
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 24 October 2022
Sec. Clinical Diabetes
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1003263
This article is part of the Research TopicSafety Evaluation of Hypoglycemic DrugsView all 5 articles
Objective: To analyze the efficacy and safety of three novel hypoglycemic agents, glucagon-like peptidyl-1 receptor agonists, dipeptidyl peptidase-4 inhibitors (DPP-4i), and sodium-glucose cotransporter two inhibitors (SGLT2i) in type 2 diabetes mellitus (T2DM) patients with severe chronic kidney disease (CKD) (defined in this study as CKD stage 3 B or above, eGFR< 45 mL/min/1.73 m²) based on important RCTs to date.
Methods: We retrieved studies published before April 15, 2022, from EMBASE, PubMed/MEDLINE, Cochrane Library and included randomized controlled trials in which the participants were patients with T2DM and severe CKD. Frequentist methods were used in the network meta-analysis.
Results: Nineteen studies of 17 trials involving 6,607 participants met our inclusion criteria. Compared with placebo and DPP-4i, SGLT2i demonstrated a significantly lower incidence of serious renal-related adverse events or renal death, and the odds ratios (OR) were 0.69 (0.58, 0.81) and 0.63 (0.40, 1.00), respectively. Compared with placebo, SGLT2i significantly reduced the incidence of all-cause death and severe AE; the ORs were 0.72 (0.55, 0.94) and 0.65 (0.47, 0.91), respectively. Compared with placebo, DPP-4i significantly reduced the level of HbA1c, and the difference between mean changes from baseline was -0.36 (-0.63, -0.09).
Conclusions: Patients with T2DM complicated by severe CKD may benefit from SGLT2i. SGLT2i can reduce the incidence of serious renal-related AEs or renal death, as well as severe side effects, and has a positive effect on the patient’s renal function and survival, even for only CKD patients can also be considered. GLP-1 RAs can be used as a supplement if blood sugar control is poor. For dialysis patients, DPP-4i can assist blood glucose control, reduce insulin dosage, and reduce the risk of hypoglycemia.
Systematic review registration: INPLASY https://inplasy.com/inplasy-2021-12-0106/, identifier INPLASY2021120106.
The International Diabetes Federation estimates that 463 million adults aged 20–79 years had diabetes mellitus (DM) globally in 2019, and this number is expected to reach 700 million by 2045. Approximately 90% of patients with DM have type 2 diabetes mellitus (T2DM) (1). Diabetic kidney disease (DKD) accounts for approximately 25% of diabetic cases and is the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) in most countries. The pathogenesis of DKD is complex, and its clinical manifestations include a continuous increase in urinary albumin excretion and a progressive decrease in estimated glomerular filtration rate (eGFR). Although there is no cure for CKD, its progress can be delayed by controlling risk factors such as hypertension, hyperglycemia, and dyslipidemia (2). T2DM in patients with severe CKD (defined in this study as CKD stage 3 B or above, eGFR< 45 mL/min/1.73 m2) is more challenging to treat since renal damage may affect drug clearance through the kidney, leading to interruption or reduction of most hypoglycemic therapies. In particular, the pharmacological effects of these drugs are difficult to predict in T2DM patients undergoing hemodialysis as the accumulation and rapid clearance of antidiabetic drugs or their metabolites during hemodialysis makes it difficult to maintain blood glucose control (3–5). Therefore, it is particularly difficult to provide a scheme for optimal glycemic control in patients with severe CKD. In addition, the control of blood glucose while protecting renal function and reducing mortality is also worthy of further research. In the past few decades, three novel hypoglycemic agents, glucagon-like peptidyl-1 receptor agonists (GLP-1RAs), dipeptidyl peptidase-4 inhibitors (DPP-4i), and sodium-glucose cotransporter 2 inhibitors (SGLT2i) have been introduced. They have become widely used in the treatment of T2DM. The 2020 Guidelines (6) of KDIGO recommended SGLT2i for patients with eGFR ≥ 30 mL/min/1.73 m2. GLP-1RAs are safe for patients with CKD but are not recommended for ESRD patients. DPP-4i reduces blood glucose and lowers the risk of hypoglycemia, but there is no evidence that it improves renal or cardiovascular outcomes. After completing several large RCTs, some new findings have been made regarding the efficacy of the three novel hypoglycemics in patients with severe DKD and ESRD. Research by Heerspink et al. (7) revealed that dapagliflozin significantly reduced the incidence of death from renal or cardiovascular causes in DKD patients with eGFR<45 mL/min/1.73 m2 than that in patients who were administered a placebo. Another study showed that dialysis patients taking saxagliptin had significantly lower HbA1c levels than controls (8). However, there is insufficient evidence to conclude whether these agents are effective for T2DM patients with severe CKD.
The purpose of this study was to analyze the efficacy and safety of the three novel hypoglycemics in T2DM patients with severe CKD, based on important RCTs to date. Due to the lack of head-to-head trials, this study used network meta-analysis.
We followed the PRISMA guidelines for network meta-analysis (9). The study protocol was published in the INPLASY database under the registration number INPLASY2021120106 (https://inplasy.com/inplasy-2021-12-0106/).
We included RCTs with parallel-group design. The participants were T2DM patients with severe CKD. If the study population in the subgroup met the above criteria, the study was considered eligible. Studies included at least one of the following treatments: GLP-1RA, DPP-4i, and SGLT2i. The results of eligible studies included changes in HbA1c levels from baseline, hypoglycemic events, renal-related adverse events (AEs) (consisting of renal replacement therapy, acute kidney injury, and acute kidney failure), or death. We excluded single-arm studies and positive control studies for the two drugs in the same category.
We searched for articles published before April 15, 2022. Electronic searches were conducted in Embase, PubMed/Medline, and the Cochrane Library. The search terms used are presented in the Supplementary Method S1 section of the supplementary materials. The two authors conducted an independent literature search, screened titles and abstracts, and read the full text to determine whether the study met the inclusion criteria. The PRISMA flow diagram was used to summarize the study selection process.
One author extracted relevant information from qualified trials, and the other author independently reviewed the data. The differences were resolved by consensus through discussion. The extraction included author, year of publication, country, stage of CKD, age, sex percentage, drug use, sample size, and follow-up. For data that were not available in digital form, we used the free software PlotDigitizer (https://plotdigitizer.com/app)to extract digital data from graphics. In some cases, we obtained standard deviations from standard errors or 95% CIs and classified data from individual patient data or percentages. We also obtained the required data from publications by different authors of the same trial. The primary outcome was the incidence of serious renal-related AEs or renal death, other outcomes were assessed as serious AEs, hypoglycemia, severe hypoglycemia, all-cause mortality, and changes in HbA1c compared with baseline. We performed a subgroup analysis of patients with ESRD undergoing hemodialysis.
This network meta-analysis was performed using a frequentist random-effects model (10). We ranked medications according to the surface under the cumulative ranking (SUCRA) (11). A higher SUCRA value indicated better performance. Odds ratio (OR) was used to pool effect sizes for adverse events, weighted mean difference was used to pool effect sizes for HbA1c change, and interval estimation was performed using 95% CIs. The level of significance was set at an α value of 0.05. We assessed the transitivity assumption by analyzing the distribution or frequency of potential effect modifiers in treatment: HbA1c at baseline, age, and proportion of men. We conducted random effects (DerSimonian- Laird estimator) pairwise meta-analyses for all comparisons, allowing for heterogeneity in effect size between studies. The proportion of the total variance within each pairwise comparison that is due to between study heterogeneity was estimated using the I² statistic. We evaluated the small-study effects by visually observing publication bias using a comparison-adjusted funnel plot (12). The mvmeta command and network packages of commands in Stata software were used for this analysis (13, 14). Pairwise meta-analysis with a random-effects model was used to perform subgroup analysis. All analyses were performed using Stata version 15.0.
The quality of the retrieved RCTs was assessed according to the Cochrane Handbook of Systematic Reviews of Interventions (15). Potential sources of bias include sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), and selective outcome reporting (reporting bias). The two authors independently conducted this assessment, and studies were graded as high risk, low risk, or uncertain risk.
Electronic retrieval of the database yielded 5,024 studies. After reading the full text, 19 studies on 17 trials covering 6607 participants met our inclusion criteria (EMPA-REG OUTCOME trial was included in two studies and the DAPA-CKD trial in two studies) (in Supplementary Figure 1).
In the 17 RCTs, SGLT2i, DPP-4i, and GLP-1RA were used as pharmacological interventions in five, nine, and three trials, respectively. The average age of the patients was 60-70 years. The baseline of patients adopted in the subgroup of this study was eGFR < 45 mL/min per 1.73m². Detailed information on each study is listed in Table 1. Supplementary Figures 2-8 show the network for all the included trials.
Among the 17 RCTs, two were open-label and had a high risk of performance bias. Two trials had a high risk of attrition bias. One trial had a high risk of selection bias because of the imbalance between the intervention and control groups in the severe CKD subgroup. Most studies had a low risk of bias. Details of the quality assessment are illustrated in Supplementary Figures 9, 10.
The mean age of the included studies was between 60 and 70 years old, with the maximum being 70.5 ± 8.5 and the minimum being 60.5 ± 9.1; The proportion of men is more than that of women, between 50% and 80%, while in one study, the proportion of men is 37%. Supplementary Figures 11, 12. In addition, the mean level distribution of baseline HbA1c is 6.5 ± 0.8 to 8.7 ± 1.5, therefore the baseline HbA1c of subjects is very similar, Supplementary Figure 13. The intervention measures of the study were similar: comparing the studied agents with placebo on the basis of background hypoglycemic therapy, and there were 2 trails without any background hypoglycemic, Table 1. The quality of the research was also balanced, as shown in Table 1. As such, the assumption of transitivity is likely to hold in our data.
We found some evidence of statistical heterogeneity (I² statistic > 50%) within pairwise comparisons, however, only one I² statistic was greater than 85%. We believe that the homogeneity assumption of both pairwise and network meta-analysis is met, Supplementary Table 1.
This study compared the incidence of various serious AEs and evaluated the safety of the three novel hypoglycemics GLP-1RA, DPP-4i, and SGLT2i in T2DM patients with severe CKD. The changes in HbA1c from baseline were also compared to observe the efficacy of these three novel hypoglycemics in reducing blood glucose.
Supplementary Table 1 presents the results of the pairwise meta-analysis and heterogeneity estimates. Briefly, Compared with placebo, SGLT2i demonstrated a significantly lower incidence of renal-related adverse events and all-cause mortality, ORs were 0.686(0.581, 0.810) and 0.719(0.548, 0.944), respectively. Compared with placebo, DPP-4i and GLP1 demonstrated significant reduction in HbA1c, with mean difference of -0.362(-0.634, -0.089) and -0.491(-0.835, -0.147), respectively.
Serious renal-related AEs or renal deaths were reported in seven studies. Compared with placebo and DPP-4i, SGLT2i demonstrated significantly lower incidence of serious renal-related AEs or renal death, ORs were 0.69 (0.58, 0.81) and 0.63 (0.40, 1.00), respectively (Figure 1). SUCRA ranking showed that SGLT2i performed best and placebo performed the worst, as shown in Table 2.
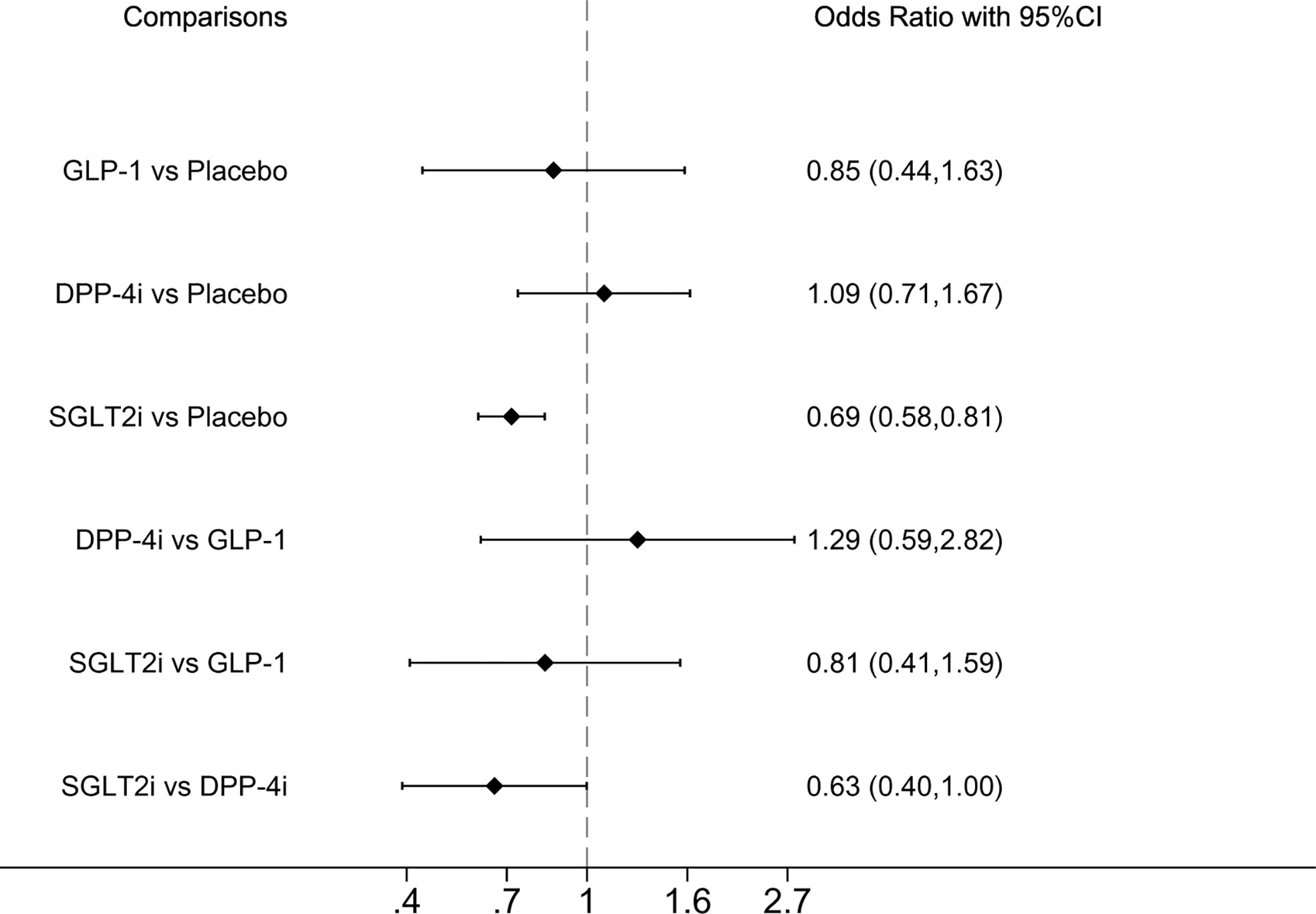
Figure 1 Forest plot of odds ratio comparing the serious renal-related adverse events or renal death between each medicine class or between each medicine class and placebo.
Serious AEs (defined as medical events that resulted in death, hospitalization, or significant disability or incapacity, and were identified by searching in the Medical Dictionary for Regulatory Activities) (23, 31) were reported in eight studies. Compared with placebo, SGLT2i demonstrated a significantly lower incidence of serious AEs OR was 0.65 (0.47, 0.91) ((Figure 2). SUCRA ranking showed that SGLT2i performed best and GLP-1 performed the worst (Table 2).

Figure 2 Forest plot of odds ratio comparing the serious adverse events between each medicine class or between each medicine class and placebo.
All-cause mortality was reported in seven studies, whereas GLP-1RA was absent. Compared with placebo, SGLT2i demonstrated a significantly lower incidence of all-cause mortality, OR of 0.72 (0.55, 0.94] ((Figure 3). SUCRA ranking showed that SGLT2i performed best and placebo performed the worst (Table 2).
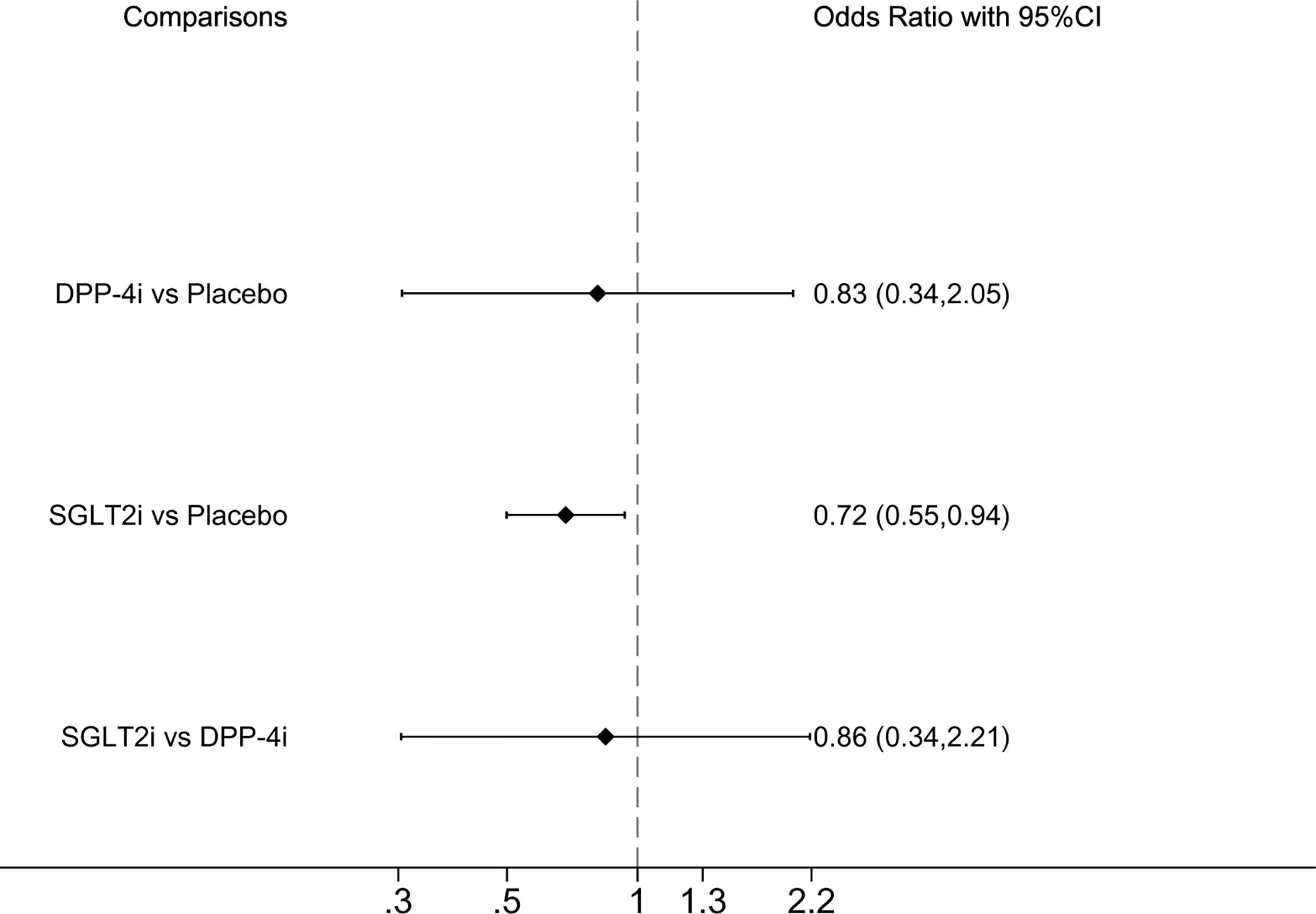
Figure 3 Forest plot of odds ratio comparing the all-cause mortality between each medicine class or between each medicine class and placebo.
There were no significant differences between the three novel hypoglycemic and placebo groups in the incidence of hypoglycemia or severe hypoglycemia, nor were there significant differences between the three novel hypoglycemics themselves (in Supplementary Figures 14, 15). This suggests that these three drugs are equally likely to cause adverse events of hypoglycemia in T2DM patients with severe CKD.
The mean changes from baseline HbA1c levels were reported in 12 studies. Compared with placebo, DPP-4i demonstrated a significant reduction in HbA1c, with a mean difference of 0.36 (-0.63, -0.09) (in Supplementary Figure 16). The SUCRA ranking showed that DPP-4i performed best and placebo performed the worst (Table 2).
Due to the lack of data on the efficacy and safety of SGLT2i and GLP-1RA in dialysis patients, we only performed subgroup analyses of DPP-4i in this population. Six studies reported mean changes from baseline HbA1c levels. Compared with placebo, DPP-4i demonstrated a significant reduction in HbA1c in patients undergoing dialysis, MD was -0.22 (-0.42, -0.03) (in Supplementary Figure 17). Three studies reported the incidence of hypoglycemia in dialysis patients treated with DPP-4i versus placebo, with no difference in the pooled results, OR was 0.88 (0.34, 2.29), in Supplementary Figure 18.
No publication bias was found by visual inspection of the funnel plots (in Supplementary Figure 19).
In this study, compared with placebo, SGLT-2i demonstrated significantly lower all-cause mortality, the incidence of serious renal-related AEs or renal death, and severe side effects. Although there was no significant difference between the three drugs, SGLT2i had the highest SUCRA ranking and the best efficacy. SGLT2i is a novel oral antidiabetic drug that is distinguished from other traditional hypoglycemics. SGLT2i can act on renal tubules and exert glucose-lowering effects by inhibiting renal tubular reabsorption of glucose and promoting urinary glucose excretion. Theoretically, the hypoglycemic effect of SGLT2i usually declines as kidney function declines (32). Thus, many physicians are skeptical about the use of SGLT2i in patients with stage 4 disease.
Nevertheless, patients with stage 4 disease were covered by all three included studies (7, 17, 21), and our findings illustrated that even CKD patients with stage 4 can still benefit from SGLT2i. However, the SUCRA ranking in this study showed that SGLT2i had the worst performance in reducing HbA1c, all-cause death, and severe kidney injury were still significantly lower. This illustrates from another perspective that the renal protection of SGLT2i is not entirely dependent on blood glucose control but is most likely through direct renal mechanisms to benefit the kidneys. Reliable evidence is still lacking regarding the renal protective effect of SGLT2i, but several hypotheses have been proposed to explain the observed phenomenon. One of the proposed mechanisms is an improved glomerular hemodynamics due to increased adenosine levels caused by an increase in membrane Na+/K+ ATPase activity, which leads to afferent arteriole constriction, thereby reducing glomerular hyperfiltration, reducing proteinuria, and preserving renal function in the long term (33–36). SGLT2i may also protect the kidneys in other ways, including enhancing oxygenation of the kidney by reducing tubular energy requirements, metabolic and anti-inflammatory effects, and directly affecting glomerular endothelial function (37, 38). Therefore, we believe that SGLT2i is not completely dependent on the function of blood sugar control and is still capable of renal protection, making it feasible to use in CKD stage 4 with T2DM patients; moreover, it can have a positive effect on the patient’s renal function and survival, even for only CKD patients can also be considered.
GLP-1 RAs (including exenatide, liraglutide, and lixisenatide) exert hypoglycemic effects by enhancing insulin secretion and inhibiting glucagon secretion through the activation of GLP-1 receptors. Lixisenatide and exenatide are eliminated mainly through glomerular filtration and subsequent proteolytic degradation in the renal tubules (39–42). However, they are not suitable for patients with advanced renal insufficiency. However, liraglutide is so unique that it binds plasma proteins extensively (98%) and is metabolized similarly to large proteins, with no specific organ identified as the main elimination site. The kidneys excrete only a small portion (6%). Liraglutide was covered by all the GLP-1RA studies we included (19, 22, 25). In our study, GLP-1 RAs did not differ from placebo concerning serious renal-related AEs or renal death in patients with severe CKD. GLP-1 RAs ranked better than DPP-4i and placebo, but lower than placebo SGLT2i in SUCRA rankings. In the SUCRA ranking, the effect of GLP-1 RAs in reducing HbA1c was second only to DPP-4i and better than SGLT2i and placebo. Notably, Idorn et al. (22) found that using GLP-1 RAs in patients with ESRD significantly reduced the basal insulin dose during treatment without worsening glycemic control. This suggests that GLP-1 RAs may be suitable for patients with ESRD, although larger studies are needed to confirm this finding. The 2020 KDIGO guidelines (6) recommend using long-acting GLP-1RA for T2DM patients with CKD who have not achieved individualized glycemic targets with metformin and SGLT2i or are unable to take these agents. We believe that GLP-1 RAs can be added to maintain blood glucose levels when SGLT2i cannot achieve the blood glucose targets in stage 3 B and stage 4 CKD patients. It not only benefits from the reno-protective effects of SGLT2i but also ensures the control of blood glucose by GLP-1 RAs.
In the subgroup analysis of this study, it was found that DPP-4i significantly reduced HbA1c and did not increase the risk of hypoglycemia in dialysis patients compared with placebo. The mechanism of DPP-4i includes stimulation of insulin and glucagon, indicating that its combination with other hypoglycemics can further reduce HbA1c. Some researchers have recently raised doubts about HbA1c as a blood glucose indicator. HbA1c is a long-term biomarker that reflects blood glucose levels over the lifespan of red blood cells. Notably, CKD is associated with diseases such as oxidative stress, metabolic acidosis, and inflammation, which, in addition to hyperglycemia, may simultaneously promote the formation of advanced glycation end products, leading to elevated HbA1c levels (43).
On the contrary, due to erythropoiesis stimulants or iron replacement therapy, blood transfusion, and anemia, the survival or age of erythrocytes is shortened, and HbA1c is lowered (43, 44). These effects were most significant in patients with advanced CKD, especially those undergoing dialysis. Despite some limitations, no better biomarkers have been found to replace HbA1c. Therefore, the 2020 KDIGO guidelines (6) recommend that HbA1c blood glucose monitoring is appropriate for all adults, children, and patients with renal failure treated by dialysis or renal transplantation. Therefore, when evaluating the hypoglycemic effect by reducing HbA1c in patients, we believe that DPP-4I is better than the other two drugs and is equally effective in dialysis patients.
To our knowledge, this is the first network meta-analysis of the efficacy and safety of three novel hypoglycemics in T2DM patients with severe CKD. Previous meta-analyses have compared the benefits of SGLT2i and GLP-1RAs in patients with CKD (eGFR < 60 ml/min/1.73 m2) (45). Two meta-analyses have assessed the efficacy and safety of only DPP-4i in T2DM patients with moderate to severe renal impairment (46, 47). This meta-analysis provides evidence for this under-lighted area. Nevertheless, this study had several limitations. First, the patients in this study were all from subgroups of RCTs included; patients with severe CKD might be less randomized. Second, only DPP-4i was evaluated in the subgroup analysis of dialysis patients because of little data, but the efficacy of GLP-1 and SGLT2i in dialysis patients was not evaluated. Third, since the included RCTs did not compare the three drugs head-to-head, this network meta-analysis did not test the inconsistency by comparing the results of indirect and direct comparisons. Therefore, the findings of this study should be interpreted with caution. Perhaps what we can expect is more head-to-head clinical trials in the future.
In conclusion, we believe that T2DM patients with severe CKD may benefit from SGLT2i. SGLT2i can reduce the incidence of serious renal-related AEs or renal death, as well as severe side effects, and has a positive effect on the patient’s renal function and survival, even for only CKD patients can also be considered. If blood glucose control is poor, GLP-1 RAs can be administered as a supplement, but this is not recommended for patients with end-stage renal disease because there is insufficient evidence to support its safety and efficacy in this population. In dialysis patients, DPP-4i can assist blood glucose control, reduce insulin dosage, and reduce the risk of hypoglycemia.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
YL, YH and XH contributed equally to this work. YL, YH, XH, WG and YM conceived and designed the study. KC and BL screened and extracted data. YL, YH and XH performed the statistical analyses. All of the authors contributed to the interpretation of data. YL, YH and XH drafted the manuscript. All of the authors revised it critically for important intellectual content. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1003263/full#supplementary-material
1. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the international diabetes federation diabetes atlas, 9(Th) edition. Diabetes Res Clin Pract (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
2. Kidney FN, Kidney D. Kdigo 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl (2013) 3(1):1–150. doi: 10.1038/kisup.2012.63-77
3. Herrington WG, Nye HJ, Aung T. Metformin use in chronic kidney disease: New evidence to guide dosing. QJM Monthly J Assoc Phys (2013) 106(11):1059–61. doi: 10.1093/qjmed/hct155
4. Schweizer A, Dejager S. Experience with vildagliptin in patients ≥75 years with type 2 diabetes and moderate or severe renal impairment. Diabetes Ther res Treat Educ Diabetes Related Disord (2013) 4(2):257–67. doi: 10.1007/s13300-013-0027-x
5. McGill JB, Sloan L, Newman J, Patel S, Sauce C, von Eynatten M, et al. Long-term efficacy and safety of linagliptin in patients with type 2 diabetes and severe renal impairment: A 1-year, randomized, double-blind, placebo-controlled study. Diabetes Care (2013) 36(2):237–44. doi: 10.2337/dc12-0706
6. Kidney Disease: Improving Global Outcomes (KDIGO) Diabetes Work Group. Kdigo 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int (2020) 98(4s):S1–s115. doi: 10.1016/j.kint.2020.06.019
7. Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, et al. Dapagliflozin in patients with chronic kidney disease. New Engl J Med (2020) 383(15):1436–46. doi: 10.1056/NEJMoa2024816
8. Abe M, Higuchi T, Moriuchi M, Okamura M, Tei R, Nagura C, et al. Efficacy and safety of saxagliptin, a dipeptidyl peptidase-4 inhibitor, in hemodialysis patients with diabetic nephropathy: A randomized open-label prospective trial. Diabetes Res Clin Pract (2016) 116:244–52. doi: 10.1016/j.diabres.2016.04.034
9. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The prisma extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann Internal Med (2015) 162(11):777–84. doi: 10.7326/m14-2385
10. Veroniki AA, Vasiliadis HS, Higgins JP, Salanti G. Evaluation of inconsistency in networks of interventions. Int J Epidemiol (2013) 42(1):332–45. doi: 10.1093/ije/dys222
11. White IR, Barrett JK, Jackson D, Higgins JP. Consistency and inconsistency in network meta-analysis: Model estimation using multivariate meta-regression. Res Synthesis Methods (2012) 3(2):111–25. doi: 10.1002/jrsm.1045
12. Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta-analysis in stata. PloS One (2013) 8(10):e76654. doi: 10.1371/journal.pone.0076654
13. White I. Multivariate random-effects meta-analysis. Stata J (2009) 9:40–56. doi: 10.1177/1536867X0900900103
14. White I. Multivariate random-effects meta-regression: Updates to mvmeta. Stata J (2011) 11:255–70. doi: 10.1177/1536867X1101100206
15. Higgins JP, Savović J, Page MJ, Elbers RJ, Sterne JA. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, editors. Cochrane handbook for systematic reviews of interventions, vol. Chichester (UK: John Wiley & Sons (2011). p. 205–28.
16. Arjona Ferreira JC, Corry D, Mogensen CE, Sloan L, Xu L, Golm GT, et al. Efficacy and safety of sitagliptin in patients with type 2 diabetes and esrd receiving dialysis: A 54-week randomized trial. Am J Kidney Dis Off J Natl Kidney Foundation (2013) 61(4):579–87. doi: 10.1053/j.ajkd.2012.11.043
17. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ, et al. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: A randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol (2014) 2(5):369–84. doi: 10.1016/s2213-8587(13)70208-0
18. Chacra A, Gantz I, Mendizabal G, Durlach L, O'Neill EA, Zimmer Z, et al. A randomised, double-blind, trial of the safety and efficacy of omarigliptin (a once-weekly dpp-4 inhibitor) in subjects with type 2 diabetes and renal impairment. Int J Clin Pract (2017) 71(6):e12955. doi: 10.1111/ijcp.12955
19. Davies MJ, Bain MC, Atkin SL, Rossing P, Scott D, Shamkhalova MS, et al Efficacy and safety of liraglutide versus placebo as add-on to glucose-lowering therapy in patients with type 2 diabetes and moderate renal Impairment (Lira-renal): A randomized clinical trial. Diabetes care (2016) 39(2):222–30. doi: 10.2337/dc14-2883
20. Heerspink HJL, Sjöström CD, Jongs N, Chertow GM, Kosiborod M, Hou FF, et al. Effects of dapagliflozin on mortality in patients with chronic kidney disease: A pre-specified analysis from the dapa-ckd randomized controlled trial. Eur Heart J (2021) 42(13):1216–27. doi: 10.1093/eurheartj/ehab094
21. Herrington WG, Preiss D, Haynes R, von Eynatten M, Staplin N, Hauske SJ, et al. The potential for improving cardio-renal outcomes by sodium-glucose Co-Transporter-2 inhibition in people with chronic kidney disease: A rationale for the empa-kidney study. Clin Kidney J (2018) 11(6):749–61. doi: 10.1093/ckj/sfy090
22. Idorn T, Knop FK, Jørgensen MB, Jensen T, Resuli M, Hansen PM, et al. Safety and efficacy of liraglutide in patients with type 2 diabetes and end-stage renal disease: An investigator-initiated, placebo-controlled, double-blind, parallel-group, randomized trial. Diabetes Care. (2015) 39(2):202–13. doi: 10.2337/dc15-1025
23. Ito M, Abe M, Okada K, Sasaki H, Maruyama N, Tsuchida M, et al. The dipeptidyl peptidase-4 (Dpp-4) inhibitor vildagliptin improves glycemic control in type 2 diabetic patients undergoing hemodialysis. Endocrine J (2011) 58(11):979–87. doi: 10.1507/endocrj.ej11-0025
24. Kothny W, Shao Q, Groop PH, Lukashevich V. One-year safety, tolerability and efficacy of vildagliptin in patients with type 2 diabetes and moderate or severe renal impairment. Diabetes Obes Metab (2012) 14(11):1032–9. doi: 10.1111/j.1463-1326.2012.01634.x
25. Mann JFE, Orsted DD, Brown-Frandsen K, Marso SP, Poulter NR, Rasmussen S, et al. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med (2017) 377(9):839–48. doi: 10.1056/NEJMoa1616011
26. Munch M, Meyer L, Hannedouche T, Kunz K, Alenabi F, Winiszewski P, et al. Effect of adding vildagliptin to insulin in haemodialysed patients with type 2 diabetes: The vilddial study, a randomized, multicentre, prospective study. Diabetes Obes Metab (2020) 22(6):978–87. doi: 10.1111/dom.13988
27. Neuen BL, Ohkuma T, Neal B, Matthews DR, de Zeeuw D, Mahaffey KW, et al. Cardiovascular and renal outcomes with canagliflozin according to baseline kidney function: Data from the canvas program. Circulation (2018) 138(15):1537–50. doi: 10.1161/circulationaha.118.035901
28. Nowicki M, Rychlik I, Haller H, Warren ML, Suchower L, Gause-Nilsson I. Saxagliptin improves glycaemic control and is well tolerated in patients with type 2 diabetes mellitus and renal impairment. Diabetes Obes Metab (2011) 13(6):523–32. doi: 10.1111/j.1463-1326.2011.01382.x
29. Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med (2019) 380(24):2295–306. doi: 10.1056/NEJMoa1811744
30. Udell JA, Bhatt DL, Braunwald E, Cavender MA, Mosenzon O, Steg PG, et al. Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes and moderate or severe renal impairment: Observations from the savor-timi 53 trial. Diabetes Care (2015) 38(4):696–705. doi: 10.2337/dc14-1850
31. Wanner C, Lachin JM, Inzucchi SE, Fitchett D, Mattheus M, George J, et al. Empagliflozin and clinical outcomes in patients with type 2 diabetes mellitus, established cardiovascular disease, and chronic kidney disease. Circulation (2018) 137(2):119–29. doi: 10.1161/circulationaha.117.028268
32. Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. Sglt2 inhibitors and the diabetic kidney. Diabetes Care (2016) 39 Suppl 2:S165–71. doi: 10.2337/dcS15-3006
33. Tsimihodimos V, Filippatos TD, Filippas-Ntekouan S, Elisaf M. Renoprotective effects of Sglt2 inhibitors: Beyond glucose reabsorption inhibition. Curr Vasc Pharmacol (2017) 15(2):96–102. doi: 10.2174/1570161114666161007163426
34. Ferrannini E. Sodium-glucose Co-transporters and their inhibition: Clinical physiology. Cell Metab (2017) 26(1):27–38. doi: 10.1016/j.cmet.2017.04.011
35. Vallon V, Thomson SC. Targeting renal glucose reabsorption to treat hyperglycaemia: The pleiotropic effects of Sglt2 inhibition. Diabetologia (2017) 60(2):215–25. doi: 10.1007/s00125-016-4157-3
36. Wanner C. Empa-reg outcome: The nephrologist's point of view. Am J Med (2017) 130(6s):S63–72. doi: 10.1016/j.amjmed.2017.04.007
37. Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI. Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int (2018) 94(1):26–39. doi: 10.1016/j.kint.2017.12.027
38. Heerspink HJL. Sodium glucose Co-transporter 2 inhibition: A new avenue to protect the kidney. Nephrol dialysis Transplant Off Publ Eur Dialysis Transplant Assoc - Eur Renal Assoc (2019) 34(12):2015–7. doi: 10.1093/ndt/gfz033
39. Nakata H, Sugitani S, Yamaji S, Otsu S, Higashi Y, Ohtomo Y, et al. Pancreatitis with pancreatic tail swelling associated with incretin-based therapies detected radiologically in two cases of diabetic patients with end-stage renal disease. Internal Med (2012) 51(21):3045–9. doi: 10.2169/internalmedicine.51.7876
40. Scheen AJ. Pharmacokinetics and clinical use of incretin-based therapies in patients with chronic kidney disease and type 2 diabetes. Clin Pharmacokinet (2015) 54(1):1–21. doi: 10.1007/s40262-014-0198-2
41. Christensen M, Knop FK, Vilsbøll T, Holst JJ. Lixisenatide for type 2 diabetes mellitus. Expert Opin Investig Drugs (2011) 20(4):549–57. doi: 10.1517/13543784.2011.562191
42. Filippatos TD, Elisaf MS. Effects of glucagon-like peptide-1 receptor agonists on renal function. World J Diabetes (2013) 4(5):190–201. doi: 10.4239/wjd.v4.i5.190
43. Little RR, Rohlfing CL, Tennill AL, Hanson SE, Connolly S, Higgins T, et al. Measurement of Hba(1c) in patients with chronic renal failure. Clinica chimica acta; Int J Clin Chem (2013) 418:73–6. doi: 10.1016/j.cca.2012.12.022
44. Tarim O, Küçükerdoğan A, Günay U, Eralp O, Ercan I. Effects of iron deficiency anemia on hemoglobin A1c in type 1 diabetes mellitus. Pediatr Int Off J Japan Pediatr Soc (1999) 41(4):357–62. doi: 10.1046/j.1442-200x.1999.01083.x
45. Yamada T, Wakabayashi M, Bhalla A, Chopra N, Miyashita H, Mikami T, et al. Cardiovascular and renal outcomes with sglt-2 inhibitors versus glp-1 receptor agonists in patients with type 2 diabetes mellitus and chronic kidney disease: A systematic review and network meta-analysis. Cardiovasc Diabetol (2021) 20(1):14. doi: 10.1186/s12933-020-01197-z
46. Chen M, Liu Y, Jin J, He Q. The efficacy and safety of dipeptidyl peptidase-4 inhibitors for treatment of type 2 diabetes mellitus patients with severe renal impairment: A meta-analysis. Renal fail (2016) 38(4):581–7. doi: 10.3109/0886022x.2016.1149682
Keywords: glucagon-like peptidyl-1 receptor agonists, dipeptidyl peptidase-4 inhibitors, sodium-glucose cotransporter two inhibitors, severe chronic kidney disease, diabetes mellitus
Citation: Li Y, Hu Y, Huyan X, Chen K, Li B, Gu W and Mu Y (2022) Comparison of efficacy and safety of three novel hypoglycemic agents in patients with severe diabetic kidney disease: A systematic review and network meta-analysis of randomized controlled trials. Front. Endocrinol. 13:1003263. doi: 10.3389/fendo.2022.1003263
Received: 26 July 2022; Accepted: 29 September 2022;
Published: 24 October 2022.
Edited by:
Sanbao Chai, International Hospital, Peking University, ChinaReviewed by:
Shanshan Wu, Affiliated Beijing Friendship Hospital, Capital Medical University, ChinaCopyright © 2022 Li, Hu, Huyan, Chen, Li, Gu and Mu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiming Mu, bXV5aW1pbmdAMzAxaG9zcGl0YWwuY29tLmNu; Weijun Gu, Z3V3ZWlqdW4zMDFAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.