
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 21 November 2022
Sec. Obesity
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1008820
Aims: The aim of the study was to evaluate the association between evening chronotype and social jetlag (SJL) with obesity, blood glucose and lipid levels in non-shift working adults.
Methods: The databases of MEDLINE, EMBASE and Cochrane Reviews were searched for studies analyzing the metabolic parameters among groups of different chronotypes or SJL until Feb 2022. Weighted mean difference (WMD) and 95% confidence intervals (CI) were used to analyze the association between these parameters and chronotypes or SJL.
Results: A total of 27 studies were included in this meta-analysis. Compared with morning chronotype, the participants with evening chronotype had higher body mass index (BMI) (WMD= 0.44 kg/m2, 95%CI, 0.30 to 0.57 kg/m2, p<0.001), higher fasting blood glucose level (WMD= 5.83mg/dl, 95%CI, 3.27to 8.38 mg/dl, p<0.001), higher total cholesterol level (WMD= 6.63mg/dl, 95%CI, 0.69 to 12.56 mg/dl, p=0.03), and lower high density lipoprotein cholesterol (HDL-C) level (WMD= -1.80mg/dl, 95%CI, -2.30 to -1.31 mg/dl, p<0.001). Compared with the participants with small SJL, the participants with large SJL had larger waist circumference (WMD= 0.80cm, 95%CI, 0.77 to 0.83cm, p<0.001).
Conclusions: Evening chronotype and SJL were associated with obesity and unfavorable metabolic parameters of glucose and lipid metabolism.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022303401.
The circadian system is controlled by the master clock located in the suprachiasmatic nuclei of the hypothalamus and is entrained by the environmental factors such as the light-dark cycle. However, people live in modern industrialized societies access to artificial light in 24 hours, and they may exhibit different preference in the circadian rhythm of sleep-wake cycle, which is called chronotype (1). Chronotypes can be basically divided into eveningness and morningness, otherwise known as night owls and early birds. Some people with an evening chronotype may cause circadian misalignment. They suffer from chronic sleep deficiency because they sleep late but still have to wake up early due to social demands. As a result, the behavioral rhythm is mismatched with their endogenous circadian rhythm. The inadequate sleep is often paid back on weekends or free days. This phenomenon leads to “social jetlag”, which is measured as the difference of mid-sleep time between work and free days and reflects the degree of circadian misalignment (2).
It was suggested that the evening chronotype and circadian misalignment might be associated with negative health problems including metabolic disorders. In healthy adults, it was reported that in-laboratory induced circadian misalignment resulted in elevated glucose levels, insulin resistance and increased systemic inflammation (3). Epidemiology studies indicated that people with a late chronotype and short sleep duration were prone to have harmful lifestyle such as sedentary behavior and unhealthy nutrition (4, 5). As a demonstrative example of circadian misalignment, shift work was associated with increased risk of obesity and metabolic syndrome (MetS) (6, 7). However, among the studies with non-shift workers, discrepancy existed in the association between chronotypes and obesity. Several large population-based cross-sectional studies did not find an association between late chronotype and adiposity (8–11). A population-based longitudinal study revealed that the evening energy intake was associated with obesity, regardless of chronotype (12). The causal relationship of evening chronotype and obesity, and the mechanism behind their association were not fully elucidated.
In addition, though a longitudinal study indicated a higher incidence of type 2 diabetes (T2DM) in people with evening chronotype (13), the studies of social jetlag (SJL) had inconsistent results. SJL was only associated with increased risk of MetS and diabetes/prediabetes among participants younger than 61 years old in a population-based cohort (14). Another cohort study found SJL was associated with obesity only in the morning chronotypes (15). While some studies demonstrated the association between late chronotype and poor glycemic control in patients with T2DM (16–18), another study did not indicate an association of SJL and HbA1c levels in diabetic patients (19).
In order to better explore the association between evening chronotype and circadian misalignment with obesity, T2DM, and MetS in non-shift workers, we performed this systemic review and meta-analysis of the published studies to compare the anthropometric and metabolic indicators between different chronotypes and SJL groups.
Comprehensive searches for published studies until Feb 2022 were performed in the electronic database of MEDLINE, EMBASE and Cochrane Reviews. The search strategy included the following terms: chronotype, social jetlag, metabolism, glucose, BMI, obesity (for a complete list, see Supplementary Table 1). The registration number for this meta-analysis in PROSPERO was CRD42022303401. The primary aim of this meta-analysis was to clarify the association between circadian misalignment, indicated by evening chronotype or large SJL, and the anthropometric and metabolic variables of adiposity and MetS in general population as well as the patients with metabolic disorders. The PECOS (Population, Exposure, Control, Outcome, Study design) strategy used to define the research question is shown in Table 1.
Chronotypes could be assessed by validated questionnaires including the Horne-Östberg Morningness-Eveningness Questionnaire (MEQ), the reduced MEQ, and the Munich Chrono Type Questionnaire (MCTQ) (1). The simplified assessment of chronotypes used in some studies included scoring from a single self-reported question “Are you a morning person or an evening person?” or the mid-sleep time on free days (MSF). Chronotypes were categorized into 2 to 5 groups in different studies. In our meta-analysis, we only compared the latest chronotype with the earliest chronotype of each study.
Circadian misalignment describes a variety of circumstances including inappropriately timed sleep and wake or misaligned central and peripheral rhythms (20). SJL is a continuous variable to reflect inappropriately timed sleep and wake, which is calculated as the absolute difference between MSF and mid-sleep time on weekdays. People with a SJL ≥1 hour were usually considered as having circadian misalignment (2). Some studies used both 1 and 2 hours as the cutoffs for categorization of different extent of SJL. We recalculated these data and used SJL≥1 hour to indicate circadian mismatch, and compared with SJL <1h group.
Studies were considered eligible for inclusion if they fulfilled the following inclusion criteria: (i) presented primary outcome as evaluating the association between chronotypes or SJL and the anthropometric and metabolic parameters of MetS in randomized controlled studies (RCTs) and observational studies (cross-sectional studies, cohort studies, or case–control studies); (ii) evaluated the metabolic parameters in different chronotypes or SJL groups separately. Studies were excluded using the following criteria: (i) performed in night shift workers; (ii) performed in children and adolescents; (iii) did not classify chronotypes or SJL in groups; (iv) did not present the continuous metabolic indicators in mean ± standard deviation (SD).
For study selection, two investigators independently screened the titles, abstracts, and full articles according to eligibility criteria. Searches were collected, and duplicates were removed. Any potentially relevant citation or any discrepancy was then reviewed in full-text, and decisions about eligibility were independently verified by a third investigator.
Full-text articles were assessed and data were extracted, including the following information: (i) author; (ii) year of publication; (iii) region; (iv) study design; (v) methods of determination of chronotype; (vi) population; (vii) baseline characteristics. The main variables for evaluation were: (i) numbers of participants with different chronotype or SJL groups; (ii) means and SD for the anthropometric and metabolic variables of two groups; (iii) numbers of MetS, T2DM and the other metabolic disorders in each group. All data from the included publications were extracted into standardized data tables and independently verified by two investigators.
Qualities of cross-sectional and cohort studies were evaluated by the Newcastle-Ottawa Scale (NOS) (21). The evaluation included selection, exposure/outcome and comparability. High quality studies were scored 6–9 stars. RCTs were evaluated using the Cochrane Collaboration tool (22).
Weighted mean differences (WMD) were calculated with 95% confidence interval (CI) for comparisons of anthropometric and metabolic parameters between morning and evening chronotypes, and large and small SJL groups by using the Inverse Variance statistical method. Odds ratios (ORs) and CIs were calculated for the comparison of the prevalence of T2DM and the other metabolic disorders between groups by the Mantel-Haenszel statistical method. Higgins I2 statistic was used to quantify the percentage of total variance in the summary estimate due to between-study heterogeneity. Random-effects models was used when an I2 value was more than 50% which represented substantial high levels of heterogeneity, while fixed-effects models were used when an I2 value was no more than 50%. Sensitivity analysis was performed by including and excluding small sample size, or low-quality studies, or studies with characteristics different from the others. Publication bias was assessed via visual inspection of the funnel plot. The subgroup analysis was made based on the definition of chronotype and different characteristics of the population. The statistical analyses were performed with the Review Manager statistical software package (Version 5.3, Nordic Cochrane Center, Copenhagen, Denmark).
A total of 27 studies were included in this meta-analysis, among which 19 were analyzed in groups of chronotypes and 8 were analyzed in the groups of SJL. Figure 1 showed the flowchart of the included studies. Baseline characteristics of the included studies were presented in Supplementary Table 2. RCT was not available in the searching of database. Qualities of the cross-sectional and cohort studies were evaluated by the NOS. Twenty-five studies were evaluated as high quality with a score of 6 to 9 points, and 2 studies were scored as 5 points.
Among the included studies, chronotypes were assessed by validated questionnaires including MEQ (17, 18, 23–30), and the reduced MEQ (12, 13, 31, 32). Some studies used one single question to assess the chronotypes (33, 34). MSF was used in 3 studies as a metric of chronotype (8, 9, 16).
Four among included studies adopted the SJL cutoffs of both 1 and 2 hours to categorize into three groups (14, 15, 19, 35). Three studies used the cutoff of 1 hour to define small and large SJL groups (36–38). One study used the median of SJL, which was 1.07 hours, to define small and large SJL groups (39).
Sixteen studies were conducted in general population or healthy people without chronic diseases, four studies in patients with T2DM, three studies in patients with chronic metabolic disorders, two studies in obese participants, and 2 in subjects who underwent bariatric surgery (Supplementary Table 2).
The data of different studies coming from a same cohort were only included once in the meta-analysis of each parameter. There were 4 studies from a large population-based cohort in Finland, two studies coming from a same cohort of Brazil, and two studies from an Italian cohort. Because only two cohort studies of chronotypes and two of SJL provided the data of obesity or metabolic parameters of the follow-up, we could not perform meta-analysis of the follow-up information and only used their baseline data. The prevalence of MetS, hypertension, or the other metabolic disorders, and the mean blood pressure, the fasting insulin levels, insulin resistance indicated by HOMA-IR were not analyzed due to insufficient data.
Compared with the participants with morning chronotype, the mean BMI of the participants with the evening chronotype was higher (WMD= 0.44 kg/m2, 95%CI, 0.30 to 0.57 kg/m2, p<0.001) (Figure 2). In subgroup analysis (Table 2), compared with the participants of morning chronotype, the participants of evening type had higher BMI in patients with obesity related chronic disease including T2DM as well as general population (Figure 2). The evening chronotype was associated with higher BMI in 12 studies assessed with qualified questionnaire (WMD= 0.59 kg/m2, 95%CI, 0.41 to 0.77 kg/m2, p<0.001), but not in the five studies with the other assessments of chronotype (WMD= 0.21 kg/m2, 95%CI, -0.13 to 0.55 kg/m2, p=0.23). Compared with the morning type, the evening type was associated with higher BMI in both subgroups with participants younger or older than the mean age of 50 years old. The mean waist circumference was not significantly different between the evening and morning chronotypes (Figure 2).
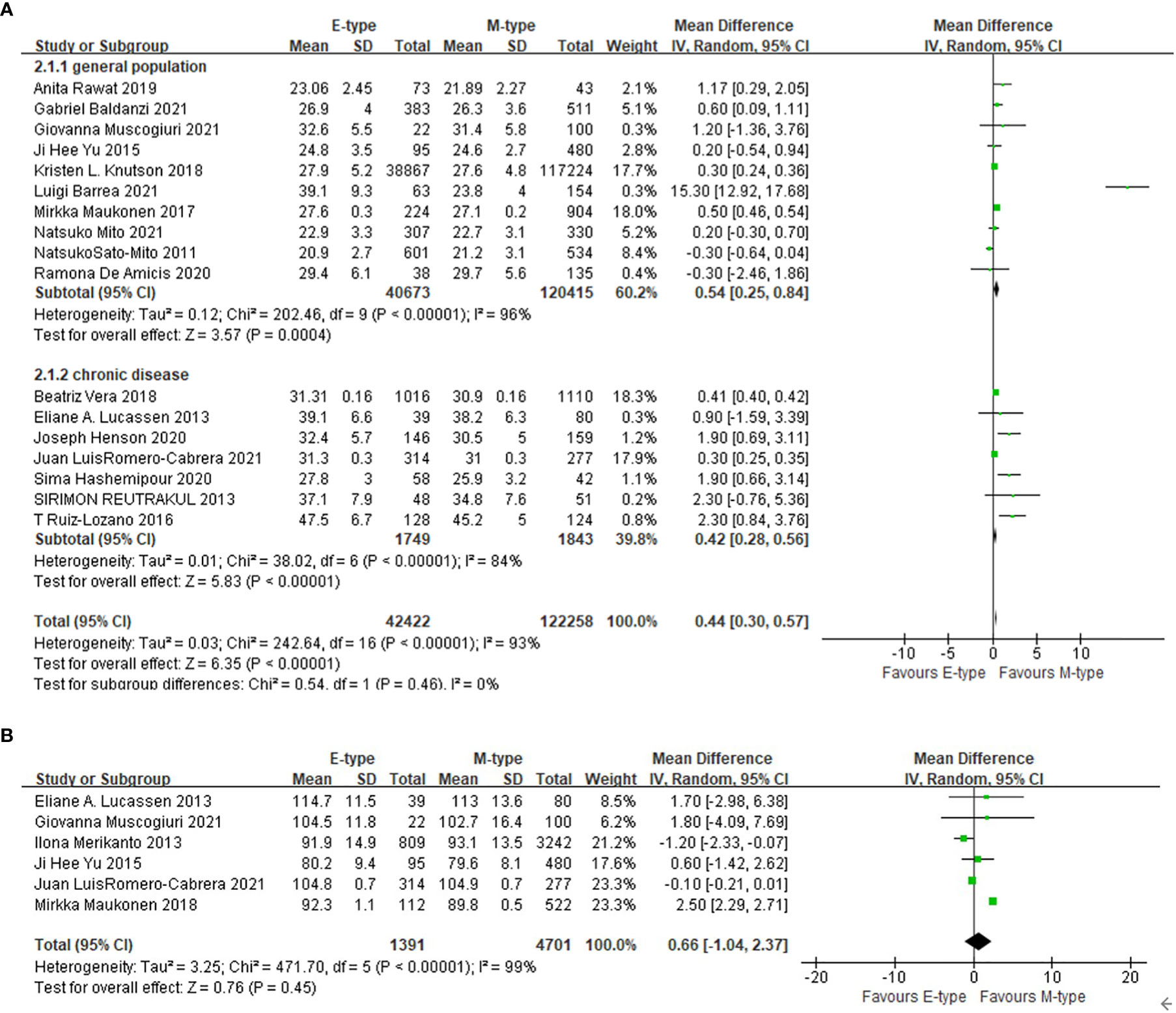
Figure 2 The association between chronotype and adiposity. (A) The association between chronotype and BMI in general population and in patients with chronic diseases. (B) The association between chronotype and waist circumference.
Compared with the participants with morning type, the participants in the evening type group had higher fasting blood glucose levels (WMD= 5.83mg/dl, 95%CI, 3.27 to 8.38 mg/dl, p<0.001). The association was still significant after excluding one study conducted in T2DM patients (WMD= 4.69mg/dl, 95%CI, 2.14 to 7.24 mg/dl, p<0.001). The prevalence of T2DM was also higher in the evening chronotype group than the morning chronotype (OR= 1.59, 95%CI, 1.01 to 2.51, p=0.04). The mean HbA1c was not statistically different between the two chronotype groups (Figure 3).
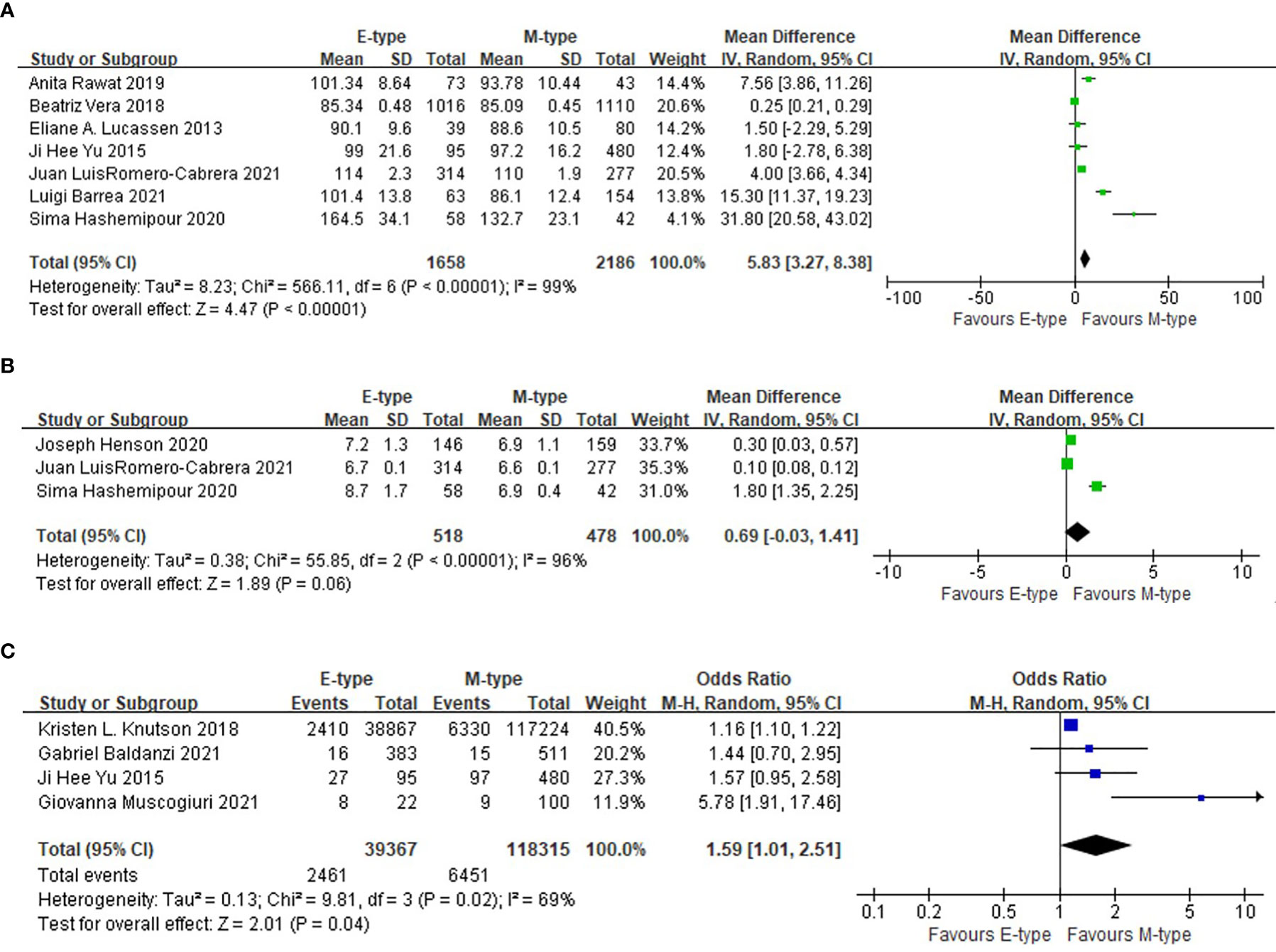
Figure 3 The association between chronotype and glycemic indicators. (A) The association between chronotype and fasting blood glucose. (B) The association between chronotype and HbA1c. (C) The association between chronotype and the prevalence of T2DM.
Compared with the participants with morning chronotype, the mean total cholesterol level in the evening chronotype group was higher (WMD= 6.63mg/dl, 95%CI, 0.69 to 12.56 mg/dl, p=0.03), and the mean high density lipoprotein cholesterol (HDL-C) was lower (WMD= -1.80mg/dl, 95%CI, -2.30 to -1.31 mg/dl, p<0.001). The triglyceride level was not statistically different between evening and morning chronotypes (Figure 4).
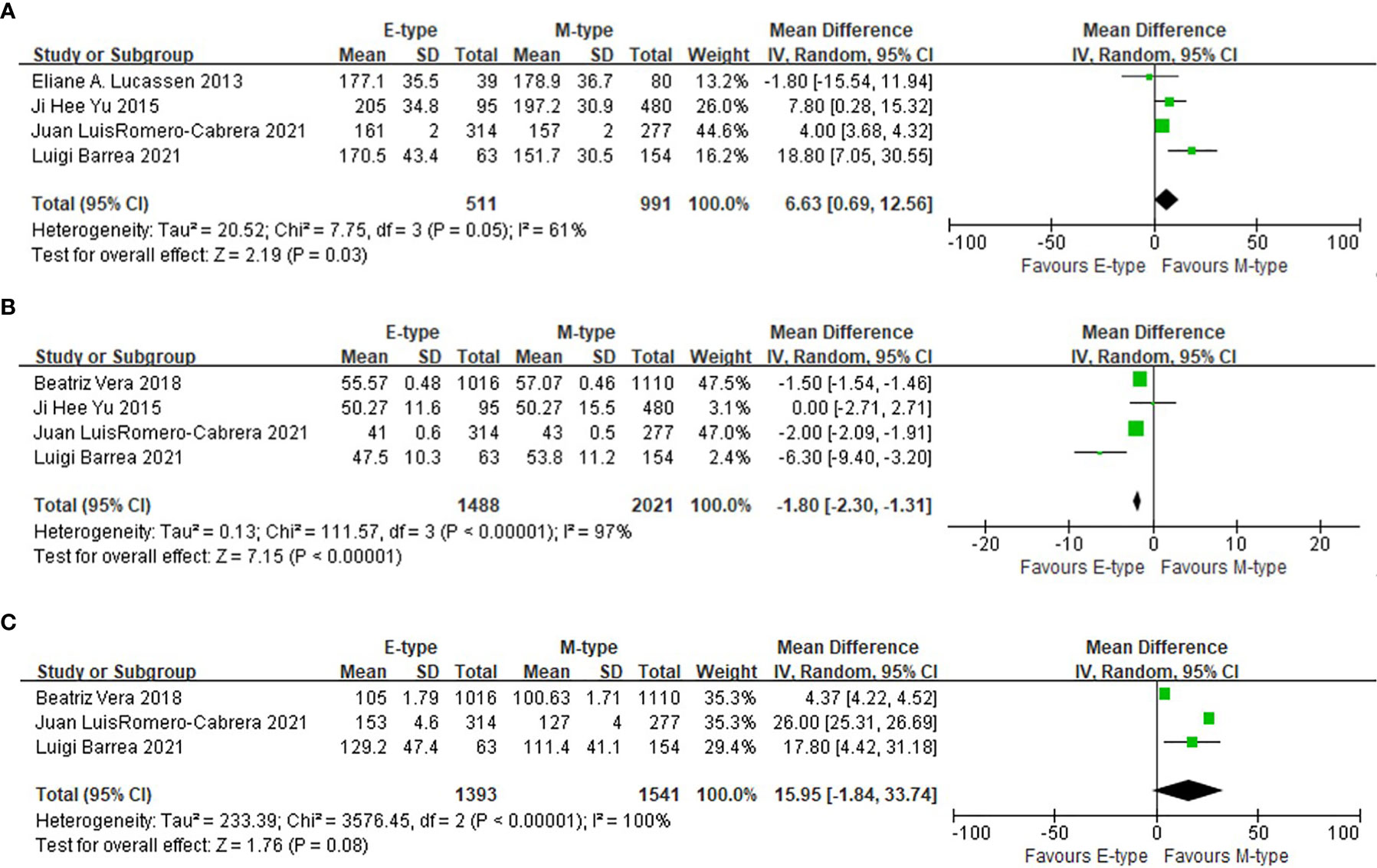
Figure 4 The association between chronotypes and lipids levels. (A) The association between chronotypes and total cholesterol. (B) The association between chronotypes and HDL-c. (C) The association between chronotypes and triglyceride.
Compared with the participants with small SJL, the participants with large SJL had larger waist circumference (WMD= 0.80cm, 95%CI, 0.77 to 0.83cm, p<0.001). However, the mean BMI, fasting blood glucose, HbA1c, and HDL-c levels were not different between small and large SJL groups (Figure 5).
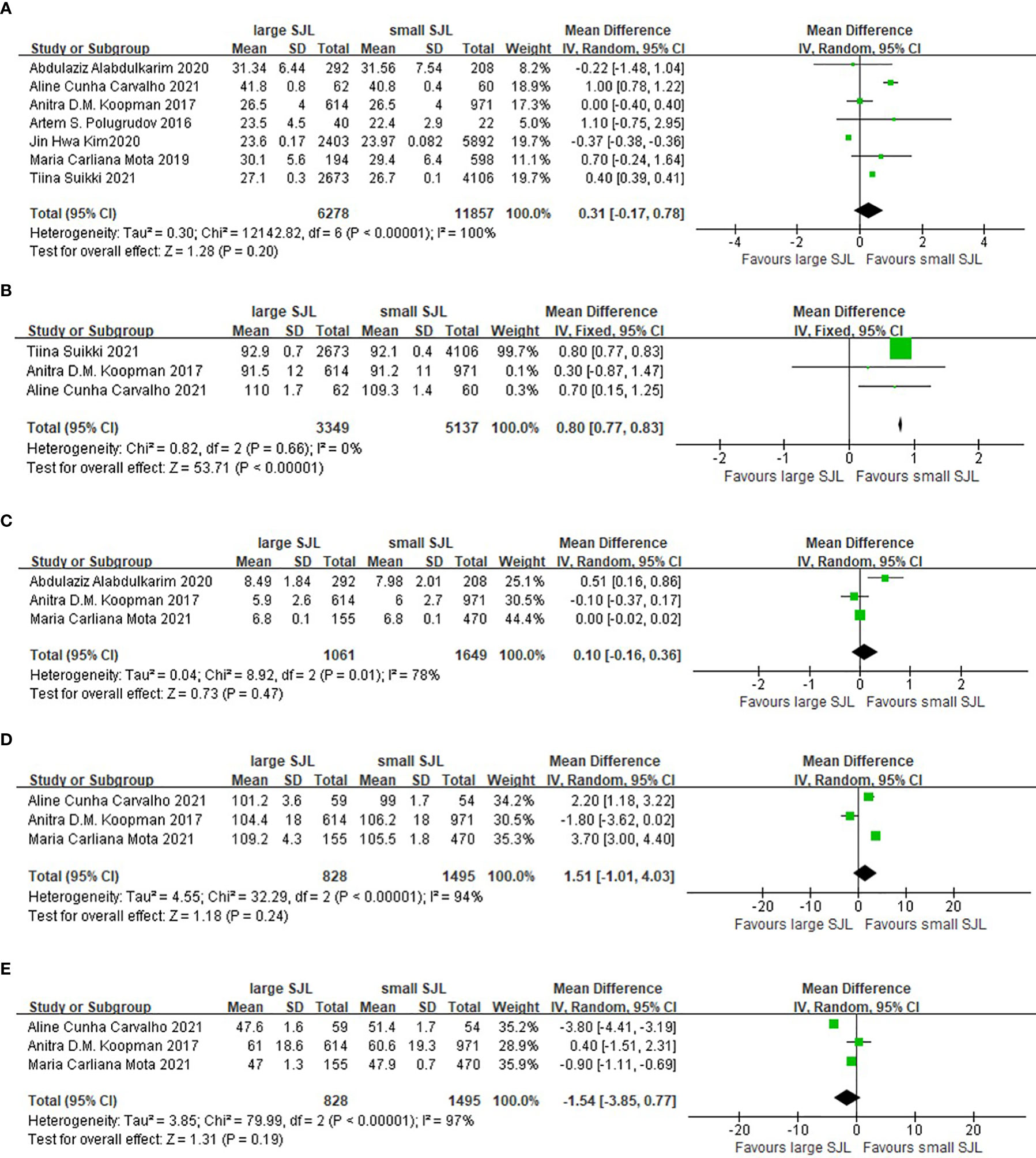
Figure 5 The association between social jetlag and metabolic parameters. (A) The association between social jetlag and BMI. (B) The association between social jetlag and waist circumference. (C) The association between social jetlag and HbA1c. (D) The association between social jetlag and fasting blood glucose. (E) The association between social jetlag and HDL-c.
According to this meta-analysis, we found that evening chronotype was associated with unfavorable metabolic indicators including higher BMI, higher fasting glucose level, higher total cholesterol level and lower HDL-c level compared with morning chronotype, and larger SJL was associated with larger waist circumference compared with smaller SJL.
Due to the exposure to artificial light and work demand in modern lifestyle, circadian misalignment is a quite common phenomenon. An extreme of circadian misalignment was observed in night shift workers. Studies revealed that shift work increased the risk of abdominal obesity, T2DM, and MetS (6, 7, 40). Among non-shift workers, people with a preference of late chronotype may experience a milder form of circadian misalignment due to the conflict of earlier wake up according to work schedules and later sleep onset in the evening.
In this meta-analysis, we found that evening chronotype was associated with higher BMI compared to morning chronotype in both the general population and participants who already had metabolic disorders including obesity, T2DM and cardiovascular disease (CVD). This result was in line with the observations in some previous cross-sectional studies (17, 31). A longitudinal study further demonstrated that evening chronotype was correlated with lower weight reduction after bariatric surgery (26). In the subgroup analysis, the association between adiposity and evening chronotype was consistent in participants with the age younger and older than 50 years old. This indicated that the association between evening chronotype and adiposity might be independent of age in adults. In line with it, a prospective study among first-year college students also found an association between evening chronotype and weight gain (41).
In addition to obesity, we also analyzed the association between the metabolic parameters of MetS including glucose and lipid levels and evening chronotype. MetS is a cluster of metabolic abnormalities that include hypertension, central obesity, insulin resistance, and dyslipidemia. These metabolic abnormalities share common pathogenies and are strongly associated with an increased risk for developing T2DM and CVD (42). Previous studies demonstrated the association between the prevalence of MetS and late chronotype in general population as well as in patients with CVD (17, 29, 30). Evening chronotype was also associated with a higher prevalence of T2DM in previous cross-sectional studies of middle-aged adults (17, 27). In addition, evening type was found to be associated with higher triglycerides and lower HDL-cholesterol levels in both cross-sectional and longitudinal studies (29, 30). Studies conducted in patients with T2DM and prediabetes demonstrated the association of late chronotype and poor glycemic control (16, 18, 43). A higher HOMA-IR reflecting insulin resistance was also observed in a population-based study (29). In our meta-analysis, the association between evening chronotype and higher fasting glucose level, higher total cholesterol level and lower HDL-c level was in accordance with the previous studies and further supported the relationship between chronotype and MetS
The mechanism of the association between late chronotype and obesity or MetS was partly due to some health-related behaviors. It was revealed that adults with evening chronotype were prone to unhealthy eating habits (5). Data from the UK Biobank project demonstrated that adults with evening chronotype consumed less fruit and vegetables than morning chronotype (44). A study in Finnish population showed greater eveningness was associated with a lower intake of whole grains, whereas a higher intake of alcohol and chocolate (45). People with evening chronotype had a lower adherence to the healthy diets including Baltic Sea diet (10) and Mediterranean diet (31). It was also found that adults with evening chronotype were more likely to skip meals and breakfast in both general population and T2DM patients (46, 47). The other harmful lifestyles including smoking and screen based sedentary behaviors were also related with evening chronotype (4, 44). Similar findings of the association between unhealthy lifestyle and evening chronotype were also found in patients with T2DM and CVD (25, 30). However, the links and/or causal relationships between chronotype and unhealthy eating habits and the other behaviors were not clear. Chronotype may be a causal factor or merely a reflection of a complex set of behaviors that affect eating patterns choice of diet, and sleep quality.
Some people with an evening chronotype may accumulate “sleep debt” during work days, which lead to a greater SJL and circadian misalignment. As a quantifiable indicator of circadian mismatch, SJL was increasingly studied recently. A study conducted in patients with obesity related chronic diseases ascertained the association between larger SJL and higher intake of total calories (37). Larger SJL, which was measured with wrist actigraphy, was associated with higher fasting insulin, waist circumference, and BMI in linear regression models (48). Cross-sectional studies demonstrated that SJL was associated with an increased prevalence of MetS (14, 49). In our meta-analysis, SJL was positively associated with waist circumference, but not with BMI, fasting glucose, HbA1c and HDL-c. The limited number of included studies decreased the significance of this meta-analysis. In addition, the association of SJL and metabolic parameters might be influenced by age and sex. The association of larger SJL and T2DM was more significant in younger (<61 years) participants (14). A longitudinal study found that higher SJL was associated with an increased risk of weight gain only in men (35). Thus, the association between SJL and adiposity or metabolic disorders still needs more investigations in different populations.
The mechanism of the association between circadian misalignment and metabolic disorders is not fully elucidated. In normal physiological state, the circadian rhythms generated by the central clock and phase-adjusted by ambient light should synchronize the clocks in the peripheral tissues to match the daily patterns of behavior, such as feeding, activity and sleep (50). In rodents, peripheral clocks were entrained by timing of food intake (51). In human, the function of peripheral tissues could also be affected by impaired control from the central circadian pacemaker and peripheral clocks. An in-laboratory study conducted in healthy young men found that the delay of meal timing can regulate blood glucose rhythms and clock gene expression in white adipose tissue (52). Thus, in people with circadian mismatch, the misalignment of the peripheral oscillators and the central circadian pacemaker might influence metabolic regulation, and lead to abnormal physiological responses to food intake.
Genetic factors may also play a role in the relationship of circadian misalignment and metabolic dysregulation. Endogenous circadian rhythms generated in circadian clocks are based on a complex program of gene expression (53). Recent accumulating evidence suggested that the clock related genes mutations participated in metabolism including lipogenesis and glucose homeostasis (54, 55). However, a population-based study did not find a relationship between chronotype-related variants and MetS (29). The genetic mechanism of the association between chronotype and metabolic disturbance was still under investigation.
The study had the strength in that it was the first meta-analysis to assess the association between evening chronotype and circadian misalignment and parameters of MetS in non-shift working adults. The limitations of the study were as follows. Firstly, the chronotypes were evaluated by different methods in these included studies causing heterogeneity. However, we have done subgroup analysis to diminish this influence. Secondly, the included studies inconsistently categorized the chronotypes into different number of groups. We only included part of the data in some studies and compared the latest chronotype with the earliest type. Thirdly, the included data were all from cross-sectional observations. We could not evaluate the causal relationship. Fourthly, patients with T2DM and the other chronic diseases were included in the meta-analysis of glycemic indicators and lipid levels. The medication of anti-diabetic or lipid-lowering agents may become confounding factors. However, most included studies were qualified in reducing selection bias between groups. Subgroup analysis of different population was also performed. Lastly, a few relevant studies did not present data in groups classified by chronotypes or SJL, so we could not include them in the meta-analysis.
In conclusion, this meta-analysis demonstrated that an evening chronotype and circadian misalignment presented as larger SJL, were associated with several unfavorable metabolic parameters relating to adiposity, glucose and lipid metabolism. In the future, more prospective studies and random controlled trials will be needed to establish causality and elucidate the underlying mechanisms.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
RZ, CL, and WY performed the study selection and data extraction. RZ and FL performed the statistical analyses. JW helped in the preparation of the tables and figures. RZ and XC wrote the manuscript. XC and LJ designed the systematic review protocol and revised the manuscript. All authors read and approved the final manuscript. All authors contributed to the article and approved the submitted version.
This work was supported by Beijing Natural Science Foundation (No.7202216), National Natural Science Foundation of China (Nos. 81970698), and National Key Research and Development Program of China (2018YFC1314100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1008820/full#supplementary-material
BMI, body mass index; CVD, cardiovascular disease; CI, confidence interval; HbA1c, glycated hemoglobin A1c; HDL-c, high density lipoprotein cholesterol; HOMA-IR, homeostatic model assessment of insulin resistance; MCTQ, Munich Chrono Type Questionnaire; MetS, metabolic syndrome; MEQ, Horne-Östberg Morningness-Eveningness Questionnaire; MSF, mid-sleep time on free days; NOS, Newcastle-Ottawa Scale; OR, Odds ratios; RCT, randomized controlled studies; SD, standard deviation; SJL, social jetlag; T2DM, type 2 diabetes mellitus; WMD, weighted mean difference.
1. Adan A, Archer SN, Hidalgo MP, Di Milia L, Natale V, Randler C. Circadian typology: A comprehensive review. Chronobiol Int (2012) 29(9):1153–75. doi: 10.3109/07420528.2012.719971
2. Wittmann M, Dinich J, Merrow M, Roenneberg T. Social jetlag: misalignment of biological and social time. Chronobiol Int (2006) 23(1-2):497–509. doi: 10.1080/07420520500545979
3. Buxton OM, Cain SW, O'Connor SP, Porter JH, Duffy JF, Wang W, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med (2012) 4(129):129ra43. doi: 10.1126/scitranslmed.3003200
4. Patterson F, Malone SK, Grandner MA, Lozano A, Perkett M, Hanlon A. Interactive effects of sleep duration and morning/evening preference on cardiovascular risk factors. Eur J Public Health (2018) 28(1):155–61. doi: 10.1093/eurpub/ckx029
5. Teixeira GP, Guimaraes KC, Soares A, Marqueze EC, Moreno CRC, Mota MC, et al. Role of chronotype in dietary intake, meal timing, and obesity: a systematic review. Nutr Rev (2022). doi: 10.1093/nutrit/nuac044
6. Brum MCB, Dantas Filho FF, Schnorr CC, Bertoletti OA, Bottega GB, da Costa Rodrigues T. Night shift work, short sleep and obesity. Diabetol Metab Syndr (2020) 12:13. doi: 10.1186/s13098-020-0524-9
7. Yang X, Di W, Zeng Y, Liu D, Han M, Qie R, et al. Association between shift work and risk of metabolic syndrome: A systematic review and meta-analysis. Nutr Metab Cardiovasc Dis (2021) 31(10):2792–9. doi: 10.1016/j.numecd.2021.06.007
8. Mito N, Fujimoto E, Sasaki S, D. Three-generation Study of Women on and G. Health Study. Association of chronotype as assessed by the midpoint of sleep with the dietary intake and health-related quality of life for elderly Japanese women. J Nutr Sci (2021) 10:e25. doi: 10.1017/jns.2021.16
9. Sato-Mito N, Sasaki S, Murakami K, Okubo H, Takahashi Y, Shibata S, et al. The midpoint of sleep is associated with dietary intake and dietary behavior among young Japanese women. Sleep Med (2011) 12(3):289–94. doi: 10.1016/j.sleep.2010.09.012
10. Maukonen M, Kanerva N, Partonen T, Kronholm E, Konttinen H, Wennman H, et al. The associations between chronotype, a healthy diet and obesity. Chronobiol Int (2016) 33(8):972–81. doi: 10.1080/07420528.2016.1183022
11. Johnsen MT, Wynn R, Bratlid T. Optimal sleep duration in the subarctic with respect to obesity risk is 8-9 hours. PloS One (2013) 8(2):e56756. doi: 10.1371/journal.pone.0056756
12. Maukonen M, Kanerva N, Partonen T, Mannisto S. Chronotype and energy intake timing in relation to changes in anthropometrics: A 7-year follow-up study in adults. Chronobiol Int (2019) 36(1):27–41. doi: 10.1080/07420528.2018.1515772
13. Merikanto I, Lahti T, Puolijoki H, Vanhala M, Peltonen M, Laatikainen T, et al. Associations of chronotype and sleep with cardiovascular diseases and type 2 diabetes. Chronobiol Int (2013) 30(4):470–7. doi: 10.3109/07420528.2012.741171
14. Koopman ADM, Rauh SP, van 't Riet E, Groeneveld L, van der Heijden AA, Elders PJ, et al. The Association between Social Jetlag, the Metabolic Syndrome, and Type 2 Diabetes Mellitus in the General Population: The New Hoorn Study. J Biol Rhythms (2017) 32(4):359–68. doi: 10.1177/0748730417713572
15. Suikki T, Maukonen M, Partonen T, Jousilahti P, Kanerva N, Mannisto S. Association between social jet lag, quality of diet and obesity by diurnal preference in Finnish adult population. Chronobiol Int (2021) 38(5):720–31. doi: 10.1080/07420528.2021.1876721
16. Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL, et al. Chronotype is independently associated with glycemic control in type 2 diabetes. Diabetes Care (2013) 36(9):2523–9. doi: 10.2337/dc12-2697
17. Yu JH, Yun CH, Ahn JH, Suh S, Cho HJ, Lee SK, et al. Evening chronotype is associated with metabolic disorders and body composition in middle-aged adults. J Clin Endocrinol Metab (2015) 100(4):1494–502. doi: 10.1210/jc.2014-3754
18. Hashemipour S, Yazdi Z, Mahabad N. Association of evening chronotype with poor control of type 2 Diabetes: Roles of sleep duration and insomnia level. Int J Endocrinol Metab (2020) 18(3):e99701. doi: 10.5812/ijem.99701
19. Alabdulkarim A, Alayed O, Aloraini O, Almozini M, Aldawsari K, Bin Khathlan YZ. The association between social jetlag and glycemic control in diabetic patients at king saud university medical city. Cureus (2020) 12(7):e9215. doi: 10.7759/cureus.9215
20. Baron KG, Reid KJ. Circadian misalignment and health. Int Rev Psychiatry (2014) 26(2):139–54. doi: 10.3109/09540261.2014.911149
21. Wells G. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2014).
22. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Rawat A, Gangwar AK, Tiwari S, Kant S, Garg RK, Singh PK. Sleep quality and insulin resistance in adolescent subjects with different circadian preference: A cross-sectional study. J Family Med Prim Care (2019) 8(7):2502–5. doi: 10.4103/jfmpc.jfmpc_400_19
24. Lucassen EA, Zhao X, Rother KI, Mattingly MS, Courville AB, de Jonge L, et al. Evening chronotype is associated with changes in eating behavior, more sleep apnea, and increased stress hormones in short sleeping obese individuals. PloS One (2013) 8(3):e56519. doi: 10.1371/journal.pone.0056519
25. Henson J, Rowlands AV, Baldry E, Brady EM, Davies MJ, Edwardson CL, et al. Physical behaviors and chronotype in people with type 2 diabetes. BMJ Open Diabetes Res Care (2020) 8(1). doi: 10.1136/bmjdrc-2020-001375
26. Ruiz-Lozano T, Vidal J, de Hollanda A, Canteras M, Garaulet M, Izquierdo-Pulido M. Evening chronotype associates with obesity in severely obese subjects: interaction with CLOCK 3111T/C. Int J Obes (Lond) (2016) 40(10):1550–7. doi: 10.1038/ijo.2016.116
27. Muscogiuri G, Barrea L, Aprano S, Framondi L, Di Matteo R, Altieri B, et al. Chronotype and cardio metabolic health in obesity: Does nutrition matter? Int J Food Sci Nutr (2021) 72(7):892–900. doi: 10.1080/09637486.2021.1885017
28. Barrea L, Muscogiuri G, Pugliese G, Graziadio C, Maisto M, Pivari F, et al. Association of the Chronotype Score with Circulating Trimethylamine N-Oxide (TMAO) Concentrations. Nutrients (2021) 13(5). doi: 10.3390/nu13051671
29. Vera B, Dashti HS, Gomez-Abellan P, Hernandez-Martinez AM, Esteban A, Scheer F, et al. Modifiable lifestyle behaviors, but not a genetic risk score, associate with metabolic syndrome in evening chronotypes. Sci Rep (2018) 8(1):945. doi: 10.1038/s41598-017-18268-z
30. Romero-Cabrera JL, Garaulet M, Jimenez-Torres J, Alcala-Diaz JF, Quintana Navarro GM, Martin-Piedra L, et al. Chronodisruption and diet associated with increased cardiometabolic risk in coronary heart disease patients: The CORDIOPREV study. Transl Res (2022) 242:79–92. doi: 10.1016/j.trsl.2021.11.001
31. De Amicis R, Galasso L, Leone A, Vignati L, De Carlo G, Foppiani A, et al. Is abdominal fat distribution associated with chronotype in adults independently of lifestyle factors? Nutrients (2020) 12(3). doi: 10.3390/nu12030592
32. Maukonen M, Kanerva N, Partonen T, Kronholm E, Tapanainen H, Kontto J, et al. Chronotype differences in timing of energy and macronutrient intakes: A population-based study in adults. Obes (Silver Spring) (2017) 25(3):608–15. doi: 10.1002/oby.21747
33. Knutson KL, von Schantz M. Associations between chronotype, morbidity and mortality in the UK Biobank cohort. Chronobiol Int (2018) 35(8):1045–53. doi: 10.1080/07420528.2018.1454458
34. Baldanzi G, Hammar U, Fall T, Lindberg E, Lind L, Elmstahl S, et al. Evening chronotype is associated with elevated biomarkers of cardiometabolic risk in the EpiHealth cohort: A cross-sectional study. Sleep (2022) 45(2). doi: 10.1093/sleep/zsab226
35. Kim JH, Lyu YS, Kim SY. Impact of social jetlag on weight change in adults: Korean national health and nutrition examination survey 2016-2017. Int J Environ Res Public Health (2020) 17(12). doi: 10.3390/ijerph17124383
36. Polugrudov AS, Panev AS, Smirnov VV, Paderin NM, Borisenkov MF, Popov SV. Wrist temperature and cortisol awakening response in humans with social jetlag in the North. Chronobiol Int (2016) 33(7):802–9. doi: 10.3109/07420528.2016.1168829
37. Mota MC, Silva CM, Balieiro LCT, Goncalves BF, Fahmy WM, Crispim CA. Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PloS One (2019) 14(2):e0212126. doi: 10.1371/journal.pone.0212126
38. Mota MC, Silva CM, Balieiro LCT, Fahmy WM, Marqueze EC, Moreno CRC, et al. Social jetlag Is associated with impaired metabolic control during a 1-year follow-up. Front Physiol (2021) 12:702769. doi: 10.3389/fphys.2021.702769
39. Carvalho AC, Mota MC, Marot LP, Mattar LA, de Sousa JAG, Araujo ACT, et al. Circadian misalignment is negatively associated with the anthropometric, metabolic and food intake outcomes of bariatric patients 6 months after surgery. Obes Surg (2021) 31(1):159–69. doi: 10.1007/s11695-020-04873-x
40. Gao Y, Gan T, Jiang L, Yu L, Tang D, Wang Y, et al. Association between shift work and risk of type 2 diabetes mellitus: A systematic review and dose-response meta-analysis of observational studies. Chronobiol Int (2020) 37(1):29–46. doi: 10.1080/07420528.2019.1683570
41. Culnan E, Kloss JD, Grandner M. A prospective study of weight gain associated with chronotype among college freshmen. Chronobiol Int (2013) 30(5):682–90. doi: 10.3109/07420528.2013.782311
42. Rochlani Y, Pothineni NV, Kovelamudi S, Mehta JL. Metabolic syndrome: pathophysiology, management, and modulation by natural compounds. Ther Adv Cardiovasc Dis (2017) 11(8):215–25. doi: 10.1177/1753944717711379
43. Anothaisintawee T, Lertrattananon D, Thamakaison S, Knutson KL, Thakkinstian A, Reutrakul S. Later chronotype is associated with higher hemoglobin A1c in prediabetes patients. Chronobiol Int (2017) 34(3):393–402. doi: 10.1080/07420528.2017.1279624
44. Patterson F, Malone SK, Lozano A, Grandner MA, Hanlon AL. Smoking, Screen-Based Sedentary Behavior, and Diet Associated with Habitual Sleep Duration and Chronotype: Data from the UK Biobank. Ann Behav Med (2016) 50(5):715–26. doi: 10.1007/s12160-016-9797-5
45. Kanerva N, Kronholm E, Partonen T, Ovaskainen ML, Kaartinen NE, Konttinen H, et al. Tendency toward eveningness is associated with unhealthy dietary habits. Chronobiol Int (2012) 29(7):920–7. doi: 10.3109/07420528.2012.699128
46. Meule A, Roeser K, Randler C, Kubler A. Skipping breakfast: morningness-eveningness preference is differentially related to state and trait food cravings. Eat Weight Disord (2012) 17(4):e304–8. doi: 10.3275/8723
47. Reutrakul S, Hood MM, Crowley SJ, Morgan MK, Teodori M, Knutson KL. The relationship between breakfast skipping, chronotype, and glycemic control in type 2 diabetes. Chronobiol Int (2014) 31(1):64–71. doi: 10.3109/07420528.2013.821614
48. Wong PM, Hasler BP, Kamarck TW, Muldoon MF, Manuck SB. Social Jetlag, Chronotype, and Cardiometabolic Risk. J Clin Endocrinol Metab (2015) 100(12):4612–20. doi: 10.1210/jc.2015-2923
49. Islam Z, Akter S, Kochi T, Hu H, Eguchi M, Yamaguchi M, et al. Association of social jetlag with metabolic syndrome among Japanese working population: The Furukawa Nutrition and Health Study. Sleep Med (2018) 51:53–8. doi: 10.1016/j.sleep.2018.07.003
50. Huang W, Ramsey KM, Marcheva B, Bass J. Circadian rhythms, sleep, and metabolism. J Clin Invest (2011) 121(6):2133–41. doi: 10.1172/JCI46043
51. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science (2010) 330(6009):1349–54. doi: 10.1126/science.1195027
52. Wehrens SMT, Christou S, Isherwood C, Middleton B, Gibbs MA, Archer SN, et al. Meal Timing Regulates the Human Circadian System. Curr Biol (2017) 27(12):1768–1775e3. doi: 10.1016/j.cub.2017.04.059
53. Montaruli A, Castelli L, Mule A, Scurati R, Esposito F, Galasso L, et al. Biological Rhythm and Chronotype: New Perspectives in Health. Biomolecules (2021) 11(4). doi: 10.3390/biom11040487
54. Panda S. Circadian physiology of metabolism. Science (2016) 354(6315):1008–15. doi: 10.1126/science.aah4967
Keywords: chronotype (morningness-eveningness), circadian misalignment, social jetlag (SJL), obesity, glucose metabolism, Lipid
Citation: Zhang R, Cai X, Lin C, Yang W, Lv F, Wu J and Ji L (2022) The association between metabolic parameters and evening chronotype and social jetlag in non-shift workers: A meta-analysis. Front. Endocrinol. 13:1008820. doi: 10.3389/fendo.2022.1008820
Received: 01 August 2022; Accepted: 03 November 2022;
Published: 21 November 2022.
Edited by:
Etienne Challet, Institut des Neurosciences Cellulaires et Intégratives, Faculté des Sciences de la Vie, Université de Strasbourg, FranceReviewed by:
Luping Ren, Hebei General Hospital, ChinaCopyright © 2022 Zhang, Cai, Lin, Yang, Lv, Wu and Ji. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Cai, ZHJfanVuZWxAc2luYS5jb20=; Linong Ji, amlsbkBiam11LmVkdS5jbg==
†ORCID: Rui Zhang, orcid.org/0000-0001-6005-8245
Xiaoling Cai, orcid.org/0000-0002-7881-0543
Chu Lin, orcid.org/0000-0002-2365-9831
Wenjia Yang, orcid.org/0000-0003-0610-5121
Fang Lv, orcid.org/0000-0002-1872-781X
Linong Ji, orcid.org/0000-0002-3262-2168
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.