
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 25 October 2022
Sec. Pediatric Endocrinology
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1009133
This article is part of the Research Topic Sexual Dimorphism and Steroid Hormone Crosstalk, Volume II View all 9 articles
 Meijuan Liu1
Meijuan Liu1 Bingyan Cao1
Bingyan Cao1 Qipeng Luo2
Qipeng Luo2 Qiao Wang1
Qiao Wang1 Min Liu1
Min Liu1 Xuejun Liang1
Xuejun Liang1 Di Wu1
Di Wu1 Wenjing Li1
Wenjing Li1 Chang Su1
Chang Su1 Jiajia Chen1
Jiajia Chen1 Chunxiu Gong1*
Chunxiu Gong1*Background: Although previous studies suggested that there is a certain level of body fat mass before puberty can be initiated, most studies have focused on girls.
Objective: To investigate the relationship between precocious puberty and physical growth in school-aged children in Beijing, China.
Methods: 7590 Chinese children (3591 girls and 3999 boys) aged 6–11 years were recruited in Beijing, China. Body mass index (BMI) categories were defined by WHO Child Growth Standards and central obesity were defined by sex-specific waist-to-height ratio cut-offs (≥0.46 for girls, ≥0.48 for boys). Sexual development was assessed using Tanner criteria.
Results: The prevalence of general obesity and central obesity among boys was higher than that in girls. Girls had a significantly higher precocious puberty rate than boys (5.93% vs. 0.87%), particularly in those aged 7 years old (9.20%). Children in the general obesity and central obesity groups have a higher prevalence of precocious puberty and earlier median ages for the attainment of Tanner B2/T2. For girls with Tanner stages≥II at 6-year-old and 7-year-old, the mean BMI was equivalent to the 50th centile of a normal 9.9-year-old and 11.9-year-old girl, respectively. The mean BMI of boys with Tanner stages≥II at 7-year-old and 8-year-old was correspondent to the 50th centile of a normal 14-year-old and 15.3-year-old boy, respectively. For girls, general obesity appears to contribute to the risk of the development of precocious puberty to a greater extent than central obesity does. For boys, central obesity, but not general obesity, was an independent risk factor for precocious puberty.
Conclusions: The prevalence of childhood obesity and precocious puberty was high in China. Precocious puberty was correlated with a large BMI. Boys had a higher threshold of BMI for puberty development than girls. Children with precocious puberty, particularly those with central obesity, should be aware of adverse cardiovascular events.
Puberty is a critical period between childhood and adulthood during which adolescents reach sexual maturity and become capable of reproduction (1). The ages of transition into a higher Tanner stage of breast, pubic hair, and testicle development are maturational milestones commonly used to describe the initiation and progression of puberty among girls and boys. In recent decades, a growing body of literature reports the earlier development of secondary sexual characteristics in children (2–6). According to the data collected from the National Health and Nutrition Examination Survey (NHANES), the mean age for menarche in the United States declined over time from 13.3 years in girls born before 1920 to 12.4 years in girls born in 1980–1984 (4). In Thailand, the median age of onset of breast development showed a steady decline, from 10.7 years in 1980 to 9.9 years in 1994, and recently to 9.4 years in 1999 (2). It is important to note, that earlier ages of puberty have been reported to be associated with a higher risk of adult disease, including obesity, type 2 diabetes mellitus, metabolic syndrome, depression, cardiovascular disease, cancer, etc. (7–9). Thus, obtaining up-to-date pubertal data and understanding modifiable factors influencing pubertal timing could have important public health implications.
Indeed, puberty is a complex physical process, which involves a complicated interplay between genetic, nutritional, environmental, and socio-economic factors (10). Although the mechanisms controlling the onset and tempo of puberty are still largely unknown, accumulating research has confirmed the important role of obesity in sexual development (11–14). In recent years, the decreasing age of pubertal onset has paralleled the rising prevalence of pediatric obesity (15), and multiple studies have demonstrated that childhood overweight/obesity is associated with earlier timing or faster progression of pubertal development (16–18). For instance, overweight and obese girls were more likely to attain pubertal milestones earlier compared with normal-weight children [overweight girls: -5.5 months, 95% confidence interval (CI): -7.1, -3.9 months; obese girls: -5.2 months, 95% CI: -7.1, -3.4 months] (17). Furthermore, previous studies indicated that body weight was involved in puberty onset and maintenance (19, 20). Girls who had earlier onset of puberty had markedly higher age-normalized body mass index (BMI) (21), whereas girls who had low body weight or body fat were often accompanied by delayed menarche and menstrual irregularities (20, 22). That is, a certain threshold of body weight and fat has to be reached before puberty can be initiated and maintained. However, most of these studies focus on girls, and results on boys seem to be inconsistent (16, 17, 23).
As well as obesity, body fat distribution has lately been found to be associated with sexual development. Boys and girls with a high-level body fat percentage (24) and waist-to-height ratio (WHtR) (25) had increased risks of earlier puberty in comparison with those with a low-level body fat percentage and WHtR. However, in the majority of studies, overweight/obesity is defined by BMI level, which was not effective for providing information on fat distribution. There are, indeed, only a few studies have examined the association between abdominal obesity and puberty onset.
With the above-mentioned points in mind, this study aimed to estimate the prevalence and distribution of childhood precocious puberty, especially in different age and weight categories; and to explore the associations between childhood general obesity/central obesity with puberty onset.
This was a cross-sectional, school-based study, carried out in Beijing, the capital of China. The data presented here were obtained from the survey on Students’ Constitution and Health. Children were excluded if they were taking medication (such as glucocorticoid) that could cause precocious puberty or had unilateral mammary development, or a preexisting diagnosis of organic disease (such as ovarian tumors), or chronic diseases (such as chronic kidney disease), or genital abnormalities (such as cryptorchidism). A total sample of 7590 students (3591 girls and 3999 boys) aged 6–11 years were enrolled in this study. For general obesity, children were classified into five categories: severe thinness (n=151), thinness (n=520), normal (n=3787), overweight (n=1346), and obesity (n=1786). For central obesity, children were classified into two categories: non-central obesity (n=4908) and central obesity (n=2682). All students completed a physical examination. Written informed consent was obtained from a parent or guardian on behalf of each child and the study was approved by the ethics committee of Beijing Children’s Hospital, Capital Medical University.
All children underwent a standardized series of physical examinations that were conducted by trained pediatric endocrinologists. Body weight (to the nearest 0.1 kg) was measured using a scale (Tanita HA 503, Tanita Corporation, Tokyo, Japan) with subjects wearing only lightweight clothing and without shoes. Height (to the nearest 0.1 cm) was assessed in the erect position without shoes by a portable Seca 213 stadiometer (Seca GmbH, Hamburg, Germany). Waist circumference (WC, to the nearest 0.1 cm) was obtained with an inelastic measuring tape at the midpoint of the horizontal line between the lowest rib margin and the iliac crest. Two measurements (measurement error ≤ 0.1 kg/0.1 cm) were taken and the average was calculated for the analysis. BMI was calculated as children’s weight (kg) divided by the square of height (m2). Children were classified into five categories: severe thinness, thinness, normal, overweight, and obesity according to sex-age-specific BMI cut-offs recommended by the WHO Child Growth Standards (http://www.who.int/growthref/en/) as follows: severe thinness if BMI<−3 standard deviation (SD), thinness if BMI<−2 SD, overweight if BMI>+1SD, and obesity if BMI>+2SD (26, 27). WHtR was calculated as WC (cm) divided by height (cm), figures equal to or greater than 0.46 and 0.48 were considered central obesity for girls and boys, respectively (28).
The puberty assessment of children was evaluated by professional pediatric endocrinology physicians. In girls, breast development was evaluated by inspection combined with palpation. In boys, testicular volume was estimated by palpation to the nearest 1 mL using Prader’s orchidometer. The pubertal stages of breast, pubic hair, and testicular volume were graded from I (prepubertal) to V (fully mature) using the Tanner staging method (29, 30). Precocious puberty was defined as the occurrence of Tanner stage II or above for breast (B2) or pubic hair development (PH2) before 8 years in girls, and Tanner stage II or above for testicle development (T2, volume ≥4 mL) or PH2 before 9 years in boys (31). Sexual development ≥B2/PH2 in girls and T2/PH2 in boys were defined as Tanner stage II or above for breast/pubic hair development in girls and testicle/pubic hair development in boys (25).
Categorical variables were presented as frequency (percentage), and continuous variables were presented as mean ± SD. Comparisons of continuous variables were performed using Student t-tests, and comparisons of categorical variables were performed using Chi-squared tests with Fisher’s exact statistic. The detection rates of Tanner stages≥II in different ages and obesity categories were directly calculated. Probit analysis was used for estimating the median age of the population at entry into Tanner stage II or greater for breast, pubic hair, and testicular development. Odds ratios (ORs) with 95% CIs were calculated to assess the associations between precocious puberty, BMI categories, and central obesity. All the raw data were analyzed by IBM SPSS Statistic package version 21 (IBM Corp., Armonk, New York, USA), p value<0.05 was considered statistically significant.
In total, 7590 students (girls: 3591, boys: 3999) aged 6–11 years were assessed in this study, and the basic characteristics were summarized in Table 1. The age distribution was similar for boys and girls (p>0.05). Boys had higher weight, height, WC, BMI, and WHtR than girls (p all <0.05). The proportion of general obesity and central obesity were markedly higher in boys than in girls (general obesity: 32.28% vs. 13.78%, central obesity: 39.93% vs. 30.21%).
The prevalence of sexual development ≥B2/PH2 in girls and T2/PH2 in boys at different ages was depicted in Figure 1. Prevalence of Tanner stages ≥II was significantly higher in girls than in boys. The prevalence of Tanner stages ≥PH2 among girls aged 9–11 years was relatively higher than those in boys (9 years: 3.41% vs. 0.39%, 10 years: 28.09% vs. 3.07%, 11 years: 56.54% vs. 15.08%). At ages 6–11 years, girls had reached a higher proportion of Tanner stages ≥B2 than boys with Tanner stages ≥T2 (6 years: 2.33% vs. 0.16%, 7 years: 9.20% vs. 0.38%, 8 years: 33.19% vs. 1.45%, 9 years: 55.79% vs. 2.58%, 10 years: 85.46% vs. 23.51%, 11 years: 93.09% vs. 56.82%). Girls had a significantly higher precocious puberty rate than boys. The overall rate of precocious puberty in girls aged <8 years and in boys aged <9 years was 5.93% and 0.87%, respectively. The highest precocious puberty rate for girls and boys was in the 7-year-old group (9.20%) and the 8-year-old group (1.45%), respectively.
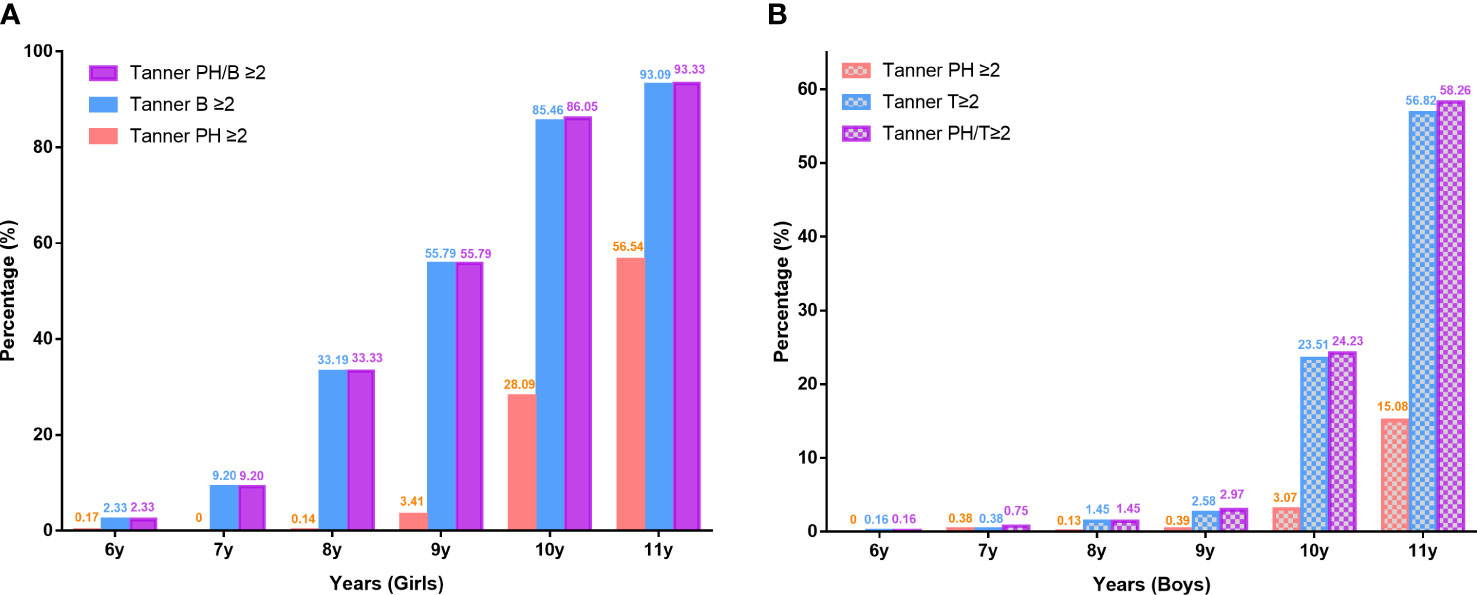
Figure 1 Prevalence of testicle, breast, and pubic hair development according to pubertal stages at different ages in girls (A) and boys (B). Ph, pubic hair; T, testicle; B, breast.
The prevalence of Tanner stages ≥II in different obesity categories in both genders was also examined. As shown in Figure 2, general obesity and central obesity had an obvious influence on sexual development in both genders at different ages. Compared with children with severe thinness/thinness, we found an increased prevalence of Tanner stages ≥II in the normal-weight group in both genders, and an especially high prevalence of Tanner stages ≥II in the overweight/obesity group. Additionally, the prevalence of Tanner stages ≥II in the central obesity group was higher than that in the normal-weight group in both genders at different ages. In both genders, the prevalence of precocious puberty in the overweight/obesity and central obesity group was higher than that in the control group (girls: 11.01% vs. 4.49%, 6.01% vs. 4.93%; boys: 1.31% vs. 0.64%, 1.48% vs. 0.49%).
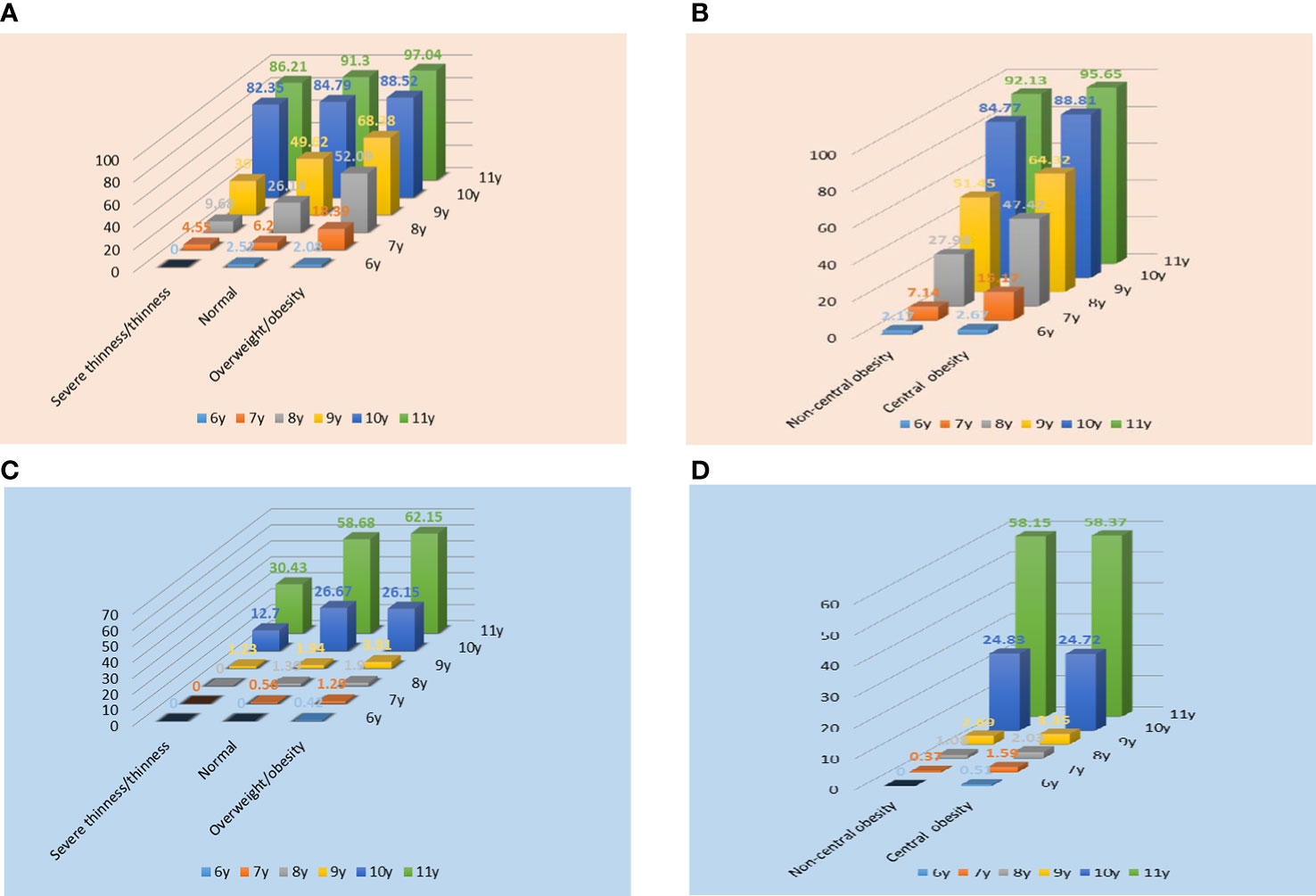
Figure 2 Prevalence of Tanner stages ≥II in different obesity categories in girls (A, B) and boys (C, D). In Figure A, C, normal refers to children with normal body mass index.
To further explore the effects of obesity on puberty onset, we then estimated the median age of both girls and boys for the attainment of Tanner B2/T2/PH2 (Table 2). In girls, the median age of B2/PH2 was 10.13 and 11.14 in the severe thinness/thinness group, 9.20 and 10.73 in the normal-weight group, 8.92 and 10.72 in the overweight/obesity group. A similar decreasing trend with increasing BMI was observed in the median ages of T2 (10.83, 10.67, 10.63 years) in boys, although the trend was not significant. This finding indicated that severe thinness/thinness had a greater impact on puberty onset than overweight/obesity. Notably, negative associations between general obesity and median ages of B2 and T2 were also found in central obesity. For girls, the median age of B2 was 9.27 (95% CI=9.17 to 9.38) and 8.84 (95% CI=8.59 to 9.09) years for children in the control group and the central obesity group. For boys, the median age of T2 in the control group (10.67, 95% CI=10.56 to 10.79) was older than that in the central obesity group (10.63, 95% CI=10.49 to 10.78). Although the trend for earlier median ages of Tanner B2/T2/PH2 was similar in girls and boys, it was more profound in girls.
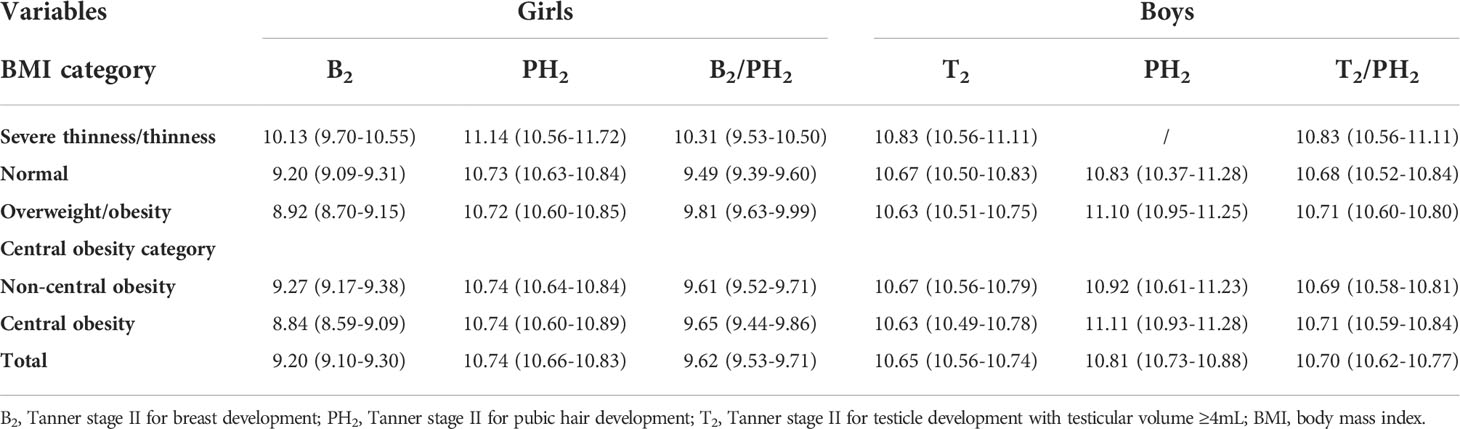
Table 2 Median age (95% CI) of attainment of different pubertal stages according to probit analysis.
In both genders, precocious puberty was correlated with a large physical growth, especially for BMI. As illustrated in Figure 3A, the mean height of girls with Tanner stages≥II at 6-year-old and 7-year-old was 125.01 ± 9.71 cm and 131.46 ± 5.69 cm, which was equivalent to the 50th centile of a normal 7.9-year-old and 8.9-year-old girl, respectively (Figure 3A). For boys with Tanner stages ≥II at 7-year-old and 8-year-old, the mean height was 133.93 ± 9.71 cm and 141.15 ± 7.07 cm, which was correspondent to the 50th centile of a normal 9.3-year-old and 10.6-year-old boy, respectively (Figure 3C). Similar phenomena were observed for BMI. For girls with Tanner stages ≥II at 6-year-old and 7-year-old, the mean BMI was 16.56 ± 4.11 kg/m2 and 17.71 ± 2.93 kg/m2, which was equivalent to the 50th centile of a normal 9.9-year-old and 11.9-year-old girl, respectively (Figure 3B). The mean BMI of boys with Tanner stages ≥II at 7-year-old and 8-year-old was 19.04 ± 3.13 kg/m2 and 20.05 ± 4.29 kg/m2, which was correspondent to the 50th centile of a normal 14-year-old and 15.3-year-old boy, respectively (Figure 3D). Taken together, it appears that physical growth, especially BMI must be kept above a certain threshold level to initiate puberty development. The threshold was much higher in boys than in girls, which was in accordance with the above observations (higher prevalence of obesity and lower rate of precocious puberty in boys).
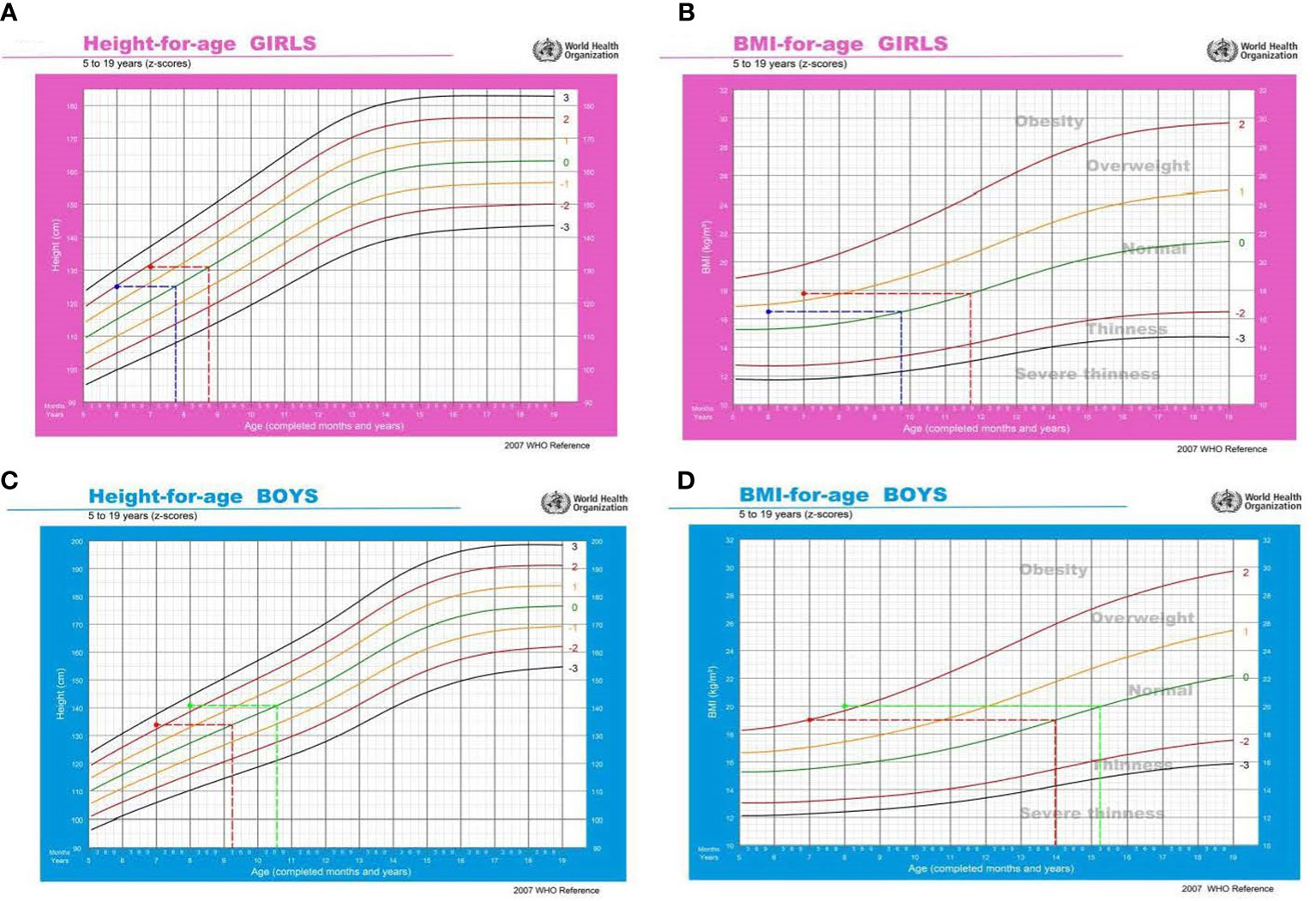
Figure 3 Relationship between precocious puberty and physical growth. In Figures (A, B), the blue line refers to girls with Tanner stages≥II at 6-year-old, and the red line refers to girls with Tanner stages≥II at 7-year-old. In Figures (C, D), the red line refers to boys with Tanner stages≥II at 7-year-old, and the green line refers to boys with Tanner stages≥II at 8-year-old.
We further explored the relationship between different types of obesity and the risk of precocious puberty. As shown in Table 3, for girls, both general obesity and central obesity were independently associated with an increased risk of precocious puberty. General obesity appears to contribute to the risk of development of precocious puberty to a greater extent than central obesity does (general obesity: OR=2.46, 95% CI 1.33–4.55; central obesity: OR=1.81, 95% CI 1.14–2.89; respectively). For boys, central obesity, but not general obesity, was an independent risk factor for precocious puberty(OR=2.93, 95% CI 1.04–8.26, p<0.05).
In this cross-sectional study with a large sample of Chinese children aged 6–11 years in Beijing, we found that the prevalence of general obesity and central obesity were 13.78% and 30.21% in girls, 32.28% and 39.93% in boys, respectively. It is clearly seen that with the rapid economic development and urbanization, the prevalence of childhood obesity in China has reached epidemic proportions. In our present study, the overall rate of precocious puberty in girls aged <8 years and in boys aged <9 years was 5.93% and 0.87%, respectively. The highest precocious puberty rate for girls and boys was in the 7-year-old group (9.20%) and 8-year-old group (1.45%), respectively. The prevalence of precocious puberty observed in this study was significantly higher than that found in previous studies. In a cross-sectional epidemiological study conducted in 6 representative geographical areas in China in 2013, breast development at 7-year-old was observed in 2.56% of girls, and testicular volume ≥4 mL at 8-year-old was observed in 0.61% of boys (32). Recently, studies conducted in other cities in China (Shanghai, Zhongshan, Chongqing, etc.) also noted the high prevalence of precocious puberty (girls: 4.8%–23.07%, boys:0.8%–9.53%) (25, 31, 33). Additionally, we found that boys showed a higher rate of obesity, but a lower rate of precocious puberty rate than girls. The reasons for the higher precocious puberty prevalence rate observed in girls are not entirely clear. We speculate that the distinct criteria used in girls (breast, not gonads) and boys (testicle, gonads) may partially be responsible for this difference in prevalence. The enlarged testes in boys were in a stricter sense of puberty initiation.
In addition to the markedly increased prevalence, the age of puberty onset has also significantly advanced. Jaruratanasirikul et al. reported that the median age of Tanner B2 varied from 9.9 years in 1994 to 9.6 years in 2012, which indicated the trend toward earlier onset of puberty in Thailand school girls (2). A similar phenomenon was also observed in mainland China. In 2009–2010, Zhu et al. conducted a cross-sectional study in 18707 children in 6 representative cities in China, they found that the median ages of onset of Tanner B2 and T2 were 9.69 (95% CI: 9.63–9.75) years and 11.25 (95% CI: 11.19–11.30) years, respectively (32). According to the data from the Shanghai Children’s Health, Education and Lifestyle Evaluation (SCHEDULE) study in 2014, the median ages of B2 and PH2 for girls were 8.62 (95% CI: 8.57–8.67) years and 10.62 (95% CI: 10.53–10.71) years, the median ages of T2 and PH2 for boys were 13.84 (95% CI: 13.40–14.40) years and 10.46 (95% CI: 10.21–10.78) years (25). The median ages of T2 and PH2 for boys in our present study were earlier than that found in previous studies in Shanghai (25), which may be attributable to the higher prevalence of central obesity in boys in our study. However, these studies only focused on the age of puberty onset without anthropometric measurements (weight, height, BMI, etc.), and comparative analysis could not be calculated for these cases. Taken together, the high prevalence and earlier age of precocious puberty observed in our results together with our publications revealed an urgent need for scaled-up effective interventions among Chinese children.
Increasing evidence suggests that obesity is closely relevant to precocious puberty, the rise in the prevalence of childhood obesity alongside the rise in early sexual development, especially in girls (17, 21, 23, 34, 35). Four findings of our study confirmed the close links between obesity and precocious puberty in girls. Firstly, this study revealed that the prevalence of precocious puberty in the overweight/obesity and central obesity group was 11.01% and 6.01%, which was significantly higher than that in the normal-weight (4.49%) and non-central obesity groups (4.93%). This finding was in line with the study of Liu et al. among 4058 children aged 6–11 years in Guangdong (31), also, the study of Wei et al. among 11000 children aged 4–12 years in Leshan (36), both of which showed girls who were overweight or obese had a higher prevalence of precocious puberty. Secondly, we observed that overweight/obesity, as well as central obesity, were associated with earlier age at attaining Tanner B2. Results from other studies support our results. In a recent cohort and sibling-matched analyses performed by Brix et al., childhood overweight and obesity were associated with earlier puberty in a dose-dependent manner in boys and girls (17). Thirdly, our study also demonstrated that girls with precocious puberty were correlated with a large BMI. For girls with precocious puberty at 6-year-old and 7-year-old, the mean BMI was 16.56 ± 4.11 kg/m2 and 17.71 ± 2.93 kg/m2, which was equivalent to the 50th centile of a normal 9.9-year-old and 11.9-year-old girl, respectively. As early as the 1970s, Frisch proposed the “critical weight hypothesis”, which stated that a certain body fat depot seems to be required for the process of initiating normal reproductive function (20). Previous studies found that the values of weight, height, WC (33), BMI, and WHtR (32) in precocious puberty children were higher than those in peer normal children. Children with high-level of body fat as well as those with a rapid increase in anthropometric profiles were more sensitive to earlier puberty onset (24). Our results, together with data reported by others, indicated the importance to maintain healthy adiposity status in preventing earlier puberty onset in children. Fourthly, in the present study, general obesity and central obesity were found to as individual risk variables affecting precocious puberty. It is noteworthy that in girls, general obesity appears to contribute to the risk of development of precocious puberty to a greater extent than central obesity does (general obesity: OR=2.46, 95% CI 1.33–4.55; central obesity: OR=1.81, 95% CI 1.14–2.89; respectively). Our results were in keeping with studies by Chen et al. in Shanghai, which demonstrated that more than for central obesity, the risk of precocious puberty in girls rose even more with general obesity (general obesity: OR=9.00, 95% CI 5.60–14.46; central obesity: OR=5.40, 95% CI 4.10–7.12; respectively) (25). However, based on the relatively small number of studies available, more findings about the effect of fat distribution on pubertal development in girls should be done in the future.
As stated above, the link between obesity and precocious puberty in girls is well established. However, observations in boys remain inconclusive (31, 37–40). In our present study, we found that the prevalence of Tanner stages ≥II was significantly higher in the general obesity and central obesity groups for boys aged 6–11 years. Furthermore, boys with general obesity and central obesity started puberty, as assessed by testicular enlargement, significantly earlier compared to normal weight and non-central obese boys. However, Our result differs from previous studies by Lee et al. in US boys, who showed a J-shaped association between weight status with the timing of puberty, that is to say, overweight boys seemed to mature earlier, but obese boys matured later than normal-weight boys (37). This disagreement may be attributable to geographic distribution and racial differences. The mean BMI of boys with Tanner stages ≥II at 7-year-old and 8-year-old was 19.04 ± 3.13 kg/m2 and 20.05 ± 4.29 kg/m2, which was correspondent to the 50th centile of a normal 14-year-old and 15.3-year-old boy, respectively. While boys with precocious puberty were also correlated with a large BMI, just like girls, the threshold of BMI for puberty development in boys was higher than that in girls. We speculate that the distinct criteria used in girls (breast, not gonads) and boys (testicle, gonads) may partially be responsible for this difference in prevalence. The manifestation of puberty initiation was stricter in boys (testicle, gonads) than in girls (breast, not gonads), as the majority of the breast is composed of fat tissue. This was also consistent with the clinical observations that boys had a significantly higher prevalence of obesity but a lower rate of precocious puberty than girls. Another interesting finding worthy of note was that in boys, central obesity was an independent risk factor, whereas general obesity was not. That is, in boys, WHtR had a greater influence than BMI on precocious puberty. Central obesity has been implicated as one of the most important risk factors in the development of various metabolic diseases including hypertension and cardiovascular diseases. Thus, children with precocious puberty, particularly those with central obesity, should be a cause for concern among physicians regarding possible adverse cardiovascular events.
Several limitations should be considered when interpreting our present findings. First, this was a cross-sectional study and the cause-effect relationship between precocious puberty and obesity could not be determined in our study. Longitudinal investigations are needed to determine the causal direction. Second, although the physical examination was conducted by trained pediatric endocrinologists, the inter-observer bias cannot be ruled out. Third, in our present study, the breast development of girls was evaluated by inspection combined with palpation. It was rather difficult to employ ultrasound diagnosis on a large scale in children taking part in the physical examination. Fourth, our results might not be representative of the population as a whole and should be interpreted with caution.
In conclusion, the prevalence of childhood general obesity, central obesity, and precocious puberty was high in China. Children with general obesity and central obesity were more vulnerable to precocious puberty, but this correlation had gender differences. Boys had a higher threshold of BMI for puberty development than girls. The causal relationship between obesity and precocious puberty and the underlying mechanisms need to be studied in the future.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from a parent or guardian on behalf of each child and the study was approved by the ethics committee of Beijing Children’s Hospital, Capital Medical University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
All authors helped to perform the research; MJL wrote the manuscript; BC contributed to the project management; QL participated in the interpretation of data. QW, ML, XL, DW, WL, CS, and JC took part in the collection of clinical samples; CG conceived and designed the project as well as revised the manuscript. All listed authors revised the paper critically and approved the final version of the submitted manuscript.
This study was funded by The Pediatric Medical Coordinated Development Center of Beijing Hospitals Authority (XTYB201808), the National Key Research and Development Program of China (2016YFC0901505, 2016YFC1305304), and the Beijing Municiple Administration of Hospital Clinical Medicine Development of Special Funding Support (No. ZYLX201821).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Liu Y, Peterson KE, Sánchez BN, Jones AD, Cantoral A, Mercado-García A, et al. Dietary intake of selenium in relation to pubertal development in Mexican children. Nutrients (2019) 11(7):1595. doi: 10.3390/nu11071595
2. Jaruratanasirikul S, Chanpong A, Tassanakijpanich N, Sriplung H. Declining age of puberty of school girls in southern Thailand. World J Pediatr (2014) 10:256–61. doi: 10.1007/s12519-014-0472-2
3. Euling SY, Herman-Giddens ME, Lee PA, Selevan SG, Juul A, Sørensen TIA, et al. Examination of US puberty-timing data from 1940 to 1994 for secular trends: Panel findings. Pediatrics (2008) 121 Suppl 3:S172–91. doi: 10.1542/peds.2007-1813D
4. McDowell MA, Brody DJ, Hughes JP. Has age at menarche changed? Results from the national health and nutrition examination survey (NHANES) 1999-2004. J Adolesc Health (2007) 40:227–31. doi: 10.1016/j.jadohealth.2006.10.002
5. Ma HM, Du ML, Luo XP, Chen SK, Liu L, Chen RM, et al. Onset of breast and pubic hair development and menses in urban chinese girls. Pediatrics (2009) 124:e269–77. doi: 10.1542/peds.2008-2638
6. Rubin C, Maisonet M, Kieszak S, Monteilh C, Holmes A, Flanders D, et al. Timing of maturation and predictors of menarche in girls enrolled in a contemporary British cohort. Paediatr Perinat Epidemiol (2009) 23:492–504. doi: 10.1111/j.1365-3016.2009.01055.x
7. Fuhrman BJ, Moore SC, Byrne C, Makhoul I, Kitahara CM, de González AB, et al. Association of the age at menarche with site-specific cancer risks in pooled data from nine cohorts. Cancer Res (2021) 81:2246–55. doi: 10.1158/0008-5472.CAN-19-3093
8. Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: The UK biobank study. Sci Rep (2015) 5:11208. doi: 10.1038/srep11208
9. Golub MS, Collman GW, Foster PM, Kimmel CA, Meyts ER, Reiter EO, et al. Public health implications of altered puberty timing. Pediatrics (2008) 121(Suppl 3):S218–30. doi: 10.1542/peds.2007-1813G
10. Veldhuis JD, Roemmich JN, Richmond EJ, Bowers CY. Somatotropic and gonadotropic axes linkages in infancy, childhood, and the puberty-adult transition. Endocr Rev (2006) 27:101–40. doi: 10.1210/er.2005-0006
11. Cheng TS, Ong KK, Biro FM. Trends toward earlier puberty timing in girls and its likely mechanisms. J Pediatr Adolesc Gynecol (2022) 35(5):527–31. doi: 10.1016/j.jpag.2022.04.009
12. Gonc EN, Kandemir N. Body composition in sexual precocity. Curr Opin Endocrinol Diabetes Obes (2022) 29:78–83. doi: 10.1097/MED.0000000000000687
13. Shalitin S, Gat-Yablonski G. Associations of obesity with linear growth and puberty. Horm Res Paediatr (2022) 95:120–36. doi: 10.1159/000516171
14. Huang A, Roth CL. The link between obesity and puberty: What is new? Curr Opin Pediatr (2021) 33:449–57. doi: 10.1097/MOP.0000000000001035
15. Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Bräuner EV, et al. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: A systematic review and meta-analysis. JAMA Pediatr (2020) 174:e195881. doi: 10.1001/jamapediatrics.2019.5881
16. Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: A systematic review and meta-analysis. Int J Environ Res Public Health (2017) 14(10):1266. doi: 10.3390/ijerph14101266
17. Brix N, Ernst A, Lauridsen L, Parner ET, Arah OA, Olsen J, et al. Childhood overweight and obesity and timing of puberty in boys and girls: cohort and sibling-matched analyses. Int J Epidemiol (2020) 49:834–44. doi: 10.1093/ije/dyaa056
18. Silventoinen K, Jelenkovic A, Palviainen T, Dunkel L, Kaprio J. The association between puberty timing and body mass index in a longitudinal setting: The contribution of genetic factors. Behav Genet (2022) 52:186–94. doi: 10.1007/s10519-022-10100-3
19. Bygdell M, Kindblom JM, Jansson JO, Ohlsson C. Revisiting the critical weight hypothesis for regulation of pubertal timing in boys. Am J Clin Nutr (2020) 113:123–8. doi: 10.1093/ajcn/nqaa304
20. Frisch RE, McArthur JW. Menstrual cycles: fatness as a determinant of minimum weight for height necessary for their maintenance or onset. Science (1974) 185:949–51. doi: 10.1126/science.185.4155.949
21. Kaplowitz PB, Slora EJ, Wasserman RC, Pedlow SE, Herman-Giddens ME. Earlier onset of puberty in girls: relation to increased body mass index and race. Pediatrics (2001) 108:347–53. doi: 10.1542/peds.108.2.347
23. Pereira A, Busch AS, Solares F, Baier I, Corvalan C, Mericq V. Total and central adiposity are associated with age at gonadarche and incidence of precocious gonadarche in boys. J Clin Endocrinol Metab (2021) 106:1352–61. doi: 10.1210/clinem/dgab064
24. Li Y, Ma T, Ma Y, Gao D, Chen L, Chen M, et al. Adiposity status, trajectories and the earlier puberty onset: Results from a longitudinal cohort study. J Clin Endocrinol Metab (2022) 107(9):2462–72. doi: 10.1210/clinem/dgac395
25. Chen C, Zhang Y, Sun W, Chen Y, Jiang Y, Song Y, et al. Investigating the relationship between precocious puberty and obesity: a cross-sectional study in shanghai, China. BMJ Open (2017) 7:e014004. doi: 10.1136/bmjopen-2016-014004
26. de Onis M, Lobstein T. Defining obesity risk status in the general childhood population: which cut-offs should we use? Int J Pediatr Obes (2010) 5:458–60. doi: 10.3109/17477161003615583
27. de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ (2007) 85:660–7. doi: 10.2471/blt.07.043497
28. Subspecialty Group of Endocrinologic, Hereditary and Metabolic Diseases, The Society of Pediatrics, Chinese Medical Association. The definition of metabolic syndrome and prophylaxis and treatment proposal in Chinese children and adolescents. Zhonghua Er Ke Za Zhi (2012) 50:420–2.
29. Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child (1969) 44:291–303. doi: 10.1136/adc.44.235.291
30. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child (1970) 45:13–23. doi: 10.1136/adc.45.239.13
31. Liu Y, Yu T, Li X, Pan D, Lai X, Chen Y, et al. Prevalence of precocious puberty among Chinese children: a school population-based study. Endocrine (2021) 72:573–81. doi: 10.1007/s12020-021-02630-3
32. Zhu M, Fu J, Liang L, Gong C, Xiong F, Liu G, et al. Epidemiologic study on current pubertal development in Chinese school-aged children. Zhejiang Da Xue Xue Bao Yi Xue Ban (2013) 42:396–402. doi: 10.3785/j.issn.1008-9292.2013.04.005
33. Zhang J, Xu J, Liu L, Xu X, Shu X, Yang Z, et al. The prevalence of premature thelarche in girls and gynecomastia in boys and the associated factors in children in southern China. BMC Pediatr (2019) 19:107. doi: 10.1186/s12887-019-1426-6
34. Atay Z, Turan S, Guran T, Furman A, Bereket A. The prevalence and risk factors of premature thelarche and pubarche in 4- to 8-year-old girls. Acta Paediatr (2012) 101:e71–5. doi: 10.1111/j.1651-2227.2011.02444.x
35. Rosenfield RL, Lipton RB, Drum ML. Thelarche, pubarche, and menarche attainment in children with normal and elevated body mass index. Pediatrics (2009) 123:84–8. doi: 10.1542/peds.2008-0146
36. Wei Q, Wu M, Li YL, Rao R, Li S, Cen Q, et al. Physical deviation and precocious puberty among school-aged children in leshan city: An investigative study. J Int Med Res (2020) 48:300060520939672. doi: 10.1177/0300060520939672
37. Lee JM, Wasserman R, Kaciroti N, Gebremariam A, Steffes J, Dowshen S, et al. Timing of puberty in overweight versus obese boys. Pediatrics (2016) 137:e20150164. doi: 10.1542/peds.2015-0164
38. Crocker MK, Stern EA, Sedaka NM, Shomaker LB, Brady SM, Ali AH, et al. Sexual dimorphisms in the associations of BMI and body fat with indices of pubertal development in girls and boys. J Clin Endocrinol Metab (2014) 99:E1519–29. doi: 10.1210/jc.2014-1384
39. Sørensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. J Clin Endocrinol Metab (2010) 95:263–70. doi: 10.1210/jc.2009-1478
Keywords: children, body mass index, central obesity, precocious puberty, gender
Citation: Liu M, Cao B, Luo Q, Wang Q, Liu M, Liang X, Wu D, Li W, Su C, Chen J and Gong C (2022) The critical BMI hypothesis for puberty initiation and the gender prevalence difference: Evidence from an epidemiological survey in Beijing, China. Front. Endocrinol. 13:1009133. doi: 10.3389/fendo.2022.1009133
Received: 01 August 2022; Accepted: 11 October 2022;
Published: 25 October 2022.
Edited by:
Artur Mazur, University of Rzeszow, PolandReviewed by:
Tetyana Chaychenko, Kharkiv National Medical University, UkraineCopyright © 2022 Liu, Cao, Luo, Wang, Liu, Liang, Wu, Li, Su, Chen and Gong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunxiu Gong, Y2h1bnhpdWdvbmdAc2luYS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.