- 1Department of Physiology, University Medical & Dental College, The University of Faisalabad, Faisalabad, Pakistan
- 2Department of Pharmacology and Therapeutics, University Medical & Dental College, The University of Faisalabad, Faisalabad, Pakistan
- 3Department of Physiology and Cell Biology, University of Health Sciences, Lahore, Pakistan
- 4Department of Internal Medicine, Faculty of Medicine Rabigh, King Abdulaziz University, Jeddah, Saudi Arabia
- 5Department of Clinical Biochemistry, Faculty of Medicine, Rabigh, King Abdulaziz University, Jeddah, Saudi Arabia
Background: Adipokines are engaged in bone physiology and regulate bone mineral density (BMD) by playing protective or cynical role in bone metabolism. The study is designed to measure and compare BMD, adipokines (retinoic acid receptor responder protein-2 RARRES2, visfatin and Intelectin-1) and their genetic variants in postmenopausal osteoporotic, osteopenic and non-osteoporotic females.
Methods: This comparative study included postmenopausal non-osteoporotic (n=72), osteopenic (n=72) and osteoporotic (n=100) females with two years of amenorrhea and age between 50 to 70 years. Gold standard DXA was used to measure BMD. Hardy-Weinberg equilibrium was established. Kruskal-Wallis test for comparisons, logistic and multivariate regression analysis were used to rule out the predictors of BMD.
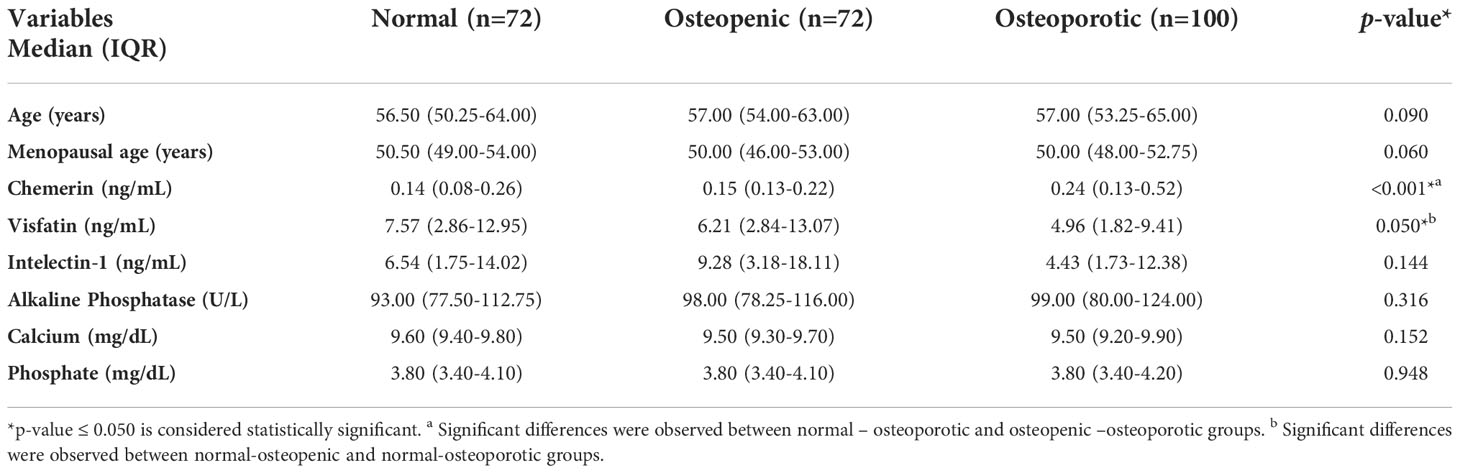
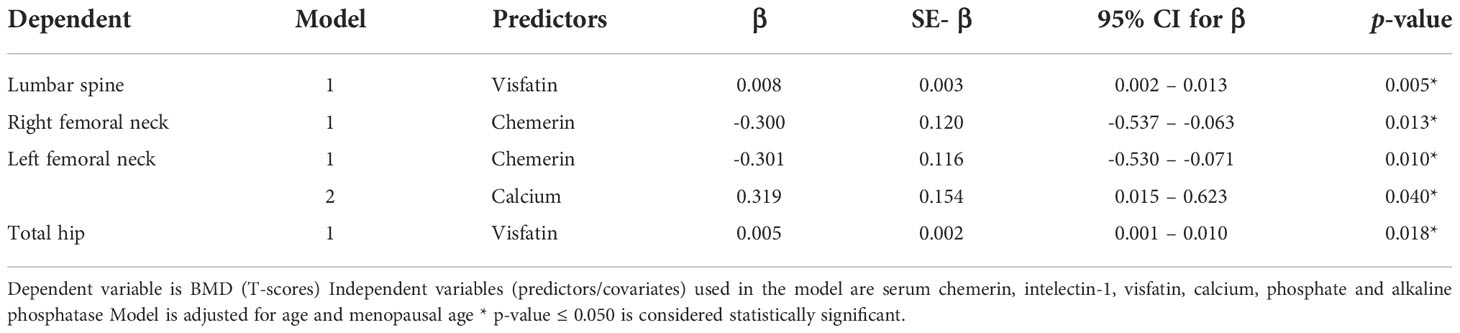
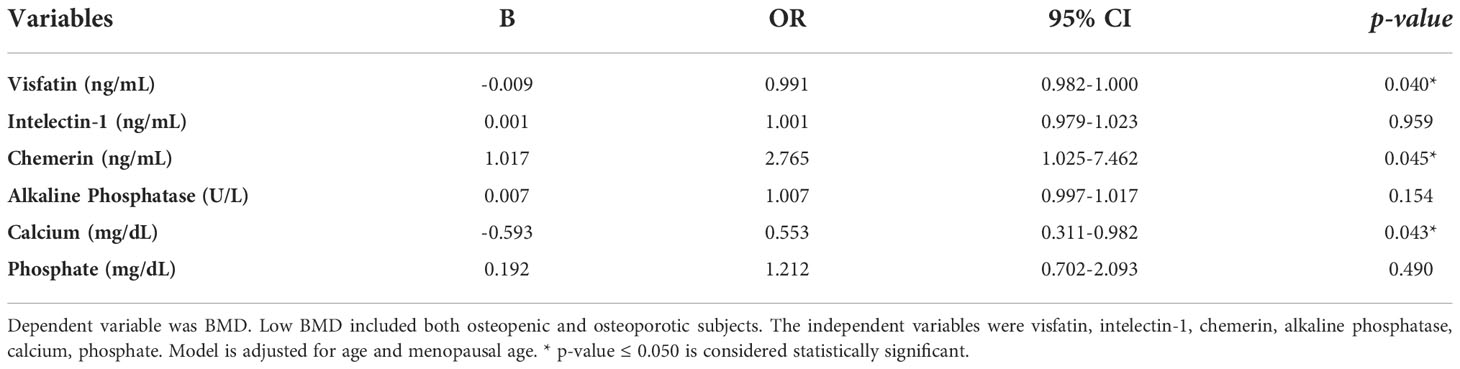
Results: On comparing the three groups, significant differences were observed in serum RARRES2 (p <0.001) and serum visfatin (p=0.050). The significant positive predictor of BMD at lumbar spine and total hip was serum visfatin. BMD at right and left femoral neck was predicted negatively by serum chemerin while BMD at left femoral neck was also predicted positively by serum calcium levels. There was significant difference in BMD at right femoral neck (p = 0.033) between rs7806429 genotypes. The odds of having low BMD increases with increasing serum levels of chemerin and decreasing serum levels of visfatin and calcium
Conclusion: The adipokines RARRES2 and visfatin are associated with BMD. RARRES2 is an independent negative and visfatin is positive predictor of BMD in postmenopausal females. BMD at right femoral neck was significantly low in RARRES2 rs7806429 TC heterozygotes.
Introduction
Osteoporosis is a multifactorial disease. It is initiated by complex interplay between various environmental, metabolic and genetic factors, leading to microarchitecture and mechanical deterioration of bones and resultant fragility fractures. Recently, research has shown the relation of adipokines with the bone mineral density (BMD) in the osteoporotic subjects (1). The adipokines (adipocytokines) are chemical messengers, which were previously thought to be released by the adipocytes only, are expressed by many tissues of the body and play a versatile part in various physiological aspects of the body including bone metabolism (2).
Retinoic acid receptor responder protein 2 (RARRES2) also called chemerin is a 14 kDa protein. It acts as a ligand for chemokine like receptor-1 (CMKLR1), a G protein coupled serpentine receptor. RARRES2 gene has six exons and is located on chromosome 7q36.1 (3). RARRES2 has a role in inflammation and adipocyte differentiation (4). It has a negative correlation with BMD and its serum levels are significantly raised in osteoporotic patients (5). It seems that it has a facilitating role in adipogenesis but inhibitory role in osteogenesis (6). In contrast, another study showed significantly decreased levels in postmenopausal osteoporotic females. However, no plausible cause of this finding was documented (7).
Single nucleotide variations in RARRES2 gene were studied in relation to various diseases but not osteoporosis. Genome wide meta-analysis showed strong association of RARRES2 rs7806429 single nucleotide variant (SNV) with circulating RARRES2 concentrations (8). We selected RARRES2 rs7806429 SNV, to observe its association with BMD and serum RARRES2 levels, which may further add in our understanding of the role of RARRES2 as potential biomarker for osteoporosis in postmenopausal females.
Visfatin also known as pre-B-cell colony-enhancing factor 1 (PBEF1) with full name of nicotinamide phosphoribosyl transferase (NAMPT) belongs to nicotinic phosphoribosyl transferase family and plays an important role in metabolism, aging and stress response. Its gene is located on chromosome 7q22.3 with an exon count of 12 (9). Human serum levels of visfatin are involved in anabolic activity of bone metabolism (10). Visfatin is believed to exert its anabolic effects on bone by altering the differentiation and functional activity of osteoblasts (11). Contrasting findings elaborating the catabolic action of visfatin on bone metabolism are also documented (12, 13). The research done so far on the potential role of visfatin related to bone is inconclusive. This indistinct division among anabolic and catabolic impacts hamper any transient utilization of visfatin as a potential marker for the diagnosis and treatment of osteoporosis. Therefore, further research is required on this front to determine the use of visfatin related to osteoporosis and diseases related to bone metabolism.
Visfatin rs2302559 polymorphism was associated with serum visfatin levels (14). As literature has shown the relation of serum visfatin levels with BMD and no study has explored the role of visfatin gene polymorphisms in relation to BMD. So, association of SNV rs2302559 has also been evaluated with BMD in this study.
Intelectin-1 (ITLN-1) also called omentin-1 is derived from the omental adipose tissue and encoded by ITLN-1 gene located on chromosome 1q23.3 and consists of eight exons and seven introns (15). It was found to have a negative role in osteoblastic differentiation and has a negative correlation with BMD (16). A study exploring the frequency of polymorphism 326A/T of gene ITLN-1 has shown that women with osteoporosis and osteopenia homozygous for AA genotype may lead to lower BMD (17).
Internationally, there are scanty, controversial and opposing data available regarding the relation of these novel adipokines (RARRES2, Intelectin-1 and visfatin) with BMD, and absolutely no published data from local population. Furthermore, other than intelectin-1 gene polymorphism 326A/T in postmenopausal Polish population (17), no polymorphisms of the selected genes have been described from local or any other population of postmenopausal females, internationally.
The short review above clearly shows that the information regarding adipokines narrated is quite scanty and equivocal when available. Therefore, present study is designed to find out serum levels of these adipokines (RARRES2, intelectin-1, visfatin) in postmenopausal osteoporotic, osteopenic and healthy postmenopausal females. In addition, genetic polymorphisms, in the genes regulating their activity were also studied in groups mentioned above. The results of this study may help us to delineate the role of these adipokines in the overall bone metabolism in postmenopausal women.
Materials and methods
The comparative study was conducted on 244 postmenopausal females after taking written informed consent. The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of University of Health Sciences, Lahore. The study subjects were recruited from Madina Teaching Hospital, following the inclusion and exclusion criteria. Postmenopausal females between the age of 50 to 70 years and two years of amenorrhea were included. Females with T-score -1.0 or higher, -1.0 to -2.5 and -2.5 or lower, measured through DXA according to WHO densitometric crtiteria (18), were placed in non-osteoporotic (n=72), osteopenic (n=72) and osteoporotic (n=100) groups respectively. Postmenopausal females with chronic renal disease, chronic liver disease, malignancies, autoimmune disease, parathyroid hormone problems, iatrogenic or premature menopause and taking medications like steroids, vitamin D or bisphosphonate therapy were not included in the study.
Bone densitometry
Bone mineral density (BMD) of postmenopausal females was evaluated by HOLOGIC-HORIZON (QDR-series), dual energy X-ray absorptiometry (DXA) system at Pakistan Institute of Nuclear Medicine (PINUM) Hospital, Faisalabad. BMD was evaluated at the lumbar spine (L2-L4), right femoral neck, left femoral neck and total hip. The results of DXA were used for final analysis and presented as T-scores. According to the criteria set by world health organization (WHO), osteoporosis in adults is diagnosed by the T-scores obtained from DXA. T-score is defined as, the comparison of measured BMD result with the average BMD of the young adults at the time of peak bone mass. T-score ≤ 2.5 standard deviations below the mean peak bone mass represent osteoporosis.
Biochemical analysis
Blood samples were collected from the subjects after an overnight fast. Four mL blood was dispensed in vacutainer without any anticoagulant, centrifuged (3000 rpm or 1000xg for 10 min) after two hours to separate the serum and stored at -70 °C until used. The biochemical and genetic analysis were performed at the postgraduate research laboratory of The University of Faisalabad.
Serum calcium, phosphate and alkaline phosphatase were measured by colorimetric method, using Roche diagnostics, cobas 6000, COBI-CD, manufactured by Hitachi high technologies corporation, Tokyo, Japan. Serum adipokines (RARRES2, Intelectin 1, Visfatin) were assessed quantitatively by ELISA using kits formulated by Elabscience Biotechnology Inc. Houston, Texas 77079, USA, and quantified by microplate data collection and analysis software Gen5™ and Gen5 Secure, manufactured by BioTek® Instruments, Inc. Winooski, Vermont 05404-0998, USA. The coefficient of variation for all assays were <10%.
Genotyping
DNA from whole blood was extracted using GeneJet whole blood genomic DNA purification mini kit, manufactured by Thermo Fisher Scientific Inc. Carlsbad, California 92008, USA. The integrity and quality of extracted DNA was assessed by using 1.0% agarose gel electrophoresis. Primers were designed, by using Tetra-primer ARMS-PCR tool and Primer3Plus tool. All the designed primers were then BLAST in the bioinformatics tool to determine homology and avoid mismatch and secondary structure formation within the primers.
RARRES2
rs7806429-F1TGGGCCTGTTTAAACTTTTTATTCCAGTT
rs7806429-F2GCAATGTATTGACAGCCAGATTAAGGG
rs7806429-R1TGAATGCCTAATATTCCATCATGTGGAT
rs7806429-R2TCAGATAAAGAATAGTAGTGGCTGGGGG
PBEF1
rs2302559-F1TATTAGTGGTGCCTGTGTACTTCGC
rs2302559-F2TATTAGTGGTGCCTGTGTACTTCGT
rs2302559-RGGTGATTCAGGGATAGATCA
ITLN-1
rs2274907-FGAGCCTTTAGGCCATGTCTCT
rs2274907-RCTCTCTTCTTCTCCAGCCCAT
The genetic regions of different genes were amplified from extracted DNA using gene specific primers under optimized conditions. After PCR amplifications, analysis of selected genetic variations were done by restriction fragment length polymorphism (RFLP)/amplification refractory mutation system (ARMS)/Allele specific PCR. Amplicons were checked using agarose gel electrophoresis. After PCR about 10 µl of amplicon was incubated with restriction enzymes. Digested samples were subjected to agarose gel electrophoresis to identify digestion patterns. The results of PCR for few single nucleotide variants (SNVs) were confirmed by sequencing. Samples were sequenced by Advance Bioscience International (ABI) China. The data obtained after sequencing were then visualized by using Chromas software to find out the variations in these sequences. Samples with ambiguous results were excluded from the final analysis. The sample size for genetic analysis was n = 243 for RARRES2 rs7806429, n = 242 for PBEF1 rs2302559 and n = 243 for ITLN-1 rs2274907.
Statistical analysis
Data were analyzed by SPSS version 26.0 (Statistical Package for Social Sciences). Normality of the data was checked by Shapiro-Wilk’s statistics. Groups were compared using Kruskal-Wallis test. Post-hoc pairwise comparisons were performed using Dunn-Bonferroni approach. Multivariate linear stepwise regression and logistic regression analysis was used to predict the BMD. The independent variables used in the model were serum RARRES2, intelectin-1, visfatin, calcium, phosphate and alkaline phosphatase. The model was adjusted for age and menopausal age. In multivariate analysis, a forward stepwise regression approach was adopted. The model added the most significant variables with the highest p-value first followed by the less significant variables. For logistic regression, BMD was included as dichotomous categorical variable. The diseased group has low BMD (including both osteopenic and osteoporotic subjects) while the non-diseased group was represented with subjects having normal BMD. The samples with any missing data were not included in the analysis. Genotypic, allelic frequencies and Hardy-Weinberg equilibrium were calculated. Association of various biochemical markers and BMD with genotype frequencies were observed using Kruskal-Wallis test. P-value ≤ 0.05 was taken as statistically significant.
Results
The study included 244 postmenopausal females, divided into three groups, Normal (n=72), Osteopenic (n=72) and Osteoporotic (n=100). The study groups were age matched. The comparison of general characteristics and biochemical parameters are presented in Table 1. Significant differences were observed between the serum levels of RARRES2 (p <0.001) and visfatin (p = 0.050), while no significant difference was observed in the serum levels of other parameters (Table 1).
Multivariate linear stepwise regression analysis was used to predict the BMD at all sites. The independent variables used in the model were serum RARRES2, intelectin-1, visfatin, calcium, phosphate and alkaline phosphatase. The model was adjusting for age and menopausal age. At the lumbar spine and hip, the independent positive predictor was serum visfatin. At right femoral neck, the independent negative predictor was serum chemerin while at left femoral neck, the independent negative predictor was serum chemerin and positive predictor was serum calcium (Table 2).
A logistic regression analysis was performed to ascertain the effects of serum visfatin, Intelectin-1, chemerin, alkaline phosphatase, calcium and phosphate on the likelihood that participants have low BMD (osteopenia and osteoporosis). Model was adjusted for age and menopausal age. The logistic regression model was statistically significant, χ2 (8) = 34.202, p < 0.001. The odds of having low BMD increases with increasing serum levels of chemerin and decreasing serum levels of visfatin and calcium (Table 3).
The comparison of RARRES2 rs7806429, PBEF1 rs2302559 and ITLN-1 rs2274907 genotypic and allelic frequencies among the study groups is presented in Table 4. For RARRES2 rs7806429, PCR product sizes were 110 bp for C allele, 156 bp for T allele, and 210 bp for two outer primers. The χ2 test of rs7806429 variant in the studied subjects suggested that it did not deviated significantly from Hardy-Weinberg equilibrium (p > 0.050). No significant differences were observed in genotype frequencies between the groups concerning rs7806429 variant (p = 0.101). For PBEF1 rs2302559, PCR product size was 247 bp. The χ2 test suggested that it did not deviated significantly from Hardy-Weinberg equilibrium (p > 0.050). No significant differences were observed in genotype frequencies between the groups concerning rs2302559 variant (p = 0.935). For ITLN-1 rs2274907, PCR product sizes were 471 bp for A allele and 274 + 197 bp for T allele. The χ2 test suggested that it did not deviated significantly from Hardy-Weinberg equilibrium (p > 0.050). No significant differences were observed in genotype frequencies between the groups concerning rs2274907 variant (p = 0.365).
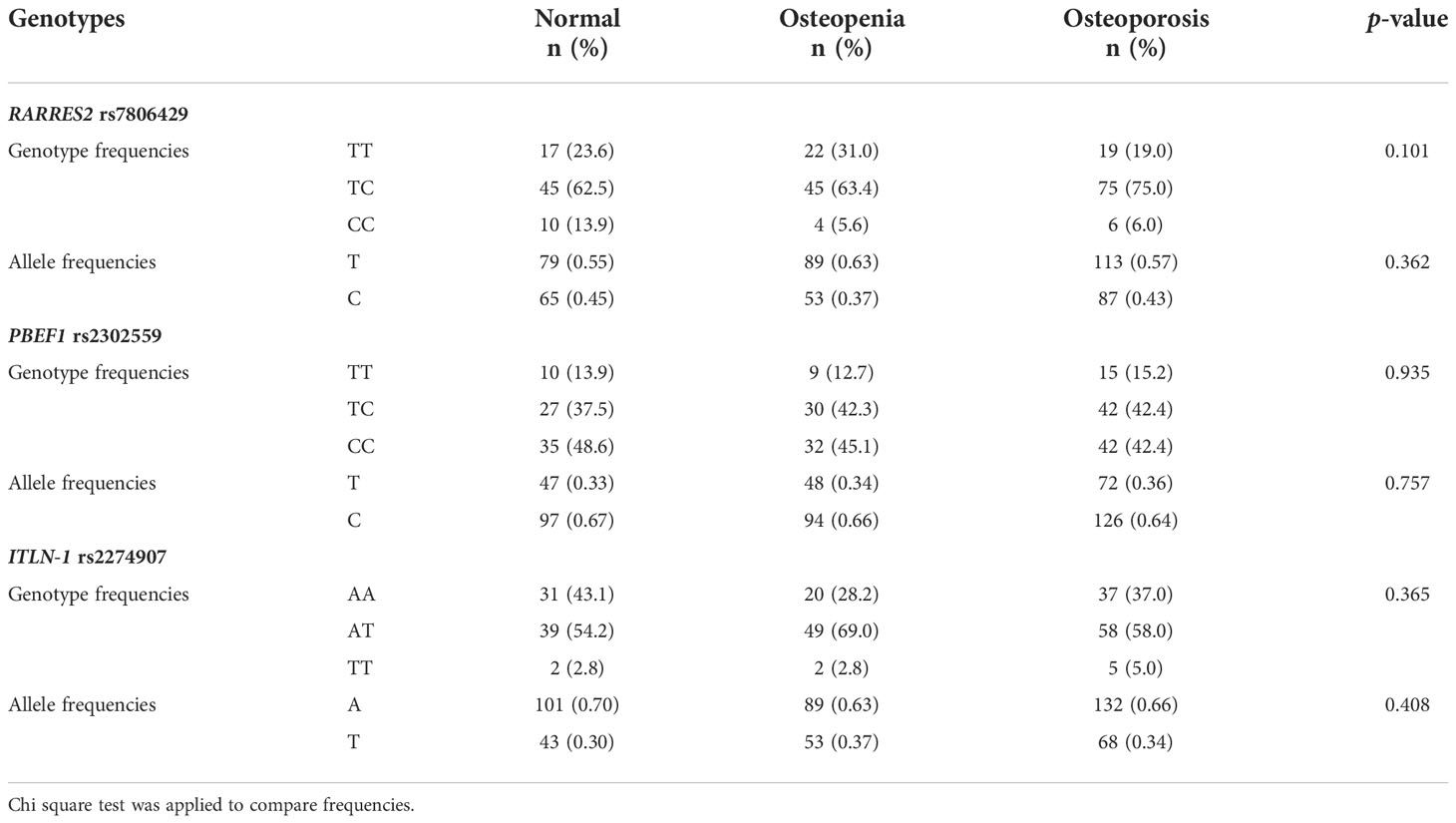
Table 4 Comparison of RARRES2 rs7806429, PBEF1 rs2302559 and ITLN-1 rs2274907 genotypic and allelic frequencies among the study groups.
The results of association analysis of RARRES2 rs7806429, PBEF1 rs2302559 and ITLN-1 rs2274907 genotypes with their respective serum parameters and BMD at various sites in the studied subjects is shown in Table 5.
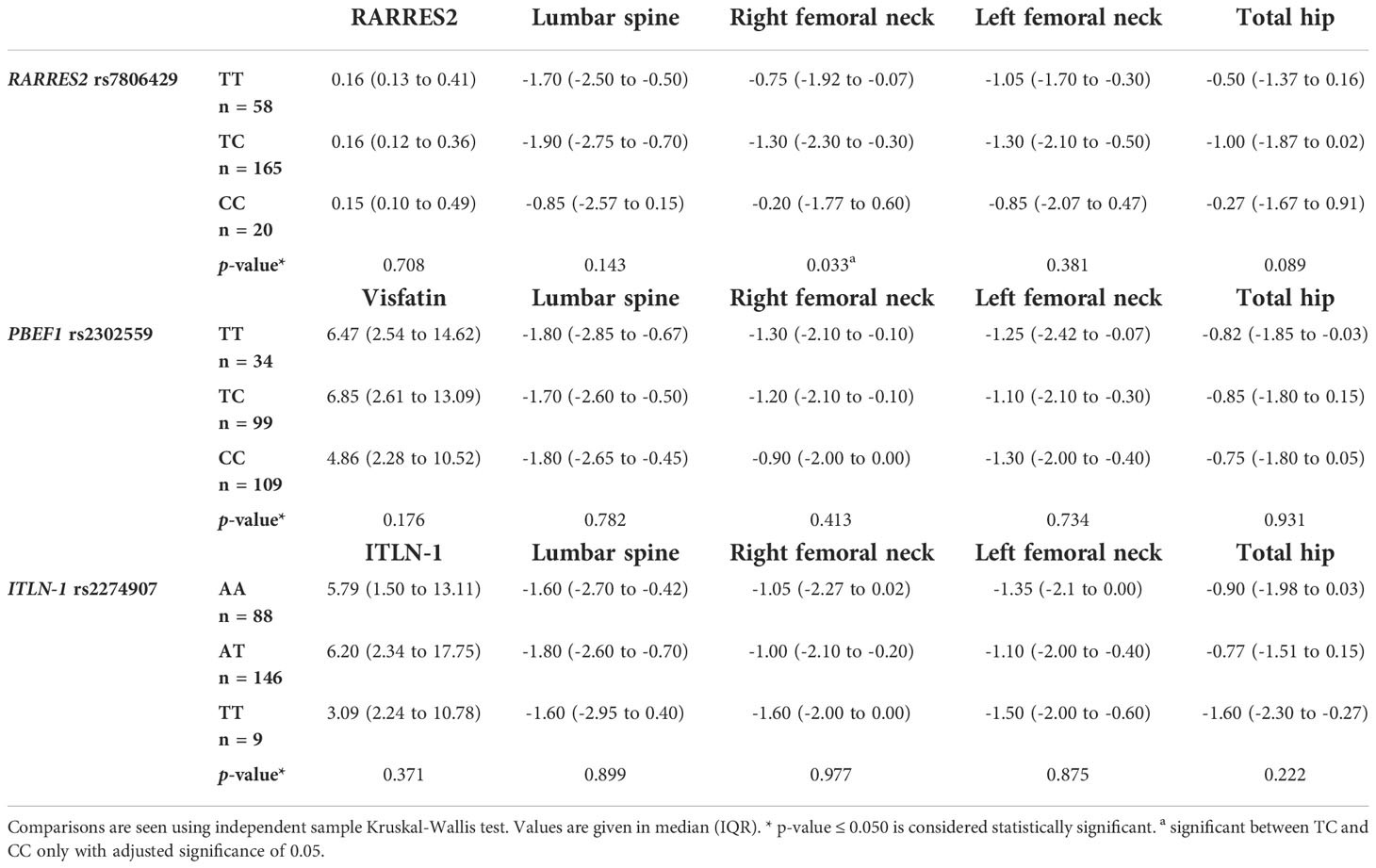
Table 5 Comparison of RARRES2 rs7806429, PBEF1 rs2302559 and ITLN-1 rs2274907 genotypes with their respective serum parameters and BMD at various sites.
There was no significant difference in the serum levels of RARRES2 between rs7806429 genotypes (p = 0.708). There was significant difference in BMD at right femoral neck (p = 0.033) between rs7806429 genotypes. Multiple comparisons after dunn-bonferroni correction showed that BMD at right femoral neck was significantly low in the rs7806429 TC heterozygotes as compared to rs7806429 CC homozygotes.
Serum visfatin levels were low in rs2302559 CC homozygotes, but the difference was not significant (p = 0.179). No significant difference was found between BMD and genotypes of visfatin rs2302559 SNV.
Serum Intelectin-1 levels were low in rs2274907 TT homozygotes, but the difference was not significant (p = 0.371). No significant difference was found between BMD and genotypes of ITLN-1 rs2274907 SNV (Table 5).
Discussion
The study included postmenopausal non-osteoporotic, osteopenic and osteoporotic females. Osteoporosis increases the porosity of bones and increase risk of fragility fractures in postmenopausal females (19).
The adipokine RARRES2 can regulate both osteogenesis and adipogenesis. In the present study, serum RARRES2 levels were significantly higher in the osteoporotic group as compared to the non-osteoporotic and osteopenic postmenopausal females. In addition, serum RARRES2 levels were found to be independent negative predictor of BMD at the right and left femoral neck. Similar results were observed in other studies in which they found significantly increased levels of RARRES2 in osteoporotic patients (5, 20). In another study conducted on Chinese obese postmenopausal women, similar findings were observed. DXA scan was performed to assess the BMD of these subjects at lumbar spine and femoral neck. They exhibited a significant negative relationship of RARRES2 with BMD at lumbar spine. However, they could not established any relationship with femoral neck BMD. They further concluded RARRES2 as an independent negative predictor of BMD. The plausible cause of this negative correlation of chemerin with BMD was low-grade inflammation which affected bone mass in these individuals (21).
Chemerin is said to act as a negative regulator of osteogenesis. A study has shown an increase in expression of osteoblast marker gene and mineralization while decrease in adipocytes differentiation in RARRES2 and its receptor knock out mice (22). In addition, hematopoietic stem cells (HSC) also secretes RARRES2 and is involved in the process of osteoclastogenesis. The study showed that RARRES2 was neutralized by introducing a blocking antibody prior to osteoblast differentiation resulting in complete loss of the process of osteoclastogenesis. Reintroduction of RARRES2 by reversing the neutralization, initiated the process of osteoclast differentiation demonstrating that RARRES2 signaling is necessary to allow HSC separation into osteoclasts (23).
A recent study conducted on mice demonstrated that RARRES2 increases the bone resorption by increasing the activity of mature osteoclasts. Interestingly, to strengthen their findings they introduced RARRES2 antagonist along with its receptor antagonist (CCX832) and observed an inhibition in osteoclastic activity and process of bone resorption. Furthermore, they elaborated that the signaling pathway involved in the action of osteoclast through RARRES2 is regulated by the extracellular signal-regulated kinase 5 (ERK5). It is the phosphorylation of this protein that initiated the signaling cascade resulting in osteoclast activation (24).
In the present study, we also evaluated the possible association of RARRES2 rs7806429 SNV with BMD in postmenopausal women. This is the first study to investigate such association. The results of association analysis of genotype rs7806429 polymorphism with serum RARRES2 levels and BMD at various sites showed that serum RARRES2 levels were not significantly associated with rs7806429 genotype distribution. While BMD at the right femoral neck was significantly high in the CC homozygotes. Further studies can clarify the role of rs7806429 SNV and RARRES2 in bone metabolism.
Visfatin, initially thought to be expressed only by the visceral adipose tissue is now known to be highly expressed by the bone marrow plasma, thus having a possibility of modulating bone metabolism (25).
In this study, serum levels of visfatin were lower in postmenopausal osteopenic and osteoporotic women as compared to non-osteoporotic women. Visfatin also appeared to be an independent positive predictor of BMD at lumbar spine and hip. The findings of this study are strongly supported by earlier studies, both in vitro and in vivo. Visfatin stimulates osteoblast proliferation, mineralization and increases the expression of type 1 collagen which is an important osteogenic marker (22). The inhibition of visfatin in mouse bone marrow mesenchymal stromal cells or visfatin knockout mouse showed reduced osteoblastogenesis, furthermore reducing serum levels of visfatin also decreases the activity of alkaline phosphatase and inhibits matrix mineralization resulting in reduce bone formation (11, 26). A study showed that serum visfatin levels have positive correlation with lumbar spine BMD and total BMD in survivors of acute lymphocytic leukemia (10).
There is quite scarce data available regarding the role of visfatin in osteoclastic function. However, most of the available data are supporting the findings of our study. Visfatin may negatively regulate the process of osteoclastogenesis. An in vitro study suggested the inhibitory role of visfatin on the differentiation of osteoclasts and generation of markers of osteoclastic activity, RANK, cathepsin-K and nuclear factor of activated T-cells c1 (NFATc1) (27). This finding was also observed in another in vitro study performed on bone marrow derived macrophages, where visfatin suppressed RANKL mediated osteoclastogenesis by suppressing the phosphorylation of several important signal transducers involved in the process of osteoclastogenesis. However, the activity of mature osteoclasts remained unaffected (28).
In contrast to our study, contradictory findings were observed in few other studies. Serum visfatin levels were negatively related with BMD (29). Serum visfatin levels were also measured in postmenopausal Iranian females and after performing multiple linear regression, they could not found any significant association of serum visfatin with bone markers (30).
Present study has also investigated the possible association of PBEF1 genetic variant rs2302559 with BMD. This is the first study to investigate such association. The frequency of TT genotype of rs2302559 variant was less in postmenopausal osteoporotic females as compared to TC and CC genotype but the association was insignificant. We did not find any association of PBEF1 genetic variant (rs2302559) with BMD and serum visfatin levels in postmenopausal females.
In this study, serum levels of Intelectin-1 were low in postmenopausal osteoporotic females as compared to non-osteoporotic females but Intelectin-1 was not a predictor of BMD at any site. Similar to our findings, a study found increase in levels of Intelectin-1 in postmenopausal women with osteoporosis and labelled it as a postmenopausal physiological compensation against bone loss (31). Intelectin-1 may have improved estrogen deficiency induced bone loss by inhibiting tumor necrosis factor-α (TNF- α) and further reduced the levels of leptin in obese Iranian women (32). Similarly, higher intelectin-1 levels were associated with lower BMD at lumbar spine only in non-diabetic postmenopausal females (33). Contradictory results were seen in older men with osteoporosis, in which it has negative correlation with BMD and bone turnover markers and this negative effect on bone was attributed to altered osteoblast differentiation via intelectin-1 (34).
In vitro studies have shown that Intelectin-1 is important in inhibiting the expression of pro-inflammatory cytokines IL-6, IL-8 and inflammatory mediators in the macrophages that are derived from the same cell lineage as osteoclasts. Intelectin-1 suppresses the accumulation of nuclear p65 and transfected nuclear factor (NF-κB) promotor activity thus reducing the activity of NF-κB pathway. They also directly reduce NF-κB activation. It also reduces the lipopolysaccharide (LPS) induced expression of the toll like receptor (TLR4) and myeloid differentiation primary response gene 88 (MyD88), thus inhibiting the TLR4/MyD88 signaling pathway (35). This pathway is important to induce the expression and secretion of various inflammatory cytokines that directly and indirectly enhance osteoclastogenesis. Intelectin-1 reduces oxidative stress by decreasing the levels of reactive oxygen species and increasing the antioxidant reduced glutathione (GSH). Intelectin-1 significantly increases the activity of nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2) thus increasing the transcription of cytoprotective proteins and inhibiting the expression of pro-inflammatory cytokines (35). All these effects are in favour of inhibiting osteoclastogenic effects and thus increased BMD.
The effects of Intelectin-1 on the osteoblasts showed the positive relation of Intelectin-1 with bone. It stimulates the proliferation of human osteoblasts by activating the phosphatidylinositol 3-kinase/serine-threonine protein kinase (PI3K/Akt) signaling pathway (36). Rao et al (2018), showed induction of inflammatory bone loss in Intelectin-1 knock out mice. They also demonstrated increase in pro-inflammatory cytokines, increase osteoclastogenesis, reduced osteogenic activity and enhanced bone destruction in the absence of Intelectin-1. Administration of recombinant intelectin-1 in the mouse model reduced the production of pro-inflammatory cytokines from macrophages thus reducing their pro-osteoclastic and anti-osteoblastic potential (37). Thus, all these findings strongly favour the bone-protecting role of Intelectin-1 against inflammation and osteoporotic bone loss.
Present study has also investigated the possible association of ITLN-1 genetic variant rs2274907 with BMD. The frequency of TT genotype of rs2274907 variant was less in postmenopausal osteoporotic females as compared to AT and AA genotype but the association was insignificant. We did not find any association of ITLN-1 rs2274907 genetic variant with BMD and serum Intelectin-1 levels in postmenopausal females. Further studies with bigger sample size are needed. Moreover, randomized controlled trials can be designed whose results can help in modifying the various factors associated with adipokines to reduce the risk of a highly prevalent disease, osteoporosis. The findings can also help in planning therapeutic and preventive aspects of a highly prevalent disease. We can go for genetic screening in offspring of the predisposed families, offer them early preventive counselling and measures resulting in higher quality of life of the sufferers. They can also act as a useful genetic marker for BMD and it would be possible to administer individual symptomatic treatment as well as early prophylactics of osteoporosis.
Limitations
The limitations of the study include small sample size, a cross sectional design and inclusion of less parameters due to funding constraints. Bone turn over markers could have also been included.
Conclusion
The adipokines RARRES2 and visfatin are associated with BMD. RARRES2 is independent negative and visfatin is positive predictor of BMD in postmenopausal females. BMD at right femoral neck was significantly low in RARRES2 rs7806429 TC heterozygotes.
Data availability statement
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of University of Health Sciences, Lahore. The patients/participants provided their written informed consent to participate in this study.
Author contributions
SuT: Conception and design, acquisition, analysis, interpretation of data, drafted the manuscript. SaT: Acquisition, analysis of data, carried out the literature search, helped in drafting the manuscript. SK: Designed and supervised the research, analysis and interpretation of data, revised the manuscript critically for important intellectual content. SA: Data analysis, interpretation, drafted and revised the manuscript critically for important intellectual content. MB: Data analysis, interpretation, drafted and revised the manuscript critically for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The research is partially supported by grant from Higher Education Commission, Pakistan under grant number: 8530/Punjab/NRPU/R&D/HEC/2017.
Acknowledgments
We are thankful to Prof. Dr. Khalid Parvez Lone (late) who supervised part of this research. We acknowledge the technical staff of Madina teaching hospital, The University of Faisalabad and Pakistan institute of nuclear medicine for helping in performing biochemical analysis and DXA measurements.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ostrowska Z, Morawiecka-Pietrzak M, Pluskiewicz W, Świętochowska E, Strzelczyk J, Gołąbek K, et al. The relationship between chemerin, bone metabolism, the RANKL/RANK/OPG system, and bone mineral density in girls with anorexia. Endokrynol Pol (2022) . 73(1):26–34. Polish. doi: 10.5603/EP.a2021.0103
2. Tariq S, Tariq S, Khaliq S, Lone KP. Serum resistin levels as predictor of low bone mineral density in postmenopausal women. Health Care Women Int (2021) . 42(1):82–91. doi: 10.1080/07399332.2020.1798965
3. RARRES2 Gene. GeneCards. RARR2 protein, RARR2 antibody (2022). Available at: https://www.genecards.org/cgi-bin/carddisp.pl?gene=RARRES2 (Accessed 4 January 2022).
4. Goralski KB, McCarthy TC, Hanniman EA, Zabel BA, Butcher EC, Parlee SD, et al. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J Biol Chem (2007) . 282:28175–88. doi: 10.1074/jbc.M700793200
5. He J, Li JC, Xie H, Xu ZH, Sun YW, Shan Q. Serum chemerin levels in relation to osteoporosis and bone mineral density: A case-control study. Dis Markers (2015) . 2015:786708. doi: 10.1155/2015/786708
6. Li Y, Chang J, Jiang T. Serum level of chemerin and bone mineral density in patients with graves disease. Zhong Nan Da Xue Xue Bao Yi Xue Ban (2017) . 42(8):947–52. doi: 10.11817/j.issn.1672-7347.2017.08.012
7. Engin-Üstün Y, Çağlayan EK, Göçmen AY, Polat MF. Postmenopausal osteoporosis is associated with serum chemerin and irisin but not with apolipoprotein m levels. J Menopausal Med (2016) . 22(2):76–9. doi: 10.6118/jmm.2016.22.2.76
8. Tönjes A, Scholz M, Breitfeld J, Marzi C, Grallert H, Gross A, et al. Genome wide meta-analysis highlights the role of genetic variation in RARRES2 in the regulation of circulating serum chemerin. PloS Genet (2014) . 10(12):e1004854. doi: 10.1371/journal.pgen.1004854
9. Gene. NAMPT nicotinamide phosphoribosyltransferase [Homo sapiens (human)] (2022). Available at: http://www.ncbi.nlm.nih.gov/gene/10135 (Accessed 06 January 2022).
10. Siviero-Miachon AA, Spinola-Castro AM, de Martino Lee ML, Calixto AR, Geloneze B, Lazaretti-Castro M, et al. Visfatin is a positive predictor of bone mineral density in young survivors of acute lymphocytic leukemia. J Bone Miner Metab (2017) . 35(1):73–82. doi: 10.1007/s00774-015-0728-5
11. He X, He J, Shi Y, Pi C, Yang Y, Sun Y, et al. Nicotinamide phosphoribosyltransferase (Nampt) may serve as the marker for osteoblast differentiation of bone marrow-derived mesenchymal stem cells. Exp Cell Res (2017) . 352(1):45–52. doi: 10.1016/j.yexcr.2017.01.021
12. Kochetkova EA, Ugai LG, Maistrovskaia YV, Nevzorova VA. Adipokines: a possible contribution to vascular and bone remodeling in idiopathic pulmonary arterial hypertension. Calcif Tissue Int (2017) . 100(4):325–31. doi: 10.1007/s00223-016-0224-5
13. Linossier MT, Amirova LE, Thomas M, Normand M, Bareille MP, Gauquelin-Koch G, et al. Effects of short-term dry immersion on bone remodeling markers, insulin and adipokines. PloS One (2017) . 12(8):e0182970. doi: 10.1371/journal.pone.0182970
14. Ooi SQ, Chan RME, Poh LKS, Loke KY, Heng CK, Chan YH, et al. Visfatin and its genetic variants are associated with obesity-related morbidities and cardiometabolic risk in severely obese children. Pediatr Obes (2014) . 9(2):81–91. doi: 10.1111/j.2047-6310.2013.00149.x
15. ITLN1 Gene. ITLN1 intelectin 1 [Homo sapiens (human)] (2022). Available at: https://www.ncbi.nlm.nih.gov/gene/55600 (Accessed 4 January 2022).
16. Yan P, Xu Y, Zhang Z, Zhu J, Miao Y, Gao C, et al. Association of circulating omentin-1 with osteoporosis in a Chinese type 2 diabetic population. Mediators Inflamm (2020) . 2020:9389720. doi: 10.1155/2020/9389720
17. Boron D, Czerny B, Bartkowiak-Wieczorek J, Sieron D, Wolski H. Omentin polymorphism and its relations to bone mineral density in women. Arch Med Res (2015) 46:173–80. doi: 10.1016/j.arcmed.2015.03.012
18. Siqueira MMLG, Casulari LA, Freitas WM, Carneiro MV Mendes LSC. Risk factors associated with fracture of the lumbosacral spine and its compromise in the quality of life of cirrhotics. Arq Gastroenterol (2022) 59(1):9–15. doi: 10.1590/S0004-2803.202200001-03
19. Tariq S, Tariq S, Khaliq S, Lone KP. Serum resistin levels and related genetic variants are associated with bone mineral density in postmenopausal women. Front Endocrinol (2022) . 13:868120. doi: 10.3389/fendo.2022.868120
20. Tariq S, Tariq S, Shahzad M. Association of serum chemerin with calcium, alkaline phosphatase and bone mineral density in postmenopausal females. Pak J Med Sci (2021) 37(2):384–8. doi: 10.12669/pjms.37.2.3907
21. Shi L, Mao C, Wang X, Liu R, Li L, Mou X, et al. Association of chemerin levels and bone mineral density in Chinese obese postmenopausal women. Medicine (2016) . 95(35):e4583. doi: 10.1097/MD.0000000000004583
22. Liu Y, Song CY, Wu SS, Liang QH, Yuan LQ, Liao EY. Novel adipokines and bone metabolism. Int J Endocrinol (2013) 2013:895045. doi: 10.1155/2013/895045
23. Muruganandan S, Dranse HJ, Rourke JL, McMullen NM, Sinal CJ. Chemerin neutralization blocks hematopoietic stem cell osteoclastogenesis. Stem Cells (2013) . 31(10):2172–82. doi: 10.1002/stem.1450
24. Ramos-Junior ES, Leite GA, Carmo-Silva CC, Taira TM, Neves KB, Colón DF, et al. Adipokine RARRES2 bridges metabolic dyslipidemia and alveolar bone loss in mice. J Bone Miner Res (2017) . 32(5):974–84. doi: 10.1002/jbmr.3072
25. Krzysik-Walker SM, Hadley JA, Pesall JE, McFarland DC, Vasilatos-Younken R, Ramachandran R. Nampt/visfatin/PBEF affects expression of myogenic regula- tory factors and is regulated by interleukin-6 in chicken skeletal muscle cells. Comp Biochem Physiol A Mol Integr Physiol (2011) . 159(4):413–21. doi: 10.1016/j.cbpa.2011.04.007
26. Ling M, Huang P, Islam S, Heruth DP, Li X, Zhang LQ, et al. Epigenetic regulation of Runx2 transcription and osteoblast differentiation by nicotinamide phosphoribosyltransferase. Cell Biosci (2017) . 7(1):1–10. doi: 10.1186/s13578-017-0154-6
27. Muruganandan S, Ionescu AM, Sinal CJ. At The crossroads of the adipocyte and osteoclast differentiation programs: Future therapeutic perspectives. Int J Mol Sci (2020) . 21(7):2277. doi: 10.3390/ijms21072277
28. Baek JM, Ahn SJ, Cheon YH, Lee MS, Oh J, Kim JY. Nicotinamide phosphoribosyltransferase inhibits receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation in vitro. Mol Med Rep (2017) 15(2):784–92. doi: 10.3892/mmr.2016.6069
29. Franco-Trepat E, Guillán-Fresco M, Alonso-Pérez A, Jorge-Mora A, Francisco V, Gualillo O, et al. Visfatin connection: Present and future in osteoarthritis and osteoporosis. J Clin Med (2019) . 8:1178. doi: 10.3390/jcm8081178
30. Kalantarhormozi MR, Assadi M, Vahdat K, Asadipooya K, Ostovar A, Darabi H, et al. Omentin-1, visfatin and adiponectin levels in relation to bone mineral density in Iranian postmenopausal women. J Bone Miner Metab (2015) . 34:422–8. doi: 10.1007/s00774-015-0688-9
31. Dikker O, Bekpinar S, Ozdemirler G, Uysal M, Vardar M, Atar S, et al. Evaluation of the relation between omentin-1 and vitamin d in postmenopausal women with or without osteoporosis. Exp Clin Endocrinol Diabetes (2018) . 126(5):316–20. doi: 10.1055/s-0043-120110
32. Moradi S, Mirzaei K, Abdurahman AA, Keshavarz SA. Adipokines may mediate the relationship between resting metabolic rates and bone mineral densities in obese women. Osteoporos Int (2017) . 28(5):1619–29. doi: 10.1007/s00198-017-3914-6
33. Ozlu T, Sarman H, Alcelik A, Isik C, Yazıcı S, Tosun M, et al. The association of omentin levels in non-diabetic postmenopausal women with bone mineral density and total body composition. Acta Med Anatolia (2015) . 3(2):51. doi: 10.15824/actamedica.00049
34. Yang L, Zhao XL, Liao B, Qin AP. Relationships between serum omentin-1 levels and bone mineral density in older men with osteoporosis. Chronic Dis Transl Med (2016) . 2:48–54. doi: 10.1016/j.cdtm.2016.02.003
35. Wang J, Gao Y, Lin F, Han K, Wang X. Omentin-1 attenuates lipopolysaccharide (LPS)-induced U937 macrophages activation by inhibiting the TLR4/MyD88/NF-κB signaling. Arch Biochem Biophys (2020) 679:108187. doi: 10.1016/j.abb.2019.108187
36. Wu SS, Liang QH, Liu Y, Cui RR, Yuan LQ, Liao EY. Omentin-1 stimulates human osteoblast proliferation through PI3K/Akt signal pathway. Int J Endocrinol (2013) 2013:368970. doi: 10.1155/2013/368970
Keywords: BMD, osteoporosis, intelectin-1, RARRES2, adipokines, visfatin/NAMPT
Citation: Tariq S, Tariq S, Khaliq S, Abualhamael SA and Baig M (2022) Association of serum levels of Visfatin, Intelectin-1, RARRES2 and their genetic variants with bone mineral density in postmenopausal females. Front. Endocrinol. 13:1024860. doi: 10.3389/fendo.2022.1024860
Received: 22 August 2022; Accepted: 01 November 2022;
Published: 30 November 2022.
Edited by:
Gudrun Stenbeck, Brunel University London, United KingdomReviewed by:
Amina Valjevac, University of Sarajevo, Bosnia and HerzegovinaMuhammad Shakil, King Edward Medical University, Pakistan
Copyright © 2022 Tariq, Tariq, Khaliq, Abualhamael and Baig. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sundus Tariq, ZHIuc3VuZHVzdGFyaXFAZ21haWwuY29t
†These authors have contributed equally to this work and share first authorship
‡ORCID: Sundus Tariq, orcid.org/0000-0003-0083-1819
Saba Tariq, orcid.org/0000-0002-6191-0601
Saba Khaliq, orcid.org/0000-0002-0345-7394
Mukhtiar Baig, orcid.org/0000-0003-0058-2031
 Sundus Tariq
Sundus Tariq Saba Tariq
Saba Tariq Saba Khaliq
Saba Khaliq Shahad Abduljalil Abualhamael4
Shahad Abduljalil Abualhamael4 Mukhtiar Baig
Mukhtiar Baig