- 1Department of General Surgery, Fudan University Affiliated Huadong Hospital, Shanghai, China
- 2Department of Gastrointestinal Surgery, Affiliated Hospital of Xuzhou Medical University, Xuzhou, Jiangsu, China
Foregut (foregut exclusions) and hindgut (rapid transit of nutrients to the distal intestine) theories are the most commonly used explanations for the metabolic improvements observed after metabolic surgeries. However, several procedures that do not comprise duodenal exclusions, such as sleeve with jejunojejunal bypass, ileal interposition, and transit bipartition and sleeve gastrectomy were found to have similar diabetes remission rates when compared with duodenal exclusion procedures, such as gastric bypass, biliopancreatic diversion with duodenal switch, and diverted sleeve with ileal interposition. Moreover, the complete exclusion of the proximal intestine could result in the malabsorption of several important micronutrients. This article reviews commonly performed procedures, with and without foregut exclusion, to better comprehend whether there is a critical need to include foregut exclusion in metabolic surgery.
Introduction
Bariatric and metabolic surgeries have resulted in significant improvements and remissions in type 2 diabetes mellitus and other metabolic comorbidities (1, 2). However, the mechanisms underlying these effects remain unclear. The foregut (proximal intestine exclusions) and hindgut theories are the classic and most commonly used explanations for the resolution of type 2 diabetes mellitus observed after metabolic surgeries (3). While the hindgut theory (rapid transit of nutrients to the distal intestine) has been widely accepted, the foregut theory is not (4, 5). Several procedures that do not comprise duodenal exclusions, such as sleeve with jejunojejunal bypass/SG-JJB, sleeve with ileal interposition/SG-II, sleeve with transit bipartition/SG-TB, and standalone sleeve gastrectomy/SG (Figure 1), have similar diabetes remission outcomes when compared with procedures comprising duodenal exclusions, such as gastric bypass/GB, biliopancreatic diversion with duodenal switch/DS, and diverted sleeve with ileal interposition/DSG-II (Figure 2) (6–13). Furthermore, the complete exclusion of the proximal intestine may result in significant micronutrient malabsorption. Thus, while being a safe metabolic procedure, the need for foregut exclusion to achieve ideal metabolic outcomes is questioned.
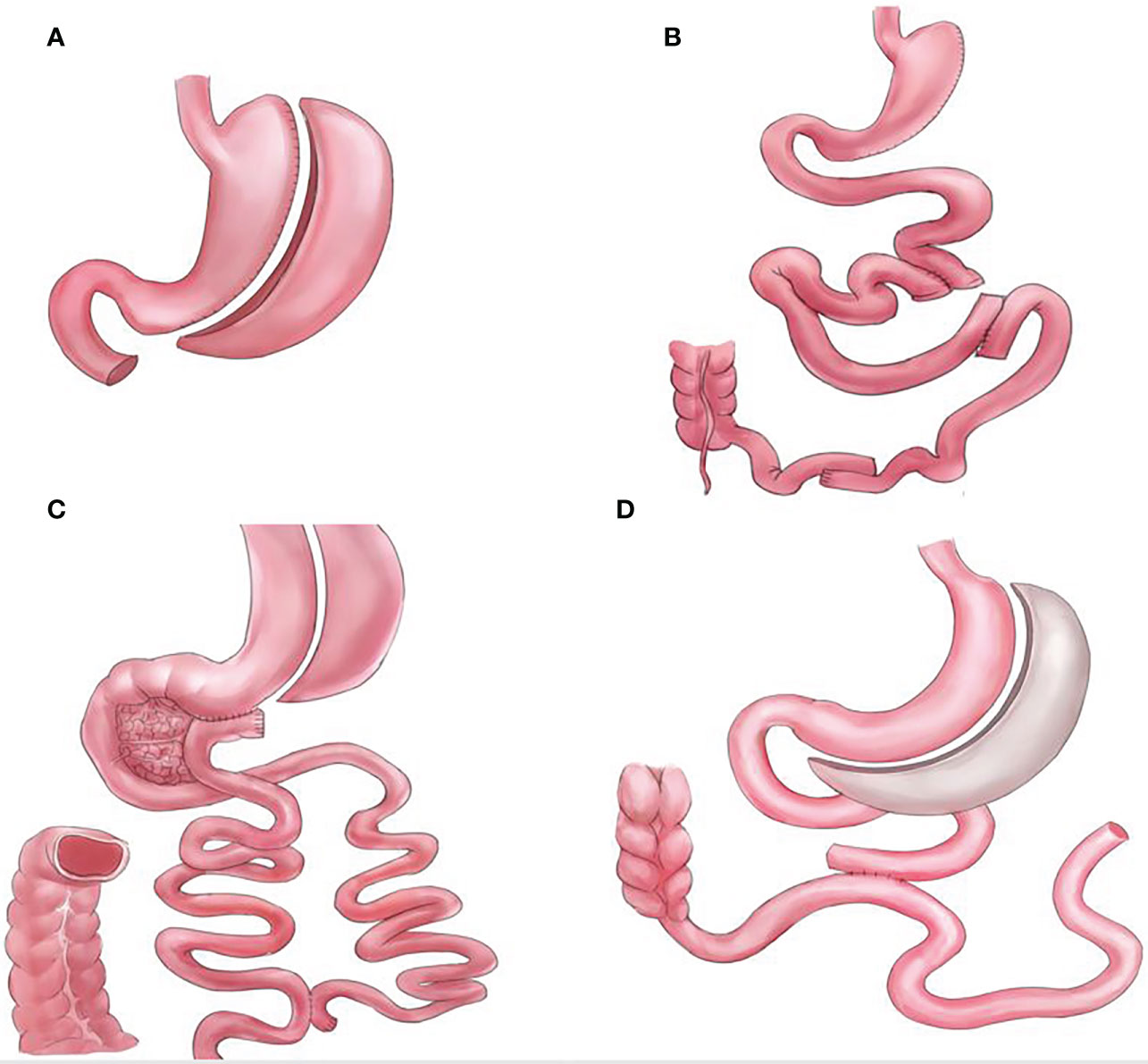
Figure 1 Graphical illustrations for procedures that do not bypass the foregut, (A) standalone sleeve gastrectomy (SG), (B) sleeve with ileal interposition (SG-II), (C) sleeve with transit bipartition (SG-TB), and (D) sleeve with jejunojejunal bypass (SG-JJB).
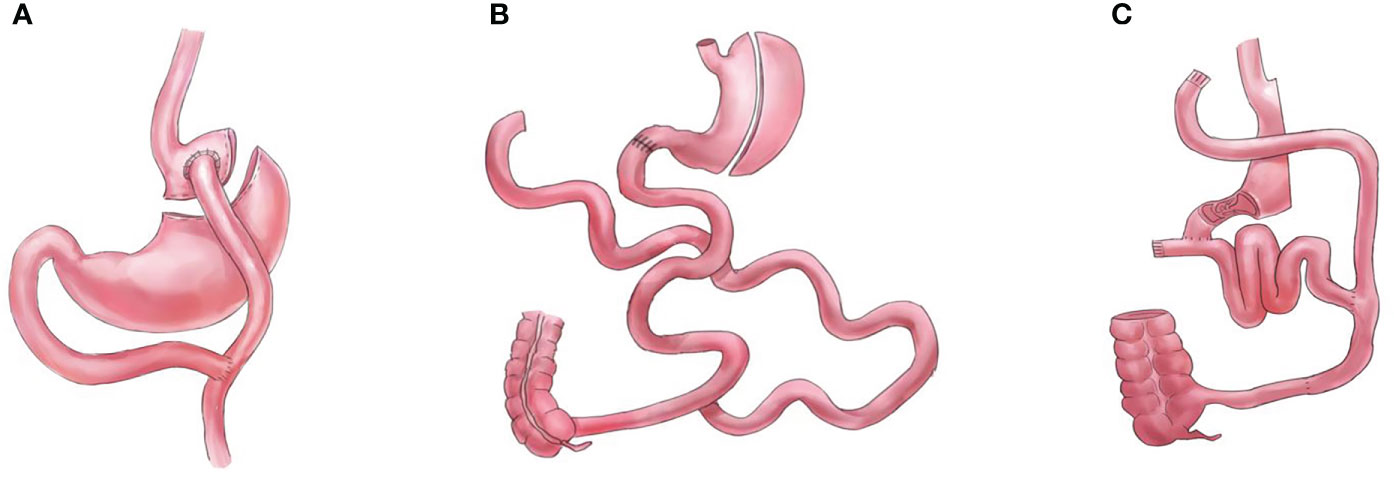
Figure 2 Graphical illustrations for procedures that completely bypass the foregut, (A) gastric bypass (GB), (B) biliopancreatic diversion with duodenal switch (DS), and (C) diverted sleeve with ileal interposition (DSG-II).
In this article, we briefly review the commonly performed metabolic procedures with and without foregut exclusion, with the hope to create a research direction to further comprehend the mechanisms of bariatric and metabolic surgeries.
Foregut hypothesis
The foregut hypothesis is one of the classic explanations for the diabetes remission observed after bariatric surgery. This hypothesis proposes that the exclusion of the proximal small intestine (duodenum and proximal jejunum) from the transit of nutrients may prevent the secretion of a “factor” that promotes insulin resistance and type 2 diabetes mellitus (3, 14, 15). However, the foregut hypothesis fails to explain how several other bariatric procedures that did not comprise duodenal exclusion, such as SG-JJB, SG-II, SG-TB, and standalone SG, still achieved excellent diabetes remission results (6, 7, 10–12). Furthermore, we are yet to identify the foregut “factor” that affects the glucose homeostasis. If the exclusion of the foregut is not required to achieve metabolic improvements, then complete exclusion of the foregut may be abandoned to achieve the ideal metabolic procedure.
Importance of foregut inclusion in bariatric and metabolic surgeries
There are significant drawbacks of excluding the foregut from nutrient transit. The proximal intestine is a major site of absorption of several important vitamins and micronutrients. For example, the risk of vitamin B12 deficiency is higher following GB than after SG and is attributable to the duodenal exclusion in gastric bypass (16). Procedures that exclude the proximal intestine, where most iron absorption occurs, are expected to increase the risk of iron deficiency (17). Reduced calcium absorption and vitamin D deficiency were also frequently observed in procedures that excluded the proximal intestine, which has the highest concentration of calcium transporters (18). Furthermore, the proximal intestine is also indirectly involved in the absorption of liposoluble vitamins and micronutrients. Procedures that bypass the proximal intestine result in the reduction of pancreatic enzyme secretion and alteration in bile salts, leading to alterations in fat assimilation (19–21).
Procedures that completely bypass the foregut
GB, DS, and DSG-II are the procedures that are normally performed according to the foregut hypothesis (bypassing the foregut). These procedures can incorporate modifications of the “one-anastomosis reconstruction”; however, such modifications do not alter the foregut exclusion components.
Multiple randomized controlled trials (RCT) have compared the efficacy of GB with procedures that do not comprise foregut exclusion. A meta-analysis of RCTs comparing the outcomes of GB and SG found that GB resulted in a superior loss of body mass index (BMI), which persisted at 3 years postoperatively (22). Interestingly, the study did not find differences in diabetes remission, hemoglobin A1c (HbA1c) level, and homeostatic model assessment of insulin resistance levels. Similarly, three RCTs (Stampede, SM-BOSS, and SLEEVE-PASS trials) found no difference between GB and SG regarding HbA1c levels and rate of diabetes remission at 5 years postoperatively (12, 13, 23). However, GB is associated with a higher risk of nutritional deficiencies than SG (16, 24). Studies have reported that reconnecting the foregut back to the configuration can solve the malnutrition issue in GB without compromising the bariatric and metabolic outcomes (25, 26).
Another classic bariatric and metabolic procedure is DS, which is regarded as the most effective procedure (27). An RCT reported that at 5 years postoperatively, DS resulted in superior weight loss and glucose improvements compared to those of GB; however, DS is associated with more nutritional complications (28). Long-term studies (10 years) have also reported the nutritional issues associated with DS, particularly with fat-soluble vitamins (29, 30). Thus, although the effectiveness of DS is undisputed, it is not commonly performed owing to the high prevalence of complications (2, 31).
DSG-II and SG-II are among the most complex procedures, as they require more anastomosis than most metabolic surgeries (32). Unlike DSG-II, which bypasses the foregut and creates malabsorption, the SSG-II procedure ensures that there is no malabsorption. However, limited data are available regarding the prevalence of nutritional deficiency between the DSG-II and SG-II procedures. However, DSG-II and SG-II resulted in similar glucose control improvements, which further questions the need for foregut exclusion (7, 33, 34). Ileal interposition procedures are not commonly performed because of their complexity and the need for a high number of anastomoses, and because a higher number of mesenteric defects are created in these procedures (32).
Procedures that do not bypass the foregut
SG, SG-TB, SG-II, and SG-JJB are the procedures that are not normally performed according to the foregut hypothesis. Similar to the GB and DS, some of these procedures can incorporate modifications of the “one-anastomosis reconstruction”; however, such modifications do not alter the foregut inclusion components.
SG can be considered the foundation of most bariatric and metabolic surgeries, as many of the procedures consist of SG. Standalone SG is currently the most commonly performed procedure worldwide, with excellent results (2, 31). As mentioned previously, when compared with GB, SG was found to be comparable regarding metabolic improvements as well as having a lower risk of nutritional deficiency (12, 13, 16, 23, 24). However, in the longer term, SG is complicated by several issues, such as weight regain, diabetes relapse, and reflux (35). Therefore, revisional surgery after SG is becoming a common practice.
SG-TB is a procedure that has been gaining significant attention in recent years (particularly its one-anastomosis form, the single-anastomosis sleeve ileal bypass/SASI). Proposed as a modification of the DS procedure, SG-TB eliminates the need to completely bypass the duodenum. The 5 year result of SG-TB was reported to be 74% excess BMI loss and 86% diabetes remission (5). SG-TB has been shown to have comparable weight loss and diabetes remission results, while having lesser risks for nutritional deficiencies when compared to GB (11, 36). When compared with DS, SG-TB was reported to have lesser weight loss results; however, there was no difference in the rate of diabetes remission (10). The authors further noted that SG-TB showed real benefits in reducing the side effects and malnutrition risks compared with DS (10). When compared with DSG-II, SG-TB showed similar weight loss and diabetes remission results; however, the differences in nutritional deficiencies between the two procedures has not been reported (9).
The SG-II procedure has been described and discussed in the previous section. Moreover, in the case of severe malnutrition after the DS procedure, conversion to SG-II (without bypassing the foregut) solved the malnutrition issues without compromising the bariatric and metabolic results (37). In conclusion, although more studies are needed, the SG-II and DSG-II demonstrated comparable weight loss and diabetes remission results; thus, questioning the need for foregut exclusion.
Other procedures that do not bypass the foregut have been reported. SG-JJB was reported to have comparable weight loss and diabetes remission rates to GB (6). A rodent model of jejunal-ileal loop bipartition has also been described, showing its effectiveness in improving glucose control (38).
SG-TB, SG-JJB, and SG-II procedures are still lacking comparative studies as well as RCTs; thus, further studies are warranted in this regard in the future. However, the results to date have been promising with regard to the notion of abandoning foregut exclusion.
Mechanisms related to the effect of bariatric surgery
Glucagon-like peptide-1 (GLP-1) is secreted by intestinal enteroendocrine L-cells and several brain cells in the brainstem following food consumption (39). GLP-1 is an incretin having the ability to enhance insulin secretion. In the brain and the stomach, GLP-1 also can promote satiety, reducing food intake. Per the hindgut theory (rapid transit of nutrients to the distal intestine), bariatric procedures that resulted in the rapid transient of nutrients showed significantly elevated GLP-1 levels following food consumption (39). A recent meta-analysis reported that postprandial GLP-1 levels were also increased following SG, possibly due to increased gastric emptying (40).
Similarly, peptide YY (PYY) was also reported to be elevated in bariatric procedures with or without duodenal exclusion (40). PYY is also secreted by the L-cells and has the ability to reduce appetite and promote satiety.
After bariatric surgery, several studies have shown that the increased level of bile acids can promote insulin secretion, increase energy expenditure, and alter the gut microbiota (41). Bile acids play a role in metabolic regulation mediated through several receptors, such as the farnesoid X receptor (FXR) and G protein-coupled bile acid receptor 1 (also known as TGR5) (41). Stimulation of FXR in insulin-resistant obese mice was shown to reduce hyperinsulinemia and improved glucose control (42). In response to bile acids, TGR5 activation promotes GLP-1 secretion in animal and human studies (43, 44). Increased bile acids can increase energy expenditure through TGR5 in the skeletal muscle and brown adipose tissue (45, 46). Bile acids and gut microbiota are affected and altered by bariatric surgery. Increased bile acid concentrations can kill and promote certain gut bacteria strains (41).
Individuals with obesity exhibit an altered gut microbiota as compared to lean controls, comprising of a decline in Bacteroidetes and an increase in Firmicutes in obese individuals (47). On the other hand, bariatric surgery resulted in the alteration of gut microbiota composition (decrease of Firmicutes/Bacteroidetes ratio), which contributes to fat mass regulation and reduced utilization of carbohydrates as energy fuel (48).
Several studies have shown that bariatric surgery induces changes in adipose tissue and improves systemic inflammation (49). Bariatric surgery induces changes in the levels of several microRNAs from the adipocyte-derived exosomes, which are correlated to the improved insulin signaling following the surgery. Several inflammatory factors, such as C-reactive protein, tumor necrosis factor-α, and interleukin-6, the hallmark for the initiation of insulin resistance, were also reduced following bariatric surgery.
Several other hypotheses explaining the mechanisms of bariatric surgery exist (41). However, all of these hypotheses can be justified through the anatomical changes leading to the distal intestine or as changes in general after bariatric surgery, further questioning the foregut hypothesis.
Discussion
The era of bariatric and metabolic surgeries has been evolving continuously. In the past, malabsorption and restriction were the primary targets of bariatric surgery for achieving an ideal healthy weight (50). However, in recent years, the era of pure metabolic surgery has been initiated, focusing on improving the metabolic potency of bariatric surgery, hence the name “metabolic surgery” (51–53). To improve the metabolic potency of a procedure, we must comprehend the mechanism of the metabolic improvements observed following metabolic procedures. However, metabolic surgery appears to have a highly complex mechanism, and more time may be needed to better understand it. Moreover, recent surgical innovations have provided us with the knowledge that could be used to improve the safety of metabolic surgery.
Classic and significant metabolic procedures, such as GB and DS, resulted in excellent metabolic outcomes (54–57). However, they also resulted in unwanted effects, such as excessive nutrient malabsorption (58). In contrast, several metabolic procedures (such as SG-JJB, SG-II, SG-TB, and SG) that maintain the foregut (either completely or partially) have been demonstrated to have efficacy that is not inferior to foregut exclusion procedures (6–13). It is imperative to acknowledge that the SG procedure has been the most performed bariatric procedure worldwide in recent years, surpassing RYGB (31). Furthermore, several RCTs have shown that SG (without duodenal exclusion) could result in comparable bariatric and metabolic outcomes compared to RYGB (with duodenal exclusion) (12, 13, 23).
Although procedures such as SG-JJB, SG-II, and SG-TB, differ in intestinal reconfiguration, they have common consequences: 1) foregut inclusion and 2) expediting nutrient flow to the distal intestine (hindgut theory). It has been recently proposed that foregut exclusions may not be necessary as long as strong stimulus to the ileum is provided (59). However, we need better comparative studies to understand not only the metabolic efficacy but also the safety of these foregut inclusion procedures.
With the foregut hypothesis being the focus of this article, it is imperative to discuss the use of duodenal-jejunal bypass liner as a treatment alternative for metabolic diseases. While it has been reported that the duodenal-jejunal bypass liner resulted in significant improvements in type 2 diabetes, the underlying mechanisms remain elusive (60). In contrast to foregut exclusion, a previous study showed that preserving foregut transit in GB and DS models still resulted in significant weight loss and glucose control improvements (25, 26, 37). Therefore, these findings created another notion, “Is bypassing the foregut necessary? Or as long as there is enough exclusion, regardless of the site of exclusion, would we still observe excellent metabolic improvements?” The goal of bariatric and metabolic surgery should be to improve the patients’ quality of life as well as improving their weight status and comorbidities, i.e. not to focus solely on the weight loss outcomes.
In conclusion, with the available studies, we cannot deny the credibility of foregut exclusion for excellent metabolic outcomes. However, the idea of abandoning complete foregut exclusion has some credibility, and more comparative studies are needed to prove this idea. Such studies should focus mainly on whether: 1) foregut inclusion resulted in non-inferior metabolic outcomes than after foregut exclusion and 2) foregut inclusion delivers better safety regarding micronutrient malabsorption than that following foregut exclusion.
Author contributions
JaW and YG propose the topic and design the manuscript flows. JaW and JY draft the manuscript. YC and JiW collect and analyse the materials for the manuscript. JW, YC, JY, JiW, and YG reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Frühbeck G. Bariatric and metabolic surgery: a shift in eligibility and success criteria. Nat Rev Endocrinol (2015) 11(8):465–77. doi: 10.1038/nrendo.2015.84
2. Welbourn R, Hollyman M, Kinsman R, Dixon J, Cohen R, Morton J, et al. Bariatric-metabolic surgery utilisation in patients with and without diabetes: Data from the IFSO global registry 2015-2018. Obes Surg (2021) 31(6):2391–400. doi: 10.1007/s11695-021-05280-6
3. Mingrone G, Castagneto-Gissey L. Mechanisms of early improvement/resolution of type 2 diabetes after bariatric surgery. Diabetes Metab (2009) 35(6 Pt 2):518–23. doi: 10.1016/S1262-3636(09)73459-7
4. Patriti A, Aisa MC, Annetti C, Sidoni A, Galli F, Ferri I, et al. How the hindgut can cure type 2 diabetes. ileal transposition improves glucose metabolism and beta-cell function in goto-kakizaki rats through an enhanced proglucagon gene expression and l-cell number. Surgery (2007) 142(1):74–85. doi: 10.1016/j.surg.2007.03.001
5. Santoro S, Castro LC, Velhote MC, Malzoni CE, Klajner S, Castro LP, et al. Sleeve gastrectomy with transit bipartition: a potent intervention for metabolic syndrome and obesity. Ann Surg (2012) 256(1):104–10. doi: 10.1097/SLA.0b013e31825370c0
6. Lin S, Li C, Guan W, Liang H. Three-year outcomes of sleeve gastrectomy plus jejunojejunal bypass: a retrospective case-matched study with sleeve gastrectomy and gastric bypass in Chinese patients with BMI ≥35 kg/m2. Obes Surg (2021) 31(8):3525–30. doi: 10.1007/s11695-021-05411-z
7. DePaula AL, Macedo AL, Schraibman V, Mota BR, Vencio S. Hormonal evaluation following laparoscopic treatment of type 2 diabetes mellitus patients with BMI 20-34. Surg Endosc (2009) 23(8):1724–32. doi: 10.1007/s00464-008-0168-6
8. Ugale S, Gupta N, Modi KD, Kota SK, Satwalekar V, Naik V, et al. Prediction of remission after metabolic surgery using a novel scoring system in type 2 diabetes - a retrospective cohort study. J Diabetes Metab Disord (2014) 13(1):89. doi: 10.1186/s40200-014-0089-y
9. Yormaz S, Yılmaz H, Ece I, Sahin M. Laparoscopic ileal interposition with diverted sleeve gastrectomy versus laparoscopic transit bipartition with sleeve gastrectomy for better glycemic outcomes in T2DM patients. Obes Surg (2018) 28(1):77–86. doi: 10.1007/s11695-017-2803-6
10. Topart P, Becouarn G, Finel JB. Is transit bipartition a better alternative to biliopancreatic diversion with duodenal switch for superobesity? comparison of the early results of both procedures. Surg Obes Relat Dis (2020) 16(4):497–502. doi: 10.1016/j.soard.2019.12.019
11. Topart P, Becouarn G, Finel JB. Comparison of 2-year results of roux-en-Y gastric bypass and transit bipartition with sleeve gastrectomy for superobesity. Obes Surg (2020) 30(9):3402–7. doi: 10.1007/s11695-020-04691-1
12. Salminen P, Helmiö M, Ovaska J, Juuti A, Leivonen M, Peromaa-Haavisto P, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss at 5 years among patients with morbid obesity: The SLEEVEPASS randomized clinical trial. JAMA (2018) 319(3):241–54. doi: 10.1001/jama.2017.20313
13. Peterli R, Wölnerhanssen BK, Peters T, Vetter D, Kröll D, Borbély Y, et al. Effect of laparoscopic sleeve gastrectomy vs laparoscopic roux-en-Y gastric bypass on weight loss in patients with morbid obesity: The SM-BOSS randomized clinical trial. JAMA (2018) 319(3):255–65. doi: 10.1001/jama.2017.20897
14. Ramos AC, Galvao Neto MP, de Souza YM, Galvao M, Murakami AH, Silva AC, et al. Laparoscopic duodenal-jejunal exclusion in the treatment of type 2 diabetes mellitus in patients with BMI < 30 kg/m2 (LBMI). Obes Surg (2009) 19:307–12. doi: 10.1007/s11695-008-9759-5
15. Rubino F, Marescaux J. Effect of duodenal-jejunal exclusion in a non-obese animal model of type 2 diabetes: a new perspective for an old disease. Ann Surg (2004) 239:1–11. doi: 10.1097/01.sla.0000102989.54824.fc
16. Alexandrou A, Armeni E, Kouskouni E, Tsoka E, Diamantis T, Lambrinoudaki I. Cross-sectional long-term micronutrient deficiencies after sleeve gastrectomy versus roux-en-Y gastric bypass: a pilot study. Surg Obes Relat Dis (2014) 10(2):262–8. doi: 10.1016/j.soard.2013.07.014
17. Ruz M, Carrasco F, Rojas P, Codoceo J, Inostroza J, Basfi-Fer K, et al. Heme- and nonheme-iron absorption and iron status 12 mo after sleeve gastrectomy and roux-en-Y gastric bypass in morbidly obese women. Am J Clin Nutr (2012) 96(4):810–7. doi: 10.3945/ajcn.112.039255
18. Saad R, Habli D, El Sabbagh R, Chakhtoura M. Bone health following bariatric surgery: An update. J Clin Densitom (2020) 23(2):165–81. doi: 10.1016/j.jocd.2019.08.002
19. Shikora SA, Kim JJ, Tarnoff ME. Nutrition and gastrointestinal complications of bariatric surgery. Nutr Clin Pract (2007) 22(1):29–40. doi: 10.1177/011542650702200129
20. Saltzman E, Philip Karl J. Nutrient deficiencies after gastric bypass surgery. Ann Rev Nutr (2013) 33:183–203. doi: 10.1146/annurev-nutr-071812-161225
21. Shankar P, Boylan M, Sriram K. Micronutrient deficiencies after bariatric surgery. Nutrition (2010) 26(11):1031–7. doi: 10.1016/j.nut.2009.12.003
22. Lee Y, Doumouras AG, Yu J, Aditya I, Gmora S, Anvari M, et al. Laparoscopic sleeve gastrectomy versus laparoscopic roux-en-Y gastric bypass: A systematic review and meta-analysis of weight loss, comorbidities, and biochemical outcomes from randomized controlled trials. Ann Surg (2021) 273(1):66–74. doi: 10.1097/SLA.0000000000003671
23. Schauer PR, Bhatt DL, Kirwan JP, Wolski K, Aminian A, Brethauer SA, et al. Bariatric surgery versus intensive medical therapy for diabetes - 5-year outcomes. N Engl J Med (2017) 376(7):641–51. doi: 10.1056/NEJMoa1600869
24. Gu L, Fu R, Chen P, Du N, Chen S, Mao D, et al. In terms of nutrition, the most suitable method for bariatric surgery: Laparoscopic sleeve gastrectomy or roux-en-Y gastric bypass? a systematic review and meta-analysis. Obes Surg (2020) 30(5):2003–14. doi: 10.1007/s11695-020-04488-2
25. Ceneviva R, Salgado Júnior W, Marchini JS. A new revisional surgery for severe protein-calorie malnutrition after roux-en-Y gastric bypass: successful duodenojejunal reconstruction using jejunal interposition. Surg Obes Relat Dis (2016) 12(2):e21–3. doi: 10.1016/j.soard.2015.09.020
26. Dolo PR, Yao L, Li C, Zhu X, Shi L, Widjaja J. Preserving duodenal-jejunal (Foregut) transit does not impair glucose tolerance and diabetes remission following gastric bypass in type 2 diabetes sprague-dawley rat model. Obes Surg (2018) 28(5):1313–20. doi: 10.1007/s11695-017-2985-y
27. O'Brien PE, Hindle A, Brennan L, Skinner S, Burton P, Smith A, et al. Long-term outcomes after bariatric surgery: a systematic review and meta-analysis of weight loss at 10 or more years for all bariatric procedures and a single-centre review of 20-year outcomes after adjustable gastric banding. Obes Surg (2019) 29(1):3–14. doi: 10.1007/s11695-018-3525-0
28. Risstad H, Søvik TT, Engström M, Aasheim ET, Fagerland MW, Olsén MF, et al. Five-year outcomes after laparoscopic gastric bypass and laparoscopic duodenal switch in patients with body mass index of 50 to 60: a randomized clinical trial. JAMA Surg (2015) 150(4):352–61. doi: 10.1001/jamasurg.2014.3579
29. Ballesteros-Pomar MD, González de Francisco T, Urioste-Fondo A, González-Herraez L, Calleja-Fernández A, Vidal-Casariego A, et al. Biliopancreatic diversion for severe obesity: Long-term effectiveness and nutritional complications. Obes Surg (2016) 26(1):38–44. doi: 10.1007/s11695-015-1719-2
30. Topart P, Becouarn G, Delarue J. Weight loss and nutritional outcomes 10 years after biliopancreatic diversion with duodenal switch. Obes Surg (2017) 27(7):1645–50. doi: 10.1007/s11695-016-2537-x
31. Welbourn R, Hollyman M, Kinsman R, Dixon J, Liem R, Ottosson J, et al. Bariatric surgery worldwide: Baseline demographic description and one-year outcomes from the fourth IFSO global registry report 2018. Obes Surg (2019) 29(3):782–95. doi: 10.1007/s11695-018-3593-1
32. Gagner M. Surgical treatment of nonseverely obese patients with type 2 diabetes mellitus: sleeve gastrectomy with ileal transposition (SGIT) is the same as the neuroendocrine brake (NEB) procedure or ileal interposition associated with sleeve gastrectomy (II-SG), but ileal interposition with diverted sleeve gastrectomy (II-DSG) is the same as duodenal switch. Surg Endosc (2011) 25(2):655–6. doi: 10.1007/s00464-010-1221-9
33. De Paula AL, Stival AR, Macedo A, Ribamar J, Mancini M, Halpern A, et al. Prospective randomized controlled trial comparing 2 versions of laparoscopic ileal interposition associated with sleeve gastrectomy for patients with type 2 diabetes with BMI 21-34 kg/m(2). Surg Obes Relat Dis (2010) 6(3):296–304. doi: 10.1016/j.soard.2009.10.005
34. DePaula AL, Stival AR, DePaula CC, Halpern A, Vencio S. Surgical treatment of type 2 diabetes in patients with BMI below 35: mid-term outcomes of the laparoscopic ileal interposition associated with a sleeve gastrectomy in 202 consecutive cases. J Gastrointest Surg (2012) 16(5):967–76. doi: 10.1007/s11605-011-1807-0
35. Nudotor RD, Prokopowicz G, Abbey EJ, Gonzalez A, Canner JK, Steele KE. Comparative effectiveness of roux-en y gastric bypass versus vertical sleeve gastrectomy for sustained remission of type 2 diabetes mellitus. J Surg Res (2021) 261:407–16. doi: 10.1016/j.jss.2020.12.024
36. Ece I, Yilmaz H, Yormaz S, Çolak B, Calisir A, Sahin M. The short-term effects of transit bipartition with sleeve gastrectomy and distal-Roux-en-Y gastric bypass on glycemic control, weight loss, and nutritional status in morbidly obese and type 2 diabetes mellitus patients. Obes Surg (2021) 31(5):2062–71. doi: 10.1007/s11695-020-05212-w
37. Almahmeed T, Pomp A, Gagner M. Laparoscopic reversal of biliopancreatic diversion with duodenal switch. Surg Obes Relat Dis (2006) 2(4):468–71. doi: 10.1016/j.soard.2006.03.023
38. Zhang X, Shen Y, Cao T, Wang Y, Qiao Z, Zhang Z, et al. A rodent model of jejunal-ileal loop bipartition (JILB): a novel malabsorptive operation. Obes Surg (2021) 31(3):1361–8. doi: 10.1007/s11695-020-05163-2
39. D'Alessio D. Is GLP-1 a hormone: Whether and when? J Diabetes Investig (2016) 7 Suppl 1(Suppl 1):50–5. doi: 10.1111/jdi.12466
40. McCarty TR, Jirapinyo P, Thompson CC. Effect of sleeve gastrectomy on ghrelin, GLP-1, PYY, and GIP gut hormones: A systematic review and meta-analysis. Ann Surg (2020) 272(1):72–80. doi: 10.1097/SLA.0000000000003614
41. Wang W, Cheng Z, Wang Y, Dai Y, Zhang X, Hu S. Role of bile acids in bariatric surgery. Front Physiol (2019) 10:374. doi: 10.3389/fphys.2019.00374
42. Cariou B, van Harmelen K, Duran-Sandoval D, van Dijk TH, Grefhorst A, Abdelkarim M, et al. The farnesoid X receptor modulates adiposity and peripheral insulin sensitivity in mice. J Biol Chem (2006) 281(16):11039–49. doi: 10.1074/jbc.M510258200
43. Thomas C, Gioiello A, Noriega L, Strehle A, Oury J, Rizzo G, et al. TGR5-mediated bile acid sensing controls glucose homeostasis. Cell Metab (2009) 10(3):167–77. doi: 10.1016/j.cmet.2009.08.001
44. Wu T, Bound MJ, Standfield SD, Gedulin B, Jones KL, Horowitz M, et al. Effects of rectal administration of taurocholic acid on glucagon-like peptide-1 and peptide YY secretion in healthy humans. Diabetes Obes Metab (2013) 15(5):474–7. doi: 10.1111/dom.12043
45. Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab (2013) 98(4):E708–12. doi: 10.1210/jc.2012-3736
46. Broeders EP, Nascimento EB, Havekes B, Brans B, Roumans KH, Tailleux A, et al. The bile acid chenodeoxycholic acid increases human brown adipose tissue activity. Cell Metab (2015) 22(3):418–26. doi: 10.1016/j.cmet.2015.07.002
47. Ciobârcă D, Cătoi AF, Copăescu C, Miere D, Crişan G. Bariatric surgery in obesity: Effects on gut microbiota and micronutrient status. Nutrients (2020) 12(1):235./ doi: 10.3390/nu12010235
48. Tremaroli V, Karlsson F, Werling M, Ståhlman M, Kovatcheva-Datchary P, Olbers T, et al. Roux-en-Y gastric bypass and vertical banded gastroplasty induce long-term changes on the human gut microbiome contributing to fat mass regulation. Cell Metab (2015) 22(2):228–38. doi: 10.1016/j.cmet.2015.07.009
49. Xu G, Song M. Recent advances in the mechanisms underlying the beneficial effects of bariatric and metabolic surgery. Surg Obes Relat Dis (2021) 17(1):231–8. doi: 10.1016/j.soard.2020.08.028
50. Buchwald H. The evolution of metabolic/bariatric surgery. Obes Surg (2014) 24(8):1126–35. doi: 10.1007/s11695-014-1354-3
51. Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F, Bhatt DL. Metabolic surgery: Weight loss, diabetes, and beyond. J Am Coll Cardiol (2018) 71(6):670–87. doi: 10.1016/j.jacc.2017.12.014
52. Buchwald H, Buchwald JN. Metabolic (Bariatric and nonbariatric) surgery for type 2 diabetes: A personal perspective review. Diabetes Care (2019) 42(2):331–40. doi: 10.2337/dc17-2654
53. Wilson R, Aminian A, Tahrani AA. Metabolic surgery: A clinical update. Diabetes Obes Metab (2021) 23 Suppl 1:63–83. doi: 10.1111/dom.14235
54. Quevedo MDP, Palermo M, Serra E, Ackermann MA. Metabolic surgery: gastric bypass for the treatment of type 2 diabetes mellitus. Transl Gastroenterol Hepatol (2017) 2:58. doi: 10.21037/tgh.2017.05.10
55. Maclellan WC, Johnson JM. Laparoscopic gastric bypass: Still the gold standard? Surg Clin North Am (2021) 101(2):161-75. doi: 10.1016/j.suc.2020.12.013
56. Hess DS, Hess DW, Oakley RS. The biliopancreatic diversion with the duodenal switch: results beyond 10 years. Obes Surg (2005) 15(3):408–16. doi: 10.1381/0960892053576695
57. Sorribas M, Casajoana A, Sobrino L, Admella V, Osorio J, Pujol-Gebellí J. Experience in biliopancreatic diversion with duodenal switch: results at 2, 5 and 10 years. Cir Esp (Engl Ed) (2021) 100(4):202–8. doi: 10.1016/j.ciresp.2021.01.008
58. Bal BS, Finelli FC, Shope TR, Koch TR. Nutritional deficiencies after bariatric surgery. Nat Rev Endocrinol (2012) 8(9):544–56. doi: 10.1038/nrendo.2012.48
59. Santoro S, Aquino CGG, Mota FC. Exclusions may be dismissed if the ileum is early and potently stimulated. Obes Surg (2021) 31(11):5049–50. doi: 10.1007/s11695-021-05526-3
Keywords: foregut hypothesis, micronutrient, type-2 diabetes, bariatric surgery, metabolic surgery
Citation: Widjaja J, Chu Y, Yang J, Wang J and Gu Y (2022) Can we abandon foregut exclusion for an ideal and safe metabolic surgery? Front. Endocrinol. 13:1014901. doi: 10.3389/fendo.2022.1014901
Received: 09 August 2022; Accepted: 24 October 2022;
Published: 10 November 2022.
Edited by:
Wah Yang, The First Affiliated Hospital of Jinan University, ChinaReviewed by:
Angelo Di Vincenzo, University of Padua, ItalyCopyright © 2022 Widjaja, Chu, Yang, Wang and Gu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Gu, yangu@shsmu.edu.cn
 Jason Widjaja
Jason Widjaja