- First Propedeutic Department of Internal Medicine, Medical School, Aristotle University of Thessaloniki, AHEPA Hospital, Thessaloniki, Greece
Hormone-dependent cancers are a major cause of morbidity and mortality in both genders. Accumulating evidence suggest that adiponectin, an adipokine with multifaceted functions, is implicated in the pathogenesis of several malignancies. In the present review, we discuss the existing data regarding this relationship. Several observational studies showed that low adiponectin levels are associated with higher risk for breast, cervical, endometrial, ovarian and prostate cancer. A relationship between adiponectin and the aggressiveness of some of these tumors has also been reported. In vitro studies reported that adiponectin inhibits the proliferation and induces apoptosis of breast, cervical, endometrial, ovarian and prostate cancer cells. Given the high prevalence of these cancers and the substantial associated morbidity and mortality, the role of agents that increase adiponectin levels and/or stimulate its activity should be evaluated for the prevention and management of these common tumors.
Introduction
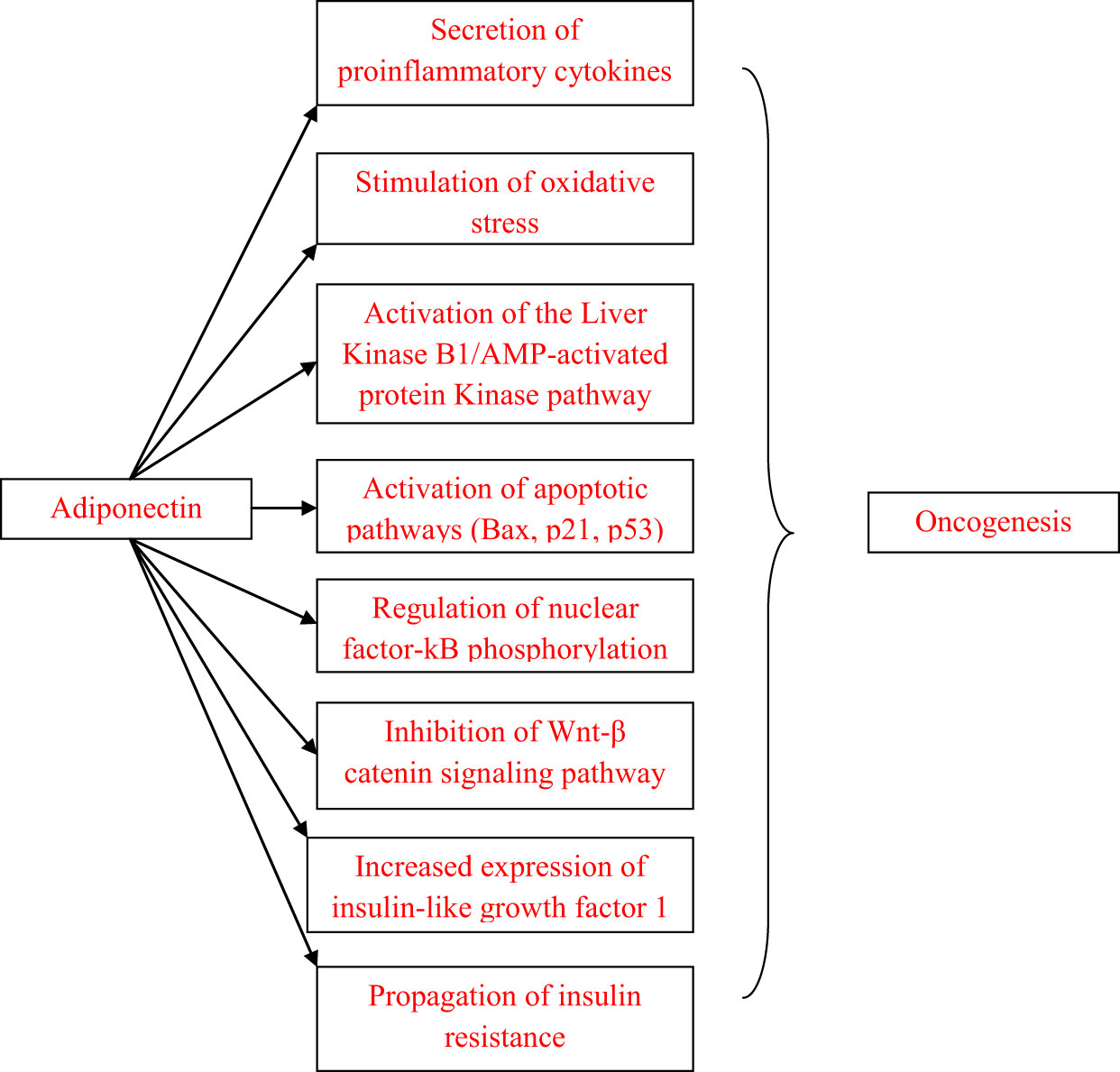
Obesity is an established risk factor for several malignancies, including colon, breast, endometrial, prostate and ovarian cancer (1–3). Metabolic abnormalities, particularly insulin resistance, as well as chronic subclinical inflammation are frequently present in obese patients and can induce adipose tissue dysfunction, which in turn appears to play a role in obesity-related oncogenesis (4). Adipose tissue is the largest endocrine system and actively participates in the regulation of metabolism, inflammation, fat distribution, appetite and bone structure (4). Adiponectin, a protein hormone secreted by adipocytes, participates in glucose regulation but also exerts anti-inflammatory, antiatherogenic and cardioprotective effects as well as effects on lipid metabolism and fatty acid oxidation (5). Accumulating preclinical and clinical data suggest that adiponectin contributes to the pathogenesis of a wide variety of malignancies, especially hormone-dependent cancers (Figure 1). This review aims to summarize the links between adiponectin and the most frequent hormone-related malignancies, namely breast, cervical, ovarian, endometrial and prostate cancer.
Adiponectin: Physiology and oncogenesis-related functions
Adiponectin is predominantly secreted by adipocytes in white adipose tissue but is also secreted in lesser amounts by other tissues such as brown adipose tissue, colon, ovaries, salivary glands, liver and skeletal muscle suggesting an autocrine and/or paracrine function upon other tissues. It is a 244-amino acid protein that includes an amino-terminal signal peptide, a species-specific variable domain, a collagen-like region of 22 Gly-X-Y repeats and a carboxyl-terminal globular domain that binds to adiponectin receptors, and is similar to the C1q (Complement factor 1q) and the trimeric topology of tumor necrosis factor α(TNF-α) (5).
The adiponectin collagen-like region allows oligomerization of the protein as a trimer though disulfide bonds and hydroxylation and glycosylation of four conserved lysine residues, while this enables the formation of middle molecular weight (MMW) examers and high molecular weight (HMW) multimers. Circulating adiponectin can take the form of full-length or HMW adiponectin in plasma, LMW and gAd (globular adiponectin), which are also present in low concentrations due to their shorter half-life (6).
Adiponectin levels are modulated by a variety of environmental, physiological and pharmacological factors such as nutrition, hormones, inflammation and medications (6). Testosterone, growth hormone (GH), glucocorticoids and prolactin decrease adiponectin secretion (7).
Three adiponectin receptors have been identified, namely adiponectin receptors 1 and 2 (AdipoR1 and AdipoR2) and T-cadherin. AdipoR1 and AdipoR2 are 375- and 311-amino acid proteins, respectively, with a molecular mass of 42.4 and 35.4 kDA, respectively. They are structurally highly related, as their protein sequence shares 67% identity, and include7 transmembrane domains, an extracellular carboxy-terminus and an intracellular amino-terminus, a structure that is opposite to that of all other G-coupled protein receptors. AdipoR1 and AdipoR2 are also highly conserved, since they share 95 and 97% identity between mice proteins, respectively (8–10).
Adiponectin exerts insulin-sensitizing effects in both skeletal muscle and in adipose tissue by enhancing APPL-1/AMPK interaction, which in turn increases the glucose uptake through glucose transporter 4 (GLUT4) (5). Therefore, low levels of adiponectin might increase the risk for oncogenesis, given that insulin resistance is associated with higher risk for several malignancies (4). Adiponectin inhibits fibroblast growth factor-2 (FGF-2)-stimulated endothelial cell proliferation and decreases vascular endothelial growth factor (VEGF)-induced endothelial cell migration. Intralesional injection of recombinant murine adiponectin into hypervascularized murine fibrocarcinomas led to a 60% reduction in tumor volumes and weights accompanied by an increase in tumor apoptosis mediated through caspase-3 activation, which suggests that adiponectin could act as a potent angiogenesis inhibitor that can activate apoptosis, and thus inhibit tumor growth (11). Adiponectin exerts its effects mainly by the Liver Kinase B1/AMP-activated protein Kinase (LKB1/AMPK) pathway, which inhibits signaling pathways involved in cell cycle initiation, cell growth and survival such as extracellular signal-regulated kinases 1/2 (ERK1/2), phosphatidyl-inositol 3-kinases (PI3K)/Protein Kinase B (Akt), c-Jun N-terminal kinase (cJNK) and signal transducer and activator of transcription-3 (STAT3) (12). Adiponectin also regulates the expression of different proteins involved in cell cycle and apoptosis, including up-regulation of p53 and Bax and down-regulation of c-myc, cyclin D1 and Bcl-2 (13). Moreover, adiponectin has anti-inflammatory effects by suppressing the phosphorylation of nuclear factor kB (NF-kB), a transcription factor involved in the regulation of the activity of various pro-inflammatory cytokines (14).
Adiponectin and breast cancer
Breast cancer is the most frequent cancer in females and accounts for 11.7% of all new cancer cases diagnosed in European countries and for 28.7% of all new cancers diagnosed in women. Among women, breast cancer accounts for 1 in 4 cancer cases and for 1 in 6 cancer deaths, ranking first in incidence in the vast majority of countries (15). Obesity is associated with increased incidence of breast cancer and with increased risk for more aggressive breast cancer (16). Regarding the relationship between adiponectin and breast cancer, lower circulating total and HMW adiponectin levels are associated with a higher risk of breast cancer, independently of body mass index (BMI) (17, 18). Recent data suggest that low adiponectin levels are also related to shorter overall mortality in patients with breast cancer (19). Moreover, in premenopausal women, lower plasma adiponectin levels predict the progression from intraepithelial neoplasia to invasive breast cancer independently of age and BMI (20). Adiponectin receptors have been identified in breast cancer cells (18). In T47D breast cancer cell lines, adiponectin inhibited cell proliferation and reduced viability, an effect partially mediated by the activation of ERK1/2 (18). In MDA-MB-231 breast cancer cells, adiponectin arrested cell cycle progression and induced apoptosis by suppressing the glycogen synthase kinase 3β/β-catenin signaling pathway (21). Adiponectin also inhibited the proliferation of MCF-7 breast cancer cells by reducing the translation of genes involved in cell cycle regulation (mitogen-activated protein kinase 3 and ATM) and apoptosis (BAG1, BAG3 and TP53) (22).
Adiponectin and cervical cancer
Cervical cancer is the fourth most frequently diagnosed cancer and the fourth leading cause of cancer-related death in women, with an estimated 604,000 new cases and 342,000 deaths worldwide in 2020 (15). Obesity is an established risk factor for cervical cancer and is also associated with more aggressive forms of this malignancy (23). There are very limited data regarding the role of adiponectin in the pathogenesis of cervical cancer. An in vitro study showed that adiponectin receptors are expressed in cervical cancer HeLa cells and that their expression increases in adiponectin-treated cells (24). Moreover, adiponectin inhibited the proliferation of HeLa cells, as evidenced by a significant increase in the cell population in G0/G1 phase, concomitant with a reduction of cell number in S and G2/M phases (24). A down-regulation of cell cycle regulators, namely cyclin D1 and c-myc, was also observed, along with an activation of apoptosis, mediated by the enhanced expression of p21, p53 and Bax and reduced expression of Bcl-2 (24).
Adiponectin and ovarian cancer
Ovarian cancer is the 8th most commonly occurring cancer in women and the 18th most common cancer overall. There were more than 313,000 new cases of ovarian cancer in 2020 (15). The relationship between obesity and ovarian tumor development has become increasingly evident, particularly in post-menopausal women (25). In case-control studies, women with low levels of adiponectin have higher risk for ovarian cancer (26, 27). Adiponectin receptors are expressed in both epithelial and granulose ovarian cancer cells (28) and their expression is associated with more advanced cancer and shorter progression-free and overall survival (29). Moreover, adiponectin inhibits the growth of OVCAR-3 and SKOV-3 ovarian cancer cells and antagonizes the proliferative effects of 17β-estradiol and insulin-like growth factor-1 on these cells by down-regulating the expression of their receptors (28). Stimulation of adiponectin receptors was also shown to induce G1 cell cycle arrest and promote apoptosis in OVCAR3, OVCAR4 and A2780 ovarian cancer cells (30).
Adiponectin and endometrial cancer
Endometrial cancer is the sixth most commonly diagnosed cancer in women with 417,000 new cases and 97,000 deaths recorded in 2020 (15). Low circulating adiponectin levels are associated with higher endometrial cancer risk, independent of other obesity-related risk factors, and this relationship appears to be stronger in premenopausal women (31, 32). Adiponectin receptors are expressed in both normal human endometrium and in endometrial cancer tissues (33). Adiponectin was shown to decrease the proliferation of KLE and RL95-2 endometrial cancer cells and also reduces their adhesion and migration by activating the adaptor molecule LKB1 (33). In HEC-1-A endometrial cancer cells, adiponectin inhibited cell growth and induced apoptosis by inactivating Akt and decreasing cyclin D1 expression (34). More recently, adiponectin was shown to promote the development of endometrial cancer by activating mitogen-activated protein kinase (MAPK) (35).
Adiponectin and prostate cancer
Epidemiological data suggest that low adiponectin levels might also be associated with higher incidence of prostate cancer (36, 37). Single nucleotide polymorphisms of genes that encode adiponectin and AdipoR1 are also related to prostate cancer incidence and aggressiveness (38, 39). Adiponectin receptor expression is lower in prostate cancer tissue than in benign prostate tissue (39, 40). In vitro studies also showed that adiponectin inhibits the proliferation of PC-3 prostate cancer cells (41) and also suppresses angiogenesis by inhibiting m-TOR activation of VEGF (42). Adiponectin also suppresses oxidative stress in human 22Rv1 and DU-145 PC cell lines by increasing the expression of NADPH oxidase-2 and -4 (43).
Conclusions
Both in vitro and observational studies suggest that adiponectin is potentially implicated in the pathogenesis of several hormone-related malignancies. Given the high prevalence of these cancers and the substantial associated morbidity and mortality, the role of agents that increase adiponectin levels and/or stimulate its activity should be evaluated for the prevention and management of these common tumors.
Author contributions
AT drafted the manuscript. KT edited and critically revised the draft. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, et al. American Society of clinical oncology position statement on obesity and cancer. J Clin Oncol (2014) 32:3568–74. doi: 10.1200/JCO.2014.58.4680
2. Calle E, Kaaks R. Overweight, obesity and cancer: Epidemiological evidence and proposed mechanisms. Nat Rev Cancer (2004) 4:579–91. doi: 10.1038/nrc1408
3. Chan DSM, Vieira AR, Aune D, Bandera EV, Greenwood DC, McTiernan A, et al. Body mass index and survival in women with breast cancer-systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol (2014) 25:1901–14. doi: 10.1093/annonc/mdu042
4. Quail DF, Dannenberg AJ. The obese adipose tissue microenvironment in cancer development and progression. Nat Rev Endocrinol (2019) 15:139–54. doi: 10.1038/s41574-018-0126-x
5. Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci (2017), 18(6):1321. doi: 10.3390/ijms18061321
6. Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, et al. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin: Implications for metabolic regulation and bioactivity. J BiolChem (2003) 278:9073–85. doi: 10.1074/jbc.M207198200
7. Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol (2000) 20:1595–9. doi: 10.1161/01.ATV.20.6.1595
8. Bokobza E, Hinault C, Tiroille V, Clavel S, Bost F, Chevalier N. The adipose tissue at the crosstalk between EDCs and cancer development. Front Endocrinol (Lausanne) (2021) 12:691658. doi: 10.3389/fendo.2021.691658
9. Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature (2003) 423:762–9. doi: 10.1038/nature01705
10. Dalamaga M, Diakopoulos KN, Mantzoros CS. The role of adiponectin in cancer: A review of current evidence. Endocr Rev (2012) 33:547–94. doi: 10.1210/er.2011-1015
11. Bråkenhielm E, Veitonmäki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, et al. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl AcadSci USA (2004) 101:2476–81. doi: 10.1073/pnas.0308671100
12. Zhou L, Deepa SS, Etzler JC, Ryu J, Mao X, Fang Q, et al. Adiponectin activates AMP-activated protein kinase in muscle cells via APPL1/LKB1-dependent and phospholipase C/Ca2+/Ca2+/calmodulin-dependent protein kinase kinase-dependent pathways. J Biol Chem (2009) 284:22426–35. doi: 10.1074/jbc.M109.028357
13. Zuo Y, Xiao T, Qiu X, Liu Z, Zhang S, Zhou N. Adiponectin reduces apoptosis of diabetic cardiomyocytes by regulating miR-711/TLR4 axis. Diabetol Metab Syndr (2022) 14:131. doi: 10.1186/s13098-022-00904-y
14. Bushra S, Al-Sadeq DW, Bari R, Sahara A, Fadel A, Rizk N. Adiponectin ameliorates hyperglycemia-induced retinal endothelial dysfunction, highlighting pathways, regulators, and networks. J Inflamm Res (2022) 15:3135–66. doi: 10.2147/JIR.S358594
15. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
16. Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med (2006) 166:2395–402. doi: 10.1001/archinte.166.21.2395
17. Yu Z, Tang S, Ma H, Duan H, Zeng Y. Association of serum adiponectin withbreast cancer: A meta-analysis of 27 case-control studies. Med (Baltimore) (2019) 98:e14359. doi: 10.1097/MD.0000000000014359
18. Körner A, Pazaitou-Panayiotou K, Kelesidis T, Kelesidis I, Williams CJ, Kaprara A, et al. Total and high-molecular-weight adiponectin in breast cancer in vitro And in vivo Studies. J Clin Endocrinol Metab (2007) 92:1041–8. doi: 10.1210/jc.2006-1858
19. Güven HE, Doğan L, Gülçelik MA, Gülçelik NE. Adiponectin: A predictor forBreast cancer survival? Eur J Breast Health (2018) 15:13–7. doi: 10.5152/ejbh.2018.4349
20. Macis D, Gandini S, Guerrieri-Gonzaga A, Johansson H, Magni P, Ruscica M, et al. Prognostic effect of circulating adiponectin in a randomized 2 x 2 trial of low-dose tamoxifen and fenretinide in premenopausal women at risk for breast cancer. J Clin Oncol (2012) 30:151–7. doi: 10.1200/JCO.2011.35.2237
21. Wang Y, Lam JB, Lam KS, Liu J, Lam MC, Hoo RL, et al. Adiponectin modulates the glycogen synthase kinase-3β/β-catenin signaling pathway and attenuates mammary tumorigenesis of MDA-MB-231 cells in nude mice. Cancer Res (2006) 66:11462–70. doi: 10.1158/0008-5472.CAN-06-1969
22. Jardé T, Caldefie-Chézet F, Goncalves-Mendes N, Mishellany F, Buechler C, Penault-Llorca F, et al. Involvement of adiponectin and leptin in breast cancer: clinical and in vitro studies. Endocr Relat Cancer (2009) 16:1197–210. doi: 10.1677/ERC-09-0043
23. Gu W, Chen C, Zhao KN. Obesity-associated endometrial and cervical cancers. Front Biosci (Elite Ed.) (2013) 5:109–18. doi: 10.2741/e600
24. Xie L, Wang Y, Wang S, Wu N, Chen Y, Yan J. Adiponectin induces growth inhibition and apoptosis in cervical cancer HeLa cells. Biologia (2011) 66:712–20. doi: 10.2478/s11756-011-0063-9
25. Nagle C, Dixon S, Jensen A, Kjaer S, Modugno F, Fereday S, et al. Obesity and survival among women with ovarian cancer: Results from the ovarian cancer association consortium. Br J Cancer (2015) 113:817–26. doi: 10.1038/bjc.2015.245
26. Otokozawa S, Tanaka R, Akasaka H, Ito E, Asakura S, Ohnishi H, et al. Associations of serum isoflavone, adiponectin and insulin levels with risk for epithelial ovarian cancer: Results of a case-control study. Asian Pac J Cancer Prev (2015) 16:4987–91. doi: 10.7314/APJCP.2015.16.12.4987
27. Li H, Sun L, Chen L, Kang Z, Hao G, Bai F. Effects of adiponectin, plasmaD-dimer, inflammation and tumor markers on clinical characteristics andprognosis of patients with ovarian cancer. J Med Biochem (2022) 41:71–8. doi: 10.5937/jomb0-26452
28. Hoffmann M, Gogola J, Ptak A. Adiponectin reverses the proliferative effects of estradiol and IGF-1 in human epithelial ovarian cancer cells by downregulating the expression of their receptors. Horm Cancer (2018) 9:166–74. doi: 10.1007/s12672-018-0331-z
29. Li X, Yu Z, Fang L, Liu F, Jiang K. Expression of adiponectin receptor-1 andPrognosis of epithelial ovarian cancer patients. Med Sci Monit (2017) 23:1514–21. doi: 10.12659/MSM.899990
30. Ramzan AA, Bitler BG, Hicks D, Barner K, Qamar L, Behbakht K, et al. Adiponectin receptor agonist AdipoRon induces apoptoticcell death and suppresses proliferation in human ovarian cancer cells. Mol Cell Biochem (2019) 461:37–46. doi: 10.1007/s11010-019-03586-9
31. Petridou E, Mantzoros C, Dessypris N, Koukoulomatis P, Addy C, Voulgaris Z, et al. Plasma adiponectin concentrations in relation to endometrial cancer: A case-control study in Greece. J Clin Endocrinol Metab (2003) 88:993–7. doi: 10.1210/jc.2002-021209
32. Cust AE, Kaaks R, Friedenreich C, Bonnet F, Laville M, Lukanova A, et al. Plasma adiponectin levels and endometrialcancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab (2007) 92:255–63. doi: 10.1210/jc.2006-1371
33. Moon HS, Chamberland JP, Aronis K, Tseleni-Balafouta S, Mantzoros CS. Direct role of adiponectin and adiponectin receptors in endometrial cancer: In vitro and ex vivo studies in humans. Mol Cancer Ther (2011) 10:2234–43. doi: 10.1158/1535-7163.MCT-11-0545
34. Cong L, Gasser J, Zhao J, Yang B, Li F, Zhao AZ. Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1-A and RL95-2. Endocr Relat Cancer (2007) 14:713–20. doi: 10.1677/ERC-07-0065
35. Yan Y, Shi H, Zhao Z, Wang S, Zhou S, Mu Y, et al. Adiponectin deficiency promotes endometrial carcinoma pathogenesis and development via activation of mitogen-activated protein kinase. J Pathol (2022) 257:146–57. doi: 10.1002/path.5874
36. Burton AJ, Gilbert R, Tilling K, Langdon R, Donovan JL, Holly JMP, et al. Circulating adiponectin and leptin and risk of overall and aggressive prostate cancer: a systematic review and meta-analysis. Sci Rep (2021) 11:320. doi: 10.1038/s41598-020-79345-4
37. Michalakis K, Williams CJ, Mitsiades N, Blakeman J, Balafouta-Tselenis S, Giannopoulos A, et al. Serum adiponectin concentrations and tissue expression of adiponectin receptors are reduced in patients with prostate cancer: a case control study. Cancer Epidemiol Biomarkers Prev (2007) 16:308–13. doi: 10.1158/1055-9965.EPI-06-0621
38. Kaklamani V, Yi N, Zhang K, Sadim M, Offit K, Oddoux C, et al. Polymorphisms of ADIPOQ and ADIPOR1 and prostate cancer risk. Metabolism (2011) 60:1234–43. doi: 10.1016/j.metabol.2011.01.005
39. Hu MB, Xu H, Hu JM, Zhu WH, Yang T, Jiang HW, et al. Genetic polymorphisms in leptin, adiponectin and their receptors affect risk and aggressiveness of prostate cancer: evidence from a meta-analysis and pooled-review. Oncotarget (2016) 7:81049–61. doi: 10.18632/oncotarget.12747
40. Mistry T, Digby JE, Chen J, Desai KM, Randeva HS. The regulation of adiponectin receptors in human prostate cancer cell lines. Biochem Biophys Res Commun (2006) 348:832–8. doi: 10.1016/j.bbrc.2006.07.139
41. Gao Q, Zheng J. Adiponectin-induced antitumor activity on prostatic cancers through inhibiting proliferation. Cell Biochem Biophys (2014) 70:461–5. doi: 10.1007/s12013-014-9941-4
42. Gao Q, Zheng J, Yao X, Peng B. Adiponectin inhibits VEGF-a in prostate cancer cells. Tumour Biol (2015) 36:4287–92. doi: 10.1007/s13277-015-3067-1
Keywords: adiponectin, adiponectin receptors, obesity, breast cancer, cervical cancer, endometrial cancer, ovarian cancer, prostate cancer
Citation: Tsankof A and Tziomalos K (2022) Adiponectin: A player in the pathogenesis of hormone-dependent cancers. Front. Endocrinol. 13:1018515. doi: 10.3389/fendo.2022.1018515
Received: 13 August 2022; Accepted: 22 September 2022;
Published: 06 October 2022.
Edited by:
Bruno M. Simões, The University of Manchester, United KingdomReviewed by:
Stefania Mai, Italian Auxological Institute (IRCCS), ItalyCopyright © 2022 Tsankof and Tziomalos. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Konstantinos Tziomalos, a3R6aW9tYWxvc0B5YWhvby5jb20=
 Alexandra Tsankof
Alexandra Tsankof Konstantinos Tziomalos
Konstantinos Tziomalos