiwan Branch, Taiwan. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1036002/full#supplementary-material
References
1. Sayın N, Kara N, Pekel G. Ocular complications of diabetes mellitus. World J Diabetes (2015) 6:92–108. doi: 10.4239/wjd.v6.i1.92
2. Vieira-Potter VJ, Karamichos D, Lee DJ. Ocular complications of diabetes and therapeutic approaches. BioMed Res Int (2016) 2016:3801570. doi: 10.1155/2016/3801570
3. Manaviat MR, Rashidi M, Afkhami-Ardekani M, Shoja MR. Prevalence of dry eye syndrome and diabetic retinopathy in type 2 diabetic patients. BMC Ophthalmol (2008) 8:10–. doi: 10.1186/1471-2415-8-10
5. Achtsidis V, Eleftheriadou I, Kozanidou E, Voumvourakis KI, Stamboulis E, Theodosiadis PG, et al. Dry eye syndrome in subjects with diabetes and association with neuropathy. Diabetes Care (2014) 37(10):e210–1. doi: 10.2337/dc14-0860
6. Bikbova G, Oshitari T, Tawada A, Yamamoto S. Corneal changes in diabetes mellitus. Curr Diabetes Rev (2012) 8(4):294–302. doi: 10.2174/157339912800840479
7. Su YC, Hung JH, Chang KC, Sun CC, Huang YH, Lee CN, et al. Comparison of sodium-glucose cotransporter 2 inhibitors vs glucagonlike peptide-1 receptor agonists and incidence of dry eye disease in patients with type 2 diabetes in Taiwan. JAMA Netw Open (2022) 5(9):e2232584. doi: 10.1001/jamanetworkopen.2022.32584
8. Lv H, Li A, Zhang X, Xu M, Qiao Y, Zhang J, et al. Meta-analysis and review on the changes of tear function and corneal sensitivity in diabetic patients. Acta Ophthalmol (2014) 92(2):e96–e104. doi: 10.1111/aos.12063
9. Kan S, Acar U, Kizilgul M, Beyazyildiz E, Cankaya AB, Apaydin M, et al. Tear film and ocular surface evaluation in gestational diabetes mellitus. Semin Ophthalmol (2018) 33(3):402–6. doi: 10.1080/08820538.2016.1250919
10. Kim YJ, Kim TG. The effects of type 2 diabetes mellitus on the corneal endothelium and central corneal thickness. Sci Rep (2021) 11(1):8324. doi: 10.1038/s41598-021-87896-3
11. Kaiserman I, Kaiserman N, Nakar S, Vinker S. Dry eye in diabetic patients. Am J Ophthalmol (2005) 139(3):498–503. doi: 10.1016/j.ajo.2004.10.022
12. Ward MF 2nd, Le P, Donaldson JC, Van Buren E, Lin FC, Lefebvre C, et al. Racial and ethnic differences in the association between diabetes mellitus and dry eye disease. Ophthal Epidemiol (2019) 26(5):295–300. doi: 10.1080/09286586.2019.1607882
13. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst Rev (2021) 10(1):89. doi: 10.1186/s13643-021-01626-4
14. Booth A, Clarke M, Dooley G, Ghersi D, Moher D, Petticrew M, et al. PROSPERO: An international prospective register of systematic reviews. Syst Rev. (2012) 1:2. doi: 10.1186/2046-4053-1-2
15. Sullivan BD, Whitmer D, Nichols KK, Tomlinson A, Foulks GN, Geerling G, et al. An objective approach to dry eye disease severity. Invest Ophthalmol Visual Sci (2010) 51(12):6125–30. doi: 10.1167/iovs.10-5390
16. Taskapili M, Serefoglu Cabuk K, Aydin R, Atalay K, Kirgiz A, Sit D, et al. The effects of hemodialysis on tear osmolarity. J Ophthalmol (2015) 2015:170361–. doi: 10.1155/2015/170361
17. Ziaragkali S, Kotsalidou A, Trakos N. Dry eye disease in routine rheumatology practice. Mediterr J Rheumatol (2018) 29(3):127–39. doi: 10.31138/mjr.29.3.127
18. Kuo YK, Lin IC, Chien LN, Lin TY, How YT, Chen KH, et al. Dry eye disease: A review of epidemiology in Taiwan, and its clinical treatment and merits. J Clin Med (2019) 8(8):1227. doi: 10.3390/jcm8081227
19. Fu R, Vandermeer BW, Shamliyan TA, O’Neil ME, Yazdi F, Fox SH, et al. Handling continuous outcomes in quantitative synthesis. (2013).
20. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol (2014) 14(1):135. doi: 10.1186/1471-2288-14-135
21. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol (2005) 5:13. doi: 10.1186/1471-2288-5-13
22. Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers’ intentions to vaccinate related to their knowledge, beliefs and attitudes? a systematic review. BMC Public Health (2013) 13(1):154. doi: 10.1186/1471-2458-13-154
25. Zou X, Wang S, Zhang P, Lu L, Zou H. Quantitative proteomics and weighted correlation network analysis of tear samples in adults and children with diabetes and dry eye. Trans Vision Sci Technol (2020) 9(13):1–15. doi: 10.1167/tvst.9.13.8
26. Gao Y, Zhang Y, Ru YS, Wang XW, Yang JZ, Li CH, et al. Ocular surface changes in type II diabetic patients with proliferative diabetic retinopathy. Int J Ophthalmol (2015) 8(2):358–64. doi: 10.3980/j.issn.2222-3959.2015.02.26
27. Han JX, Wang H, Liang HH, Guo JX. Correlation of the retinopathy degree with the change of ocular surface and corneal nerve in patients with type 2 diabetes mellitus. Int J Ophthalmol (2021) 14(5):750–8. doi: 10.18240/ijo.2021.05.17
28. Li B, Sheng M, Xie L, Liu F, Yan G, Wang W, et al. Tear proteomic analysis of patients with type 2 diabetes and dry eye syndrome by two-dimensional nano-liquid chromatography coupled with tandem mass spectrometry. Invest Ophthalmol Visual Sci (2014) 55(1):177–86. doi: 10.1167/iovs.13-12080
29. Lin X, Xu B, Zheng Y, Coursey TG, Zhao Y, Li J, et al. Meibomian gland dysfunction in type 2 diabetic patients. J Ophthalmol (2017) 2017:3047867. doi: 10.1155/2017/3047867
30. Liu R, Ma B, Gao Y, Ma B, Liu Y, Qi H. Tear inflammatory cytokines analysis and clinical correlations in diabetes and nondiabetes with dry eye. Am J Ophthalmol (2019) 200:10–5. doi: 10.1016/j.ajo.2018.12.001
31. Liu X, Gu YS, Xu YS. Changes of tear film and tear secretion after phacoemulsification in diabetic patients. J Zhejiang University: Sci B (2008) 9(4):324–8. doi: 10.1631/jzus.B0710359
32. Lyu Y, Zeng X, Li F, Zhao S. The effect of the duration of diabetes on dry eye and corneal nerves. Contact Lens Anterior Eye (2019) 42(4):380–5. doi: 10.1016/j.clae.2019.02.011
33. Qu JH, Tian L, Zhang XY, Sun XG. Early central and peripheral corneal microstructural changes in type 2 diabetes mellitus patients identified using in vivo confocal microscopy: A case-control study. Med (United States) (2017) 96(38):e7960. doi: 10.1097/MD.0000000000007960
34. Yang M, Zhao T, Liu J, Wang Z. Study on factors contributing to xerophthalmia among type-2 diabetes patients. Int J Clin Exp Med (2018) 11(4):4183–7.
35. Yu L, Chen X, Qin G, Xie H, Lv P. Tear film function in type 2 diabetic patients with retinopathy. Ophthalmologica (2008) 222(4):284–91. doi: 10.1159/000140256
36. Yu T, Shi WY, Song AP, Gao Y, Dang GF, Ding G. Changes of meibomian glands in patients with type 2 diabetes mellitus. Int J Ophthalmol (2016) 9(12):1740–4. doi: 10.18240/ijo.2016.12.06
37. Yusufu M, Liu X, Zheng T, Fan F, Xu J, Luo Y. Hydroxypropyl methylcellulose 2% for dry eye prevention during phacoemulsification in senile and diabetic patients. Int Ophthalmol (2018) 38(3):1261–73. doi: 10.1007/s10792-017-0590-7
38. Zeng X, Lv Y, Gu Z, Jia Z, Zhang C, Lu X, et al. The effects of diabetic duration on lacrimal functional unit in patients with type II diabetes. J Ophthalmol (2019) 2019:8127515. doi: 10.1155/2019/8127515
39. Zhang M, Xiang YH. Analysis of ocular surface dysfunction in patients with type 2 diabetes mellitus. Int Eye Sci (2020) 20(11):1853–7.
40. Wang Y, Li D, Su W, Dai Y. Clinical features, risk factors, and therapy of epithelial keratitis after cataract surgery. J Ophthalmol (2021) 2021:6636228. doi: 10.1155/2021/6636228
41. Zhang C, Xi L, Zhao S, Wei R, Huang Y, Yang R, et al. Interleukin-1β and tumour necrosis factor-α levels in conjunctiva of diabetic patients with symptomatic moderate dry eye: case-control study. BMJ Open (2016) 6(8):e010979. doi: 10.1136/bmjopen-2015-010979
42. Zhang K, Zhang S, Yu J, Lu Y, Zhu X. Changes of the tear film lipid layer thickness after cataract surgery in patients with diabetes mellitus. Acta Ophthalmol (2021) 99(2):e202–e8. doi: 10.1111/aos.14565
43. Fan F, Li X, Li K, Jia Z. To find out the relationship between levels of glycosylated hemoglobin with meibomian gland dysfunction in patients with type 2 diabetes. Ther Clin Risk Manage (2021) 17:797–807. doi: 10.2147/TCRM.S324423
44. Liang S, Niu W, Wang H, Yang L. Alterations of ocular surface parameters in type 2 diabetic patients. Diabetes Metab Syndrome Obes: Targets Ther (2021) 14:3787–93. doi: 10.2147/DMSO.S323770
45. Akinci A, Cetinkaya E, Aycan Z. Dry eye syndrome in diabetic children. Eur J Ophthalmol (2007) 17(6):873–8. doi: 10.1177/112067210701700601
46. Inanc M, Kiziltoprak H, Hekimoglu R, Tekin K, Ozalkak S, Koc M, et al. Alterations of tear film and ocular surface in children with type 1 diabetes mellitus. Ocular Immunol Inflamm (2020) 28(3):362–9. doi: 10.1080/09273948.2019.1571212
47. Çakır BK, Katırcıoğlu Y, Ünlü N, Duman S, Üstün H. Ocular surface changes in patients treated with oral antidiabetic drugs or insulin. Eur J Ophthalmol (2016) 26(4):303–6. doi: 10.5301/ejo.5000710
48. Celikay O, Kosker M, Çalışkan S, Cakal E, Pinarli FA, Gurdal C. Ocular surface assessment in maturity-onset diabetes of the young. Int J Diabetes Develop Countries (2021) 41(1):136–40. doi: 10.1007/s13410-020-00843-2
49. Ozdemir M, Buyukbese MA, Cetinkaya A, Ozdemir G. Risk factors for ocular surface disorders in patients with diabetes mellitus. Diabetes Res Clin Pract (2003) 59(3):195–9. doi: 10.1016/S0168-8227(02)00244-9
50. Gunay M, Celik G, Yildiz E, Bardak H, Koc N, Kirmizibekmez H, et al. Ocular surface characteristics in diabetic children. Curr Eye Res (2016) 41(12):1526–31. doi: 10.3109/02713683.2015.1136421
51. Aksoy Aydemir G, Aydemir E, Asik A. Changes in tear meniscus analysis of children who have type 1 diabetes mellitus, with and without vitamin d deficiency. Cornea (2021) 41(11):1412–7. doi: 10.1097/ICO.0000000000002908
52. Koca S, Koca SB, İnan S. Ocular surface alterations and changes of meibomian glands with meibography in type 1 diabetic children. Int Ophthalmol (2022) 42(5):1613–21. doi: 10.1007/s10792-021-02155-8
53. Akil H, Buluş AD, Andiran N, Alp MN. Ocular manifestations of type 1 diabetes mellitus in pediatric population. Indian J Ophthalmol (2016) 64(9):654–8. doi: 10.4103/0301-4738.194336
54. Stuard WL, Titone R, Robertson DM. Tear levels of insulin-like growth factor binding protein 3 correlate with subbasal nerve plexus changes in patients with type 2 diabetes mellitus. Invest Ophthalmol Visual Sci (2017) 58(14):6105–12. doi: 10.1167/iovs.17-22425
55. Beckman KA. Characterization of dry eye disease in diabetic patients versus nondiabetic patients. Cornea (2014) 33(8):851–4. doi: 10.1097/ICO.0000000000000163
56. Dogru M, Kaderli B, Gelisken O, Yücel A, Avci R, Goto E, et al. Ocular surface changes with applanation contact lens and coupling fluid use after argon laser photocoagulation in noninsulin-dependent diabetes mellitus. Am J Ophthalmol (2004) 138(3):381–8. doi: 10.1016/j.ajo.2004.04.008
57. Dogru M, Katakami C, Inoue M. Tear function and ocular surface changes in noninsulin-dependent diabetes mellitus. Ophthalmology (2001) 108(3):586–92. doi: 10.1016/S0161-6420(00)00599-6
58. Saito J, Enoki M, Hara M, Morishige N, Chikama TI, Nishida T. Correlation of corneal sensation, but not of basal or reflex tear secretion, with the stage of diabetic retinopathy. Cornea (2003) 22(1):15–8. doi: 10.1097/00003226-200301000-00004
59. Gekka M, Miyata K, Nagai Y, Nemoto S, Sameshima T, Tanabe T, et al. Corneal epithelial barrier function in diabetic patients. Cornea (2004) 23(1):35–7. doi: 10.1097/00003226-200401000-00006
60. Inoue K, Kato S, Ohara C, Numaga J, Amano S, Oshika T. Ocular and systemic factors relevant to diabetic keratoepitheliopathy. Cornea (2001) 20(8):798–801. doi: 10.1097/00003226-200111000-00004
61. Trindade M, Castro de Vasconcelos J, Ayub G, Grupenmacher AT, Gomes Huarachi DR, Viturino M, et al. Ocular manifestations and neuropathy in type 2 diabetes patients with charcot arthropathy. Front Endocrinol (2021) 12. doi: 10.3389/fendo.2021.585823
62. Alves M, Reinach PS, Paula JS, Vellasco e Cruz AA, Bachette L, Faustino J, et al. Comparison of diagnostic tests in distinct well-defined conditions related to dry eye disease. PloS One (2014) 9(5):e97921. doi: 10.1371/journal.pone.0097921
63. De Freitas GR, Ferraz GAM, Gehlen M, Skare TL. Dry eyes in patients with diabetes mellitus. Prim Care Diabetes (2021) 15(1):184–6. doi: 10.1016/j.pcd.2020.01.011
64. Garcia DM, de Oliveira FR, Módulo CM, Faustino J, Barbosa AP, Alves M, et al. Is sjögren’s syndrome dry eye similar to dry eye caused by other etiologies? discriminating different diseases by dry eye tests. PloS One (2018) 13(12):e0208420. doi: 10.1371/journal.pone.0208420
65. Ferdousi M, Petropoulos IN, Kalteniece A, Azmi S, Ponirakis G, Efron N, et al. No relation between the severity of corneal nerve, epithelial, and keratocyte cell morphology with measures of dry eye disease in type 1 diabetes. Invest Ophthalmol Vis Sci (2018) 59(13):5525–30. doi: 10.1167/iovs.18-25321
66. Cousen P, Cackett P, Bennett H, Swa K, Dhillon B. Tear production and corneal sensitivity in diabetes. J Diabetes Complications (2007) 21(6):371–3. doi: 10.1016/j.jdiacomp.2006.05.008
67. Kesarwani D, Rizvi SWA, Khan AA, Amitava AK, Vasenwala SM, Siddiqui Z. Tear film and ocular surface dysfunction in diabetes mellitus in an Indian population. Indian J Ophthalmol (2017) 65(4):301–4. doi: 10.4103/ijo.IJO_939_15
68. Manchikanti V, Kasturi N, Rajappa M, Gochhait D. Ocular surface disorder among adult patients with type II diabetes mellitus and its correlation with tear film markers: A pilot study. Taiwan J Ophthalmol (2021) 11(2):156–60. doi: 10.4103/tjo.tjo_56_20
69. Onyekwelu OM, Aribaba OT, Onyekwelu VI, Idowu OO, Salami MO, Badmos KB, et al. Correlation between clinical and cytological parameters of dry eye among diabetics in a Nigerian tertiary hospital. Int Ophthalmol (2020) 40(8):2055–64. doi: 10.1007/s10792-020-01382-9
70. Baek J, Doh SH, Chung SK. Assessment of the tear meniscus using optical coherence tomography in patients with type 2 diabetes mellitus. Cornea (2015) 34(12):1534–40. doi: 10.1097/ICO.0000000000000651
71. Yoon KC, Im SK, Seo MS. Changes of tear film and ocular surface in diabetes mellitus. Korean J Ophthalmol (2004) 18(2):168–74. doi: 10.3341/kjo.2004.18.2.168
72. Andersen J, Baun O, Aamand HE. Tear secretion in juvenile diabetics with and without autonomic neuropathy. Acta Ophthalmol (1985) 63(1):93–6. doi: 10.1111/j.1755-3768.1985.tb05223.x
73. Goebbels M. Tear secretion and tear film function in insulin dependent diabetics. Br J Ophthalmol (2000) 84(1):19–21. doi: 10.1136/bjo.84.1.19
74. Oriowo OM. Profile of central corneal thickness in diabetics with and without dry eye in a Saudi population. Optometry (2009) 80(8):442–6. doi: 10.1016/j.optm.2008.12.008
75. Stolwijk TR, van Best JA, Lemkes HH, de Keizer RJ, Oosterhuis JA. Determination of basal tear turnover in insulin-dependent diabetes mellitus patients by fluorophotometry. Int Ophthalmol (1991) 15(6):377–82. doi: 10.1007/BF00137948
76. Symeonidis C, Papakonstantinou E, Galli A, Tsinopoulos I, Mataftsi A, Batzios S, et al. Matrix metalloproteinase (MMP-2, -9) and tissue inhibitor (TIMP-1, -2) activity in tear samples of pediatric type 1 diabetic patients: MMPs in tear samples from type 1 diabetes. Graefe’s Arch Clin Exp Ophthalmol (2013) 251(3):741–9. doi: 10.1007/s00417-012-2221-3
77. Chang SW, Hsu HC, Hu FR, Chen MS. Corneal autofluorescence and epithelial barrier function in diabetic patients. Ophthal Res (1995) 27(2):74–9. doi: 10.1159/000267600
78. Sandra Johanna GP, Antonio LA, Andrés GS. Correlation between type 2 diabetes, dry eye and meibomian glands dysfunction. J Optomet (2019) 12(4):256–62. doi: 10.1016/j.optom.2019.02.003
79. Aljarousha M, Badarudin NE, Che Azemin MZ. Comparison of dry eye parameters between diabetics and non-diabetics in district of kuantan, pahang. Malaysian J Med Sci (2016) 23(3):72–7.
80. Derakhshan A, Abrishami M, Khajedaluee M, Omidtabrizi A, Moghaddam SG. Comparison between tear film osmolar cocentration and other tear film function parameters in patients with diabetes mellitus. Korean J Ophthalmol (2019) 33(4):326–32. doi: 10.3341/kjo.2013.0146
81. Eissa IM, Khalil NM, El-Gendy HA. A controlled study on the correlation between tear film volume and tear film stability in diabetic patients. J Ophthalmol (2016) 2016:5465272. doi: 10.1155/2016/5465272
82. Tóth N, Silver DM, Balla S, Káplár M, Csutak A. In vivo corneal confocal microscopy and optical coherence tomography on eyes of participants with type 2 diabetes mellitus and obese participants without diabetes. Graefes Arch Clin Exp Ophthalmol (2021) 259(11):3339–50. doi: 10.1007/s00417-021-05251-8
83. Najafi L, Malek M, Valojerdi AE, Aghili R, Khamseh ME, Fallah AE, et al. Dry eye and its correlation to diabetes microvascular complications in people with type 2 diabetes mellitus. J Diabetes Its Complications (2013) 27(5):459–62. doi: 10.1016/j.jdiacomp.2013.04.006
84. Alves Mde C, Carvalheira JB, Módulo CM, Rocha EM. Tear film and ocular surface changes in diabetes mellitus. Arq Bras Oftalmol (2008) 71(6 Suppl):96–103. doi: 10.1590/s0004-27492008000700018
85. Craig JP, Wang MT. Factors predisposing the Asian eye to dry eye disease. Invest Ophthalmol Visual Sci (2019) 60(9):2746–.
86. Uchino M. What we know about the epidemiology of dry eye disease in Japan. Invest Ophthalmol Visual Sci (2018) 59(14):DES1–6. doi: 10.1167/iovs.17-23491
87. Butovich IA, Suzuki T, Wojtowicz J, Bhat N, Yuksel S. Comprehensive profiling of Asian and Caucasian meibomian gland secretions reveals similar lipidomic signatures regardless of ethnicity. Sci Rep (2020) 10(1):14510. doi: 10.1038/s41598-020-71259-5
88. de Paiva CS. Effects of aging in dry eye. Int Ophthalmol Clin (2017) 57(2):47–64. doi: 10.1097/IIO.0000000000000170
89. Sharma A, Hindman HB. Aging: A predisposition to dry eyes. J Ophthalmol (2014) 2014:781683. doi: 10.1155/2014/781683
90. Obata H, Yamamoto S, Horiuchi H, Machinami R. Histopathologic study of human lacrimal gland. statistical analysis with special reference to aging. Ophthalmology (1995) 102(4):678–86. doi: 10.1016/S0161-6420(95)30971-2
91. Bukhari AA, Basheer NA, Joharjy HI. Age, gender, and interracial variability of normal lacrimal gland volume using MRI. Ophthal Plast Reconstr Surg (2014) 30(5):388–91. doi: 10.1097/IOP.0000000000000117
92. Chhadva P, McClellan AL, Alabiad C, Feuer WJ, Batawi H, Galor A. Impact of eyelid laxity on symptoms and signs of dry eye disease. Cornea (2016) 35:531 5. doi: 10.1097/ICO.0000000000000786
93. Hykin PG, Bron AJ. Age-related morphological changes in lid margin and meibomian gland anatomy. Cornea (1992) 11(4):334–42. doi: 10.1097/00003226-199207000-00012
Keywords: tear function, diabetes mellitus, dry eye, keratoconjunctivitis sicca, glycemic control
Citation: Kuo Y-K, Shao S-C, Lin E-T, Pan L-Y, Yeung L and Sun C-C (2022) Tear function in patients with diabetes mellitus: A systematic review and meta-analysis. Front. Endocrinol. 13:1036002. doi: 10.3389/fendo.2022.1036002
Received: 03 September 2022; Accepted: 29 September 2022;
Published: 21 October 2022.
Edited by:
Pranav Kumar Prabhakar, Lovely Professional University, IndiaReviewed by:
Prity Sahay, All India Institute of Medical Sciences, IndiaPranay Pankaj, Jaipur National University, India
Copyright © 2022 Kuo, Shao, Lin, Pan, Yeung and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chi-Chin Sun, YXJ2aW4uc3VuQG1zYS5oaW5ldC5uZXQ=
 Yu-Kai Kuo1,2
Yu-Kai Kuo1,2 Shih-Chieh Shao
Shih-Chieh Shao Li-Yen Pan
Li-Yen Pan Chi-Chin Sun
Chi-Chin Sun