- Department of Obstetrics, Gynecology and Reproductive Sciences, Yale School of Medicine, New Haven, CT, United States
Despite the clinically recognized association between endometriosis and infertility, the mechanisms implicated in endometriosis-associated infertility are not fully understood. Endometriosis is a multifactorial and systemic disease that has pleiotropic direct and indirect effects on reproduction. A complex interaction between endometriosis subtype, pain, inflammation, altered pelvic anatomy, adhesions, disrupted ovarian reserve/function, and compromised endometrial receptivity as well as systemic effects of the disease define endometriosis-associated infertility. The population of infertile women with endometriosis is heterogeneous, and diverse patients’ phenotypes can be observed in the clinical setting, thus making difficult to establish a precise diagnosis and a single mechanism of endometriosis related infertility. Moreover, clinical management of infertility associated with endometriosis can be challenging due to this heterogeneity. Innovative non-invasive diagnostic tools are on the horizon that may allow us to target the specific dysfunctional alteration in the reproduction process. Currently the treatment should be individualized according to the clinical situation and to the suspected level of impairment. Here we review the etiology of endometriosis related infertility as well as current treatment options, including the roles of surgery and assisted reproductive technologies.
Background
Endometriosis is a complex and systemic clinical syndrome that can negatively impact on women’s reproductive health and quality of life (1). Chronic inflammation and hormonal dependance are the main underlying pathophysiologic mechanisms that drive endometriosis, and the association of these two key biological features make the natural history of this disease distinct.
A possible relationship between endometriosis and infertility was first suggested in the Corpus Hippocraticum, as women suffering from dysmenorrhea were urged to conceive as quickly as possible to increase the chance of become pregnant (2). Today, nearly 10% of women in their reproductive age suffer from endometriosis and about one third of them experience infertility, almost twice the rate observed among women without the disease (3). Up to 50% of infertile women are found to suffer from endometriosis (4).
Despite the clinically recognized association between endometriosis and infertility, the mechanisms implicated in endometriosis-associated infertility are unclear and this condition is currently considered multifactorial. In addition, the diagnosis of endometriosis is currently underestimated due to the almost exclusive reliance on surgical findings, which delays diagnosis until symptoms require surgical intervention. The ability to identify endometriosis also critically depend on surgeon’s expertise and may preclude early recognition and treatment. The average time to diagnostics ranges from 4 to 11 years is reported in these patients, and this delay has a significant impact on health-care utilization and costs (5, 6). Indeed, the absence of macroscopic lesions or clinical features does not exclude the diagnosis of endometriosis, as infertility is often the only health concern. Furthermore, only one-half of women with endometriosis-associated infertility show typical lesions (7). In women with infertility, an early diagnosis of endometriosis is crucial from the perspective of fertility because the burden of the disease could be even more deleterious when compounded by the effect of increasing age on ovarian reserve.
The focus of this review is to provide an update of pathophysiology of endometriosis-associated infertility. We will also discuss current medical and surgical strategies, and the role of fertility preservation and of assisted reproductive technologies (ART) in patients with endometriosis.
Pathogenesis of endometriosis
Understanding the pathogenesis of endometriosis is crucial as it may have meaningful clinical and therapeutical implications. To date, none of the proposed theories have been able to comprehensively explain the natural history of the disease and its associated diverse clinical presentations. The common thread to all theories is a complex dysregulated hormonal signaling, enhanced proinflammatory microenvironment that has the potential to drive the initiation, maintenance, and progression of the disease (Figure 1).
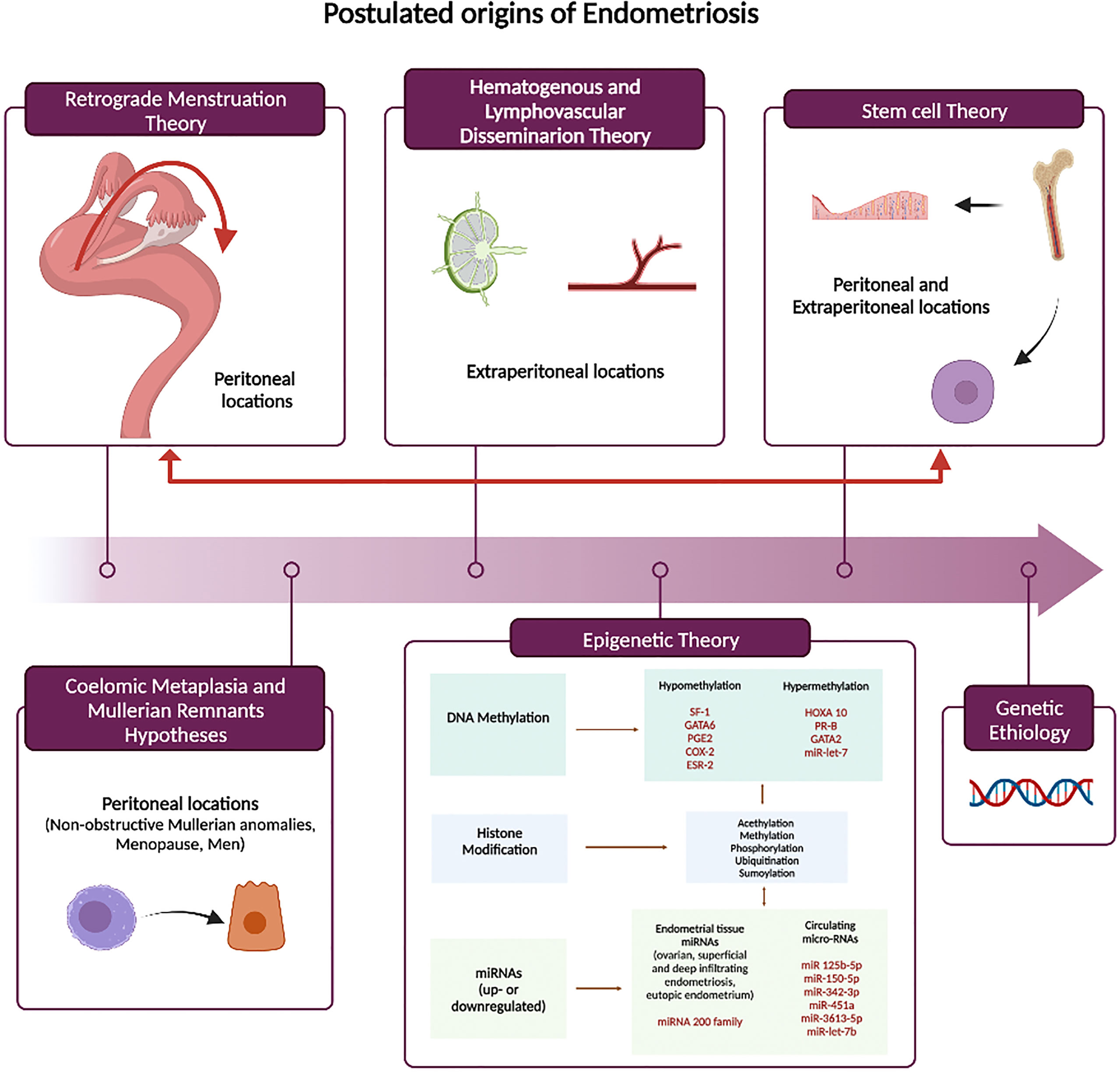
Figure 1 Theories of Endometriosis Pathogenesis. SF-1, steroidogenic factor 1;GATA6, GATA binding protein 6;PGE2, Prostaglandin E2;COX2, cyclooxygenase-2;ESR-2, estrogen receptor alpha; HOXA10, homebox protein A10;PR-B, progesterone receptor isoform B; GATA2, GATA binding protein 2.
Retrograde menstruation theory
The most widely accepted pathogenetic hypothesis is based on retrograde menstruation as proposed by Sampson in 1927 (8–10). Viable endometrial tissue moves into the pelvic cavity through the fallopian tubes at the time of menses, adheres to the peritoneal mesothelial cells, proliferates, and finally invades pelvic structures. Retrograde menstruation is a physiological event that occurs in approximately 90% of women (11–13). Viable endometrial tissue has been identified in the shed menstrual endometrium (13). However, the differences in its morpho-histological, hormonal, and biological composition compared with the eutopic endometrium of healthy women still remain matter of investigation. Endometrial reflux seems to be enhanced in women with endometriosis and possibly driven by the action of prostaglandins that may cause disorganized myometrial contraction (14–16). Moreover, the incidence of endometrial reflux is much higher in women with congenital anomalies causing menstrual outflow obstruction (17). This theory has been well supported by animal models of endometriosis. Normal endometrial tissue placed into the peritoneal cavity recapitulates the disease, including the effects on eutopic endometrium, suggesting that an abnormal endometrium is not a prerequisite for initiation and development of endometriosis (18–21).
Early age at menarche, long duration and heavy menstrual flow are all well-recognized epidemiological risk factors for the development of endometriosis. The anatomical predominance of endometriosis in the right side of both hemipelvis and diaphragm, further supports this theory (22). This asymmetry has been attributed to both a physiological process (the clockwise intraperitoneal current) and an anatomical factor (the presence of the sigmoid colon and falciform ligament). However, the retrograde menstruation hypothesis is not sufficient to explain the development of rare forms of the disease.
Coelomic metaplasia and mullerian remnants hypotheses
The coelomic metaplasia and mullerian remnants hypothesis are both based on the concept that endometriotic lesions originate in-situ from embryological remnants or by metaplasia. Based on the mullerian remnants hypothesis (“mullerianosis”) (23), endometriosis is a consequence of the aberrant migration and differentiation of embryonic cell rests originating from the Mullerian ducts during organogenesis. This hypothesis can explain the presence of endometriosis in in adolescents before or shortly after menarche and in fetuses (24–26). Embryological studies (24) support the presence of Mullerian remnants in the cul-de-sac area, uterosacral ligaments, and medial broad ligaments. Alternatively, both germinal ovarian epithelium and peritoneum may undergo a Mullerian metaplasia and differentiate into endometrium (27). This latter theory would explain the presence of endometriosis in ovary, sigmoid colon, appendix, or more distal sites such as the diaphragm and pleura (28), although direct infiltration through diaphragmatic fenestrations is possible. Additionally, both hypotheses may explain rare cases of endometriosis in women with Mayer-Rokitansky-Kuster-Hauser syndrome and other non-obstructive Mullerian anomalies (29–31), in the absence of menstruation (menopause) (32) and in men (33–36).
Hematogenous and lymphovascular dissemination
Sampson recognized that retrograde menstruation does not explain uncommon extraperitoneal locations and diverse clinical presentations with symptoms remote form the pelvis (37). He first suggested hematogenous or lymphatic dissemination of endometrial like-tissue as an alternative theory. This hypothesis implies that endometrial cells enter the uterine vasculature or lymphatic system at menstruation, and they spread to ectopic sites (38). In murine models of surgically induced endometriosis, endometriosis-derived cells are capable of migration and micrometastasis to different extra-pelvic organs including lung, spleen, liver and brain (39). Clinically, this theory has been supported by the presence of endometrial tissue in the uterine vasculature (37) and by evidence of emboli in sentinel lymph nodes (40).
Stem cell theory
In the last few years, it has become clear that altered stem cell trafficking contribute to the etiology and pathophysiology of endometriosis. The first evidence on the contribution of bone marrow derived stem cells (BMDCs) in the regeneration of the endometrium was reported in 2004 (41); subsequent studies have confirmed the bone marrow contribution to endometrium (42, 43). Both progenitor cells within the endometrium and multipotent cells from bone marrow contribute to endometrial homeostasis. The BMDSCs travel through the circulatory system and contribute to the composition of eutopic endometrium (41). After travelling to endometrium these BMDSCs can become restricted to an endometrial cell lineage, contributing to the pool of both stromal and epithelial endometrial progenitor cells. Some become located in the basal layer of the eutopic endometrium and regenerate on a monthly basis under the influence of estrogens. Furthermore, women with endometriosis have a higher number of these pluripotential cells compared with healthy women during menses (44).
During menstruation, women with endometriosis shed more basalis cells, including progenitor cells, than healthy individuals, these cells can more easily generate endometrium in ectopic locations than differentiated cells and further expand on Sampson’s theory of retrograde menstruation (45).
BMDSCs can directly differentiate into endometrium without first being localized in the uterus. Ectopic differentiation of circulating stem cells has been proposed as a pathogenetic mechanism of endometriosis. Mesenchymal extrauterine stem cells derived from bone marrow and other sources may also be involved in the pathogenesis of the disease both in the peritoneal cavity as well as distant sites. Their inappropriate differentiation to endometrial cells at ectopic locations is likely the principal source of extraperitoneal endometriosis (46, 47).
The ability of BMDSCs to contribute to endometriotic lesion and differentiate into endometrial phenotypes help to explain how ectopic tissue can occur in locations outside the peritoneal cavity and in non-peritoneal-derived cells, such as lungs (48–50), central nervous system (51) and in men (33–36). Furthermore, BMDSC are attracted by eutopic endometrium under injury and inflammatory conditions (52). Endometriotic lesions, through the production and release of pro-inflammatory cytokines and chemokines (47) and under estrogenic influence (53) recruit more stem cells to further promote lesion growth. Additionally circulating endothelial progenitor cells contribute to the vascularization of endometriotic lesions. Stem cells are also capable of trafficking between endometriotic lesions and the eutopic endometrium, and therefore likely contribute to the impaired uterine receptivity in these women (54). These cells, derived from endometriosis, migrate as mesenchymal stem cells (MSC), engraft the uterine stroma, however activation epithelial Wnt signaling that likely distorts the epithelial-stromal dialog needed for optimal endometrial development and receptivity.
Genetic etiology
Familiar clusters of endometriosis have been found in humans (55) and nonhuman primates (56, 57). However, no distinct inheritance pattern has been established and the notion that multiple genes contribute to endometriosis is widely accepted. Studies on monozygotic twins show that endometriosis has an estimated total heritability of approximately 51% (58–60). Daughters of mothers with surgically confirmed endometriosis have more than double risk of developing the disease (61). Moreover, familial inherited of endometriosis tends to be more severe with an earlier onset of symptoms compared with sporadic cases (55, 62). Meta-analyses of genome-wide association studies of diverse populations have identified a robust association of endometriosis with certain risk loci involved in sex steroid hormone pathways, indicating a possible role in the development of advanced stages of endometriosis (63–68). However, none are common and in total these genetic variants account for only a small fraction disease risk. In general, a multitude of genetic variants with only weak individual effects care likely responsible for the increased hereditary risk of endometriosis (69).
In the context of infertility associated with endometriosis, a recent cross-sectional study including 213 infertile women with endometriosis who underwent IVF procedures, found that single nucleotide variants of FSHB and FSHR separately interfered with the hormonal profile (both FSH and LH levels) and ultimately with the number of oocytes retrieved in these patients at any stage of the disease (70).
The epigenetic theory
There is a growing body of evidence that epigenetics has a key role in the pathogenesis of endometriosis. Epigenetic modifications involve dynamic and reversible changes in the chromatin structure influencing gene expression in a heritable fashion. Epigenetic phenomena are likely to have implications for diagnosis, prognosis and for the possibility of developing targeted therapeutic strategies. The hallmarks of epigenetic gene regulation are DNA methylation (hypo and hypermethylation), histone modifications, and microRNA production, which lead to expression or suppression of specific proteins. Comparative studies of both ectopic lesions and eutopic endometrium stromal cells have provided data on the role of epigenetic factors in the etiopathogenesis of endometriosis and its related infertility (19, 71–73).
DNA methylation is one of the most common epigenetic modifications and active in endometrium. Numerous studies have revealed a direct correlation with the expression of genes influencing the implantation process in eutopic endometrium of women with endometriosis. Homebox protein-A10 (HOXA10) is a gene that has a well characterized and essential role in generating a receptive endometrium. Hypermethylation of the HOXA gene promoter has been demonstrated both in animals and in the eutopic endometrium of women with endometriosis compared to healthy controls (19, 74, 75). As promoter hypermethylation is generally associated with gene silencing, the reduced HOXA10 gene expression in the endometrium of women with endometriosis is, at least in part, responsible for the impaired uterine receptivity. Conversely, one recent study (76) found hypomethylation of the HOXA10 gene in the endometrium of women with a previous history of endometriosis and under hormonal treatment at the time of surgery, opening the possibility that long-term therapy may reverse epigenetic signatures classically seen in the disease. Aberrant DNA methylation patterns have also been found in endometriotic tissue compared with eutopic endometrium. The promoter of progesterone receptor (PR) isoform B gene is hypermethylated in endometriosis, with subsequent reduced PR-B expression (77–79) contributing to the relatively persistent progesterone resistance. Similarly, the different level of expression and methylation (hypo or hyper) of certain transcriptional factors (GATA6, GATA 2 and steroidogenic factor 1(SF1)) may account for estrogen dependency and progesterone resistance by changing the expression of both estrogen receptor-beta and progesterone receptor (80–82). Lastly, the invasive proprieties of endometriotic cells have also found to be regulated by hypermethylation in endometriosis (83–85). Let-7 microRNA is hypermethylated in endometriosis leading to decreased Let-7 expression and disinhibition of KRAS and other genes that drive endometriosis growth and invasion (86).
Little is known about the role of histone modifications in the pathogenesis of endometriosis, and results are often conflicting. A marked histone hypoacetylation has been shown in endometriotic stromal cells of both eutopic and ectopic tissue of affected women compared to healthy endometrium (87) and HDAC enzymes seems to play a key role in this process (88–91). Also, acetylation levels of H3 and H4 histones are lower in ectopic lesions and eutopic endometrium of women with endometriosis compared with healthy women (92, 93).
MicroRNAs (MiRNAs) are small RNA molecules of approximately 22 bases. They interact with mRNA and change gene expression by inhibiting translation or inducing mRNA degradation. Their increased expression causes repression of translation from the mRNA while decreased MiRNA expression can lead to upregulation of protein production from mRNA. They also target and regulate both methylation and acetylation processes, thereby modifying the epigenome. Unlike other epigenetic mechanisms, miRNAs regulate gene expression at a post-transcriptional level, and they are found both intra- and extracellularly (94). They target genes involved in hormone metabolism, cell cycle proliferation, migration, and invasion, immune- inflammatory response, epithelial-mesenchymal transition (EMT), apoptosis and angiogenesis (95). Differential expression of more than 100 miRNAs has been found in paired endometriotic lesions and eutopic endometrium of women with and without endometriosis (96, 97). In addition, different expression profiles were detected and reported as characteristic to each lesion subtype (ovarian, superficial, and deep infiltrating endometriosis) (98). Moreover, miRNA signatures in endometrium are likely to change with the respect of the different phases of the menstrual cycle (99, 100). The most frequently detected miRNA both in endometriomas and endometriotic lesions, found to be downregulated in six studies was miR-200 family, known to play a crucial role in the EMT, a relevant process in the establishment of endometriotic lesions (101, 102). Other miRNA reported to be differentially regulated (up- or downregulated) in endometriotic lesions in more than two studies were miR-1, -29c, -34c, -100, -141, -145, -183, -196b, -200a, -200b, -200c, -202, -365, and -375 (103). Several of these are also known to be involved in EMT, as well as, cell proliferation, cell adhesion, invasion and angiogenesis and demonstrating binding to target mRNAs is an important step to validate their role in the pathogenesis of the disease. Extracellular miRNAs are found in all body fluids, including the circulation (both serum and plasma) (104). Circulating microRNAs can potentially impact endometriotic lesion development by mediating intercellular communication between eutopic endometrium and ectopic implants (105).
Pathophysiology of endometriosis associated infertility
Translational animal models of endometriosis associated infertility
Considering the limited knowledge of endometriosis pathophysiology, research has long focused on finding animal models to study suspected pathogenic mechanisms and to find novel targets for therapy. As with human endometriosis, animal models of endometriosis reveal an impact on fecundity in terms of impaired folliculogenesis, ovulation, fertilization, implantation or embryonic developement (106). Non-human primates have been extensively used as experimental models for endometriosis because of their phylogenetic proximity to humans. They menstruate cyclically and therefore they can develop endometriosis spontaneously, resembling the human disease based on retrograde menstruation. To date, 11 species of menstruating non-human primates have been reported (107). Ectopic lesions are laparoscopically and histologically identical and at a similar pelvic sites (108). However, spontaneous endometriosis develops slowly, at a lower rate compared to humans and might be multifactorial. Therefore, alternative methods of artificially induced endometriosis have been introduced in these species: cervical repositioning (109), cervical occlusion (110) or surgical induction (18, 21). The use of the non-human primate model of endometriosis, either inducible or spontaneous, seems to provide an excellent tool to investigate not only the pathogenesis of disease but also its associated infertility and impaired endometrial function. These models offer the opportunity to investigate the effect of endometriosis on the eutopic endometrium because of their similar reproductive physiology and endometrial pattern compared to humans. However, high costs, restricted facilities and ethical challenges are limiting their use for experimental purposes. Conversely, rodents do not menstruate and therefore they do not develop endometriosis spontaneously. Only homologuos models of surgically induced endometriosis have been used so far in this setting. Heterologous mouse models consisting of immunodeficient mouses do not seem offer obvious advantages in the study of endometriosis associated infertility. Ectopic transplanted tissue grows and behaves in a hormone-dependent manner, and they exhibit similar histological patterns compared with human endometriotic lesions (106). Despite of these limitations, the rodent model offers a low cost option and the opportunity to perform studies on large homogeneous groups of genetically similar animals. Transgenerational and long-term studies can also be performed because there is no rejection of the transplanted ectopic tissue. For an accurate model of the human condition, an intact hypothalamic-pituitary-ovarian axis in the animal recipient is essential for the evaluation of endometriosis and its related infertility. Another challenge is the difficulty in developing models that recapitulate all subtypes of endometriotic disease and therefore individualize and target. For example, there is a lack of specific in vivo models which resemble characteristics of ovarian endometriosis and its related infertility. To date, few animal models of ovarian endometriosis have been successfully implemented (108, 111, 112). Spontaneous ovarian endometriosis in non-human primates is also not as common as in humans (108). Lastly, a major limitation of these models is the concomitant establishment of other subtypes of the disease within the peritoneal cavity causing potential confounding effects. To capture the full extent of human disease it is possible to transplant human endometriosis into an immunodeficient mouse. This model may best recapitulate human disease (113).
Role of pain
For a successful natural conception, the feasibility of sexual intercourse is an important prerequisite, and one that is often neglected, however this is a potentially relevant mechanism involved in endometriosis-associated infertility. Pain may be a factor involved in endometriosis-related infertility when superficial dyspareunia (pain occurring in or around the vaginal introitus) makes intercourse difficult to achieve or deep dyspareunia makes intercourse difficult to sustain, leading to avoidance to sexual activity. However, only a few studies have focused on the relationship between superficial dyspareunia and endometriosis and is often concomitant with deep dyspareunia (114, 115). One cross-sectional study conducted on 300 women with histologically confirmed endometriosis reported that the severity of superficial dyspareunia was associated with increased odds of infertility concerns (116). Endometriosis is associated with a 9-fold increased risk of deep dyspareunia mostly due to the infiltrative form and severe stages of the disease affecting the posterior vaginal fornix, the pouch of Douglas, the uterosacral ligaments, and the rectum (117–119). Although relatively frequent, dyspareunia, is not the exclusive sexual complaint in women with endometriosis. Systematic reviews have highlighted that about two thirds of women with endometriosis have some form of sexual dysfunction not limited to deep dyspareunia (120–122). Chronic, nonmenstrual pelvic pain associated with the disease might influence sexual life by reducing desire, frequency of sexual intercourse, arousal, or orgasm. This will have a significant negative impact on intimate relationships, emotional well-being, and quality of life in general. In this regard, a holistic approach, rather than just a mechanistic approach, is mandatory given the complex nature of human sexuality.
Mechanical factors
Pelvic adhesions and anatomical distortion potentially affect the conception process in endometriosis. Inflammation, fibrosis, adhesions, and surgical sequela are the main pathophysiologic processes involved. Anatomical distortion and mechanical factors may impair oocyte release from the ovary, inhibit tubal ovum pick up or ovum transport, and/or block sperm transfer into the fallopian tube. Interestingly, no term pregnancies occurred in a non-human primate animal model of induced endometriosis when adnexal adhesions were noted on the same side as the ovulation occured (123).
Ovarian reserve
The ovary is the most common location of endometriosis. Ovarian reserve is one of the main prognostic factors regarding fertility and is in large part related to a woman’s age. Ovarian reserve is defined as the supply of non-growing, unrecruited primordial follicles (124); currently, a clinical tool that accurately predicts ovarian reserve does not exist. Despite concerns over their role and its specificity in clinical practice, antral follicle count (AFC) and serum anti-Mullerian hormone levels (AMH) are currently the most widely used indices of ovarian function (125). AMH is best used in identifying women who may be poor responders to gonadotropin stimulation in the setting of assisted reproductive technologies (ART) (126). AMH concentrations are not greatly affected by the menstrual cycle or oral contraceptives, making measurement possible at any time.
At present, the pathophysiologic mechanism of diminished ovarian reserve in endometriosis remains unclear. Nevertheless, there is a growing molecular, histological, and morphological evidence that endometriomas have a detrimental effect on ovarian function. Whether the endometrioma reduces the amount of functional tissue available by space-occupying effect (mechanical stretching damage) or by a direct toxic effect remains unknown.
An endometrioma is a peculiar benign cyst without a real capsule; therefore, there is exchange of cysts contents with the adjacent healthy ovarian cortex. Unlikely other benign cysts, the fluid of endometriotic cyst is able to induce oxidative stress in viable cells and potentially cause damage to healthy tissue. Molecular comparative analysis of the cystic fluid revealed high concentrations of free iron which is able to mediate the production of reactive oxygen species (ROS) that are highly diffusible through cellular compartments. An increase in the iron concentration in the follicular fluid from follicles in contact with the endometrioma was found in comparison with the contralateral healthy ovary (127). Moreover, proteolytic enzymes, inflammatory and adhesion molecules were also found in the endometriotic cyst fluid microenviroment (128). Thus, the release of toxic cysts contents in the adjacent ovarian parenchyma may lead to oxidative stress, fibrosis, loss of cortical stroma, smooth muscle cell metaplasia, impaired vascularization, and, at later stage, reduced follicular maturation and atresia in early follicles (128). Notably, the demonstration of increased oxidative stress affecting the normal ovarian cortex surrounding an endometrioma strongly suggest a ROS-induced fibrogenic response, leading to inhibition of angiogenesis and to follicular damage (129).
Maneschi et al. (130) first found a reduced follicular number and activity prior to surgery compared in histopathological specimens of endometriomas compared to other benign cysts; these findings were later confirmed in other similar studies evaluating follicular density (131–133). Another histopathological study also found increased fibrotic tissue surrounding endometrioma in comparison with that of other benign cysts (134). Interestingly, focal inflammation in the ovarian cortex of affected ovaries was suggested to cause enhanced follicular recruitment and atresia as a result of fibrosis and loss of cortex-specific stroma that maintains the follicular niche (135). Hence, excessive primordial follicle activation was proposed as a mechanism of “burn-out” of the follicular reservoir in ovarian endometriosis (136). Primordial follicle activation is an irreversible process and results in follicular depletion. The PI3K/PTEN/Akt/FOXO3 and PI3K/Akt/mTOR signaling pathways are the best-characterized regulators of primordial follicle activation during the initial recruitment. Takeuchi et al. (136) demonstrated that the number of primordial follicles was diminished, whereas primary, secondary, antral and growing follicle numbers increased in human ovaries with endometrioma, and this effect was mediated by the PI3K-PTEN-Akt-Foxo3 pathway. Similarly luteinized granulosa cells of women with endometriosis are characterized by increased apoptosis (137), however the specific putative mechanism leading to cell loss has not been yet identified. One recent study (138) found that proteins involved in apoptotic pathways were significantly increased in cortical tissue surrounding small endometriotic cysts (<3cm) but not in those surrounding other benign cysts.
Clinical findings confirm this trend; one recent, large, prospective cohort study including 106,633 premenopausal, laparoscopically confirmed endometriosis patients found higher risk for early natural menopause compared to those without endometriosis, especially in nulliparous women and in those who never used oral contraceptives (139). In two recent meta-analysis both serum AMH and AFC were found to be reduced in patients with unoperated endometriomas compared to patients with other benign ovarian cysts without endometriosis (140, 141). Moreover, in a prospective longitudinal study, a time-dependent effect was recently where serum AMH decline in women with untreated endometriomas faster than in age-matched healthy controls (142). Five meta-analyses (143–146) evaluating reproductive outcomes of women with endometrioma who had not undergone previous adnexal surgery found a reduced responsiveness to ovarian stimulation as measured by higher cycle cancellation rate, lower number of oocytes retrieved and lower number of formed embryos despite similar pregnancy and live birth rates. Besides their overall effect, important questions have been raised concerning endometrioma and the effect of size and bilaterality. Indeed, several comparative studies (147–150) in patients with unilateral endometriomas undergoing IVF showed that the affected and the healthy ovary produce a similar number of codominant follicles and oocytes perhaps indicating more than a local effect in the affected ovary; a single visible endometrioma maybe a marker of bilateral disease or alternatively there may be a systemic effect of the single endometrioma on both ovaries. Women with bilateral endometriomas demonstrate an even lower response to stimulation, however clinical pregnancy rate are not affected (151–153), likely overcome by the availability of multiple eggs and embryos
Another key concern is whether surgery has a negative impact on residual ovarian function. Despite the many studies that have been performed to evaluate the impact of surgical treatment of ovarian endometrioma on ovarian reserve, the data are still inconclusive. The potential detrimental impact of adnexal surgery on ovarian reserve has been elucidated in several histological studies confirming that cystectomy is generally associated with inadvertent removal of healthy ovarian tissue and primordial follicles adjacent to the cyst’s pseudocapsule (154, 155); this effect increases proportionally with cyst diameter (156), and ultimately is poorly correlated with the level of expertise in reproductive surgery (157, 158). Unlike other benign cysts, in which a well-defined capsule is present, endometrioma is not surrounded by a capsule (154) and technical difficulties may arise due to the absence of a clear cleavage plane. However, the damage inflicted by surgery may also be due to the related local inflammation or vascular compromise secondary to excessive manipulation of the cortex with subsequent tearing, bleeding, and the need for electrosurgical coagulation (159). Five meta-analyses showed a significant reduction in serum AMH concentrations after surgical treatment of endometriomas (160–162) and this effect is persistent post-operatively up to 18 months (162) and more pronounced in case of bilateral adnexal surgery (163–165). In contrast, two meta-analyses showed that ovarian reserve evaluated by AFC is not decreased after surgical treatment of endometriomas (162, 166). Concerning reproductive outcomes following IVF treatment, two recent meta-analyses (167, 168) showed a lower number of oocytes retrieved in women who had surgical treatment for endometrioma compared to women with expectant management; this finding was previously confirmed separately in case of unilateral treatment, compared with the contralateral normal ovary without endometrioma in the same patient (144). However, two meta-analyses (144, 169) concluded that women who had surgical treatment before IVF/ICSI had a similar live birth rate, clinical pregnancy rate, miscarriage rate, number of oocytes retrieved, and cancellation rate per cycle compared with those with untreated endometrioma. Lastly, according to a recent cohort study (170), the SAFE (surgery and ART for endometriomas) trial, about 50% of women with endometrioma were able to conceive spontaneously within 6 to 12 months after surgery. On the other hand, higher FSH and LH levels between the 2nd and the 5th day of the cycle prior to IVF required higher doses of gonadotropins for ovarian stimulation, and lower number of oocytes were retrieved after surgery for endometrioma in the remaining cohort of patients addressed for IVF, compared with women with unexplained infertility.
Despite all these efforts, further clinical analysis implementing standardization of endometrioma size, bilaterality, surgical technique, post-operative time-interval evaluations and clinical measurements are needed to help in elucidating both contributions and the magnitude of the effect.
Oocyte quality, embryo transport, sperm function and motility, sperm-oocyte interaction
The possible effect of ovarian endometriosis on oocyte quality is still under debate. Deeper understanding of the impact of the disease on oocyte quality is crucial as fertility preservation techniques are gaining attention in the counseling and treatment of this patients. Only few studies have investigated the impact of endometriosis on embryological competence. A recent meta-analysis including 22 studies indicate that endometriosis does not affect embryo morphology: Women with endometriosis have comparable high-quality embryo rate, cleavage rate, and embryo formation rate, regardless the stage of the disease (171). Results from several meta-analyses analyzing IVF outcomes, are controversial due to the high heterogeneity of the included studies (172–175). One recent large cohort study including 3818 embryos in cleavage stage found similar fertilization rate and embryo quality, despite a reduction in viable pregnancy rate. Conversely, one recent retrospective analysis using time-lapse technology observed altered relative kinetics in embryos from patients with endometriosis, supporting poorer embryo quality (176). Lastly, from the oocyte donation perspective, reduced pregnancy and implantation rates are observed when oocytes come from donors with endometriosis (177–179), supporting an effect of endometriosis on embryo quality. In contrast no difference was seen in recipients of donated oocytes based on the presence or absence of endometriosis. However, the cases used in these studies do not reflect the general population of women with endometriosis. The recipients in oocyte donation programs are relatively older compared with the majority of women with endometriosis seeking for pregnancy. With diminishing ovarian reserve and menopause endometriosis typically resolves. A history of endometriosis in a recipient of donor oocytes may not reflect current disease status. Therefore, use of results from oocyte donation does not provide a valid model to evaluate implantation and pregnancy rates in young women with infertility related to endometriosis.
Dysregulation of steroidogenesis and alterations of intrafollicular microenvironment are the main pathophysiological processes investigated in the context of endometriosis. E2 is crucial for follicular maturation and oocyte competence; follicular fluid also plays an important role in the reproductive performance of oocytes. Alterations in the normal physiology of the granulosa cells such as increased apoptosis and dysregulations of molecular pathways involved in its development and have been intensively studied. Granulosa cells of women with endometriosis are characterized by a decreased expression of P540 aromatase, a key enzyme in estrogen production. Similarly, some evidence also indicates an altered progesterone secretion from granulosa cells that might affect normal oocyte maturation (180, 181). Symmetrical lower E2 levels and higher progesterone levels were found in the follicular fluid of patients with endometriosis compared to controls (182). Moreover, follicular fluid has been shown to be subject to an important oxidative stress (183–188). An imbalance in ROS and antioxidant systems in the oocyte microenvironment could promote abnormal oocyte development, causing DNA damage, which would result in lower oocyte quality. In another study, cryopreserved human oocytes exposed to endometriotic fluid from patients with advanced stages of the disease had excess cellular fragmentation of derived embryos that may lead to impaired embryo development by inducing apoptosis in surrounding blastomeres or by altering blastomere division (189).
An altered systemic and peritoneal immune and inflammatory profile that characterize women with endometriosis has also been proposed to directly influence the follicular fluid composition. Altered levels of pro-inflammatory cytokines and growth factors (IL1B, TNFa, IL2, IL8, IL12, IL6, RANTES) have been reported in the follicular fluid of women with endometriosis compared to controls (190–192). Follicular fluid is released into the peritoneal cavity at each ovulation. Three studies have shown spindle and chromosome damage after incubating murine (193, 194) and bovine (185, 195) oocytes in metaphase II with both peritoneal fluid and follicular fluid derived from infertile women with endometriosis. A reduced implantation rate in normal rabbits was observed when the peritoneal fluid from rabbits with surgically induced endometriosis was transferred (196). On the other hand, intraperitoneal injection of peritoneal fluid from women with endometriosis significantly reduced implantation rates in rabbits as well as in hamsters (197, 198).
Gamete transport is also affected by the inflammatory microenvironment, anatomical distortions and uterotubal dysperistalsis associated with endometriosis (15). The endometriotic pro-inflammatory peritoneal fluid microenvironment may also affect sperm function (199–201) by inducing sperm DNA fragmentation (201), disrupt sperm membrane permeability or integrity (202), reduced sperm mobility (203), impaired sperm-oocyte interaction (204) and abnormal sperm acrosome reaction (205).
Impaired ovulation
Clinical data concerning spontaneous ovulation rate in these women is poor and controversial (206, 207). Prolactin levels are significantly higher in women with endometriosis when compared to those of women without endometriosis. Hyperprolactinemia prevents luteinizing hormone pulsatility and interferes with hypothalamic function by blocking estrogen receptors, thus producing anovulation. Another potential cause of ovulation failure in women with endometriosis is the luteinized unruptured follicle syndrome (208), a condition challenging to estimate in clinical settings in which the dominant follicle undergoes luteinization but fails to rupture at or to release the oocyte. Altered patterns of estrogen and progesterone secretion leading to an abnormal luteal phase may also compromise ovulation in these women (209). An association between endometriosis, luteinized unruptured follicle syndrome, and impaired fertility was observed in non-human primates animal models of endometriosis (123, 210) as well as in a mouse model of surgically-induced endometriosis (211, 212).
Endometrial receptivity
The implantation rate is clearly diminished in women with endometriosis during both natural cycles and ART treatments, even in patients with minimal disease (213–215). However, data from clinical studies suggesting that endometriosis leads to implantation defects implicating the endometrium is still conflicting (216, 217). Two recent reports showed similar outcomes in terms of implantation rates through ART cycles when compared to healthy controls (218, 219).
Defective implantation could be due to a reduced endometrial receptivity or decidualization capacity in these women.
The eutopic endometrium of women with endometriosis displays several molecular and functional, abnormalities compared to healthy women’s endometrium (220–223). Gradual and profound alterations have also been detected in the endometrium of endometriosis-induced baboons (224, 225). However, the mechanism and specific signal that leads to alterations in the endometrial microenvironment of women with endometriosis is not fully characterized and is still unknown whether changes in the endometrial pattern are the cause for the infertility and for presence of ectopic lesions or vice versa.
Endometrial receptivity and decidualization is dependent upon hormonally regulated molecular processes. Estradiol (E2) and progesterone (P4) responsive signaling pathways are regulated in an epithelial and stromal compartment-specific manner in the endometrium. Progesterone is the main hormone responsible for the transient receptive endometrial phenotype, essential for embryo implantation. The endometrial response to P4 is characterized by inhibition of estrogen-dependent proliferation of epithelial cells, secretory maturation of the glands, and transformation of stromal cells into specialized decidual cells. Functional dysregulation of steroid hormone signaling in endometriosis, such as upregulation of E2-induced cell proliferation, inflammation and progesterone resistance, seems to play an important role in impairing endometrial receptivity in these patients (226, 227). The shift toward estrogen dominance promotes inflammation, angiogenesis, cell proliferation, and immunosuppression. Both total endometrial PR expression and PR-A/PR-B isoforms ratios are dysregulated in the endometrium of women with endometriosis (222, 228–230) and in mice with induced endometriosis (19). Moreover, progesterone receptor expression levels are lower in women with endometriosis associated-infertility (231), whereas estrogen receptor 1 (ESR-1) levels are increased in the mid- secretory phase endometrium of these women compared to controls (232, 233).
From a histological perspective, Noyes et al. in 1950 have been proposed eight morphological criteria to evaluate endometrial receptivity, and for many decades they were adopted as the main diagnostic tool for detection of endometrial receptivity defects. However, these criteria have been questioned in recent years and several randomized control trials (RCT) (234, 235) have invalidated their use based on data demonstrating that histological dating of the endometrium does not discriminate between fertile and infertile women. Similarly, the negative predictive value of the endometrial thickness and the endometrial pattern as ultrasonographic parameters (236–239) in predicting endometrial receptivity are insufficient (240).
A transition from an anatomical and histological to a molecular perspective led to the genome-wide screening of all transcribed genes. Transcriptomic analysis of both eutopic and ectopic endometrium from women with or without endometriosis found dysregulations of selected genes that are implicated in the implantation process (241). Interestingly, HOXA10 is a progesterone target in the endometrium. The homebox gene family is critical in the development of the female reproductive tract during embryonic stages as well as in the regulation of endometrial receptivity during adulthood in response to steroid hormones (242). Decreased HOXA10 and HOXA 11 expression has shown to be involved in impaired endometrial receptivity, resulting in decreased implantation rates (75, 243). Patients with endometriosis do not show the normal physiologic rise in HOXA10 and HOXA11 during the mid-luteal phase of the menstrual cycle (75, 243, 244). HOX gene expression is also subjected to epigenetic modifications that leads to long-lasting alterations in endometrial receptivity (245). HOXA10 hypermethylation is an important mechanism responsible for its diminished expression (74). Both murine and baboon endometriosis models showed hypermethylation of the promoter region of HOXA10 and decreased expression of HOXA10 genes in the eutopic endometrium (19, 246). In humans, hypermethylation of HOXA10 was also identified in the endometrium of women with endometriosis (71, 74). Lastly, under normal conditions, high expression of the HOXA10 gene suppresses the transcription of the EMX2 gene, which is also essential in regulating endometrial receptivity and implantation. With the diminished expression of HOXA10 in endometriosis, the increased level of endometrial EMX2 directly affects endometrial cell proliferation and function during the peri-implantation period, resulting in aberrant implantation (247).
Integrins are cell adhesion molecules expressed in the endometrium during the receptive window and therefore involved in successful implantation. Interestingly, B3-integrin subunit is a direct downstream target gene of both HOXA10 and ESR-1 (248) and its aberrant expression have been described in the endometrium of women with endometriosis. Moreover, in the clinical setting, ART is less effective in patients with lower expression level of B3-integrin in the eutopic endometrium (249). Other transcriptional factors involved in regulation and mediation of progesterone signaling (IGFBP1, GATA2, FOXO1, ARID1A, NOTCH1and WNT4), required for successful implantation are also reduced in endometrium of women with endometriosis. In human endometrial stromal cells, silencing of GATA2, diminishes markers of decidualization (82) and interestingly the expression level is significantly reduced in the endometrium of women with endometriosis (80). Defects in decidual response have been also investigated in the eutopic endometrium of women with endometriosis. Compromised decidualization of cultured stromal cells was found in fresh shed endometrium as well as in the eutopic endometrium of women with endometriosis compared with matched healthy controls (250, 251). Several pathways have found to be aberrant in endometriosis (251–256) contributing to the unfavorable environment and promoting aberrant effects on the maternal/embryo interface. Increased activation of PI3K/AKT (252) pathway and decreased NOTCH signaling (256) contributes to inactivates FOXO1, an important mediator of decidualization involved in the progesterone signaling. Moreover, AKT pathway has been shown to downregulate and upregulate ESR-2 and ESR-1, respectively, with the net effect of promoting estrogen dominance (257, 258). Lastly, IGFBP1, a downstream target gene of HOXA 10 and a marker of decidualization, is reduced in the endometrium of women with endometriosis (251), and it is also downregulated in the eutopic endometrium of mice with induced endometriosis (19).
It is still poorly understood how the immune system contributes to and influences the endometrial microenvironment and the implantation window. Data is conflicting on the immune cell population of both ectopic and eutopic endometrium of women with endometriosis and controls, especially regarding absolute numbers, markers, activation states and cycle dependence due to heterogeneity of studies (259). Eutopic endometrium microenvironment of women with endometriosis seems to be more pro-inflammatory than controls and aberrant functions of certain immune population may lead to an inhospitable environment for embryo implantation. Interestingly, type I classically activated macrophages, that secretes proinflammatory factors, are the main population in eutopic endometrium of women with endometriosis, across all cycle phases, compared with normal controls (260–262). This proinflammatory predominance may decrease embryo nidation. The relative less cytotoxicity of natural killers together with their higher immaturity in the eutopic endometrium of women with endometriosis was significantly correlated with the infertility status in the same group (263). Concerning the role of B cells, anti-endometrial antibodies may also play a role in impaired implantation by affecting directly endometrial function for embryo receptivity (264). Finally, circulating and endometrial/decidual regulatory T cells (Tregs) have shown to be reduced in women with recurrent pregnancy loss, recurrent implantation failure and endometriosis (265).
Adenomyosis and other uterine factors
Endometriosis and adenomyosis often co-exist, especially in infertile women (266, 267). Additionally, the concomitant presence of both conditions seems to worsen fertility outcomes (267). In baboons, endometriosis was found to be statistically significantly associated to adenomyosis and the latter was found to be strongly associated with primary infertility (268). Several pathogenetic hypotheses have been postulated regarding adenomyosis and its associated infertility, including junctional zone thickness and subsequent perturbed uterine peristalsis that may alter utero-tubal transport, as well as biochemical, functional and epigenetic alterations in both eutopic and ectopic endometrium (269). The eutopic endometrial microenviroment in adenomyosis differs from the endometrium of unaffected women (270, 271). However, it remains conflicting whether these changes are of clinical significance and, in particular, in the setting of assisted reproductive technologies. According to the most recent meta-analysis in the field (213, 272) the presence of adenomyosis was associated with lower clinical pregnancy rate, higher risk of miscarriage following ART, and (independently of the mode of conception) with adverse pregnancy and neonatal outcomes. However, because of the limited number of comparative studies and their heterogeneous design, the real influence of adenomyosis alone on fertility is still controversial and poorly understood. One recent retrospective cohort study including more than 2000 subjects who underwent ART and by excluding those with decreased ovarian reserve and coexistence of endometriosis and fibroids, found that adenomyosis has a negative effect on IVF outcomes including an increased risk of miscarriage and a reduced live birth rate (273). There are several major limitations in investigating the impact of adenomyosis on infertility. First, there are major diagnostic limitations related with coexistence of endometriosis and adenomyosis, making the interpretation of the available literature difficult. Second, with the advent of 3D ultrasound and the use of magnetic resonance imaging the diagnosis of adenomyosis can now be relatively reliable without the need of histological examination of the surgical specimen (274). However, there is no consensus regarding diagnostic features of adenomyosis using imaging making the interpretation of observational studies challenging; different imaging criteria to define adenomyosis are commonly used. Lastly, adenomyosis frequently coexists with other gynecological disorders and potential confounders, such as uterine leyomiomas. As with adenomyosis, uterine fibroids, in particular submucous leyomiomas, has been associated with lower implantation rates and increased risk for early pregnancy loss (275, 276). The main pathophysiological processes implicated in endometriosis associated infertility are summarized in Figure 2.
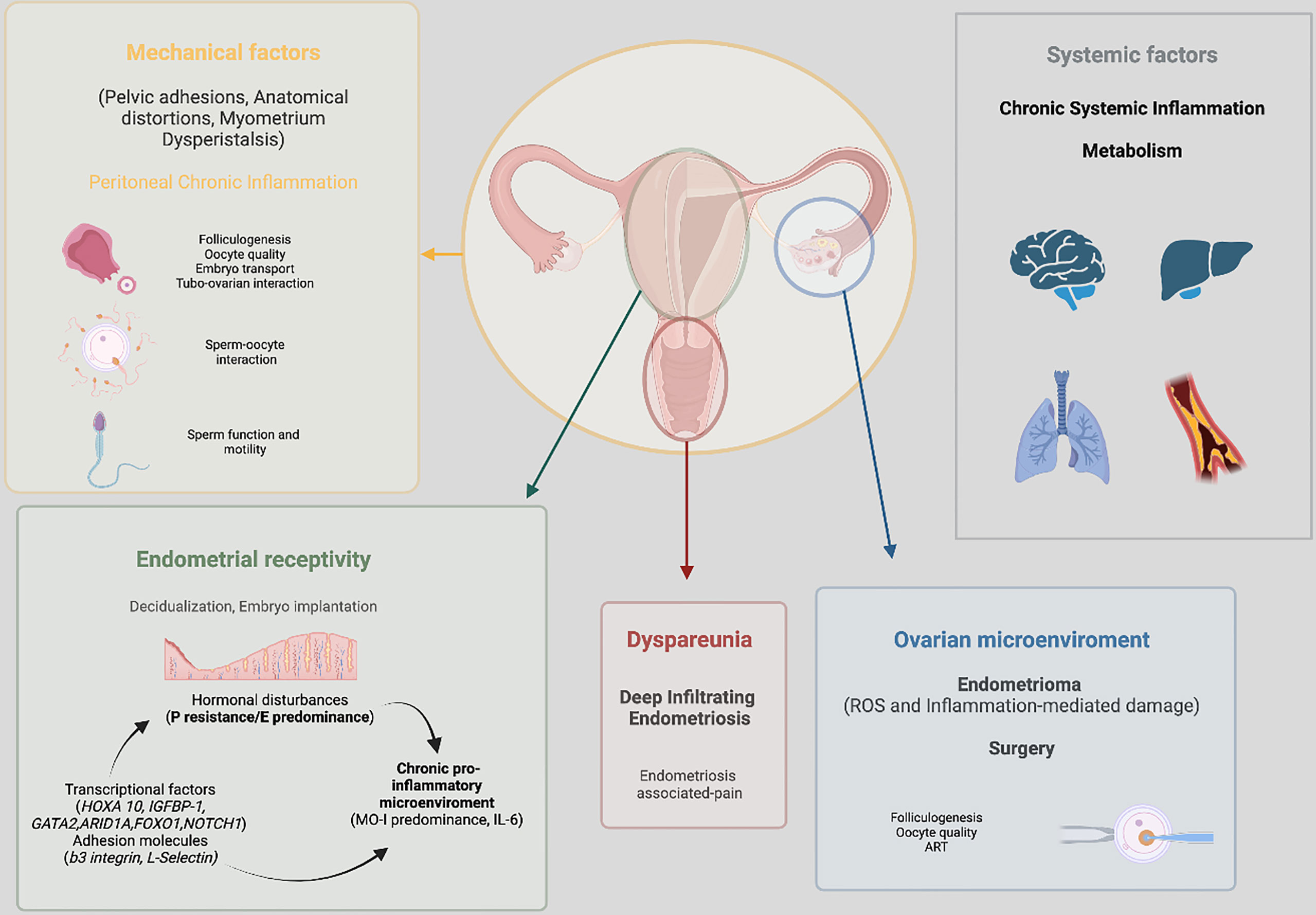
Figure 2 Summary of the main pathophysiological processes implicated in endometriosis associated infertility.
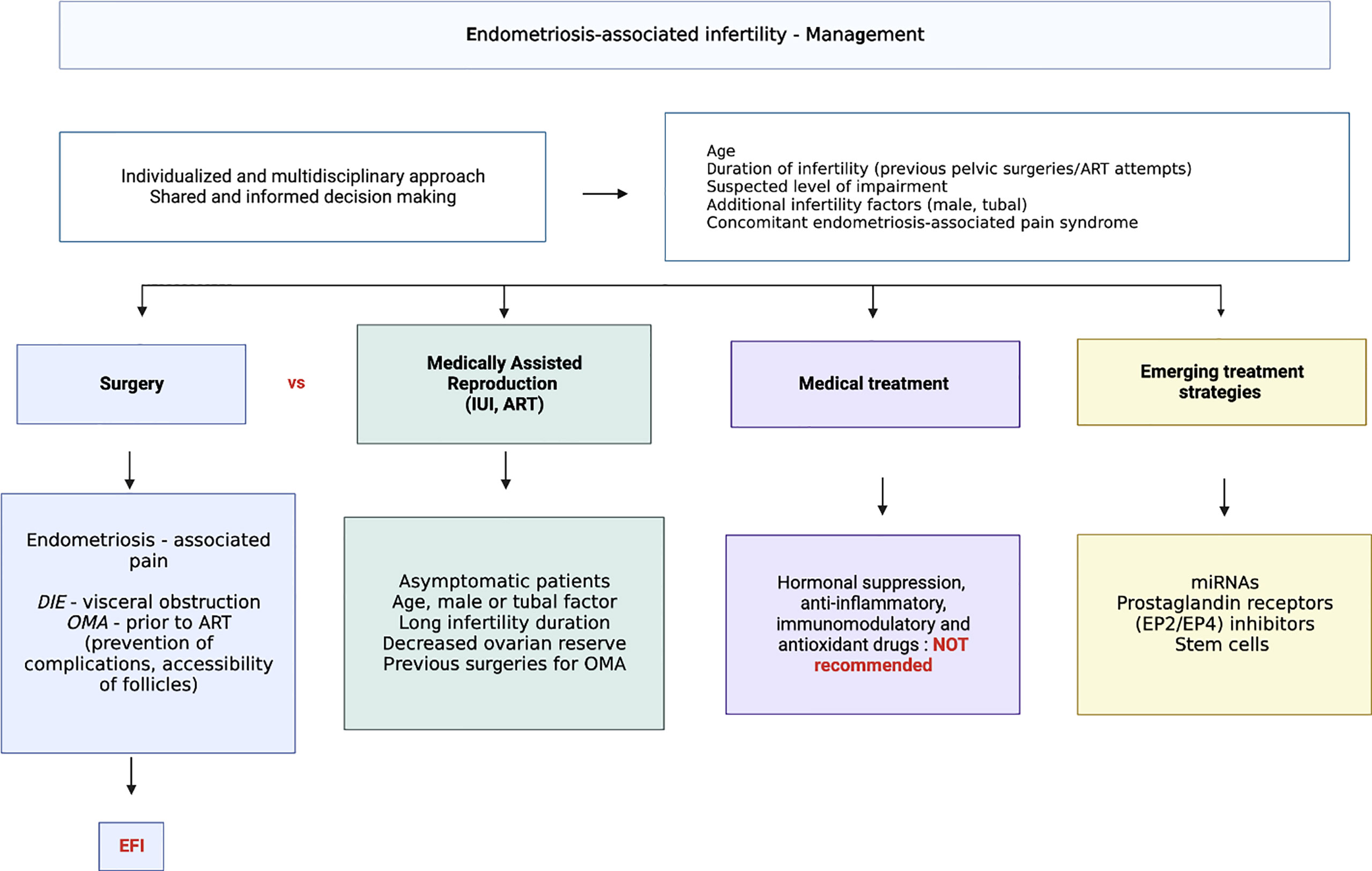
Management of endometriosis associated infertility
Clinical management of infertility associated with endometriosis is challenging due to lack of high-quality scientific evidence and conflicting available guidelines (277). The complexity in therapeutic decision-making is mainly related to the heterogeneous population of infertile women with endometriosis which includes diverse patient’s phenotypes. This often requires innovative diagnostic and therapeutic tools to target the specific dysfunctional step of the reproduction process. Therefore, care of women with endometriosis-associated infertility is best undertaken in referral centers where a multidisciplinary approach can be offered and where both surgery and IVF services are present.
From the patient perspective, a shared and informed decision is mandatory because different treatment options may involve both clinical and personal aspects. The treatment must be individualized according to the clinical situation and to the suspected level of impairment. Factors such as woman’s age, ovarian reserve, duration of infertility, additional infertility factors (male, tubal), ASRM stage, previous surgical treatment for endometriosis, concomitant pain, and indications for IVF-ET must be considered because they will influence the choice of treatment and may also have socio-economic implications.
In American and European guidelines (278, 279), the management of endometriosis is still based on the disease stage defined according to the revised American Society of Reproductive Medicine (rASRM) classification. Despite the high consensus and multiple revisions, the currently used classifications system has several limitations, including failure in predicting fertility outcomes and in accounting for the different types of endometriosis. For this reason, Adamson and Pasta developed a validated and predictive endometriosis staging system, the Endometriosis Fertility Index (EFI), to estimate the non-ART pregnancy rate (natural intercourse or IUI) in women with surgically documented endometriosis (280). This scoring system, which takes into account patient-related factors (age, length of infertility, history of previous pregnancy) and surgical factors (rASRM total score, endometriosis lesions and “least function score” from the tubes and ovaries), is highly accurate (281) and reproducible (282) and represents an important clinical decision tool to counsel patients on their reproductive options after surgery.
While ART can correct many defects that prevent conception, implantation failure is not easily identified, and in most cases, there are no available treatments. The endometrial status is rarely investigated during the standard work-up of infertile women performed in infertility clinics worldwide, even prior to ART (283). Thus, in the era of precision medicine and tailored therapy, a reliable endometrial receptivity assay would be of huge clinical and economical benefit for the patient’s selection process. Starting from functional analysis of the endometrium, Kliman et al. (284) introduced an innovative endometrial functional diagnostic tool (endometrial function test EFT®) based on the use of antibodies two cyclins, as expression patterns of this type of mitotic cycle regulators have been associated with implantation success or failure. Moreover, revolutionary diagnostic tests based on transcriptomic and bioinformatic technologies, that can inform clinicians about the status of endometrial receptivity, have been proposed for diagnostic and prognostic purposes. The endometrial receptivity array (ERA) (285) is a and reproducible (286) microarray-based machine-learning predictive model for assessing endometrial status in the work-up for infertile patients based on the specific signature of 238 differentially expressed genes in the receptive endometrium. The ReceptivaDX™ test a new screening and diagnostic test has been proposed (287) based on the findings that B-cell-lymphoma 6 (BCL6) overexpression in the secretory endometrium of these women contribute to the progesterone resistance (288) and could potentially serve as a surrogate inflammatory marker for a dysfunctional endometrium in endometriosis associated with infertility (289, 290).
Given the important diagnostic delay, strenuous research has been made to identify potential non-invasive diagnostic tool in endometriosis and to date, remains one of the major research priorities in this disease. Several potential biomarkers have been evaluated; however, none has demonstrated sufficient sensitivity and specificity for clinical use.
Cancer antigen-125 (CA-125), a high-molecular-weight glycoprotein antigen expressed in some derivatives of the celomic epithelium, has been previously reported to be elevated in serum of women with advanced forms of the disease (291, 292); however, its overall sensivity is reported to be extremely low (53%) (293).
Based on their pivotal role in the etiopathogenesis of endometriosis, a substantial body of work has shed light on circulating/exosomal miRNAs as potential leading biomarkers for early diagnosis, prognosis, and surveillance for endometriosis. They are they are found both intra- and extracellularly, contained and released via exosomes (294).
MiRNAs are attractive due to their simple structure and their stability at the post-translational level and in extracellular biofluids. Conversely, their highly variable content and their low abundance in extracellular biofluids, make detection very demanding. Real time PCR (qRT-PCR) remains the gold standard for miRNA quantification (295). Next-generation sequencing platforms are also used for miRNA sequencing, and they showed high sensivity and excellent reproducibility (296), nevertheless a great performance variation exists among the different platforms. MicroRNA have several targets in cells and each single mRNA transcript may be subjected to regulation by various miRNAs, Thus, the same pathway maybe regulated by a panel of miRNA.
Many studies have investigated the role of circulating miRNA in endometriosis (104, 297–312). To date, more than 60 miRNAs were found to be differentially expressed in the circulation (plasma and/or serum) of women with endometriosis (313). Very few (20%) miRNAs have been replicated in more than one study (314). While generally miRNAs are not highly evolutionarily conserved, serum let-7 family miRNA showed similar dysregulation in a murine model of endometriosis (315).
In general, serum-derived miRNAs seems to yield higher sensitivity and specificity (92 and 95,5% respectively) compared with plasma-derived miRNAs and the highest biomarkers potential was found to be represented by a panel of serum-derived miRNAs comprised of miR-125-5p, miR-150-5P, mir-342-3p, miR-451a, miR-3613-5P, and let-7b (area under the curve (AUC) of 0.94) (313). Cosar et al. (302) reported a logistic regression model combining miR-125-5p, miR-451a, and miR-3613-3p. Later, the same group (300) confirmed a significant diagnostic value of a combination of six miRNAs, with a final AUC>0.9 across two independent clinical data sets.
The reason for the limited consistency of results across studies is related with the dynamic nature/behavior of miRNA expression which is influenced by lack of standardization in the study protocols, such as sample collection (menstrual phase, circadian rhythm), miRNA analysis method, case-control matching and subject’s background (age, ethnicity, health status), stage (minimal-mild vs. moderate/severe) and type of endometriosis (ovarian, peritoneal, deep infiltrating). In addition, different cutoff points were considered to define a meaningful change in expression. An important variable is whether their expression is influenced by the menstrual cycle phase.
Results need to be replicated on large series of well-phenotyped patients and under stringent conditions of sampling. The availability of a reliable non-invasive test for endometriosis will allow more accurate and accessible diagnosis as well as the potential for identification and treatment of endometriosis related infertility. Current and emerging treatment options for endometriosis associated infertility are summarized in Figure 3.
Reproductive surgery
Surgical indications should be guided by the presence or absence of pain, patient’s age, history of previous surgery for endometriosis, presence of other infertility factors, ovarian reserve, and estimated EFI. In general, it is clear that multiple surgeries should not be attempted to improve fecundity. Current guidelines suggest fertility counseling before surgery which should include AMH measurement (278, 279).
r-ASRM stages I and II
Stages I and II are not visible during the clinical and ultrasound examination and they are diagnosed mainly during diagnostic laparoscopy. A recent meta-analysis (315) of moderate quality evidence, including three RCTs on rASRM stage I/II endometriosis, concluded that operative laparoscopy increases natural viable intrauterine pregnancy rates compared to diagnostic laparoscopy only (OR 1.89, 95% CI 1.25-2.86; I2 = 0%). Similar findings were later confirmed in a network-metanalysis by Hodgson et al. (316) comparing operative laparoscopy with placebo (OR 1.63; 95% CI 1.13-2.35). Only one meta-analysis (317) analyzed the live birth rate outcome and concluded that laparoscopic surgery in this disease stages has an overall advantage in improving the chances of live birth (RR 1.52, 95% CI 1.26-1.84). Based on this evidence, operative laparoscopy is currently an option for endometriosis-associated infertility in rASRM stage I/II (278, 279) when is performed for other indications such as pain. The absolute benefit is modest with a number of women needed to be treated of 12 to achieve one additional pregnancy.
r-ASRM stages III and IV
The situation is more complex for advanced disease where high quality evidence regarding the role of surgery for infertile patients is lacking and the risk of major complications due to the surgery itself must be considered. There are no RCT to determine whether clinical pregnancy rates are improved after surgery in patients with stage III-IV of the disease. Women with advanced disease are normally counseled toward surgery in case of significant pain symptoms, large endometriomas, or ureter and bowel clinical involvement.
Apart from the deep infiltrating endometriosis (DIE)-induced alteration of pelvic anatomy and adhesions, evidence supporting a direct link between DIE and infertility is weak and the lack of high-quality data preclude firm conclusions on the effect of surgery. Moreover, DIE alone is found in only 6% of endometriosis patients (318) and surgery-related major complications in this context must be taken into account. According to three independent systematic reviews, pregnancy rate after surgery for rectovaginal endometriosis varies from 24% to 44% (319–321). An additional systematic review (322) of heterogeneous prospective and retrospective studies reported postoperative spontaneous pregnancy rates in women with DIE with and without bowel involvement of approximately 50% and 20%, respectively. Lastly, a recent meta-analysis including observational studies on both rectovaginal and rectosigmoid DIE patients showed a statistically significant benefit of surgery before IVF (323) in terms of live birth and pregnancy rates per patient and per cycle. Nonetheless results need to be interpreted with caution before attributing this rate of success entirely to surgery (324). Interestingly it has been shown in a recent study that extensive surgery in women with deep and intraperitoneal endometriosis, when compared with intraperitoneal surgery only, does not modify the fertility outcome (325). At present, operative laparoscopy for DIE represent a well-established indication in endometriosis associated-pain and in case of visceral obstruction, and is a treatment option in symptomatic patients wishing to conceive (278, 279).
Potential benefits and harms of adnexal surgery for endometrioma must be considered in this context because of its direct effect on ovarian reserve and the risk of recurrence. There is still no consensus regarding the optimal indication for surgery depending on cyst diameter due to the lack of comparative studies. According to guidlines (278, 279), key indications for surgical management of the “asymptomatic” ovarian endometrioma in patients with infertility is to improve the accessibility of follicles and to prevent potential complications (endometrioma rupture, contamination) prior to ART.
A recent meta-analysis (326) conducted by Alborzi et al. of eight prospective studies comparing pregnancy rate from four different approaches of OMA (surgery + ART, surgery + spontaneous pregnancy, aspiration with or without sclerotherapy + ART, and ART alone) found no significant difference between the four study groups. Another meta-analysis (167) analyzing surgical versus expectant management of endometriomas reported similar live birth rates per cycle after IVF in both groups. Surgery is generally not recommended for the sole purpose of enhancing fertility in an otherwise asymptomatic patient.
Medically assisted reproduction (IUI, ART)
The utility of Intrauterine Insemination (IUI) with or without ovarian stimulation in patients with endometriosis is supported by only one single RCT (327) including patients undergoing ovarian stimulation with gonadotrophins and IUI versus expectant management. The study found a 5-6 times higher live birth rate per cycle in the treatment group. IUI in combination with controlled ovarian stimulation (clomiphene citrate) is currently recommended only in infertile women with ASRM stage I/II (328).
Currently, up to 25% of in vitro fertilization (IVF)-embryo transfer procedures are performed in patients with endometriosis (329). Despite its high implementation, both the influence of endometriosis on pregnancy rates after ART, and the effectiveness of ART treatments in women with endometriosis are still a matter of debate. Main indications for ART remain tubal impairment, male factor, low EFI, and failure of other treatments. There are currently no RCTs evaluating the efficacy of this treatment option versus no intervention in women with endometriosis, and only indirect evidence is available from studies comparing ART outcomes in women with endometriosis to women without the disease. Several meta-analyses (172–175) have investigated ART outcomes of women with and without endometriosis, but results appear conflicting due to the low quality and the high heterogeneity of the selected studies. According to a recent meta-analysis (213), endometriosis consistently leads to reduced oocyte yield and a reduced fertilization rate. Milder forms of endometriosis are most likely to affect fertilization and implantation processes as discussed earlier, whereas advanced stages of the disease may influence all stages of reproduction.
Reasons for the suspected suboptimal performance of ART in endometriosis may include the affected ovarian responsiveness during the ART cycles (low ovarian reserve), impaired endometrial receptivity, and altered folliculogenesis.
Finally, a specific protocol of controlled ovarian stimulation for ART in women with endometriosis is not currently recommended as both antagonist and agonist protocols are still widely used and no difference in pregnancy or live birth rates has already been demonstrated (330).
Medical approach
Based on a presumed altered steroidogenesis in endometriosis associated infertility, the use hormonal suppression has been investigated. Based on current recommendations (278, 279) ovarian suppression (danazol, GnRH agonists, progestogens, OCP) should not be offered alone or in combination with surgery in endometriosis-related infertility because there is no evidence of its benefit on pregnancy outcomes (331). A recent Cochrane review (332), comparing the effectiveness of different timing of hormonal suppression in the setting of surgery, concluded that postsurgical medical therapy compared with no treatment or placebo may increase pregnancy rates and reduce disease recurrence, and that it should be recommended in women who cannot, or decide not to conceive immediately after surgery.
The role of downregulation with GnRH agonists prior to ART has been extensively investigated and several meta-analysis have been performed; however results are still contradictory. It has been proposed that medical treatment with gonadotropins prior to IVF may result in improved fertility outcomes in terms of both oocyte quality and endometrial receptivity.
An updated Cochrane review by Georgiu et al. (333) that included 8 RCT concluded that the effect of long-term GnRH agonist pre-treatment for at least 3 months versus no pre-treatment is uncertain in terms of live birth rate (primary outcome), clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, mean number of oocytes and mean number of embryos.
Another more recent meta-analysis (334) investigated the effectiveness of three different down-regulating protocols based on the use of GnRH-agonist (ultra-long, long and short protocol) in infertile women with endometriosis prior to ART. The authors concluded that the ultra-long protocol may improve the clinical pregnancy rate especially in patients with stages III-IV endometriosis based on data from two RCTs. Conversely, more recently, two RCTs (335, 336) failed to demonstrate a beneficial effect of the ultra-long protocol in terms of live birth rate, clinical pregnancy rate, or embryo quality; instead, it was associated with a longer duration of ovarian stimulation, a higher consumption of gonadotropins, and a lower ovarian estradiol production (335). No studies have been conducted to evaluate the efficacy of GnRH antagonists for the treatment of endometriosis-related infertility. A large multicenter RCT (337) is ongoing in the US with the aim to investigate for the first time the pre-IVF treatment with a GnRH antagonist (Elagolix) in women with endometriosis (PREGnant). Compared with GnRH-agonists, the rapid reversibility and recovery of the hormone secretion once the treatment is concluded using GnRH antagonist may allow for better outcomes at the time of ART.
Data addressing pretreatment with continuous oral contraceptives are very limited and do not allow firm conclusion (338, 339). Comparative studies between different hormonal suppression treatment strategies are lacking.
Lastly, assuming that endometriosis is a chronic inflammatory condition, the effect of several anti-inflammatory, immunomodulatory and antioxidant agents has been investigated in the context of inflammation and altered redox balance in the follicular fluid microenvironment and of suspected impaired oocyte quality (340). Pentoxifilline has been the most studied anti-inflammatory and antioxidant agent in endometriosis-associated infertility, and it has also been shown to enhance sperm motility and improve semen parameters in men with oligoasthenospermia. However, according to a recent Cochrane review (341) including 5 RCT, there is no conclusive evidence on its effectiveness and safety in endometriosis-associated-infertility.
Emerging treatment strategies
By elucidating the cellular and molecular mechanisms involved in endometriosis-associated infertility and based on the assumption that current hormonal suppression-based treatment cause important side effects rather than effectively improve fertility (342), there is a fundamental need to identify potential signaling pathways for non-hormonal targets for endometriosis associated with infertility.
Non-coding RNAs (ncRNAs) have rapidly emerged as important regulatory molecules in cancer and several reproductive diseases such as recurrent pregnancy loss and endometriosis (95). The use of ncRNAs as a therapeutic tool is still in its infancy; however, the US-FDA has recently approved three RNAi therapies (343, 344). In the context of impaired endometrial receptivity and progesterone resistance, Petracco et al. (345) identified a putative miR135 binding site in HOXA10 gene showing that miR135a and miR135b are expressed in normal endometrium and increased in the endometrium of women with endometriosis; they likely act by regulating targets of progesterone action in the endometrium. Furthermore, miR-451 was found to be the most highly downregulated in the mid-secretory phase of eutopic endometrium of baboons (346) and women with endometriosis (347) compared to controls, leading to an increased expression of transcription factors involved in regulation and mediation of progesterone signaling such as GATA2 and YWHAZ. Similarly, H19 is one of the first long noncoding RNA identified; it is expressed in a menstrual cycle-dependent fashion, confined to the stroma, and acts as a decoy for several tumor-suppressor miRNA. Moreover, it is positively regulated by E2 and negatively regulated by progesterone in the mouse. It was recently found that in the eutopic endometrium of patients with endometriosis, downregulation of H19 will increase let-7 activity, contributing to a decreased proliferation of endometrial stromal cells (through IGFR1 expression inhibition) and contributing to the impaired endometrial preparation and receptivity (through reduction of stromal cell proliferation) (348). Finally, further studies have investigated the role of certain miRNA within progesterone resistance during the luteal phase in women with endometriosis; they reveal that miR-30b, miR-30d, miR-29c and miR-194-3p are up-regulated whereas mi-494 and miR-923 are down regulated in receptive endometrium (230, 349) of both humans and baboons (350). Lastly, upregulation of miR-196 and MEK/ERK signaling proteins was reported in infertile women with minimal/mild endometriosis mediating downregulation of PGR expression and decidualization in eutopic endometrium (351). MicroRNA based therapies are promising new fertility treatments.
In the context of a pro-inflammatory endometrium, one recent study (352) investigated the pharmacological effects of selective inhibition of prostaglandin receptors (EP2/EP4) by using a chimeric mouse model of endometriosis and found that endometrial functional receptivity can potentially be restored the interaction among prostaglandin E2 (PGE2), estrogens and progesterone. The results indicate that inhibition of EP2/EP4 decreases PGE2, estrogen biosynthesis and signaling, pro-inflammatory cytokine production, and increases P4 signaling in eutopic endometrium of women with endometriosis.
Stem cell therapy has shown to be promising as a new therapeutic target for infertility, especially Asherman’s syndrome (353). The interaction between BMDSC and endometrial MSC has generated considerable interest because of their tropism toward inflamed foci.
Stem cell properties of self-renewal and differentiation made attractive their use to replace potential damaged tissues and inflammation by reducing intrauterine adhesions and fibrosis (354, 355), improving endometrial thickness (356) and promote endometrial regeneration. To date, the use of stem cell for treating endometriosis, and in particular endometriosis-associated infertility offers an attractive option because of its tropic and immunomodulatory proprieties.
Endometriotic lesions recruit stem cells away from the uterus resulting in inadequate endometrial repair and regeneration as endometriosis more effective in recruiting BMDSCs than eutopic endometrium (357). Lesions highly express CXCL12, a chemoattractant for BMDSCs expressed in many organs, as well as by endometrial stromal cells (357). Inhibiting its receptor (CXCR4)was shown to impact the migration of BMDSCs to the uterus (358). Endometriosis related-chronic inflammation likely acts by continually recruiting BMDSCs to the lesions as demonstrated in animal (52). Moreover, physiologic estradiol levels can increase CXCL12 and CXCR4 expression by endometrial stromal cells and BMDSCs respectively in vitro, thereby increasing the chemoattractiveness between the two and consequently, migration (358).
Interestingly, Badoxifene, a selective antagonist ER antagonist, showed to reduce both ectopic endometriotic lesions and the BMDCs engraftment to them, redirecting these cells to the eutopic endometrium (54, 359, 360). This phenomenon might be able to create a new endometrium partially free of epigenetic defects.
The route of stem cell administration will be a crucial component of any stem cell based therapy. Significantly greater levels of stem cell incorporate in uteri of mice when cells were administered systemically by intravenous injection as compared with local injections into the uterus (361). Interestingly, mice that received a systemic infusion of BMDSCs prior to uterine injury were also more likely than twice to achieve a pregnancy, suggesting functional repair of damaged endometrium was due to BMDSs activity (355). BMDSCs maybe superior to ESC in the treatment of uterine injury; they may allow a more complete repair due to their superior versatility, developing into a large number of cell types required for endometrial function (355).
Fertility preservation
The need for reproductive counseling utilizing a multidisciplinary medical team has become more evident in endometriosis not only prior to surgery but also at diagnosis, based on the assumption that fertility is likely to be compromised in these women.
Several options are currently available to preserve fertility, including embryo or oocyte cryopreservation and ovarian tissue cryopreservation which are no longer considered experimental procedures (362). Vitrification or planned oocyte cryopreservation technology has grown enormously during the last few years. Several meta-analyses demonstrated that clinical outcomes after vitrification are superior to the standard slow-freezing/thawing. Moreover, comparable results between vitrificated and fresh oocytes were reported (363, 364).
Many questions in terms of efficiency, effectiveness and risks remain unanswered, and the strength of evidence to support fertility preservation in endometriosis, regardless disease severity, is still limited (365). Systematically offering FP to patients with endometriosis might have a dramatic effect on the public healthcare expenditure and may expose patient to unnecessary clinical risks (366, 367). In the context of ovarian endometriosis, subgroups that would particularly benefit from fertility preservation are women with bilateral endometriomas and those scheduled for surgery for contralateral recurrence after unilateral endometrioma surgery or in whom spontaneous conception is unlikely after ovarian surgery (365–368). One of the advantages related with an earlier approach is the opportunity to preserve oocyte at a young age. However, lack of reliable data regarding the effectiveness limit full scale adoption. Women at young age may have a greater risk of recurrence, and when there is not an immediate desire for pregnancy, offering FP could be a beneficial option. Ovarian tissue cryopreservation represents an alternative in case where ovarian hyperstimulation is contraindicated. The first case of autologous ovarian transplantation with cryopreserved tissue was reported in 2000 by Oktay et al. (369). Later updates by the same group in 2010 (370) and Donnez et al. in 2005 (371) reported a similar successful approach in endometriosis cases and to date, other few cases have been reported (372–378).
Fertility sparing surgery
Optimizing fertility in patients with endometriosis requires reducing potential iatrogenic harm to the ovarian reserve. In this context, the role of adnexal sparing surgery, when indicated, is crucial for treating symptomatic women with endometriosis-associated pain for improving accessibility of follicles prior to ART due to endometriosis-associated infertility. Thus, skilled surgeons with expertise in reproductive pathophysiology are required to avoid potential insults to the healthy parenchyma and ovarian vascular network.
Decrease of normal ovarian cortex (ovarian reserve) and disease recurrence are the two main risks associated with surgery for endometrioma, regardless the surgical technique. Several surgical approaches for endometrioma have been proposed: Excisional, ablative or a combination of both. Lower rates of spontaneous pregnancy and higher rates of recurrence are associated with ablative surgery compared with cystectomy, whereas cystectomy was found to be deleterious for residual ovarian function (162, 379, 380). Since the meta-analysis of Dan et al. in 2013 (380), RCTs comparing ablative versus excisional techniques in terms of ovarian reserve markers and ovarian residual volume have been performed (381–386). Data from animal studies (387) and RCT (381, 382, 388) on the use of laser and plasma energy is encouraging and may result in less inadvertent tissue removal and thermal injury compared with cystectomy and bipolar electrosurgery. Fertility sparing surgery performed in the context of endometriosis-associated infertility also requires the systematic evaluation and optimization of tubal anatomy and patency. Concomitant adhesiolysis with restoration of pelvic anatomy is also recommended when anatomical distortion is present.
Conclusions
The mechanisms involved in endometriosis-associated infertility are still not completely understood and this condition is multifactorial. Endometriosis-associated pain and inflammation, altered pelvic anatomy and adhesions, disrupted ovarian function, and compromised endometrial receptivity all play a major role in endometriosis infertility in women with endometriosis. Identifying innovative, non-invasive diagnostic tools in endometriosis that also predict a higher risk of infertility remains one of the major research and clinical priorities in this disease; delayed diagnosis allows for disease progression which is clearly detrimental from the perspective of fertility.
Treatment options of infertility associated with endometriosis are still limited. Surgery and ART remain the mainstay of effective therapy. All medical therapies currently approved for use in this disease prevent or diminish fertility and therefore are not helpful in treating this condition. Future non-hormonal medical therapies are needed that can enhance fertility by interfering with the pathways outlined above.
Endometriosis-associated infertility requires a multidisciplinary, personalized, shared and holistic approach based on patient’s unique characteristics, endometriosis subtype and level of impairment.
Author contributions
GB and HT contributed to manuscript writing and editing. HT revised the manuscript for important intellectual content; all authors approved the final version of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet (2021) 397:839–52. doi: 10.1016/S0140-6736(21)00389-5
2. Nezhat C, Nezhat F, Nezhat C. Endometriosis: Ancient disease, ancient treatments. Fertil Steril (2012) 98:6S. doi: 10.1016/j.fertnstert.2012.08.001
3. Macer ML, Taylor HS. Endometriosis and infertility: A review of the pathogenesis and treatment of endometriosis-associated infertility. Obstet Gynecol Clin North Am (2012) 39:535–49. doi: 10.1016/j.ogc.2012.10.002
4. Healy DL, Trounson AO, Andersen AN. Female infertility: causes and treatment. Lancet (1994) 343(8912):1539–44. doi: 10.1016/s0140-6736(94)92941-6
5. Agarwal SK, Chapron C, Giudice LC, Laufer MR, Leyland N, Missmer SA, et al. Clinical diagnosis of endometriosis: A call to action. Am J Obstet Gynecol (2019) 220:354.e1–354.e12. doi: 10.1016/j.ajog.2018.12.039
6. Eisenberg VH, Decter DH, Chodick G, Shalev V, Weil C. Burden of endometriosis: Infertility, comorbidities, and healthcare resource utilization. J Clin Med (2022) 11:1133. doi: 10.3390/jcm11041133
7. Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril (1991) 55:759–65. doi: 10.1016/s0015-0282(16)54244-7
8. Sampson JA. Perforating hemorrhage (chocolate) cysts of the ovary: Their importance and especially their relation to pelvic adenomas of endometrial type (“adenomyoma” of the uterus, rectovaginal septum, sigmoid, etc.). Arch Surg (1921) 3:245–323. doi: 10.1001/archsurg.1921.011100800030011
9. Sampson JA. Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol (1927) 14:422–69. doi: 10.1016/S0002-9378(15)30003-X
10. Sampson JA. The development of the implantation theory for the origin of peritoneal endometriosis. Am J Obstet Gynecol (1940) 40:549–57. doi: 10.1016/S0002-9378(40)91238-8
11. Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol (1984) 64:151–4.
12. Kruitwagen RF, Poels LG, Willemsen WN, de Ronde IJ, Jap PH, Rolland R. Endometrial epithelial cells in peritoneal fluid during the early follicular phase. Fertil Steril (1991) 55:297–303. doi: 10.1016/s0015-0282(16)54119-3
13. Koks CAM, Dunselman GAJ, de Goeij AFPM, Arends JW, Evers JLH. Evaluation of a menstrual cup to collect shed endometrium for. Vitro Stud Fertil Steril (1997) 68:560–4. doi: 10.1016/s0015-0282(97)00250-1
14. Leyendecker G, Kunz G, Herbertz M, Beil D, Huppert P, Mall G, et al. Uterine peristaltic activity and the development of endometriosis. Ann N Y Acad Sci (2004) 1034:338–55. doi: 10.1196/annals.1335.036
15. Leyendecker G, Kunz G, Wildt L, Beil D, Deininger H. Uterine hyperperistalsis and dysperistalsis as dysfunctions of the mechanism of rapid sperm transport in patients with endometriosis and infertility. Hum Reprod (1996) 11:1542–51. doi: 10.1093/oxfordjournals.humrep.a019435
16. Rakhila H, Bourcier N, Akoum A, Pouliot M. Abnormal expression of prostaglandins E2 and F2α receptors and transporters in patients with endometriosis. BioMed Res Int (2015) 2015:808146. doi: 10.1155/2015/808146
17. Kawano Y, Hirakawa T, Nishida M, Yuge A, Yano M, Nasu K, et al. Functioning endometrium and endometrioma in a patient with Mayer- rokitanski-Kuster-Hauser syndrome. Jpn Clin Med (2014) 5:43–5. doi: 10.4137/JCM.S12611
18. Tal A, Tal R, Pluchino N, Taylor HS. Endometrial cells contribute to preexisting endometriosis lesions in a mouse model of retrograde menstruation. Biol Reprod (2019) 100:1453–60. doi: 10.1093/biolre/ioz039
19. Lee B, Du H, Taylor HS. Experimental murine endometriosis induces DNA methylation and altered gene expression in eutopic endometrium. Biol Reprod (2009) 80:79–85. doi: 10.1095/biolreprod.108.070391
20. D'Hooghe TM. Clinical relevance of the baboon as a model for the study of endometriosis. Fertil Steril (1997) 68:613–25. doi: 10.1016/s0015-0282(97)00277-x
21. Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann N Y Acad Sci (2002) 955:308–17. doi: 10.1111/j.1749-6632.2002.tb02791.x
22. Vercellini P, Abbiati A, Viganò P, Somigliana ED, Daguati R, Meroni F, et al. Asymmetry in distribution of diaphragmatic endometriotic lesions: Evidence in favour of the menstrual reflux theory. Hum Reprod (2007) 22:2359–67. doi: 10.1093/humrep/dem224
23. Batt RE, Smith RA, Buck GL, Martin DC, Chapron C, Koninckx PR, et al. Müllerianosis. Histol Histopathol (2007) 22:1161–6. doi: 10.14670/HH-22.1161
24. Signorile PG, Baldi F, Bussani R, D’Armiento M, De Falco M, Baldi A. Ectopic endometrium in human foetuses is a common event and sustains the theory of müllerianosis in the pathogenesis of endometriosis, a disease that predisposes to cancer. J Exp Clin Cancer Res (2009) 28:49. doi: 10.1186/1756-9966-28-49
25. Batt RE, Mitwally MFM. Endometriosis from thelarche to midteens: Pathogenesis and prognosis, prevention and pedagogy. J Pediatr Adolesc Gynecol (2003) 16:337–47. doi: 10.1016/j.jpag.2003.09.008
26. Tandoi I, Somigliana E, Riparini J, Ronzoni S, Vigano' P, Candiani M. High rate of endometriosis recurrence in young women. J Pediatr Adolesc Gynecol (2011) 24:376–9. doi: 10.1016/j.jpag.2011.06.012
27. Gruenwald P. Origin of endometriosis from the mesenchyme of the celomic walls. Am J Obstet Gynecol (1942) 44:470–4. doi: 10.1016/S0002-9378(42)90484-8
28. Suginami H. A reappraisal of the coelomic metaplasia theory by reviewing, endometriosis occurring in unusual sites and instances. Am J Obstet Gynecol (1991) 165:214–8. doi: 10.1016/0002-9378(91)90254-o
29. Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod (2006) 21:542–4. doi: 10.1093/humrep/dei344
30. Mok-Lin EY, Wolfberg A, Hollinquist H, Laufer MR. Endometriosis in a patient with Mayer-Rokitansky-Küster-Hauser syndrome and complete uterine agenesis: Evidence to support the theory of coelomic metaplasia. J Pediat Adolesc Gynecol (2010) 23:e35–7. doi: 10.1016/j.jpag.2009.02.010
31. Troncon JK, Zani ACT, Vieira ADD, Poli-Neto OB, Nogueira AA, Rosa-E-Silva JC. Endometriosis in a patient with mayer-rokitansky-küster-hauser syndrome. Case Rep Obstet Gynecol (2014) 2014:376231. doi: 10.1155/2014/376231
32. Asencio FA, Ribeiro HA, Ribeiro PA, Mario M, Adamyan L, Ussia A, et al. Case reports and systematic review of estrogen independent symptomatic postmenopausal endometriosis. Gynecol Surg (2018) 16:3. doi: 10.1186/s10397-019-1056-x
33. Giannarini G, Scott CA, Moro U, Grossetti B, Pomara G, Selli C. Cystic endometriosis of the epididymis. Urology (2006) 68:203.e1–3. doi: 10.1016/j.urology.2006.01.017
34. Jabr FI, Mani V. An unusual cause of abdominal pain in a male patient: Endometriosis. Avicenna J Med (2014) 4:99–101. doi: 10.4103/2231-0770.140660
35. Rei C, Williams T, Feloney M. Endometriosis in a man as a rare source of abdominal pain: A case report and review of the literature. Case Rep Obstet Gynecol (2018) 2018:2083121. doi: 10.1155/2018/2083121
37. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol (1927) 3:93–110.43.
38. Vinatier D, Orazi G, Cosson M, Dufour P. Theories of endometriosis. Eur J Obstet Gynecol Reprod Biol (2001) 96:21–34. doi: 10.1016/s0301-2115(00)00405-x
39. Samani EN, Mamillapalli R, Li F, Mutlu L, Hufnagel D, Krikun G, et al. Micrometastasis of endometriosis to distant organs in a murine model. Oncotarget (2017) 10:2282–91. doi: 10.18632/oncotarget.16889
40. Mechsner S, Weichbrodt M, Riedlinger WF, Bartley J, Kaufmann AM, Schneider A, et al. Estrogen and progestogen receptor positive endometriotic lesions and disseminated cells in pelvic sentinel lymph nodes of patients with deep infiltrating rectovaginal endometriosis: A pilot study. Hum Reprod (2008) 23:2202–9. doi: 10.1093/humrep/den259
41. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA (2004) 292(1):81–5. doi: 10.1001/jama.292.1.81
42. Mints M, Jansson M, Sadeghi B, Westgren M, Uzunel M, Hassan M, et al. Endometrial endothelial cells are derived from donor stem cells in a bone marrow transplant recipient. Hum Reprod (2008) 23:139–43. doi: 10.1093/humrep/dem342
43. Ikoma T, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, et al. Bone marrow-derived cells from male donors can compose endometrial glands in female transplant recipients. Am J Obstet Gynecol (2009) 201(6):608.e1–8. doi: 10.1016/j.ajog.2009.07.026
44. Gargett CE, Schwab KE, Deane JA. Endometrial stem/progenitor cells: The first 10 years. Hum Reprod Update (2016) 22:137–63. doi: 10.1093/humupd/dmv051
45. Leyendecker G, Herbertz M, Kunz G, Mall G. Endometriosis results from the dislocation of basal endometrium. Hum Reprod (2002) 17:2725–36. doi: 10.1093/humrep/17.10.2725
46. Figueira PG, Abrão MS, Krikun G, Taylor HS. Stem cells in endometrium and their role in the pathogenesis of endometriosis. Ann N Y Acad Sci (2011) 1221:10–7. doi: 10.1111/j.1749-6632.2011.05969.x
47. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells (2007) 25:2082–6. doi: 10.1634/stemcells.2006-0828
48. Nezhat C, King LP, Paka C, Odegaard J, Beygui R. Bilateral thoracic endometriosis affecting the lung and diaphragm. JSLS (2012) 16:140–2. doi: 10.4293/108680812X13291597716384
49. Ciriaco P, Muriana P, Carretta A, Ottolina J, Candiani M, Negri G. Catamenial pneumothorax as the first expression of thoracic endometriosis syndrome and pelvic endometriosis. J Clin Med (2022) 11:1200. doi: 10.3390/jcm11051200
50. Hwang SM, Lee CW, Lee BS, Park JH. Clinical features of thoracic endometriosis: A single center analysis. Obstet Gynecol Sci (2015) 58:223–31. doi: 10.5468/ogs.2015.58.3.223
51. Sarma D, Iyengar P, Marotta TR, terBrugge KG, Gentili F, Halliday W. Cerebellar endometriosis. AJR (2004) 182:1543–6. doi: 10.2214/ajr.182.6.1821543
52. Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and gran- ulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem Cells Dev (2012) 21:3324–31. doi: 10.1089/scd.2011.0193
53. Kulak J Jr, Fischer C, Komm B, Taylor HS. Treatment with bazedoxifene, a selective estrogen receptor modulator, causes regression of endometriosis in a mouse model. Endocrinology (2011) 152:3226–32. doi: 10.1210/en.2010-1010
54. Santamaria X, Massasa EE, Taylor HS. Migration of cells from experimental endometriosis to the uterine endometrium. Endocrinology (2012) 153:5566–74. doi: 10.1210/en.2012-1202
55. Kennedy SH, Mardon H, Barlow DH. Familial endometriosis. J Assist Reprod Genet (1995) 12:32–4. doi: 10.1007/BF02214126
56. Hadfield RM, Yudkin PL, Coe CL, Scheffler J, Uno H, Barlow DH, et al. Risk factors for endometriosis in the rhesus monkey (Macaca mulatta): A case- control study. Hum Reprod Update (1997) 3:109–15. doi: 10.1093/humupd/3.2.109
57. Zondervan KT, Weeks DE, Colman R, Cardon LR, Hadfield R, Schleffler J, et al. Familial aggregation of endometriosis in a large pedigree of rhesus macaques. Hum Reprod (2004) 19:448–55. doi: 10.1093/humrep/deh052
58. Hadfield RM, Mardon HJ, Barlow DH, Kennedy SH. Endometriosis in monozygotic twins. Fertil Steril (1997) 68:941–2. doi: 10.1016/s0015-0282(97)00359-2
59. Saha R, Pettersson HJ, Svedberg P, Olovsson M, Bergqvist A, Marions L, et al. Heritability of endometriosis. Fertil Steril (2015) 104:947–52. doi: 10.1016/j.fertnstert.2015.06.035
60. Treloar SA, O’Connor DT, O’Connor VM, Martin NG. Genetic influences on endometriosis in an Australian twin sample. Fertil Steril (1999) 71:701–10. doi: 10.1016/s0015-0282(98)00540-8
61. Dalsgaard T, Hjordt Hansen MV, Hartwell D, Lidegaard O. Reproductive prognosis in daughters of women with and without endometriosis. Hum Reprod (2013) 28:2284–8. doi: 10.1093/humrep/det231
62. Malinak LR, Buttram VC Jr, Elias S, Simpson JL. Heritage aspects of endometriosis. II. clinical characteristics of familial endometriosis. Am J Obstet Gynecol (1980) 137:332–37. doi: 10.1016/0002-9378(80)90918-7
63. Uno S, Kyo S, Maida Y, Ozaki S, Takakura M, Nakao S, et al. A genome-wide association study identifies genetic variants in the CDKN2BAS locus associated with endometriosis in Japanese. Nat Genet (2010) 42:707–10. doi: 10.1038/ng.612
64. Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet (2011) 43:51–4. doi: 10.1038/ng.731
65. Albertsen HM, Chettier R, Farrington P, Ward K. Genome-wide association study link novel loci to endometriosis. PloS One (2013) 8:e58257. doi: 10.1371/journal.pone.0058257
66. Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, et al. Genome-wide association meta-analysis identifies new endometriosis risk loci. Nat Genet (2012) 44:1355–9. doi: 10.1038/ng.2445
67. Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, et al. Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nat Commun (2017) 8:15539. doi: 10.1038/ncomms15539
68. Pagliardini L, Gentilini D, Sanchez AM, Candiani M, Viganò P, Di Blasio AM. Replication and meta-analysis of previous genome-wide association studies confirm vezatin as the locus with the strongest evidence for association with endometriosis. Hum Reprod (2015) 30:987–93. doi: 10.1093/humrep/dev022
69. Montgomery GW, Nyholt DR, Zhao ZZ, Treloar SA, Painter JN, Missmer SA, et al. The search for genes contributing to endometriosis risk. Hum Reprod Update (2008) 14:447–57. doi: 10.1093/humupd/dmn016
70. Bianco B, Loureiro FA, Trevisan CM, Peluso C, Christofolini DM, Montagna E, et al. Effects of FSHR and FSHB variants on hormonal profile and reproductive outcomes of infertile women with endometriosis. Front Endocrinol (Lausanne) (2021) 12:760616. doi: 10.3389/fendo.2021.760616
71. Naqvi H, Ilagan Y, Krikun G, Taylor HS. Altered genome-wide methylation in endometriosis. Reprod Sci (2014) 21:1237–43. doi: 10.1177/1933719114532841
72. Guo SW. Epigenetics of endometriosis. Mol Hum Reprod (2009) 15:587–607. doi: 10.1093/molehr/gap064
73. Nasu K, Kawano Y, Tsukamoto Y, Takano M, Takai N, Li H, et al. Aberrant DNA methylation status of endometriosis: Epigenetics as the pathogenesis, biomarker and therapeutic target. J Obstet Gynaecol Res (2011) 37:683–95. doi: 10.1111/j.1447-0756.2011.01663.x
74. Wu Y, Halverson G, Basir Z, Strawn E, Yan P, Guo SW. Aberrant methylation at HOXA10 may be responsible for its aberrant expression in the endometrium of patients with endometriosis. Am J Obstet Gynecol (2005) 193:371–80. doi: 10.1016/j.ajog.2005.01.034
75. Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod (1999) 14(5):1328–31. doi: 10.1093/humrep/14.5.1328
76. Kulp JL, Mamillapalli R, Taylor HS. Aberrant HOXA10 methylation in patients with common gynecologic disorders: Implications for reproductive outcomes. Reprod Sci (2016) 23:455–63. doi: 10.1177/1933719116630427
77. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Promoter hypermethylation of progesterone receptor isoform b (PR-b) in endometriosis. Epigenetics (2006) 1:106–11. doi: 10.4161/epi.1.2.2766
78. Nie J, Liu X, Guo SW. Promoter hypermethylation of progesterone receptor isoform b (PR-b) in adenomyosis and its rectification by a histone deacetylase inhibitor and a demethylation agent. Reprod Sci (2010) 17:995–1005. doi: 10.1177/1933719110377118
79. Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform a but not b is expressed in endometriosis. J Clin Endocrinol Metab (2000) 85:2897–902. doi: 10.1210/jcem.85.8.6739
80. Rubel CA, Wu SP, Lin L, Wang T, Lanz RB, Li X, et al. A Gata2-dependent transcription network regulates uterine progesterone responsiveness and endometrial function. Cell Rep (2016) 17:1414–25. doi: 10.1016/j.celrep.2016.09.093
81. Xue Q, Lin Z, Yin P, Milad MP, Cheng YH, Confino E, et al. Transcriptional activation of steroidogenic factor-1 by hypomethylation of the 5′ CpG island in endometriosis. J Clin Endocrinol Metab (2007) 92:3261–7. doi: 10.1210/jc.2007-0494
82. Dyson MT, Roqueiro D, Monsivais D, Ercan CM, Pavone ME, Brooks DC, et al. Genome-wide DNA methylation analysis predicts an epigenetic switch for GATA factor expression in endometriosis. PloS Genet (2014) 10:e1004158. doi: 10.1371/journal.pgen.1004158
83. Wu Y, Strawn E, Basir Z, Halverson G, Guo SW. Aberrant expression of deoxyribonucleic acid methyltransferases DNMT1, DNMT3A, and DNMT3B in women with endometriosis. Fertil Steril (2007) 87:24–32. doi: 10.1016/j.fertnstert.2006.05.077
84. Dyson MT, Kakinuma T, Pavone ME, Monsivais D, Navarro A, Malpani SS, et al. Aberrant expression and localization of deoxyribonucleic acid methyltransferase 3B in endometriotic stromal cells. Fertil Steril (2015) 104:953–63. doi: 10.1016/j.fertnstert.2015.06.046
85. Wu Y, Starzinski-Powitz A, Guo SW, Trichostatin A. A histone deacetylase inhibitor, attenuates invasiveness and reactivates e-cadherin expression in immortalized endometriotic cells. Reprod Sci (2007) 14:374–82. doi: 10.1177/1933719107302913
86. Meixell DA, Mamillapalli R, Taylor HS. Methylation of microribonucleic acid let-7b regulatory regions in endometriosis. F S Sci (2022) 3(2):197–203. doi: 10.1016/j.xfss.2022.03.001
87. Kawano Y, Nasu K, Li H, Tsuno A, Abe W, Takai N, et al. Application of the histone deacetylase inhibitors for the treatment of endometriosis: Histone modifications as pathogenesis and novel therapeutic target. Hum Reprod (2011) 26:2486–98. doi: 10.1093/humrep/der203
88. Wilting RH, Yanover E, Heideman MR, Jacobs H, Horner J, van der Torre J, et al. Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J (2010) 29:2586–97. doi: 10.1038/emboj.2010.136
89. Colón-Díaz M, Báez-Vega P, García M, Ruiz A, Monteiro JB, Fourquet J, et al. {HDAC}1 and HDAC 2 are differentially expressed in endometriosis. Reprod Sci (2012) 19:483–92. doi: 10.1177/1933719111432870
90. Kim TH, Yoo JY, Choi KC, Shin JH, Leach RE, Fazleabas AT, et al. Loss of HDAC3 results in nonreceptive endometrium and female infertility. Sci Transl Med (2019) 11:eaaf7533. doi: 10.1126/scitranslmed.aaf7533
91. Samartzis EP, Noske A, Samartzis N, Fink D, Imesch P. The expression of histone deacetylase 1, but not other class I histone deacetylases, is significantly increased in endometriosis. Reprod Sci (2013) 20:1416–22. doi: 10.1177/1933719113488450
92. Monteiro JB, Colón-Díaz M, García M, Gutierrez S, Colón M, Seto E, et al. Endometriosis is characterized by a distinct pattern of histone 3 and histone 4 lysine modifications. Reprod Sci (2014) 21:305–18. doi: 10.1177/1933719113497267
93. La Ferlita A, Battaglia R, Andronico F, Caruso S, Cianci A, Purrello M, et al. Non-coding RNAs in endometrial physiopathology. Int J Mol Sci (2018) 19:2120. doi: 10.3390/ijms19072120
94. Fehlmann T, Ludwig N, Backes C, Meese E, Keller A. Distribution of microRNA biomarker candidates in solid tissues and body fluids. RNA Biol (2016) 13:1084–8. doi: 10.1080/15476286.2016.1234658
95. Santamaria X, Taylor H. MicroRNA and gynecological reproductive diseases. Fertil Steril (2014) 101:1545–51. doi: 10.1016/j.fertnstert.2014.04.044
96. Bjorkman S, Taylor HS. MicroRNAs in endometriosis: Biological function and emerging biomarker candidates. Biol Reprod (2019) 100:1135–46. doi: 10.1093/biolre/ioz014
97. Wei S, Xu H, Kuang Y. Systematic enrichment analysis of microRNA expression profiling studies in endometriosis. Iran J Basic Med Sci (2015) 18:423–9.
98. Haikalis ME, Wessels JM, Leyland NA, Agarwal SK, Foster WG. MicroRNA expression pattern differs depending on endometriosis lesion type. Biol Reprod (2018) 98:623–33. doi: 10.1093/biolre/ioy019
99. Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in microRNA expression in human endometrium. Biol Reprod (2010) 82:791–801. doi: 10.1095/biolreprod.109.081059
100. Sha AG, Liu JL, Jiang XM, Ren JZ, Ma CH, Lei W, et al. Genome-wide identification of micro-ribonucleic acids associated with human endometrial receptivity in natural and stimulated cycles by deep sequencing. Fertil Steril (2011) 96:150.e5–5.e5. doi: 10.1016/j.fertnstert.2011.04.072
101. Yang YM, Yang WX. Epithelial-to-mesenchymal transition in the devel- opment of endometriosis. Oncotarget (2017) 8:41679–89. doi: 10.18632/oncotarget.16472
102. Viganò P, Ottolina J, Bartiromo L, Bonavina G, Schimberni M, Villanacci R, et al. Cellular components contributing to fibrosis in endometriosis: A literature review. J Minim Invasive Gynecol (2020) 27:287–95. doi: 10.1016/j.jmig.2019.11.011
103. Saare M, Rekker K, Laisk-Podar T, Rahmioglu N, Zondervan K, Salumets A, et al. Challenges in endometriosis miRNA studies — From tissue heterogeneity to disease specific miRNAs. Biochim Biophys Acta Mol Basis Dis (2017) 1863:2282–92. doi: 10.1016/j.bbadis.2017.06.018
104. Weber JA, Baxter DH, Zhang S, Huang DY, How Huang K, Jen Lee M, et al. The microRNA spectrum in 12 body fluids. Clin Chem (2010) 56:1733–41. doi: 10.1373/clinchem.2010.147405
105. Panir K, Schjenken JE, Robertson SA, Hull ML. Non-coding RNAs in endometriosis: A narrative review. Hum Reprod Update (2018) 24:497–515. doi: 10.1093/humupd/dmy014
106. Grümmer R. Translational animal models to study endometriosis-associated infertility. Semin Reprod Med (2013) 31(2):125–32. doi: 10.1055/s-0032-1333477
107. Story L, Kennedy S. Animal studies in endometriosis: A review. ILAR J (2004) 45(2):132–8. doi: 10.1093/ilar.45.2.132
108. Dick EJ Jr, Hubbard GB, Martin LJ, Leland MM. Record review of baboons with histologically confirmed endometriosis in a large established colony. J Med Primatol (2003) 32:39–47. doi: 10.1034/j.1600-0684.2003.00008.x
109. TE LINDE RW, SCOTT RB. Experimental endometriosis. Am J Obstet Gynecol (1950) 60:1147–73. doi: 10.1016/0002-9378(50)90517-5
110. D’Hooghe TM, Bambra CS, Suleman MA, Dunselman GA, Evers HL, Koninckx PR. Development of a model of retrograde menstruation in baboons (Papio anubis). Fertil Steril (1994) 62:635–8.
111. Kaplan CR, Eddy CA, Olive DL, Schenken RS. Effect of ovarian endometriosis on ovulation in rabbits. Am J Obstet Gynecol (1989) 160:40–4. doi: 10.1016/0002-9378(89)90083-5
112. Hayashi S, Nakamura T, Motooka Y, Ito F, Jiang L, Akatsuka S, et al. Novel ovarian endometriosis model causes infertility via iron-mediated oxidative stress in mice. Redox Biol (2020) 37:101726. doi: 10.1016/j.redox.2020.101726
113. Grechukhina O, Petracco R, Popkhadze S, Massasa E, Paranjape T, Chan E, et al. A polymorphism in a let-7 microRNA binding site of KRAS in women with endometriosis. EMBO Mol Med (2012) 4:206–17. doi: 10.1002/emmm.201100200
114. Yong PJ, Sadownik L, Brotto LA. Concurrent deep-superficial dyspareunia:prevalence, associations, and outcomes in a multidisciplinary vulvodynia program. J Sex Med (2015) 12:219 –27. doi: 10.1111/jsm.12729
115. Mabrouk M, Del Forno S, Spezzano A, Raimondo D, Arena A, Zanello M, et al. Painful love: Superficial dyspareunia and three dimensional transperineal ultrasound evaluation of pelvic floor muscle in women with endometriosis. J Sex Marital Ther (2020) 46:187–196.14. doi: 10.1080/0092623X.2019.1676852
116. Wahl KJ, Orr NL, Lisonek M, Noga H, Bedaiwy MA, Williams C, et al. Deep dyspareunia, superficial dyspareunia, and infertility concerns among women with endometriosis: A cross-sectional study. Sex Med (2020) 8:274–81. doi: 10.1016/j.esxm.2020.01.002
117. Vercellini P, Meana M, Hummelshoj L, Somigliana E, Vigano` P, Fedele L. Priorities for endometriosis research: A proposed focus on deep dyspareunia. Reprod Sci (2011) 18:114–8. doi: 10.1177/1933719110382921
118. Vercellini P, Somigliana E, Consonni D, Frattaruolo MP, De Giorgi O, Fedele L. Surgical versus medical treatment for endometriosis-associated severe deep dyspareunia: I. effect on pain during intercourse and patient satisfaction. Hum Reprod (2012) 27:3450–9. doi: 10.1093/humrep/des313
119. Denny E, Mann C. Endometriosis-associated dyspareunia: The impact on women’s lives. J Fam Plann Reprod Health Care (2007) 33:189–93. doi: 10.1783/147118907781004831
120. Barbara G, Facchin F, Buggio L, Somigliana E, Berlanda N, Kustermann A, et al. What is known and unknown about the association between endometriosis and sexual functioning: A systematic review of the literature. Reprod Sci (2017) 24:1566–76. doi: 10.1177/1933719117707054
121. Barbara G, Facchin F, Meschia M, Berlanda N, Frattaruolo MP, Vercellini P. When love hurts. A systematic review on the effects of surgical and pharmacological treatments for endometriosis on female sexual functioning. Acta Obstet Gynecol Scand (2017) 96:668–87. doi: 10.1111/aogs.13031
122. Pluchino N, Wenger JM, Petignat P, Tal R, Bolmont M, Taylor HS, et al. Sexual function in endometriosis patients and their partners: Effect of the disease and consequences of treatment. Hum Reprod Update (2016) 22:762–74. doi: 10.1093/humupd/dmw031
123. Schenken RS, Asch RH, Williams RF, Hodgen GD. Etiology of infertility in monkeys with endometriosis: luteinized unruptured follicles, luteal phase defects, pelvic adhesions, and spontaneous abortions. Fertil Steril (1984) 41:122–30. doi: 10.1016/s0015-0282(16)47552-7
124. Gleicher N, Weghofer A, Barad DH. Defining ovarian reserve to better understand ovarian aging. Reprod Biol Endocrinol (2011) 9:23. doi: 10.1186/1477-7827-9-23
125. Nelson SM. Biomarkers of ovarian response: current and future applications. Fertil Steril (2013) 99:963–9. doi: 10.1016/j.fertnstert.2012.11.051
126. Santoro N. Using antimüllerian hormone to predict fertility. JAMA (2017) 318:1333–4. doi: 10.1001/jama.2017.14954
127. Sanchez AM, Papaleo E, Corti L, Santambrogio P, Levi S, Viganò P, et al. Iron availability is increased in individual human ovarian follicles in close proximity to an endometrioma compared with distal ones. Hum Reprod (2014) 29:577–83. doi: 10.1093/humrep/det466
128. Sanchez AM, Viganò P, Somigliana E, Panina-Bordignon P, Vercellini P, Candiani M. The distinguishing cellular and molecular features of the endometriotic ovarian cyst: From pathophysiology to the potential endometrioma-mediated damage to the ovary. Hum Reprod Update (2014) 20:217–30. doi: 10.1093/humupd/dmt053
129. Matsuzaki S, Schubert B. Oxidative stress status in normal ovarian cortex surrounding ovarian endometriosis. Fertil Steril (2010) 93:2431–2. doi: 10.1016/j.fertnstert.2009.08.068
130. Maneschi F, Marasá L, Incandela S, Mazzarese M, Zupi E. Ovarian cortex surrounding benign neoplasms: A histologic study. Am J Obstet Gynecol (1993) 169:388–93. doi: 10.1016/0002-9378(93)90093-x
131. Kitajima M, Dolmans MM, Donnez O, Masuzaki H, Soares M, Donnez J. Enhanced follicular recruitment and atresia in cortex derived from ovaries with endometriomas. Fertil Steril (2014) 101:1031–7. doi: 10.1016/j.fertnstert.2013.12.049
132. Kuroda M, Kuroda K, Arakawa A, Fukumura Y, Kitade M, Kikuchi I, et al. Histological assessment of impact of ovarian endometrioma and laparoscopic cystectomy on ovarian reserve. J Obstet Gynaecol Res (2012) 38:1187–93. doi: 10.1111/j.1447-0756.2012.01845.x
133. Pavone ME, Hirshfeld-Cytron J, Tingen C, Thomas C, Thomas J, Lowe MP, et al. Human ovarian tissue cortex surrounding benign and malignant lesions. Reprod Sci (2014) 2:582–9. doi: 10.1177/1933719113506498
134. Schubert B, Canis M, Darcha C, Artonne C, Pouly JL, Déchelotte P, et al. Human ovarian tissue from cortex surrounding benign cysts: A model to study ovarian tissue cryopreservation. Hum Reprod (2005) 20:1786–92. doi: 10.1093/humrep/dei002
135. Dolmans M-M, Martinez-Madrid B, Gadisseux E, Guiot Y, Yuan WY, Torre A, et al. Short-term transplantation of isolated human ovarian follicles and cortical tissue into nude mice. Reproduction (2007) 134(2):253–62. doi: 10.1530/REP-07-0131
136. Takeuchi A, Koga K, Satake E, Makabe T, Taguchi A, Miyashita M, et al. Endometriosis triggers excessive activation of primordial follicles via PI3K-PTEN-Akt- Foxo3 pathway. J Clin Endocrinol Metab (2019) 104:5547–54. doi: 10.1210/jc.2019-00281
137. Sanchez AM, Somigliana E, Vercellini P, Pagliardini L, Candiani M, Vigano P. Endometriosis as a detrimental condition for granulosa cell steroidogenesis and development: From molecular alterations to clinical impact. J Steroid Biochem Mol Biol (2016) 155:35–46. doi: 10.1016/j.jsbmb.2015.07.023
138. Di Nisio V, Rossi G, Di Luigi G, Palumbo P, D'Alfonso A, Iorio R, et al. Increased levels of proapoptotic markers in normal ovarian cortex surrounding small endometriotic cysts. Reprod Biol (2019) 19:225–9. doi: 10.1016/j.repbio.2019.08.002
139. Thombre Kulkarni M, Shafrir A, Farland LV, Terry KL, Whitcomb BW, Eliassen AH, et al. Association between laparoscopically confirmed endometriosis and risk of early natural menopause. JAMA Netw Open (2022) 5:e2144391. doi: 10.1001/jamanetworkopen.2021.44391
140. Muzii L, Di Tucci C, Di Feliciantonio M, Galati G, Di Donato V, Musella A, et al. Antimüllerian hormone is reduced in the presence of ovarian endometriomas: A systematic review and meta-analysis. Fertil Steril (2018) 110:932–40.e1. doi: 10.1016/j.fertnstert.2018.06.025
141. Tian Z, Zhang Y, Zhang C, Wang Y, Zhu HL. Antral follicle count is reduced in the presence of endometriosis: A systematic review and meta-analysis. Reprod BioMed Online (2021) 42:237–47. doi: 10.1016/j.rbmo.2020.09.014
142. Kasapoglu I, Ata B, Uyaniklar O, Seyhan A, Orhan A, Yildiz Oguz S, et al. Endometrioma-related reduction in ovarian reserve (ERROR): A prospective longitudinal study. Fertil Steril (2018) 110:122–7. doi: 10.1016/j.fertnstert.2018.03.015
143. Gupta S, Agarwal A, Agarwal R, Loret de Mola JR. Impact of ovarian endometrioma on assisted reproduction outcomes. Reprod BioMed Online (2006) 13:349–60. doi: 10.1016/s1472-6483(10)61439-3
144. Hamdan M, Dunselman G, Li TC, Cheong Y. The impact of endometrioma on IVF/ICSI outcomes: A systematic review and meta-analysis. Hum Reprod Update (2015) 21:809–25. doi: 10.1093/humupd/dmv035
145. Alshehre SM, Narice BF, Fenwick MA, Metwally M. The impact of endometrioma on in vitro fertilisation/intra-cytoplasmic injection IVF/ICSI reproductive outcomes: A systematic review and meta-analysis. Arch Gynecol Obstet (2021) 303:3–16. doi: 10.1007/s00404-020-05796-9
146. Yang C, Geng Y, Li Y, Chen C, Gao Y. Impact of ovarian endometrioma on ovarian responsiveness and IVF: A systematic review and meta-analysis. Reprod BioMed Online (2015) 31:9–19. doi: 10.1016/j.rbmo.2015.03.005
147. Benaglia L, Pasin R, Somigliana E, Vercellini P, Ragni G, Fedele L. Unoperated ovarian endometriomas and responsiveness to hyperstimulation. Hum Reprod (2011) 26:1356–61. doi: 10.1016/j.fertnstert.2013.01.110
148. Almog B, Shehata F, Sheizaf B, Tan SL, Tulandi T. Effects of ovarian endometrioma on the number of oocytes retrieved for in vitro fertilization. Fertil Steril (2011) 95:525–7. doi: 10.1016/j.fertnstert.2010.03.011
149. Esinler I, Bozdag G, Arikan I, Demir B, Yarali H. Endometrioma ≤3 cm in diameter per se does not affect ovarian reserve in intracytoplasmic sperm injection cycles. Gynecol Obstet Invest (2012) 74:261–4. doi: 10.1159/000339630
150. Filippi F, Benaglia L, Paffoni A, Restelli L, Vercellini P, Somigliana E, et al. Ovarian endometriomas and oocyte quality: Insights from in vitro fertilization cycles. Fertil Steril (2014) 101:988–93.e1. doi: 10.1016/j.fertnstert.2014.01.008
151. Benaglia L, Bermejo A, Somigliana E, Faulisi S, Ragni G, Fedele L, et al. In vitro fertilization outcome in women with unoperated bilateral endometriomas. Fertil Steril (2013) 99:1714–9. doi: 10.1016/j.fertnstert.2013.01.110
152. Ferrero S, Scala C, Tafi E, Racca A, Venturini PL, Leone Roberti Maggiore U. Impact of large ovarian endometriomas on the response to superovulation for in vitro fertilization: A retrospective study. Eur J Obstet Gynecol Reprod Biol (2017) 213:17–21. doi: 10.1016/j.ejogrb.2017.04.003
153. Karadağ C, Yoldemir T, Demircan Karadağ S, Turgut A. The effects of endometrioma size and bilaterality on ovarian reserve. J Obstet Gynaecol (2020) 40:531–6. doi: 10.1080/01443615.2019.1633518
154. Muzii L, Bianchi A, Crocè C, Manci N, Panici PB. Laparoscopic excision of ovarian cysts: Is the stripping technique a tissue-sparing procedure? Fertil Steril (2002) 77:609–14. doi: 10.1016/s0015-0282(01)03203-4
155. Hachisuga T, Kawarabayashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod (2002) 17:432–5. doi: 10.1093/humrep/17.2.432
156. Roman H, Tarta O, Pura I, Opris I, Bourdel N, Marpeau L, et al. Direct proportional relationship between endometrioma size and ovarian parenchyma inadvertently removed during cystectomy, and its implication on the management of enlarged endometriomas. Hum Reprod (2010) 25:1428–32. doi: 10.1093/humrep/deq069
157. Muzii L, Marana R, Angioli R, Bianchi A, Cucinella G, Vignali M, et al. Histologic analysis of specimens from laparoscopic endometrioma excision performed by different surgeons: Does the surgeon matter? Fertil Steril (2011) 95:2116–9. doi: 10.1016/j.fertnstert.2011.02.034
158. Biacchiardi CP, Piane LD, Camanni M, Deltetto F, Delpiano EM, Marchino GL, et al. Laparoscopic stripping of endometriomas negatively affects ovarian follicular reserve even if performed by experienced surgeons. Reprod BioMed Online (2011) 23:740–6. doi: 10.1016/j.rbmo.2011.07.014
159. Garcia-Velasco JA, Somigliana E. Management of endometriomas in women requiring IVF: To touch or not to touch. Hum Reprod (2009) 24:496–501. doi: 10.1093/humrep/den398
160. Somigliana E, Berlanda N, Benaglia L, Viganò P, Vercellini P, Fedele L. Surgical excision of endometriomas and ovarian reserve: A systematic review on serum antimüllerian hormone level modifications. Fertil Steril (2012) 98:1531–8. doi: 10.1016/j.fertnstert.2012.08.009
161. Raffi F, Metwally M, Amer S. The impact of excision of ovarian endometrioma on ovarian reserve: A systematic review and meta-analysis. J Clin Endocrinol Metab (2012) 97:3146–54. doi: 10.1210/jc.2012-1558
162. Younis JS, Shapso N, Ben-Sira Y, Nelson SM, Izhaki I. Endometrioma surgery-a systematic review and meta-analysis of the effect on antral follicle count and anti-müllerian hormone. Am J Obstet Gynecol (2022) 226:33–51.e7. doi: 10.1016/j.ajog.2021.06.102
163. Nankali A, Kazeminia M, Jamshidi PK, Shohaimi S, Salari N, Mohammadi M, et al. The effect of unilateral and bilateral laparoscopic surgery for endometriosis on anti-mullerian hormone (AMH) level after 3 and 6 months: A systematic review and meta-analysis. Health Qual Life Outcomes (2020) 18:314. doi: 10.1186/s12955-020-01561-3
164. Moreno-Sepulveda J, Romeral C, Niño G, Pérez-Benavente A. The effect of laparoscopic endometrioma surgery on anti-müllerian hormone: A systematic review of the literature and meta-analysis. JBRA Assist Reprod (2022) 26:88–104. doi: 10.5935/1518-0557.20210060
165. Younis JS, Shapso N, Fleming R, Ben-Shlomo I, Izhaki I. Impact of unilateral versus bilateral ovarian endometriotic cystectomy on ovarian reserve: A systematic review and meta-analysis. Hum Reprod Update (2019) 25:375–91. doi: 10.1093/humupd/dmy049
166. Muzii L, Di Tucci C, Di Feliciantonio M, Marchetti C, Perniola G, Panici PB. The effect of surgery for endometrioma on ovarian reserve evaluated by antral follicle count: A systematic review and meta-analysis. Hum Reprod (2014) 29:2190–8. doi: 10.1093/humrep/deu199
167. Wu CQ, Albert A, Alfaraj S, Taskin O, Alkusayer GM, Havelock J, et al. Live birth rate after surgical and expectant management of endometriomas after In vitro fertilization: A systematic review, meta-analysis, and critical appraisal of current guidelines and previous meta-analyses. J Minim Invasive Gynecol (2019) 26:299–311.e3. doi: 10.1016/j.jmig.2018.08.029
168. Tao X, Chen L, Ge S, Cai L. Weigh the pros and cons to ovarian reserve before stripping ovarian endometriomas prior to IVF/ICSI: A meta-analysis. PloS One (2017) 12:e0177426. doi: 10.1371/journal.pone.0177426
169. Nickkho-Amiry M, Savant R, Majumder K, Edi-O'sagie E, Akhtar M. The effect of surgical management of endometrioma on the IVF/ICSI outcomes when compared with no treatment? a systematic review and meta-analysis. Arch Gynecol Obstet (2018) 297:1043–57. doi: 10.1007/s00404-017-4640-1
170. Ban Frangež H, Vrtacnik Bokal E, Štimpfel M, Divjak Budihna T, Gulino FA, Garzon S, et al. Reproductive outcomes after laparoscopic surgery in infertile women affected by ovarian endometriomas, with or without in vitro fertilisation: Results from the SAFE (surgery and ART for endometriomas) trial. J Obstet Gynaecol (2022) 42(5):1293–300. doi: 10.1080/01443615.2021.1959536
171. Dongye H, Ji X, Ma X, Song J, Yan L. The impact of endometriosis on embryo quality in in-vitro Fertilization/Intracytoplasmic sperm injection: A systematic review and meta-analysis. Front Med (Lausanne) (2021) 8:669342. doi: 10.3389/fmed.2021.669342
172. Barnhart K, Dunsmoor-Su R, Coutifaris C. Effect of endometriosis on in vitro fertilization. Fertil Steril (2002) 77:1148–55. doi: 10.1016/s0015-0282(02)03112-6
173. Harb HM, Gallos ID, Chu J, Harb M, Coomarasamy A. The effect of endometriosis on in vitro fertilisation outcome: A systematic review and meta-analysis. BJOG (2013) 120:1308–20. doi: 10.1111/1471-0528.12366
174. Hamdan M, Omar SZ, Dunselman G, Cheong Y. Influence of endometriosis on assisted reproductive technology outcomes: A systematic review and meta-analysis. Obstet Gynecol (2015) 125:79–88. doi: 10.1097/AOG.0000000000000592
175. Barbosa MA, Teixeira DM, Navarro PA, Ferriani RA, Nastri CO, Martins WP. Impact of endometriosis and its staging on assisted reproduction outcome: systematic review and meta-analysis. Ultrasound Obstet Gynecol (2014) 44:261–78. doi: 10.1002/uog.13366
176. Freis A, Dietrich JE, Binder M, Holschbach V, Strowitzki T, Germeyer A. Relative morphokinetics assessed by time-lapse imaging are altered in embryos from patients with endometriosis. Reprod Sci (2018) 25:1279–85. doi: 10.1177/1933719117741373
177. Díaz I, Navarro J, Blasco L, Simón C, Pellicer A, Remohí J. Impact of stage III-IV endometriosis on recipients of sibling oocytes: Matched case-control study. Fertil Steril (2000) 74:31–4. doi: 10.1016/s0015-0282(00)00570-7
178. Simón C, Gutiérrez A, Vidal A, de los Santos MJ, Tarín JJ, Remohí J, et al. Outcome of patients with endometriosis in assisted reproduction: Results from in-vitro fertilization and oocyte donation. Hum Reprod (1994) 9:725–9. doi: 10.1093/oxfordjournals.humrep.a138578
179. Remohí J, Ardiles G, García-Velasco JA, Gaitán P, Simón C, Pellicer A. Endometrial thickness and serum oestradiol concentrations as predictors of outcome in oocyte donation. Hum Reprod (1997) 12:2271–6. doi: 10.1093/humrep/12.10.2271
180. Gomes FM, Navarro PA, de Abreu LG, Ferriani RA, dos Reis RM, de Moura MD. Effect of peritoneal fluid from patients with minimal/mild endometriosis on progesterone release by human granulosa-lutein cells obtained from infertile patients without endometriosis: A pilot study. Eur J Obstet Gynecol Reprod Biol (2008) 138:60–5. doi: 10.1016/j.ejogrb.2007.12.008
181. Skrzypczak J. Morphology and steroidogenesis of cultured granulosa cells from endometrioidally changed ovaries. Exp Clin Endocrinol Diabetes (1995) 103:228–32. doi: 10.1055/s-0029-1211355
182. Du YB, Gao MZ, Shi Y, Sun ZG, Wang J. Endocrine and inflammatory factors and endometriosis-associated infertility in assisted reproduction techniques. Arch Gynecol Obstet (2013) 287:123–30. doi: 10.1007/s00404-012-2567-0
183. Regiani T, Cordeiro FB, da Costa Ldo V, Salgueiro J, Cardozo K, Carvalho VM, et al. Follicular fluid alterations in endometriosis: label-free proteomics by MS(E) as a functional tool for endometriosis. Syst Biol Reprod Med (2015) 61:263–76. doi: 10.3109/19396368.2015.1037025
184. Da Broi MG, de Albuquerque FO, de Andrade AZ, Cardoso RL, Jordão Junior AA, Navarro PA. Increased concentration of 8-hydroxy-2'-deoxyguanosine in follicular fluid of infertile women with endometriosis. Cell Tissue Res (2016) 366:231–42. doi: 10.1007/s00441-016-2428-4
185. Da Broi MG, Malvezzi H, Paz CC, Ferriani RA, Navarro PA. Follicular fluid from infertile women with mild endometriosis may compromise the meiotic spindles of bovine metaphase II oocytes. Hum Reprod (2014) 29:315–23. doi: 10.1093/humrep/det378
186. Nasiri N, Moini A, Eftekhari-Yazdi P, Karimian L, Salman-Yazdi R, Arabipoor A. Oxidative stress statues in serum and follicular fluid of women with endometriosis. Cell J (2017) 18:582–7. doi: 10.22074/cellj.2016.4724
187. Prieto L, Quesada JF, Cambero O, Pacheco A, Pellicer A, Codoceo R, et al. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil Steril (2012) 98:126–30. doi: 10.1016/j.fertnstert.2012.03.052
188. Giacomini E, Sanchez AM, Sarais V, Beitawi SA, Candiani M, Viganò P. Characteristics of follicular fluid in ovaries with endometriomas. Eur J Obstet Gynecol Reprod Biol (2017) 209:34–8. doi: 10.1016/j.ejogrb.2016.01.032
189. Paffoni A, Bolis V, Ferrari S, Benaglia L, Vercellini P, Somigliana E. The gametotoxic effects of the endometrioma content: Insights from a parthenogenetic human model. Reprod Sci (2019) 26:573–9. doi: 10.1177/1933719118777637
190. Singh AK, Dutta M, Chattopadhyay R, Chakravarty B, Chaudhury K. Intrafollicular interleukin-8, interleukin-12, and adrenomedullin are the promising prognostic markers of oocyte and embryo quality in women with endometriosis. J Assist Reprod Genet (2016) 33:1363–72. doi: 10.1007/s10815-016-0782-5
191. Choi YS, Cho S, Seo SK, Park JH, Kim SH, Lee BS. Alteration in the intrafollicular thiol-redox system in infertile women with endometriosis. Reproduction (2015) 149:155–62. doi: 10.1530/REP-14-0438
192. Xu H, Schultze-Mosgau A, Agic A, Diedrich K, Taylor RN, Hornung D. Regulated upon activation, normal T cell expressed and secreted (RANTES) and monocyte chemotactic protein 1 in follicular fluid accumulate differentially in patients with and without endometriosis undergoing in vitro fertilization. Fertil Steril (2006) 86:1616–20. doi: 10.1016/j.fertnstert.2006.05.043
193. Mansour G, Abdelrazik H, Sharma RK, Radwan E, Falcone T, Agarwal A. L-carnitine supplementation reduces oocyte cytoskeleton damage and embryo apoptosis induced by incubation in peritoneal fluid from patients with endometriosis. Fertil Steril (2009) 91:2079–86. doi: 10.1016/j.fertnstert.2008.02.097
194. Mansour G, Sharma RK, Agarwal A, Falcone T. Endometriosis-induced alterations in mouse metaphase II oocyte microtubules and chromosomal alignment: A possible cause of infertility. Fertil Steril (2010) 94:1894–9. doi: 10.1016/j.fertnstert.2009.09.043
195. Jianini BTGM, Giorgi VS, Da Broi MG, de Paz CC, Rosa E Silva JC, Ferriani RA, et al. Peritoneal fluid from infertile women with Minimal/Mild endometriosis compromises the meiotic spindle of metaphase II bovine oocytes. Reprod Sci (2017) 24:1304–11. doi: 10.1177/1933719116687658
196. Hahn DW, Carraher RP, Foldesy RG, McGuire JL. Experimental evidence for failure to implant as a mechanism of infertility associated with endometriosis. Am J Obstet Gynecol (1986) 155:1109–13. doi: 10.1016/0002-9378(86)90360-1
197. Steinleitner A, Lambert H, Kazensky C, Danks P. Peritoneal fluid from endometriosis patients affects reproductive outcome in an in vivo model. Fertil Steril (1990) 53(5):926–9. doi: 10.1016/s0015-0282(16)53533-x
198. Illera MJ, Juan L, Stewart CL, Cullinan E, Ruman J, Lessey BA. Effect of peritoneal fluid from women with endometriosis on implantation in the mouse model. Fertil Steril (2000) 74:41–8. doi: 10.1016/s0015-0282(00)00552-5
199. Aeby TC, Huang T, Nakayama RT. The effect of peritoneal fluid from patients with endometriosis on human sperm function in vitro. Am J Obstet Gynecol (1996) 174:1779–83. doi: 10.1016/S0002-9378(96)70210-7
200. Liu Y, Luo L, Zhao H. Changes of cytokines levels in peritoneal fluids of patients with endometriosis and its effect on reproductive activity. J Tongji Med Univ (2000) 20:163–5. doi: 10.1007/BF02887062
201. Mansour G, Aziz N, Sharma R, Falcone T, Goldberg J, Agarwal A. The impact of peritoneal fluid from healthy women and from women with endometriosis on sperm DNA and its relationship to the sperm deformity index. Fertil Steril (2009) 92:61–7. doi: 10.1016/j.fertnstert.2008.05.048
202. Said TM, Agarwal A, Falcone T, Sharma RK, Bedaiwy MA, Li L. Infliximab may reverse the toxic effects induced by tumor necrosis factor alpha in human spermatozoa: An in vitro model. Fertil Steril (2005) 83:1665–73. doi: 10.1016/j.fertnstert.2004.11.068
203. Oral E, Arici A, Olive DL, Huszar G. Peritoneal fluid from women with moderate or severe endometriosis inhibits sperm motility: The role of seminal fluid components. Fertil Steril (1996) 66:787–92. doi: 10.1016/s0015-0282(16)58637-3
204. Reeve L, Lashen H, Pacey AA. Endometriosis affects sperm-endosalpingeal interactions. Hum Reprod (2005) 20:448–51. doi: 10.1093/humrep/deh606
205. Arumugam K. Endometriosis and infertility: Raised iron concentration in the peritoneal fluid and its effect on the acrosome reaction. Hum Reprod (1994) 9:1153–7. doi: 10.1093/oxfordjournals.humrep.a138649
206. Benaglia L, Somigliana E, Vercellini P, Abbiati A, Ragni G, Fedele L. Endometriotic ovarian cysts negatively affect the rate of spontaneous ovulation. Hum Reprod (2009) 24:2183–6. doi: 10.1093/humrep/dep202
207. Leone Roberti Maggiore U, Scala C, Venturini PL, Remorgida V, Ferrero S. Endometriotic ovarian cysts do not negatively affect the rate of spontaneous ovulation. Hum Reprod (2015) 30:299–307. doi: 10.1093/humrep/deu308
208. Dmowski WP, Rao R, Scommegna A. The luteinized unruptured follicle syndrome and endometriosis. Fertil Steril (1980) 33:30–4. doi: 10.1016/s0015-0282(16)44473-0
209. Cahill DJ, Hull MG. Pituitary-ovarian dysfunction and endometriosis. Hum Reprod Update (2000) 6:56–66. doi: 10.1093/humupd/6.1.56
210. Schenken RS, Werlin LB, Williams RF, Prihoda TJ, Hodgen GD. Histologic and hormonal documentation of the luteinized un-ruptured follicle syndrome. Am J Obstet Gynecol (1986) 154(4):839–47. doi: 10.1016/0002-9378(86)90469-2
211. Moon CE, Bertero MC, Curry TE, London SN, Muse KN, Sharpe KL, et al. The presence of luteinized unruptured follicle syndrome and altered folliculogenesis in rats with surgically induced endometriosis. Am J Obstet Gynecol (1993) 169:676–82. doi: 10.1016/0002-9378(93)90642-v
212. Pal AK, Biswas S, Goswami SK, Kabir SN. Effect of pelvic endometrial implants on overall reproductive functions of female rats. Biol Reprod (1999) 60:954–8. doi: 10.1095/biolreprod60.4.954
213. Horton J, Sterrenburg M, Lane S, Maheshwari A, Li TC, Cheong Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum Reprod Update (2019) 25:592–632. doi: 10.1093/humupd/dmz012
214. Pellicer A, Oliveira N, Ruiz A, Remohí J, Simon C. Exploring the mechanism(s) of endometriosis-related infertility: An analysis of embryo development and implantation in assisted reproduction. Hum Reprod (1995) 10:91–7. doi: 10.1093/humrep/10
215. Arici A, Oral E, Bukulmez O, Duleba A, Olive DL, Jones EE. The effect of endometriosis on implantation: Results from the Yale university in vitro fertilization and embryo transfer program. Fertil Steril (1996) 65:603–7. doi: 10.1016/s0015-0282(16)58162-x
216. Miravet-Valenciano J, Ruiz-Alonso M, Gómez E, Garcia-Velasco JA. Endometrial receptivity in eutopic endometrium in patients with endometriosis: It is not affected, and let me show you why. Fertil Steril (2017) 108:28–31. doi: 10.1016/j.fertnstert.2017.06.002
217. Lessey BA, Kim JJ. Endometrial receptivity in the eutopic endometrium of women with endometriosis: It is affected, and let me show you why. Fertil Steril (2017) 108(1):19–27. doi: 10.1016/j.fertnstert.2017.05.031
218. Bishop LA, Gunn J, Jahandideh S, Devine K, Decherney AH, Hill MJ. Endo- metriosis does not impact live-birth rates in frozen embryo transfers of euploid blastocysts. Fertil Steril (2021) 115:416–22. doi: 10.1016/j.fertnstert.2020.07.050
219. Kamath MS, Subramanian V, Antonisamy B, Sunkara SK. Endometriosis and oocyte quality: An analysis of 13 614 donor oocyte recipient and autologous IVF cycles. Hum Reprod Open (2022) 2022:hoac025. doi: 10.1093/hropen/hoac025
220. May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: A systematic review of putative biomarkers. Hum Reprod Update (2011) 17:637–53. doi: 10.1093/humupd/dmr013
221. Aghajanova L, Velarde MC, Giudice LC. Altered gene expression profiling in endometrium: Evidence for progesterone resistance. Semin Reprod Med (2010) 28:51–8. doi: 10.1055/s-0029-1242994
222. Igarashi TM, Bruner-Tran KL, Yeaman GR, Lessey BA, Edwards DP, Eisenberg E, et al. Reduced expression of progesterone receptor-b in the endometrium of women with endometriosis and in cocultures of endometrial cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Fertil Steril (2005) 84:67–74. doi: 10.1016/j.fertnstert.2005.01.113
223. Park JS, Lee JH, Kim M, Chang HJ, Hwang KJ, Chang KH. Endometrium from women with endometriosis shows increased proliferation activity. Fertil Steril (2009) 92:1246–9. doi: 10.1016/j.fertnstert.2009.04.025
224. Fazleabas AT. Progesterone resistance in a baboon model of endometriosis. Semin Reprod Med (2010) 28:75–80. doi: 10.1055/s-0029-1242997
225. Jones CJ, Denton J, Fazleabas AT. Morphological and glycosylation changes associated with the endometrium and ectopic lesions in a baboon model of endometriosis. Hum Reprod (2006) 21:3068–80. doi: 10.1093/humrep/del310
226. Al-Sabbagh M, Lam EW, Brosens JJ. Mechanisms of endometrial progesterone resistance. Mol Cell Endocrinol (2012) 358:208–15. doi: 10.1016/j.mce.2011.10.035
227. Patel BG, Rudnicki M, Yu J, Shu Y, Taylor RN. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet Gynecol Scand (2017) 96:623–32. doi: 10.1111/aogs.13156
228. Bedaiwy MA, Dahoud W, Skomorovska-Prokvolit Y, Yi L, Liu JH, Falcone T, et al. Abundance and localization of progesterone receptor isoforms in endometrium in women with and without endometriosis and in peritoneal and ovarian endometriotic implants. Reprod Sci (2015) 22:1153–61. doi: 10.1177/1933719115585145
229. Wölfler MM, Küppers M, Rath W, Buck VU, Meinhold-Heerlein I, Classen-Linke I. Altered expression of progesterone receptor isoforms a and b in human eutopic endometrium in endometriosis patients. Ann Anat (2016) 206:1–6. doi: 10.1016/j.aanat.2016.03.004
230. Pei T, Liu C, Liu T, Xiao L, Luo B, Tan J, et al. miR-194-3p represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Endocrinology (2018) 159:2554–62. doi: 10.1210/en.2018-00374
231. Moberg C, Bourlev V, Ilyasova N, Olovsson M. Levels of oestrogen receptor, progesterone receptor and αB-crystallin in eutopic endometrium in relation to pregnancy in women with endometriosis. Hum Fertil (Camb) (2015) 18:30–7. doi: 10.3109/14647273.2014.922705
232. Lessey BA, Palomino WA, Apparao KB, Young SL, Lininger RA. Estrogen receptor-alpha (ER-alpha) and defects in uterine receptivity in women. Reprod Biol Endocrinol (2006) 4 Suppl:S9. doi: 10.1186/1477-7827-4-S1-S9
233. Osiński M, Wirstlein P, Wender-Ożegowska E, Mikołajczyk M, Jagodziński PP, Szczepańska M. HSD3B2, HSD17B1, HSD17B2, ESR1, ESR2 and AR expression in infertile women with endometriosis. Ginekol Pol (2018) 89:125–34. doi: 10.5603/GP.a2018.0022
234. Coutifaris C, Myers ER, Guzick DS, Diamond MP, Carson SA, Legro RS, et al. Histological dating of timed endometrial biopsy tissue is not related to fertility status. Fertil Steril (2004) 82:1264–72. doi: 10.1016/j.fertnstert.2004.03.069
235. Murray MJ, Meyer WR, Zaino RJ, Lessey BA, Novotny DB, Ireland K, et al. A critical analysis of the accuracy, reproducibility, and clinical utility of histologic endometrial dating in fertile women. Fertil Steril (2004) 81:1333–43. doi: 10.1016/j.fertnstert.2003.11.030
236. Gonen Y, Casper RF. Prediction of implantation by the sonographic appearance of the endometrium during controlled ovarian stimulation for in vitro fertilization (IVF). J In Vitro Fert Embryo Transf (1990) 7:146–52. doi: 10.1007/BF01135678
237. Isaacs JD Jr, Wells CS, Williams DB, Odem RR, Gast MJ, Strickler RC. Endometrial thickness is a valid monitoring parameter in cycles of ovulation induction with menotropins alone. Fertil Steril (1996) 65:262–6. doi: 10.1016/s0015-0282(16)58082-0
238. Oliveira JB, Baruffi RL, Mauri AL, Petersen CG, Borges MC, Franco JG Jr. Endometrial ultrasonography as a predictor of pregnancy in an in-vitro fertilization programme after ovarian stimulation and gonadotrophin-releasing hormone and gonadotrophins. Hum Reprod (1997) 12:2515–8. doi: 10.1093/humrep/12.11.2515
239. Reuter KL, Cohen S, Furey L, Baker S. Sonographic appearance of the endometrium and ovaries during cycles stimulated with human menopausal gonadotropin. J Reprod Med (1996) 41:509–14.
240. Momeni M, Rahbar MH, Kovanci E. A meta-analysis of the relationship between endometrial thickness and outcome of in vitro fertilization cycles. J Hum Reprod Sci (2011) 4:130–7. doi: 10.4103/0974-1208.92287
241. Kao LC, Germeyer A, Tulac S, Lobo S, Yang JP, Taylor RN, et al. Expression profiling of endometrium from women with endometriosis reveals candidate genes for disease-based implantation failure and infertility. Endocrinology (2003) 144:2870–81. doi: 10.1210/en.2003-0043
242. Du H, Taylor HS. The role of hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harb Perspect Med (2015) 6:a023002. doi: 10.1101/cshperspect.a023002
243. Zanatta A, Rocha AM, Carvalho FM, Pereira RM, Taylor HS, Motta EL, et al. The role of the Hoxa10/HOXA10 gene in the etiology of endometriosis and its related infertility: A review. J Assist Reprod Genet (2010) 27:701–10. doi: 10.1007/s10815-010-9471-y
244. Gendron RL, Paradis H, Hsieh-Li HM, Lee DW, Potter SS, Markoff E. Abnormal uterine stromal and glandular function associated with maternal reproductive defects in hoxa-11 null mice. Biol Reprod (1997) 56:1097–105. doi: 10.1095/biolreprod56.5.1097
245. Esfandiari F, Favaedi R, Heidari-Khoei H, Chitsazian F, Yari S, Piryaei A, et al. Insight into epigenetics of human endometriosis organoids: DNA methylation analysis of HOX genes and their cofactors. Fertil Steril (2021) 115:125–37. doi: 10.1016/j.fertnstert.2020.08.1398
246. Kim JJ, Taylor HS, Lu Z, Ladhani O, Hastings JM, Jackson KS, et al. Altered expression of HOXA10 in endometriosis: Potential role in decidualization. Mol Hum Reprod (2007) 13:323–32. doi: 10.1093/molehr/gam005
247. Daftary GS, Taylor HS. EMX2 gene expression in the female reproductive tract and aberrant expression in the endometrium of patients with endometriosis. J Clin Endocrinol Metab (2004) 89:2390–6. doi: 10.1210/jc.2003-031389
248. Lessey BA, Castelbaum AJ, Sawin SW, Buck CA, Schinnar R, Bilker W, et al. Aberrant integrin expression in the endometrium of women with endometriosis. J Clin Endocrinol Metab (1994) 79:643–9. doi: 10.1210/jcem.79.2.7519194
249. Joshi NR, Kohan-Ghadr HR, Roqueiro DS, Yoo JY, Fru K, Hestermann E, et al. Genetic and epigenetic changes in the eutopic endometrium of women with endometriosis: Association with decreased endometrial αvβ3 integrin expression. Mol Hum Reprod (2021) 27:gaab018. doi: 10.1093/molehr/gaab018
250. Shih AJ, Adelson RP, Vashistha H, Khalili H, Nayyar A, Puran R, et al. Single-cell analysis of menstrual endometrial tissues defines phenotypes associated with endometriosis. BMC Med (2022) 20:315. doi: 10.1186/s12916-022-02500-3
251. Klemmt PA, Carver JG, Kennedy SH, Koninckx PR, Mardon HJ. Stromal cells from endometriotic lesions and endometrium from women with endometriosis have reduced decidualization capacity. Fertil Steril (2006) 85:564–72. doi: 10.1016/j.fertnstert.2005.08.046
252. Yin X, Pavone ME, Lu Z, Wei J, Kim JJ. Increased activation of the PI3K/AKT pathway compromises decidualization of stromal cells from endometriosis. J Clin Endocrinol Metab (2012) 97:E35–43. doi: 10.1210/jc.2011-1527
253. Osteen KG, Bruner-Tran KL, Eisenberg E. Reduced progesterone action during endometrial maturation: A potential risk factor for the development of endometriosis. Fertil Steril (2005) 83:529–37. doi: 10.1016/j.fertnstert.2004.11.026
254. Ahn JI, Yoo JY, Kim TH, Kim YI, Ferguson SD, Fazleabas AT, et al. cAMP- response element–binding 3–like protein 1 (CREB3L1) is required for de- cidualization and its expression is decreased in women with endometriosis. Curr Mol Med (2016) 16:276–87. doi: 10.2174/1566524016666160225153659
255. Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroido- genic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol Reprod (2008) 80:105–14. doi: 10.1095/biolreprod.108.070300
256. Su RW, Strug MR, Joshi NR, Jeong JW, Miele L, Lessey BA, et al. Decreased notch pathway signaling in the endometrium of women with endometriosis impairs decidualization. J Clin Endocrinol Metab (2015) 100:E433–42. doi: 10.1210/jc.2014-3720
257. Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H. Phosphatidylinositol 3-kinase/AKT–mediated activation of es- trogen receptor alpha: A new model for antiestrogen resistance. J Biol Chem (2001) 276:9817–24. doi: 10.1074/jbc.M010840200
258. Sanchez M, Sauve K, Picard N, Tremblay A. The hormonal response of es- trogen receptor beta is decreased by the phosphatidylinositol 3-kinase/Akt pathway via a phosphorylation-dependent release of CREB-binding protein. J Biol Chem (2007) 282:4830–40. doi: 10.1074/jbc.M607908200
259. Vallvé-Juanico J, Houshdaran S, Giudice LC. The endometrial immune environment of women with endometriosis. Hum Reprod Update (2019) 25:564–91. doi: 10.1093/humupd/dmz018
260. Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod (2009) 24:325–32. doi: 10.1093/humrep/den393
261. Khan KN, Masuzaki H, Fujishita A, Kitajima M, Sekine I, Ishimaru T. Differential macrophage infiltration in early and advanced endometriosis and adjacent peritoneum. Fertil Steril (2004) 81:652–61. doi: 10.1016/j.fertnstert.2003.07.037
262. Takebayashi A, Kimura F, Kishi Y, Ishida M, Takahashi A, Yamanaka A, et al. Subpopulations of macrophages within eutopic endometrium of endometriosis patients. Am J Reprod Immunol (2015) 73:221–31. doi: 10.1111/aji.12331
263. Giuliani E, Parkin KL, Lessey BA, Young SL, Fazleabas AT. Characterization of uterine NK cells in women with infertility or recurrent pregnancy loss and associated endometriosis. Am J Reprod Immunol (2014) 72:262–9. doi: 10.1111/aji.1225
264. Odukoya O, Bansal A, Cooke I. Serum endometrial IgG antibodies and soluble CD23 concentrations in patients with endometriosis. Acta Obstet Gynecol Scand (1996) 75:927–31. doi: 10.3109/00016349609055030
265. Hey-Cunningham AJ, Riaz A, Fromm PD, Kupresanin F, Markham R, McGuire HM. Circulating and endometrial regulatory T cell and related populations in endometriosis and infertility: Endometriosis is associated with blunting of endometrial cyclical effects and reduced proportions in moderate-severe disease. Reprod Sci (2022) 29:229–42. doi: 10.1007/s43032-021-00658-4
266. Capezzuoli T, Vannuccini S, Fantappie F, Orlandi G, Rizzello F, Coccia ME, et al. Ultrasound findings in infertile & women with endometriosis: Evidence of concomitant uterine disorders. Gynecol Endocrinol (2020) 36:808–812. doi: 10.1080/09513590.2020.1736027
267. Kunz G, Beil D, Huppert P, Noe M, Kissler S, Leyendecker G. Adenomyosis in endometriosis–prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum Reprod (2005) 20:2309–16. doi: 10.1093/humrep/dei021
268. Barrier BF, Malinowski MJ, Dick EJ Jr, Hubbard GB, Bates GW. Adenomyosis in the baboon is associated with primary infertility. Fertil Steril; (2004) 82:1091–4. doi: 10.1016/j.fertnstert.2003.11.065
269. Campo S, Campo V, Benagiano G. Adenomyosis and infertility. Reprod BioMed Online (2012) 24(1):35–46. doi: 10.1016/j.rbmo.2011.10.003
270. Benagiano G, Brosens I, Habiba M. Structural and molecular features of the endomyometrium in endometriosis and adenomyosis. Hum Reprod Update (2014) 20(3):386–402. doi: 10.1093/humupd/dmt052
271. Fischer CP, Kayisili U, Taylor HS. HOXA10 expression is decreased in endometrium of women with adenomyosis. Fertil Steril (2011) 95(3):1133–6. doi: 10.1016/j.fertnstert.2010.09.060
272. Nirgianakis K, Kalaitzopoulos DR, Schwartz ASK, Spaanderman M, Kramer BW, Mueller MD, et al. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: A systematic review and meta-analysis. Reprod BioMed Online (2021) 42(1):185–206. doi: 10.1016/j.rbmo.2020.09.023
273. Liang T, Zhang W, Pan N, Han B, Li R, Ma C. Reproductive outcomes of In vitro fertilization and fresh embryo transfer in infertile women with adenomyosis: A retrospective cohort study. Front Endocrinol (Lausanne) (2022) 13:865358. doi: 10.3389/fendo.2022.865358
274. Bazot M, Darai E. Role of transvaginal sonography and magnetic resonance imaging in the diagnosis of uterine adenomyosis. Fertil Steril (2018) 109:389–97. doi: 10.1016/j.fertnstert.2018.01.024
275. Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: A systematic literature review from conception to delivery. Am J Obstet Gynecol (2008) 198:357–66. doi: 10.1016/j.ajog.2007.12.039
276. Pritts EA, Parker WH, Olive DL. Fibroids and infertility: An updated system- atic review of the evidence. Fertil Steril (2009) 91:1215–23. doi: 10.1016/j.fertnstert.2008.01.051
277. Hirsch M, Begum MR, Paniz É, Barker C, Davis CJ, Duffy J. Diagnosis and management of endometriosis: A systematic review of international and national guidelines. BJOG (2018) 125:556–64. doi: 10.1111/1471-0528.14838
278. Practice Committee of the American Society for Reproductive Medicine. Endometriosis and infertility: A committee opinion. Fertil Steril (2012) 98:591–8. doi: 10.1016/j.fertnstert.2012.05.031
279. Becker CM, Bokor A, Heikinheimo O, Horne A, Jansen F, Kiesel L, et al. ESHRE guideline: Endometriosis. Hum Reprod Open (2022) 2:hoac009. doi: 10.1093/hropen/hoac009
280. Adamson GD, Pasta DJ. Endometriosis fertility index: The new, validated endometriosis staging system. Fertil Steril (2010) 94:1609–15. doi: 10.1016/j.fertnstert.2009.09.035
281. Vesali S, Razavi M, Rezaeinejad M, Maleki-Hajiagha A, Maroufizadeh S, Sepidarkish M. Endometriosis fertility index for predicting non-assisted reproductive technology pregnancy after endometriosis surgery: A systematic review and meta-analysis. BJOG (2020) 127:800–9. doi: 10.1111/1471-0528.16107
282. Tomassetti C, Bafort C, Meuleman C, Welkenhuysen M, Fieuws S, D'Hooghe T. Reproducibility of the endometriosis fertility index: A prospective inter-/intra-rater agreement study. BJOG (2020) 127:107–14. doi: 10.1111/1471-0528.15880
283. Practice Committee of the American Society for Reproductive Medicine, Practice Committee of the American Society for Reproductive Medicine. Fertility evaluation of infertile women: A committee opinion. Fertil Steril (2021) 116:1255–65. doi: 10.1016/j.fertnstert.2021.08.038
284. Kliman HJ, Honig S, Walls D, Luna M, McSweet JC, Copperman AB. Optimization of endometrial preparation results in a normal endometrial function test (EFT) and good reproductive outcome in donor ovum recipients. J Assist Reprod Genet (2006) 23:299–303. doi: 10.1007/s10815-006-9061-1
285. Díaz-Gimeno P, Horcajadas JA, Martínez-Conejero JA, Esteban FJ, Alama P, Pellicer A, et al. A genomic diagnostic tool for human endometrial receptivity based on the transcriptomic signature. Fertil Steril (2011) 95:50–60. doi: 10.1016/j.fertnstert.2010.04.063
286. Díaz-Gimeno P, Ruiz-Alonso M, Blesa D, Bosch N, Martínez-Conejero JA, Alamá P, et al. The accuracy and reproducibility of the endometrial receptivity array is superior to histology as a diagnostic method for endometrial receptivity. Fertil Steril (2013) 99:508–17. doi: 10.1016/j.fertnstert.2012.09.046
287. Nezhat C, Rambhatla A, Miranda-Silva C, Asiaii A, Nguyen K, Eyvazzadeh A, et al. BCL-6 overexpression as a predictor for endometriosis in patients undergoing In vitro fertilization. JSLS (2020) 24:e2020.00064. doi: 10.4293/JSLS.2020.00064
288. Almquist LD, Likes CE, Stone B, Brown KR, Savaris R, Forstein DA, et al. Endometrial BCL6 testing for the prediction of in vitro fertilization outcomes: A cohort study. Fertil Steril (2017) 108:1063–9. doi: 10.1016/j.fertnstert.2017.09.017
289. Evans-Hoeker E, Lessey BA, Jeong JW, Savaris RF, Palomino WA, Yuan L, et al. Endometrial BCL6 overexpression in eutopic endometrium of women with endometriosis. Reprod Sci (2016) 23:1234–41. doi: 10.1177/1933719116649711
290. Sansone AM, Hisrich BV, Young RB, Abel WF, Bowens Z, Blair BB, et al. Evaluation of BCL6 and SIRT1 as non-invasive diagnostic markers of endometriosis. Curr Issues Mol Biol (2021) 43:1350–60. doi: 10.3390/cimb43030096
291. Muyldermans M, Cornillie FJ, Koninckx PR. CA125 and endometriosis. Hum Reprod Update (1995) 1:173–87. doi: 10.1093/humupd/1.2.173
292. Barbieri RL, Niloff JM, Bast RC Jr, Scaetzl E, Kistner RW, Knapp RC. Elevated serum concentrations of CA-125 in patients with advanced endometriosis. Fertil Steril (1986) 45:630–4. doi: 10.1016/s0015-0282(16)49333-7
293. Hirsch M, Duffy J, Davis CJ, Nieves Plana M, Khan KS. Diagnostic accuracy of cancer antigen 125 for endometriosis: A systematic review and meta-analysis. BJOG (2016) 123:1761–8. doi: 10.1111/1471-0528.14055
294. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol (2007) 9:654–9. doi: 10.1038/ncb1596
295. Schmittgen TD, Lee EJ, Jiang J, Sarkar A, Yang L, Elton TS, et al. Real-time PCR quantification of precursor and mature microRNA. Methods (2008) 44:31–8. doi: 10.1016/j.ymeth.2007.09.006
296. 't Hoen PA, Ariyurek Y, Thygesen HH, Vreugdenhil E, Vossen RH, de Menezes RX, et al. Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucleic Acids Res (2008) 36:e141. doi: 10.1093/nar/gkn705
297. Cho S, Mutlu L, Grechukhina O, Taylor HS. Circulating microRNAs as potential biomarkers for endometriosis. Fertil Steril (2015) 103:1252–60.e1. doi: 10.1016/j.fertnstert.2015.02.013
298. Suryawanshi S, Vlad AM, Lin HM, Mantia-Smaldone G, Laskey R, Lee M, et al. Plasma microRNAs as novel biomarkers for endometriosis and endometriosis-associated ovarian cancer. Clin Cancer Res (2013) 19:1213–24. doi: 10.1158/1078-0432.CCR-12-2726
299. Jia SZ, Yang Y, Lang J, Sun P, Leng J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum Reprod (2013) 28:322–30. doi: 10.1093/humrep/des413
300. Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS. Accurate diagnosis of endometriosis using serum microRNAs. Am J Obstet Gynecol (2020) 223:557.e1–557.e11. doi: 10.1016/j.ajog.2020.02.050
301. Wang L, Huang W, Ren C, Zhao M, Jiang X, Fang X, et al. Analysis of serum microRNA profile by solexa sequencing in women with endometriosis. Reprod Sci (2016) 23:1359–70. doi: 10.1177/1933719116641761
302. Cosar E, Mamillapalli R, Ersoy GS, Cho S, Seifer B, Taylor HS. Serum microRNAs as diagnostic markers of endometriosis: A comprehensive array-based analysis. Fertil Steril (2016) 106:402–9. doi: 10.1016/j.fertnstert.2016.04.013
303. Rekker K, Saare M, Roost AM, Kaart T, Sõritsa D, Karro H, et al. Circulating miR-200-family micro-RNAs have altered plasma levels in patients with endometriosis and vary with blood collection time. Fertil Steril (2015) 104:938–46.e2. doi: 10.1016/j.fertnstert.2015.06.029
304. Hsu CY, Hsieh TH, Tsai CF, Tsai HP, Chen HS, Chang Y, et al. miRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J Pathol (2014) 232:330–43. doi: 10.1002/path.4295
305. Wang WT, Zhao YN, Han BW, Hong SJ, Chen YQ. Circulating microRNAs identified in a genome-wide serum microRNA expression analysis as noninvasive biomarkers for endometriosis. J Clin Endocrinol Metab (2013) 98:281–289. doi: 10.1210/jc.2012-2415
306. Misir S, Hepokur C, Oksasoglu B, Yildiz C, Yanik A, Aliyazicioglu Y. Circulating serum miR-200c and miR-34a-5p as diagnostic biomarkers for endometriosis. J Gynecol Obstet Hum Reprod (2021) 50:102092. doi: 10.1016/j.jogoh.2021.102092
307. Hossein Razi M, Eftekhar M, Ghasemi N, Hasan Sheikhha M, Dehghani Firoozabadi A. Expression levels of circulatory mir-185-5p, vascular endothelial growth factor, and platelet-derived growth factor target genes in endometriosis. Int J Reprod BioMed (2020) 18:347–58. doi: 10.18502/ijrm.v13i5.7155
308. Papari E, Noruzinia M, Kashani L, Foster WG. Identification of candidate microRNA markers of endometriosis with the use of next-generation sequencing and quantitative real-time polymerase chain reaction. Fertil Steril (2020) 113:1232–41. doi: 10.1016/j.fertnstert.2020.01.026
309. Pateisky P, Pils D, Szabo L, Kuessel L, Husslein H, Schmitz A, et al. Hsa-miRNA-154-5p expression in plasma of endometriosis patients is a potential diagnostic marker for the disease. Reprod BioMed Online (2018) 37:449–66. doi: 10.1016/j.rbmo.2018.05.007
310. Bashti O, Noruzinia M, Garshasbi M, Abtahi M. MiR-31 and miR-145 as potential non-invasive regulatory biomarkers in patients with endometriosis. Cell J (2018) 20:84–9. doi: 10.22074/cellj.2018.4915
311. Nothnick WB, Falcone T, Joshi N, Fazleabas AT, Graham A. Serum miR-451a levels are significantly elevated in women with endometriosis and recapitulated in baboons (Papio anubis) with experimentally-induced disease. Reprod Sci (2017) 24:1195–202. doi: 10.1177/1933719116681519
312. Nisenblat V, Sharkey DJ, Wang Z, Evans SF, Healey M, Ohlsson Teague EMC, et al. Plasma miRNAs display limited potential as diagnostic tools for endometriosis. J Clin Endocrinol Metab (2019) 104:1999–2022. doi: 10.1210/jc.2018-01464
313. Agrawal S, Tapmeier T, Rahmioglu N, Kirtley S, Zondervan K, Becker C. The miRNA mirage: How close are we to finding a non-invasive diagnostic biomarker in endometriosis? A systematic review. Int J Mol Sci (2018) 19:599. doi: 10.3390/ijms19020599
314. Leonova A, Turpin VE, Agarwal SK, Leonardi M, Foster WG. A critical appraisal of the circulating levels of differentially expressed microRNA in endometriosis. Biol Reprod (2021) 105:1075–85. doi: 10.1093/biolre/ioab134
315. Seifer BJ, Su D, Taylor HS. Circulating miRNAs in murine experimental endometriosis. Reprod Sci (2017) 24:376–81. doi: 10.1177/1933719116667228
316. Hodgson RM, Lee HL, Wang R, Mol BW, Johnson N. Interventions for endometriosis-related infertility: A systematic review and network meta-analysis. Fertil Steril (2020) 113:374–382.e2. doi: 10.1016/j.fertnstert.2019.09.031
317. Jin X, Ruiz Beguerie J. Laparoscopic surgery for subfertility related to endometriosis: A meta-analysis. Taiwan J Obstet Gynecol (2014) 53:303–8. doi: 10.1016/j.tjog.2013.02.004
318. Somigliana E, Infantino M, Candiani M, Vignali M, Chiodini A, Busacca M, et al. Association rate between deep peritoneal endometriosis and other forms of the disease: Pathogenetic implications. Hum Reprod (2004) 19:168–71. doi: 10.1093/humrep/deg513
319. Meuleman C, Tomassetti C, D'Hoore A, Van Cleynenbreugel B, Penninckx F, Vergote I, et al. Surgical treatment of deeply infiltrating endometriosis with colorectal involvement. Hum Reprod Update (2011) 17:311–26. doi: 10.1093/humupd/dmq057
320. Vercellini P, Somigliana E, Viganò P, Abbiati A, Barbara G, Crosignani PG. Surgery for endometriosis-associated infertility: A pragmatic approach. Hum Reprod (2009) 24:254–69. doi: 10.1093/humrep/den379
321. Vercellini P, Barbara G, Buggio L, Frattaruolo MP, Somigliana E, Fedele L. Effect of patient selection on estimate of reproductive success after surgery for rectovaginal endometriosis: literature review. Reprod BioMed Online (2012) 24:389–95. doi: 10.1016/j.rbmo.2012.01.003
322. Iversen ML, Seyer-Hansen M, Forman A. Does surgery for deep infiltrating bowel endometriosis improve fertility? A systematic review. Acta Obstet Gynecol Scand (2017) 96:688–93. doi: 10.1111/aogs.13152
323. Casals G, Carrera M, Domínguez JA, Abrão MS, Carmona F. Impact of surgery for deep infiltrative endometriosis before In vitro fertilization: A systematic review and meta-analysis. J Minim Invasive Gynecol (2021) 28:1303–12.e5. doi: 10.1016/j.jmig.2021.02.007
324. Somigliana E, Garcia-Velasco JA. Treatment of infertility associated with deep endometriosis: Definition of therapeutic balances. Fertil Steril (2015) 104:764–70. doi: 10.1016/j.fertnstert.2015.08.003
325. Douay-Hauser N, Yazbeck C, Walker F, Luton D, Madelenat P, Koskas M. Infertile women with deep and intraperitoneal endometriosis: Comparison of fertility outcome according to the extent of surgery. J Minim Invasive Gynecol (2011) 18:622–8. doi: 10.1016/j.jmig.2011.06.004
326. Alborzi S, Zahiri Sorouri Z, Askari E, Poordast T, Chamanara K. The success of various endometrioma treatments in infertility: A systematic review and meta-analysis of prospective studies. Reprod Med Biol (2019) 18:312–22. doi: 10.1002/rmb2.12286
327. Tummon IS, Asher LJ, Martin JS, Tulandi T. Randomized controlled trial of superovulation and insemination for infertility associated with minimal or mild endometriosis. Fertil Steril (1997) 68:8–12. doi: 10.1016/s0015-0282(97)81467-7
328. Werbrouck E, Spiessens C, Meuleman C, D'Hooghe T. No difference in cycle pregnancy rate and in cumulative live-birth rate between women with surgically treated minimal to mild endometriosis and women with unexplained infertility after controlled ovarian hyperstimulation and intrauterine insemination. Fertil Steril (2006) 86:566–71. doi: 10.1016/j.fertnstert.2006.01.044
329. Vassilopoulou L, Matalliotakis M, Zervou MI, Matalliotaki C, Spandidos DA, Matalliotakis I. Endometriosis and in vitro fertilisation. Exp Ther Med (2018) 16:1043–51. doi: 10.3892/etm.2018.6307
330. Pabuccu R, Onalan G, Kaya C. GnRH agonist and antagonist protocols for stage I-II endometriosis and endometrioma in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril (2007) 88:832–9. doi: 10.1016/j.fertnstert.2006.12.046
331. Hughes E, Brown J, Collins JJ, Farquhar C, Fedorkow DM, Vandekerckhove P. Ovulation suppression for endometriosis. Cochrane Database Syst Rev (2007) 2007:CD000155. doi: 10.1002/14651858.CD000155.pub2
332. Chen I, Veth VB, Choudhry AJ, Murji A, Zakhari A, Black AY, et al. Pre- and postsurgical medical therapy for endometriosis surgery. Cochrane Database Syst Rev (2020) 11:Cd003678. doi: 10.1002/14651858.CD003678.pub3
333. Georgiou EX, Melo P, Baker PE, Sallam HN, Arici A, Garcia-Velasco JA, et al. Long-term GnRH agonist therapy before in vitro fertilisation (IVF) for improving fertility outcomes in women with endometriosis. Cochrane Database Syst Rev (2019) 2019:CD013240. doi: 10.1002/14651858.CD013240.pub2
334. Cao X, Chang HY, Xu JY, Zheng Y, Xiang YG, Xiao B, et al. The effectiveness of different down-regulating protocols on in vitro fertilization-embryo transfer in endometriosis: A meta-analysis. Reprod Biol Endocrinol (2020) 18:16. doi: 10.1186/s12958-020-00571-6
335. Tomassetti C, Beukeleirs T, Conforti A, Debrock S, Peeraer K, Meuleman C, et al. The ultra-long study: A randomized controlled trial evaluating long-term GnRH downregulation prior to ART in women with endometriosis. Hum Reprod (2021) 36:2676–86. doi: 10.1093/humrep/deab163
336. Kaponis A, Chatzopoulos G, Paschopoulos M, Georgiou I, Paraskevaidis V, Zikopoulos K, et al. Ultralong administration of gonadotropin-releasing hormone agonists before in vitro fertilization improves fertilization rate but not clinical pregnancy rate in women with mild endometriosis: A prospective, randomized, controlled trial. Fertil Steril (2020) 113:828–35. doi: 10.1016/j.fertnstert.2019.12.018
337. Taylor H, Li HJ, Carson S, Flores V, Pal L, Robbins J, et al. Pre-IVF treatment with a GnRH antagonist in women with endometriosis (PREGNANT): Study protocol for a prospective, double-blind, placebo-controlled trial. BMJ Open (2022) 12(6):e052043. doi: 10.1136/bmjopen-2021-052043
338. Tamura H, Yoshida H, Kikuchi H, Josaki M, Mihara Y, Shirafuta Y, et al. The clinical outcome of dienogest treatment followed by in vitro fertilization and embryo transfer in infertile women with endometriosis. J Ovarian Res (2019) 12:123. doi: 10.1186/s13048-019-0597-y
339. Guo H, Li J, Shen X, Cong Y, Wang Y, Wu L, et al. Efficacy of different progestins in women with advanced endometriosis undergoing controlled ovarian hyperstimulation for in vitro fertilization-a single-center non-inferiority randomized controlled trial. Front Endocrinol (Lausanne) (2020) 11:129. doi: 10.3389/fendo.2020.00129
340. Corachán A, Pellicer N, Pellicer A, Ferrero H. Novel therapeutic targets to improve IVF outcomes in endometriosis patients: A review and future prospects. Hum Reprod Update (2021) 27:923–72. doi: 10.1093/humupd/dmab014
341. Grammatis AL, Georgiou EX, Becker CM. Pentoxifylline for the treatment of endometriosis-associated pain and infertility. Cochrane Database Syst Rev (2021) 8:CD007677. doi: 10.1002/14651858
342. Taylor HS. Emerging therapies for endometriosis. Fertil Steril (2021) 115:317–8. doi: 10.1016/j.fertnstert.2020.11.005
343. Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, Kristen AV, et al. Patisiran, an RNAi therapeutic, for hereditary transthyretin amyloidosis. N Engl J Med (2018) 379:11–21. doi: 10.1056/NEJMoa1716153
344. Balwani M, Sardh E, Ventura P, Peiró PA, Rees DC, Stölzel U, et al. Phase 3 trial of RNAi therapeutic givosiran for acute intermittent porphyria. N Engl J Med (2020) 382:2289–301. doi: 10.1056/NEJMoa1913147
345. Petracco R, Grechukhina O, Popkhadze S, Massasa E, Zhou Y, Taylor HS. MicroRNA 135 regulates HOXA10 expression in endometriosis. J Clin Endocrinol Metab (2011) 96:E1925–33. doi: 10.1210/jc.2011-1231
346. Joshi NR, Su RW, Chandramouli GV, Khoo SK, Jeong JW, Young SL, et al. Altered expression of microRNA-451 in eutopic endometrium of baboons (Papio anubis) with endometriosis. Hum Reprod (2015) 30:2881–91. doi: 10.1093/humrep/dev229
347. Gao S, Liu S, Gao ZM, Deng P, Wang DB. Reduced microRNA-451 expression in eutopic endometrium contributes to the pathogenesis of endometriosis. World J Clin cases (2019) 7:2155–64. doi: 10.12998/wjcc.v7.i16.2155
348. Ghazal S, McKinnon B, Zhou J, Mueller M, Men Y, Yang L, et al. H19 lncRNA alters stromal cell growth via IGF signaling in the endometrium of women with endometriosis. EMBO Mol Med (2015) 7:996–1003. doi: 10.15252/emmm.201505245
349. Altmäe S, Martinez-Conejero JA, Esteban FJ, Ruiz-Alonso M, Stavreus-Evers A, Horcajadas JA, et al. MicroRNAs miR-30b, miR-30d, and miR-494 regulate human endometrial receptivity. Reprod Sci (2013) 20:308–17. doi: 10.1177/1933719112453507
350. Joshi NR, Miyadahira EH, Afshar Y, Jeong JW, Young SL, Lessey BA, et al. Progesterone resistance in endometriosis is modulated by the altered expression of MicroRNA-29c and FKBP4. J Clin Endocrinol Metab (2017) 102:141–9. doi: 10.1210/jc.2016-2076
351. Zhou M, Fu J, Xiao L, Yang S, Song Y, Zhang X, et al. miR-196a overexpression activates the MEK/ERK signal and represses the progesterone receptor and decidualization in eutopic endometrium from women with endometriosis. Hum Reprod (2016) 31:2598–608. doi: 10.1093/humrep/dew223
352. Arosh JA, Lee J, Balasubbramanian D, Stanley JA, Long CR, Meagher MW, et al. Molecular and preclinical basis to inhibit PGE2 receptors EP2 and EP4 as a novel nonsteroidal therapy for endometriosis. Proc Natl Acad Sci U.S.A. (2015) 112:9716–21. doi: 10.1073/pnas.1507931112
353. Simoni M, Taylor HS. Therapeutic strategies involving uterine stem cells in reproductive medicine. Curr Opin Obstet Gynecol (2018) 30:209–16. doi: 10.1097/GCO.0000000000000457
354. Nagori CB, Panchal SY, Patel H. Endometrial regeneration using autologous adult stem cells followed by conception by in vitro fertilization in a patient of severe asherman's syndrome. J Hum Reprod Sci (2011) 4:43–8. doi: 10.4103/0974-1208.82360
355. Alawadhi F, Du H, Cakmak H, Taylor HS. Bone marrow-derived stem cell (BMDSC) transplantation improves fertility in a murine model of asherman's syndrome. PloS One (2014) 9:e96662. doi: 10.1371/journal.pone.0096662
356. Santamaria X, Cabanillas S, Cervelló I, Arbona C, Raga F, Ferro J, et al. Autologous cell therapy with CD133+ bone marrow-derived stem cells for refractory asherman's syndrome and endometrial atrophy: A pilot cohort study. Hum Reprod (2016) 31:1087–96. doi: 10.1093/humrep/dew042
357. Moridi I, Mamillapalli R, Cosar E, Ersoy GS, Taylor HS. Bone marrow stem cell chemotactic & activity is induced by elevated CXCl12 in endometriosis. Reprod Sci (2017) 24:526–533. doi: 10.1177/1933719116672587
358. Wang X, Mamillapalli R, Mutlu L, Du H, Taylor HS. Chemoattraction of bone marrow- derived stem cells towards human endometrial stromal cells is mediated by estradiol regulated CXCL12 and CXCR4 expression. Stem Cell Res (2015) 15:14–22. doi: 10.1016/j.scr.2015.04.004
359. Sakr S, Naqvi H, Komm B, Taylor HS. Endometriosis impairs bone marrow-derived stem cell recruitment to the uterus whereas bazedoxifene treatment leads to endometriosis regression and improved uterine stem cell engraftment. Endocrinology (2014) 155:1489–97. doi: 10.1210/en.2013-1977
360. Naqvi H, Sakr S, Presti T, Krikun G, Komm B, Taylor HS. Treatment with bazedoxifene and conjugated estrogens results in regression of endome- triosis in a murine model. Biol Reprod (2014) 90:121. doi: 10.1095/biolreprod.113.114165
361. Liu Y, Tal R, Pluchino N, Mamillapalli R, Taylor HS. Systemic administration of bone marrow-derived cells leads to better uterine engraftment than use of uterine-derived cells or local injection. J Cell Mol Med (2018) 22:67–76. doi: 10.1111/jcmm.13294
362. Practice Committee of the American Society for Reproductive Medicine. Electronic address:QXNybUBhc3JtLm9yZw==. Fertility preservation in patients undergoing gonadotoxic therapy or gonadectomy: A committee opinion. Fertil Steril (2019) 112:1022–33. doi: 10.1016/j.fertnstert.2019.09.013
363. Potdar N, Gelbaya TA, Nardo LG. Oocyte vitrification in the 21st century and post-warming fertility outcomes: A systematic review and meta-analysis. Reprod BioMed Online (2014) 29:159–76. doi: 10.1016/j.rbmo.2014.03.024
364. Rienzi L, Gracia C, Maggiulli R, LaBarbera AR, Kaser DJ, Ubaldi FM, et al. Oocyte, embryo and blastocyst cryopreservation in ART: systematic review and meta-analysis comparing slow-freezing versus vitrification to produce evidence for the development of global guidance. Hum Reprod Update (2017) 23:139–55. doi: 10.1093/humupd/dmw038
365. Calagna G, Della Corte L, Giampaolino P, Maranto M, Perino A. Endometriosis and strategies of fertility preservation: A systematic review of the literature. Eur J Obstet Gynecol Reprod Biol (2020) 254:218–25. doi: 10.1016/j.ejogrb.2020.09.045
366. Somigliana E, Vigano P, Filippi F, Papaleo E, Benaglia L, Candiani M, et al. Fertility preservation in women with endometriosis: For all, for some, for none? Hum Reprod (2015) 30:1280–6. doi: 10.1093/humrep/dev078
367. Streuli I, Benard J, Hugon-Rodin J, Chapron C, Santulli P, Pluchino N. Shed- ding light on the fertility preservation debate in women with endometriosis: A SWOT analysis. Eur J Obstet Gynecol Reprod Biol (2018) 229:172–8. doi: 10.1016/j.ejogrb.2018.08.577
368. Cobo A, García-Velasco JA, Remohí J, Pellicer A. Oocyte vitrification for fertility preservation for both medical and nonmedical reasons. Fertil Steril (2021) 115:1091–101. doi: 10.1016/j.fertnstert.2021.02.006
369. Oktay K, Karlikaya G. Ovarian function after transplantation of frozen, banked autologous ovarian tissue. N Engl J Med (2000) 342:1919. doi: 10.1056/NEJM200006223422516
370. Oktay K, Oktem O. Ovarian cryopreservation and transplantation for fertility preservation for medical indications: Report of an ongoing experience. Fertil Steril (2010) 93:762–8. doi: 10.1016/j.fertnstert.2008.10.006
371. Donnez J, Squifflet J, Dolmans MM, Martinez-Madrid B, Jadoul P, Van Langendonckt A. Orthotopic transplantation of fresh ovarian cortex: A report of two cases. Fertil Steril (2005) 84:1018. doi: 10.1016/j.fertnstert.2005.06.011
372. Elizur SE, Chian RC, Holzer HE, Gidoni Y, Tulandi T, Tan SL. Cryopreservation of oocytes in a young woman with severe and symptomatic endometriosis: A new indication for fertility preservation. Fertil Steril (2009) 91:293.e1–3. doi: 10.1016/j.fertnstert.2007.06.040
373. Garcia-Velasco JA, Domingo J, Cobo A, Martínez M, Carmona L, Pellicer A. Five years' experience using oocyte vitrification to preserve fertility for medical and nonmedical indications. Fertil Steril (2013) 99:1994–9. doi: 10.1016/j.fertnstert.2013.02.004
374. Raad J, Sonigo C, Tran C, Sifer C, Durnerin IC, Grynberg M. Oocyte vitrification for preserving fertility in patients with endometriosis: First observational cohort study… and many unresolved questions. Letter to Editor Eur J Obstet Gynecol Reprod Biol (2018) 220:140–1. doi: 10.1016/j.ejogrb.2017.12.001
375. Kuroda K, Ikemoto Y, Ochiai A, Ozaki R, Matsumura Y, Nojiri S, et al. Combination treatment of preoperative embryo cryopreservation and endoscopic surgery (Surgery-ART hybrid therapy) in infertile women with diminished ovarian reserve and uterine myomas or ovarian endometriomas. J Minim Invasive Gynecol (2019) 26:1369–75. doi: 10.1016/j.jmig.2019.02.008
376. Cobo A, Giles J, Paolelli S, Pellicer A, Remohí J, García-Velasco JA. Oocyte vitrification for fertility preservation in women with endometriosis: An observational study. Fertil Steril (2020) 113:836–44. doi: 10.1016/j.fertnstert.2019.11.017
377. Kim SJ, Kim SK, Lee JR, Suh CS, Kim SH. Oocyte cryopreservation for fertility preservation in women with ovarian endometriosis. Reprod BioMed Online (2020) 40:827–34. doi: 10.1016/j.rbmo.2020.01.028
378. Hong YH, Lee HK, Kim SK, Lee JR, Suh CS. The significance of planned fertility preservation for women with endometrioma before an expected ovarian cystectomy. Front Endocrinol (Lausanne) (2021) 12:794117. doi: 10.3389/fendo.2021.794117
379. Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev (2008) 2008:CD004992. doi: 10.1002/14651858.CD004992.pub3
380. Dan H, Limin F. Laparoscopic ovarian cystectomy versus fenestration/coagulation or laser vaporization for the treatment of endometriomas: A meta-analysis of randomized controlled trials. Gynecol Obstet Invest (2013) 76:75–82. doi: 10.1159/000351165
381. Candiani M, Ottolina J, Posadzka E, Ferrari S, Castellano LM, Tandoi I, et al. Assessment of ovarian reserve after cystectomy versus “one-step” laser vaporization in the treatment of ovarian endometrioma: A small randomized clinical trial. Hum Reprod (2018) 33:2205–11. doi: 10.1093/humrep/dey305.
382. Rius M, Gracia M, Ros C, Martínez-Zamora MÁ, deGuirior C, Quintas L, et al. Impact of endometrioma surgery on ovarian reserve: A prospective, randomized, pilot study comparing stripping with CO2 laser vaporization in patients with bilateral endometriomas. J Int Med Res (2020) 48:300060520927627. doi: 10.1177/0300060520927627
383. Shaltout MF, Elsheikhah A, Maged AM, Elsherbini MM, Zaki SS, Dahab S, et al. A randomized controlled trial of a new technique for laparoscopic management of ovarian endometriosis preventing recurrence and keeping ovarian reserve. J Ovarian Res (2019) 20:12:66. doi: 10.1186/s13048-019-0542-0
384. Sweed MS, Makled AK, El-Sayed MA, Shawky ME, Abd-Elhady HA, Mansour AM, et al. Ovarian reserve following laparoscopic ovarian cystectomy vs cyst deroofing for endometriomas. J Minim Invasive Gynecol (2019) 26:877–82. doi: 10.1016/j.jmig.2018.06.022
385. Muzii L, Achilli C, Bergamini V, Candiani M, Garavaglia E, Lazzeri L, et al. Comparison between the stripping technique and the combined excisional/ablative technique for the treatment of bilateral ovarian endometriomas: A multicentre RCT. Hum Reprod (2016) 31:339–44. doi: 10.1093/humrep/dev31
386. Araujo RSDC, Maia SB, Baracat CMF, Fernandes CQBA, Ribeiro HSAA, Ribeiro PAAG. Ovarian function following use of various hemostatic techniques during treatment for unilateral endometrioma: A randomized controlled trial. Int J Gynaecol Obstet (2021) 20:410. doi: 10.1002/ijgo.13912
387. Pedroso J, Gutierrez M, Volker KW. Comparative thermal effects of J-plasma, monopolar, argon and laser electrosurgery in a porcine tissue model. J Minim Invasive Gynecol (2014) 21:S59. doi: 10.1016/j.jmig.2014.08.210
388. Tsolakidis D, Pados G, Vavilis D, Athanatos D, Tsalikis T, Giannakou A, et al. The impact on ovarian reserve after laparoscopic ovarian cystectomy versus three-stage management in patients with endometriomas: A prospective randomized study. Fertil Steril (2010) 94:71–7. doi: 10.1016/j.fertnstert.2009.01.138
Keywords: endometriosis, infertility, pathogenesis, ovarian reserve, endometrial receptivity, in-vitro fertilization (IVF), stem cell
Citation: Bonavina G and Taylor HS (2022) Endometriosis-associated infertility: From pathophysiology to tailored treatment. Front. Endocrinol. 13:1020827. doi: 10.3389/fendo.2022.1020827
Received: 16 August 2022; Accepted: 06 October 2022;
Published: 26 October 2022.
Edited by:
Lusine Aghajanova, Stanford Healthcare, United StatesReviewed by:
Michael Strug, Stanford University, United StatesAntonio Simone Laganà, University of Palermo, Italy
Copyright © 2022 Bonavina and Taylor. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hugh S. Taylor, aHVnaC50YXlsb3JAeWFsZS5lZHU=
 Giulia Bonavina
Giulia Bonavina Hugh S. Taylor
Hugh S. Taylor