
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
PERSPECTIVE article
Front. Endocrinol., 28 September 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1021800
This article is part of the Research TopicDaily Challenges Around Physical Exercise, Nutrition and Medication in Type 1 DiabetesView all 9 articles
The scientific literature shows that exercise has many benefits for individuals with type 1 diabetes. Yet, several barriers to exercise in this population exist, such as post-exercise hypoglycaemia or hyperglycaemia. Several studies suggest that the timing of exercise may be an important factor in preventing exercise-induced hypoglycaemia or hyperglycaemia. However, there is a paucity of evidence solely focused on summarising findings regarding exercise timing and the impact it has on glucose metabolism in type 1 diabetes. This report suggests that resistance or high-intensity interval exercise/training (often known as HIIT) may be best commenced at the time of day when an individual is most likely to experience a hypoglycaemic event (i.e., afternoon/evening) due to the superior blood glucose stability resistance and HIIT exercise provides. Continuous aerobic-based exercise is advised to be performed in the morning due to circadian elevations in blood glucose at this time, thereby providing added protection against a hypoglycaemic episode. Ultimately, the evidence concerning exercise timing and glycaemic control remains at an embryonic stage. Carefully designed investigations of this nexus are required, which could be harnessed to determine the most effective, and possibly safest, time to exercise for those with type 1 diabetes.
In individuals with type 1 diabetes, exercise is advised for condition management, for general health and well-being, and for reducing the risk of several chronic conditions (1). Nevertheless, many individuals with type 1 diabetes are unable to meet physical activity guidelines, with one recent study reporting that 49% of volunteers do not achieve published recommendations (2). Numerous factors explain this trend, such as lack of time and/or social support, as well as specific type 1 diabetes associated risks with exercise, including exercise-induced hypoglycaemia or hyperglycaemia (1). Hypoglycaemia, for example, can be a risk for several hours following exercise and is a well-recognised barrier to exercise in this cohort (3). Improving personal knowledge of insulin pharmacokinetics may be one approach to help mitigate this barrier (3). For example, reducing insulin basal and/or bolus doses prior to exercise can prevent hypoglycaemia during aerobic exercise, however aggressive reductions can cause hyperglycaemia (4). Another key factor in mitigating this barrier is through developing an understanding of coherent exercise strategies to reduce the risk of hypoglycaemia (3). A certain strategy may involve the consideration of exercise timing to augment the functioning of the biological clock, which is a key regulator of glucose homeostasis (5). For instance, skeletal muscle insulin sensitivity displays circadian rhythmicity that could arguably have implications for insulin administration/timing on blood glucose management around scheduled exercise (6–8). Various studies have thus sought to ascertain an optimal time of day to exercise for glucose management in type 1 diabetes (9–12). In this short Perspectives report, we aim to summarise findings in this important domain and identify practical approaches, based on observed circadian responses, to combat barriers to exercise in those with type 1 diabetes.
The term “circadian” is a Latin derivative of the phrase “Circa Diem” and translates as “about a day”, referring to the ~24-hr diurnal cycle. Almost every cell in the human body exhibits circadian rhythmicity, which is regulated by the molecular clock mechanism (13). The molecular clock controls metabolic, physiological, and behavioural oscillations throughout the 24-hr period, via an autoregulatory transcriptional-translational feedback loop (13). People with type 1 diabetes have disturbed molecular clocks, which are partly responsible for increased mortality rates in those with the condition (14). For example, normal blood pressure exhibits a circadian rhythm, dipping during sleep, which is important for cardiovascular health (15). However, individuals with type 1 diabetes have an increased prevalence of non-dipping blood pressure, thereby increasing the risks of hypertension (16) and nephropathy (17). As a result, these complications ultimately contribute to the development of cardiovascular disease as a leading cause of mortality in type 1 diabetes (18). Moreover, social jetlag, a prominent circadian misalignment, is independently associated with long-term blood glucose levels (glycosylated haemoglobin - HbA1c) (19), and this may, to some extent, underpin observations that those with type 1 diabetes engaging in shift work (defined as work scheduled outside standard daytime hours and night work of at least 3-hours duration between ~23:00–06:00) present with higher HbA1c compared to those that do not follow shift patterns (20). Appropriate timing of exercise has been postulated to reset the molecular clock and circadian rhythms (21) and, hence, arguably has the potential to improve cardiometabolic health in type 1 diabetes by restoring crucial biological and physiological processes beyond that of a less carefully selected exercise bout.
At present, the data suggests that fasted morning exercise triggers a rise in blood glucose following exercise, when compared to postprandial afternoon exercise (9–12). In contrast, recent studies show a decline in glucose following afternoon exercise (10, 11), which may explain the higher rates of hypoglycaemia (10.7 events per individual) following afternoon exercise compared to morning exercise (5.6 events per individual) (9). Mechanistically, morning exercise (~07:00-08:00) may increase blood glucose via circadian-mediated elevations in cortisol (Figure 1) and growth hormone as part of the ‘Dawn Phenomenon’ (22); this may have a glucose-sparing effect by stimulating lipolysis. However, in the afternoon (~16:00-17:00), growth hormone and cortisol decline, thereby increasing the risk of hypoglycaemia, as gluconeogenesis and glucagon concentration decrease, respectively (9).
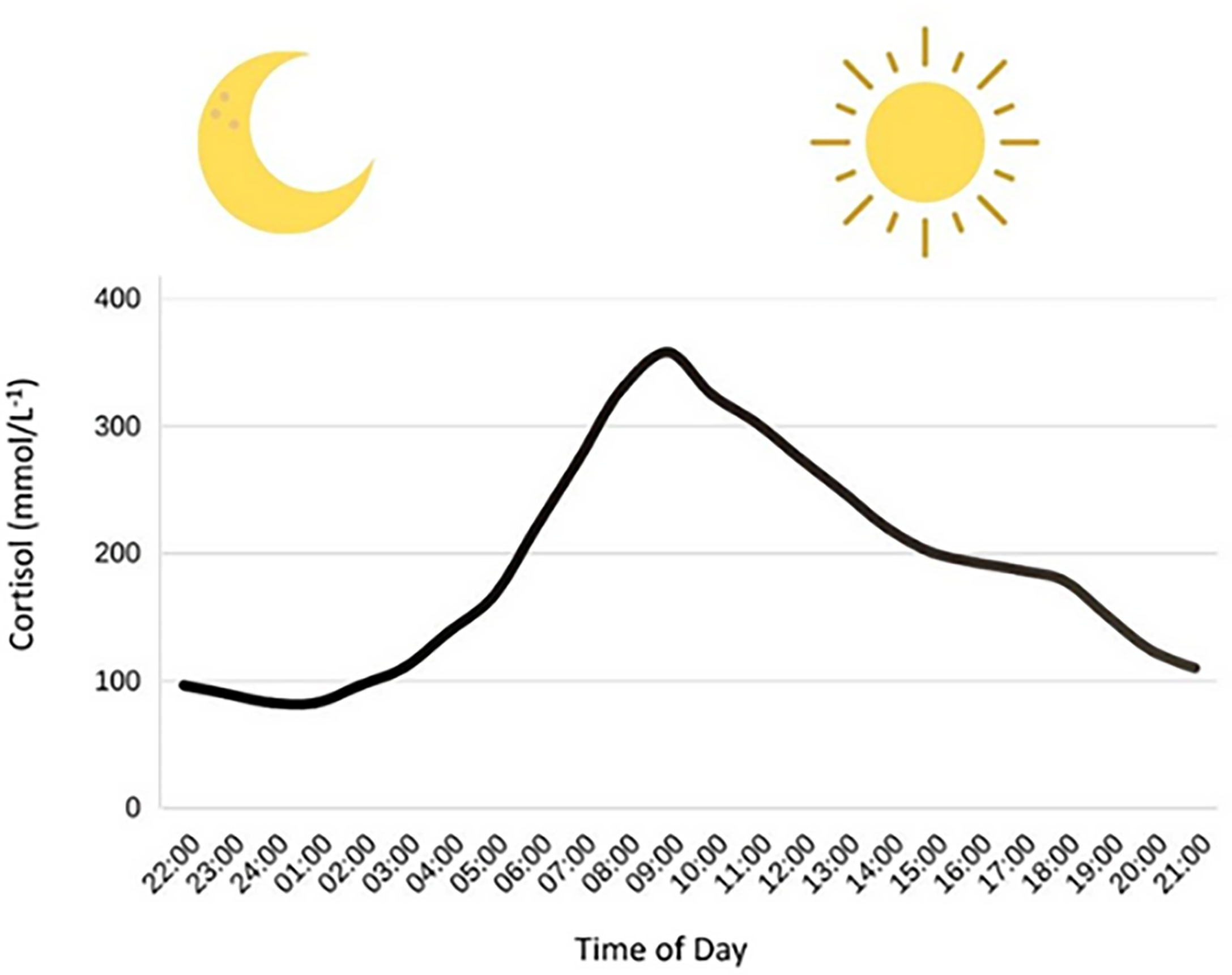
Figure 1 Circadian variation in cortisol concentrations. Cortisol has a nadir around (01:00) and then has a steep rise in the morning hours, eventually hitting a peak at around (09:00). After this peak, cortisol concentrations decline throughout the rest of the day. Figure adapted from Debono et al. (23).
Figure 1. Circadian variation in cortisol concentrations. Cortisol has a nadir around (01:00) and then has a steep rise in the morning hours, eventually hitting a peak at around (09:00). After this peak, cortisol concentrations decline throughout the rest of the day. Figure adapted from Debono et al.s (23).
Unlike the observations regarding exercise-induced hypoglycaemia in the afternoon (9–11), evidence for the time-of-day effects of exercise-induced hyperglycaemia appears equivocal. Two studies have reported no difference in the number of hyperglycaemic events between exercise timing conditions (9, 10). In contrast, Toghi-Eshghi and Yardley found more hyperglycaemic episodes (12 versus 5) six hours post-exercise in the morning relative to the afternoon (11). Similarly, Ruegemer et al. observed a hyperglycaemic response to morning exercise but not afternoon exercise among participants; the morning hyperglycaemia was mild and short-lived, with minimal impact on overall blood glucose (12). These heterogeneous findings may be explained by differences in exercise intensity and exercise modality. With respect to the latter, a meta-analysis by Garcia-Garcia et al. (24) showed that rapid decays of blood glucose were found during continuous moderate intensity exercise, whereas resistance exercise was associated with more constrained decreases. Aerobic exercise in type 1 diabetes relies heavily on blood glucose as a fuel source, whereas blood glucose during resistance exercise is better protected given that intramuscular glycogen is the primary fuel used (1). Thus, resistance exercise has been shown to provide greater blood glucose stability when compared to aerobic exercise (24). Specifically, Yardley et al. (25) observed a decrease in plasma glucose from 8.4 ± 2.7 to 6.8 ± 2.3 mmol∙L-1 during resistance exercise (45-minutes long, consisting of 7 exercises, performing 3 sets of 8 repetitions maximum with 90-seconds rest between sets) compared to 9.2 ± 3.4 to 5.8 ± 2.0 mmol∙L-1 during aerobic exercise (45-minutes at 60% of V̇O2max) in type 1 diabetes. All exercise sessions in this study were performed at 16:00 and in the fed state (25). Therefore, it is conceivable that morning aerobic exercise results in lower elevations in peripheral blood glucose (12) compared to morning resistance exercise, which appears to intensify the circadian mediated rise in peripheral blood glucose (11), at least when the exercise is performed in a fasted state. An increase in blood glucose during resistance exercise in type 1 diabetes seems to occur only when performed in a fasted state (11, 26–28). A meta-analysis investigating metabolic responses to fed and fasted exercise found that fasted exercise was associated with higher levels of free fatty acids than fed exercise (29). Free fatty acids can induce acute and chronic insulin resistance (30), which may, coupled with the Dawn Phenomenon, underpin the increased blood glucose during fasted exercise, and consequently, the observed post-exercise hyperglycaemia in the fasted morning exercise groups in the studies by Toghi-Eshghi and Yardley (11) and Ruegemer et al. (12). However, further direct studies are warranted to explore the mechanistic basis of such observations in those with type 1 diabetes.
Exercise intensity is also an important consideration in type 1 diabetes, with high-intensity interval exercise and training (HIIT) proving safer than continuous exercise at reducing the risk of hypoglycaemia (31). This may, in part, be due to HIIT causing similar physiological glucose responses as resistance training, again due to greater reliance on intramuscular glycogen and phosphagens over blood glucose as energy (1). Interestingly, Yardley (10) used a HIIT protocol (10-second sprints every 2 minutes for 24 minutes), yet, observed no differences between exercise timing conditions for hyperglycaemic events. Mechanistically, this exercise protocol might be expected to increase hyperglycaemic episodes in the morning, similar to the observations following the resistance exercise used by Toghi-Eshghi and Yardley (11). However, a key difference between studies is that Yardley (10) notably included a 10-minute warm-up and an 11-minute cooldown at 50% V̇O₂peak, possibly explaining the reductions in hyperglycaemic effects observed in the morning exercise group compared to Toghi-Eshghi and Yardley (11), who did not include a cooldown protocol. A low-intensity aerobic exercise cool-down may counteract post-exercise hyperglycaemia, however, current evidence for this approach is limited. Mechanistically a cooldown may counteract post-exercise hyperglycaemia via increased glucose (32) and lactate oxidation. Lactate is a substrate for gluconeogenesis and without a cooldown, may instead be converted to glucose in the liver resulting in subsequent hyperglycaemia (33, 34). One study has shown that performing running exercise (at 60% V̇O2peak), albeit for 45minutes, after a resistance bout improves glycaemic stability throughout exercise and reduces the duration and severity of post-exercise hypoglycaemia (35). Consequently, future research should seek to investigate the integration and effectiveness of realistic cool-down strategies, taking into consideration exercise duration and intensity.
A point worth mentioning at this juncture is that the sample sizes used among studies are relatively consistent, with most having a sample of between 6-12 participants, apart from Gomez et al. (9) who recruited 32 participants. This increases the weight of the findings by Gomez et al. (9) as small samples are known to disrupt the effect of statistical findings compared to larger samples. Although, only Yardley (10) used a power calculation in which they calculated a sample of 12 participants with an 0.86 power and an alpha of 0.05. Also, the consistent exercise times implemented across studies (07:00 versus 16:00/17:00) allow for valid comparisons between studies. Finally, randomised controlled crossover study designs were ubiquitously used, which are considered the gold standard due to participants acting as their own control, thereby reducing risks of interindividual confounding factors.
In addition to the direct effects of exercise on blood glucose, there may also be indirect effects incurred through the impact exercise has on factors such as diet and sleep. For instance, exercise is known to cause changes in the regulation of appetite, with an acute exercise bout showing a reduction in appetite in both lean and obese individuals (36), possibly through suppression of the hunger hormone ghrelin and increases in satiety hormones peptide YY (PYY), pancreatic polypeptide (PP), and glucagon-like peptide 1 (GLP-1) (37). Gut hormones related to hunger and satiety are known to display diurnal oscillations, with ghrelin displaying peaks in the evening and showing troughs in the morning (38). Considering that humans consume the majority of their daily calories in the evening, and also happen to eat more types of sweet and refined sugar foods in the latter part of the day (39), it is reasonable to theorise that performing an exercise bout (with appetite reducing effects) at this time may reduce caloric intake and improve dietary quality. Therefore, improving dietary intake and/or quality could lead to improved blood glucose in type 1 diabetes, as was shown by Nansel, Lipsky, and Liu (40). Furthermore, an evening exercise bout with its insulin sensitising effects may improve insulin sensitivity at a time in which it is typically worsened (41). Increased insulin sensitivity is an important therapeutic target to reduce the risk of macro- (42) and microvascular (43) complications in type 1 diabetes.
The timing of exercise may additionally impact sleep quality. Sleep quality is an important consideration given that one night of partial sleep deprivation in type 1 diabetes has been shown to cause peripheral insulin resistance (44). The literature investigating the effects of exercise timing on sleep quality demonstrates contrasting findings: Alley et al. found that the timing of resistance exercise had no impact on sleep outcomes (45), and this was supported by Burgess et al. (46) who found that regardless of the time of day exercise is performed, it does not impact sleep quality. Opposingly, Yamanaka et al. (47) found that morning and evening exercise, respectively, differentially impact body temperature and cardiac activity during sleep and stated that morning aerobic exercise may improve sleep quality relative to an identical exercise stimulus performed in the evening, due to allowing time for the exercise-induced stimulation of the sympathetic nervous system to diminish. However, whether these variations manifest in perturbations in health remain unknown as the study failed to investigate any health markers. Furthermore, a systematic review and meta-analysis found no evidence that evening exercise impacts sleep, except for vigorous exercise performed ≤ 1 h before bedtime (48). Therefore, individuals should also consider whether evening exercise impacts their sleep, and perhaps modify exercise time accordingly. It therefore seems likely that performing exercise at any time of day (with the exception being HIIT ≤ 1 h before bedtime) improves sleep outcomes when compared to no exercise at all in those with type 1 diabetes (49), and thus identifies another mechanism through exercise can elicit desirable effects for those with type 1 diabetes.
Exercise in type 1 diabetes is clinically endorsed for various reasons, most notably to improve cardiometabolic health (18). Physical activity recommendations from the American Diabetes Association (ADA) include ≥150 minutes of moderate physical activity and ≥2 resistance exercise sessions per week (50). Partaking in resistance or HIIT exercise, instead of continuous exercise, at the time of day an individual is most likely to experience a hypoglycaemic event (i.e., afternoon/evening) may be more prudent and advised because of the superior blood glucose stability these exercise modalities provide (24, 25, 31). Contrastingly, if continuous exercise is the preferred mode of exercise, it may be best commenced in the morning due to known elevations in type 1 diabetes blood glucose concentration at this time, potentially providing added protection against a hypoglycaemic episode during this heavily glucose reliant exercise form (24, 25). Additionally, individuals with type 1 diabetes who regularly exercise are advised to strategically consume carbohydrates before, during and after exercise to protect against a hypoglycaemic episode. The quantity of carbohydrates to be taken depends on factors such as the exercise undertaken (considering mode, duration, and intensity etc.), and present blood glucose/insulin concentrations. For example, when insulin levels are low, it is recommended that an individual should consume 30-60g of carbohydrate per hour to prevent hypoglycaemia during exercise. Whereas, up to 75g of carbohydrate per hour is advised under high insulin conditions (1). Reductions in insulin basal and/or bolus dose can be used in addition to, or alongside, carbohydrate intake for the prevention of exercise-induced hypoglycaemia. Various insulin adjustment strategies can be used, one of which involves reducing the pre-exercise bolus by 30-50% up to 90 minutes before aerobic exercise (51) – decisions around such approaches should be made in consultation with the designated clinical/endocrinology teams and informed by a patient’s/individual’s past experiences and response to exercise. Aerobic exercise may require greater reductions in insulin doses and/or more carbohydrate intake than a HIIT session, whereas resistance training may require increased insulin in the post-exercise recovery phase (1). The order of resistance and aerobic exercise is also an important consideration if both/multiple exercise modalities are completed in the same session. Performing resistance exercise prior to aerobic exercise can keep blood glucose concentration buoyant (35), possibly due to an increase in counter-regulatory hormones that mitigate glucose clearance. This approach can reduce the possibility of a hypoglycaemic episode and may therefore be a practical approach for those individuals who suffer from hypoglycaemia as a function of exercise. For further practical advice on how to manage exercise with type 1 diabetes, we recommend the excellent consensus statement by Riddell et al. (1).
All the experimental studies outlined within compared fasted morning exercise with postprandial afternoon exercise. Consequently, experiments were not only investigating the timing of exercise and possible circadian effects, but also implicitly shedding light on the timing of exercise in relation to the fasted and the fed/postprandial state that ultimately requires further scrutiny. This brief synopsis considers peripheral glucose concentration mainly in relation to exercise timing, which is why studies that compared blood glucose responses at the same time of day in a fasted or fed state, such as Yamanouchi et al. (52), were not considered for commentary. We acknowledge that glucose management in this clinical population is multifactorial and inherently complex with exercise regimes requiring careful, individualised tailoring with respect to factors such as carbohydrate intake, insulin adjustments and other personal circumstances. Further research in type 1 diabetes is warranted, including long-term studies exploring the impact of exercise type (resistance vs. aerobic; continuous vs HIIT) and exercise timing (based on personal preference, or even chronotype) on key metabolic and endocrine predictors for those with type 1 diabetes (Figure 2). Of particular interest is research on HIIT exercise in relation to exercise timing, as HIIT exercise has been shown to provide similar physiological adaptations as continuous training, while simultaneously reducing the extent of glycogen breakdown, thus providing additional protection against exercise-induced hypoglycaemia (1, 31). Personalised exercise prescription and support may likely hold the key to sustaining adherence and yielding the fullest health benefits for those with type 1 diabetes. Indeed, Lascar et al. (53), highlighted that one-to-one advice from a health and fitness advisor had large appeal to individuals with type 1 diabetes, primarily due to the perception that one-to-one advice would be tailored to individual needs.
Figure 2 Future research in type 1 diabetes should aim to investigate the impact of exercise timing on different exercise modalities in relation to biological factors such as chronotype. Core blood glucose monitoring (CGM) is a metabolic predictor of specific interest.
There is increasing scientific interest in how circadian rhythms and molecular clocks interact with a plethora of conditions, such as type 1 diabetes. Disrupted circadian rhythms may contribute to poorer long-term blood glucose management (i.e., increased HbA1c) and increased cardiovascular risk in type 1 diabetes. Due to this interaction, clinicians and other professionals who are interested and involved in those with type 1 diabetes may consider factors such as exercise timing to maximise the therapeutic outcomes of an exercise bout. Furthermore, prescribing a suitable time to exercise may reduce the occurrence of a hypoglycaemic event, and thus assist in removing a common perceived barrier to exercise in type 1 diabetes. We state that the benefits of exercise in type 1 diabetes far outweigh the inherent risks, but also remain cognisant of the numerous challenges in trying to safely incorporate consistent activity into a daily schedule: carefully choosing the time of day to exercise might be one such approach to facilitate a more active lifestyle in type 1 diabetes.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
J W, RF, and CM devised the concept of the report. RF conducted the systemic searching of appropriate literature and was responsible for the drafting of the original piece of work alongside CM. All authors contributed to the article and approved the submitted version.
RF is funded by a Department for Education (DfE) NI scholarship to support this work as part of his PhD programme.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Riddell MC, Gallen IW, Smart CE, Taplin CE, Adolfsson P, Lumb AN, et al. Exercise management in type 1 diabetes: A consensus statement. Lancet Diabetes Endocrinol (2017) 5(5):377–90. doi: 10.1016/S2213-8587(17)30014-1
2. Beattie CM, Stein JA, Heinrich K. Physical activity behavior comparisons between adults with and without type 1 diabetes. Health Behav Res (2021) 4(1):3. doi: 10.4148/2572-1836.1087
3. Brazeau AS, Rabasa-Lhoret R, Strychar I, Mircescu H. Barriers to physical activity among patients with type 1 diabetes. Diabetes Care (2008) 31(11):2108–9. doi: 10.2337/dc08-0720
4. Zander E, Bruns W, Wulfert P, Besch W, Lubs D, Chlup R, et al. Muscular exercise in type I-diabetics. i. different metabolic reactions during heavy muscular work in dependence on actual insulin availability. Exp Clin Endocrinol Diabetes (1983) 82(4):78–90. doi: 10.1055/s-0029-1210259
5. Kalsbeek A, Yi CX, La Fleur SE, Fliers E. The hypothalamic clock and its control of glucose homeostasis. Trends Endocrinol Metab (2010) 21(7):402–10. doi: 10.1016/j.tem.2010.02.005
6. Morris CJ, Yang JN, Garcia JI, Myers S, Bozzi I, Wang W, et al. Endogenous circadian system and circadian misalignment impact glucose tolerance via separate mechanisms in humans. Proc Natl Acad Sci (2015) 112(17):2225–34. doi: 10.1073/pnas.1418955112
7. Wefers J, van Moorsel D, Hansen J, Connell NJ, Havekes B, Hoeks J, et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc Natl Acad Sci (2018) 115(30):7789–94. doi: 10.1073/pnas.1722295115
8. Basse AL, Dalbram E, Larsson L, Gerhart-Hines Z, Zierath JR, Treebak JT. Skeletal muscle insulin sensitivity show circadian rhythmicity which is independent of exercise training status. Front Physiol (2018) 9:1198. doi: 10.3389/fphys.2018.01198
9. Gomez AM, Gomez C, Aschner P, Veloza A, Munoz O, Rubio C, et al. Effects of performing morning versus afternoon exercise on glycemic control and hypoglycemia frequency in type 1 diabetes patients on sensor-augmented insulin pump therapy. J Diabetes Sci Technol (2015) 9(3):619–24. doi: 10.1177/1932296814566233
10. Yardley JE. Fasting may alter blood glucose responses to high-intensity interval exercise in adults with type 1 diabetes: A randomized, acute crossover study. Can J Diabetes (2020) 44(8):727–33. doi: 10.1016/j.jcjd.2020.09.007
11. Toghi-Eshghi SR, Yardley JE. Morning (fasting) vs afternoon resistance exercise in individuals with type 1 diabetes: A randomized crossover study. J Clin Endocrinol Metab (2019) 104(11):5217–24. doi: 10.1210/jc.2018-02384
12. Ruegemer J, Squires RW, Marsh HM, Haymond MW, Cryer PE, Rizza RA, et al. Differences between prebreakfast and late afternoon glycemic responses to exercise in IDDM patients. Diabetes Care (1990) 13(2):104–10. doi: 10.2337/diacare.13.2.104
13. Sato T, Greco CM. Expanding the link between circadian rhythms and redox metabolism of epigenetic control. Free Radical Biol Med (2021) 170:50–8. doi: 10.1016/j.freeradbiomed.2021.01.009
14. Afsar B. Disruption of circadian blood pressure, heart rate and the impact on glycemic control in type 1 diabetes. Diabetes Metab Syndrome (2015) 9(4):359–63. doi: 10.1016/j.dsx.2014.05.002
15. Sayk F, Becker C, Teckentrup C, Fehm H, Struck J, Wellhoener JP, et al. To dip or not to dip: On the physiology of blood pressure decrease during nocturnal sleep in healthy humans. Hypertension (2007) 49(5):1070–6. doi: 10.1161/HYPERTENSIONAHA.106.084343
16. Dost A, Klinkert C, Kapellen T, Lemmer A, Naeke A, Grabert M, et al. Arterial hypertension determined by ambulatory blood pressure profiles: Contribution to microalbuminuria risk in a multicenter investigation in 2,105 children and adolescents with type 1 diabetes. Diabetes Care (2008) 31(4):720–5. doi: 10.2337/dc07-0824
17. Stella P, Tabak AG, Zgibor JC, Orchard TJ. Late diabetes complications and non-dipping phenomenon in patients with type 1 diabetes. Diabetes Res Clin Pract (2006) 71(1):14–20. doi: 10.1016/j.diabres.2005.05.001
18. Katz M, Giani E, Laffel L. Challenges and opportunities in the management of cardiovascular risk factors in youth with type 1 diabetes: Lifestyle and beyond. Curr Diabetes Rep (2015) 15(12):1–11. doi: 10.1007/s11892-015-0692-4
19. Larcher S, Gauchez A, Lablanche S, Pepin J, Benhamou P, Borel A. Impact of sleep behavior on glycemic control in type 1 diabetes: The role of social jetlag. Eur J Endocrinol (2016) 175(5):411–9. doi: 10.1530/EJE-16-0188
20. Young J, Waclawski E, Young JA, Spencer J. Control of type 1 diabetes mellitus and shift work. Occup Med (2012) 63(1):70–2. doi: 10.1093/occmed/kqs176
21. Gabriel BM, Zierath JR. Circadian rhythms and exercise–re-setting the clock in metabolic disease. Nat Rev Endocrinol (2019) 15(4):197–206. doi: 10.1038/s41574-018-0150-x
22. Schmidt MI, Hadji-Georgopoulos A, Rendell M, Margolis S, Kowarski A. The dawn phenomenon, an early morning glucose rise: Implications for diabetic intraday blood glucose variation. Diabetes Care (1981) 4(6):579–85. doi: 10.2337/diacare.4.6.579
23. Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J Clin Endocrinol Metab (2009) 94(5):1548–54. doi: 10.1210/jc.2008-2380
24. Garcia-Garcia F, Kumareswaran K, Hovorka R, Hernando ME. Quantifying the acute changes in glucose with exercise in type 1 diabetes: A systematic review and meta-analysis. Sports Med (2015) 45(4):587–99. doi: 10.1007/s40279-015-0302-2
25. Yardley JE, Kenny GP, Perkins BA, Riddell MC, Balaa N, Malcolm J, et al. Resistance versus aerobic exercise: Acute effects on glycemia in type 1 diabetes. Diabetes Care (2013) 36(3):537–42. doi: 10.2337/dc12-0963
26. Turner D, Luzio S, Gray BJ, Dunseath G, Rees ED, Kilduff LP, et al. Impact of single and multiple sets of resistance exercise in type 1 diabetes. Scand J Med Sci Sports (2015) 25(1):99–109. doi: 10.1111/sms.12202
27. Turner D, Luzio S, Gray BJ, Bain SC, Hanley S, Richards A, et al. Algorithm that delivers an individualized rapid-acting insulin dose after morning resistance exercise counters post-exercise hyperglycaemia in people with type 1 diabetes. Diabetic Med (2016) 33(4):506–10. doi: 10.1111/dme.12870
28. Turner D, Gray BJ, Luzio S, Dunseath G, Bain SC, Hanley S, et al. Similar magnitude of post-exercise hyperglycemia despite manipulating resistance exercise intensity in type 1 diabetes individuals. Scand J Med Sci Sports (2016) 26(4):404–12. doi: 10.1111/sms.12472
29. Aird TP, Davies RW, Carson BP. Effects of fasted vs fed-state exercise on performance and post-exercise metabolism: A systematic review and meta-analysis. Scand J Med Sci Sports (2018) 28(5):1476–93. doi: 10.1111/sms.13054
30. Boden G. Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care (2002) 5(5):545–9. doi: 10.1097/00075197-200209000-00014
31. Hasan S, Shaw SM, Gelling LH, Kerr CJ, Meads CA. Exercise modes and their association with hypoglycemia episodes in adults with type 1 diabetes mellitus: A systematic review. BMJ Open Diabetes Res Care (2018) 6(1):e000578. doi: 10.1136/bmjdrc-2018-000578
32. Jenni S, Oetliker C, Allemann S, Ith M, Tappy L, Wuerth S, et al. Fuel metabolism during exercise in euglycaemia and hyperglycaemia in patients with type 1 diabetes mellitus–a prospective single-blinded randomised crossover trial. Diabetologia (2008) 51(8):1457–65. doi: 10.1007/s00125-008-1045-5
33. Adeva-Andany M, López-Ojén M, Funcasta-Calderón R, Ameneiros-Rodríguez E, Donapetry-García C, Vila-Altesor M, et al. Comprehensive review on lactate metabolism in human health. Mitochondrion (2014) 17:76–100. doi: 10.1016/j.mito.2014.05.007
34. Miller BF, Fattor JA, Jacobs KA, Horning MA, Navazio F, Lindinger MI, et al. Lactate and glucose interactions during rest and exercise in men: effect of exogenous lactate infusion. J Physiol (2002) 544(3):963–75. doi: 10.1113/jphysiol.2002.027128
35. Yardley JE, Kenny GP, Perkins BA, Riddell MC, Malcolm J, Boulay P, et al. Effects of performing resistance exercise before versus after aerobic exercise on glycemia in type 1 diabetes. Diabetes Care (2012) 35(4):669–75. doi: 10.2337/dc11-1844
36. Douglas JA, King JA, Clayton DJ, Jackson AP, Sargeant JA, Thackray AE, et al. Acute effects of exercise on appetite, ad libitum energy intake and appetite-regulatory hormones in lean and overweight/obese men and women. Int J Obes (2017) 41(12):1737–44. doi: 10.1038/ijo.2017.181
37. Schubert MM, Sabapathy S, Leveritt M, Desbrow B. Acute exercise and hormones related to appetite regulation: A meta-analysis. Sports Med (2014) 44(3):387–403. doi: 10.1007/s40279-013-0120-3
38. Qian J, Morris CJ, Caputo R, Garaulet M, Scheer FA. Ghrelin is impacted by the endogenous circadian system and by circadian misalignment in humans. Int J Obes (2019) 43(8):1644–9. doi: 10.1038/s41366-018-0208-9
39. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab (2015) 22(5):789–98. doi: 10.1016/j.cmet.2015.09.005
40. Nansel TR, Lipsky LM, Liu A. Greater diet quality is associated with more optimal glycemic control in a longitudinal study of youth with type 1 diabetes. Am J Clin Nutr (2016) 104(1):81–7. doi: 10.3945/ajcn.115.126136
41. Morgan LM, Aspostolakou F, Wright J, Gama R. Diurnal variations in peripheral insulin resistance and plasma non-esterified fatty acid concentrations: A possible link? Ann Clin Biochem (1999) 36(4):447–50. doi: 10.1177/000456329903600407
42. Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: “Double diabetes” in the diabetes control and complications trial. Diabetes Care (2007) 30(3):707–12. doi: 10.2337/dc06-1982
43. Bjornstad P, Snell-Bergeon JK, Rewers M, Jalal D, Chonchol MB, Johnson RJ, et al. Early diabetic nephropathy: A complication of reduced insulin sensitivity in type 1 diabetes. Diabetes Care (2013) 36(11):3678–83. doi: 10.2337/dc13-0631
44. Donga E, Van Dijk M, Van Dijk JG, Biermasz NR, Lammers GJ, Van Kralingen K, et al. Partial sleep restriction decreases insulin sensitivity in type 1 diabetes. Diabetes Care (2010) 33(7):1573–7. doi: 10.2337/dc09-2317
45. Alley JR, Mazzochi JW, Smith CJ, Morris DM, Collier SR. Effects of resistance exercise timing on sleep architecture and nocturnal blood pressure. J Strength Condition Res (2015) 29(5):1378–85. doi: 10.1519/JSC.0000000000000750
46. Burgess VN, Antonio J, Bland HW, Wagner R, Tartar JL, Melton BF. The effect of timing and type of exercise on the quality of sleep in trained individuals. Int J Exercise Sci (2020) 13(7):837.
47. Yamanaka Y, Hashimoto S, Takasu NN, Tanahashi Y, Nishide SY, Honma S, et al. Morning and evening physical exercise differentially regulate the autonomic nervous system during nocturnal sleep in humans. Am J Physiol-Regulatory Integr Comp Physiol (2015) 309(9):1112–21. doi: 10.1152/ajpregu.00127.2015
48. Stutz J, Eiholzer R, Spengler CM. Effects of evening exercise on sleep in healthy participants: a systematic review and meta-analysis. Sports Med (2019) 49(2):269–87. doi: 10.1007/s40279-018-1015-0
49. Alarcón-Gómez J, Chulvi-Medrano I, Martin-Rivera F, Calatayud J. Effect of high-intensity interval training on quality of life, sleep quality, exercise motivation and enjoyment in sedentary people with type 1 diabetes mellitus. Int J Environ Res Public Health (2021) 18(23):12612. doi: 10.3390/ijerph182312612
50. Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: A position statement of the American diabetes association. Diabetes Care (2016) 39(11):2065–79. doi: 10.2337/dc16-1728
51. Franc S, Daoudi A, Pochat A, Petit MH, Randazzo C, Petit C, et al. Insulin-based strategies to prevent hypoglycaemia during and after exercise in adult patients with type 1 diabetes on pump therapy: The DIABRASPORT randomized study. Diabetes Obes Metab (2015) 17(12):1150–7. doi: 10.1111/dom.12552
52. Yamanouchi K, Abe R, Takeda A, Atsumi Y, Shichiri M, Sato Y. The effect of walking before and after breakfast on blood glucose levels in patients with type 1 diabetes treated with intensive insulin therapy. Diabetes Res Clin Pract (2002) 58(1):11–8. doi: 10.1016/S0168-8227(02)00099-2
Keywords: exercise, circadian, glucose metabolism, type 1 diabetes mellitus, molecular clock
Citation: Fitzpatrick R, Davison G, Wilson JJ, McMahon G and McClean C (2022) Exercise, type 1 diabetes mellitus and blood glucose: The implications of exercise timing. Front. Endocrinol. 13:1021800. doi: 10.3389/fendo.2022.1021800
Received: 17 August 2022; Accepted: 12 September 2022;
Published: 28 September 2022.
Edited by:
Klemen Dovc, University Medical Centre Ljubljana, SloveniaReviewed by:
Hidetaka Hamasaki, Hamasaki Clinic, JapanCopyright © 2022 Fitzpatrick, Davison, Wilson, McMahon and McClean. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ross Fitzpatrick, Rml0enBhdHJpY2stUjExQHVsc3Rlci5hYy51aw==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.