
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 07 October 2022
Sec. Cardiovascular Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1017177
This article is part of the Research Topic Adrenal Neuroendocrine System and Cardiometabolic Health: Pathophysiology and Clinical Implications View all 6 articles
 Lin Gan
Lin Gan Nanfang Li*
Nanfang Li* Mulalibieke Heizhati
Mulalibieke Heizhati Mengyue Lin
Mengyue Lin Qing Zhu
Qing Zhu Xiaoguang Yao
Xiaoguang Yao Ting Wu
Ting Wu Menghui Wang
Menghui Wang Qin Luo
Qin Luo Delian Zhang
Delian Zhang Wen Jiang
Wen Jiang Junli Hu
Junli HuObjective: To evaluate the association of plasma aldosterone concentration (PAC) with incident cardiovascular disease (CVD) and all-cause mortality in hypertensive patients with suspected obstructive sleep apnea (OSA) and calculate the optimal cut-off value of PAC for this specific population.
Patients and methods: Participants with PAC at baseline in UROSAH in 2011-2013 were enrolled and followed up till 2021. Composite outcome included CVD and all-cause mortality. Cox proportional hazards model was used to evaluate the relationship between PAC and the composite outcome. Time-dependent ROC curve was used to determine the optimal cut-off value of PAC. Besides, we conducted subgroup analyses and sensitivity analyses.
Results: 3173 hypertensive participants aged 18-84 years comprised analytical sample. During a median follow-up of 7.3 years and 22640 person-years, 69 deaths and 343 cases of incident CVD occurred. The incidence of composite outcome was increased with elevation in tertile of PAC. Compared with the first tertile, the risk of CVD and all-cause death was higher in third tertile (HR=1.81, 95%CI: 1.39-2.35, P<0.001). Time-dependent ROC curve showed optimal threshold for PAC was 12.5ng/dl. Whether renin was suppressed or not (≤0.5 or >0.5ng/ml per h), elevated PAC was associated with an increased risk of CVD. Our results remained stable and consistent in sensitivity analyses.
Conclusion: Higher PAC was associated with increased risk of CVD and all-cause mortality in hypertensives with suspected OSA, even in the absence of primary aldosteronism (PA). Hypertensives with PAC≥12.5ng/dl showed a significantly increased risk of CVD, indicating that special attention and treatment were required in this specific population.
Cardiovascular disease (CVD) is the leading cause of death and disability-adjusted life years globally, causing one-third of deaths (1, 2). Hypertension is the leading modifiable risk factor for CVD, affecting more than 30% of adults worldwide (3, 4). Due to ageing of the population and lifestyle-related risk factors, burdens of both hypertension and CVD have been increasing. In addition, management of traditional risk factors does not seem to reduce CVD morbidity and mortality, therefore exploring new potential risk factors, such as increased aldosterone levels, may improve the risk prediction of CVD and provide new targets for prevention and or treatment to reduce CVD deaths.
Aldosterone, a key hormone in renin-angiotensin-aldosterone system (RAAS), plays a pivotal role in maintaining homeostasis of fluid and regulation of blood pressure. Long-term activation of the RAAS can lead to excessive PAC, thereby promoting and perpetuating hypertension, congestive heart failure, chronic kidney disease, and metabolic disease (5). Furthermore, excessive PAC is closely related to an increase in event and mortality of CVD (6, 7). In experimental models, excessive circulating and tissue aldosterone have been found to have adverse effects on cardiovascular system through some mechanisms, such as oxidative stress, endothelial dysfunction, inflammation, and interstitial fibrosis (8–10).
Several population-based studies observed that PAC is positively correlated with blood pressure, waist circumference, insulin resistance and other risk factors of CVD (11–14). PAC is also associated with markers of subclinical atherosclerosis including coronary artery calcification and ankle-brachial index (15, 16). However, the reported effects of PAC on CVD and mortality are not always consistent in epidemiological or clinical studies. Some studies have found that elevated PAC leads to development of CVD and all-cause mortality (17, 18), whereas others report that PAC is not associated with CVD (19, 20). In addition, the MESA study has found serum aldosterone concentration is associated with increased risk of all-cause mortality when renin was suppressed (15). The inconsistencies may be, in part, explained by instability of PAC, which can be affected by medications, salt intake, disease state and or ethnicity (21–23). In addition, there is a paucity of study on the relationship between PAC and CVD and all-cause mortality in hypertensive patients. Therefore, to clarify the association of PAC and cardiovascular events and the optimal threshold of PAC in hypertensive patients, we conducted this longitudinal study among hypertensive patients with suspected OSA.
Participants for current study were the hypertensive patients with PAC measurement at baseline in Urumqi Research on Sleep Apnea and Hypertension (UROSAH).
UROSAH is a single-center observational study to assess the association of OSA with long term cardiovascular outcomes in patients with hypertension. Hypertensive patients aged 18 years and over who visited Hypertension Center of People’s Hospital of Xinjiang Uygur Autonomous Region China between Jan 2011 and Dec 2013 were reviewed. Inclusion criteria of UROSAH were following: hypertensive patients who were suspected of OSA. Exclusion criteria in the present study were as follows: 1. patients with acute severe cardiovascular and cerebrovascular diseases in recent 3 months of enrollment; 2. patients with acute asthma, chronic obstructive pulmonary disease, interstitial lung disease, pulmonary tuberculosis and other respiratory diseases; 3. patients who were using steroids, bronchodilators and antihistamines at the time of hospitalization; 4. patients with malignant tumor, acute infection and autoimmune diseases; 5. renal and renovascular hypertension, pheochromocytoma, Cushing’s syndrome and other common secondary hypertension; 6. patients with failure to sleep study. In total, 3605 hypertensive patients with suspected OSA were included. In addition, patients who were lost to follow-up and without PAC data at baseline were excluded from the current study.
Finally, 3173 individuals comprised analytical sample (Figure 1). The current study was approved by Ethics Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region.
2169 (68.4%) hypertensives completed the measurement of PAC and PRA without interfering agents. That is, diuretics (including mineralocorticoid antagonists) were discontinued for at least 6 weeks, and dihydropyridine calcium antagonist, β-receptor blockers, angiotensin-converting enzyme inhibitors (ACEIs), and angiotensin receptor blockers (ARBs) were ceased for at least 4 weeks, or any antihypertensive medications were not taken at least 2 weeks before PAC and PRA measurement. When necessary, patients were allowed to take α-blockers (doxazosin or terazosin) and/or non-dihydropyridine calcium channel blocker (slow-release verapamil). 1004 (31.6%) participants completed the measurement of PAC and PRA under existence of interfering agents. All blood samples were collected in the morning after patients had been ambulant for at least 2h and rested for 15 min in sitting position. PAC was measured by radioimmunoassay using a commercially available kit (Beckman Coulter, Brea, CA, USA), the intra- and inter-assay coefficients of variation were 5.2 and 8.3, respectively. PRA was measured by using an iodine angiotensin I radioimmunoassay kit (Northern Biotechnology Institutes, Beijing, China), and the intra- and inter-assay coefficients of variation were 9.5 and 13.4, respectively. ARR was then calculated by dividing the PAC by PRA. The suspicious PA were defined as ARR≥20 (ng/dl)/(ng/ml per h) and PAC≥12ng/dl. The saline infusion test (SIT) was performed in suspect PA patients and the diagnosis of PA after SIT was based on Endocrine Society Guideline (24).
All patients underwent in-laboratory overnight PSG (Compumedics E series, Australia) examination. OSA was defined as an apnea-hypopnea index (AHI)≥5 events per hour; further, the severity of OSA was defined as follows: mild OSA (5≤AHI<15), moderate OSA (15≤AHI<30), and severe OSA (AHI≥30).
Other information as covariates, including age, gender, body mass index (BMI), waist circumference, cigarette consumption (yes/no), alcohol intake (yes/no), duration of hypertension, diabetes mellitus (DM, yes/no), apnea-hypopnea index (AHI), office systolic blood pressure (SBP), office diastolic blood pressure (DBP), uric acid (UA), serum creatinine (Scr), alanine aminotransferase (ALT), aspartate aminotransferase (AST), fasting plasma glucose (FPG), total cholesterol (TC), triglycerides (TG), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), lipoprotein a (Lp a), homocysteine (Hcy), serum potassium, serum sodium, and information on lipid regulating, antiplatelet and antihypertensive agents were collected as well.
Seated BP at hospitalization was measured in the upper arm after patients rested quietly for 10 mins at least with a mercury sphygmomanometer using international recommendations (25). The mean value of two measurements was recorded and used for analysis. BMI was measured in units of weight(kg)/height2(m). Estimated glomerular filtration rate(eGFR) was calculated using CKD-EPI equation (26).
Composite outcome were incident CVD and all-cause mortality. CVD included non-fatal stroke (ischemic stroke, hemorrhagic stroke, transient ischemic attack), coronary events (acute myocardial infarction, coronary revascularization, hospitalized unstable angina), hospitalized heart failure, and aortic dissection. All participants were followed up through medical records, outpatient and or inpatient visits, and telephone communication. All events were certified by medical documents and confirmed by the clinical event committee. The deadline for follow-up was Jan 2021.
Continuous variables with a normal distribution were presented as mean ± standard deviation and compared between groups using analysis of variance (ANOVA). Non-normally distributed continuous variables were presented as median (interquartile range) and compared between groups using Kruskal-Wallis H test. Categorical variables were expressed as frequency and percentage, and chi-square test was used for comparison between groups.
Kaplan-Meier curve was used to evaluate the relationship of PAC, PRA, and ARR with the composite outcomes, and log-rank test was used for comparison. To evaluate the validity of the proportional hazard assumption, the assumption was evaluated using log-minus-log-survival function and was found valid. Cox proportional hazard regression models were used to evaluate hazard ratios (HR) and 95% confidence intervals (CI) with inclusion of PAC as categorical (tertiles) or continuous variables (per 1, 5, 10 unit). Based on least absolute shrinkage and selection operator (LASSO) regression and univariate COX regression analysis, multivariate modeling was performed through the following sequential adjustments:
Model 1 (based on LASSO regression) was adjusted for age, DM at baseline, duration of hypertension≥5 years, cigarette consumption, alcohol intake, waist circumference, SBP, DBP, eGFR, TG, HDL-C, Lp(a), serum potassium, and PRA;
Model 2 (based on univariate COX regression analysis) was adjusted for gender, age, BMI, DM at baseline, duration of hypertension≥5 years, cigarette consumption, alcohol intake, AHI≥15 event per hour, waist circumference, SBP, DBP, eGFR, TC, TG, HDL-C, LDL-C, Lp(a), serum potassium, and PRA;
Model 3 was Model 2 plus antihypertensive agents, statins, antiplatelet agents, and regular CPAP treatment.
Considering that composite outcomes would change over time, we used time-dependent ROC curve with highest Youden index to determine the cut-off for PAC. Additionally, to identify potential effects of variables on the relationship between PAC and composite outcomes, stratified analyses were further performed according to gender, age (≤55 or >55 years), cigarette consumption (yes or no), alcohol intake (yes or no), BMI (<28 or ≥28kg/m2), AHI (<15 or ≥15 event per hour), SBP (<135 or ≥135mmHg), DBP (<85 or≥85mmHg), PRA (≤0.5 or >0.5ng/ml per h), and interfering agents (yes or no). We performed sensitivity analyses to further evaluate whether the association was present and stable by excluding patients with follow-up time less than 12 months, and with CVD at baseline. In view of the positive association between PA and CVD, we performed sensitivity analyses after exclusion of participants with suspicious PA (defined as ARR≥20 and PAC≥12), and with diagnosed PA. Furthermore, considering the potential influence of renal dysfunction on PAC and composite outcomes, we conducted a sensitivity analysis after exclusion of participants with eGFR<60 (ml/min per 1.73m2) at baseline. Statistical analyses were conducted in SPSS version 25.0 for Windows (SPSS Inc., Chicago, Illinois, USA) and R version 4.0.5.
A total of 3173 hypertensives were included, aged 48.2 ± 10.8 years. Among them, 2097(66.1%) were men, 1066(33.1%) were cigarette consumers, and 1051(33.1%) were alcohol takers. Median (interquartile range) was 13.1(9.0,19.1) ng/dl for PAC, 1.4(0.6,2.8) ng/ml/h for PRA, and 9.8(4.9,23.2) ng/dl per ng/ml/h for ARR. Mean SBP and DBP were 139.8 ± 19.5mmHg and 92.1 ± 13.9mmHg, respectively.
Participants were divided into three groups as T1 (<10.35), T2 (10.35–16.69) and T3 (>16.69) using tertiles of PAC at baseline. Participants in the third tertile of PAC were more likely to be younger, and had higher SBP and DBP. Additionally, participants with higher PAC had lower eGFR, HDL-C and serum potassium. Details of participant characteristics by tertiles of PAC at baseline were shown in Table 1.
As shown in Table 2, during a median follow-up period of 7.3 years (interquartile range, 6.4-8.2) and 22640.25 person-years, 69 deaths and 343 incident CVD occurred with incidence of 18.2/1000 person-years in all participants. Incidence in third tertile was 23.9/1000 person-years.

Table 2 Follow-up time and incidence of primary outcomes in total patients and tertiled by plasma aldosterone concentration.
Kaplan-Meier curves for composite outcomes according to PAC, PRA, and ARR tertiles were presented in Figure 2. The curve depicted an increased risk of composite outcomes in participants in the top tertile of PAC (P log-rank<0.001).
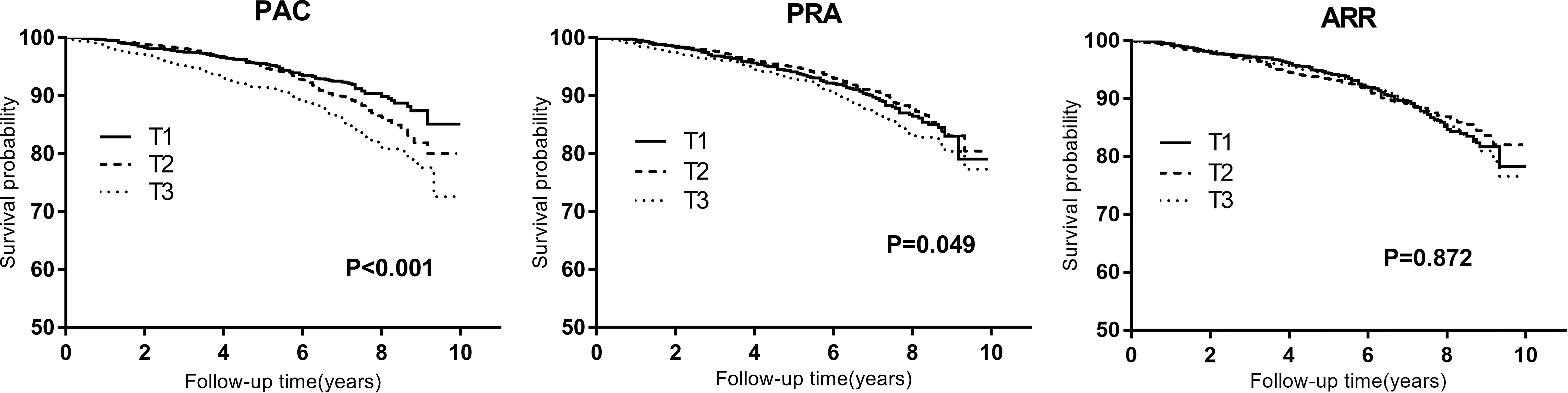
Figure 2 Kaplan-Meier curves for composite outcomes across tertiles of plasma aldosterone concentration (PAC), plasma renin activity (PRA), and aldosterone-to-renin ratio (ARR). T1 to T3 indicate ascending tertiles; T1 below 10.35(n=1058), T2 10.35–16.69 (n=1057), T3 above 16.69 (ng/dl) (n=1058) for PAC; T1 below 0.80(n=1055), T2 0.80–2.16 (n=1064), T3 above2.16 (ng/ml per h) (n=1054) for PRA; T1 below 6.05(n=1057), T2 6.05–16.33 (n=1060), T3 above 16.33(ng/dl)/(ng/ml per h) (n=1056) for ARR.
Cox regression showed that high PAC was associated with increased risk of CVD and all-cause death in unadjusted model, with HR of 1.86 (T3 vs T1:HR=1.86, 95%CI, 1.45-2.38, P<0.001), which remained significant in full adjusted model (Table 3). Compared with patients in first tertile, adjusted HR was 1.81 for those in third tertile (95%CI,1.39-2.35, P<0.001). Every 1, 5 and 10 unit increase of PAC was associated with a 1%, 7%, and 14% higher risk of CVD and all-cause death, respectively, in full adjusted model (Model3). Sensitivity analyses by excluding those with follow-up less than 12 months, reduced eGFR (<60 ml/min per 1.73m2), those with CVD at baseline, suspicious PA patients, or diagnosed PA patients did not change the association between PAC and composite outcomes (Tables S2 through S6).
Time-dependent ROC curve with the highest Youden index was used to determine the optimal cut-off value of PAC for predicting 8-year CVD and all-cause death, then we determined that the optimal threshold for PAC was 12.5ng/dl (AUC=0.573, 95%CI: 0.539-0.607; +LR=1.401, -LR=0.632; Se=0.669, Sp=0.524).
We divided all patients into two groups using the observed cut-off as <12.5ng/dl and ≥12.5ng/dl groups. In binary variables, the incidence of composite outcomes in the patients with PAC ≥12.5ng/dl was significantly higher than those with PAC <12.5ng/dl, and HRs for unadjusted model and full adjusted model 3 were 1.65 (95%CI,1.34-2.03, P<0.001), and 1.61 (95%CI, 1.30-1.99 P<0.001), respectively.
Stratified analyses were performed to evaluate the association of PAC as a binary variable (≥12.5 versus <12.5ng/dl) with the risk of incident composite outcomes. Gender, age, cigarette consumption, alcohol intake, BMI, AHI, SBP, DBP, PRA, and interfering agents on PAC had no significant effect modification on the PAC composite outcomes association (P for interaction>0.05) (Figure 3). Whether renin is suppressed or not, elevated PAC is independently associated with increased CVD risk.
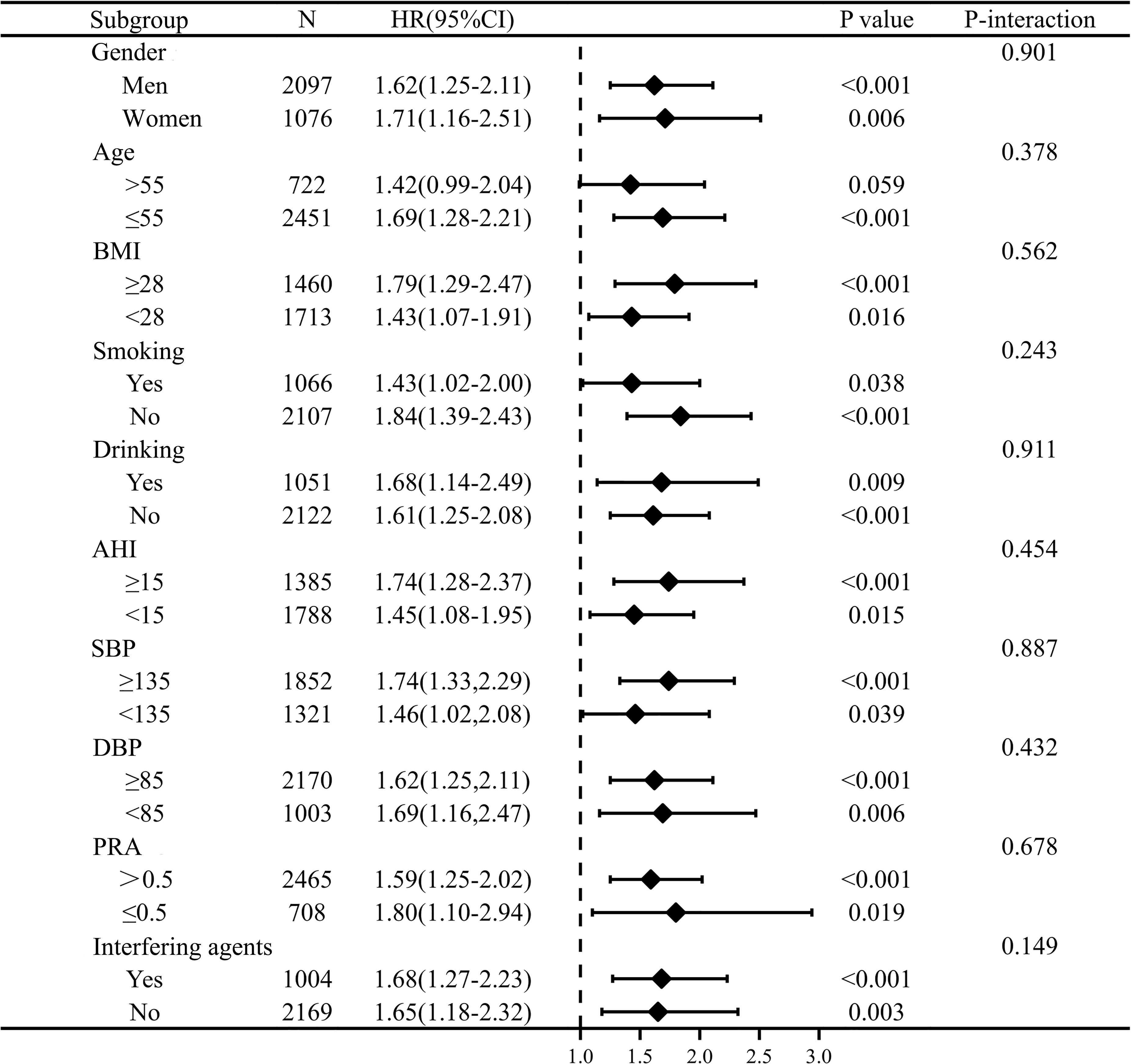
Figure 3 Stratification analysis on relationship of PAC with outcome. PAC was converted to binary variables according to the cut-off value of 12.5ng/dl via time-dependent receiver operating characteristic curve. The model was adjusted for gender, age, BMI, DM at baseline, duration of hypertension≥5, cigarette consumption, alcohol intake, AHI≥15, waist circumference, SBP, DBP, eGFR, TC, TG, HDL-C, LDL-C, Lp(a), serum potassium, PRA, and antihypertensive agents, statins, antiplatelet agents, regular CPAP treatment.
Recent studies have focused on deleterious effects of circulating aldosterone on cardiovascular morbidity and mortality in various populations with inconsistent results among studies and with few data in hypertensives, a group of population with elevated risk for CVD and a huge burden worldwide due to increasing prevalence. To our knowledge, this is the first study in this specific population with inclusion of conditions like OSA and PA. The main observations of the current retrospective cohort study encompassed the following. Higher PAC is associated with higher risks of CVD and all-cause death in hypertensives, independent of established CVD risk factors, of antihypertensive, antiplatelet and lipid regulating treatments, of PRA and even independent of OSA and PA. The optimal cut-off value of PAC for predicting the occurrence of CVD is 12.5ng/dl.
Recently, with recommendation and promotion of screening for PA in hypertensives, damage of PAC to cardiovascular system have gradually acquired more attention. Patients with PA are more likely to develop CVD than patients with essential hypertension, in which PAC may play important roles (7, 8, 27). Current results of UROSAH add some evidence for ongoing debate and or uncertainty and extend previous findings to hypertensive patients. That is, higher PAC is associated with increased risk of CVD and all-cause mortality. Consistent with current observations, LURIC study found that higher PAC is associated with increased CVD and all-cause mortality in elderly patients referred to coronary angiography (17); OPERA study also observed higher PAC is associated with the occurrence of death, heart failure, and acute renal failure in patients with acute myocardial infarction (28). Nonetheless, considering that these results were derived from patients with cardiac insufficiency or ACS in the above studies, it is unclear whether PAC directly damages cardiovascular system, or it is just a marker of RAAS overactivation, and catecholamine surges. Another study from community dwelling African Americans, which included 60% of hypertensive patients, reported that elevated PAC may play important roles in developing CVD and all-cause death (18). Nonetheless, differences in PAC levels among ethnic or racial entities also affect the stability and extrapolation of the results.
Some previous findings have suggested that PAC were associated with cardiac structural and increased risk of all-cause mortality in renin-suppressed group (15, 29). Our stratified analysis shows whether renin is suppressed or not (≤0.5 or >0.5ng/ml per h), elevated PAC is independently associated with an increased risk of CVD. Similarly, sensitivity analysis by exclusion of suspicious PA or diagnosed PA did not change the association between PAC and CVD. These findings imply that attention should still be paid to the harm caused by elevated PAC in hypertensive patients in the absence of PA. Considering the interfering effects of medications on PAC and PRA measurement (24), we performed analysis by presence of interfering agents (yes or no) at PAC measurement and observed that it had no effect on the results (P for interaction=0.149). Considering the impact of PA and of interfering medications on PAC measurement is an important part of our study, since most previous studies failed to analyze. This may also be important for the generalization of current results to patients with essential hypertension. Furthermore, the age stratified analysis found that there was a weakened association between PAC and composite outcomes in the subgroup of age >55 years (30), which may be related to the small number of participants in this group(n=722), whereas further studies are needed.
We conducted this study in hypertensive patients with suspected OSA, and the bidirectional relationship between aldosterone and OSA (31, 32) may limit the generalizability of current results to general hypertensive population. However, hypertension and OSA commonly co-exist. Prevalence of OSA among hypertensive patients is estimated to be about 30%-50% (33), and it is still increasing worldwide (34). In addition, to eliminate the influence of OSA, AHI was taken into account as one of the confounders in Cox regression. Furthermore, stratified analysis by presence of OSA still showed elevated PAC is still a risk factor for CVD in hypertensives with and without OSA.
Currently, few studies have explored the cut-off value of PAC to determine hypertensive population at high risk of developing CVD. A Japanese retrospective cross-sectional study of PA patients observed that patients with PAC≥125pg/ml show a significantly increased risk of CVD and recommended PA-specific treatments to these patients (27). We believe that it is necessary to calculate the optimal cut-off value, which may be helpful for guiding the treatment and management strategy of hypertensive patients. Due to aldosterone escape, interruption of RAAS by ACEI and ARB may not be able to attenuate harmful effects of aldosterone (23). Previous studies have found that mineralocorticoid receptor antagonists (MRAs) are effective in improving the prognosis of patients with heart failure and treating resistant hypertension (35–37), and are also a key treatment for PA patients. Whether to use MRAs to antagonize damage of PAC is a question worth pondering for hypertensive patients with PAC≥12.5ng/dl, at least in Chinese population based on current observation, whereas more studies are warranted.
The current study has several strengths. First, we evaluated the relationship between PAC and cardiovascular events in larger-scale hypertensive patients through longitudinal follow-up. Second, we considered the potential effects of the antihypertensive, antiplatelet and lipid regulating agents, renin, and OSA in the Cox regression. Third, we considered the potential influence of OSA, suspicious PA or diagnosed PA in stratified analyses and sensitivity analyses. Fourth, we evaluated the impact of interfering agents at PAC measurement on the results, which was not accomplished in other studies. Nevertheless, several limitations need to be discussed. First, we lacked 24-hour urine sodium data to assess whether sodium intake had an impact on the relationship between PAC and CVD. Second, we calculated cut-off value of PAC, above which CVD risk was increased. However, the extrapolation of the cut-off value may be affected by the study population and region. Third, we also lacked data on changes in medications, lifestyle and PAC levels during follow-up period, which might affect our results. Fourth, the different detection methods of aldosterone concentration are not consistent. Compared with LC/MS method, radioimmunoassay has errors for detection of lower concentrations of aldosterone, most probably from cross reactivity with soluble aldosterone metabolite, which may limit the extrapolation of our results.
In conclusion, higher PAC is associated with increased risk of CVD and all-cause mortality, even in the absence of PA. Hypertensive patients with PAC≥12.5ng/dl have a significantly increased risk of CVD and special attention and treatment need to be considered.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by Ethics Committee of the People’s Hospital of Xinjiang Uygur Autonomous Region. The patients/participants provided their written informed consent to participate in this study.
LG contributed to the study design and statistical analysis; LG, NL, MH, ML and QZ analyzed the data together and drafted the manuscript; XY, TW, MW, QL, DZ, WJ, and JH participated in data collection. All authors have read and approval the final manuscript.
This research was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (grant number: 2020-RW330-002).
We thank all participants and staff of the UROSAH study for their important contributions.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1017177/full#supplementary-material
1. Joseph P, Leong D, Mckee M, Anand SS, Schwalm JD, Teo K, et al. Reducing the global burden of cardiovascular disease, part 1: The epidemiology and risk factors. Circ Res (2017) 121(6):677. doi: 10.1161/CIRCRESAHA.117.308903
2. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global burden of cardiovascular diseases and risk factors, 1990-2019: Update from the GBD 2019 study. J Am Coll Cardiol (2020) 76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010
3. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol (2020) 16(4):223–37. doi: 10.1038/s41581-019-0244-2
4. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet (2019) 394(10204):1145–58. doi: 10.1016/S0140-6736(19)30427-1
5. Patel S, Rauf A, Khan H, Abu-Izneid T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. BioMed Pharmacother (2017) 94:317–25. doi: 10.1016/j.biopha.2017.07.091
6. Monticone S, D'Ascenzo F, Moretti C, Williams TA, Veglio F, Gaita F, et al. Cardiovascular events and target organ damage in primary aldosteronism compared with essential hypertension: a systematic review and meta-analysis. Lancet Diabetes Endocrinol (2018) 6(1):41–50. doi: 10.1016/S2213-8587(17)30319-4
7. Hundemer GL, Curhan GC, Yozamp N, Wang M, Vaidya A. Cardiometabolic outcomes and mortality in medically treated primary aldosteronism: a retrospective cohort study. Lancet Diabetes Endocrinol (2018) 6(1):51–9. doi: 10.1016/S2213-8587(17)30367-4
8. Briet M, Schiffrin EL. Aldosterone: effects on the kidney and cardiovascular system. Nat Rev Nephrol (2010) 6(5):261–73. doi: 10.1038/nrneph.2010.30
9. Brown NJ. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat Rev Nephrol (2013) 9(8):459–69. doi: 10.1038/nrneph.2013.110
10. Bollati M, Lopez C, Bioletto F, Ponzetto F, Ghigo E, Maccario M. Parasiliti-Caprino M. atrial fibrillation and aortic ectasia as complications of primary aldosteronism: Focus on pathophysiological aspects. Int J Mol Sci (2022) 23(4):2111. doi: 10.3390/ijms23042111
11. Xanthakis V, Vasan RS. Aldosterone and the risk of hypertension. Curr Hypertens Rep (2013) 15(2):102–7. doi: 10.1007/s11906-013-0330-y
12. Bochud M, Nussberger J, Bovet P, Maillard MR, Elston RC, Paccaud F, et al. Plasma aldosterone is independently associated with the metabolic syndrome. Hypertension (2006) 48(2):239–45. doi: 10.1161/01.HYP.0000231338.41548.fc
13. Kumagai E, Adachi H, Jacobs DR Jr., Hirai Y, Enomoto M, Fukami A, et al. Plasma aldosterone levels and development of insulin resistance: prospective study in a general population. Hypertension (2011) 58(6):1043–8. doi: 10.1161/HYPERTENSIONAHA.111.180521
14. Buglioni A, Cannone V, Sangaralingham SJ, Heublein DM, Scott CG, Bailey KR, et al. Aldosterone predicts cardiovascular, renal, and metabolic disease in the general community: A 4-year follow-up. J Am Heart Assoc (2015) 4(12):e002505. doi: 10.1161/JAHA.115.002505
15. Inoue K, Goldwater D, Allison M, Seeman T, Kestenbaum BR, Watson KE. Serum aldosterone concentration, blood pressure, and coronary artery calcium: The multi-ethnic study of atherosclerosis. Hypertension (2020) 76(1):113–20. doi: 10.1161/HYPERTENSIONAHA.120.15006
16. Bernini G, Galetta F, Franzoni F, Bardini M, Taurino C, Bernardini M, et al. Arterial stiffness, intima-media thickness and carotid artery fibrosis in patients with primary aldosteronism. J Hypertens (2008) 26(12):2399–405. doi: 10.1097/HJH.0b013e32831286fd
17. Tomaschitz A, Pilz S, Ritz E, Meinitzer A, Boehm BO, Marz W. Plasma aldosterone levels are associated with increased cardiovascular mortality: the ludwigshafen risk and cardiovascular health (LURIC) study. Eur Heart J (2010) 31(10):1237–47. doi: 10.1093/eurheartj/ehq019
18. Joseph JJ, Echouffo-Tcheugui JB, Kalyani RR, Yeh HC, Bertoni AG, Effoe VS, et al. Aldosterone, renin, cardiovascular events, and all-cause mortality among African americans: The Jackson heart study. JACC Heart Fail (2017) 5(9):642–51. doi: 10.1016/j.jchf.2017.05.012
19. Kumar A, Patel DR, Brennan DM, Wolski KE, Lincoff AM, Ruotolo G, et al. Plasma aldosterone levels are not associated with cardiovascular events among patients with high-risk vascular disease: Insights from the ACCELERATE trial. J Am Heart Assoc (2019) 8(23):e013790. doi: 10.1161/JAHA.119.013790
20. Wang TJ, Gona P, Larson MG, Tofler GH, Levy D, Newton-Cheh C, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med (2006) 355(25):2631–9. doi: 10.1056/NEJMoa055373
21. Conlin PR. Interactions of high salt intake and the response of the cardiovascular system to aldosterone. Cardiol Rev (2005) 13(3):118–24. doi: 10.1097/01.crd.0000148845.74124.c0
22. Rossi GP, Bisogni V, Bacca AV, Belfiore A, Cesari M, Concistre A, et al. The 2020 Italian society of arterial hypertension (SIIA) practical guidelines for the management of primary aldosteronism. Int J Cardiol Hypertens (2020) 5:100029. doi: 10.1016/j.ijchy.2020.100029
23. Te Riet L, van Esch JH, Roks AJ, van den Meiracker AH, Danser AH. Hypertension: renin-angiotensin-aldosterone system alterations. Circ Res (2015) 116(6):960–75. doi: 10.1161/CIRCRESAHA.116.303587
24. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, et al. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J Clin Endocrinol Metab (2016) 101(5):1889–916. doi: 10.1210/jc.2015-4061
25. Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, et al. Human blood pressure determination by sphygmomanometry. Circulation (1993) 88(5 Pt 1):2460–70. doi: 10.1161/01.CIR.88.5.2460
26. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med (2009) 150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006
27. Ohno Y, Sone M, Inagaki N, Yamasaki T, Ogawa O, Takeda Y, et al. Prevalence of cardiovascular disease and its risk factors in primary aldosteronism: A multicenter study in Japan. Hypertension (2018) 71(3):530–7. doi: 10.1161/HYPERTENSIONAHA.117.10263
28. Beygui F, Montalescot G, Vicaut E, Rouanet S, Van Belle E, Baulac C, et al. Aldosterone and long-term outcome after myocardial infarction: A substudy of the french nationwide observatoire sur la prise en charge hospitaliere, l'Evolution a un an et les caRacteristiques de patients presentant un infArctus du myocarde avec ou sans onde q (OPERA) study. Am Heart J (2009) 157(4):680–7. doi: 10.1016/j.ahj.2008.12.013
29. Brown JM, Wijkman MO, Claggett BL, Shah AM, Ballantyne CM, Coresh J, et al. Cardiac structure and function across the spectrum of aldosteronism: the atherosclerosis risk in communities study. Hypertension (2022) 79(9):1984–1993. doi: 10.1161/HYPERTENSIONAHA.122.19134
30. Hannemann A, Friedrich N, Ludemann J, Volzke H, Rettig R, Peters J, et al. Reference intervals for aldosterone, renin, and the aldosterone-to-renin ratio in the population-based study of health in pomerania (SHIP-1). Horm Metab Res (2010) 42(6):392–9. doi: 10.1055/s-0030-1247545
31. Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens (2012) 26(5):281–7. doi: 10.1038/jhh.2011.47
32. Chee MR, Hoo J, Libianto R, Gwini SM, Hamilton G, Narayan O, et al. Prospective screening for primary aldosteronism in patients with suspected obstructive sleep apnea. Hypertension (2021) 77(6):2094–103. doi: 10.1161/HYPERTENSIONAHA.120.16902
33. Konecny T, Kara T, Somers VK. Obstructive sleep apnea and hypertension: an update. Hypertension (2014) 63(2):203–9. doi: 10.1161/HYPERTENSIONAHA.113.00613
34. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med (2019) 7(8):687–98. doi: 10.1016/S2213-2600(19)30198-5
35. Pitt B, Zannad F, Remme WJ, Cody R, Castaigne A, Perez A, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. randomized aldactone evaluation study investigators. N Engl J Med (1999) 341(10):709–17. doi: 10.1056/NEJM199909023411001
36. Zannad F, McMurray JJ, Krum H, van Veldhuisen DJ, Swedberg K, Shi H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med (2011) 364(1):11–21. doi: 10.1056/NEJMoa1009492
Keywords: aldosterone, cardiovascular disease, all-cause mortality, hypertension, renin suppression
Citation: Gan L, Li N, Heizhati M, Lin M, Zhu Q, Yao X, Wu T, Wang M, Luo Q, Zhang D, Jiang W and Hu J (2022) Higher plasma aldosterone is associated with increased risk of cardiovascular events in hypertensive patients with suspected OSA: UROSAH data. Front. Endocrinol. 13:1017177. doi: 10.3389/fendo.2022.1017177
Received: 11 August 2022; Accepted: 23 September 2022;
Published: 07 October 2022.
Edited by:
Peng Li, University of Michigan, United StatesReviewed by:
Tatsuo Shimosawa, International University of Health and Welfare (IUHW), JapanCopyright © 2022 Gan, Li, Heizhati, Lin, Zhu, Yao, Wu, Wang, Luo, Zhang, Jiang and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nanfang Li, bG5hbmZhbmcyMDE2QHNpbmEuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.