- 1Diabetes, Endocrinology and Metabolism Section, Department of Internal Medicine I, St. Josef Hospital, Ruhr University Bochum, Bochum, Germany
- 2Diabetes Centre Bochum/Hattingen, Klinik Blankenstein, Hattingen, Germany
- 3Centre for Rare Endocrine Diseases, Ruhr Centre for Rare Diseases (CeSER), Ruhr University Bochum and Witten/Herdecke University, Bochum, Germany
- 4Centre for Diabetes Technology, Catholic Hospitals Bochum, Ruhr University Bochum, Bochum, Germany
- 5Department for Electrophysiology, Medical Hospital I, Klinikum Vest, Recklinghausen, Germany
- 6Singapore Institute for Clinical Sciences (SICS), Agency for Science, Technology and Research (ASTAR), Singapore, Singapore
- 7Department of Endocrinology, Tan Tock Seng Hospital, Singapore, Singapore
- 8Metabolic Disorders Research Programme, Lee Kong Chian School of Medicine, Singapore, Singapore
- 9Cardiovascular and Metabolic Disorders Program, Duke-National University of Singapore Medical School, Singapore, Singapore
Editorial on the Research Topic:
Thyroid hormones and cardiac arrhythmia
The thyro-cardiac axis: Growing attention to a long-known connection
Among the premier effects of thyroid hormones in vertebrates are actions on the cardiovascular system (1–5). Cardiovascular complications rank among the key causes of death in thyroid emergencies (6).
While our knowledge of the link between thyroid disease and cardiac arrhythmia dates back more than two centuries, attention to this connection continues to rise. The number of publications meeting the search formula “thyroid AND heart” is approaching the mark of 10,000 results (Figure 1), and it is still exponentially growing. A considerable proportion of these publications covers cardiac arrhythmia.
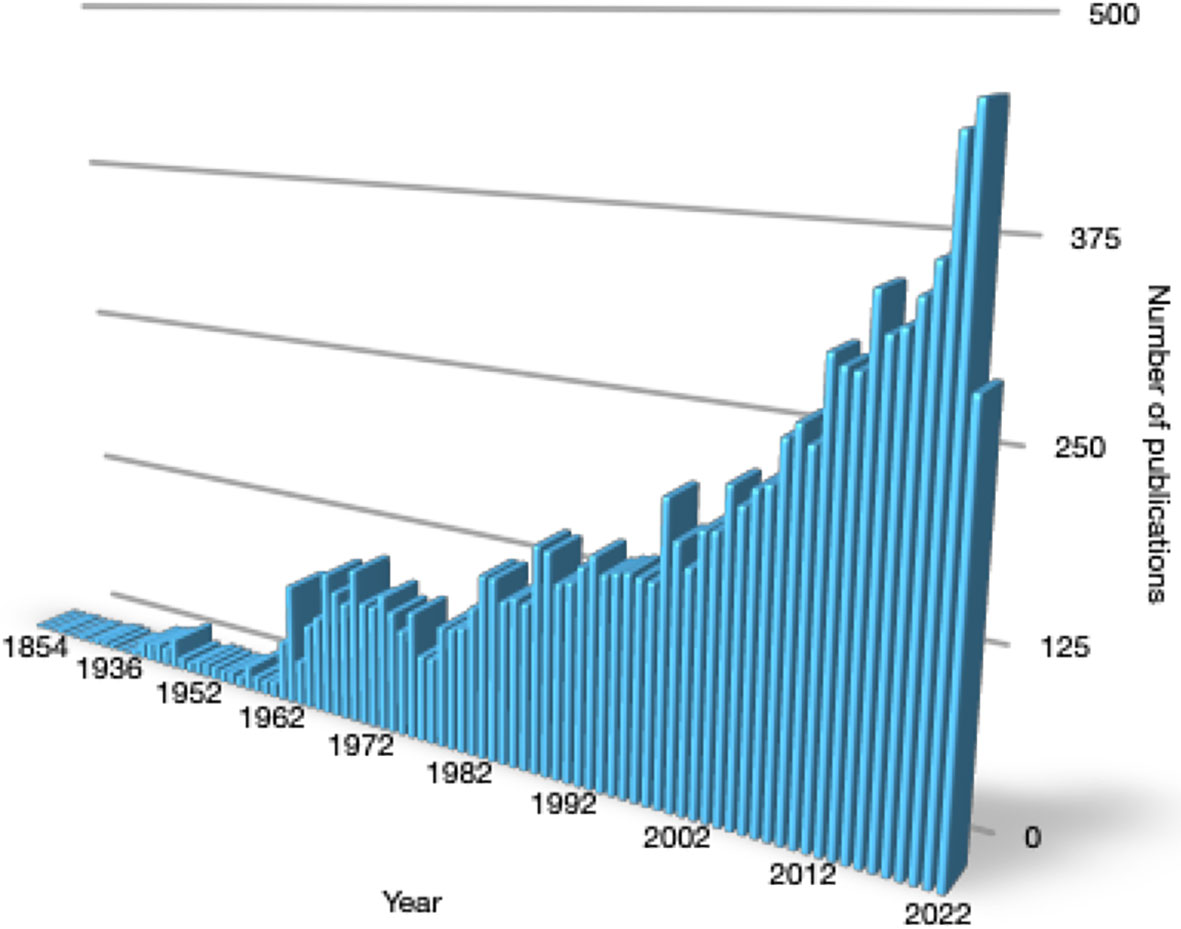
Figure 1 Yearly number of publications meeting the search term “thyroid AND heart” from 1854 to August 2022 in PubMed.
Therapeutic algorithms protocolized by cardiovascular medicine benefitted the prognosis of patients with chronic heart disease, and preventive programs have reduced cardiovascular morbidity at the population level. However, a substantial residual hazard persists. Therefore, the rediscovery of thyroid function as a major determinant of cardiovascular health is timely to address this gap of fundamental significance.
This Research Topic was initiated to provide a forum on recent developments in the thyro-cardiac nexus and to foster a deeper physiological understanding of this vitally important intersection. A series of articles summarising the state of current evidence deliver new perspectives on recent developments.
Thyroid dysfunction and supraventricular arrhythmia
The resting heart rate is strongly modulated by thyroid hormone. This is the reason, why it became integral to established scoring systems for the diagnosis of myxoedema coma and thyroid storm (7–10). Thyrotoxicosis may also potentiate ectopic beats and atrial fibrillation (AF) (11–14). In adults, AF is a common condition conveying significant health hazard. Current guidelines recommend assessing the thyroid status in subjects with AF and, vice versa, screening for AF in hyperthyroid patients (15).
Subclinical hyperthyroidism and even FT4 concentration in its highest quartile in persons with normal TSH are associated with increased incidence of AF (16). Gencer et al. review this observation and provide recommendations for the management of new-onset thyroid-related AF.
Unlike in adults, AF is exceedingly rare in children and adolescents. Subramonian et al. describe the case of a 15-year-old female with AF and thyrotoxic tachymyopathy due to Graves’ disease, being reverted after successful treatment of the thyroid condition.
Subclinical hyperthyroidism is also associated with an increased risk for the recurrence of AF after catheter ablation, as demonstrated in the retrospective cohort study by Li et al.
Thyroid function and ventricular arrhythmic events
In the ventricles, thyrotoxicosis promotes triggered activity and the formation of re-entry circuits giving rise to ventricular tachycardia, flutter and fibrillation (17, 18). These forms of malignant arrhythmia contribute to the high fatality rate of untreated thyroid storm.
This is exemplified by the case of a young woman with Graves’ disease and overt hyperthyroidism described by Fu et al. She suffered a cardiac arrest due to ventricular fibrillation but ultimately recovered following multimodal intensive care treatment including targeted temperature management with an intravascular cooling system.
Allostatic load – confounder or mediator of risk?
The hypothalamus-pituitary-thyroid axis can dynamically adapt to physiological requirements. In type 1 allostatic load (e.g. in starvation or critical illness) the set point of the feedback loop is lowered, so that the concentrations of T3 (and occasionally TSH and T4) are down-regulated (19). A largely opposite endocrine pattern is observed in type 2 allostatic load (e. g. in psychosocial stress or certain psychiatric diseases) (19, 20).
Allostatic responses may raise considerable problems in the differential diagnosis of thyroid function. We (Dietrich et al.) demonstrated this for TSH, which correlates to a marker of type 2 allostatic load. Therefore, the prevalence of subclinical hypothyroidism may be overestimated in chronic psychosocial stress (21–24).
Low-T3 syndrome as the key component of thyroid allostasis in critical illness, tumours, uraemia and starvation (TACITUS) or non-thyroidal illness syndrome (NTIS) can be maladaptive and heralds a poor prognosis in severe illnesses. Two independent studies (Gao et al. and Abdu et al.) could demonstrate that this also applies to myocardial infarction with nonobstructive coronary arteries (MINOCA), a major subtype of type 2 myocardial infarction.
Clinical evidence from prospective studies
Overt thyroid dysfunction is an established risk factor for mortality and major adverse cardiovascular events (MACE) (25–29). For minor disorders of thyroid function (e.g. subclinical hypo- and hyperthyroidism and within-reference range variations of thyroid hormones), the evidence was less clear. A systematic review including 32 studies covering more than 1.3 million subjects and a subsequent meta-analysis (Müller et al.) found cardiovascular death (CVD) to be predicted by both subclinical hypo- and hyperthyroidism. Circulating FT4 concentration was positively associated with the hazard ratio for CVD and MACE. The results suggest a monotonic association of FT4 to cardiovascular risk, but a complex U-shaped pattern linking TSH to cardiovascular endpoints, supporting the assumption of heterogeneous pathophysiological mechanisms.
Physiological evidence and molecular mechanisms
In the clinical setting, the risk for malignant arrhythmia can be estimated e.g. by measuring certain time intervals in the electrocardiogram (ECG). In addition to the QT interval, an established biomarker of thyroid function (30), we could show that the Tp-e and JT intervals, which are not affected by QRS duration, correlate to TSH, FT4 and FT3 concentration (Aweimer et al.).
The impact of thyroid hormones on cardiac electrophysiology is mediated by a plethora of mechanisms (31). This applies to normal automaticity, triggered activity, disorders of impulse conduction and re-entry mechanisms. At a molecular level, thyroid hormones act via four distinct signalling types involving different time scales ranging from minutes to hours, where the expression of multiple genes is profoundly modulated (Müller et al).
Aspects of therapy and prevention
Given the risk conferred by even slight deviations of thyroid hormones from an individual optimum, it is of paramount importance to restore euthyroidism as fast as possible in the case of thyroid emergencies (6). For thyroid storm, Lim et al. describe several options like pharmacotherapy and therapeutic plasma exchange, supported by adjuvant extra-corporal systems, including continuous renal replacement therapy (CRRT) and extracorporeal membrane oxygenation (ECMO). Irreversible brain damage in thyroid storm may be prevented by intravascular cooling devices, as demonstrated by Fu et al. For thyroid-related AF, Gencer et al. provide a comprehensive flow chart focussing on adjuvant non-endocrine treatment modalities.
Special caveats apply to substitution therapy with levothyroxine in hypothyroidism. In certain stages of thyroid cancer, TSH-suppressive therapy is recommended by current guidelines (32), but it may confer increased cardiovascular risk, as reviewed by Gluvic et al., thereby implying the need to evaluate its benefit:risk ratio and hence individualize the degree of TSH suppression that optimizes the health outcome.
Prospectus
This collection of articles provides a current overview of the interface between thyroid function and cardiac rhythmology from different perspectives. According to the available evidence, even slight deviations of thyroid hormones confer significant risks for MACE, including malignant arrhythmia. Advanced diagnostical strategies should address the whole feedback loop to avoid misinterpretation by allostatic load, hysteresis and other effects, and modern therapeutic measures should involve multimodal approaches (21–23, 33–37). This is of particular importance due to the biological potency and relatively long plasma half-life of thyroid hormones.
The findings presented in this Research Topic also affect the ongoing debate about cardiometabolic medicine (38–40), strongly supporting the integration of thyroidology and cardiovascular rhythmology in this emerging subspecialty.
Author contributions
JD, PM, and ML wrote some of the papers in this Research Topic and participated as guest editors for manuscripts, where they were not co-authors themselves. All authors listed have made a substantial, direct, and intellectual contribution to this editorial and approved it for publication.
Acknowledgments
JD, PM and ML thank all authors, reviewers, and external editors for their valuable contributions to this Research Topic.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Corona G, Croce L, Sparano C, Petrone L, Sforza A, Maggi M, et al. Thyroid and heart, a clinically relevant relationship. J Endocrinol Invest (2021) 44(12):2535–44. doi: 10.1007/s40618-021-01590-9
2. Stojkovic M, Zarkovic M. Subclinical thyroid dysfunction and the risk of cardiovascular disease. Curr Pharm Des (2020) 26(43):5617–27. doi: 10.2174/1381612826666201118094747
3. Biondi B, Palmieri EA, Lombardi G, Fazio S. Effects of subclinical thyroid dysfunction on the heart. Ann Intern Med (2002) 137(11):904–14. doi: 10.7326/0003-4819-137-11-200212030-00011
4. Razvi S, Jabbar A, Pingitore A, Danzi S, Biondi B, Klein I, et al. Thyroid hormones and cardiovascular function and diseases. J Am Coll Cardiol (2018) 71(16):1781–96. doi: 10.1016/j.jacc.2018.02.045
5. Jabbar A, Pingitore A, Pearce SH, Zaman A, Iervasi G, Razvi S. Thyroid hormones and cardiovascular disease. Nat Rev Cardiol (2017) 14(1):39–55. doi: 10.1038/nrcardio.2016.174
6. Dietrich JW. Thyreotoxische krise und myxödemkoma. Der Nuklearmediziner (2016) 39(02):124–31. doi: 10.1055/s-0042-105786
7. Akamizu T, Satoh T, Isozaki O, Suzuki A, Wakino S, Iburi T, et al. Diagnostic criteria, clinical features, and incidence of thyroid storm based on nationwide surveys. Thyroid (2012) 22(7):661–79. doi: 10.1089/thy.2011.0334
8. Burch HB, Wartofsky L. Life-threatening thyrotoxicosis. Thyroid storm Endocrinol Metab Clin North Am (1993) 22(2):263–77. doi: 10.1016/S0889-8529(18)30165-8
9. Chiong YV, Bammerlin E, Mariash CN. Development of an objective tool for the diagnosis of myxedema coma. Transl Res (2015) 166(3):233–43. doi: 10.1016/j.trsl.2015.01.003
10. Popoveniuc G, Chandra T, Sud A, Sharma M, Blackman MR, Burman KD, et al. A diagnostic scoring system for myxedema coma. Endocr Pract (2014) 20(8):808–17. doi: 10.4158/EP13460.OR
11. Aguilar M, Rose RA, Takawale A, Nattel S, Reilly S. New aspects of endocrine control of atrial fibrillation and possibilities for clinical translation. Cardiovasc Res (2021) 117(7):1645–61. doi: 10.1093/cvr/cvab080
12. Komatsu R, Karimi N, Zimmerman NM, Sessler DI, Bashour CA, Soltesz EG, et al. Biochemically diagnosed hypothyroidism and postoperative complications after cardiac surgery: a retrospective cohort analysis. J Anesth (2018) 32(5):663–72. doi: 10.1007/s00540-018-2533-5
13. Meng X, Wang XL, Zhang ZY, Zhang K, Gao J, Zheng JL, et al. Association between thyroid dysfunction and incidence of atrial fibrillation in patients with hypertrophic obstructive cardiomyopathy. Front Endocrinol (Lausanne) (2022) 13:875003. doi: 10.3389/fendo.2022.875003
14. Selmer C, Faber J. Mild thyroid dysfunction: A potential target in prevention of atrial fibrillation? Circulation (2017) 136(22):2117–8. doi: 10.1161/CIRCULATIONAHA.117.031283
15. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): The task force for the diagnosis and management of atrial fibrillation of the European society of cardiology (ESC) developed with the special contribution of the European heart rhythm association (EHRA) of the ESC. Eur Heart J (2021) 42(5):373–498. doi: 10.1093/eurheartj/ehaa612
16. Baumgartner C, da Costa BR, Collet TH, Feller M, Floriani C, Bauer DC, et al. Thyroid function within the normal range, subclinical hypothyroidism, and the risk of atrial fibrillation. Circulation (2017) 136(22):2100–16. doi: 10.1161/CIRCULATIONAHA.117.028753
17. Müller P, Dietrich JW, Lin T, Bejinariu A, Binnebössel S, Bergen F, et al. Usefulness of serum free thyroxine concentration to predict ventricular arrhythmia risk in euthyroid patients with structural heart disease. Am J Cardiol (2020) 125(8):1162–9. doi: 10.1016/j.amjcard.2020.01.019
18. Sharp NA, Neel DS, Parsons RL. Influence of thyroid hormone levels on the electrical and mechanical properties of rabbit papillary muscle. J Mol Cell Cardiol (1985) 17(2):119–32. doi: 10.1016/s0022-2828(85)80015-8
19. Chatzitomaris A, Hoermann R, Midgley JE, Hering S, Urban A, Dietrich B, et al. Thyroid allostasis-adaptive responses of thyrotropic feedback control to conditions of strain, stress, and developmental programming. Front Endocrinol (Lausanne) (2017) 8:163. doi: 10.3389/fendo.2017.00163
20. Aweimer A, El-Battrawy I, Akin I, Borggrefe M, Mugge A, Patsalis PC, et al. Abnormal thyroid function is common in takotsubo syndrome and depends on two distinct mechanisms: results of a multicentre observational study. J Intern Med (2021) 289(5):675–87. doi: 10.1111/joim.13189
21. Abbey EJ, McGready J, Ferrucci L, Simonsick EM, Mammen JSR. Thyroid hormone supplementation and all-cause mortality in community-dwelling older adults: Results from the Baltimore longitudinal study of aging. J Am Geriatr Soc (2021) 69(5):1283–90. doi: 10.1111/jgs.17015
22. Abbey EJ, McGready J, Sokoll LJ, Simonsick EM, Mammen JSR. Free thyroxine distinguishes subclinical hypothyroidism from other aging-related changes in those with isolated elevated thyrotropin. Front Endocrinol (Lausanne) (2022) 13:858332. doi: 10.3389/fendo.2022.858332
23. Alonso-Ventura V, Civeira F, Alvarado-Rosas A, Lou-Bonafonte M, Calmarza P, Moreno-Franco B, et al. A cross-sectional study examining the parametric thyroid feedback quantile index and its relationship with metabolic and cardiovascular diseases. Thyroid (2022). doi: 10.1089/thy.2022.0025
24. Mammen JS, McGready J, Ladenson PW, Simonsick EM. Unstable thyroid function in older adults is caused by alterations in both thyroid and pituitary physiology and is associated with increased mortality. Thyroid (2017) 27(11):1370–7. doi: 10.1089/thy.2017.0211
25. Laulund AS, Nybo M, Brix TH, Abrahamsen B, Jorgensen HL, Hegedus L. Duration of thyroid dysfunction correlates with all-cause mortality. OPENTHYRO Register Cohort PloS One (2014) 9(10):e110437. doi: 10.1371/journal.pone.0110437
26. Lillevang-Johansen M, Abrahamsen B, Jorgensen HL, Brix TH, Hegedus L. Over- and under-treatment of hypothyroidism is associated with excess mortality: A register-based cohort study. Thyroid (2018) 28(5):566–74. doi: 10.1089/thy.2017.0517
27. Liu X, Wong CKH, Chan WWL, Tang EHM, Woo YC, Liu SYW, et al. Attaining biochemical euthyroidism early after total thyroidectomy in graves' disease may lower long-term morbidity risk. BJS Open (2022) 6(4). doi: 10.1093/bjsopen/zrac079
28. Yeap BB, Alfonso H, Hankey GJ, Flicker L, Golledge J, Norman PE, et al. Higher free thyroxine levels are associated with all-cause mortality in euthyroid older men: the health in men study. Eur J Endocrinol (2013) 169(4):401–8. doi: 10.1530/EJE-13-0306
29. Marrakchi S, Kanoun F, Idriss S, Kammoun I, Kachboura S. Arrhythmia and thyroid dysfunction. Herz (2015) 40 Suppl 2:101–9. doi: 10.1007/s00059-014-4123-0
30. Isaksen JL, Skov MW, Graff C, Ellervik C, Kanters JK. Electrocardiography in euthyroid individuals: a Danish general population study. Minerva Endocrinol (2022) 47(1):1119–30. doi: 10.23736/s2724-6507.20.03170-3
31. Lin H, Mitasikova M, Dlugosova K, Okruhlicova L, Imanaga I, Ogawa K, et al. Thyroid hormones suppress epsilon-PKC signalling, down-regulate connexin-43 and increase lethal arrhythmia susceptibility in non-diabetic and diabetic rat hearts. J Physiol Pharmacol (2008) 59(2):271–85.
32. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
33. Fitzgerald SP, Falhammar H. Redefinition of successful treatment of patients with hypothyroidism. is TSH the best biomarker of euthyroidism? Front Endocrinol (Lausanne) (2022) 13:920854. doi: 10.3389/fendo.2022.920854
34. Hoermann R, Midgley JEM, Larisch R, Dietrich JW. Individualised requirements for optimum treatment of hypothyroidism: complex needs, limited options. Drugs Context (2019) 8:212597. doi: 10.7573/dic.212597
35. Leow MK. A review of the phenomenon of hysteresis in the hypothalamus-Pituitary-Thyroid axis. Front Endocrinol (Lausanne) (2016) 7:64. doi: 10.3389/fendo.2016.00064
36. Li E, Yen PM, Dietrich JW, Leow MK. Profiling retrospective thyroid function data in complete thyroidectomy patients to investigate the HPT axis set point (PREDICT-IT). J Endocrinol Invest (2021) 44(5):969–77. doi: 10.1007/s40618-020-01390-7
37. Jonklaas J, Razvi S. Reference intervals in the diagnosis of thyroid dysfunction: treating patients not numbers. Lancet Diabetes Endocrinol (2019) 7(6):473–83. doi: 10.1016/S2213-8587(18)30371-1
38. Eckel RH, Blaha MJ. Cardiometabolic medicine: A call for a new subspeciality training track in internal medicine. Am J Med (2019) 132(7):788–90. doi: 10.1016/j.amjmed.2019.02.027
39. Reiter-Brennan C, Cainzos-Achirica M, Soroosh G, Saxon DR, Blaha MJ, Eckel RH. Cardiometabolic medicine - the US perspective on a new subspecialty. Cardiovasc Endocrinol Metab (2020) 9(3):70–80. doi: 10.1097/XCE.0000000000000224
Keywords: thyroid function, cardiac arrhythmia, cardiovascular mortality, sudden cardiac death, MACE, hypothyroidism, thyrotoxicosis, cardiometabolic medicine
Citation: Dietrich JW, Müller P and Leow MKS (2022) Editorial: Thyroid hormones and cardiac arrhythmia. Front. Endocrinol. 13:1024476. doi: 10.3389/fendo.2022.1024476
Received: 21 August 2022; Accepted: 24 August 2022;
Published: 06 September 2022.
Edited and Reviewed by:
Terry Francis Davies, Icahn School of Medicine at Mount Sinai, United StatesCopyright © 2022 Dietrich, Müller and Leow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Johannes W. Dietrich, am9oYW5uZXMuZGlldHJpY2hAcnVoci11bmktYm9jaHVtLmRl
†ORCID: Johannes W. Dietrich, orcid.org/0000-0002-1185-3549
 Johannes W. Dietrich
Johannes W. Dietrich Patrick Müller
Patrick Müller Melvin Khee Shing Leow
Melvin Khee Shing Leow