
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 28 October 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1016613
Background: There is a relative lack of data that systematically investigates the breadth and validity of the association between bariatric surgery and health-related outcomes. We aimed to evaluate the quantity, validity, and credibility of evidence regarding the association between bariatric surgery and health-related outcomes using an umbrella review of meta-analyses.
Methods: We systematically searched PubMed, Embase, and the Web of Science databases from inception until December 2, 2021, to identify meta-analyses of observational or interventional studies that investigated the association between bariatric surgery and multiple health outcomes. We extracted the summary effect size and 95% confidence interval (CI) data. The Assessment of Multiple Systematic Reviews (AMSTAR-2) and Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) guidelines were used for methodological and evidence quality assessments, respectively.
Results: Twenty-eight studies with 82 different health-related outcomes were included in this umbrella review. Beneficial effects of bariatric surgery have been observed in cancer incidence, mortality, cardiovascular risk, polycystic ovary syndrome (PCOS), anxiety symptoms, depressive symptoms, gestational diabetes mellitus, gestational hypertension, large for gestational age (LGA), macrosomia, post-term birth, risk of kidney stones, albuminuria, urinary incontinence, fecal incontinence, Barrett’s esophagus, and diabetic retinopathy. However, adverse effects of bariatric surgery were observed for maternal anemia, perinatal mortality, congenital anomalies, preterm birth, neonatal intensive care unit (NICU) admission, intrauterine growth restriction, small for gestational age (SGA), fracture risk, upper limb fracture, suicide, self-harm, and alcohol use disorder (AUD).
Conclusions: Current evidence suggests that bariatric surgery improves the majority of health-related outcomes; however, caution is advised given it may increase the risk of adverse mental effects, perinatal problems, and fractures.
Obesity has become a global problem and its prevalence has rapidly increased in recent decades (1). Bariatric surgery has been found to be effective in promoting weight loss and obesity-related comorbidities, such as diabetes and hypertension (2). In 2018, approximately 252,000 bariatric procedures were performed in the United States, and the safety and efficacy of bariatric surgery have been confirmed through long-term clinical follow-up (3).
Despite its accepted safety, the rate of bariatric surgery remains < 1% among the eligible population in the United States (4). This low rate may be driven by questions regarding the long-term effectiveness of bariatric surgery (5). In addition, bariatric surgery may be harmful despite its benefits in terms of weight loss and diabetes remission. Compared with usual care for patients with obesity, the risk of suicide increased by 1.98 times in patients after bariatric surgery (6). A study involving 2,458 participants who were followed up for an average of 4.9 years indicated that suicidal ideation was 5.3% preoperatively and 3.8% one-year postoperatively (7). Incidences of suicide and attempted suicide occurred after an average of 3.8–3.9 years post-surgery (7). A previous meta-analysis suggested that patients had a higher risk of self-harm after bariatric surgery (8). Additionally, the incidence of AUD (9) and fracture risk (10) increased after surgery. Several meta-analyses have indicated an association between bariatric surgery and health-related outcomes; however, their results have been controversial. For example, a meta-analysis involving seven studies suggested that bariatric surgery was associated with a lower risk of cancer incidence (11). In contrast, another meta-analysis involving three studies indicated that bariatric surgery did not significantly reduce the risk of prostate and esophageal cancer (12). Furthermore, outcomes such as sexual function and PCOS have typically received less attention (13, 14).
An umbrella review is a reassessment of systematic reviews and meta-analyses on all health outcomes associated with a particular exposure (15). It provides the highest level of evidence and leads to more reliable conclusions concerning a medical research topic (16). Herein, we performed an umbrella review to identify and evaluate the association between bariatric surgery and health-related outcomes, systematically assessed the quality and strength of the evidence across all health outcomes, and identified studies with the strongest evidence.
We systematically searched PubMed, Embase, and Web of Science from inception until December 2, 2021, to identify meta-analyses of observational or interventional studies that investigated the association between bariatric surgery and any health-related outcomes. Detailed search terms are available in Supplementary File 1. To avoid missing relevant meta-analyses during the initial search, we manually searched the reference lists of eligible publications and applicable clinical guidelines.
The inclusion criteria were as follows (1): investigating the association between bariatric surgery and health-related outcomes; (2) each outcome consisting of at least three studies; (3) studies reporting effect sizes: odds ratio (OR), relative risk (RR), and hazard ratio (HR); (4) summary effect size with 95% (CI); and (5) published in English. The improvement or remission of diabetes after gastrectomy was initially reported > 50 years ago (17). A range of national and international guidelines and position statements state that bariatric surgery can lead to immediate and long-lasting diabetes remission in patients with diabetes and obesity (18–20). Owing to the well-known role of bariatric surgery in the treatment of diabetes, studies that investigated diabetes as the outcome of interest were excluded. We also excluded meeting abstracts, narrative reviews, studies with no data on health outcomes, systematic review protocols, animal studies, and other basic studies. When several meta-analyses investigated the same outcome, we selected the newest meta-analysis with the largest number of studies (21, 22). Two authors independently reviewed the studies. All differences were discussed and resolved by consensus.
Two authors independently extracted all data. Disagreements were resolved by consensus. When the meta-analysis included multiple outcomes, each outcome was extracted separately. The following items were extracted from each meta-analysis: health-related outcomes, first author and year of publication, follow-up, type of bariatric surgery, number of studies and participants in the meta-analysis, design of the original studies, metric of effect size, effects model of meta-analysis, effect size with 95% CI, P-value of heterogeneity or value of I2, and publication bias measures. If the numbers of cases and controls were not reported, we extracted these from the original study.
Methodological quality was assessed for each meta-analysis using the AMSTAR-2, a methodological quality tool used to evaluate systematic reviews and meta-analyses of randomized and non-randomized studies (23). The AMSTAR-2 consists of 16 items, seven of which are critical domains. Each review was graded on whether the critical or non-critical items had methodological defects. Grades were divided into “high”, “moderate”, “low”, and “critically low”. The AMSTAR-2 provides good agreement, reliability, construct validity, and feasibility for methodological quality assessments.
The quality of health-related outcomes was assessed using the GRADE system, which offers a transparent and structured process for developing and presenting evidence summaries (24). Typically, the evidence quality of each outcome is divided into four categories (“high”, “moderate”, “low”, and “very low”) according to assessment of the risk of bias, inconsistency, indirectness, imprecision, and publication bias (25).
The aim of an umbrella review is not to repeat the searches, assess study eligibility, evaluate the risk of bias, or conduct meta-analyses of the included reviews, but rather to provide an overall picture of the findings for particular questions (16). Therefore, we only extracted the existing effect size and 95% CI for each outcome rather than searching for the original studies and reanalyzing the summary estimates. The measures of heterogeneity were based on the P-value of heterogeneity or value of I2; P-value < 0.1 or I2 ≥ 50% were regarded as having significant heterogeneity. Publication bias was assessed using Egger’s test, Begg’s test, or funnel chart in the related meta-analysis, which indicated statistically significant publication bias when the P-value was < 0.1.
A total of 4,401 potentially eligible articles were identified: 2,256 from PubMed; 1,080 from the Web of Science; and 1,065 from Embase. Eleven additional records were identified by reviewing the references of the selected studies. The flowchart of the selection process is shown in Figure 1. After screening titles, abstracts, and full texts, 28 studies with 82 different health-related outcomes were included in the umbrella review (Figure 2). The associations between bariatric surgery and health-related outcomes are presented in Supplementary File 2.
Nine meta-analyses examined the association between cancer risk and bariatric surgery; however, the results were inconclusive. Compared with patients with obesity who did not undergo bariatric surgery, patients who underwent bariatric surgery had a 44% decrease in total cancer incidence (OR = 0.56; 95% CI: 0.46 to 0.68) (11) and a reduction in obesity-related cancer (OR= 0.43, 95% CI: 0.27 to 0.69) (26) (Figure 3). Specifically, bariatric surgery decreased the risk of colorectal cancer (RR = 0.64; 95% CI: 0.42 to 0.98) (27), endometrial cancer (RR = 0.33; 95% CI: 0.21 to 0.51) (28), breast cancer (OR = 0.50; 95% CI: 0.37 to 0.67) (29), and ovarian cancer (OR = 0.47; 95% CI: 0.27 to 0.81) (28) (Figure 3). No association was found between bariatric surgery and pancreatic cancer (OR = 0.70, 95% CI: 0.24 to 2.01) (11), prostate cancer (OR = 0.82, 95% CI: 0.39 to 1.73) (12), or esophageal cancer (OR = 0.79, 95% CI: 0.43 to 1.44) (12) (Figure 4).
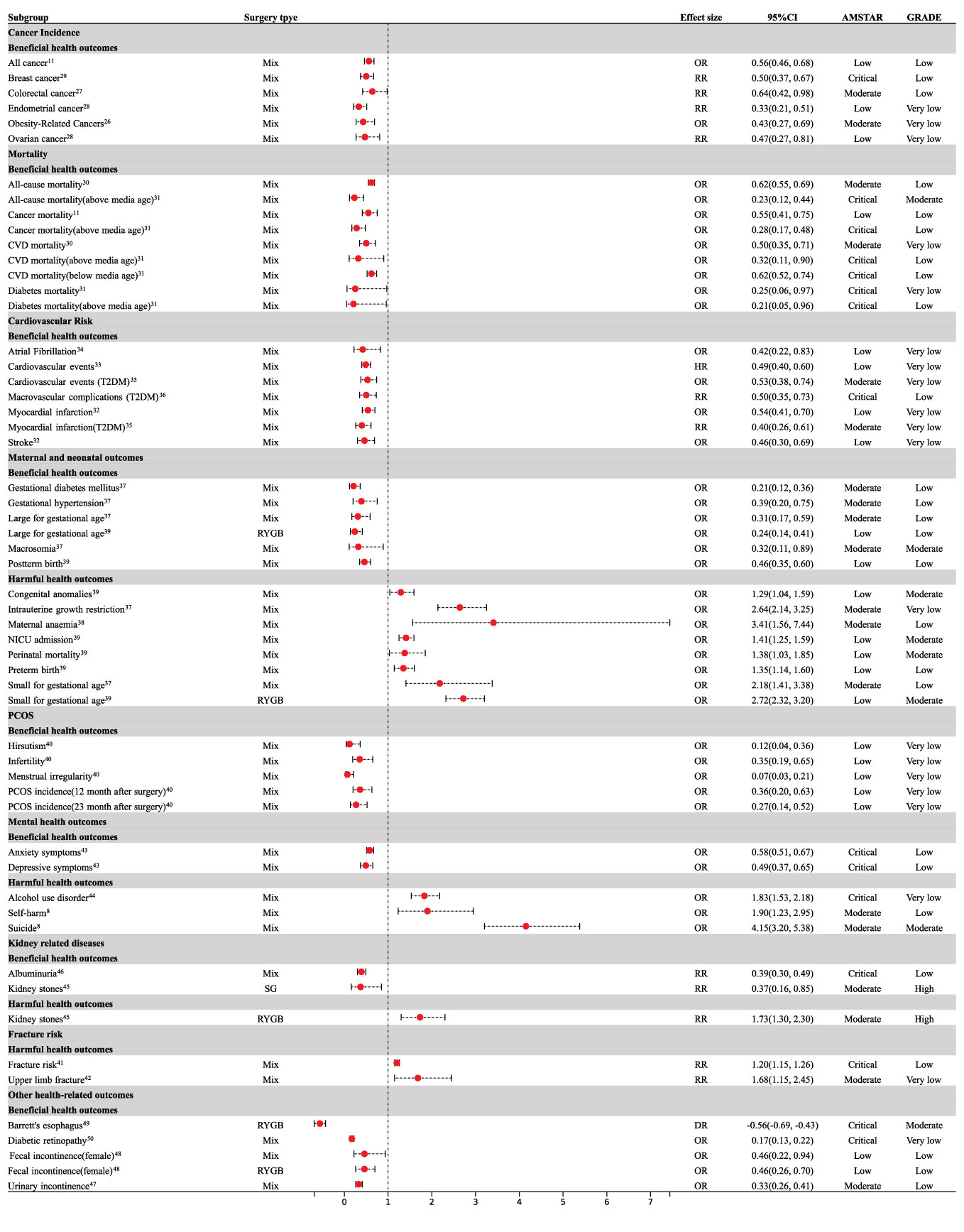
Figure 3 Forest plot of beneficial and harmful health outcomes associated with bariatric surgery. CI, confidence interval; CVD, cardiovascular disease; T2DM, type 2 diabetes mellitus; PCOS, polycystic ovary syndrome; RYGB, Roux-en-Y gastric bypass; SG, Sleeve Gastrectomy; NICU, neonatal intensive care unit.

Figure 4 Forest plot of non-significant associations with bariatric surgery. CI, confidence interval; T2DM, type 2 diabetes mellitus; RYGB, Roux-en-Y gastric bypass; SG, Sleeve Gastrectomy; NICU, neonatal intensive care unit.
There was sufficient evidence that bariatric surgery significantly reduced all-cause mortality (OR = 0.62, 95% CI: 0.55 to 0.69) (30), cardiovascular disease (CVD) mortality (OR = 0.50, 95% CI: 0.35 to 0.71) (30), diabetes-related mortality (OR = 0.25, 95% CI: 0.06 to 0.97) (31), and mortality associated with cancer (OR = 0.55, 95% CI: 0.41 to 0.75) (11) (Figure 3). Median age at the time of the bariatric surgery was 39 years. Compared to patients with obesity who did not undergo bariatric surgery, bariatric surgery was consistently associated with a decreased risk of all-cause mortality (OR = 0.23, 95% CI: 0.12 to 0.44), CVD mortality (OR = 0.32, 95% CI: 0.11 to 0.90), diabetes-related mortality (OR = 0.21, 95% CI: 0.05 to 0.96), and cancer mortality (OR = 0.28, 95% CI: 0.17 to 0.48) above the median age (31) (Figure 3). Below the median age, bariatric surgery was not associated with a decrease in all-cause, diabetes, or cancer mortality (Figure 4), however, CVD mortality was reduced (31) (Figure 3).
Patients who underwent bariatric surgery had a reduced risk of stroke (OR = 0.46; 95% CI: 0.30 to 0.69) (32), cardiovascular events (OR = 0.49; 95% CI: 0.40 to 0.60) (33), myocardial infarction (OR = 0.54; 95% CI: 0.41 to 0.70) (32), and atrial fibrillation (OR = 0.42; 95% CI: 0.22 to 0.83) (34) compared to those who did not undergo bariatric surgery (Figure 3). Bariatric surgery was also associated with a significant reduction in cardiovascular events (HR=0.53; 95% CI: 0.38 to 0.74), myocardial infarction (RR = 0.40; 95% CI: 0.26 to 0.61) (35), and macrovascular complications (RR = 0.50; 95% CI: 0.35 to 0.73) (36) in patients with type 2 diabetes mellitus over five years of follow-up (Figure 3). However, bariatric surgery did not reduce the incidence of stroke (RR = 0.53; 95% CI: 0.28 to 1.01) in patients with type 2 diabetes mellitus (35) (Figure 4).
With regard to maternal outcomes, bariatric surgery reduced the rates of gestational diabetes mellitus (OR = 0.21; 95% CI: 0.12 to 0.36) and gestational hypertension (OR = 0.39; 95% CI: 0.20 to 0.75) (37) (Figure 3); however, bariatric surgery increased the risk of maternal anemia (OR = 3.41; 95% CI: 1.56 to 7.44) when compared with control subjects who were matched for pre-surgery body mass index (38) (Figure 3). Bariatric surgery was not associated with preeclampsia (OR = 0.59; 95% CI: 0.32 to 1.09) or cesarean delivery (OR = 0.63; 95% CI: 0.39 to 1.02) (37) (Figure 4).
Regarding neonatal outcomes, bariatric surgery was significantly related to an increased risk of perinatal mortality (OR = 1.38; 95% CI: 1.03 to 1.85), congenital anomalies (OR = 1.29; 95% CI: 1.04 to 1.59), preterm birth (OR = 1.35; 95% CI: 1.14 to 1.60), NICU admission (OR = 1.41; 95% CI: 1.25 to 1.59), intrauterine growth restriction (OR = 2.64; 95% CI: 2.14 to 3.25), and SGA (OR = 2.18; 95% CI: 1.41 to 3.38) (37, 39) (Figure 3). The bariatric surgery subgroup analysis demonstrated an increased risk of SGA in patients following Roux-en-Y gastric bypass (RYGB) (OR = 2.72; 95% CI: 2.32 to 3.20) (39) (Figure 3). Bariatric surgery significantly reduced the risk of LGA (OR = 0.31; 95% CI: 0.17 to 0.59), macrosomia (OR = 0.32; 95% CI: 0.11 to 0.89), and post-term birth (OR = 0.46; 95% CI: 0.35 to 0.60) (37, 39) (Figure 3). Subgroup analysis by type of surgery demonstrated that RYGB reduced LGA (OR = 0.24; 95% CI: 0.14 to 0.41) (39) (Figure 3). SG was not associated with SGA (OR = 0.88; 95% CI: 0.58 to 1.34) and LGA (OR = 0.59; 95% CI: 0.30 to 1.14) (39) (Figure 4). RYGB was not associated with perinatal mortality (OR = 1.48; 95% CI: 0.87 to 2.51), NICU admission (OR =1.83; 95% CI: 0.84 to 4.00), post-term birth (OR = 0.55; 95% CI: 0.29 to 1.04), or preterm birth (OR = 1.14; 95% CI: 0.89 to 1.46) (39) (Figure 4).
For PCOS, bariatric surgery led to a significantly lower risk of menstrual irregularity (OR = 0.07; 95% CI: 0.03 to 0.21), hirsutism (OR = 0.12; 95% CI: 0.04 to 0.36), infertility (OR = 0.35; 95% CI: 0.19 to 0.65), and the incidence of PCOS was significantly decreased at the 12-month (OR=0.36; 95% CI: 0.20 to 0.63) and 23-month (OR = 0.27; 95% CI: 0.14 to 0.52) follow-ups in comparison to pre-surgery (40) (Figure 3).
The meta-analysis followed up for an average of 2–4.9 years indicated that participants undergoing bariatric surgeries were associated with a higher risk of fractures (RR = 1.20; 95% CI: 1.15 to 1.26) (41), especially upper limb fractures (RR = 1.68; 95% CI: 1.15 to 2.45) (42) in comparison to patients with obesity who did not undergo bariatric surgery (Figure 3), however, there was no significant effect on spine fracture (RR = 1.45; 95% CI: 0.91 to 2.31) (42) (Figure 4).
Bariatric surgery improved depression (OR = 0.49; 95% CI: 0.37 to 0.65) and anxiety (OR = 0.58; 95% CI: 0.51 to 0.67) in patients with obesity after surgery (43) (Figure 3). Bariatric surgery appeared to increase the risk of suicide (OR = 4.15; 95% CI: 3.20 to 5.38) and self-harm (OR = 1.90; 95% CI: 1.23 to 2.95) during a follow-up of eight–ten years (8) (Figure 3). Bariatric surgery had no significant effect on AUD after one- and two-years follow-up (Figure 4); however, AUD incidence significantly increased (OR = 1.83; 95% CI: 1.53 to 2.178) after three years of follow-up (44) (Figure 3).
Bariatric surgery was not significantly associated with the risk of kidney stones (RR =1.22; 95% CI: 0.63 to 2.35) (45) (Figure 4). However, the bariatric surgery subgroup analysis demonstrated an increased risk of kidney stones in patients after RYGB (RR = 1.73; 95% CI: 1.30 to 2.30) and a decreased risk of kidney stones after sleeve gastrectomy (RR = 0.37;95% CI: 0.16 to 0.85) (45) (Figure 3). Bariatric surgery significantly reduced albuminuria (RR = 0.39;95% CI: 0.30 to 0.49) (46) (Figure 3).
For urinary incontinence, 13.4 months after surgery, the risk of urinary incontinence (OR = 0.33; 95% CI: 0.26 to 0.41) in women was significantly lower than that before surgery (47) (Figure 3). There was a significant reduction in fecal incontinence in women after bariatric surgery (OR = 0.46; 95% CI: 0.22 to 0.94) (48) (Figure 3). RYGB improved fecal incontinence (OR = 0.46; 95% CI: 0.26 to 0.70) (48) (Figure 3). Bariatric surgery significantly improved Barrett’s esophagus (RD = -0.56; 95% CI: - 0.69 to - 0.43) > 1 year after surgery (49) (Figure 3). Participants who underwent bariatric surgery had a significantly lower risk of diabetic retinopathy (RR = 0.17; 95% CI: 0.13 to 0.22) (50) (Figure 3). There was no significant effect on pelvic organ prolapse (OR = 0.48; 95% CI: 0.22 to 1.07) or fecal incontinence (OR = 0.80; 95% CI: 0.53 to 1.21) (47) (Figure 4). There was no effect on fecal incontinence following SG (OR = 0.21; 95% CI: 0.04 to 1.16) or gastric banding (OR = 0.84; 95% CI: 0.45 to 1.56) (48) (Figure 4).
The methodological quality of the 28 studies assessed using AMSTAR-2 is presented in Supplementary File 3. Nine studies (32%) were rated as critically low, eight studies (29%) as low, and eleven studies (39%) as moderate. The quality of evidence for each outcome was assessed using the GRADE system. Two outcomes were rated as high quality (2%), fifteen outcomes (18%) as moderate, thirty-five (43%) as low, and thirty (37%) as very low (Supplementary File 4).
In this umbrella review, we assessed 28 studies with 82 different health outcomes. According to the existing evidence, bariatric surgery benefits a sequence of health outcomes. Beneficial associations were found for the risk of various cancers, CVD, mortality, PCOS, urinary incontinence, and fecal incontinence. Moreover, we highlighted that bariatric surgery could be harmful in certain populations, leading to poor mental health outcomes, fractures, kidney stones, and adverse perinatal outcomes.
Bariatric surgery is associated with a lower incidence of female-specific cancers such as ovarian, breast, and endometrial cancers. Obesity contributes to approximately 6% of all cancers (51). Furthermore, it accounts for 14% and 20% of all cancer-related deaths in men and women, respectively, in the United States (51). Feigelson et al. conducted a cohort study including 17,998 bariatric surgery patients and 53,889 matched controls with ten years of follow-up, indicating that bariatric surgery was associated with a reduced risk of breast cancer in both premenopausal and postmenopausal women (52). According to a study involving 1,867 participants with obesity with a mean follow-up of 18.1 years in Sweden, bariatric surgery may reduce the incidence of female-specific cancers (53). The following mechanisms that lead to female-specific cancers may contribute to this association: hyperestrogenemia, insulin resistance, and chronic inflammation (54). Breast and endometrial cancers are highly sensitive to estrogen, and respond rapidly to changes. Adipose tissue expresses high levels of the estrogen-synthesizing enzyme aromatase; therefore, it is an important source of estrogen (55). Women with obesity are more likely to develop insulin resistance, which reduces the concentration of sex hormone-binding globulin in the body, resulting in an increase in bioavailability of estrogen (56, 57). Increased estrogen levels and bioavailability are associated with breast, ovarian, and endometrial cancers. The lower mortality after metabolic surgery may be due to improved statuses of diabetes, hypertension, and CVD (58).
In this umbrella review, bariatric surgery was found to be associated with a reduction in the prevalence of depression and anxiety. A prospective meta-analysis of 68 studies found that the postoperative prevalence of depression decreased by 8–74% (59). Psychosocial and physiological factors can lead to depression and anxiety. Increased body image satisfaction in patients with obesity after bariatric surgery results in improved self-esteem and self-worth (59), and these positive thoughts can improve symptoms of depression and anxiety. In contrast, insulin and leptin resistance affect the normal function of brain tissue, which can lead to depression (60). Bariatric surgery can improve insulin resistance and leptin secretion, thereby improving depression (61). However, this umbrella review also showed that bariatric surgery was associated with an increased risk of suicide, self-harm, and AUD. Recent studies have shown that patients with preoperative suicide-related psychiatric disorders and gastric bypass are more likely to commit suicide (62). Patients may have unrealistic expectations of life after surgery, and disappointment can lead to mental illness and suicide. By contrast, surgical trauma precipitates the remaining underlying psychiatric vulnerability in patients with a history of psychiatric disorders (63). Gastric bypass is more likely to cause nutritional deficiencies and serious complications than other types of procedures, thereby affecting patients’ quality of life and contributing to suicide (64). In addition, alcohol intake after gastric bypass surgery results in higher blood alcohol concentrations and an increased incidence of alcohol abuse, which can lead to impulsive behavior (65).
Despite the beneficial effects of bariatric surgery on pregnancy outcomes, some adverse effects persist. As many as 80% of patients undergoing bariatric surgery are women of childbearing age (66). Additionally, PCOS and fertility in women with obesity have been shown to improve after bariatric surgery. Therefore, pregnancy after bariatric surgery is becoming increasingly common. Poor perinatal outcomes included perinatal mortality, congenital anomalies, preterm birth, NICU admission, intrauterine growth restriction, and SGA. It has been hypothesized that adverse perinatal outcomes are mainly related to malnutrition. Malabsorption surgery bypasses the small intestine, the main site of vitamin and mineral absorption (67, 68). Studies have shown that 11–77% of women undergoing bariatric surgery develop anemia during pregnancy (69). The rate of folate deficiency after gastric bypass surgery is reported to be 16% (70), and 60–97% of pregnant patients post-bariatric surgery have vitamin D deficiencies (71, 72). Another study showed a median surgery-to-conception interval of 1.1 years, suggesting that many women may continue trying to lose weight when pregnant (73), leading to nutrient deficiencies. Patients willing to bear children after surgery must be fully informed about the possible benefits and risks of pregnancy, the appropriate interval between bariatric surgery and pregnancy, and effective postoperative nutritional support.
Our study has several strengths. First, we reviewed the association between bariatric surgery and 82 health-related outcomes. We summarized the outcomes that were significantly improved or deteriorated after bariatric surgery, which could increase understanding of the impact of bariatric surgery on multiple health-related outcomes and improve postoperative management. However, this study had some limitations. Different bariatric procedures are associated with different efficacies of weight loss and postoperative complications (74). Bariatric surgery applied in most outcomes were combined with mixed procedures. Due to the lack of relevant data, we did not analyze the impact of different bariatric procedures on health-related outcomes separately. The association between different bariatric procedures and multiple health-related outcomes should be investigated in the future. In addition, the methodological quality and the quality of evidence for each outcome of the included papers are not high. It is necessary to follow up on the health-related outcomes of bariatric surgery in real time to summarize the latest evidence.
Bariatric surgery benefits most health-related outcomes and is worth promoting in patients with obesity. However, after bariatric surgery, caution should be exercised due to the increased risk of adverse mental and perinatal effects, fractures, and kidney stones. Furthermore, studies investigating ways to improve the postoperative management of patients that underwent bariatric surgery are required.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
JL was the project leader in the current study and wrote the manuscript. YJC and JZ searched the databases and screened the articles. YC and ZC extracted the data and conducted the statistical analyses. Finally, YY and YW reviewed and revised the manuscript. All authors contributed to the manuscript and approved the submitted version.
This study was supported by the Sichuan Science & Technology Program (No. 2022YFS0167), West China Nursing Discipline Development Special Fund Project, Sichuan University (No. HXHL21002), West China Nursing Discipline Development Special Fund Project, Sichuan University (No. HXHL20044).
The authors would like to acknowledge all authors of the original studies that were included in this meta-analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1016613/full#supplementary-material
1. Collaboration, N.C.D.R.F. Trends in adult body-mass index in 200 countries from 1975 to 2014: A pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet (2016) 387:1377–96. doi: 10.1016/s0140-6736(16)30054-x
2. Schauer P, Bhatt D, Kirwan J, Wolski K, Aminian A, Brethauer S, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med (2017) 376:641–51. doi: 10.1056/NEJMoa1600869
3. Arterburn DE, Telem DA, Kushner RF, Courcoulas AP. Benefits and risks of bariatric surgery in adults: A review. JAMA (2020) 324:879–87. doi: 10.1001/jama.2020.12567
4. Campos G, Khoraki J, Browning M, Pessoa B, Mazzini G, Wolfe L. Changes in utilization of bariatric surgery in the united states from 1993 to 2016. Ann Surg (2020) 271:201–09. doi: 10.1097/sla.0000000000003554
5. Chao G, Bonham A, Ross R, Stricklen A, Ghaferi A. Patient-reported comorbidity assessment after bariatric surgery: A potential tool to improve longitudinal follow-up. Ann Surg (2021). doi: 10.1097/sla.0000000000004841
6. Konttinen H, Sjöholm K, Jacobson P, Svensson P, Carlsson L, Peltonen M. Prediction of suicide and nonfatal self-harm after bariatric surgery: a risk score based on sociodemographic factors, lifestyle behavior, and mental health: a nonrandomized controlled trial. Ann Surg (2021) 274:339–45. doi: 10.1097/sla.0000000000003742
7. Gordon K, King W, White G, Belle S, Courcoulas A, Ebel F, et al. A longitudinal examination of suicide-related thoughts and behaviors among bariatric surgery patients. Surg Obes Relat Dis (2019) 15:269–78. doi: 10.1016/j.soard.2018.12.001
8. Castaneda D, Popov V, Wander P, Thompson C. Risk of suicide and self-harm is increased after bariatric surgery-a systematic review and meta-analysis. Obes Surg (2019) 29:322–33. doi: 10.1007/s11695-018-3493-4
9. King W, Chen J, Mitchell J, Kalarchian M, Steffen K, Engel S, et al. Prevalence of alcohol use disorders before and after bariatric surgery. JAMA (2012) 307:2516–25. doi: 10.1001/jama.2012.6147
10. Ahlin S, Peltonen M, Sjöholm K, Anveden Å, Jacobson P, Andersson-Assarsson J, et al. Fracture risk after three bariatric surgery procedures in swedish obese subjects: up to 26 years follow-up of a controlled intervention study. J Intern Med (2020) 287:546–57. doi: 10.1111/joim.13020
11. Zhang K, Luo Y, Dai H, Deng Z. Effects of bariatric surgery on cancer risk: evidence from meta-analysis. Obes Surg (2020) 30:1265–72. doi: 10.1007/s11695-019-04368-4
12. Wiggins T, Antonowicz SS, Markar SR. Cancer risk following bariatric surgery-systematic review and meta-analysis of national population-based cohort studies. Obes Surg (2019) 29:1031–39. doi: 10.1007/s11695-018-3501-8
13. Xu J, Wu Q, Zhang Y, Pei C. Effect of bariatric surgery on male sexual function: a meta-analysis and systematic review. Sex Med (2019) 7:270–81. doi: 10.1016/j.esxm.2019.06.003
14. Li YJ, Han Y, He B. Effects of bariatric surgery on obese polycystic ovary syndrome: a systematic review and meta-analysis. Surg Obes Relat Dis (2019) 15:942–50. doi: 10.1016/j.soard.2019.03.032
15. Poole R, Kennedy O, Roderick P, Fallowfield J, Hayes P, Parkes J. Coffee consumption and health: umbrella review of meta-analyses of multiple health outcomes. BMJ (2017) 359:j5024. doi: 10.1136/bmj.j5024
16. Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc (2015) 13:132–40. doi: 10.1097/xeb.0000000000000055
17. Friedman M, Sancetta A, Magovern G. The amelioration of diabetes mellitus following subtotal gastrectomy. Surg Gynecol Obstet (1955) 100:201–4.
18. Rubino F, Kaplan L, Schauer P, Cummings D. The diabetes surgery summit consensus conference: recommendations for the evaluation and use of gastrointestinal surgery to treat type 2 diabetes mellitus. Ann Surg (2010) 251:399–405. doi: 10.1097/SLA.0b013e3181be34e7
19. American Diabetes Association. Standards of medical care in diabetes-2019 abridged for primary care providers. Clin Diabetes (2019) 37:11–34. doi: 10.2337/cd18-0105
20. American Diabetes Association. 7. obesity management for the treatment of type 2 diabetes: standards of medical care in diabetes-2018. Diabetes Care (2018) 41:S65–s72. doi: 10.2337/dc18-S007
21. Theodoratou E, Tzoulaki I, Zgaga L, Ioannidis J. Vitamin d and multiple health outcomes: umbrella review of systematic reviews and meta-analyses of observational studies and randomised trials. BMJ (2014) 348:g2035. doi: 10.1136/bmj.g2035
22. Radua J, Ramella-Cravaro V, Ioannidis J, Reichenberg A, Phiphopthatsanee N, Amir T, et al. What causes psychosis? an umbrella review of risk and protective factors. World Psychiatry (2018) 17:49–66. doi: 10.1002/wps.20490
23. Shea B, Reeves B, Wells G, Thuku M, Hamel C, Moran J, et al. Amstar 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (2017) 358:j4008. doi: 10.1136/bmj.j4008
24. Guyatt G, Oxman A, Kunz R, Woodcock J, Brozek J, Helfand M, et al. Grade guidelines: 7. rating the quality of evidence–inconsistency. J Clin Epidemiol (2011) 64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017
25. Guyatt G, Oxman A, Akl E, Kunz R, Vist G, Brozek J, et al. Grade guidelines: 1. introduction-grade evidence profiles and summary of findings tables. J Clin Epidemiol (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
26. Yang XW, Li PZ, Zhu LY, Zhu S. Effects of bariatric surgery on incidence of obesity-related cancers: a meta-analysis. Med Sci Monit (2015) 21:1350–7. doi: 10.12659/msm.893553
27. Almazeedi S, El-Abd R, Al-Khamis A, Albatineh AN, Al-Sabah S. Role of bariatric surgery in reducing the risk of colorectal cancer: a meta-analysis. Br J Surg (2020) 107:348–54. doi: 10.1002/bjs.11494
28. Ishihara BP, Farah D, Fonseca MCM, Nazario A. The risk of developing breast, ovarian, and endometrial cancer in obese women submitted to bariatric surgery: a meta-analysis. Surg Obes Relat Dis (2020) 16:1596–602. doi: 10.1016/j.soard.2020.06.008
29. Lovrics O, Butt J, Lee Y, Lovrics P, Boudreau V, Anvari M, et al. The effect of bariatric surgery on breast cancer incidence and characteristics: a meta-analysis and systematic review. Am J Surg (2021) 222:715–22. doi: 10.1016/j.amjsurg.2021.03.016
30. Wiggins T, Guidozzi N, Welbourn R, Ahmed AR, Marker SR. Association of bariatric surgery with all-cause mortality and incidence of obesity-related disease at a population level: A systematic review and meta-analysis. PloS Med (2020) 17:e1003206. doi: 10.1371/journal.pmed.1003206
31. Pontiroli AE, Ceriani V, Tagliabue E. Compared with controls, bariatric surgery prevents long-term mortality in persons with obesity only above median age of cohorts: A systematic review and meta-analysis. Obes Surg (2020) 30:2487–96. doi: 10.1007/s11695-020-04530-3
32. Kwok CS, Pradhan A, Khan MA, Anderson SG, Keavney BD, Myint PK, et al. Bariatric surgery and its impact on cardiovascular disease and mortality: A systematic review and meta-analysis. Int J Cardiol (2014) 173:20–8. doi: 10.1016/j.ijcard.2014.02.026
33. Sutanto A, Wungu C, Susilo H, Sutanto H. Reduction of major adverse cardiovascular events (mace) after bariatric surgery in patients with obesity and cardiovascular diseases: a systematic review and meta-analysis. Nutrients (2021) 13:3568. doi: 10.3390/nu13103568
34. Chokesuwattanaskul R, Thongprayoon C, Bathini T, Sharma K, Watthanasuntorn K, Lertjitbanjong P, et al. Incident atrial fibrillation in patients undergoing bariatric surgery: A systematic review and meta-analysis. Intern Med J (2020) 50:810–7. doi: 10.1111/imj.14436
35. Yan G, Wang J, Zhang J, Gao K, Zhao Q, Xu X. Long-term outcomes of macrovascular diseases and metabolic indicators of bariatric surgery for severe obesity type 2 diabetes patients with a meta-analysis. PloS One (2019) 14:e0224828. doi: 10.1371/journal.pone.0224828
36. Hussain S, Khan M, Jamali M, Siddiqui A, Gupta G, Hussain M, et al. Impact of bariatric surgery in reducing macrovascular complications in severely obese t2dm patients. Obes Surg (2021) 31:1929–36. doi: 10.1007/s11695-020-05155-2
37. Kwong W, Tomlinson G, Feig DS. Maternal and neonatal outcomes after bariatric surgery; a systematic review and meta-analysis: do the benefits outweigh the risks? Am J Obstet Gynecol (2018) 218:573–80. doi: 10.1016/j.ajog.2018.02.003
38. Galazis N, Docheva N, Simillis C, Nicolaides KH. Maternal and neonatal outcomes in women undergoing bariatric surgery: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol (2014) 181:45–53. doi: 10.1016/j.ejogrb.2014.07.015
39. Akhter Z, Rankin J, Ceulemans D, Ngongalah L, Ackroyd R, Devlieger R, et al. Pregnancy after bariatric surgery and adverse perinatal outcomes: a systematic review and meta-analysis. PloS Med (2019) 16:e1002866. doi: 10.1371/journal
40. Skubleny D, Switzer N, Gill RS, Dykstra M, Shi X, De Gara C, et al. The impact of bariatric surgery on polycystic ovary syndrome: a systematic review and meta-analysis. Obes Surg (2016) 26:169–76. doi: 10.1007/s11695-015-1902-5
41. Chaves Pereira de Holanda N, de Lima Carlos I, Chaves de Holanda Limeira C, Cesarino de Sousa D, Serra de Lima Junior F, Telis de Vilela Araújo A, et al. Fracture risk after bariatric surgery: A systematic literature review and meta-analysis. Endocr Pract (2022) 28:58–69. doi: 10.1016/j.eprac.2021.09.007
42. Zhang Q, Chen Y, Li J, Chen D, Cheng Z, Xu S, et al. A meta-analysis of the effects of bariatric surgery on fracture risk. Obes Rev (2018) 19:728–36. doi: 10.1111/obr.12665
43. Fu R, Zhang Y, Yu K, Mao D, Su H. Bariatric surgery alleviates depression in obese patients: a systematic review and meta-analysis. Obes Res Clin Pract (2022) 16:10–6. doi: 10.1016/j.orcp.2021.11.002
44. Azam H, Shahrestani S, Phan K. Alcohol use disorders before and after bariatric surgery: A systematic review and meta-analysis. Ann Transl Med (2018) 6:148. doi: 10.21037/atm.2018.03.16
45. Thongprayoon C, Cheungpasitporn W, Vijayvargiya P, Anthanont P, Erickson SB. The risk of kidney stones following bariatric surgery: A systematic review and meta-analysis. Ren Fail (2016) 38:424–30. doi: 10.3109/0886022X.2015.1137186
46. Huang H, Lu J, Dai X, Li Z, Zhu L, Zhu S, et al. Improvement of renal function after bariatric surgery: A systematic review and meta-analysis. Obes Surg (2021) 31:4470–84. doi: 10.1007/s11695-021-05630-4
47. Montenegro M, Slongo H, Juliato CRT, Minassian VA, Tavakkoli A, Brito LGO. The impact of bariatric surgery on pelvic floor dysfunction: A systematic review. J Minim Invasive Gynecol (2019) 26:816–25. doi: 10.1016/j.jmig.2019.01.013
48. Mohamed F, Jeram M, Coomarasamy C, Lauti M, Wilson D, MacCormick A. Does bariatric surgery improve faecal incontinence? A systematic review and meta-analysis. Obes Surg (2021) 31:2942–53. doi: 10.1007/s11695-021-05360-7
49. Adil M, Al-Taan O, Rashid F, Munasinghe A, Jain V, Whitelaw D, et al. A systematic review and meta-analysis of the effect of roux-en-y gastric bypass on barrett's esophagus. Obes Surg (2019) 29:3712–21. doi: 10.1007/s11695-019-04083-0
50. Yu C, Park L, Pinto A, Ma O, Lee Y, Gupta R, et al. The impact of bariatric surgery on diabetic retinopathy: A systematic review and meta-analysis. Am J Ophthalmol (2021) 225:117–27. doi: 10.1016/j.ajo.2020.12.033
51. Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med (2003) 348:1625–38. doi: 10.1056/NEJMoa021423
52. Feigelson H, Caan B, Weinmann S, Leonard A, Powers J, Yenumula P, et al. Bariatric surgery is associated with reduced risk of breast cancer in both premenopausal and postmenopausal women. Ann Surg (2020) 272:1053–59. doi: 10.1097/sla.0000000000003331
53. Anveden Å, Taube M, Peltonen M, Jacobson P, Andersson-Assarsson J, Sjöholm K, et al. Long-term incidence of female-specific cancer after bariatric surgery or usual care in the swedish obese subjects study. Gynecol Oncol (2017) 145:224–29. doi: 10.1016/j.ygyno.2017.02.036
54. MacKintosh M, Derbyshire A, McVey R, Bolton J, Nickkho-Amiry M, Higgins C, et al. The impact of obesity and bariatric surgery on circulating and tissue biomarkers of endometrial cancer risk. Int J Cancer (2019) 144:641–50. doi: 10.1002/ijc.31913
55. McCawley G, Ferriss J, Geffel D, Northup C, Modesitt S. Cancer in obese women: potential protective impact of bariatric surgery. J Am Coll Surg (2009) 208:1093–98. doi: 10.1016/j.jamcollsurg.2009.01.045
56. Iyengar N, Zhou X, Gucalp A, Morris P, Howe L, Giri D, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res (2016) 22:2283–89. doi: 10.1158/1078-0432.Ccr-15-2239
57. Renehan A, Tyson M, Egger M, Heller R, Zwahlen M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet (2008) 371:569–78. doi: 10.1016/s0140-6736(08)60269-x
58. Doumouras AG, Hong D, Lee Y, Tarride JE, Paterson JM, Anvari M. Association between bariatric surgery and all-cause mortality: A population-based matched cohort study in a universal health care system. Ann Intern Med (2020) 173:694–703. doi: 10.7326/m19-3925
59. Dawes A, Maggard-Gibbons M, Maher A, Booth M, Miake-Lye I, Beroes J, et al. Mental health conditions among patients seeking and undergoing bariatric surgery: A meta-analysis. JAMA (2016) 315:150–63. doi: 10.1001/jama.2015.18118
60. Milaneschi Y, Simmons W, van Rossum E, Penninx B. Depression and obesity: evidence of shared biological mechanisms. Mol Psychiatry (2019) 24:18–33. doi: 10.1038/s41380-018-0017-5
61. Gu L, Lin K, Du N, Ng D, Lou D, Chen P. Differences in the effects of laparoscopic sleeve gastrectomy and laparoscopic roux-en-y gastric bypass on gut hormones: Systematic and meta-analysis. Surg Obes Relat Dis (2021) 17:444–55. doi: 10.1016/j.soard.2020.10.018
62. Kauppila JH, Santoni G, Tao W, Lynge E, Jokinen J, Tryggvadóttir L, et al. Risk factors for suicide after bariatric surgery in a population-based nationwide study in five nordic countries. Ann Surg (2022) 275:e410–e14. doi: 10.1097/sla.0000000000004232
63. Fazel S, Wolf A, Palm C, Lichtenstein P. Violent crime, suicide, and premature mortality in patients with schizophrenia and related disorders: A 38-year total population study in sweden. Lancet Psychiatry (2014) 1:44–54. doi: 10.1016/s2215-0366(14)70223-8
64. Mitchell J, Crosby R, de Zwaan M, Engel S, Roerig J, Steffen K, et al. Possible risk factors for increased suicide following bariatric surgery. Obesity (2013) 21:665–72. doi: 10.1002/oby.20066
65. Klockhoff H, Näslund I, Jones A. Faster absorption of ethanol and higher peak concentration in women after gastric bypass surgery. Br J Clin Pharmacol (2002) 54:587–91. doi: 10.1046/j.1365-2125.2002.01698.x
66. Rottenstreich A, Elazary R, Goldenshluger A, Pikarsky A, Elchalal U, Ben-Porat T. Maternal nutritional status and related pregnancy outcomes following bariatric surgery: a systematic review. Surg Obes Relat Dis (2019) 15:324–32. doi: 10.1016/j.soard.2018.11.018
67. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition (2010) 26:1031–7. doi: 10.1016/j.nut.2009.12.003
68. Cruz S, Matos A, da Cruz S, Pereira S, Saboya C, Ramalho A. Relationship between the nutritional status of vitamin a per trimester of pregnancy with maternal anthropometry and anemia after roux-en-y gastric bypass. Nutrients (2017) 9:989. doi: 10.3390/nu9090989
69. Mead NC, Sakkatos P, Sakellaropoulos GC, Adonakis GL, Alexandrides TK, Kalfarentzos F. Pregnancy outcomes and nutritional indices after 3 types of bariatric surgery performed at a single institution. Surg Obes Relat Dis (2014) 10:1166–73. doi: 10.1016/j.soard.2014.02.011
70. Devlieger R, Guelinckx I, Jans G, Voets W, Vanholsbeke C, Vansant G. Micronutrient levels and supplement intake in pregnancy after bariatric surgery: a prospective cohort study. PloS One (2014) 9:e114192. doi: 10.1371/journal.pone.0114192
71. Medeiros M, Matos A, Pereira S, Saboya C, Ramalho A. Vitamin d and its relation with ionic calcium, parathyroid hormone, maternal and neonatal characteristics in pregnancy after roux-En-Y gastric bypass. Arch Gynecol Obstet (2016) 293:539–47. doi: 10.1007/s00404-015-3861-4
72. Cruz S, de Matos A, da Cruz S, Pereira S, Saboya C, Ramalho A. Maternal anthropometry and its relationship with the nutritional status of vitamin d, calcium, and parathyroid hormone in pregnant women after roux-En-Y gastric bypass. Obes Surg (2018) 28:3116–24. doi: 10.1007/s11695-018-3331-8
73. Johansson K, Cnattingius S, Näslund I, Roos N, Trolle Lagerros Y, Granath F, et al. Outcomes of pregnancy after bariatric surgery. N Engl J Med (2015) 372:814–24. doi: 10.1056/NEJMoa1405789
Keywords: bariatric surgery, health outcomes, metabolic surgery, obesity, umbrella review
Citation: Liao J, Yin Y, Zhong J, Chen Y, Chen Y, Wen Y and Cai Z (2022) Bariatric surgery and health outcomes: An umbrella analysis. Front. Endocrinol. 13:1016613. doi: 10.3389/fendo.2022.1016613
Received: 12 August 2022; Accepted: 11 October 2022;
Published: 28 October 2022.
Edited by:
Evan P. Nadler, Children’s National Hospital, United StatesReviewed by:
Jason David Fraser, Children’s Mercy Hospital, United StatesCopyright © 2022 Liao, Yin, Zhong, Chen, Chen, Wen and Cai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhaolun Cai, Y2Fpemhhb2x1bkBmb3htYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.