- 1Department of Medical Imaging, The First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Radiology, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 3Department of Radiology, The Quzhou Affiliated Hospital of Wenzhou Medical University, Quzhou People’s Hospital, Quzhou, China
- 4Department of Radiology, Hangzhou Ninth People’s Hospital, Hangzhou, China
- 5Department of Radiology, Zhejiang Xiaoshan Hospital, Hangzhou, China
- 6Department of Radiology, Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province, Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, Hangzhou, China
Objectives: To investigate the value of mean attenuation value (AVmean), minimum attenuation value (AVmin), and CT histogram (CTH) for the differential diagnosis of adrenal adenoma and non-adenoma in two medical centers.
Methods: The plain CT data of 403 cases of adrenal adenoma and 141 cases of non-adenoma in center A were retrospectively analyzed, and compared with data of 86 cases of adenoma and 71 cases of non-adenoma in center B. All cases were confirmed by pathology or clinical follow-up. The diagnostic efficacy of AVmean ≤ 10 Hounsfield units (HU), AVmin ≤ 0 HU, and CTH negative pixels ≥ 10% for adrenal adenoma, and AVmin and CTH for adenoma with AVmean > 10Hu were compared between the two medical centers.
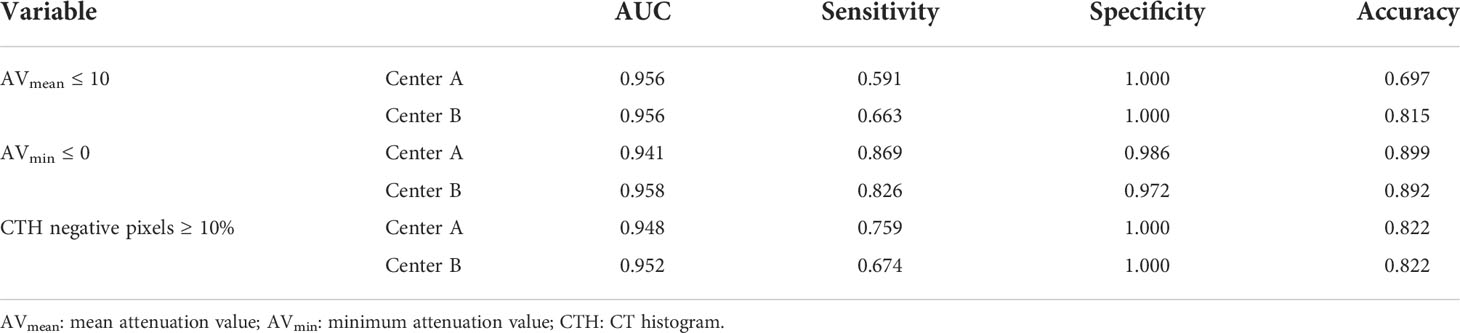
Results: In medical centers A and B, the AUC of AVmean for the differential diagnosis of adenoma and non-adenoma was 0.956 and 0.956, respectively, and the corresponding sensitivity, specificity, and accuracy were, 0.591 and 0.663, 1.000 and 1.000, 0.697, and 0.815, respectively, when the threshold was ≤ 10 HU. The AUC of AVmin was 0.941 and 0.958, respectively, and the corresponding sensitivity, specificity, and accuracy were 0.869 and 0.826, 0.986, and 0.972, 0.899, and 0.892, respectively, when the threshold was ≤ 0 HU. The AUC of CTH negative pixels was 0.948 and 0.952, respectively, and the corresponding sensitivity, specificity, and accuracy were 0.759 and 0.674, 1.000 and 1.000, 0.822, and 0.822, respectively, when the threshold was ≥ 10%. Among adenoma with AVmean >10 HU, the best threshold of AVmin in center A and center B were -0.250HU and 2.375HU, and the corresponding AUC, sensitivity and specificity were 0.858 and 0.846, 0.691 and 0.586, 0.986 and 0.958; the best threshold of CTH in center A and center B were 0.895% and 0.775%, and the corresponding AUC, sensitivity and specificity were 0.873 and 0.822, 0.818 and 0.724, 0.837 and 0.915.
Conclusion: AVmean, AVmin, and CTH are all important parameters for differentiating adrenal adenoma from non-adenoma. Even for adenomas with AVmean > 10 HU, AVmin and CTH still had high diagnostic efficiency. The three parameters are complementary, assisting clinicians to develop personalized treatments.
Introduction
The detection rate of adrenal incidentalomas gradually increases with the application of cross-sectional imaging, accounting for approximately 4-10% of all abdominal computed tomography (CT) examinations (1–4). Adrenal incidentalomas include solid tumors, such as adrenal adenoma, adrenal pheochromocytoma, adrenocortical carcinoma, and adrenal metastasis, of which adrenal adenoma accounts for 70-80% (4, 5). Most adrenal adenomas do not have endocrine functions and only need to clinical follow-up (1, 2). However, adrenal pheochromocytomas mainly secrete catecholamines, leading to the elevated blood pressure, and adrenal cortical carcinomas can invade surrounding tissues and distant metastasis, while adrenal metastasis involves precise clinical staging of the primary tumors, all of which require early clinical intervention. Therefore, it is extremely important to clarify the nature of adrenal incidentalomas to develop personalized treatments.
Plain CT, adrenal washout CT, and chemical shift magnetic resonance imaging (MRI) are important imaging methods to identify adrenal incidentalomas, of which plain CT is a simple, economic, and more commonly used method. In plain CT, adrenal adenomas mainly have a low-density due to different proportions of intracellular lipids. Therefore, the amount of lipid components in tumors can be reflected by mean attenuation value (AVmean), minimum attenuation value (AVmin), and CT histogram (CTH), and an adenoma can be therefore diagnosed. To our knowledge, AVmean is the most commonly used parameter for the diagnosis of adrenal adenoma. At present, the widely accepted threshold value for AVmean is ≤ 10 Hounsfield units (HU). Although its specificity of the diagnosis of adenoma can reach 93.3-100%, its sensitivity is somewhat insufficient and varies greatly, ranging from 47.6% to 82.2% (6–10). CTH is another method to diagnose adrenal adenoma. The widely accepted threshold for CTH is negative pixels ≥ 10%, which is similar to the specificity of AVmean ≤ 10 Hu, and its sensitivity is improved to some extent, ranging from 82.9% to 92.2% (7–9). However, the acquisition of CTH is complicated and requires a special post-processing workstation, which is difficult to be popularized and applied in imaging diagnostic terminals. In 2015, we, for the first time, used AVmean ≤ 0 HU in the differential diagnosis of adrenal adenoma and non-adenoma (11), and the results showed that the sensitivity and accuracy of AVmean ≤ 0 HU were significantly higher than those of AVmean ≤ 10 HU, and the specificity was slightly lower than that of AVmean ≤ 10 HU. It is noteworthy that if AVmean, CTH, and AVmin can be combined to achieve complementary advantages, it may improve the differential diagnosis of adrenal adenoma by CT plain to a certain extent, avoid unnecessary radiation exposure and adverse reactions of contrast agents, and reduce the patient’s visiting time and medical expenses.
In the present study, AVmean, AVmin, and CTH were used to conduct a comparative study of adrenal adenoma and non-adenoma in two medical centers based on plain CT data, in order to explore the diagnostic efficacy and stability of these three parameters, so as to provide an important basis for clinical treatment decisions.
Methods
Study population
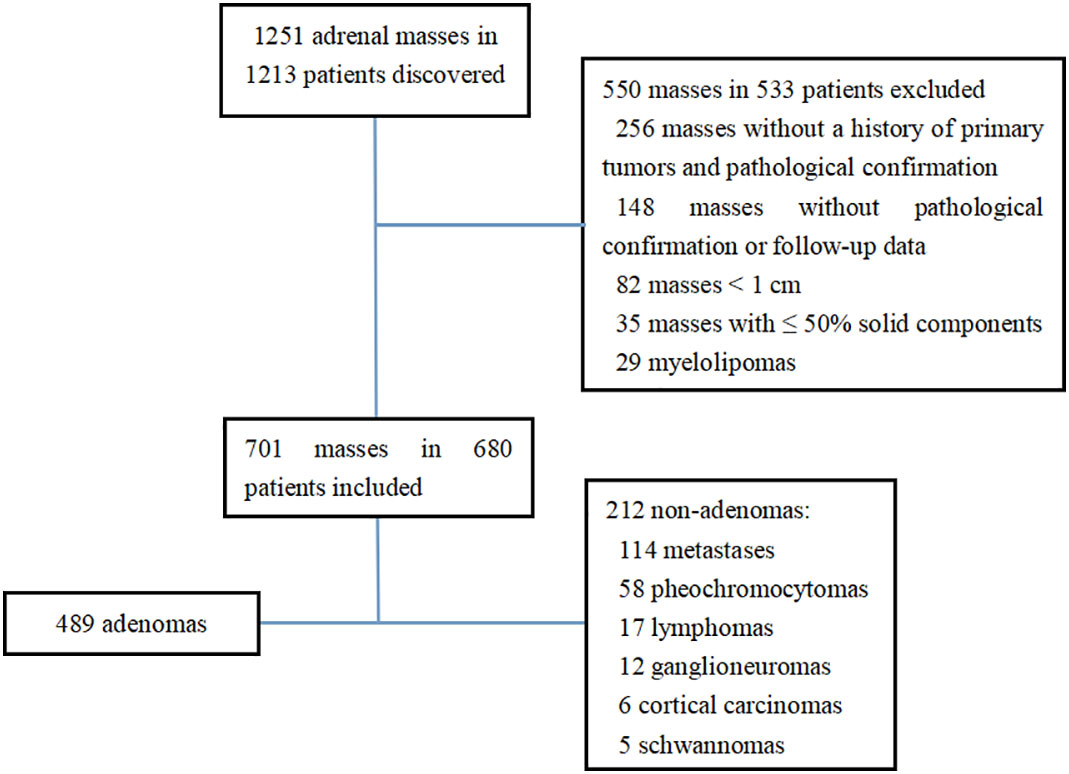
This study was performed in accordance with the ethical standards of the two institutions. Due to the retrospective design of the study, informed consent from the patients was waived according to national policy. The plain CT data of adrenal tumors in medical centers A and B from January 2012 to December 2021 were retrospectively analyzed. Initially, 1251 adrenal masses in 1213 patients were found in the two medical centers. The inclusion criteria were as follows: 1) patients who were confirmed by pathology or clinical follow-up; 2) the minimum diameter of the tumor was ≥ 1.0 cm; 3) patients who had tumors with > 50% solid components. A total of 550 masses in 533 patients were excluded, including 256 masses without a history of primary tumors and without pathological confirmation, 82 masses with tumor size < 1 cm, 148 masses with a history of primary tumors, while without pathological confirmation or follow-up data, and 35 masses with ≤ 50% solid components. Finally, a total of 680 patients with 701 masses met the inclusion criteria, including 328 men and 352 women, who aged 18-90 years old, with a median age of 56 (47.75, 64.25) years old (Figure 1). There were 636 tumors confirmed by pathology, including 489 cases of adrenal adenoma, 58 cases of pheochromocytoma, 49 cases of metastases, 17 cases of lymphoma, 12 cases of ganglioneuroma, 6 cases of cortical carcinoma, and 5 cases of schwannoma. Besides, 65 cases were confirmed by clinical follow-up, all of which were metastases. Clinical follow-up confirmation refers to a history of the primary tumors, and a rapid enlargement of the mass within 6 months or a rapid shrinkage after chemotherapy (8).
CT examination
All cases were scanned by plain CT. In medical centers A, a GE light speed 16-slice spiral CT or Optima540 16-slice spiral CT was used. The scanning pitch was 1.0 and 1.75 respectively, the layer thickness and layer distance of the former were 3.75mm, and the layer thickness and layer distance of the latter were 5mm. In medical center B, a GE optima 16-slice spiral CT was used, with scanning pitch of 1.75, layer thickness of 5mm and layer spacing of 5mm.
Image assessment
The images were jointly analyzed by two radiologists who had worked for 8 and 6 years on the GE Advantage Windows 4.6 workstation (General Electric Inc., Boston, MA, USA). The largest slice of the tumor was selected for the measurement of CTH, and the circular or oval region of interest (ROI) was used as large as possible (7, 12). The histogram analysis software was used to depict the CT value distribution curve in ROI. The X-axis represents the distribution range of CT value in ROI, and the Y-axis represents the frequency of each CT value. The software automatically obtained the percentage of negative pixels in ROI. In the present study, AVmean and AVmin were measured by a 4-point method, that is, the maximum transverse diameter and the longitudinal diameter were made on the maximum axial plane of the tumor to obtain the intersection point, the midpoint from the intersection point to the edge of the tumor was taken as the measurement point, and the ROI was 19 ~ 24 mm² (11) (Figures 2–5). When each ROI was selecting, it was attempted to avoid necrotic, hemorrhagic, and calcified areas. All measurements were made in triplicate, and the average value was calculated as the final value.
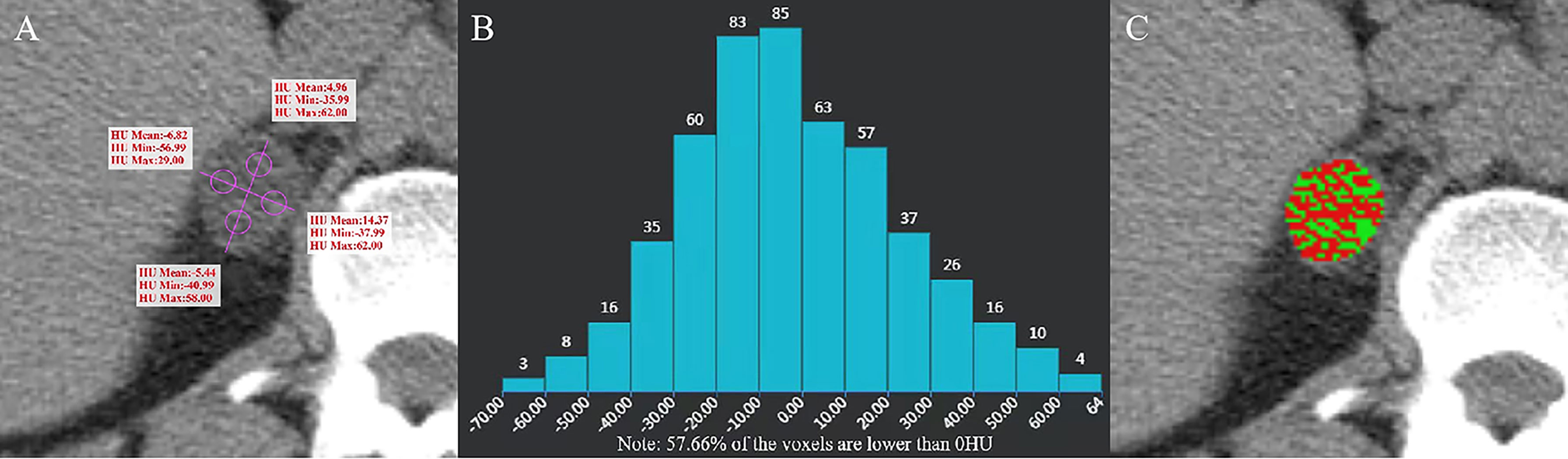
Figure 2 Adenoma of the right adrenal gland in a 47-year-old man. AVmean = 1.768 HU, AVmin = -42.99 HU (A), and CTH negative pixels = 57.66% (B). The CT value in the red area is ≤ 10 HU, and the CT value in the green area is > 10 HU (C).
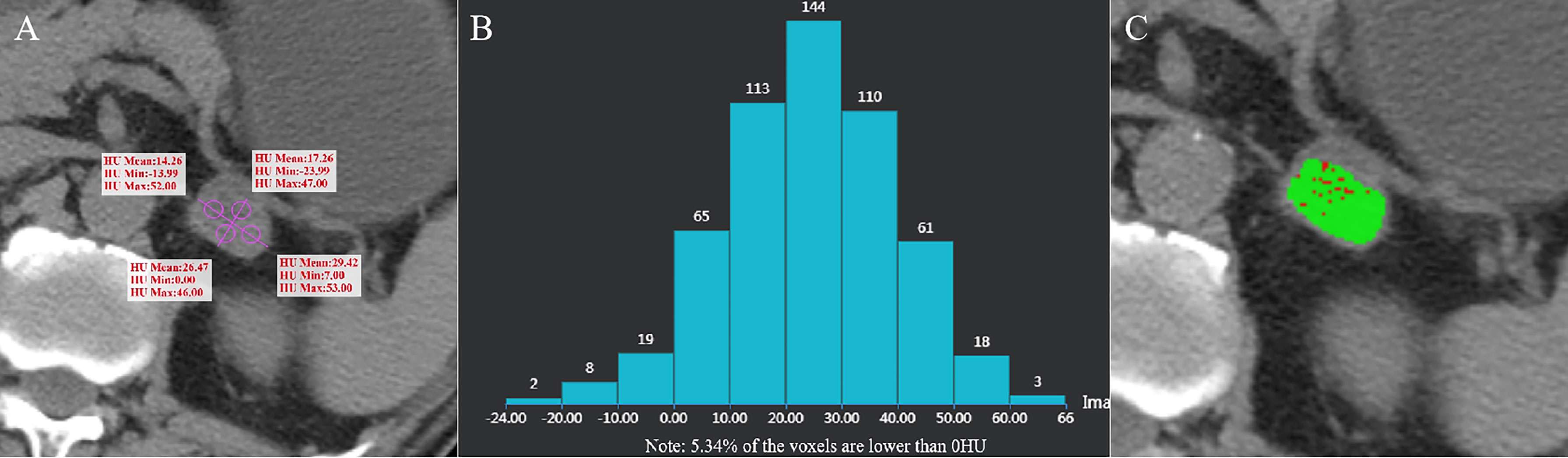
Figure 3 Adrenal adenoma of the left adrenal gland in a 62-year-old man. AVmean =21.853 HU (A), AVmin = -7.745 HU, and CTH negative pixel =5.34% (B). The CT value in the red area is ≤ 10 HU, and the CT value in the green area is > 10 HU (C).
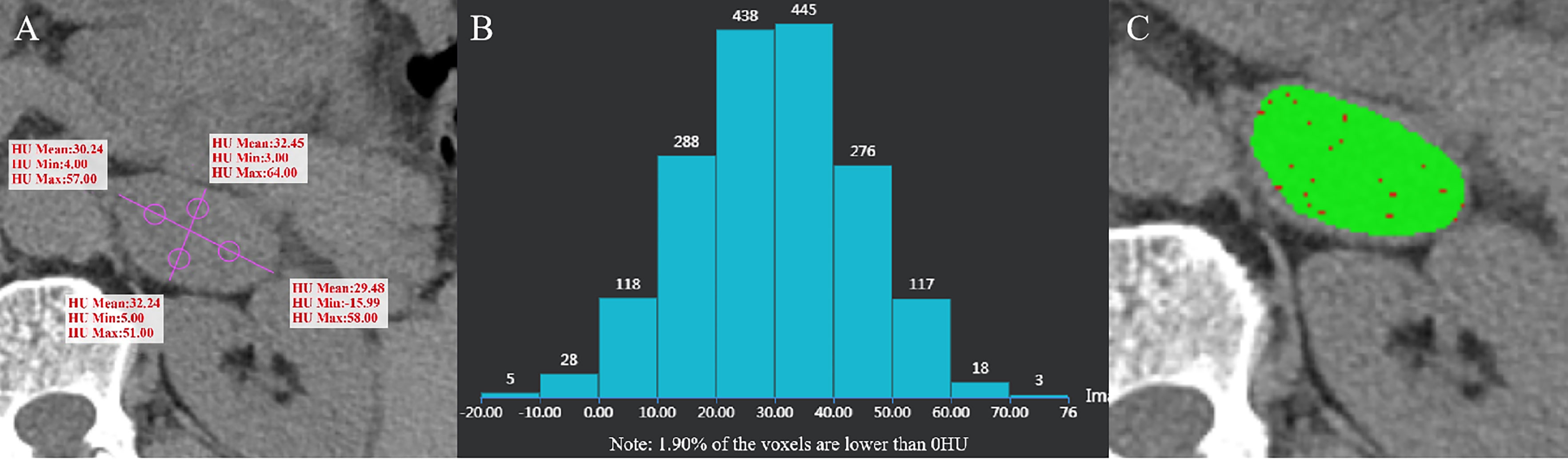
Figure 4 Ganglioneuroma of the left adrenal gland in a 55-year-old man. AVmean = 31.103 HU, AVmin = -0.75 HU (A), and CTH negative pixel =1.9% (B). The CT value in the red area is ≤10 HU, and the CT value in the green area is > 10 HU (C).
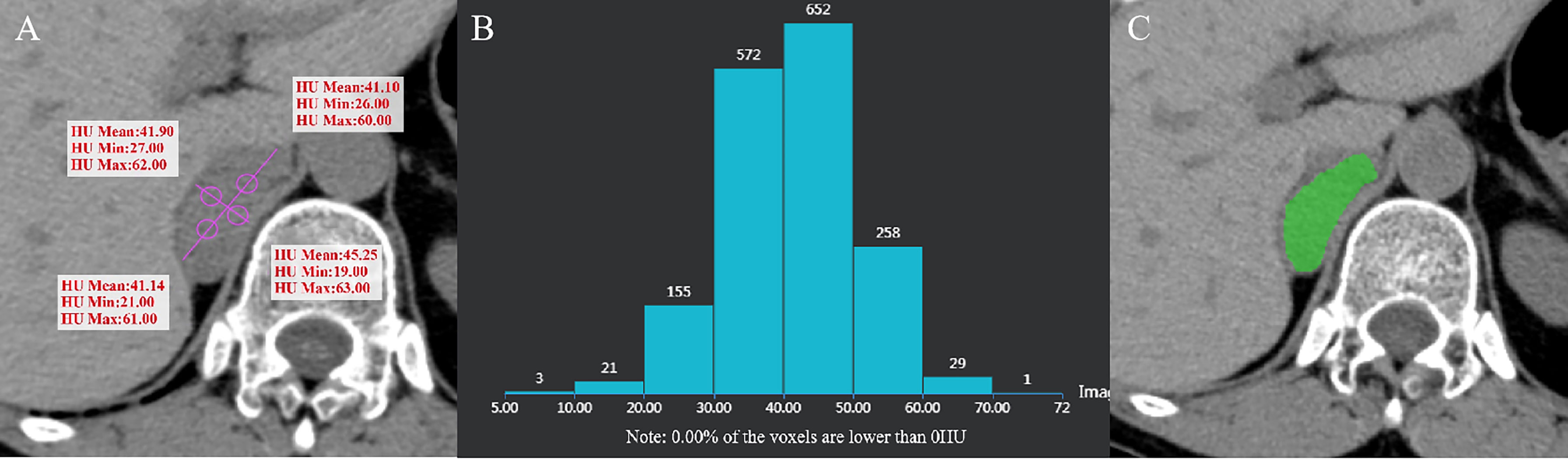
Figure 5 Pheochromocytoma of the right adrenal gland in a 64-year-old woman. AVmean = 41.345 HU, AVmin = 23.25 HU (A), and CTH negative pixel = 0 (B). CT value in the green area is > 10 HU (C).
Statistical analysis
Statistical analysis was performed using SPSS 25.0 software (IBM Corp., Armonk, NY, USA). The independent-samples t-test or the Mann-Whitney U test was used to compare AVmean, AVmin, and CTH between adrenal adenomas and non-adenomas. AVmean ≤ 10 HU, AVmin ≤ 0 HH, and CTH negative pixels ≥ 10% were analyzed by the Chi-squared test or the Fisher’s exact test. Receiver operating characteristic (ROC) curves of AVmean, AVmin, and CTH in two medical centers were plotted to distinguish adrenal adenoma from non-adenoma, and value of area under the curve (AUC) was calculated. The sensitivity, specificity, and accuracy of AVmean ≤ 10 HU, AVmin ≤ 0 HU, and CTH negative pixels ≥ 10% for diagnosing adrenal adenoma, and AVmin and CTH for adenoma with AVmean > 10Hu were compared between the two medical centers. P value < 0.05 was considered statistically significant.
Results
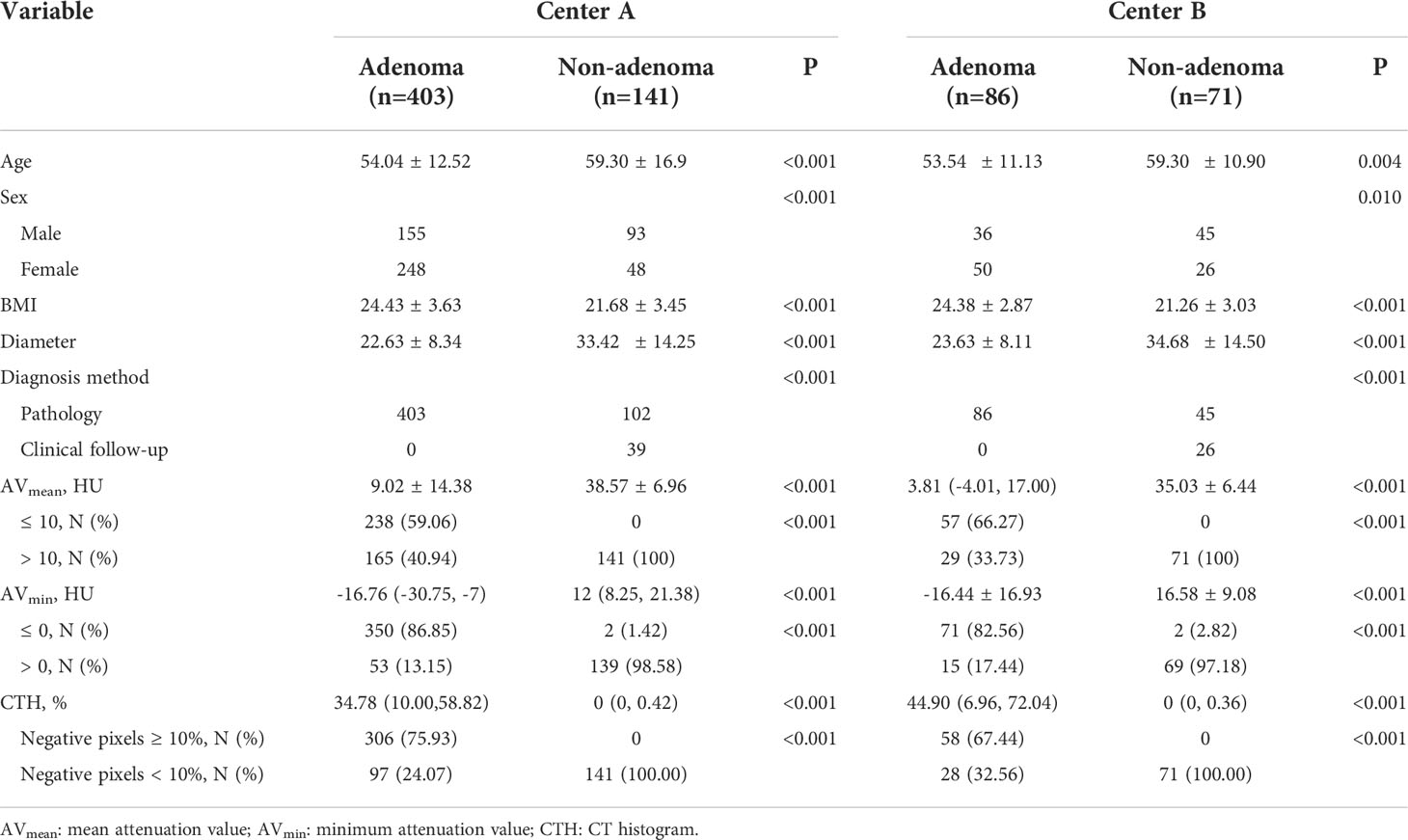
The distribution of gender, age and BMI in adenomas and non-adenomas was shown in Table 1. Among 489 cases of adrenal adenoma in the two medical centers, there were 297 cases of the left-sided adrenal adenoma and 192 cases of the right-sided adrenal adenoma, with a diameter of 10.0 ~ 51.2 mm (mean, 22.8 ± 8.2 mm) (Figures 2, 3). Among 212 cases of non-adenoma, there were 110 cases of the left-sided adrenal adenoma and 112 cases of the right-sided adrenal adenoma, with a diameter of 10.0 ~ 68.5 mm (mean, 34.4 ± 13.9 mm) (Figures 4, 5).
In center A and B, AVmean, AVmin, and CTH of adenoma and non-adenoma were shown in Table 1, and these parameters were significantly lower among the adrenal adenomas (all P < 0.001). The proportions of AVmean ≤ 10 HU, AVmin ≤ 0 HU, and CTH negative pixels ≥ 10% were also presented in Table 1, and these parameters have significant difference in the number of adenomas and non-adenomas (all P < 0.001). In center A, there were 2 cases of non-adenoma with AVmin ≤ 0 HU, including 1 case of lung cancer with adrenal metastasis and 1 case of ganglioneuroma (Figure 4). In center B, there were 2 cases of non-adenoma with minAVs ≤ 0 HU, all of which were ganglioneuroma.
In both the centers, showing AVmean ≤ 10 HU, AVmin ≤ 0 HU, and CTH negative pixels ≥ 10% (all P<0.001), and In center A and B, the AUC of AVmean, AVmin, CTH negative pixels for the differential diagnosis of adenoma and non-adenoma was 0.941~0.956 and 0.952~0.958, respectively. The corresponding sensitivity, specificity, and accuracy of AVmean ≤ 10 HU, AVmin ≤ 0 HU, and CTH negative pixels≥10% were shown in Table 2.
Among adenoma with AVmean >10 HU, the best threshold of AVmin in center A and center B were -0.250HU and 2.375HU, and the corresponding AUC, sensitivity and specificity were 0.858 and 0.846, 0.691 and 0.586, 0.986 and 0.958; the best threshold of CTH in center A and center B were 0.895% and 0.775%, and the corresponding AUC, sensitivity and specificity were 0.873 and 0.822, 0.818 and 0.724, 0.837 and 0.915 (Table 3).
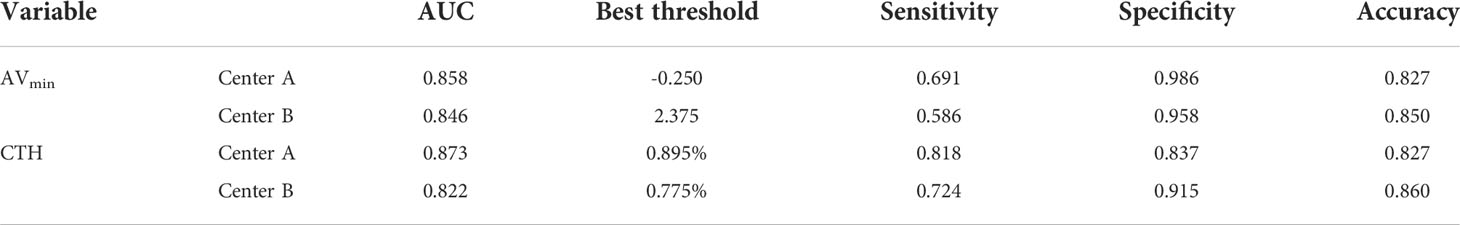
Table 3 Comparison of diagnostic efficacy of AVmin and CTH for adenoma with AVmean >10 HU in two centers.
Discussion
In the present study, we conducted a control study on cases with adrenal adenoma and non-adenoma from two medical centers using plain CT scan with AVmean, AVmin, and CTH. The results showed that sensitivity and accuracy of AVmean ≤ 10 HU were the lowest, those of AVmin ≤ 0 Hu were the highest, and those of CTH negative pixels ≥ 10% were in the middle. Moreover, specificity of AVmean ≤ 10 HU and CTH negative pixels ≥ 10% was equal to 1.000, and that of AVmin ≤ 0 HU was slightly lower (0.972). The sensitivity, specificity, and accuracy of the AVmin ≤ 0 HU and CTH negative pixels ≥ 10% for the diagnosis of adrenal adenoma in center A were similar to those in center B. The specificity of AVmean ≤ 10 HU in the diagnosis of adrenal adenoma in center A was consistent with that in center B, while the sensitivity was quite different (0.591 vs. 0.663), which could be related to the different inclusion criteria for surgical resection considered in the two medical centers. When AVmin in center A and center B was less than -0.250HU and 2.375HU, respectively, and CTH in center A and center B was greater than 0.895% and 0.775%, respectively, adenomas with AVmean >10 HU could be effectively diagnosed, and AVmin had high specificity, CTH had high sensitivity.
It is noteworthy that AVmean, AVmin, and CTH are closely correlated together. CTH was first proposed by Bae et al. for the differential diagnosis of adrenal masses (9). It can display all pixel values in ROI of tumors and record them in the form of histograms. The X-axis corresponds to the CT values, and the Y-axis represents the pixel frequency of each CT value. CTH can fully reflect the heterogeneity of pixels in different ROIs. The incidence of adenoma can be evaluated by the percentage of negative pixels in ROI, that is, the larger the percentage, the greater the incidence of adenoma. At present, the widely accepted threshold is CTH negative pixels ≥10%, with a sensitivity of 82.9-92.2% and a specificity of 98.2-100% for the diagnosis of adenoma (7–9). Moreover, AVmean is the average value of all pixels in the ROI, which is equivalent to the average value of all pixels in CTH. The lower the negative pixel value and the larger the proportion in ROI, the greater the incidence of adenoma. The broadly accepted threshold is AVmean ≤ 10 HU, with a sensitivity of 47.6-82.2% and a specificity of 93.3-100% for the diagnosis of adenoma (6–10). AVmean is the minimum value of pixels in ROI, which is equivalent to the minimum value of pixels in CTH. The smaller the AVmean and the larger distribution areas, the greater the incidence of adenoma. We previously used AVmean ≤ 0 HU as the threshold for diagnosing adenoma, with a sensitivity of 90.1% and a specificity of 96.8% (11).
The premise of diagnosing adenoma with CTH negative pixels ≥ 10% and AVmean ≤ 10 HU requires the ratio of negative pixels to total pixels in the ROI to reach a certain value. Therefore, when the number of negative pixels in the tumor is small and the distribution is sparse, and the ratio of negative pixels to total pixels is lower than this value, the above-mentioned two criteria will be difficult to diagnose adrenal adenoma, especially for AVmean ≤ 10 HU. However, AVmin ≤ 0 HU is different, because even if the negative pixels in the tumor are limited and sparse, as long as the ROI contains negative pixels, it can be judged as an adrenal adenoma. The present study showed that using AVmin and CTH was effective in differentiating adenoma with AVmean > 10 HU, the former with high specificity and the latter with high sensitivity. Besides, using AVmin ≤ 0 HU as the best threshold to diagnose adenoma, 44 cases (10.92%) and 13 cases (18.31%) of adenoma with CTH negative pixels < 10% could be diagnosed in center A and center B. It should be noted that 4 cases (1.89%) with AVmin ≤ 0 HU were non-adenoma in the two centers, which may be related to sparse visible components (e.g., nucleus) in the stroma of ganglioneuroma, edema in some cases, and small necrotic areas in the measurement of metastatic tumors that could not be distinguished by naked eyes.
Park et al. reported different results due to different measurement locations and ROIs (13). At present, in the selection of ROI for AVmean and CTH, scholars mainly use circular or oval shape, and the measured area is 1/2-2/3 of the largest layer (3, 6, 9), or take the largest area as far as possible (1, 2, 7, 8, 12). There are some shortcomings in this measurement method. Firstly, the definition of measurement area is extremely wide. When measurement is carried out on the largest cross-sectional area of the tumor, from more than 1/2 to cover the whole cross-sectional area as much as possible, several measurement methods can be used, which are corresponded to different measured areas, and different measured areas may cause certain differences. Secondly, it is difficult to avoid necrosis (especially in the central area of tumor that is prone to necrosis), hemorrhage, calcification, etc., when measuring the area of the largest slice of more than 1/2 tumor. Necrosis may increase the percentage of negative pixels, resulting in an increase in the false positive diagnostic rate of AVmean and CTH, while hemorrhage and calcification may increase the percentage of positive pixels, that is, AVmean ≥ 10 HU and/or CTH negative pixels < 10%, resulting in difficulties in the diagnosis. In order to avoid these shortcomings, the present study improved our previous measurement method (11), changing 5 points to 4 points, that is, eliminating the measurement points in the central area of the tumor. This newly proposed measurement method have the following advantages: 1) the measurement method is clearly defined, and it is highly repeatable; 2) the central area of the tumor which is prone to necrosis can be avoided; 3) multi-point measurement can reflect the heterogeneity of different points in tumor; 4) calculation of average value of four measurement points can reduce the measurement error.
There were four deficiencies in the present study. Firstly, 65 cases of adrenal metastasis were clinically confirmed rather than the pathology. However, the majority of the patients who were diagnosed with adrenal metastasis have lost the opportunity of surgery, and it was no longer necessary to perform invasive surgery or puncture, thus, we strictly adopted the clinical criteria for judging metastatic tumors proposed by Halefoglu (8). Secondly, adrenal washout CT and chemical shift MRI are of great significance in the differential diagnosis of adrenal masses, especially when plain CT scan is not predominant in the diagnosis of for lipid-poor adenomas and non-adenomatous lesions (14–16). However, this study aimed to explore the clinical value of three measurement methods in plain CT scan. In the future studies, we will combine these three measurement methods with washout CT and chemical shift MRI for a more comprehensive analysis. Thirdly, the study aimed to compare the differential diagnostic efficacy of three CT parameters for adrenal tumors. Functional diagnosis (cortisol or aldosterone assessment) of the masses has not been analyzed, and comprehensive analysis combined with these laboratory parameters will be our future direction. Finally, there might be selection bias due to the retrospective analysis of the data. Hence, additional prospective multicenter controlled studies are required to eliminate the above-mentioned limitations.
In conclusion, AVmean ≤ 10 HU, AVmin ≤ 0 HU, and CTH negative pixels ≥10% have high diagnostic efficiency for adrenal adenomas and high consistency between the two medical centers. Even for adenomas with AVmean> 10 HU, AVmin and CTH could still distinguish them to a great extent. The complementarity of these three parameters could enhance the role of plain CT scan in the differential diagnosis of adrenal adenoma, and assist clinicians to develop personalized treatments.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by Affiliated Hangzhou First People’s Hospital, Zhejiang University School of Medicine, The Quzhou Affiliated Hospital of Wenzhou Medical University, and Quzhou People’s Hospital. The ethics committee waived the requirement of written informed consent for participation.
Author contributions
ZD, MZ, and ZH conceived and designed the study. ZH, MW, and XZ collected the clinical and image data of all cases. ZH, MW, and PW reviewed, analyzed, and classified the imaging data. PW and HZ performed statistical analysis. ZH and MW wrote the first draft of the manuscript. All authors contributed to manuscript revision and approved the submitted version.
Funding
This study was supported by the National Natural Science Foundation of China (81871337), the Medical Science Research Program of Zhejiang Province (2020RC091, 2021RC024), the Key Laboratory of Clinical Cancer Pharmacology and Toxicology Research of Zhejiang Province (2020E10021).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nagayama Y, Inoue T, Oda S, Tanoue S, Nakaura T, Morinaga J, et al. Unenhanced dual-layer spectral-detector CT for characterizing indeterminate adrenal lesions. Radiology (2021) 301(2):369–78. doi: 10.1148/radiol.2021202435
2. Nagayama Y, Inoue T, Kato Y, Tanoue S, Kidoh M, Oda S, et al. Relative enhancement ratio of portal venous phase to unenhanced CT in the diagnosis of lipid-poor adrenal adenomas. Radiology (2021) 301(2):360–8. doi: 10.1148/radiol.2021210231
3. Liu T, Sun H, Zhang H, Duan J, Hu Y, Xie S. Distinguishing adrenal adenomas from non-adenomas with multidetector CT: evaluation of percentage washout values at a short time delay triphasic enhanced CT. Br J Radiol (2019) 91(1094):20180429. doi: 10.1259/bjr.20180429
4. An YY, Yang GZ, Lin B, Zhang N, Hou HT, Zhu FM, et al. Differentiation of lipid-poor adenoma from pheochromocytoma on biphasic contrast-enhanced CT. Abdom Radiol (NY) (2021) 46(9):4353–61. doi: 10.1007/s00261-021-03121-9
5. Elmohr MM, Fuentes D, Habra MA, Bhosale PR, Qayyum AA, Gates E, et al. Machine learning-based texture analysis for differentiation of large adrenal cortical tumours on CT. Clin Radiol (2019) 74(10):818. doi: 10.1016/j.crad.2019.06.021
6. Kirsch MJ, Kohli MW, Long KL, Pitt SC, Schneider DF, Sippel RS, et al. Utility of the 10 hounsfield unit threshold for identifying adrenal adenomas: Can we improve? Am J Surg (2020) 220(4):920–4. doi: 10.1016/j.amjsurg.2020.04.021
7. Szász P, Kučera P, Čtvrtlík F, Langová K, Hartmann I, Tüdös Z. Diagnostic value of unenhanced CT attenuation and CT histogram analysis in differential diagnosis of adrenal tumors. Medicina (Kaunas) (2020) 56(11):597. doi: 10.3390/medicina56110597
8. Halefoglu AM, Bas N, Yasar A, Basak M. Differentiation of adrenal adenomas from nonadenomas using CT histogram analysis method: a prospective study. Eur J Radiol (2010) 73(3):643–51. doi: 10.1016/j.ejrad.2008.12.010
9. Bae KT, Fuangtharnthip P, Prasad SR, Joe BN, Heiken JP. Adrenal masses: CT characterization with histogram analysis method. Radiology (2003) 228(3):735–42. doi: 10.1148/radiol.2283020878
10. Ceccato F, Tizianel I, Voltan G, Maggetto G, Merante Boschin I, Quaia E, et al. Attenuation value in adrenal incidentalomas: A longitudinal study. Front Endocrinol (Lausanne) (2021) 12:794197. doi: 10.3389/fendo.2021.794197
11. Zhu M, Qu J, Han Z. Evaluate the efficacy of minimum attenuation value in differentiation of adrenal adenomas from nonadenomas on unenhanced CT. Clin Imaging (2016) 40(1):86–9. doi: 10.1016/j.clinimag.2015.09.006
12. Tüdös Z, Kučera P, Szász P, Hartmann I, Langová K, Škarda J, et al. Influence of slice thickness on result of CT histogram analysis in indeterminate adrenal masses. Abdom Radiol (NY) (2019) 44(4):1461–9. doi: 10.1007/s00261-018-1835-2
13. Park SY, Park BK, Park JJ, Kim CK. CT sensitivities for large (≥3 cm) adrenal adenoma and cortical carcinoma. Abdom Imaging (2015) 40(2):310–7. doi: 10.1007/s00261-014-0202-1
14. Platzek I, Sieron D, Plodeck V, Borkowetz A, Laniado M, Hoffmann RT. Chemical shift imaging for evaluation of adrenal masses: a systematic review and meta-analysis. Eur Radiol (2019) 29(2):806–17. doi: 10.1007/s00330-018-5626-5
15. Schieda N, Siegelman ES. Update on CT and MRI of adrenal nodules. AJR Am J Roentgenol (2017) 208(6):1206–17. doi: 10.2214/AJR.16.17758
Keywords: adrenal gland neoplasms, adrenal adenoma, computed tomography, attenuation value, CT histogram
Citation: Han Z, Wu M, Wei P, Zhu H, Zhang X, Ding Z and Zhang M (2022) Differential diagnostic value of plain CT scan in adrenal adenoma and non-adenoma: A two-center control study of mean attenuation value, minimum attenuation value, and CT histogram. Front. Endocrinol. 13:1007870. doi: 10.3389/fendo.2022.1007870
Received: 31 July 2022; Accepted: 24 October 2022;
Published: 09 November 2022.
Edited by:
Valentina Morelli, Istituto Auxologico Italiano, ItalyReviewed by:
Guido Zavatta, University of Bologna, ItalyMiao Zhang, University of Texas MD Anderson Cancer Center, United States
Weiwei Zhan, Shanghai Jiaotong University School of Medicine, China
Copyright © 2022 Han, Wu, Wei, Zhu, Zhang, Ding and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongxiang Ding, aGFuZ3pob3Vkeng3M0AxMjYuY29t; Ming Zhang, emhhbmdtaW5nMDFAeGp0dS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Zhijiang Han
Zhijiang Han Mengwei Wu3†
Mengwei Wu3† Peiying Wei
Peiying Wei Hanlin Zhu
Hanlin Zhu Zhongxiang Ding
Zhongxiang Ding Ming Zhang
Ming Zhang