
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 16 December 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1024500
This article is part of the Research TopicAdvances in Diabetic Kidney Disease: Pathophysiology, Clinical Characteristics, and Future DirectionsView all 10 articles
Background: In sepsis patients, Type 2 Diabetes Mellitus (T2DM) was associated with an increased risk of kidney injury. Furthermore, kidney damage is among the dangerous complications, with a high mortality rate in sepsis patients. However, the underlying predictive model on the prediction of major adverse kidney events within 30 days (MAKE30) in sepsis patients with T2DM has not been reported by any study.
Methods: A total of 406 sepsis patients with T2DM were retrospectively enrolled and divided into a non-MAKE30 group (261 cases) and a MAKE30 group (145 cases). In sepsis patients with T2DM, univariate and multivariate logistic regression analyses were conducted to identify independent predictors of MAKE30. Based on the findings of multivariate logistic regression analysis, the corresponding nomogram was constructed. The nomogram was evaluated using the calibration curve, Receiver Operating Characteristic (ROC) curve, and decision curve analysis. A composite of death, new Renal Replacement Therapy (RRT), or Persistent Renal Dysfunction (PRD) comprised MAKE30. Finally, subgroup analyses of the nomogram for 30-day mortality, new RRT, and PRD were performed.
Results: In sepsis patients with T2DM, Mean Arterial Pressure (MAP), Platelet (PLT), cystatin C, High-Density Lipoprotein (HDL), and apolipoprotein E (apoE) were independent predictors for MAKE30. According to the ROC curve, calibration curve, and decision curve analysis, the nomogram model based on those predictors had satisfactory discrimination (AUC = 0.916), good calibration, and clinical application. Additionally, in sepsis patients with T2DM, the nomogram model exhibited a high ability to predict the occurrence of 30-day mortality (AUC = 0.822), new RRT (AUC = 0.874), and PRD (AUC = 0.801).
Conclusion: The nomogram model, which is available within 24 hours after admission, had a robust and accurate assessment for the MAKE30 occurrence, and it provided information to better manage sepsis patients with T2DM.
Sepsis is a life-threatening organ dysfunction due to a dysregulated host response to infection induced by bacterial, viral, or fungal infection (1). Sepsis is currently the leading cause of mortality for patients, accounting for over 10% of the mortality within hospitals (1, 2). Furthermore, sepsis is associated with a mortality rate of 25–30% (3). It was reported that between 17–20% of the patients with sepsis had diabetes mellitus (4, 5). With the wide adoption of Western food and lifestyles, it is projected that the prevalence of T2DM will exceed 700 million worldwide and may soon reach pandemic levels (6). Due to immune system dysfunction, diabetic patients have an increased propensity to develop infections and are at a higher risk for sepsis (2 - 6 times) (7, 8).
Additionally, sepsis is particularly harmful to the kidney, which makes sepsis-induced acute kidney injury (S-AKI) a risk factor for increased mortality rate (9). Moreover, a growing body of research has revealed that T2DM was associated with an increased risk of S-AKI, but did not increase the overall mortality of sepsis patients (5, 8, 10). The renal damage may be due to increased activation of NF-kappa B, TGF-β and oxidant levels as a result of consistent hyperglycaemia milieu, or it may result from end-organ damage by atherosclerosis (8). Nevertheless, S-AKI cannot be used to assess clinically major renal adverse events such as death, and dialysis dependency, among others. The National Institute of Diabetes and Digestive and Kidney Diseases workgroup on clinical trials in Acute Kidney Injury (AKI) recommended the use of MAKE30 as an endpoint in 2012 (11). Effects of the short-term or longer-term evolution of AKI were captured by MAKE30, a composite of death, new RRT, or PRD (11). Furthermore, MAKE30 was a composite and objective clinical outcome measure for sepsis patients that reflected comprehensive renal outcomes better than just a single complication, AKI. Therefore, early MAKE30 prediction and prompt personalized management may enhance the clinical prognosis.
It is crucial to select appropriate endpoints in clinical trials. Although there is consensus that serum creatinine and urine volume are employed as AKI predictors, the serum creatinine and urine volume have a significant variation until 50% of renal function is lost, indicating that none of them are particularly meaningful to patients. In addition, researchers are beginning to understand that patient-centered outcome, such as mortality, dialysis, and chronic kidney disease development, are more important for patients. With the help of MAKE30, it is possible to detect short-term effects on AKI, improve target therapy, and facilitate the conduct of clinical trials. The MAKE30 has been used as an adequate test endpoint in several clinical trials including the SALT, SMART, and pediatric sepsis trials (12–14). There are no trustworthy or reliable prediction models, however, to identify MAKE30 in sepsis patients with T2DM. We hypothesized that a nomogram model, based on routine biomarkers available within 24 hours of admission, may be of significant clinical value to predict MAKE30 because conventional biochemical indications are intrinsically unstable when used as a single index. To identify high-risk individuals likely to develop MAKE30 in sepsis patients with T2DM, the goal of our study was to identify the risk variables for MAKE30 and build an early prediction model.
Between January 2015 and December 2021, 406 sepsis patients with T2DM participated in a retrospective cohort study (project number: 81773128), and anonymized clinical data are obtained from the Biobank of First Affiliated Hospital of Xi’an Jiaotong University (Xi’an, China).
All sepsis patients with T2DM (18 years old and above) were evaluated for study enrollment. The sepsis 3.0 criteria were used for sepsis diagnosis (1). Furthermore, T2DM patients were identified if one of the following conditions was met: (1) self-reported diagnosis of T2D, (2) fasting plasma glucose (FPG) ≥7.0 mmol/L or (3) having received T2DM medications according to the 2022 American Diabetes Association (ADA) criteria (15). The following were the exclusion criteria: (1) below 18 years old; (2) hospitalization less than 24 hours; (3) A history of chronic kidney disease (stage 4-5) or renal transplantation, or current hemodialysis; (4) Hematological disorders. Participants were then divided into MAKE30 group and non-MAKE30 group based on their diagnostic criteria (16). Any one of the following criteria can be used to diagnose MAKE30 composite endpoints: (1) death; (2) receiving RRT for the first time; (3) a PRD (defined as a final inpatient serum creatinine value greater than or equal to 200% of baseline). These three components of MAKE30 were removed 30 days after inclusion or at hospital release, whichever came first. Furthermore, baseline serum creatinine was determined as follows: (1) If available, the lowest value was measured between 12 months and 24 hours prior to hospitalization; (2) When measured values were unavailable, an estimate was made using the formula previously described [creatinine(μmol/l)= 88.4 × (0.74 − 0.2 + 0.003 × age) in females, and creatinine (μmol/l) = 88.4 × (0.74 + 0.003 × age) in males] (16, 17).
The general information immediately available within 24 hours after admission included age, gender, temperature, Heart Rate (HR), Respiratory Rate (RR), MAP, source of infection, and Sequential Organ Failure Assessment (SOFA). The level of White Blood Cells (WBC), Neutrophil Percentage (NEUT%), lymphocyte, monocyte, PLT, Procalcitonin (PCT), Prothrombin Time Activity (PTA), Thrombin Time (TT), International Normalized Ratio (INR), Fibrinogen Degradation Products (FDP), D-Dimer (D-D), Fibrinogen (FIB), Activated Partial Thromboplastin Time (APTT), Prothrombin Time (PT), Globin (GLB); Albumin (ALB); Total Bilirubin (TBiL); Lipoprotein(a) (Lp(a)); apoE, apolipoprotein B (apoB), apolipoprotein A (apoA), Low-Density Lipoprotein (LDL), HDL, Triglycerides (TGs), Total Cholesterol (TC), urinary glucose, Uric Acid (UA), cystatin C, Blood Urea Nitrogen (BUN), Creatinine (Cr), and Total Cholesterol (TC) were also recorded within 24 hours after admission.
Mean ± standard deviation was used to express the continuous variables that conformed to the normal distribution, median (interquartile range) was used to express non-normally distributed continuous variables, and categorical variables were expressed by the percentages. To further identify the independent predictors of MAKE30, univariate and multivariate logistic regression models were used. The corresponding nomogram was constructed using the output from multivariate logistic regression analysis, and then we constructed an online dynamic nomogram using the “DynNom” package. The ROC curves were used to evaluate the accuracy of independent predictors of MAKE30. The calibration curves were drawn to assess the consistency of the observed results and predicted probability. Decision Curve Analysis (DCA) was performed to assess the clinical net benefit of the predictive model. Finally, an analysis of the secondary outcomes using ROC curves was performed to assess the discrimination of the nomogram for 30-day mortality.
SPSS 26.0 software and R version 4.1.2 were used for the statistical analysis, at P < 0.05.
In the final analyses, 406 sepsis patients with T2DM in total were selected (Figure 1). Of the 406, 145 (35.7%) patients reached MAKE30 during hospitalization. For the individual components, the mortality incidence was 92 (22.7%), new RRT was 45 (11.1%), and PRD was 37 (9.11%). Venn diagram shows the relationship among MAKE30 components more intuitively (Figure 2A). The median age of participants was 62 years (range, 57 to 71), with 39.2% of patients being female. Patients with MAKE30 were significantly older than those with non-MAKE30 (Table 1). Moreover, patients with MAKE30 had significantly higher SOFA scores than those with non-MAKE30 (10, 8-13 vs. 5, 3-7; P < 0.001). There were significant differences in the MAP (67, 63-90 vs. 93, 80-112; P < 0.001), HR, and infection sources between the MAKE30 group and the non-MAKE30 group. Nevertheless, gender, temperature, and RR were not significantly different at P > 0.05.
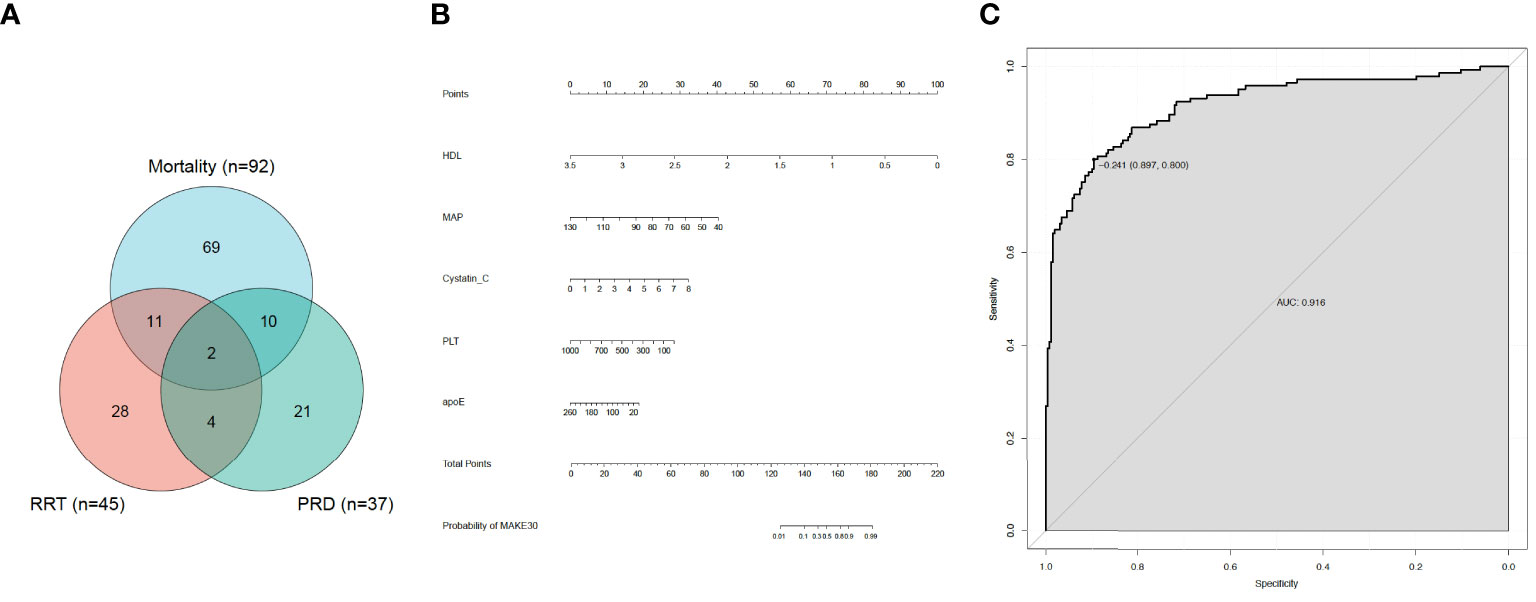
Figure 2 MAKE30 in sepsis patients with type 2 diabetes mellitus. (A) Venn diagram of major adverse kidney events within 30 days (MAKE30) components; (B) Nomogram to estimate the risk of MAKE30 in sepsis patients with type 2 diabetes mellitus; (C) The ROC curve of the nomogram for predicting MAKE30 in sepsis patients with type 2 diabetes mellitus.
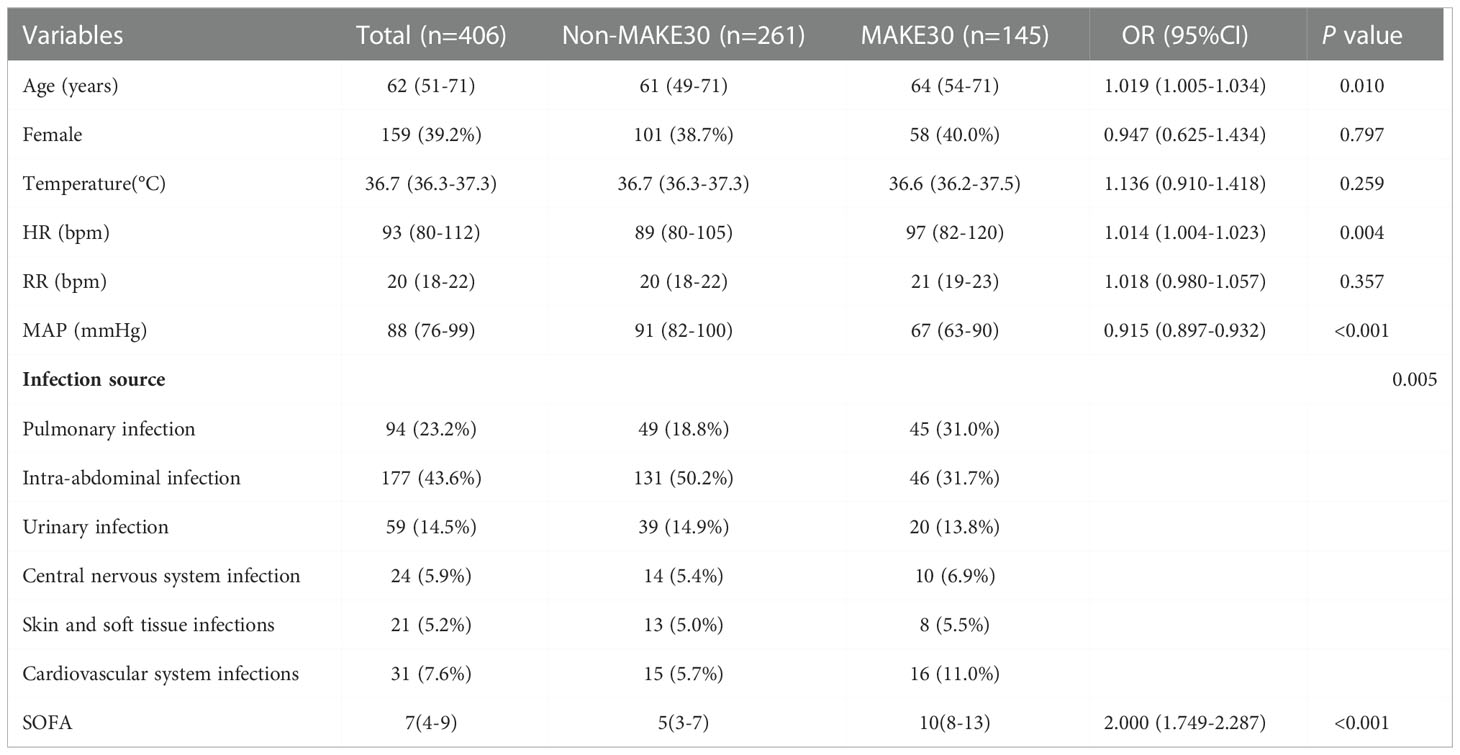
Table 1 The demographic and clinical data of sepsis patients with diabetes between the non-MAKE30 and MAKE30 groups.
All the clinical biochemical indicators were available within 24 hours of admission as shown in Table 2. Univariate analyses revealed that the level of NEUT, PCT, TT, INR, FDP, D-D, APTT, PT, TBiL, UA, cystatin C (2.0, 1.1-2.9 vs. 1.1, 0.9-1.5; P < 0.001), BUN, and Cr significantly increased in the MAKE30 group compared with the non-MAKE30 group. On the contrary, the level of PLT (97, 58-151 vs. 162, 95-251; P < 0.001), PTA, apoE (35.5, 30.3-55.0 vs. 49.5, 34.8-75.2; P < 0.001), apoB, apoA, LDL, HDL (0.39, 0.29-0.51 vs. 0.70, 0.58-0.90; P < 0.001), and TGs were significantly lower in the S-AKI group, compared with the non-MAKE30 group.
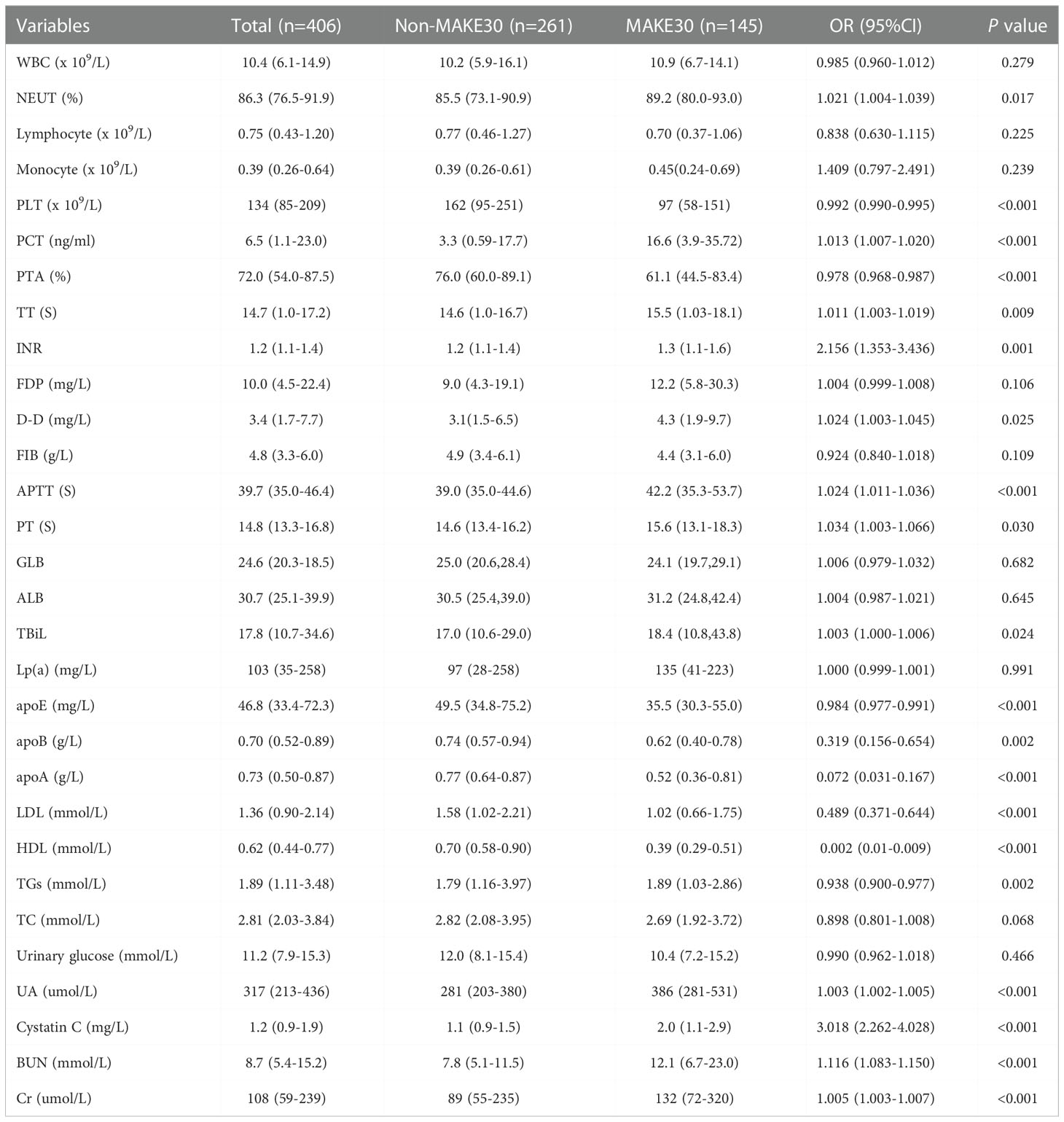
Table 2 Univariate analyses of clinical biochemical indicators between non-MAKE30 and MAKE30 groups in sepsis patients with T2DM.
The significant different variables, including age, HR, MAP, NEUT, PLT, PCT, PTA, TT, INR, FDP, D-D, APTT, PT, TBiL, apoE, apoB, apoA, LDL, HDL, TGs, UA, BUN, cystatin C and Cr were used in multivariate logistic regression analyses. Then, it revealed that in sepsis patients with T2DM, MAP (0.928,0.906-0.950), PLT (0.995,0.992-0.999), HDL (0.009,0.002-0.036), apoE (0.988,0.979-0.997), and cystatin C (1.960,0.360-2.826) were independent predictors for MAKE30 (Table 3). Furthermore, a nomogram based on these traits was created to predict MAKE30 in sepsis patients with T2DM (Figure 2B). Moreover, we provided an online version of this nomogram using the “DynNom” package for widespread use by physicians and researchers (https://diabetes-s-aki.shinyapps.io/DynNomapp/).
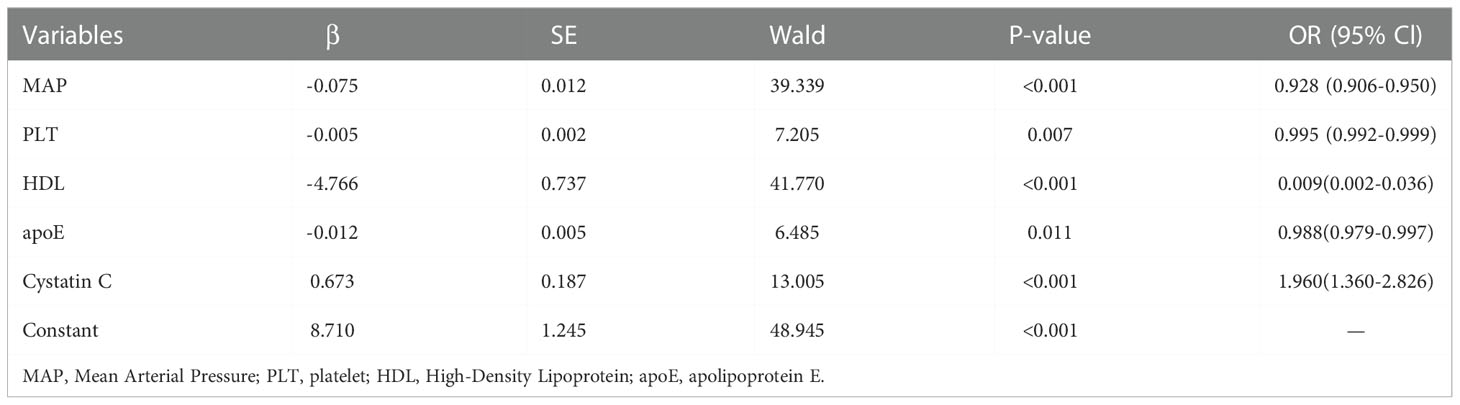
Table 3 Multivariate logistic regression analyses of independent predictors for MAKE30 in sepsis patients with T2DM.
In sepsis patients with T2DM, the diagnostic value of the nomogram model for MAKE30 was assessed using the ROC curve. The model had a good ability to predict MAKE30 (AUC = 0.916) as demonstrated in Figure 2C. Moreover, the predictive probabilities based on the calibration curve were consistent with the observation results, indicating a successful calibration (Figure 3A). In DCA, it was found that the nomogram has a superior overall net benefit within a wide and practical range of threshold probabilities, implying a high potential for clinical application (Figure 3B). Hence, in sepsis patients with T2DM, the nomogram model may be a robust MAKE30 predictor.
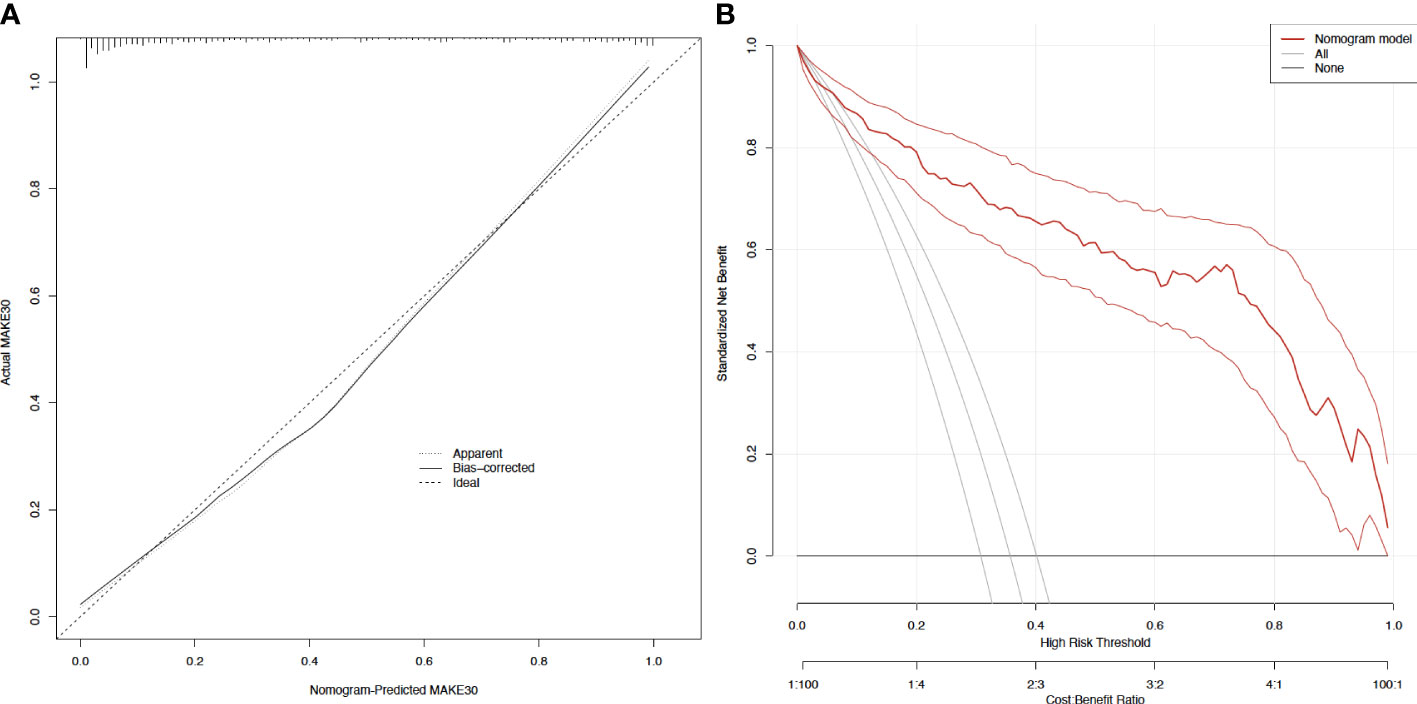
Figure 3 Calibration curves and decision curve analysis of the nomogram for predicting MAKE30 (A) Calibration curves of the nomogram; (B) Decision curve analysis of the nomogram.
Figure 4 illustrates how ROC curve analyses revealed that the nomogram model also had a high ability to predict the occurrence of 30-day mortality (AUC = 0.822), new RRT (AUC = 0.874), and PRD (AUC = 0.801) in sepsis patients with T2DM.
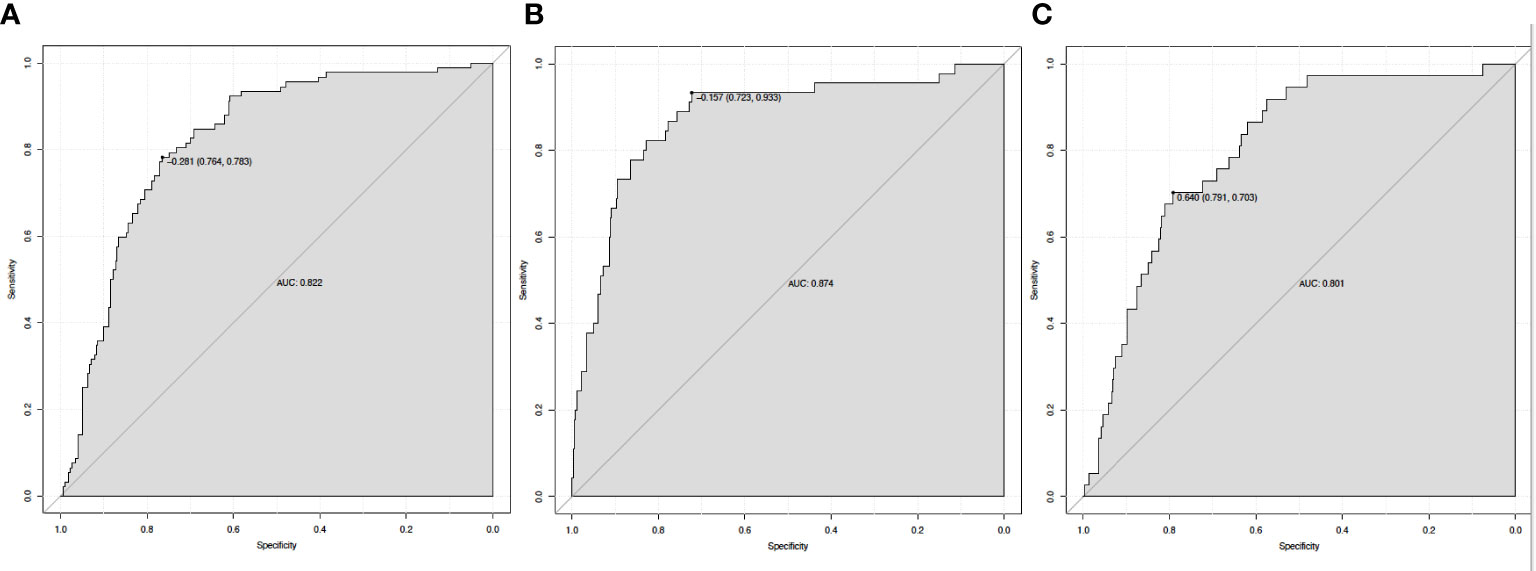
Figure 4 The ROC curve of the nomogram for predicting 30-day mortality (A), new RRT (B), and PRD (C). RRT, renal replacement therapy; PRD, persistent renal dysfunction.
We constructed a simple nomogram model to predict MAKE30 in sepsis patients with T2DM based on those independent predictors (MAP, PLT, Cystatin C, HDL, and apoE) within 24 hours of admission. The most important thing was that the model worked well and was simple to apply. In addition, doctors must use a quick and precise nomogram model to anticipate MAKE30 to make tailored management decisions, which will improve the outcome and reduce mortality in sepsis patients with T2DM. Since sepsis is one of the most common causes of kidney injury, it is a prominent clinical problem in critically ill patients (18). Furthermore, more sepsis patients with T2DM 145 (35.7%) reached a MAKE30 composite outcome, than patients with acute pancreatitis (16%) and sepsis patients (28.3%) (16, 17).
The classical theories suggest that the primary pathogenic mechanisms causing kidney damage include decreased renal blood flow, secondary tubular epithelial cell death, or acute tubular necrosis. In the current study, sepsis with T2DM and a low level of MAP is an independent risk factor for MAKE30, which indicates renal tissue ischemia. However, more studies have revealed that various mechanisms, such as inflammation, microcirculatory dysfunction, and metabolic reprogramming, contribute to the pathogenesis of kidney injury, and that hemodynamic instability was not a necessary pathogenetic factor for kidney injury (19, 20). It is well known that during the sepsis period, inflammatory mediators are released in the intravascular compartment, causing damage to the vascular endothelium. Then, the coagulation system subsequently hyperactivated as a result of damaged vascular endothelium activating platelets first, which significantly caused the subsequent formation of micro-thrombi contributing to kidney injury (20, 21). Intimate and complex relationships existed between the inflammatory response and coagulation failure, both of which have a significant impact on the etiology of kidney damage in sepsis patients with type 2 diabetes. We also found that PLT was an independent predictor of MAKE30. Previous studies have demonstrated shown that PLT is important for controlling hemostasis as well as for interacting with other immune cells to modulate the immune and inflammatory response (22). Lv et al. also revealed that platelets contributed to kidney injury by inducing renal cell apoptosis (23).
In addition, we demonstrated that in sepsis patients with T2DM, cystatin C was an independent predictor of MAKE30. Protease inhibitors belonging to the cystatin superfamily, serum cystatin C, can pass freely through the glomerular filtration and then be completely reabsorbed and degraded by the renal tubules (24). It is not significantly influenced by biological factors such as age, gender, muscle mass, diet, infection, and tumors, in contrast to Creatinine (Cr). Serum Cr may not be able to detect very early changes in renal function, particularly in sepsis patients with early tubular necrosis (18). Nevertheless, serum cystatin C was able to predict the occurrence of AKI, one to two days earlier than serum Cr (18, 25). Cystatin C has been suggested as a potential predictor for the early diagnosis of S-AKI or was an alternative to the gold standard “creatinine” in growing number of studies in recent years (18, 26). Regardless of the underlying precipitating factors, previous studies have demonstrated that cystatin C played a significant role in the diagnosis and prediction of kidney injury in sepsis or other critical clinical conditions (25, 27). Moreover, in critically ill neonates, cystatin C could be used as a powerful predictor of kidney injury (28). When it was immediately estimated within 24 hours after admission for early detection of kidney injury, hence, serum or urine, cystatin C was very valuable (26).In addition, it was found that serum cystatin C was associated with the recovery, death, or renal replacement therapy of kidney injury (27, 29).
Future interventions may be directed at altering the apolipoproteins and cholesterol of sepsis patients, which was revealed by omic methods (30, 31). Proinflammatory cytokines can also alter the hepatic synthesis of apolipoprotein and acute phase reactants in the liver, such as tumor necrosis factor-alpha (TNF-α), interleukin-6 (IL-6), and interleukin-1 (IL-1) (32). This study revealed that apoE was an independent predictor for MAKE30. Increasing evidence has demonstrated that apoE is important in the pathophysiological course of anti-infection and anti-inflammation like sepsis (31). Previous studies have demonstrated that apoE 3 could protect porcine proximal tubular cells from gentamicin-induced injury (33), and apoE ϵ4 was protective against the development of kidney injury (34).
Decreased HDL concentrations are commonplace during an acute sepsis episode and are proportional to the degree of inflammation (32). Studies have shown that HDL levels decreased by 40–70% throughout the inflammatory process, which resulted in patients with sepsis having a bad prognosis (35). Furthermore, in patients with sepsis, reduced HDL levels were independently associated with an increased risk of kidney injury onset and decreased Glomerular Filtration Rate (GFR) (36). Chien et al. indicated that to avoid disease progression, multi-organ dysfunction, and renal injury in sepsis, HDL levels were a prognostic factor for making a personalized management plan (37). Reduced cholesterol may be the outcome of elevated erythropoietin brought on by hypoxia signaling activation in patients with ischemia-induced kidney impairment (38). Since kidneys are significant in the recycling of senescent HDL particles, clinical studies have demonstrated that low levels of HDL were associated with increased risk of kidney injury (35). Moreover, polymorphisms in HDL metabolism genes such as rs1800777 (allele A) in the CETP gene, were strongly associated with an increased risk of kidney injury during sepsis (39). Increased HDL levels may activate the endothelial nitric oxide synthase (eNOS) pathway, reducing the production of adhesion molecules, leucocytes activation, and neutrophil infiltration, reducing vascular impairment and renal parenchymal damage (35). The loss of HDL and its components is influenced by renal tubular injury influences tubular reabsorption function and catabolism (35). Extra renal synthesis and metabolism of HDL components can also be influenced by kidney injury (40). Therefore, in sepsis patients with T2DM, lower HDL is significantly associated with MAKE30 prediction
In this study, we developed a simple nomogram model (based on MAP, PLT, Cystatin C, HDL, and apoE) to predict MAKE30 in sepsis patients with T2DM. After verification, it demonstrated good performance in discrimination, calibration, and clinical application. This nomogram can also be used to determine the appropriate treatment options for high-risk patients. The application of the nomogram model is demonstrated by the following example: assuming a sepsis patient with T2DM with a MAP of 70 mmHg, a Cystatin C of 3 mg/L, an HDL of 1 mmol/L, a PLT of 200 x 109/L, and an apoE of 40 mg/L. The score assigned to each parameter on the “Points” axis is obtained as shown in Figure 2B. The sum of points for each parameter is used to calculate the final score [27 (MAP) + 12 (Cystatin C) + 72 (HDL) + 23 (PLT) + 15 (apoE) = 149]. This score corresponds to about 33% risk of developing MAKE30. Another option is to use the online version (https://diabetes-s-aki.shinyapps.io/DynNomapp/) to obtain the same result easily. Finally, we also discovered that in sepsis patients with T2DM, the nomogram model had perfect predictive power for predicting 30-day mortality (AUC = 0.822), new RRT (AUC = 0.874), and PRD (AUC = 0.801).
Nevertheless, this study had some limitations. Firstly, since it was a single-center study, there was a chance of selection bias influencing the results. Secondly, it was a seven-year retrospective study, so there was significant advancement in the management decision-making process, affecting sepsis development. Thirdly, the model was constructed from a training group with a significantly smaller testing sample. Therefore, additional multi-center prospective studies with an adequate cohort size would be needed to assess its potential and validate the results.
In conclusion, our study demonstrated that in sepsis patients with T2DM, the levels of MAP, PLT, Cystatin C, HDL, and apoE available within 24 hours after admission played a critical role in MAKE30 prediction. Moreover, the predictive nomogram model based on those predictors performed well in the discrimination, calibration, and clinical application for MAKE30, which is crucial for clinicians to make timely personalized management decisions.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
This study was conducted following the Declaration of Helsinki and was approved by the Ethical Committee of the First Affiliated Hospital of Xi’an Jiaotong University. All patient data were analyzed in anonymity. Patient consent was waived by the ethics committee, as no individual data were published, nor was any intervention performed on patients.
QX conceived of the study and drafted the manuscript. TX, RC, and XZ participated in the statistical analysis. HW participated the design of the study. SW, CL, and JZ participated in its design and coordination and helped to draft the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by National Nature Science Foundation of China (No. 82072145); Clinical Research Fund of the First Affiliated Hospital of Xi’an Jiaotong University (Establishment of a Model for Early Prediction, Diagnosis and Monitoring of Sepsis: A Cross Regional Multicenter Cohort Study; No. XJTU1AF-CRF-2020-003).
We appreciate the nurses and physicians who assisted with the study. We thank the Biobank of First Affiliated Hospital of Xi’an Jiaotong University for providing clinical data.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1024500/full#supplementary-material
1. Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA (2016) 315(8):801–10. doi: 10.1001/jama.2016.0287
2. Frydrych LM, Fattahi F, He K, Ward PA, Delano MJ. Diabetes and sepsis: Risk, recurrence, and ruination. Front Endocrinol (Lausanne) (2017) 8:271. doi: 10.3389/fendo.2017.00271
3. Yue S, Li S, Huang X, Liu J, Hou X, Wang Y, et al. Construction and validation of a risk prediction model for acute kidney injury in patients suffering from septic shock. Dis Markers (2022) 2022:9367873. doi: 10.1155/2022/9367873
4. Trevelin SC, Carlos D, Beretta M, da Silva JS, Cunha FQ. Diabetes mellitus and sepsis: A challenging association. Shock (2017) 47(3):276–87. doi: 10.1097/SHK.0000000000000778
5. Esper AM, Moss M, Martin GS. The effect of diabetes mellitus on organ dysfunction with sepsis: an epidemiological study. Crit Care (2009) 13(1):R18. doi: 10.1186/cc7717
6. Bhutani J, Bhutani S. Worldwide burden of diabetes. Indian J Endocrinol Metab (2014) 18(6):868–70. doi: 10.4103/2230-8210.141388
7. Costantini E, Carlin M, Porta M, Brizzi MF. Type 2 diabetes mellitus and sepsis: state of the art, certainties and missing evidence. Acta Diabetol (2021) 58(9):1139–51. doi: 10.1007/s00592-021-01728-4
8. Jiang L, Cheng M. Impact of diabetes mellitus on outcomes of patients with sepsis: an updated systematic review and meta-analysis. Diabetol Metab Syndr (2022) 14(1):39. doi: 10.1186/s13098-022-00803-2
9. Poston JT, Koyner JL. Sepsis associated acute kidney injury. BMJ (2019) 364:k4891. doi: 10.1136/bmj.k4891
10. Sathananthan M, Sathananthan A, Jeganathan N. Characteristics and outcomes of patients with and without type 2 diabetes mellitus and pulmonary sepsis. J Intensive Care Med (2020) 35(9):836–43. doi: 10.1177/0885066619833910
11. Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, et al. et al: Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol (2012) 7(5):844–50. doi: 10.2215/CJN.12791211
12. Self WH, Semler MW, Wanderer JP, Ehrenfeld JM, Byrne DW, Wang L, et al. et al: Saline versus balanced crystalloids for intravenous fluid therapy in the emergency department: study protocol for a cluster-randomized, multiple-crossover trial. Trials (2017) 18(1):178. doi: 10.1186/s13063-017-1923-6
13. Semler MW, Self WH, Wanderer JP, Ehrenfeld JM, Wang L, Byrne DW, et al. et al: Balanced crystalloids versus saline in critically ill adults. N Engl J Med (2018) 378(9):829–39. doi: 10.1056/NEJMoa1711584
14. Weiss SL, Balamuth F, Thurm CW, Downes KJ, Fitzgerald JC, Laskin BL. Major adverse kidney events in pediatric sepsis. Clin J Am Soc Nephrol (2019) 14(5):664–72. doi: 10.2215/CJN.12201018
15. American Diabetes Association Professional Practice C, American Diabetes Association Professional Practice C, Draznin B, Aroda VR, Bakris G, Benson G, et al. 2. classification and diagnosis of diabetes: Standards of medical care in diabetes-2022. Diabetes Care (2022) 45(Suppl 1):S17–38. doi: 10.2337/dc22-S002
16. Zhang X, Ye B, Mao W, Liu L, Li G, Zhou J, et al. Major adverse kidney events within 30 days in patients with acute pancreatitis: a tertiary-center cohort study. HPB (Oxford) (2022) 24(2):169–75. doi: 10.1016/j.hpb.2021.05.012
17. Mele A, Cerminara E, Habel H, Rodriguez-Galvez B, Oldner A, Nelson D, et al. Fluid accumulation and major adverse kidney events in sepsis: a multicenter observational study. Ann Intensive Care (2022) 12(1):62. doi: 10.1186/s13613-022-01040-6
18. Al-Amodi HS, Abdelsattar S, Kasemy ZA, Bedair HM, Elbarbary HS, Kamel HFM. Potential value of TNF-alpha (-376 G/A) polymorphism and cystatin c (CysC) in the diagnosis of sepsis associated acute kidney injury (S-AK I) and prediction of mortality in critically ill patients. Front Mol Biosci (2021) 8:751299. doi: 10.3389/fmolb.2021.751299
19. Peerapornratana S, Manrique-Caballero CL, Gomez H, Kellum JA. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int (2019) 96(5):1083–99. doi: 10.1016/j.kint.2019.05.026
20. Fani F, Regolisti G, Delsante M, Cantaluppi V, Castellano G, Gesualdo L, et al. Recent advances in the pathogenetic mechanisms of sepsis-associated acute kidney injury. J Nephrol (2018) 31(3):351–9. doi: 10.1007/s40620-017-0452-4
21. Jansen MPB, Florquin S, Roelofs J. The role of platelets in acute kidney injury. Nat Rev Nephrol (2018) 14(7):457–71. doi: 10.1038/s41581-018-0015-5
22. Joffre J, Hellman J, Ince C, Ait-Oufella H. Endothelial responses in sepsis. Am J Respir Crit Care Med (2020) 202(3):361–70. doi: 10.1164/rccm.201910-1911TR
23. Lv D, Zhang Y, Wang C, Gu Y, Zhang Y, Li X. Platelets derived transthyretin participate in the development of sepsis associated acute kidney injury by inducing oxidative stress and apoptosis of renal tubular epithelial cells. Shock (2022) 57(5):722–31. doi: 10.1097/SHK.0000000000001908
24. Bellos I, Fitrou G, Daskalakis G, Papantoniou N, Pergialiotis V. Serum cystatin-c as predictive factor of preeclampsia: A meta-analysis of 27 observational studies. Pregnancy Hypertens (2019) 16:97–104. doi: 10.1016/j.preghy.2019.03.006
25. Herget-Rosenthal S, Marggraf G, Hüsing J, Göring F, Pietruck F, Janssen O, et al. Early detection of acute renal failure by serum cystatin c. Kidney Int (2004) 66(3):1115–22. doi: 10.1111/j.1523-1755.2004.00861.x
26. Bian Z, Zhu R, Chen S. The predict value of serum/urocystatin c on acute kidney injury in elderly patients with sepsis. Exp Gerontol (2021) 155:111576. doi: 10.1016/j.exger.2021.111576
27. Leem AY, Park MS, Park BH, Jung WJ, Chung KS, Kim SY, et al. Value of serum cystatin c measurement in the diagnosis of sepsis-induced kidney injury and prediction of renal function recovery. Yonsei Med J (2017) 58(3):604–12. doi: 10.3349/ymj.2017.58.3.604
28. Hidayati EL, Utami MD, Rohsiswatmo R, Tridjaja B. Cystatin c compared to serum creatinine as a marker of acute kidney injury in critically ill neonates. Pediatr Nephrol (2021) 36(1):181–6. doi: 10.1007/s00467-020-04668-3
29. Nejat M, Pickering JW, Walker RJ, Endre ZH. Rapid detection of acute kidney injury by plasma cystatin c in the intensive care unit. Nephrol Dial Transplant (2010) 25(10):3283–9. doi: 10.1093/ndt/gfq176
30. Sharma NK, Tashima AK, Brunialti MKC, Ferreira ER, Torquato RJS, Mortara RA, et al. Proteomic study revealed cellular assembly and lipid metabolism dysregulation in sepsis secondary to community-acquired pneumonia. Sci Rep (2017) 7(1):15606. doi: 10.1038/s41598-017-15755-1
31. Shao Y, Zhao T, Zhang W, He J, Lu F, Cai Y, et al. Presence of the apolipoprotein e-ϵ4 allele is associated with an increased risk of sepsis progression. Sci Rep (2020) 10(1):15735. doi: 10.1038/s41598-020-72616-0
32. Golucci A, Marson FAL, Ribeiro AF, Nogueira RJN. Lipid profile associated with the systemic inflammatory response syndrome and sepsis in critically ill patients. Nutrition (2018) 55-56:7–14. doi: 10.1016/j.nut.2018.04.007
33. Takamoto K, Kawada M, Ikeda D, Yoshida M. Apolipoprotein E3 (apoE3) safeguards pig proximal tubular LLC-PK1 cells against reduction in SGLT1 activity induced by gentamicin c. Biochim Biophys Acta (2005) 1722(3):247–53. doi: 10.1016/j.bbagen.2004.12.006
34. MacKensen GB, Swaminathan M, Ti LK, Grocott HP, Phillips-Bute BG, Mathew JP, et al. Preliminary report on the interaction of apolipoprotein e polymorphism with aortic atherosclerosis and acute nephropathy after CABG. Ann Thorac Surg (2004) 78(2):520–6. doi: 10.1016/j.athoracsur.2004.02.106
35. Stasi A, Franzin R, Fiorentino M, Squiccimarro E, Castellano G, Gesualdo L. Multifaced roles of HDL in sepsis and SARS-CoV-2 infection: Renal implications. Int J Mol Sci (2021) 22(11):5980. doi: 10.3390/ijms22115980
36. Roveran Genga K, Lo C, Cirstea M, Zhou G, Walley KR, Russell JA, et al. Two-year follow-up of patients with septic shock presenting with low HDL: the effect upon acute kidney injury, death and estimated glomerular filtration rate. J Intern Med (2017) 281(5):518–29. doi: 10.1111/joim.12601
37. Chien JY, Jerng JS, Yu CJ, Yang PC. Low serum level of high-density lipoprotein cholesterol is a poor prognostic factor for severe sepsis. Crit Care Med (2005) 33(8):1688–93. doi: 10.1097/01.CCM.0000171183.79525.6B
38. Li J, Yang M, Yu Z, Tian J, Du S, Ding H. Kidney-secreted erythropoietin lowers lipidemia via activating JAK2-STAT5 signaling in adipose tissue. EBioMedicine (2019) 50:317–28. doi: 10.1016/j.ebiom.2019.11.007
39. Genga KR, Trinder M, Kong HJ, Li X, Leung AKK, Shimada T, et al. CETP genetic variant rs1800777 (allele a) is associated with abnormally low HDL-c levels and increased risk of AKI during sepsis. Sci Rep (2018) 8(1):16764. doi: 10.1038/s41598-018-35261-2
Keywords: sepsis, type 2 diabetes mellitus, major adverse kidney events within 30 days, nomogram model, early warning
Citation: Xin Q, Xie T, Chen R, Wang H, Zhang X, Wang S, Liu C and Zhang J (2022) Predictive nomogram model for major adverse kidney events within 30 days in sepsis patients with type 2 diabetes mellitus. Front. Endocrinol. 13:1024500. doi: 10.3389/fendo.2022.1024500
Received: 24 August 2022; Accepted: 06 December 2022;
Published: 16 December 2022.
Edited by:
Mohammad H. Abukhalil, Al-Hussein Bin Talal University, JordanReviewed by:
Lu Ke, Nanjing University, ChinaCopyright © 2022 Xin, Xie, Chen, Wang, Zhang, Wang, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shufeng Wang, ZGF3bkBtYWlsLnhqdHUuZWR1LmNu; Chang Liu, bGl1Y2hhbmdkb2N0b3JAMTYzLmNvbQ==; Jingyao Zhang, eW91MTJvdXlAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.