
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol., 23 September 2022
Sec. Reproduction
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1014558
This article is part of the Research TopicThe Mechanism and Clinical Application of Neuroendocrine Hormones in Infertility-related DiseasesView all 5 articles
 Juanjuan Yu1†
Juanjuan Yu1† Peiqin Chen1,2†
Peiqin Chen1,2† Yifan Luo1†
Yifan Luo1† Mu Lv1
Mu Lv1 Liqun Lou1
Liqun Lou1 Qimeng Xiao1
Qimeng Xiao1 Luxia Wang3
Luxia Wang3 Juan Chen3
Juan Chen3 Mingzhu Bai4*
Mingzhu Bai4* Zhenbo Zhang1,5*
Zhenbo Zhang1,5*Objective: This study aimed to examine the efficacy of HRT with gonadotropin-releasing hormone agonist (GnRH-a) pre-treatment in women with male-factor infertility who underwent a frozen embryo transfer (FET) programme.
Design: Between January 2016 and October 2020, 2733 women with male-factor infertility who underwent the HRT protocol as the endometrial preparation method were enrolled at two Reproductive Medicine Centres. Patients were divided into two groups based on whether they had GnRH-a pre-treatment before HRTs: the GnRHa-HRT group and the HRT group. The inverse probability of treatment weighting (IPTW) method was conducted to balance patient baseline characteristics between treatment cohorts to reduce selection bias. The live birth rate was considered regarded as the primary pregnancy outcome.
Results: Multivariate logistic regression adjusted for confounding factors, the GnRHa-HRT group showed a notably higher rate of live birth (OR 2.154, 95% CI 1.636~2.835, P<0.001) when compared to the HRT group. Additionally, the rate of miscarriage was significantly lower in the GnRHa-HRT group. The GnRHa-HRT group had significantly higher rates of biochemical pregnancy, clinical pregnancy, multiple pregnancy, and term birth.
Conclusion: The endometrial preparation protocol of HRT with GnRH-a pre-treatment could obviously increase the live birth rate for women with male-factor infertility undergoing the FET programme.
Infertility is one of the major health problems worldwide, affecting 8-12% of couples at reproductive ages (1–3). According to the World Health Organization, 50% of couples have low fertility due to male-factors (4, 5). Successful pregnancy in couples with male infertility often requires embryo transfer, including fresh embryo transfer and frozen embryo transfer (FET). Recently, improvements in cryopreservation techniques (vitrification) and the development of effective ovarian stimulation protocols have markedly increased options for elective FET protocols (6, 7). FET cycle practice facilitates the elective single-embryo transfer and lessens the effect of steroid hormones used for ovarian stimulation and embryo at the time of egg collection when compared to fresh embryo transfer (8). At the same time, it gives endometrial receptivity enough time to be regulated. And a series of studies have indicated that pregnancy rates with FET procedures in assisted reproductive patients are higher than those with fresh cycle procedures (9–11). Therefore, the use of FET is being used more widely.
During a FET cycle, the determination of the appropriate endometrial preparation protocol is critical to maximizing the success of assisted reproduction technology (ART). There are three common methods of endometrial preparation: hormone replacement therapy (HRT), natural cycle, and stimulation cycle. HRTs are currently the most widely utilized method due to its wide range of applications, no frequent follow-up, and low cycle cancellation rate. Patients with or without normal ovaries and with or without normal menstrual cycles can participate in the HRT program (12). Several studies have concluded that HRT cycles are comparable to natural cycles in terms of pregnancy outcomes (13, 14).
To avoid hormone disruptors in subsequent therapy when using HRTs, gonadotropin-releasing hormone agonist (GnRH-a) pre-treatment can downregulate any hormones produced by the ovaries. However, it is yet unclear whether HRT with GnRH-a pre-treatment can improve reproductive outcomes. A recent clinical study suggests that pre-treatment of GnRH-a significantly improves the live birth rate in patients with multiple failed embryo implantation (15). Moreover, some studies indicate that the use of GnRH-a can improve endometrial receptivity (16–18). Studies have shown that HRT with GnRH-a pre-treatment improves pregnancy outcomes in patients with endometriosis and adenomyosis (19–21), but not in those with polycystic ovary syndrome (PCOS) (22, 23).
The cases number of endometriosis, adenomyosis, and PCOS infertility are only a part of the population, but male-factors make up the majority of ART patients. However, the specific disease for which the GnRH-a preconditioning HRT regimen is applicable is unclear. And endometrial preparation for male factor infertility has received limited attention. To address this issue, we divided eligible patients with male-factor infertility into the HRT group and the HRT with GnRH-a pre-treated group. Then we analysed the differences in the pregnancy outcomes between the two groups.
The retrospective cohort study was conducted at two reproductive medicine centres, Shanghai General Hospital and Xuzhou Maternity and Child Health Care Hospital. 2733 cases of endometrial preparation protocols have been analysed for HRTs during the FET programme between 1 January 2016, and 1 October 2020. The two reproduction centres specialize in treating patients with male factor infertility, so there have been many cases with male factor infertility. In this study, we defined male factors infertility as moderate to severe oligo-astheno-terato-spermia and azoospermia. Comprehensive assessment of male factor infertility based on semen analysis. Semen quality data were analysed according to the fifth edition of WHO guideline (24).
The inclusion criteria were as follows:1) A FET programme and endometrial preparation method using HRT protocol with or without GnRH-a. 2) Male-factor is identified as the cause of infertility. 3) The embryos transferred from female patients are of high-quality. The embryos with 7-12 cells and grade I-II were selected at cleavage stage, and the embryos with 4BB, 4BA, 4AB or 4AA grade were selected at blastocyst stage (25–27). 4) Endometrial thickness ≥8mm on the transfer day.
Exclusion criteria were as follows: 1) The woman’s age was<20 or ≥40 years old. 2) The causes of infertility combined with other diseases such as ovulation factors, fallopian tube factors, endometriosis, adenomyosis, etc. 3) Uterine abnormalities in women, such as congenital uterine malformations, fibroids that affect pregnancy, endometrial polyps, etc. 4) Infertile women with recurrent pregnancy loss or recurrent implantation failure history. 5) Any chromosomal abnormality in either spouse. 6) The female patients had a severe chronic or acute systemic disease. A detailed flow chart of patient selection can be found in Figure 1.
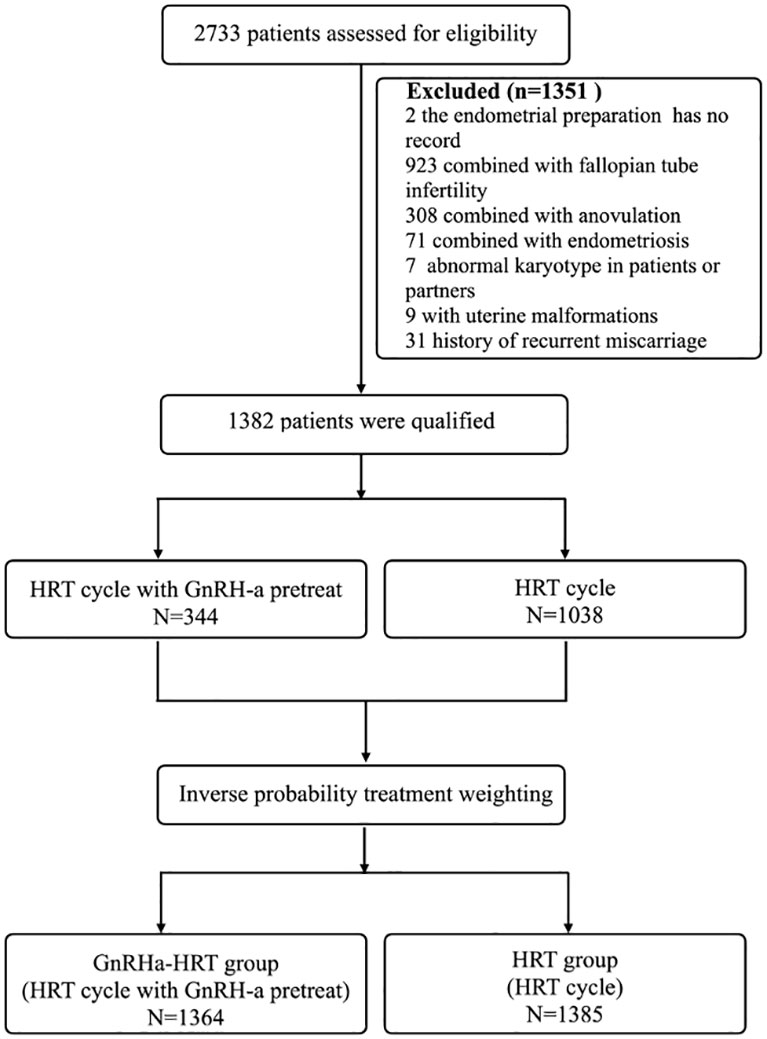
Figure 1 Trial profile. HRT, hormone replacement therapy; GnRH-a, gonadotropin-releasing hormone agonist.
According to the indications of endometrial preparation methods and patients’ informed consent, eligible patients were divided into an HRT group (HRT group) and an HRT cycle with GnRH-a pre-treatment group (GnRHa-HRT group).
In the HRT protocols, as shown in Figure 2, estradiol tablets (Femoston; Abbott Biologicals B.V., The Netherlands) 6mg once daily oral use from the next day after menstruation. On the 7th day of medication, endometrial thickness was examined by vaginal ultrasound, estradiol tablets were changed to 8 mg/d if necessary, and the duration of medication was extended. Blood samples were collected when the endometrial thickness was ≥8mm. The endometrial transformation was performed when the E2 level was≥ 200pg/mL and the progesterone level was <1.4ng/mL. On day 10 of estrogen medication, dydrogesterone tablets (Duphphaston; Abbott Biologicals B.V., The Netherlands) 30mg orally once daily and progesterone (Crinone; Fleet Laboratories Limited, UK) 90mg once daily vaginally were added. According to the embryo transfer stage, if the cleavage embryo is on day 3, it will be transplanted on day 4 after the progesterone was administered.
In the GnRHa-HRT group, the patient was injected with 3.75mg GnRH-a (Diphereline, Ipsen Pty Ltd., France) on the second day of menstruation, and the HRT protocol will be started on day 28 later.
After endometrium preparation, vitrified embryos were thawed for transfer. In all FET cycles, no more than two embryos were transferred. According to the cryopreserved embryos of the patients, blastocysts transfer was preferred, and cleavage embryo was selected if there was no blastocysts. Assisted hatching is routinely performed before embryo transfer. The embryo transfer was performed via the flexible catheter (Frydman,1321600) under transabdominal ultrasound guidance. Luteal support was continued until a negative pregnancy test was obtained on the 14th day after embryo transfer. If pregnancy was achieved, hormone administration continued until 12 weeks’ gestation.
Levels of serum human chorionic gonadotropin (β-HCG) serum were determined in all patients on day 14 after embryo transfer. Biochemical pregnancy was taken into account if the serum β-HCG level was ≥5 IU/L. Clinical pregnancy was considered if a gestational sac was found on ultrasound on the 28th day after embryo transfer. Biochemical abortion was defined as positive blood β-hCG 14 days after embryo transfer, but no gestational sac was detected on ultrasound 28 days after embryo transfer. A clinical pregnancy that ends in miscarriage must do so before the 28th week of intrauterine pregnancy, whether spontaneous or therapeutic and foetus’ weight of <1000g. The ratio of live birth cycles to all cycles was defined as the live birth rate. The delivery of any surviving neonate at 28 weeks of gestation and beyond was referred to as a live birth.
All analyses were conducted using R (R Project for Statistical Computing, Austria), v 4.1.2. The inverse probability of treatment weighting (IPTW) approach was utilized for generating a propensity model (28). Individuals are weighted as the inverse of their probability in each group as the predicted probability per sample. The balance of baseline characteristics between groups was evaluated using the standardized mean difference (SMD), with SMD <0.1 considered balanced (29, 30). Univariate and multivariate logistic regression models were used to analyze the relationship between treatment groups and pregnancy outcomes. Multiple logistic regression was performed to balance confounders: maternal age, body mass index, type of infertility, duration of infertility, endometrial thickness, number of embryos transferred, and embryo type. Patient characteristics and pregnancy outcomes were represented as mean ± standard deviation (SD) or number (%) of patients. All P-values are two-tailed. The P-value for statistically significant comparisons was less than 0.05. The IPTW analyses were performed via the “RISCA” R package, v1.0.1 (31). P-value and SMD of baseline characteristics were calculated using the “TableOne” R package, v 0.13.2 (32). The function svyglm of the “survey” R package, v 4.1-1, is used to perform univariate and multivariate logistic regression analysis (33). R scripts can be provided if needed.
2733 patients enrolled in this retrospective study. Of these, 2 cases were lost to follow-up, 923 cases were combined with fallopian tube infertility, 308 cases were combined with anovulation, 71 cases were combined with endometriosis, 7 cases were abnormal karyotypes in patients or partners, 9 cases were with uterine malformations, and 31 cases were history of recurrent miscarriage. 1351 patients were excluded, leaving1382 to be enrolled. Of the total of 1382 women, 1038 patients underwent the HRT group, and 344 underwent the GnRHa-HRT group (Figure 1).
The basic conditions of female patients with male-factor infertility in the HRT group and the GnRHa-HRT group are shown in Table 1. Before IPTW, the baseline characteristics of the two groups were statistically different. Female patients in the GnRHa-HRT group were older, had a higher body mass index (BMI), had longer infertility years, and had a thicker endometrium. And there were statistically distinct types and numbers of embryos between the two groups.
After IPTW, there were no significant differences in terms of maternal age, body mass index, endometrial thickness, type of infertility and duration of infertility, number of transferred embryos, or embryo type between the two groups (all SMD<0.1 and P>0.05, Table 1).
Pregnancy outcomes between the two groups were analyzed using univariate and multivariate logistic models, as shown in Table 2. The GnRHa-HRT group had a significantly higher live birth rate than the HRT group (OR 2.154, 95%CI 1.636~2.835, P<0.001), according to the results of both univariate and multivariate logistic regression analysis.
In multivariate logistic regression adjusted for confounding factors, the rate of miscarriage was significantly lower in the GnRHa-HRT group (OR 0.432, 95% CI 0.245~0.762, P=0.004). And the GnRHa-HRT group had a higher rate of biochemical pregnancy (OR 1.853, 95% CI 1.398~2.457, P<0.001), clinical pregnancy (OR 1.845, 95% CI 1.401~2.430; P<0.001), multiple pregnancy (OR 1.908, 95% CI 1.283~2.837, P=0.001), and term delivery (OR 1.745, 95% CI 1.142~2.667, P=0.010). The rate of biochemical abortion (OR 0.718, 95% CI 0.390~1.325, P=0.289), ectopic pregnancy (OR 1.171, 95% CI 0.230~5.969, P=0.849), and preterm delivery (OR 0.977, 95% CI 0.542~1.759, P=0.937) have no statistical difference between two groups.
This work showed that the GnRHa-HRT group had significantly improved pregnancy outcomes for male-factor infertility, including higher rates of live birth, biochemical pregnancy, and clinical pregnancy while reducing the miscarriage rate. Additionally, pregnancy complications such as biochemical pregnancy, ectopic pregnancy, and preterm birth did not significantly differ between the two groups.
The success of every ART mainly depends on the implantation of the embryo. Studies have shown that embryo quality, endometrial receptivity, and embryo-endometrial dialogue are three determining factors in successful implantation (34–38). We strictly performed the inclusion criteria in order to better rule out the influence of embryo quality. In addition, we excluded cases of female factors affecting infertility-related disorders. Therefore, we supposed that GnRH-a not only improves endometrial receptivity but also promotes dialogue between embryo and endometrium.
Endometrial thickness is one of the markers of endometrial receptivity. Before adjustment, The endometrium in the GnRHa-HRT group was thicker than that in the HRT group, which is in line with many previous researches (15, 39, 40). Some recent studies suggested that it is oestrogen supplementation without GnRH-a suppression that may lead to an increase in luteinizing hormone (LH), which ultimately has a detrimental effect on endometrial receptivity (23, 41, 42). Moreover, establishing a dialogue between endometrium and embryo, as well as immune tolerance/protection from the host, requires not only hormonal regulation but also several endogenous molecules produced by endometrium and/or embryo (34). A review found that male-factor infertility is related to spontaneous abortion rates (43). Several studies have shown that GnRH-a not only promotes the expression of protective endometrial receptivity markers such as LIF, MEIS1, and HOXA10 but also increases and develops pinopodes well (17) (44). In this study, the clinical pregnancy rate was higher and the miscarriage rate was lower in the GnRHa-HRT group, suggesting that GnRH-a plays an essential role in synchronizing endometrial and embryo development and promoting the dialogue between endometrium and embryo.
Although we excluded the low-quality embryo, male infertility can affect the quality of the embryo, which cannot be fully judged from the morphology of the embryo (45–48). Examples of factors that have negative effects on the quality of embryo include the effects of Y chromosome microdeletions, DNA fragmentation, sperm aneuploidy, the role of proseminins and histones, sperm epigenetic profiles, and sperm chromatin structure (45). We speculated that good endometrial-embryo dialogue can correct certain aspects of the embryo, making it easier to implant.
The following are the study’s limitations. 1) The impact of aneuploid embryos cannot be excluded. The evaluation of embryo quality in this study was mainly based on embryo morphology rather than preimplantation genetic testing for aneuploidy.2) Neonatal outcomes were not compared to fully assess the effect of GnRH-a on reproductive outcomes in male-factor infertility. 3) Although IPTW was used to balance baseline conditions, other unknown confounders, such as basal hormone levels, may also have affected the results. For more accurate findings, multicentre prospective clinical trials are required.
We have reason to assume that our findings are reliable given the IPTW-adjusted population baseline, multivariate logistic regression balancing confounders, and the substantial number of cases in this study. This paper will serve as a reference for the preparation of the endometrium for the treatment of female patients with male-factor infertility.
In conclusion, this study found that a regimen of GnRH-a preconditioning with HRT for endometrial preparation improved pregnancy outcomes in patients with male-factor infertility patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of Shanghai General Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
JY and PC conducted the statistical analysis of the data and wrote the manuscript. YL was responsible for the collation of data. ML, LL, QX, JC, and LW were responsible for data acquisition and interpretation. ZZ contributed to the conception. MB contributed to the design of the work and rigorously revised the manuscript’s important intellectual content. All authors checked the lasted version. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (grants NO. 81902630, 81872111, and 81672562); the Shanghai Municipal Science and Technology Committee of Shanghai’s Excellent Academic Leader Program (grants NO. 19XD1423100), and the project of Outstanding Medical Doctor for ZZ.
We would like to thank Jing Lu for her help in data analysis.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem (2018) 62:2–10. doi: 10.1016/j.clinbiochem.2018.03.012
2. Carson SA, Kallen AN. Diagnosis and management of infertility: A review. JAMA (2021) 326(1):65–76. doi: 10.1001/jama.2021.4788
3. Boivin J, Bunting L, Collins JA, Nygren KG. International estimates of infertility prevalence and treatment-seeking: Potential need and demand for infertility medical care. Hum Reprod (2007) 22(6):1506–12. doi: 10.1093/humrep/dem046
4. Sharlip ID, Jarow JP, Belker AM, Lipshultz LI, Sigman M, Thomas AJ, et al. Best practice policies for male infertility. Fertil Steril (2002) 77(5):873–82. doi: 10.1016/s0015-0282(02)03105-9
5. Practice Committee of the American Society for Reproductive M. Diagnostic evaluation of the infertile male: a committee opinion. Fertil Steril (2015) 103(3):e18–25. doi: 10.1016/j.fertnstert.2014.12.103
6. Rezazadeh Valojerdi M, Eftekhari-Yazdi P, Karimian L, Hassani F, Movaghar B. Vitrification versus slow freezing gives excellent survival, post warming embryo morphology and pregnancy outcomes for human cleaved embryos. J Assist Reprod Genet (2009) 26(6):347–54. doi: 10.1007/s10815-009-9318-6
7. Bosch E, De Vos M, Humaidan P. The future of cryopreservation in assisted reproductive technologies. Front Endocrinol (Lausanne) (2020) 11:67. doi: 10.3389/fendo.2020.00067
8. Shapiro BS, Daneshmand ST, Garner FC, Aguirre M, Hudson C, Thomas S. Evidence of impaired endometrial receptivity after ovarian stimulation for in vitro fertilization: A prospective randomized trial comparing fresh and frozen-thawed embryo transfer in normal responders. Fertil Steril (2011) 96(2):344–8. doi: 10.1016/j.fertnstert.2011.05.050
9. European IVFmCddftESoHR, Embryology, Wyns C, Bergh C, Calhaz-Jorge C, De Geyter C, Kupka MS, et al. ART in Europe, 2016: Results generated from European registries by ESHRE. Hum Reprod Open (2020) 2020(3):hoaa032. doi: 10.1093/hropen/hoaa032
10. Wei DM, Liu JY, Sun Y, Shi YH, Zhang B, Liu JQ, et al. Frozen versus fresh single blastocyst transfer in ovulatory women: A multicentre, randomised controlled trial. Lancet (2019) 393(10178):1310–8. doi: 10.1016/s0140-6736(18)32843-5
11. Laval M, Garlantézec R, Guivarc’h-Levêque A. Birthweight difference of singletons conceived through in vitro fertilization with frozen versus fresh embryo transfer: An analysis of 5406 embryo transfers in a retrospective study 2013-2018. J Gynecol Obstet Hum Reprod (2020) 49(1):101644. doi: 10.1016/j.jogoh.2019.101644
12. Azimi Nekoo E, Chamani M, Shahrokh Tehrani E, Hossein Rashidi B, Davari Tanha F, Kalantari V. Artificial endometrial preparation for frozen-thawed embryo transfer with or without pretreatment with depot gonadotropin releasing hormone agonist in women with regular menses. J Family Reprod Health (2015) 9(1):1–4.
13. Mackens S, Santos-Ribeiro S, van de Vijver A, Racca A, Van Landuyt L, Tournaye H, et al. Frozen embryo transfer: A review on the optimal endometrial preparation and timing. Hum Reprod (2017) 32(11):2234–42. doi: 10.1093/humrep/dex285
14. Groenewoud ER, Cohlen BJ, Al-Oraiby A, Brinkhuis EA, Broekmans FJ, de Bruin JP, et al. A randomized controlled, non-inferiority trial of modified natural versus artificial cycle for cryo-thawed embryo transfer. Hum Reprod (2016) 31(7):1483–92. doi: 10.1093/humrep/dew120
15. Xia L, Tian L, Zhang S, Huang J, Wu Q. Hormonal replacement treatment for frozen-thawed embryo transfer with or without GnRH agonist pretreatment: A retrospective cohort study stratified by times of embryo implantation failures. Front Endocrinol (Lausanne) (2022) 13:803471. doi: 10.3389/fendo.2022.803471
16. Xu B, Geerts D, Hu S, Yue J, Li Z, Zhu G, et al. The depot GnRH agonist protocol improves the live birth rate per fresh embryo transfer cycle, but not the cumulative live birth rate in normal responders: a randomized controlled trial and molecular mechanism study. Hum Reprod (2020) 35(6):1306–18. doi: 10.1093/humrep/deaa086
17. Ruan HC, Zhu XM, Luo Q, Liu AX, Qian YL, Zhou CY, et al. Ovarian stimulation with GnRH agonist, but not GnRH antagonist, partially restores the expression of endometrial integrin beta3 and leukaemia-inhibitory factor and improves uterine receptivity in mice. Hum Reprod (2006) 21(10):2521–9. doi: 10.1093/humrep/del215
18. Orvieto R, Meltzer S, Rabinson J, Zohav E, Anteby EY, Nahum R. GnRH agonist versus GnRH antagonist in ovarian stimulation: the role of endometrial receptivity. Fertil Steril (2008) 90(4):1294–6. doi: 10.1016/j.fertnstert.2007.10.022
19. Park CW, Choi MH, Yang KM, Song IO. Pregnancy rate in women with adenomyosis undergoing fresh or frozen embryo transfer cycles following gonadotropin-releasing hormone agonist treatment. Clin Exp Reprod Med (2016) 43(3):169–73. doi: 10.5653/cerm.2016.43.3.169
20. Niu Z, Chen Q, Sun Y, Feng Y. Long-term pituitary downregulation before frozen embryo transfer could improve pregnancy outcomes in women with adenomyosis. Gynecol Endocrinol (2013) 29(12):1026–30. doi: 10.3109/09513590.2013.824960
21. Surrey ES, Silverberg KM, Surrey MW, Schoolcraft WB. Effect of prolonged gonadotropin-releasing hormone agonist therapy on the outcome of in vitro fertilization-embryo transfer in patients with endometriosis. Fertil Steril (2002) 78(4):699–704. doi: 10.1016/S0015-0282(02)03373-3
22. Liu X, Shi J, Bai H, Wen W. Pretreatment with a GnRH agonist and hormone replacement treatment protocol could not improve live birth rate for PCOS women undergoing frozen-thawed embryo transfer cycles. BMC Pregnancy Childbirth (2021) 21(1):835. doi: 10.1186/s12884-021-04293-4
23. Luo L, Chen M, Wen Y, Zhang L, Zhou C, Wang Q. Pregnancy outcome and cost-effectiveness comparisons of artificial cycle-prepared frozen embryo transfer with or without GnRH agonist pretreatment for polycystic ovary syndrome: A randomised controlled trial. BJOG (2021) 128(4):667–74. doi: 10.1111/1471-0528.16461
24. World Health O. WHO laboratory manual for the examination and processing of human semen. 5th ed. Genevavix: World Health Organization (2010), 271 p.: ill. (some col.); 25 cm. p.
25. Gardner DK, Schoolcraft WB eds. In vitro culture of human blastocysts. In: 11th world congress on in vitro fertilization and human reproductive genetics. Sydney, Australia Towards Reproduct Certainty (1999)
26. Fisch JD, Sher G, Adamowicz M, Keskintepe L. The graduated embryo score predicts the outcome of assisted reproductive technologies better than a single day 3 evaluation and achieves results associated with blastocyst transfer from day 3 embryo transfer. Fertil Steril (2003) 80(6):1352–8. doi: 10.1016/j.fertnstert.2003.05.013
27. Fisch JD, Rodriguez H, Ross R, Overby G, Sher G. The graduated embryo score (GES) predicts blastocyst formation and pregnancy rate from cleavage-stage embryos. Hum Reprod (2001) 16(9):1970–5. doi: 10.1093/humrep/16.9.1970
28. Mao H, Li L, Greene T. Propensity score weighting analysis and treatment effect discovery. Stat Methods Med Res (2019) 28(8):2439–54. doi: 10.1177/0962280218781171
29. Vuvan V, Vicenzino B, Mellor R, Heales LJ, Coombes BK. Unsupervised isometric exercise versus wait-and-See for lateral elbow tendinopathy. Med Sci Sports Exerc (2020) 52(2):287–95. doi: 10.1249/MSS.0000000000002128
30. Feld E, Harton J, Meropol NJ, Adamson BJS, Cohen A, Parikh RB, et al. Effectiveness of first-line immune checkpoint blockade versus carboplatin-based chemotherapy for metastatic urothelial cancer. Eur Urol (2019) 76(4):524–32. doi: 10.1016/j.eururo.2019.07.032
31. Vincenzi B, Napolitano A, Fiocco M, Mir O, Rutkowski P, Blay JY, et al. Adjuvant imatinib in patients with GIST harboring exon 9 KIT mutations: Results from a multi-institutional European retrospective study. Clin Cancer Res (2022) 28(8):1672–9. doi: 10.1158/1078-0432.CCR-21-1665
32. Panos A, Mavridis D. TableOne: An online web application and r package for summarising and visualising data. Evid Based Ment Health (2020) 23(3):127–30. doi: 10.1136/ebmental-2020-300162
33. Lumley T, Scott A. Fitting regression models to survey data. Stat Sci (2017) 32(2):265–78. doi: 10.1214/16-sts605
34. Salilew-Wondim D, Schellander K, Hoelker M, Tesfaye D. Oviductal, endometrial and embryonic gene expression patterns as molecular clues for pregnancy establishment. Anim Reprod Sci (2012) 134(1-2):9–18. doi: 10.1016/j.anireprosci.2012.08.006
35. Craciunas L, Gallos I, Chu J, Bourne T, Quenby S, Brosens JJ, et al. Conventional and modern markers of endometrial receptivity: A systematic review and meta-analysis. Hum Reprod Update (2019) 25(2):202–23. doi: 10.1093/humupd/dmy044
36. Simon C, Moreno C, Remohi J, Pellicer A. Cytokines and embryo implantation. J Reprod Immunol (1998) 39(1-2):117–31. doi: 10.1016/s0165-0378(98)00017-5
37. Edwards RG. Implantation, interception and contraception. Hum Reprod (1994) 9(6):985–95. doi: 10.1093/oxfordjournals.humrep.a138673
38. Franasiak JM, Forman EJ, Hong KH, Werner MD, Upham KM, Treff NR, et al. The nature of aneuploidy with increasing age of the female partner: A review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril (2014) 101(3):656–63 e1. doi: 10.1016/j.fertnstert.2013.11.004
39. Simon A, Hurwitz A, Zentner BS, Bdolah Y, Laufer N. Transfer of frozen-thawed embryos in artificially prepared cycles with and without prior gonadotrophin-releasing hormone agonist suppression: A prospective randomized study. Hum Reprod (1998) 13(1O):2712–7. doi: 10.1093/humrep/13.10.2712
40. Qi Q, Luo J, Wang Y, Xie Q. Effects of artificial cycles with and without gonadotropin-releasing hormone agonist pretreatment on frozen embryo transfer outcomes. J Int Med Res (2020) 48(6):300060520918474. doi: 10.1177/0300060520918474
41. Hill MJ, Miller KA, Frattarelli JL. A GnRH agonist and exogenous hormone stimulation protocol has a higher live-birth rate than a natural endogenous hormone protocol for frozen-thawed blastocyst-stage embryo transfer cycles: An analysis of 1391 cycles. Fertil Steril (2010) 93(2):416–22. doi: 10.1016/j.fertnstert.2008.11.027
42. Wang Y, Hu WH, Wan Q, Li T, Qian Y, Chen MX, et al. Effect of artificial cycle with or without GnRH-a pretreatment on pregnancy and neonatal outcomes in women with PCOS after frozen embryo transfer: A propensity score matching study. Reprod Biol Endocrinol (2022) 20(1):56. doi: 10.1186/s12958-022-00929-y
43. Lin MH, Kuo-Kuang Lee R, Li SH, Lu CH, Sun FJ, Hwu YM. Sperm chromatin structure assay parameters are not related to fertilization rates, embryo quality, and pregnancy rates in in vitro fertilization and intracytoplasmic sperm injection, but might be related to spontaneous abortion rates. Fertil Steril (2008) 90(2):352–9. doi: 10.1016/j.fertnstert.2007.06.018
44. Guo S, Li Z, Yan L, Sun Y, Feng Y. GnRH agonist improves pregnancy outcome in mice with induced adenomyosis by restoring endometrial receptivity. Drug Des Devel Ther (2018) 12:1621–31. doi: 10.2147/DDDT.S162541
45. Colaco S, Sakkas D. Paternal factors contributing to embryo quality. J Assist Reprod Genet (2018) 35(11):1953–68. doi: 10.1007/s10815-018-1304-4
46. Shoukir Y, Chardonnens D, Campana A, Sakkas D. Blastocyst development from supernumerary embryos after intracytoplasmic sperm injection: A paternal influence? Hum Reprod (1998) 13(6):1632–7. doi: 10.1093/humrep/13.6.1632
47. Miller JE, Smith TT. The effect of intracytoplasmic sperm injection and semen parameters on blastocyst development in vitro. Hum Reprod (2001) 16(5):918–24. doi: 10.1093/humrep/16.5.918
Keywords: male-factor infertility, frozen embryo transfer, endometrial preparation, gonadotropin-releasing hormone agonist, hormone replacement treatment, inverse probability of treatment weighting, live birth rate
Citation: Yu J, Chen P, Luo Y, Lv M, Lou L, Xiao Q, Wang L, Chen J, Bai M and Zhang Z (2022) GnRH-agonist pretreatment in hormone replacement therapy improves pregnancy outcomes in women with male-factor infertility. Front. Endocrinol. 13:1014558. doi: 10.3389/fendo.2022.1014558
Received: 08 August 2022; Accepted: 06 September 2022;
Published: 23 September 2022.
Edited by:
Zhiqin Bu, Zhengzhou University, ChinaReviewed by:
Marzieh Ghasemi, Zahedan university of Medical Sciences, IranCopyright © 2022 Yu, Chen, Luo, Lv, Lou, Xiao, Wang, Chen, Bai and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mingzhu Bai, YmFpbWluZ3podWlAc2luYS5jbg==; Zhenbo Zhang, emhhbmd6aGVuYm96emJAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.