
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 09 December 2022
Sec. Cancer Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1026737
This article is part of the Research Topic A Year in Review: Discussions in Cancer Endocrinology View all 5 articles
Purpose: Current staging criteria for papillary thyroid cancer (PTC) do not include the number of metastatic lymph nodes (LNs), which is highly predictive of survival in multiple cancers. The LN metastasis burden is particularly relevant for older adults with thyroid cancer because of their poor prognosis. We examined a modified staging system for this population utilizing node number (Nn).
Methods: Overall, 14,341 patients aged 55 years or older with stage I-IVB PTC were identified in the 2004–2015 Surveillance, Epidemiology and End Results database. Cox regression models were conducted to test the relationship between positive LN number and PTC-specific survival (PTCSS). Independent training/validation sets were used to derive and validate a new revised TNnM grouping. The 8th edition American Joint Committee on Cancer TNM staging system was compared with TNnM stage by calculating the 10-year PTCSS rates, Harrell’s concordance index (C-index), and Akaike’s information criterion (AIC).
Results: An increase in number of LN metastases was identified as an independent, negative prognostic factor for PTCSS in multivariate analysis. 10-year PTCSS for stage I-IVB based on the AJCC 8th edition TNM were 98.83%, 93.49%, 71.21%, 72.95%, and 58.52%, respectively, while 10-year PTCSS for the corresponding stage in the TNnM were 98.59%, 92.2%, 83.26%, 75.24%, and 56.73%, respectively. The revised TNnM stage was superior, with a higher C-index and a lower AIC in both the training and validation cohorts.
Conclusion: The TNnM staging system for PTC patients ≥ 55 years could be associated with improved outcomes. External validation studies of this system are warranted.
Papillary thyroid cancer (PTC) is the most common endocrine malignancy, and its incidence has risen rapidly worldwide over the last 30 years (1, 2). In the United States, in 2021, approximately 42% of patients who were newly diagnosed with thyroid cancer were ≥ 55 years of age (3), and this proportion is projected to grow as the population ages (4). Older patients with PTC present more often with more advanced diseases, larger median tumor sizes, higher rates of node positivity, and higher propensities for distant metastatic spread when compared to their younger counterparts (5–7). In addition, the older age group accounts for the overwhelming majority of thyroid cancer deaths (6–8). Given the poor overall prognosis of PTC in older adults, there is a strong need for more precise staging methods to predict patient survival and help tailor individualized treatment approaches.
According to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system for PTC, among patients who are diagnosed at 55 years of age or older, positive nodal involvement in the absence of gross extrathyroidal extension or distant metastases is considered as stage II disease. However, this stage group classification considers only one lymph node (LN) factor: N0 (no positive nodes) or NX (regional nodes cannot be assessed) versus an N1 (positive nodes) status, and it does not account for the number of metastatic LNs (9), which is a well-established predictor of mortality and included in the AJCC staging for a variety of other cancers (10–15). Prior studies have proposed modifications to the definitions of the AJCC (8th edition) staging manual for PTC. The results from these studies suggest that incorporation of the following additional prognostic factors into the AJCC 8th edition can create prognostically accurate cancer staging systems: multiple age cutoffs, American Thyroid Association risk stratification system, and comorbidities (16–18). However, to date, there is a paucity of data on the prognostic performance of proposed stage schemes by the inclusion of involved node number. Moreover, while a previous study has demonstrated the prognostic significance of positive LN numbers in younger patients with PTC (19), few studies have examined the impact of the number of nodal metastases in older adults. Thus, whether the positive node number is optimal for PTC prognosis of this population or whether replacement of the current nodal classification (e.g., N0, N1a, N1b) with LN number would result in better overall prognostic stage groupings remains unknown.
The number of metastatic LNs detected is dependent on the extent of LN dissection and the intensity of pathologic evaluation. In this condition, in addition to the AJCC N stage, some studies have suggested that LN status should be described by LN density (LND) (20, 21), which is the number of positive LNs divided by the total number of harvested nodes. LND correlates with the total LN yield and is a surrogate of adequacy of neck dissection. Several articles have shown that use of the LND allowed investigators to better stratify the prognosis after resection of malignant neoplasms of the head and neck (22, 23). However, debate exists about whether the most accurate prognostic factor in PTC patients is the number of positive LNs or the LND.
In this study, given that the relative importance of other LN factors, such as number and LND, has not been systemically investigated previously in older patients with PTC, we use population-based data to evaluate the association of the numerical metastatic nodal disease burden with PTC-specific survival (PTCSS) among patients with PTC who were aged ≥ 55 years at the time of diagnosis and propose modified N category-based AJCC TNM staging systems that may provide patients with more accurate survival estimates.
We conducted a retrospective cohort study using the Surveillance, Epidemiology, and End Results (SEER) database, which collects data on cancer occurrences in 18 geographic regions covering 28% of the US population (24). Individuals who were aged ≥ 55 years with a diagnosis of PTC (International Classification of Diseases for Oncology, third edition histology codes 8050/3, 8260/3, and 8340-8344/3), between 2004 and 2015, were assessed (n = 44,414). 9,305 patients were excluded due to previous cancers. Patients not undergoing surgery of a primary site, removal of less than a thyroid lobe, or with unknown surgery status were eliminated (n = 2,036). Patients who had no node examined (n = 17,845), incomplete information on stage of disease (n = 195) or LN data (n = 640), or missing vital status (n = 52) were also excluded, leaving 14,341 eligible individuals for the study (Figure 1). To establish the prognostic model, the cohort was split into a training set containing the sample diagnosed with PTC between 2004 and 2009, and a validation set including the participants diagnosed between 2010 and 2015. The research protocol used de-identified data and was exempted by the Southern Medical University from institutional review board review.
Variables obtained within the SEER database included patient age at the time of diagnosis, sex (male or female), race (white, black, other, or unknown), period of diagnosis, tumor size, tumor extension, nodal stage, presence of distant metastases (yes or no), number of nodes removed, number of nodal metastases, extent of thyroidectomy (thyroid lobectomy or total thyroidectomy), RAI therapy (yes or no), survival months, and cause of death. The LN density (LND) was defined as the ratio of the number of positive nodes to the number of nodes removed. Lobectomy was defined as removal of a thyroid lobe with or without isthmectomy, and total thyroidectomy included total, near-total, or subtotal thyroid resection. RAI was documented as if patients received radioisotopes. Stages were classified based on the guidelines of the AJCC 8th edition. PTCSS was defined as the time interval (in months) from surgery until death from PTC.
We summarized baseline patient data using descriptive statistics. We estimated survival curves via the Kaplan–Meier method, and we used log-rank tests to compare differences in survival. For correlation of prognostic factors with survival in PTC, we carried out univariate and multivariable analyses by employing Cox regression models. Prognostic factors were kept in the multivariate model only if statistically significant on univariable analysis. Using X-tile software 3.6.1 version via an internal cross-validation method based on the minimum P values from log-rank χ2 statistics (Rimm Lab, New Haven, CT) (25), we identified the optimal cut-off points for positive node number and defined a new Nn category. To propose revised TNnM stages, first, we divided the entire training cohort into 16 data subsets using varying T, Nn, and M category combinations (eTable in the supplement). Then, these subsets were amalgamated into 5 homogeneous categories consistent with those of the current TNM stage groupings, based on the principle of similar 10-year PTCSS estimate within the same stage grouping and maximum differences in 10-year PTCSS estimate among different stage groupings. A concordance index (C-index) was computed to compare the discrimination ability of the proposed TNnM and AJCC 8th edition system (26). Internal validation using standard bootstrapping techniques (1000 replications) was performed, and optimism-corrected estimates of the C-index were calculated for each system. Akaike’s information criterion (AIC) was also reported for each model. The AIC provided a relative measure of model quality; smaller values correspond with a better fitting model. As a general guideline, differences ≥ 10 indicate substantial improvement in the fit of the model (27). Significance between the C-index of the revised TNnM and current TNM stage models was determined using the Z testing method in the “CsChange” package in R (28). Analyses were conducted using R, version 4.0.3 (R Foundation). Two-sided P value < 0.05 was considered statistically significant.
Between January 2004 and December 2015, a total of 14,341 patients who were aged 55 years or older with stage I to IVB PTC met inclusion criteria (Figure 1), thereof 4,971 patients in the training set and 9,370 patients in the validation set. Patient baseline characteristics for the two groups are displayed in Table 1. The nodal staging of the entire study cohort was N0, N1a, N1b, and unknown was 62.5%, 20.9%, 14.4%, and 3.4%, respectively. The mean number of positive LNs, LN count, and LND was 1.6, 6.5, and 0.2, respectively, for the training cohort and 1.8, 8.0, and 0.2, respectively, for the validation cohort.
During a median follow-up of 125 months (range, 0 to 179 months), a total of 372 patients (7.5%) aged 55 years or older died of thyroid cancer in the training cohort. Factors correlated with PTCSS are listed in Table 2. In the univariate analysis, an increasing number of metastatic nodes was associated with significantly compromised PTCSS, with a hazard ratio (HR) of 1.09 (95% confidence interval [CI] 1.08–1.11; P < 0.001). On multivariable analysis, increasing involved node number remained a significant independent predictor of poor PTCSS (HR 1.02, 95% CI 1.00–1.04; P = 0.041). Additionally, the results also revealed that both stage N1a and N1b were independent risk factors for survival (both P < 0.001). However, LND had no independent impact on survival (P = 0.8).
Using X-tile, we have identified optimal cut-off points of the positive node number, which were 0, 1, and 4 in the training set (Figure 2). Based on this result, we proposed a revised Nn staging criteria, in which N0n indicates no evidence of LN metastasis, N1n indicates 1 to 4 metastatic nodes, and N2n indicates > 4 metastatic nodes. These patients in the training cohort were classified into 16 TNnM groups corresponding to different combinations of 8th edition AJCC T/M and Nn classification (1): T1N0nM0 (2), T2N0nM0 (3), T3N0nM0 (4), T4aN0nM0 (5), T4bN0nM0 (6), T1N1nM0 (7), T2N1nM0 (8), T3N1nM0 (9), T4aN1nM0 (10), T4bN1nM0 (11), T1N2nM0 (12), T2N2nM0 (13), T3N2nM0 (14), T4aN2nM0 (15), T4bN2nM0, and (16) T-anyNn-anyM1 (eTable in the supplement). Review of the 10-year PTCSS estimates for the above 16 groups showed that the patients could be amalgamated into the following proposed stage groupings: stage I (T1-2N0nM0 or T1N1nM0); stage II (T3N0nM0 or T2N1nM0 or T1-2N2nM0); stage III (T4N0nM0 or T3N1nM0); stage IVA (T4N1nM0 or T3N2nM0); stage IVB (T4N2nM0 or T-anyNn-anyM1) (Table 3). Under the proposed TNnM groupings of the 4,971 patients, 3,388 (68.2%) were stage I, 719 (14.5%) stage II, 215 (4.3%) stage III, 265 (5.3%) stage IVA, and 384 (7.7%) stage IVB (Table 3). The adjusted multivariable survival analysis showed a significant trend for an increased risk of PTC-specific mortality among older patients in each successive TNnM stage grouping (P for trend < 0.001).
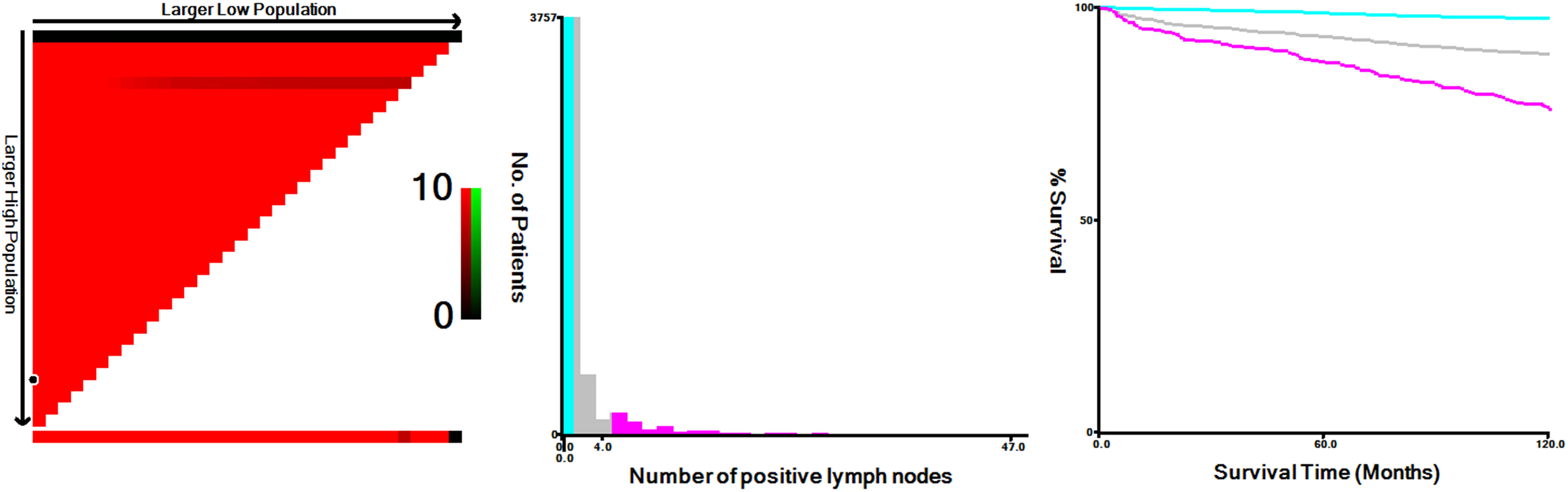
Figure 2 X-tile analysis of 10-year disease-specific survival using patients’ data in SEER registry. The optimal cut-off values for the positive node number were 0, 1 and 4 in the training cohort (χ2 = 333.0712, P < 0.0001).
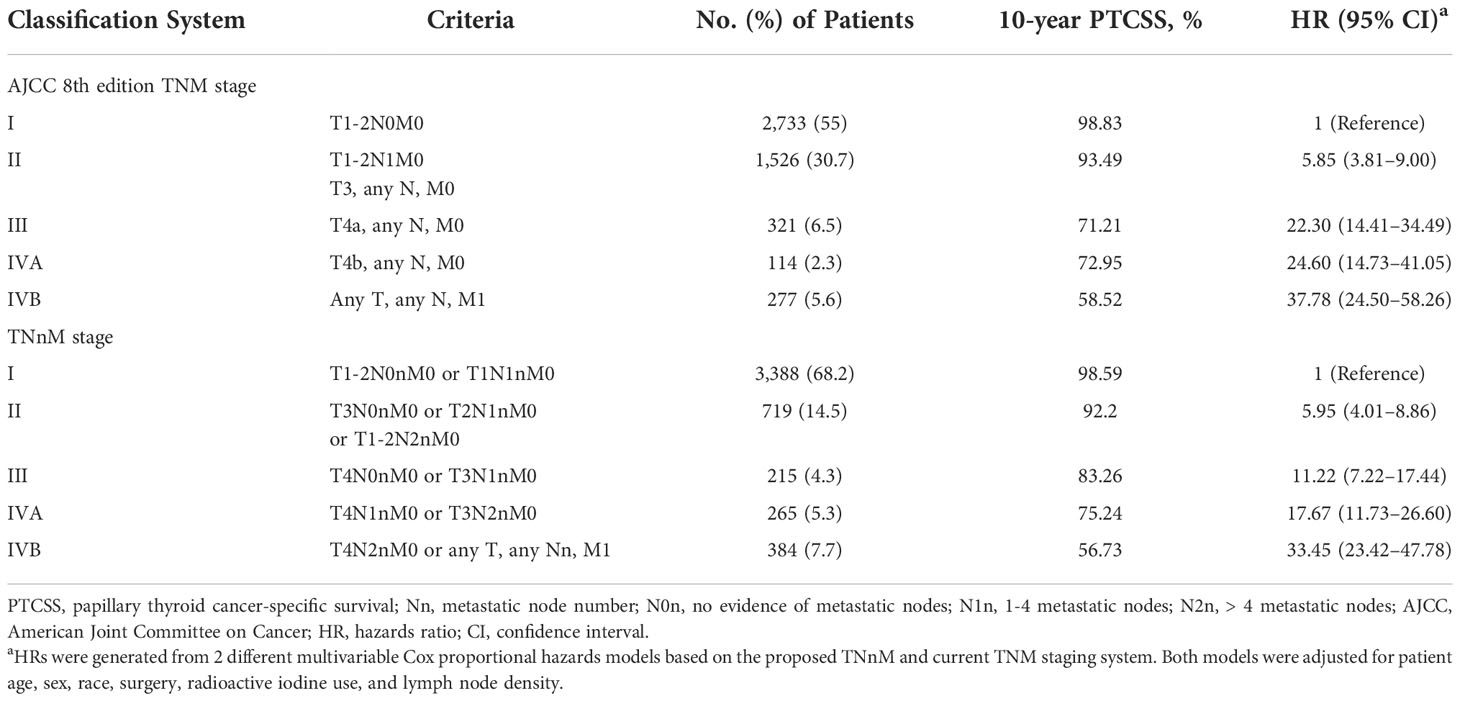
Table 3 Survival for AJCC 8th edition TNM and TNnM staging systems for patients aged ≥ 55 years with papillary thyroid cancer.
Kaplan–Meier survival graphs for the TNnM and current TNM staging are illustrated in Figure 3. The 10-year PTCSS rates for I-IVB AJCC stages were 98.83, 93.49, 71.21, 72.95, and 58.52%, respectively (log-rank P < 0.001, Figure 3A), while that for I-IVB stages of the proposed TNnM groupings were 98.59, 92.2, 83.26, 75.24, and 56.73%, respectively (log-rank P < 0.001, Figure 3B). Of note, no significant difference was observed in the survival rate between the current AJCC stage III and IVA disease (log-rank P = 0.86). In contrast, the revised TNnM system demonstrated an improvement in separation of stage III versus IVA disease (log-rank P = 0.008).
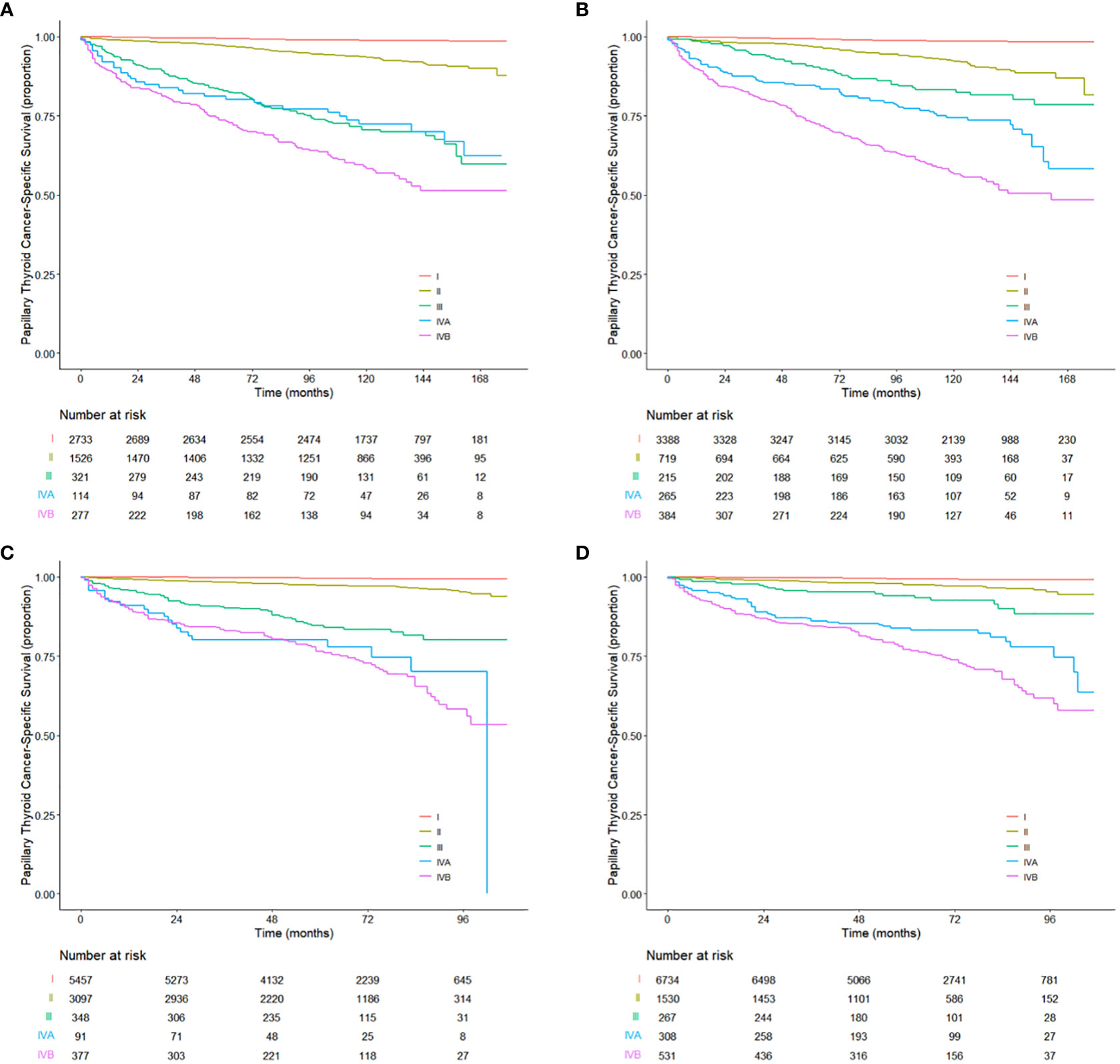
Figure 3 Kaplan-Meier estimates for the patients aged 55 years or older from (A) the training cohort and (C) the validation cohort using the current TNM staging system. Kaplan-Meier estimates for the patients aged 55 years or older from (B) the training cohort and (D) the validation cohort using the revised TNnM staging system.
Furthermore, the revised TNnM stage grouping had better PTCSS predictive ability than the AJCC 8th edition system (indicated by the higher C-index: 0.871, 95% CI 0.852–0.889 and 0.855, 95% CI 0.836–0.873, for the models with the TNnM and AJCC, respectively; difference in C-index 0.016, 95% CI 0.007–0.026, P < 0.001). The optimism-corrected, bootstrapped C-index values were similar after the internal validation (0.869 vs. 0.853). The revised TNnM system also provided a better fit for the data (indicated by the lower AIC value: 5347 vs. 5458).
The utility of the revised TNnM staging system was subsequently evaluated in an independent validation cohort of 9,370 patients aged ≥ 55 years with PTC from the 2010–2015 SEER database, using the same analyses as for the training cohort. The median follow-up period for the validation data set was 64 months (range, 0 to 107 months). There were 288 death (3.1%) events attributed to PTC. Notably, when stratifying patients by the 8th edition AJCC system, no significant difference in PTCSS was seen in patients with stage IVA disease compared with those with stage IVB diseases (HR 1.10, 95% CI 0.69–1.76; P = 0.7; Figure 3C). However, the survival curves between stage IVA and IVB disease in the revised TNnM system exhibited were clearly distinguishable (HR 1.52, 95% CI 1.10–2.09; P = 0.01; Figure 3D). The proposed TNnM system outperformed the current AJCC TNM system in the validation population, with a higher C-index in discrimination (0.900 vs. 0.886; difference in C-index 0.014, 95% CI 0.03–0.025, P = 0.015) and better statistical model fit with lower AIC (4263 vs. 4339).
An accurate staging system is critical to convey the extent of disease, help guide treatment selection, and inform prognosis. Currently, the 8th edition TNM classification of differentiated thyroid cancer proposed by the AJCC is being followed in clinical practice. This system for PTC incorporates LN positive and the anatomic location of positive LNs (9), but does not consider the total number of tumor-positive nodes as a staging variable, which is characterized as a predominant prognostic factor in most head and neck cancer patients (14, 19, 29). Thus, it is necessary to develop alternative stage groupings including positive node number that could be used to further refine prognostic information.
In this large study of 14,341 patients 55 years or older who underwent surgery for PTC, we showed an increasing number of LN metastases to be significantly associated with compromised PTCSS. Furthermore, we re-defined the regional LN classification according to the total number of metastatic positive nodes: N0n, no node metastasis; N1n, 1–4 metastatic nodes, and N2n, > 4 metastatic nodes. We further used objective criteria to create a new, internally validated TNnM staging with performance superior to that of the traditional 8th edition TNM staging. To our knowledge, no modified disease-specific staging schemes that incorporate the number of metastatic nodes exist for older patients with AJCC stage I to IVB PTC. Once externally validated in large cohorts, this system could then have the potential to become an evidence-based adjunct aiding in personalized treatment, discussions of prognosis, and stratification of future clinical trials for PTC patients aged 55 years or older.
Our findings support a previous study by Adam et al. (19), who showed that in a cohort of 30,193 individuals with PTC, the number of metastatic cervical LNs is a critical predictor of overall mortality. However, the study by Adam et al. included only patients who were diagnosed at < 45 years of age without distant metastases, and their study was conducted over an earlier and longer period than our own (1988–2006 vs. 2004–2015). Similar risk estimates between increasing positive node numbers and compromised survival have also been observed in two recent studies (30, 31). The results of our study add to these findings by its adjustment for important LN parameters, such as nodal stage and LNR, and by focusing exclusively on patients who were aged ≥ 55 years, at a relatively high risk of mortality from thyroid cancer, and accounted for a growing proportion of patients with PTC with increasing life expectancy. Our work suggests that the number of LNs involved would have a substantial proposed revision for the current AJCC staging system for the older population.
The TNnM stage is consistent with previous studies which have demonstrated the significance of cervical LN features in prognosis for patients with PTC. In a retrospective analysis of 2,542 patients with PTC from the MD Anderson between 2000 and 2015 (21), a new modified TNM staging schema was proposed, incorporating LN ratio in the current AJCC system, which stratified the risk groups better with respect to overall survival and PTCSS than the traditional categorical TNM system. A limitation of that analysis was that it did not adjust for the total number of nodal metastases, which may, at least partly, affect the relationship between LN ratio and patient survival. In addition, this study did not report in detail how to derive the LN ratio-based staging system, but only listed the final stage groupings. A recent study from the Republic of Korea that included 745 N1b PTC patients without distant metastases found an increase in predictive ability (32), with a C-index of 0.84 and 0.87 for the 8th edition TNM classification and the alternative prognostic grouping using lateral LN ratio and largest LN size, respectively. The result that positive LN number rather than LN ratio was statistically associated with survival in our series is in disagreement with the above two studies. One possible explanation could be variances in the population chosen, the therapeutic standards used, the duration of the follow-ups, and the methodologies and statistics considered.
Existing staging guidelines for PTC remove nodal factors (e.g., location, size, or number of nodes involved) from stage III or IV disease; such revisions may not be regarded as clinically appropriate for older patients. Indeed, the AJCC 8th edition system failed to clearly distinguish PTCSS rates of the patients between stages III and IVA in this study. Similar to our findings, Tam et al. performed a retrospective review of 2,579 patients with differentiated thyroid cancer (33), 56 had AJCC 8th edition stage III tumors, and 67 had stage IV tumors. They did not find any statistically significant differences in disease-specific survival between stage III and IV patients. Using a new revised N classification according to the positive node number, the proposed TNnM staging system could resolve all overlaps of survival estimates in the AJCC stages in both the derivation and validation data sets. In addition, C-index and AIC confirm the predictive superiority of this proposed TNnM system over the current 8th edition AJCC TNM system.
Recently, several cohort studies on the Asian population have also tried to modify the eighth edition of the TNM staging system for PTC by incorporating a variety of clinicopathologic characteristics of metastatic LNs. Li et al. and colleagues (34) conducted a single-center retrospective study, including 6,165 Chinese individuals, which found that PTC patients with extranodal extension present worse PTCSS and incorporating extranodal extension in TNM classification identifies poor-risk patients more accurately. Moreover, two studies have demonstrated that sub-classification based on the location of the metastatic LNs in older patients with stage I/II can predict disease-specific mortality more precisely than the current AJCC TNM system in the Korean population (35, 36). In a study involving Japanese patients (37), investigators revealed that the revised TNM staging employing the size of positive nodes could be more useful for the prediction of PTCSS. However, until now, few data have been available on variation in the prognostic implication of nodal metastases, as well as that of concurrent predictors such as location, size, number of LNs involved, percentage of LNs involved, and extranodal extension, between Asian and the U.S. populations and among different countries. Additional international multicenter studies are necessary to assess the prognostic impact of LN characteristics in PTC.
The prognostic significance of LN involvement might differ according to the patient age at diagnosis. For patients with PTC aged 55 years or older, the data have consistently demonstrated decreased survival in the context of cervical LN metastases (38, 39). Our findings reported in the present study further corroborate that older patients ≥ 55 years presenting with N1 disease at presentation have poor PTCSS compared with patients with N0 disease. In younger patients with PTC, however, the overall impact of nodal metastases on long-term survival is debatable. Based on the current TNM staging system, patients < 55 years with PTC without distant metastases are all classified as having stage I disease even if they harbor lateral neck disease or extensive cervical LN metastases (9). However, recent large-scale cohort studies have reported N1b disease and an increasing number of positive nodes to be prognostic in patients less than 55 years of age at diagnosis of PTC (19, 35). In addition, large data examining the independent impact of LND on long-term PTCSS for patients less than 55 years are lacking.
Our study had several strengths. The SEER database provided a large volume of contemporary patients who were aged ≥ 55 years, which was associated with an increase in statistical power. In addition, the database contains cases from 18 registry areas in the United States, thereby helping to reduce the bias that could be derived from a single institutional study. Thus, the generalizability of the results was satisfactory. Owing to these notable strengths, the SEER registry data have been frequently utilized to study thyroid cancer staging (40–42). Our study also had some limitations. First, some selection biases were inevitable because of the retrospective and observational nature of this study. It was, therefore, difficult to draw an absolute conclusion that the TNnM staging system is superior to the 8th edition TNM classification for older patients with PTC. Second, our proposed staging system should be externally validated in a new cohort, although rigorous internal validation was conducted. Third, we were unable to control for potentially important variables that could have affected survival outcomes, such as vascular invasions, sizes of the largest positive LN, and extra-nodal extensions (43–45), owing to their absence from the data set. Finally, participants in this investigation were all aged ≥ 55 years and diagnosed with PTC, so further confirmation of the proposed TNnM staging system’s generalization into young populations and other thyroid cancer subtypes is needed.
We established the critical importance of metastatic cervical LN burden in delineating the prognosis among PTC patients aged ≥ 55 years, with increased positive nodes conferring worse PTCSS. Moreover, we present a proposed TNnM staging scheme that incorporates metastatic node number into the current AJCC system to improve long-term PTCSS prediction. After external validation, the proposed system should be considered in future reviews of the AJCC 8th edition.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Study concepts and design: Y-GS. Data acquisition: FC, Q-LS. Quality control of data and algorithms: Y-GS, J-YT, X-CH. Data analysis and interpretation: Y-GS, X-CH. Statistical analysis: Y-GS, FC. Manuscript preparation, edition, and review: All authors. All authors contributed to the article and approved the submitted version.
We sincerely thank the SEER database for providing platforms and valuable data sets. We would like to thank Editage (www.editage.com ) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1026737/full#supplementary-material
1. Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol (2020) 16(1):17–29. doi: 10.1038/s41574-019-0263-x
2. Deng Y, Li H, Wang M, Li N, Tian T, Wu Y, et al. Global burden of thyroid cancer from 1990 to 2017. JAMA Netw Open (2020) 3(6):e208759. doi: 10.1001/jamanetworkopen.2020.8759
3. National Cancer Institute. Surveillance, epidemiology, and end results program: SEER fact sheets-thyroid cancer. Available at: https://seer.cancer.gov/statfacts/html/thyro.html (Accessed April, 2022).
4. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin (2007) 57(1):43–66. doi: 10.3322/canjclin.57.1.43
5. Lerch H, Schober O, Kuwert T, Saur HB. Survival of differentiated thyroid carcinoma studied in 500 patients. J Clin Oncol (1997) 15(5):2067–75. doi: 10.1200/JCO.1997.15.5.2067
6. Park HS, Roman SA, Sosa JA. Treatment patterns of aging americans with differentiated thyroid cancer. Cancer (2010) 116(1):20–30. doi: 10.1002/cncr.24717
7. Orosco RK, Hussain T, Brumund KT, Oh DK, Chang DC, Bouvet M. Analysis of age and disease status as predictors of thyroid cancer-specific mortality using the surveillance, epidemiology, and end results database. Thyroid (2015) 25(1):125–32. doi: 10.1089/thy.2014.0116
8. Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the united states, 1974-2013. JAMA (2017) 317(13):1338–48. doi: 10.1001/jama.2017.2719
9. Perrier ND, Brierley JD, Tuttle RM. Differentiated and anaplastic thyroid carcinoma: major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin (2018) 68(1):55–63. doi: 10.3322/caac.21439
10. Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67(6):472–92. doi: 10.3322/caac.21409
11. Tarantino I, Warschkow R, Hackert T, Schmied BM, Büchler MW, Strobel O, et al. Staging of pancreatic cancer based on the number of positive lymph nodes. Br J Surg (2017) 104(5):608–18. doi: 10.1002/bjs.10472
12. Giuliano AE, Connolly JL, Edge SB, Mittendorf EA, Rugo HS, Solin LJ, et al. Breast cancer-major changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin (2017) 67(4):290–303. doi: 10.3322/caac.21393
13. Katsumata S, Aokage K, Ishii G, Nakasone S, Sakai T, Okada S, et al. Prognostic impact of the number of metastatic lymph nodes on the eighth edition of the TNM classification of NSCLC. J Thorac Oncol (2019) 14(8):1408–18. doi: 10.1016/j.jtho.2019.04.016
14. Ebrahimi A, Gupta R, Luk P, Low TH, McDowell L, Magarey MJR, et al. Number of nodal metastases and the American joint committee on cancer staging of head and neck cutaneous squamous cell carcinoma: A multicenter study. Oral Oncol (2020) 111:104855. doi: 10.1016/j.oraloncology.2020.104855
15. Nguyen AT, Luu M, DJ L, Hamid O, Mallen-St Clair J, Faries MB, et al. Quantitative metastatic lymph node burden and survival in merkel cell carcinoma. J Am Acad Dermatol (2021) 84(2):312–20. doi: 10.1016/j.jaad.2019.12.072
16. Kim YN, Kim M, Ahn HS, Kim K, Park SY, Kim HI, et al. Refining the tumor-node-metastasis staging system for individualized treatment of differentiated thyroid carcinoma. Oral Oncol (2019) 89:8–13. doi: 10.1016/j.oraloncology.2018.12.014
17. Ghaznavi SA, Ganly I, Shaha AR, English C, Wills J, Tuttle RM. Using the American thyroid association risk-stratification system to refine and individualize the American joint committee on cancer eighth edition disease-specific survival estimates in differentiated thyroid cancer. Thyroid (2018) 28(10):1293–300. doi: 10.1089/thy.2018.0186
18. Karadaghy OA, Kallogjeri D, Piccirillo JF. Development of a new clinical severity staging system for patients with nonmetastatic papillary thyroid carcinoma. JAMA Otolaryngol Head Neck Surg (2017) 143(12):1173–180. doi: 10.1001/jamaoto.2017.0550
19. Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol (2015) 33(21):2370–5. doi: 10.1200/JCO.2014.59.8391
20. Schneider DF, Chen H, Sippel RS. Impact of lymph node ratio on survival in papillary thyroid cancer. Ann Surg Oncol (2013) 20(6):1906–11. doi: 10.1245/s10434-012-2802-8
21. Amit M, Tam S, Boonsripitayanon M, Cabanillas ME, Busaidy NL, Grubbs EG, et al. Association of lymph node density with survival of patients with papillary thyroid cancer. JAMA Otolaryngol Head Neck Surg (2018) 144(2):108–14. doi: 10.1001/jamaoto.2017.2416
22. Sano D, Yabuki K, Takahashi H, Arai Y, Chiba Y, Tanabe T, et al. Is lymph-node ratio a superior predictor than lymph node status for recurrence-free and overall survival in patients with head and neck squamous cell carcinoma? Ann Surg Oncol (2014) 21(6):1912–8. doi: 10.1245/s10434-014-3634-5
23. Machens A, Lorenz K, Weber F, Dralle H. Superiority of metastatic lymph node ratio over number of node metastases and TNM/AJCC n classification in predicting cancer-specific survival in medullary thyroid cancer. Head Neck (2022) 44(12):2717–26. doi: 10.1002/hed.27181
24. National Cancer Institute. Surveillance, epidemiology, and end results. Available at: http://www.seer.cancer.gov (Accessed April 15, 2021).
25. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res (2004) 10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713
26. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med (1996) 15(4):361–87. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
27. Burnham KP, Anderson DR. Model selection and multimodel inference: A practical information-theoretic approach. New York, NY: Springer Verlag New York (2002).
28. Ganz P, Heidecker B, Hveem K, Jonasson C, Kato S, Segal MR, et al. Development and validation of a protein-based risk score for cardiovascular outcomes among patients with stable coronary heart disease. JAMA (2016) 315(23):2532–41. doi: 10.1001/jama.2016.5951
29. Zumsteg ZS, Luu M, Kim S, Tighiouart M, Mita A, KS S, et al. Quantitative lymph node burden as a 'very-high-risk' factor identifying head and neck cancer patients benefiting from postoperative chemoradiation. Ann Oncol (2019) 30(1):76–84. doi: 10.1093/annonc/mdy490
30. Rajeev P, Ahmed S, Ezzat TM, Sadler GP, Mihai R. The number of positive lymph nodes in the central compartment has prognostic impact in papillary thyroid cancer. Langenbecks Arch Surg (2013) 398(3):377–82. doi: 10.1007/s00423-012-1041-6
31. Wang LY, Palmer FL, Nixon IJ, Tuttle RM, Shah JP, Patel SG, et al. Lateral neck lymph node characteristics prognostic of outcome in patients with clinically evident N1b papillary thyroid cancer. Ann Surg Oncol (2015) 22(11):3530–6. doi: 10.1245/s10434-015-4398-2
32. Kim HI, Kim K, Park SY, Choe JH, Kim JH, Kim JS, et al. Refining the eighth edition AJCC TNM classification and prognostic groups for papillary thyroid cancer with lateral nodal metastasis. Oral Oncol (2018) 78:80–6. doi: 10.1016/j.oraloncology.2018.01.021
33. Tam S, Boonsripitayanon M, Amit M, Fellman BM, Li Y, Busaidy NL, et al. Survival in differentiated thyroid cancer: comparing the AJCC cancer staging seventh and eighth editions. Thyroid (2018) 28(10):1301–10. doi: 10.1089/thy.2017.0572
34. Genpeng L, Pan Z, Tao W, Rixiang G, Jingqiang Z, Zhihui L, et al. Prognostic implications of extranodal extension in papillary thyroid carcinomas: A propensity score matching analysis and proposal for incorporation into current tumor, lymph node, metastasis staging. Surgery (2022) 171(2):368–76. doi: 10.1016/j.surg.2021.07.018
35. Kim M, Jeon MJ, Oh HS, Park S, Song DE, Sung TY, et al. Prognostic implication of N1b classification in the eighth edition of the tumor-node-metastasis staging system of differentiated thyroid cancer. Thyroid (2018) 28(4):496–503. doi: 10.1089/thy.2017.0473
36. Kim M, Kim HK, Kim HI, Kim EH, Jeon MJ, Yi HS, et al. Modification of the eight-edition tumor-node-metastasis staging system with N1b for papillary thyroid carcinoma: A multi-institutional cohort study. Oral Oncol (2018) 86:48–52. doi: 10.1016/j.oraloncology.2018.09.008
37. Ito Y, Miyauchi A, Kihara M, Masuoka H, Higashiyama T, Miya A. Subclassification of tumor extension and nodal metastasis in papillary thyroid cancer to improve prognostic accuracy of the eighth edition of the tumor-node-metastasis classification. World J Surg (2020) 44(2):336–45. doi: 10.1007/s00268-019-05120-w
38. Zaydfudim V, Feurer ID, Griffin MR, Phay JE. The impact of lymph node involvement on survival in patients with papillary and follicular thyroid carcinoma. Surgery (2008) 144(6):1070–7. doi: 10.1016/j.surg.2008.08.034
39. Nixon IJ, Wang LY, Palmer FL, Tuttle RM, Shaha AR, Shah JP, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery (2014) 156(1):137–46. doi: 10.1016/j.surg.2014.03.027
40. Adam MA, Thomas S, Roman SA, Hyslop T, Sosa JA. Rethinking the current American joint committee on cancer TNM staging system for medullary thyroid cancer. JAMA Surg (2017) 152(9):869–76. doi: 10.1001/jamasurg.2017.1665
41. Pontius LN, Oyekunle TO, Thomas SM, Stang MT, Scheri RP, Roman SA, et al. Projecting survival in papillary thyroid cancer: a comparison of the seventh and eighth editions of the American joint commission on Cancer/Union for international cancer control staging systems in two contemporary national patient cohorts. Thyroid (2017) 27(11):1408–16. doi: 10.1089/thy.2017.0306
42. Chen L, Qian K, Guo K, Zheng X, Sun W, Sun T, et al. A novel n staging system for predicting survival in patients with medullary thyroid cancer. Ann Surg Oncol (2019) 26(13):4430–8. doi: 10.1245/s10434-019-07871-1
43. Wreesmann VB, Nixon IJ, Rivera M, Katabi N, Palmer F, Ganly I, et al. Prognostic value of vascular invasion in well-differentiated papillary thyroid carcinoma. Thyroid (2015) 25(5):503–8. doi: 10.1089/thy.2015.0052
44. Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as thepresence of extranodal extension. Thyroid (2012) 22(11):1144–52. doi: 10.1089/thy.2012.0043
Keywords: papillary thyroid cancer, number of positive lymph nodes, TNM staging system, prognosis, SEER
Citation: Sun Y-G, Chen F, Sun Q-L, Tian J-Y and He X-C (2022) The number of metastatic lymph nodes optimizes staging in patients aged 55 years or older with papillary thyroid cancer. Front. Endocrinol. 13:1026737. doi: 10.3389/fendo.2022.1026737
Received: 24 August 2022; Accepted: 28 November 2022;
Published: 09 December 2022.
Edited by:
Valentina Guarnotta, University of Palermo, ItalyReviewed by:
Khawla S. Al-Kuraya, King Faisal Specialist Hospital & Research Centre, Saudi ArabiaCopyright © 2022 Sun, Chen, Sun, Tian and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun-Gang Sun, c3VubnlyaXNlQGkuc211LmVkdS5jbg==
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.