
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Endocrinol. , 12 October 2022
Sec. Neuroendocrine Science
Volume 13 - 2022 | https://doi.org/10.3389/fendo.2022.1000758
This article is part of the Research Topic Neuroendocrine Research in Health and Disease Volume II View all 7 articles
Non-invasive transcutaneous auricular vagus nerve stimulation (taVNS) as a newly developed technique involves stimulating the cutaneous receptive field formed by the auricular branch of the vagus nerve in the outer ear, with resulting activation of vagal connections to central and peripheral nervous systems. Increasing evidence indicates that maladaptive neural plasticity may underlie the pathology of several pediatric neurodevelopmental and psychiatric disorders, such as autism spectrum disorder, attention deficit hyperactivity disorder, disruptive behavioral disorder and stress-related disorder. Vagal stimulation may therefore provide a useful intervention for treating maladaptive neural plasticity. In the current review we summarize the current literature primarily on therapeutic use in adults and discuss the prospects of applying taVNS as a therapeutic intervention in specific pediatric neurodevelopmental and other psychiatric disorders. Furthermore, we also briefly discuss factors that would help optimize taVNS protocols in future clinical applications. We conclude from these initial findings that taVNS may be a promising alternative treatment for pediatric disorders which do not respond to other interventions.
Neural plasticity is a key mechanism involved in childhood brain development which both regulates and optimizes the function of neural circuitry controlling cognition and behavior. It can also help the brain to recover from injury (1–3). Maladaptive neuroplasticity may underlie the pathology of neurodevelopmental and other psychiatric disorders, such as autism spectrum disorder (ASD), anxiety, and depression (4, 5). Non-invasive brain stimulation (NIBS) techniques are increasingly used to promote neurological or psychiatric rehabilitation by modulating neural plasticity (6). In the last two decades, transcutaneous auricular vagus nerve stimulation (taVNS) has in particular attracted attention in clinical applications since Ventureyra (7) first proposed it as a non-invasive alternative to vagal nerve stimulation (VNS) for treatment of epilepsy (7). To date, taVNS has been used to help alleviate symptoms not only of epilepsy but also splanchnic diseases (e.g., heart failure) (8), stroke (9, 10) and tinnitus (11, 12) as well as some psychiatric disorders (e.g., major depressive disorder (MDD) (13–15). Increasing evidence from animal studies and clinical trials primarily in adult humans suggest that the therapeutic effects of invasive and noninvasive VNS may stem from its role in modulating maladaptive brain plasticity (10, 15–18). This may particularly be particular relevance in the case in developing child and adolescent brains given evidence from brain imaging that they are more highly plastic relative to adults (2, 19–21). Indeed, children and adolescents show accelerated neural plasticity compared to adults after brain stimulation (22).
There is a high prevalence of ASD (around 1%), attention-deficit/hyperactivity disorder (ADHD, 4%), disruptive behavioral disorder (DBD, 6.1%), obsessive-compulsive disorder (OCD, between 2% ~ 4%), depression and anxiety-related disorders (around 5%) in pediatric populations worldwide (23–26). Furthermore, overlapping clinical behavioral manifestations across these disorders and comorbid conditions are often reported (27–29). For example, social dysfunction is often seen in ASD, ADHD and obsessive-compulsive disorder (OCD) (30–32). Impulsivity and inattention are not only reported in ADHD, but also ASD and DBD (33, 34). The high frequency of comorbidities could be a result of shared pathophysiology and associated mechanisms. Importantly,, taVNS has been shown to have modulatory effects on cortical and subcortical brain regions that are associated with the neuropathology of these disorders and to help regulate some social-emotional functions that are impaired in them (35–39). These findings support the use of taVNS as a promising non-pharmaceutical treatment to mitigate symptoms of these disorders.
Currently, behavioral training is the most commonly used intervention technique for the aforementioned intractable neurodevelopmental and other psychiatric disorders that are prevalent during childhood/adolescence (40). For instance, language and social skill training are commonly used for children with ASD (41, 42). Additionally, cognitive behavioral therapy is frequently adopted as a treatment for depression (43). Intensive behavioral therapies may successfully improve behavioral outcomes in patients with these disorders by promoting adaptive plasticity in dysregulated neural circuitry (44, 45). However, these behavioral interventions are lengthy and time consuming, and a proportion of children fail to benefit. On the other hand, taVNS as a non-invasive technique has been recently reported to improve clinical outcomes in some intractable disorders, such as major depression disorder and post-traumatic stress disorder (46–49). In sum, these may suggest taVNS as a potential adjunctive non-invasive technique to help increase the benefit of behavioral interventions.
Here in the current review, we have therefore summarized current preliminary evidence for the effects of taVNS on different clinical behavioral manifestations targeting pediatric neurodevelopmental and other psychiatric disorders, including ASD, ADHD, OCD, DBD, depression and anxiety-related disorders and also briefly illustrate the underlying mechanisms of taVNS effects from the perspective of anatomical and neuroendocrine aspects of vagus nerve stimulation. In addition, we briefly discuss feasibility issues and several factors and that would help optimize taVNS protocols to improve therapeutic effects when applied in clinical situations in the future.
The vagus nerve is the tenth cranial nerve that starts at the level of the brainstem and establishes a mutual connection between the brain and major body organs (Figure 1A). Afferent fibers of the vagus nerve send sensory (visceral and somatic) impulses to the vagal nuclei connections, the nucleus of the solitary tract (NST) and spinal nucleus of the trigeminal nerve (SNT), located in the medulla. Components of sensory information are further relayed to higher order brain regions (e.g. hippocampus, amygdala, thalamus and neocortex), thereby allowing the vagus nerve to modulate activity in widespread subcortical and cortical brain areas (50, 51). Thus, signals generated in the vagus nerve have the potential to affect a broad range of brain functions (see Figure 1B, for more detailed information regarding the physiology of the vagus nerve see (52)).
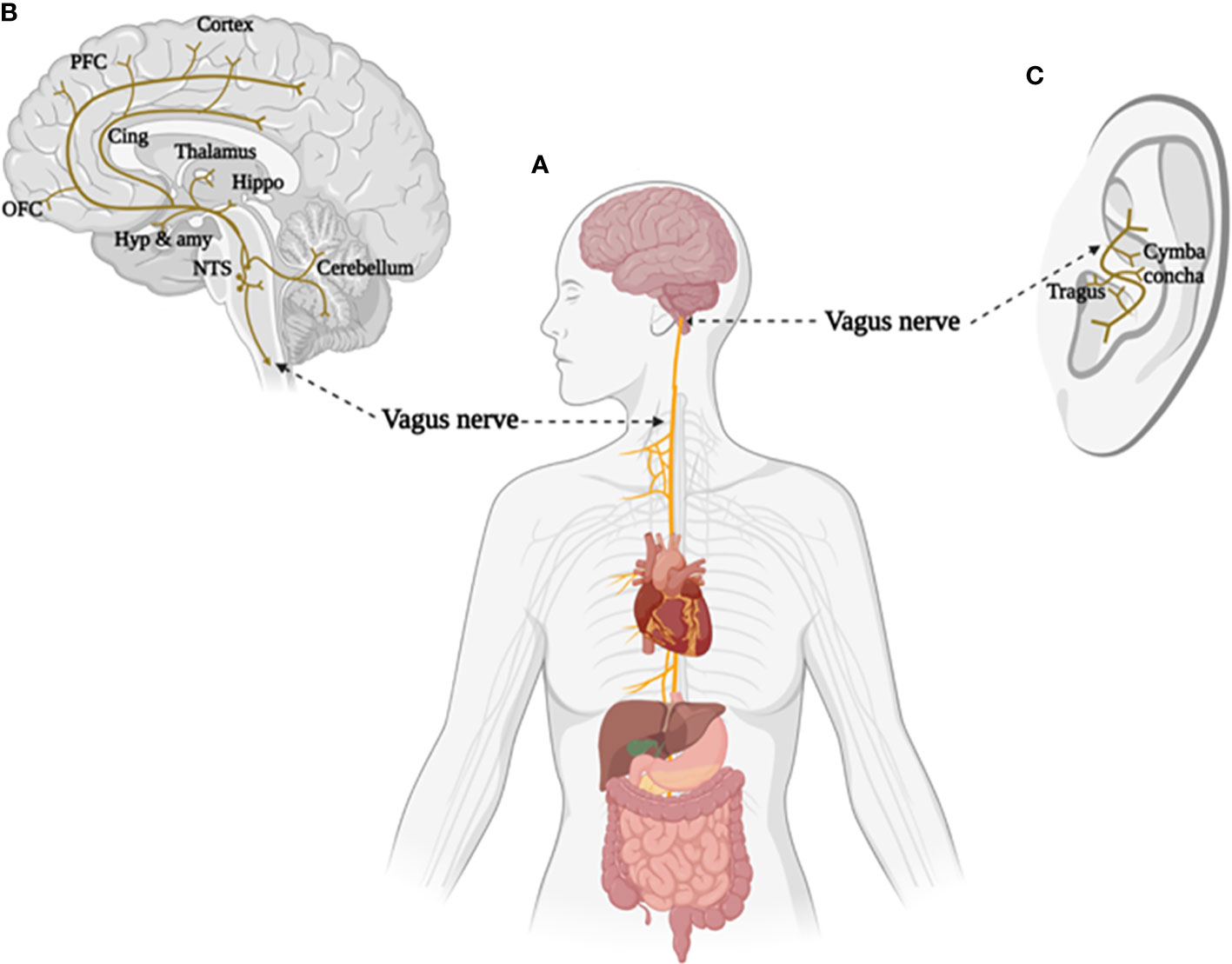
Figure 1 The brain and body projections of vagus nerve. (A) Illustration of the connection between brain and major body organs via the vagus nerve. (B) Areas of the brain involved in the afferent vagal pathway. Nucleus tractus solitarius (NTS), hypothalamus (Hyp), amygdala (amy), hippocampus (Hippo), cingulate cortex (Cing), orbital frontal cortex (OFC), and prefrontal cortex (PFC). (C) Distribution of the vagus nerve in the external ear. Created with BioRender.com.
Interest in artificial VNS for therapeutic purpose has increased given the crucial role that the vagus plays in determining brain-body interactions. Evidence from animal models and clinical studies has demonstrated a potential for invasive VNS in modulating neural and physiological changes contributing to a number of chronic diseases (53, 54). Therefore, a large variety of disorders, such as epilepsy, migraine, inflammation maladaptive and metabolic syndrome are possible potential targets for VNS therapy (55). Anatomical evidence from humans and other animal species indicates that the tragus, concha, and cymba concha in the external auditory canal are the only places in the body with a cutaneous afferent vagus nerve distribution, making non-invasive transcutaneous stimulation of the vagus nerve possible (51, 56) (Figure 1C).
A number of brain imaging studies have shown that taVNS modulates brain function primarily by its direct afferent projections to specific brain structures, including the brainstem and other higher order relays of vagal afferents (visceral and somatic), such as the amygdala, hypothalamus and prefrontal cortex (50, 51, 57). Although the pathways by which taVNS exerts its various effects are still poorly understood, its potential for regulation of neurotransmission and promoting neuroplasticity is important in the context of neurodevelopmental and other psychiatric disorders. For instance, treatment effects of taVNS on stroke and tinnitus via its modulatory role in motor and sensory neural plasticity have been increasingly reported (9–12). Moreover, taVNS is also associated with the release of noradrenaline in the brain, as well as the inhibitory transmitter GABA, which potentially leads to VNS-mediated seizure reduction and antidepressant effects (58). Additionally, VNS inhibits excitatory glutamate release (59) and also increases the release of neurotrophic factors as well as stimulating cellular proliferation and neurogenesis in the brain, which correlate not only with antidepressant effects but also neuronal plasticity, memory, learning and cognitive processes (60).
Currently, taVNS has already been approved in Europe as a treatment for epilepsy and depression in 2020, for chronic pain in 2012 and for anxiety in 2019,and was also approved by the US Food and Drug Administration (FDA) for therapeutic use in depression and anxiety in 2006 (61, 62). Further, studies in healthy populations have demonstrated that taVNS can enhance cognitive performance (58) and brain-body functions (52), suggesting its potential therapeutic role in a number of disorders. We have therefore summarized the reported effects of taVNS on specific clinical symptoms in the following sections (also see in Table 1).
The common features of pediatric depressive disorders are pervasive sadness, irritability, or anhedonia, along with impairments in a range of cognitive domains such as episodic memory, emotion regulation, sustained attention and capacity for inhibition (98–102). Adolescence is a critical period for the development of depression, and the worldwide prevalence of any depressive disorder in this age group is 2.6% (24). However, around 40% of adolescents with depression do not respond to current psychotherapy or pharmacotherapy interventions and more innovative treatments are needed (103).
The effects of VNS on mood were first observed in patients with epilepsy, and subsequently it was approved for the treatment of refractory depression (104). Several studies have now also used taVNS as a noninvasive alternative of VNS, and found beneficial effects on mood in adult MDD patients (17, 49). Other studies have shown effects on clinical severity. For example, after one month of treatment, scores on the Hamilton Depression Rating Scale were significantly reduced in a taVNS compared to control group in adult MDD patients, and this was associated with increased default mode network functional connectivity under taVNS (13). Kraus and colleagues (38) found that taVNS compared to sham stimulation could decrease BOLD-signals in limbic brain areas and improve subjective well-being ratings (38). Indeed, a range of beneficial taVNS effects have now been reported in a number of clinical trials on MDD patients (14, 15, 17, 49, 105). Recently, evidence from healthy populations also indicates that a prolonged period of effort exertion with concurrent taVNS in comparison to sham stimulation could boost mood recovery, indicating that taVNS may help improve affect after a mood challenge (66).
Previous research has shown that emotion regulation deficits may play an important role in contributing to sustained sad mood in depressive patients (98, 106). In line with this, Koenig and colleagues reported that taVNS decreased attention to sad stimuli in adolescents with MDD when they performed in different emotion recognition tasks (68). Furthermore, in healthy subjects, taVNS reduced the ability to recognize sadness in dynamic bodily expressions (67). Similarly, a recent study indicates that participants receiving active taVNS, compared to sham, were better at using cognitive reappraisal strategy to down-regulate their response to negative emotional pictures (65). Moreover, taVNS could improve impaired cognitive flexibility in depressive patients by enhancing divergent thinking in healthy participants (63). Lack of pleasure (i.e., no interest in reaction to pleasurable stimuli or experiences and lack of anticipation of pleasure) is another main symptom of depression. One recent study demonstrated that acute taVNS facilitated reward-seeking by boosting invigoration, suggesting that taVNS may enhance pursuit of prospective rewards (64). Thus, all the above results suggest that taVNS could be a useful add-on to current therapies for depressive disorders (e.g., emotion regulation, cognitive flexibility, lack of pleasure) in pediatric as well as adult populations.
Anxiety disorders are among the most prevalent psychiatric conditions in children and adolescents worldwide but are often untreated in pediatric populations (107, 108). Excessive fear and anxiety are shared features of anxiety disorders, and uncontrollable and excessive worrying is a typical symptom of generalized anxiety disorder (GAD) in particular (109).
Burger and colleagues suggested that attentional engagement to threat and negative thought intrusions could be reduced by active taVNS in high trait worrying adults, providing preclinical support for future application of taVNS in the treatment of pediatric GAD (69, 70). Fear extinction is also a fundamental step in exposure therapies for anxiety and stress-related disorders (e.g., post-traumatic stress disorder (PTSD)) and low levels of vagal activity have been found in anxiety patients. Thus, VNS could be a non-pharmacological alternative for improving extinction memory (110–112). Studies have now shown that taVNS has beneficial effects on the modulation of fear extinction. For instance, extinction of declarative fear and explicit fear extinction learning could be facilitated by active taVNS compared to sham stimulation (71, 72). Additionally, it has been found that an extinction training together with taVNS resulted in rapid anxiolytic effects as well as an inhibition of fear potentiated startle response (73). Furthermore, associated memory performance and recollection-based memory can be enhanced by taVNS, suggesting its potential role in promoting extinction memory retention beyond its effect on extinction learning (74, 75). Additionally, it has also been found that neurobiological dysfunctions in post-traumatic stress disorder (PTSD), such as increased norepinephrine and sympathetic activity and abnormal inflammatory function, could be modulated by vagal activity (for more detailed discussion see (113)). Thus, taVNS may also be a potential anxiolytic intervention for treatment of pediatric as well as adult anxiety related disorders.
Social dysfunction is one of the key characteristics of ASD, and also occurs in ADHD and OCD (114, 115). Impaired emotion recognition is also often observed in these disorders (116). The symptoms of these disorders can often be severe and cause problems in everyday life as well as stress and economic burden for individuals and their families. So far, no effective and reliable treatment has been established for ASD in particular, and there is an urgent need for developing novel effective therapies.
Pre-clinical studies have demonstrated that taVNS can improve emotion recognition in healthy populations. For example, emotion recognition based on the eye region alone (76), whole faces (77) or body movement (67) is enhanced by active taVNS compared to sham stimulation. Further, taVNS can also generally increase emotion recognition in healthy adolescents independent of the type of task (68).Ventura-Bort and colleagues (81) reported that taVNS increased memory performance for emotional but not neutral materials and facilitated early attentional discrimination between emotional and neutral scenes. This may indicate a role of taVNS in increasing the salience of emotional stimuli. In line with this, taVNS has been recently reported to bias visual attention towards salient facial features, which are important for emotional recognition, and increasing endogenous release of the hypothalamic neuropeptide, oxytocin (78). Previously, it had already been found that plasma oxytocin concentrations in rats increased immediately after iVNS (117). A large number of studies have demonstrated as important role for oxytocin in facilitating social cognition and reward (118), taVNS effects on oxytocin may play a key role in helping to increase the salience of social cues (119). Some clinical trials in children with ASD have also shown it can improve social symptoms (120–122). Interoception, which is regarded as a fundamental basis for emotional processing, can also be improved under taVNS, with is evidenced by increased cardiac interoceptive accuracy in a heartbeat discrimination task (79). Furthermore, researchers also found that taVNS modulates attention to direct gaze (salient social cue) irrespective of the expressed emotion in a Rapid Serial Visual Presentation task (80). This finding suggests that taVNS may enhance perception of gaze direction, thereby increasing joint attention, making the observer more sensitive to socially relevant facial cues. In addition, a few studies have reported that massage, which increases vagal activity, can improve social responses and relationships between parents and children with ASD (123, 124). Overall, therefore, the above studies suggest that taVNS has a great potential in improving social cognition and responses (i.e., emotional processing, eye contact) in individuals with neurodevelopment disorders (for more details see Table 1).
The main features of ADHD are a persistent pattern of inattention and/or hyperactivity-impulsivity that interfere with functioning or development (125). However, impulsive behaviors are also seen in children with ASD and DBD and ones with oppositional defiant disorder (ODD) or conduct disorder (CD). Response inhibition deficits often relate to impulsivity, and together they greatly increase the likelihood that these children will develop antisocial personality disorder or substance use disorders and face incarceration in adulthood (126–128).
Several published meta-analyses of functional MRI studies on ADHD patients have demonstrated abnormal neural activity (129–131) in the executive control and dorsal attentional networks (132–134) which can also be activated by taVNS (50). Other preclinical studies in healthy populations have demonstrated beneficial effects of taVNS on behavioral and executive control, which further suggest its potential therapeutic application in disorders involving problematic impulse control (58). For example, Beste and colleagues (83) investigated the effects of taVNS on different aspects of inhibitory control (i.e., backward inhibition and response inhibition), and reported enhanced response control after active taVNS (83). Subsequently, Fisher and colleagues (2018) demonstrated that taVNS increased adaption to conflict in a response conflict task (the Simon task) (84). Furthermore, response selection during sequential action (85), automatic motor inhibition (135) and self-control in delay discounting (90) have all been reported to be improved by taVNS. It has also been suggested that the effects of taVNS on improving response control in the above studies may be due to its modulatory role in reducing resources required for cognitive control (89). Additionally, emerging evidence has shown that cognitive flexibility, general adaptive control and sustained attention can be enhanced by taVNS, indicating its potential use in alleviating inattention symptoms in pediatric as well as adult ADHD patients (87, 88).
Children with ASD often suffer from gastrointestinal problems which are associated with vagal activity (136–138). As shown in Figure 1, gastrointestinal tract dysfunction could be regulated by stimulating the vagus nerve which plays a key role in the interaction between brain and peripheral organs. Hong and colleagues (93) found that taVNS led to significant reduction in action potential frequency and increased action potential amplitude in the stomach compared to controls, and raised levels of gastrin 3 h after stimulation (93). Subsequently, Teckentrup and colleagues (64) reported that taVNS reduced gastric activity frequency without acutely altering resting energy expenditure (94). A recent study also indicated that gastric motility could be increased by high frequency taVNS (95). These three tentative studies indicate that taVNS may have potential treating of gastrointestinal dysregulations in ASD.
Additionally, a key feature of ASD is restricted verbal and nonverbal communication, and a failure in spoken language development (139). Two recent studies have shown that taVNS could improve novel orthography acquisition and enhance speech category learning in healthy populations. Thus taVNS as an adjunct to language training could be a novel therapeutic strategy for children with ASD (91, 92).
Comorbidity of depression and anxiety in youth is often reported in clinical situations (140, 141), and GAD and MDD have a high rate of comorbidity (142, 143). A possible explanation is that they share some diagnostic symptoms, such as sleeping problems, difficulty concentrating, being easily fatigued, and psychomotor agitation (144). It has been reported that taVNS treatment is effective in improving sleep quality and prolonging sleep duration in primary insomnia patients via the regulation of a broad brain network (i.e., default mode network, salience network and sensorimotor network) (96, 97, 145, 146). He and colleagues (97) have also reported that 4 weeks of taVNS treatment improved chronic insomnia symptoms by decreasing Pittsburgh Sleep Quality Index (PSQI) and Flinders Fatigue Scale (FFS) scores, and increasing the reduced neuroexcitability of the dorsolateral prefrontal cortex. Altered dorsolateral prefrontal cortex excitability was associated with symptom improvements and may therefore predict the efficacy of taVNS treatment effects. Together, this preliminary evidence indicates that taVNS may be expected to play an active role in the treatment sleep problems common in patients with depression and anxiety-related disorders as well as in ASD.
Furthermore, auricular electroacupuncture (EA) on vagally innervated regions, which can mimic taVNS, is reported to be effective in treatment of insomnia and relief of acute and chronic pain as well (147). Recently, Li and colleagues found that taVNS combined with cranial EA can be applied for the treatment of depression with chronic pain (148).
In sum, therefore taVNS may represent a potential therapeutic intervention for a number of different clinical behavioral manifestations targeting pediatric neurodevelopmental and other psychiatric disorders, including ASD, ADHD, OCD, DBD, depression and anxiety-related disorders (see Figure 2).

Figure 2 Illustration of the potential effects of taVNS on clinical symptoms and corresponding disorders. Depressive disorder (DD), Generalized anxiety disorder (GAD), Post-traumatic stress disorder (PTSD), Obsessive-compulsive disorder (OCD), Autism spectrum disorder (ASD), Conduct disorder (CD), Attention-deficit/hyperactivity disorder (ADHD), and Oppositional defiant disorder (ODD).
Anatomical evidence indicates that the external ear is the only part of our body where the vagus nerve has a peripheral termination (51), and that taVNS produces its functional effects by stimulation of the auricular branch of the vagus nerve (ABVN) (52). Therefore, both the auricular anatomy of vagus nerve and its corresponding physiological properties influence appropriate localization and stimulation parameters for taVNS devices (149), and in turn affect the safety and effectiveness of this technique. Here we detail some of the key factors that need to be considered for optimizing taVNS application protocols in clinical pediatric cases.
The cymba concha (100% innervated by ABVN) and tragus (45% innervated by ABVN) are the two most frequently chosen auricular regions in taVNS studies (149). However, there is some controversy regarding the optimal positions in the ear for attachment of electrodes for taVNS (150, 151). Notably, it is essential to confirm that it is the vagus nerve rather than other auricular nerves (great auricular nerve, auriculotemporal nerve and lesser occipital nerve) which is activated via taVNS. Evidence from an fMRI study has demonstrated that stimulation of cymba concha induced the strongest activation of the NTS, which is the recipient of most afferent vagal projections located in the brainstem, compared to the ear canal, inner tragus and earlobe (57). Additionally, stimulating the inner tragus relative to the earlobe demonstrated increased activation in brain regions receiving projections from the brainstem (50).The tragus may also have some practical advantages over the cymba concha (150) given that it appears to be easier to apply electrical stimulation by attaching a clip electrode to the tragus rather than by inserting or affixing electrodes to the concha.
Importantly, the current knowledge of auricular vagal nerve anatomy needs to be extended by more anatomical studies on the human ear since to date there is only one dissection study performed on 7 German cadavers (14 ears) (56). Optimal localizations of electrodes need to be informed by more precise future studies.
The left ear has been favored most in taVNS studies since it is thought to avoid any risk of incurring possible cardiac arrhythmic effects associated with activation of efferent vagal fibers connected to the right ear (152). However, a study has reported that stimulation of right ear has more beneficial effects on the modulation of heart rate variability (HRV) when compared to left ear (153). A systematic review has also concluded that right-ear stimulation does not increase the risk of aversive effects (154). In addition, bilateral taVNS has been used in a number of studies (64, 155–158) with no obvious adverse events being reported. Currently, studies on the neurophysiological effects underlying different stimulation sites are scarce and more evidence should be provided in future studies, particularly in terms of establishing potential risks in pediatric populations.
The vagal nerve consists of different types of fibers sub-serving specific functions. The myelinated A-fibers which convey somatic afferent information are supposed to be the main target for taVNS (159). Consideration of the signaling properties of Aβ fibers which exclusively send somatic and touch impulses to the central nervous system should be the main focus when deciding optimal stimulation patterns for taVNS. A relatively high frequency of 20-25Hz and short pulse widths are able to recruit thick Aβ fibers (6-12 mm), resulting in activation of the parasympathetic system, while low frequency of 0-0.5Hz and elongated pulse widths are required to stimulate thin fibers, such as myelinated Aδ (1-5 mm) or non-myelinated C fibers (0.4-2 mm), resulting more in activation of the sympathetic system (149).
Currently, there is no consensus on stimulus parameter settings in the taVNS field (61). Variable combinations of frequency, pulse width and intensity have been used given that taVNS devices have been used in a wide range of applications in both clinical and healthy populations (58, 160–162). Although several studies have been carried out to establish optimal stimulation parameters for VNS (163–165), only one has systematically investigated the effects of varying parameters of taVNS (pulse width: 0.1 ms, 0.2 ms, 0.5 ms; frequency: 1 Hz, 10 Hz, 25 Hz) in 20 healthy individuals, and concluded that a combination of 0.5 ms pulse width and 10 Hz frequency induced the greatest effects on heart rate (166). Generally, frequencies of 25 Hz or 20 Hz combined with pulse widths of 0.25 – 1 ms have most commonly been used in previous clinical and preclinical studies (154, 162). In addition, stimulation intensity is often fixed at 0.5mA (37, 72, 82, 83, 167), but in other cases is tailored to individuals' sensitivity/tolerance (50, 57, 153, 168, 169). Furthermore, use of alternating on and off periods of stimulation every 30 s have often been adopted in taVNS procedures to help reduce habituation (63, 77, 85, 88, 170, 171). Overall, therefore, stimulation parameters for taVNS devices still need to be optimized by future studies, particularly for use in pediatric populations.
Although several studies have tried to investigate the underlying neural mechanisms of taVNS effects, inconsistent findings have been observed due to the variations among stimulation protocols and participants (61). Consequently, no reliable biomarker(s) have been established which could indicate the efficacy of taVNS in general. At present, heart rate variability, some noradrenergic process markers, such as salivary alpha amylase (sAA), P300 amplitude of event-related potentials (ERPs) and pupil dilation are mostly recorded to demonstrate effective vagal activation (for details see review from (172)). However, given the failure of observing increased noradrenergic activity in active taVNS compared to sham stimulation in several studies (86, 167, 169, 173–175), we may need to consider cautiously three possible explanations for the null effects of taVNS. Firstly, suboptimal stimulation parameters. In these studies, pulse width and frequency were kept fixed, although intensity was flexible to adjusted according to individuals pain threshold. However, evidence from animal studies has indicated that it is a combination of intensity and pulse width rather than intensity alone that determines the activation of noradrenergic system (163). Closed-loop taVNS (CL-taVNS) where feedback from rapidly changing bio-signals is used to simultaneously adjust stimulation parameters may be a good choice in future studies to improve treatment efficacy for different disorders (176). Currently, only two CL-taVNS systems exist. The first of these is respiratory-gated auricular vagal afferent nerve stimulation (RAVANS), which works on the principle that inhalation induces transient inhibition of vagal nerve activity, and has shown therapeutic benefits on pain in patients with pelvic pain and migraine (177, 178). A second system is motor-activated auricular vagus nerve stimulation (MAAVNS) (179, 180), which uses electromyography (EMG) to record motor activities as an input signal to guide the administration of taVNS targeting specific motor activity. This is now applied in neonates for oromotor neurorehabilitation (181). In principle, other biomarkers may also be available for developing new CL-taVNS systems in future according to specific clinical purpose. Secondly, unlike invasive VNS that involves the simultaneous activation of afferent and efferent fibers of vagus nerve, taVNS that only stimulates a small branch of afferent vagus nerve fibers may be insufficient to effectively induce measurable central effects on noradrenergic network and the related biomarkers. Thirdly, the earlobe may not be an optimal site to apply sham stimulation given that earlobe stimulation may be associated with the release of other neurotransmitters (e.g., acetylcholine) that also have an impact on the biomarkers of noradrenergic activation (i.e., pupil size, sAA and cortisol). Alternatively, the ear scapha could be a potential site of sham stimulation (182), but central effects of stimulating this site need to be further investigated. Taken together, this also suggests more studies are required to help optimize protocols and stimulation parameters for obtaining reliable results in the future clinical studies.
A systematic review including 1322 participants from 51 studies reported that the most common side effects of taVNS were local skin irritation from electrode placement, headache and nasopharyngitis, although symptoms were usually mild and temporary. Moreover, frequency (Hz) and pulse width (ms) of stimulation were not correlated with the occurrence of side effects (154). In addition, taVNS has been used to treat oral feeding dysfunction in premature newborns (≤33 weeks) (181) and pediatric nephrotic syndrome in young patients (183) without observing adverse events related to stimulation. These suggest that applying taVNS in pediatric populations should represent little risk of significant side effects, although more future trials are included to assess potential short- or longer-term adverse effects.
Tolerance of wearing taVNS electrodes clips in young children, particularly those with ASD, is clearly an issue that needs consideration and it is important that electrode clips are both small and comfortable and that stimulation is not painful. Badran and colleagues have adopted a customized ear-clip the size of which is suitable for newborns to make the taVNS treatment possible (stimulation frequency at 25 Hz, pulse width at 500 μs, and current intensity at 0.1 mA below perceptual threshold) (181). Further, it has also been reported that taVNS could be successfully used in the treatment of pediatric nephrotic syndrome in young children and adolescents (4-17 years, at a frequency of 30 Hz with individual pulse widths of 300 μs, and pulse amplitude intensity was adjusted to the participant's tolerance) (183). However, future studies on children will need to consider use of positive reinforcement to increase cooperation behaviors, adopting CL-taVNS approaches and perhaps in some cases administering taVNS during natural sleep. It is worth noting that many research studies have been performed where young children with disorders are trained to tolerate procedures such as MRI, and to accept wearing EEG or fNIRS electrodes on their head.
Although research on taVNS has progressively increased in the past two decades, this field is still in its infancy. A number of precautions should be considered for establishing the potential use of taVNS protocols in pediatric populations: (1) More reliable biomarkers of taVNS need to be established, especially the causal link between taVNS and increased vagal activity. Currently, some noradrenergic related activities and parasympathetic functions have been proposed to be the candidates for indicating effective vagus nerve stimulation (i.e., pupil diameter, salivary alpha-amylase and heart rate variability), but inconsistent results have often been reported. Thus, stimulation sites and parameters should be further optimized to enhance treatment efficacy. (2) Long-term and acute effects of taVNS should be carefully investigated, especially for translational purpose, and potential long-term effects need to be investigated in clinical conditions. This information may also help for optimizing individualized treatment. (3) Treatment procedures and outcome measurements can focus on one clinical condition, which may help promote the validation of beneficial effects of the taVNS technique. (4) More preclinical evidence on taVNS effects from pediatric populations is required given that the majority of current studies are from adult populations. (5) The application and side effects of taVNS in young children with neurodevelopment and psychiatric disorders should be investigated in randomized clinical trials. Studies exploring treatment effect of taVNS in children are scarce, and although some have reported no adverse events during the treatment period (181, 183), more future work is urgently needed.
Early intervention is critical to enhancing the quality of life for any child who suffers from symptoms of neurodevelopmental or other psychiatric disorders. For neurodevelopmental disorders in particular there is considerable evidence supporting early therapeutic intervention as having the most effective outcome (184–187) reflecting the fact that developmental changes in the brain are most prevalent at this stage and capacity for brain plasticity changes in response to therapy is highest. In general, taVNS has a tremendous potential as a non-invasive adjunctive treatment targeting specific behavioral manifestations including social dysfunction, impulsivity and inattention, anxiety and fear, and depression in several pediatric neurodevelopment and psychiatric disorders, although standardized stimulation protocols (i.e., stimulation region and stimulation parameters) still need to be established.
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
This work was supported by the Fundamental Research Funds for the Central Universities, UESTC (grant number ZYGX2020J027 - WZ), China Postdoctoral Science Foundation (grant number 2018M643432 - WZ), Guangdong Basic and Applied Basic Research Foundation (grant number 2021A1515110511 - WZ), Natural Science Foundation of Sichuan Province (grant number 2022NSFSC1375 - WZ), National Natural Science Foundation of China (NSFC) (grant number 31530032 - KK), and Key Scientific and Technological projects of Guangdong Province (grant number 2018B030335001 - KK).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Jäncke L. The plastic human brain. Restor Neurol Neurosci (2009) 27:521–38. doi: 10.3233/RNN-2009-0519
2. Kolb B, Gibb R. Brain plasticity and behaviour in the developing brain. J Can Acad Child Adolesc Psychiatry (2011) 20:265–76.
3. Poldrack RA. Imaging brain plasticity: Conceptual and methodological issues— a theoretical review. NeuroImage (2000) 12:1–13. doi: 10.1006/nimg.2000.0596
4. Bourgeron T. From the genetic architecture to synaptic plasticity in autism spectrum disorder. Nat Rev Neurosci (2015) 16:551–63. doi: 10.1038/nrn3992
5. Cramer SC, Sur M, Dobkin BH, O’Brien C, Sanger TD, Trojanowski JQ, et al. Harnessing neuroplasticity for clinical applications. Brain (2011) 134:1591–609. doi: 10.1093/brain/awr039
6. George MS, Caulfield KA, Wiley M. Shaping plasticity with non-invasive brain stimulation in the treatment of psychiatric disorders: Present and future. Handb Clin Neurol (2022) 184:497–507. doi: 10.1016/B978-0-12-819410-2.00028-X
7. Ventureyra ECG. Transcutaneous vagus nerve stimulation for partial onset seizure therapy. Child’s Nerv. Syst. (2000) 16:101–2. doi: 10.1007/s003810050021
8. Jiang Y, Po SS, Amil F, Dasari TW. Non-invasive low-level tragus stimulation in cardiovascular diseases. Arrhythm Electrophysiol Rev (2020) 9:40–6. doi: 10.15420/aer.2020.01
9. Baig SS, Kamarova M, Ali A, Su L, Dawson J, Redgrave JN, et al. Transcutaneous vagus nerve stimulation (tVNS) in stroke: the evidence, challenges and future directions. Autonomic Neurosci (2022) 237:102909. doi: 10.1016/j.autneu.2021.102909
10. Capone F, Miccinilli S, Pellegrino G, Zollo L, Simonetti D, Bressi F, et al. Transcutaneous vagus nerve stimulation combined with robotic rehabilitation improves upper limb function after stroke. Neural Plasticity (2017) 2017:e7876507. doi: 10.1155/2017/7876507
11. Lehtimäki J, Hyvärinen P, Ylikoski M, Bergholm M, Mäkelä JP, Aarnisalo A, et al. Transcutaneous vagus nerve stimulation in tinnitus: a pilot study. Acta Oto-Laryngologica (2013) 133:378–82. doi: 10.3109/00016489.2012.750736
12. Shim HJ, Kwak MY, An Y-H, Kim DH, Kim YJ, Kim HJ. Feasibility and safety of transcutaneous vagus nerve stimulation paired with notched music therapy for the treatment of chronic tinnitus. J Audiol Otol (2015) 19:159–67. doi: 10.7874/jao.2015.19.3.159
13. Fang J, Egorova N, Rong P, Liu J, Hong Y, Fan Y, et al. Early cortical biomarkers of longitudinal transcutaneous vagus nerve stimulation treatment success in depression. NeuroImage: Clin (2017) 14:105–11. doi: 10.1016/j.nicl.2016.12.016
14. Hein E, Nowak M, Kiess O, Biermann T, Bayerlein K, Kornhuber J, et al. Auricular transcutaneous electrical nerve stimulation in depressed patients: a randomized controlled pilot study. J Neural Transm (2013) 120:821–7. doi: 10.1007/s00702-012-0908-6
15. Wang Z, Fang J, Liu J, Rong P, Jorgenson K, Park J, et al. Frequency-dependent functional connectivity of the nucleus accumbens during continuous transcutaneous vagus nerve stimulation in major depressive disorder. J Psychiatr Res (2018) 102:123–31. doi: 10.1016/j.jpsychires.2017.12.018
16. Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, et al. Reversing pathological neural activity using targeted plasticity. Nature (2011) 470:101–4. doi: 10.1038/nature09656
17. Liu J, Fang J, Wang Z, Rong P, Hong Y, Fan Y, et al. Transcutaneous vagus nerve stimulation modulates amygdala functional connectivity in patients with depression. J Affect Disord (2016) 205:319–26. doi: 10.1016/j.jad.2016.08.003
18. Huffman WJ, Subramaniyan S, Rodriguiz RM, Wetsel WC, Grill WM, Terrando N. Modulation of neuroinflammation and memory dysfunction using percutaneous vagus nerve stimulation in mice. Brain Stimul. (2019) 12:19–29. doi: 10.1016/j.brs.2018.10.005
19. Rapoport J, Gogtay N. Brain neuroplasticity in healthy, hyperactive and psychotic children: Insights from neuroimaging. Neuropsychopharmacol. : Off Publ Am Coll Neuropsychopharmacol (2008) 33:181–97. doi: 10.1038/sj.npp.1301553
20. Anderson V, Spencer-Smith M, Wood A. Do children really recover better? neurobehavioural plasticity after early brain insult. Brain : J Neurol (2011) 134:2197–221. doi: 10.1093/brain/awr103
21. Power JD, Schlaggar BL. Neural plasticity across the lifespan. WIREs Dev Biol (2017) 6:e216. doi: 10.1002/wdev.216
22. Brunoni AR, Nitsche MA, Bolognini N, Bikson M, Wagner T, Merabet L, et al. Clinical research with transcranial direct current stimulation (tDCS): Challenges and future directions. Brain Stimul (2012) 5:175–95. doi: 10.1016/j.brs.2011.03.002
23. James SC, Farrell LJ, Zimmer-Gembeck MJ. “Description and prevalence of OCD in children and adolescents.,”. Wiley Handb Obsessive Compulsive Disord John (2017), pp. 5–23. doi: 10.1002/9781118890233.ch1
24. Polanczyk GV, Salum GA, Sugaya LS, Caye A, Rohde LA. Annual research review: A meta-analysis of the worldwide prevalence of mental disorders in children and adolescents. J Child Psychol Psychiatry (2015) 56:345–65. doi: 10.1111/jcpp.12381
25. Boileau B. A review of obsessive-compulsive disorder in children and adolescents. Dialogues Clin Neurosci (2011) 13:401–11. doi: 10.31887/DCNS.2011.13.4/bboileau
26. Zeidan J, Fombonne E, Scorah J, Ibrahim A, Durkin MS, Saxena S, et al. Global prevalence of autism: A systematic review update. Autism Res (2022) 15:778–90. doi: 10.1002/aur.2696
27. Kaat AJ, Lecavalier L. Disruptive behavior disorders in children and adolescents with autism spectrum disorders: A review of the prevalence, presentation, and treatment. Res Autism Spectr Disord (2013) 7:1579–94. doi: 10.1016/j.rasd.2013.08.012
28. Mannion A, Leader G. Comorbidity in autism spectrum disorder: A literature review. Res Autism Spectr Disord (2013) 7:1595–616. doi: 10.1016/j.rasd.2013.09.006
29. Mulraney M, Schilpzand EJ, Hazell P, Nicholson JM, Anderson V, Efron D, et al. Comorbidity and correlates of disruptive mood dysregulation disorder in 6–8-year-old children with ADHD. Eur Child Adolesc Psychiatry (2016) 25:321–30. doi: 10.1007/s00787-015-0738-9
30. Anholt GE, Cath DC, van Oppen P, Eikelenboom M, Smit JH, van Megen H, et al. Autism and ADHD symptoms in patients with OCD: Are they associated with specific OC symptom dimensions or OC symptom severity? J Autism Dev Disord (2010) 40:580–9. doi: 10.1007/s10803-009-0922-1
31. Baribeau DA, Dupuis A, Paton TA, Hammill C, Scherer SW, Schachar RJ, et al. Structural neuroimaging correlates of social deficits are similar in autism spectrum disorder and attention-deficit/hyperactivity disorder: analysis from the POND network. Transl Psychiatry (2019) 9:1–14. doi: 10.1038/s41398-019-0382-0
32. Baribeau DA, Doyle-Thomas KAR, Dupuis A, Iaboni A, Crosbie J, McGinn H, et al. Examining and comparing social perception abilities across childhood-onset neurodevelopmental disorders. J Am Acad Child Adolesc Psychiatry (2015) 54:479–86.e1. doi: 10.1016/j.jaac.2015.03.016
33. Ogundele MO. Behavioural and emotional disorders in childhood: A brief overview for paediatricians. World J Clin Pediatr (2018) 7:9–26. doi: 10.5409/wjcp.v7.i1.9
34. Pisano S, Masi G. Recommendations for the pharmacological management of irritability and aggression in conduct disorder patients. Expert Opin Pharmacother (2020) 21:5–7. doi: 10.1080/14656566.2019.1685498
35. Cheng W, Rolls ET, Gu H, Zhang J, Feng J. Autism: reduced connectivity between cortical areas involved in face expression, theory of mind, and the sense of self. Brain (2015) 138:1382–93. doi: 10.1093/brain/awv051
36. Courchesne E, Campbell K, Solso S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Res (2011) 1380:138–45. doi: 10.1016/j.brainres.2010.09.101
37. Dietrich S, Smith J, Scherzinger C, Hofmann-Preiß K, Freitag T, Eisenkolb A, et al. A novel transcutaneous vagus nerve stimulation leads to brainstem and cerebral activations measured by functional MRI / funktionelle magnetresonanztomographie zeigt aktivierungen des hirnstamms und weiterer zerebraler strukturen unter transkutaner vagusnervstimulation. Biomed Eng / Biomedizinische Technik (2008) 53:104–11. doi: 10.1515/BMT.2008.022
38. Kraus T, Hösl K, Kiess O, Schanze A, Kornhuber J, Forster C. BOLD fMRI deactivation of limbic and temporal brain structures and mood enhancing effect by transcutaneous vagus nerve stimulation. J Neural Transm (2007) 114:1485–93. doi: 10.1007/s00702-007-0755-z
39. Townsend J, Westerfield M, Leaver E, Makeig S, Jung T-P, Pierce K, et al. Event-related brain response abnormalities in autism: evidence for impaired cerebello-frontal spatial attention networks. Cogn Brain Res (2001) 11:127–45. doi: 10.1016/S0926-6410(00)00072-0
40. Engineer CT, Hays SA, Kilgard MP. Vagus nerve stimulation as a potential adjuvant to behavioral therapy for autism and other neurodevelopmental disorders. J Neurodev Disord (2017) 9:20. doi: 10.1186/s11689-017-9203-z
41. Hampton LH, Kaiser AP. Intervention effects on spoken-language outcomes for children with autism: a systematic review and meta-analysis. J Intellectual Disability Res (2016) 60:444–63. doi: 10.1111/jir.12283
42. Zhang Q, Wu R, Zhu S, Le J, Chen Y, Lan C, et al. Facial emotion training as an intervention in autism spectrum disorder: A meta-analysis of randomized controlled trials. Autism Res (2021) 14:2169–82. doi: 10.1002/aur.2565
43. Oud M, de WL, Vermeulen-Smit E, Bodden D, Nauta M, Stone L, et al. Effectiveness of CBT for children and adolescents with depression: A systematic review and meta-regression analysis. Eur Psychiatry (2019) 57:33–45. doi: 10.1016/j.eurpsy.2018.12.008
44. Bryck RL, Fisher PA. Training the brain: Practical applications of neural plasticity from the intersection of cognitive neuroscience, developmental psychology, and prevention science. Am Psychol (2012) 67:87–100. doi: 10.1037/a0024657
45. Kilgard MP. Harnessing plasticity to understand learning and treat disease. Trends Neurosci (2012) 35:715–22. doi: 10.1016/j.tins.2012.09.002
46. Bremner JD, Gurel NZ, Jiao Y, Wittbrodt MT, Levantsevych OM, Huang M, et al. Transcutaneous vagal nerve stimulation blocks stress-induced activation of interleukin-6 and interferon-γ in posttraumatic stress disorder: A double-blind, randomized, sham-controlled trial. Brain Behav Immun Health (2020) 9:100138. doi: 10.1016/j.bbih.2020.100138
47. Fang J, Rong P, Hong Y, Fan Y, Liu J, Wang H, et al. Transcutaneous vagus nerve stimulation modulates default mode network in major depressive disorder. Biol Psychiatry (2016) 79:266–73. doi: 10.1016/j.biopsych.2015.03.025
48. Lamb DG, Porges EC, Lewis GF, Williamson JB. Non-invasive vagal nerve stimulation effects on hyperarousal and autonomic state in patients with posttraumatic stress disorder and history of mild traumatic brain injury: Preliminary evidence. Front Med (Lausanne) (2017) 4:124. doi: 10.3389/fmed.2017.00124
49. Tu Y, Fang J, Cao J, Wang Z, Park J, Jorgenson K, et al. A distinct biomarker of continuous transcutaneous vagus nerve stimulation treatment in major depressive disorder. Brain Stimul (2018) 11:501–8. doi: 10.1016/j.brs.2018.01.006
50. Badran BW, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, Brown JC, et al. Neurophysiologic effects of transcutaneous auricular vagus nerve stimulation (taVNS) via electrical stimulation of the tragus: A concurrent taVNS/fMRI study and review. Brain Stimul (2018) 11:492–500. doi: 10.1016/j.brs.2017.12.009
51. Butt MF, Albusoda A, Farmer AD, Aziz Q. The anatomical basis for transcutaneous auricular vagus nerve stimulation. J Anat (2020) 236:588–611. doi: 10.1111/joa.13122
52. Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, et al. Current directions in the auricular vagus nerve stimulation I – a physiological perspective. Front Neurosci (2019) 13:854. doi: 10.3389/fnins.2019.00854
53. Beekwilder JP, Beems T. Overview of the clinical applications of vagus nerve stimulation. J Clin Neurophysiol (2010) 27:130–8. doi: 10.1097/WNP.0b013e3181d64d8a
54. Oleson T. Auriculotherapy stimulation for neuro-rehabilitation. NeuroRehabilitation (2002) 17:49–62. doi: 10.3233/NRE-2002-17107
55. Groves DA, Brown VJ. Vagal nerve stimulation: a review of its applications and potential mechanisms that mediate its clinical effects. Neurosci Biobehav Rev (2005) 29:493–500. doi: 10.1016/j.neubiorev.2005.01.004
56. Peuker ET, Filler TJ. The nerve supply of the human auricle. Clin Anat (2002) 15:35–7. doi: 10.1002/ca.1089
57. Yakunina N, Kim SS, Nam E-C. Optimization of transcutaneous vagus nerve stimulation using functional MRI. Neuromodulation: Technol at Neural Interface (2017) 20:290–300. doi: 10.1111/ner.12541
58. Colzato L, Beste C. A literature review on the neurophysiological underpinnings and cognitive effects of transcutaneous vagus nerve stimulation: challenges and future directions. J Neurophysiol (2020) 123:1739–55. doi: 10.1152/jn.00057.2020
59. Chen S-P, Ay I, de Morais AL, Qin T, Zheng Y, Sadhegian H, et al. Vagus nerve stimulation inhibits cortical spreading depression. Pain (2016) 157:797–805. doi: 10.1097/j.pain.0000000000000437
60. Mercante B, Ginatempo F, Manca A, Melis F, Enrico P, Deriu F. Anatomo-physiologic basis for auricular stimulation. Med Acupuncture (2018) 30:141–50. doi: 10.1089/acu.2017.1254
61. Farmer AD, Strzelczyk A, Finisguerra A, Gourine AV, Gharabaghi A, Hasan A, et al. International consensus based review and recommendations for minimum reporting standards in research on transcutaneous vagus nerve stimulation (Version 2020). Front Hum Neurosci (2021) 0:568051. doi: 10.3389/fnhum.2020.568051
62. Yap JYY, Keatch C, Lambert E, Woods W, Stoddart PR, Kameneva T. Critical review of transcutaneous vagus nerve stimulation: Challenges for translation to clinical practice. Front Neurosci (2020) 14:284. doi: 10.3389/fnins.2020.00284
63. Colzato LS, Ritter SM, Steenbergen L. Transcutaneous vagus nerve stimulation (tVNS) enhances divergent thinking. Neuropsychologia (2018) 111:72–6. doi: 10.1016/j.neuropsychologia.2018.01.003
64. Neuser MP, Teckentrup V, Kühnel A, Hallschmid M, Walter M, Kroemer NB. Vagus nerve stimulation boosts the drive to work for rewards. Nat Commun (2020) 11:3555. doi: 10.1038/s41467-020-17344-9
65. De Smet S, Baeken C, Seminck N, Tilleman J, Carrette E, Vonck K, et al. Non-invasive vagal nerve stimulation enhances cognitive emotion regulation. Behav Res Ther (2021) 145:103933. doi: 10.1016/j.brat.2021.103933
66. Ferstl M, Teckentrup V, Lin WM, Kräutlein F, Kühnel A, Klaus J, et al. Non-invasive vagus nerve stimulation boosts mood recovery after effort exertion. psychol Med (2021) 1–11. doi: 10.1017/S0033291720005073
67. Steenbergen L, Maraver MJ, Actis-Grosso R, Ricciardelli P, Colzato LS. Recognizing emotions in bodies: Vagus nerve stimulation enhances recognition of anger while impairing sadness. Cognit Affect Behav Neurosci (2021) 21:1246–61. doi: 10.3758/s13415-021-00928-3
68. Koenig J, Parzer P, Haigis N, Liebemann J, Jung T, Resch F, et al. Effects of acute transcutaneous vagus nerve stimulation on emotion recognition in adolescent depression. Psychol Med (2021) 51:511–20. doi: 10.1017/S0033291719003490
69. Burger AM, van der Does W, Brosschot JF, Verkuil B. From ear to eye? no effect of transcutaneous vagus nerve stimulation on human pupil dilation: A report of three studies. Biol Psychol (2020) 152:107863. doi: 10.1016/j.biopsycho.2020.107863
70. Burger AM, van der Does W, Thayer JF, Brosschot JF, Verkuil B. Transcutaneous vagus nerve stimulation reduces spontaneous but not induced negative thought intrusions in high worriers. Biol Psychol (2019) 142:80–9. doi: 10.1016/j.biopsycho.2019.01.014
71. Burger AM, Verkuil B, Fenlon H, Thijs L, Cools L, Miller HC, et al. Mixed evidence for the potential of non-invasive transcutaneous vagal nerve stimulation to improve the extinction and retention of fear. Behav Res Ther (2017) 97:64–74. doi: 10.1016/j.brat.2017.07.005
72. Burger AM, Verkuil B, Van Diest I, van der Does W, Thayer JF, Brosschot JF. The effects of transcutaneous vagus nerve stimulation on conditioned fear extinction in humans. Neurobiol Learn Memory (2016) 132:49–56. doi: 10.1016/j.nlm.2016.05.007
73. Szeska C, Richter J, Wendt J, Weymar M, Hamm AO. Promoting long-term inhibition of human fear responses by non-invasive transcutaneous vagus nerve stimulation during extinction training. Sci Rep (2020) 10:1529. doi: 10.1038/s41598-020-58412-w
74. Jacobs HIL, Riphagen JM, Razat CM, Wiese S, Sack AT. Transcutaneous vagus nerve stimulation boosts associative memory in older individuals. Neurobiol Aging (2015) 36:1860–7. doi: 10.1016/j.neurobiolaging.2015.02.023
75. Giraudier M, Ventura-Bort C, Weymar M. Transcutaneous vagus nerve stimulation (tVNS) improves high-confidence recognition memory but not emotional word processing. Front Psychol (2020) 11:1276. doi: 10.3389/fpsyg.2020.01276
76. Colzato LS, Sellaro R, Beste C. Darwin Revisited: The vagus nerve is a causal element in controlling recognition of other’s emotions. Cortex (2017) 92:95–102. doi: 10.1016/j.cortex.2017.03.017
77. Sellaro R, de Gelder B, Finisguerra A, Colzato LS. Transcutaneous vagus nerve stimulation (tVNS) enhances recognition of emotions in faces but not bodies. Cortex (2018) 99:213–23. doi: 10.1016/j.cortex.2017.11.007
78. Zhu S, Qing Y, Zhang Y, Zhang X, Ding F, Zhang R, et al. Transcutaneous auricular vagus nerve stimulation increases eye-gaze on salient facial features and oxytocin release. Psychophysiology (2022) 59:e14107. doi: 10.1111/psyp.14107
79. Villani V, Tsakiris M, Azevedo RT. Transcutaneous vagus nerve stimulation improves interoceptive accuracy. Neuropsychologia (2019) 134:107201. doi: 10.1016/j.neuropsychologia.2019.107201
80. Maraver MJ, Steenbergen L, Hossein R, Actis-Grosso R, Ricciardelli P, Hommel B, et al. Transcutaneous vagus nerve stimulation modulates attentional resource deployment towards social cues. Neuropsychologia (2020) 143:107465. doi: 10.1016/j.neuropsychologia.2020.107465
81. Ventura-Bort C, Wirkner J, Wendt J, Hamm AO, Weymar M. Establishment of emotional memories is mediated by vagal nerve activation: Evidence from non-invasive taVNS. J Neurosci (2021) 41:7636–48 doi: 10.1523/JNEUROSCI.2329-20.2021
82. Steenbergen L, Sellaro R, Stock A-K, Verkuil B, Beste C, Colzato LS. Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during action cascading processes. Eur Neuropsychopharmacol (2015) 25:773–8. doi: 10.1016/j.euroneuro.2015.03.015
83. Beste C, Steenbergen L, Sellaro R, Grigoriadou S, Zhang R, Chmielewski W, et al. Effects of concomitant stimulation of the GABAergic and norepinephrine system on inhibitory control – a study using transcutaneous vagus nerve stimulation. Brain Stimul. (2016) 9:811–8. doi: 10.1016/j.brs.2016.07.004
84. Fischer R, Ventura-Bort C, Hamm A, Weymar M. Transcutaneous vagus nerve stimulation (tVNS) enhances conflict-triggered adjustment of cognitive control. Cognit Affect Behav Neurosci (2018) 18:680–93. doi: 10.3758/s13415-018-0596-2
85. Jongkees BJ, Immink MA, Finisguerra A, Colzato LS. Transcutaneous vagus nerve stimulation (tVNS) enhances response selection during sequential action. Front Psychol (2018) 9:1159. doi: 10.3389/fpsyg.2018.01159
86. Keute M, Demirezen M, Graf A, Mueller NG, Zaehle T. No modulation of pupil size and event-related pupil response by transcutaneous auricular vagus nerve stimulation (taVNS). Sci Rep (2019) 9:11452. doi: 10.1038/s41598-019-47961-4
87. Keute M, Barth D, Liebrand M, Heinze H-J, Kraemer U, Zaehle T. Effects of transcutaneous vagus nerve stimulation (tVNS) on conflict-related behavioral performance and frontal midline theta activity. J Cognit Enhanc (2020) 4:121–30. doi: 10.1007/s41465-019-00152-5
88. Borges U, Knops L, Laborde S, Klatt S, Raab M. Transcutaneous vagus nerve stimulation may enhance only specific aspects of the core executive functions. A Randomized Crossover Trial. Front Neurosci (2020) 14:523. doi: 10.3389/fnins.2020.00523
89. Pihlaja M, Failla L, Peräkylä J, Hartikainen KM. Reduced frontal nogo-N2 with uncompromised response inhibition during transcutaneous vagus nerve stimulation–more efficient cognitive control? Front Hum Neurosci (2020) 14:561780. doi: 10.3389/fnhum.2020.561780
90. Steenbergen L, Colzato LS, Maraver MJ. Vagal signaling and the somatic marker hypothesis: The effect of transcutaneous vagal nerve stimulation on delay discounting is modulated by positive mood. Int J Psychophysiol (2020) 148:84–92. doi: 10.1016/j.ijpsycho.2019.10.010
91. Llanos F, McHaney JR, Schuerman WL, Yi HG, Leonard MK, Chandrasekaran B. Non-invasive peripheral nerve stimulation selectively enhances speech category learning in adults. NPJ Sci Learn (2020) 5:1–11. doi: 10.1038/s41539-020-0070-0
92. Thakkar VJ, Engelhart AS, Khodaparast N, Abadzi H, Centanni TM. Transcutaneous auricular vagus nerve stimulation enhances learning of novel letter-sound relationships in adults. Brain Stimul (2020) 13:1813–20. doi: 10.1016/j.brs.2020.10.012
93. Hong G-S, Pintea B, Lingohr P, Coch C, Randau T, Schaefer N, et al. Effect of transcutaneous vagus nerve stimulation on muscle activity in the gastrointestinal tract (transVaGa): a prospective clinical trial. Int J Colorectal Dis (2019) 34:417–22. doi: 10.1007/s00384-018-3204-6
94. Teckentrup V, Neubert S, Santiago JCP, Hallschmid M, Walter M, Kroemer NB. Non-invasive stimulation of vagal afferents reduces gastric frequency. Brain Stimul. (2020) 13:470–3. doi: 10.1016/j.brs.2019.12.018
95. Steidel K, Krause K, Menzler K, Strzelczyk A, Immisch I, Fuest S, et al. Transcutaneous auricular vagus nerve stimulation influences gastric motility: A randomized, double-blind trial in healthy individuals. Brain Stimul (2021). doi: 10.1016/j.brs.2021.06.006
96. Wu X, Zhang Y, Luo W, Mai R, Hou X, Xia Z, et al. Brain functional mechanisms determining the efficacy of transcutaneous auricular vagus nerve stimulation in primary insomnia. Front Neurosci (2021) 15:609640. doi: 10.3389/fnins.2021.609640
97. He J-K, Jia B-H, Wang Y, Li S-Y, Zhao B, Zhou Z-G, et al. Transcutaneous auricular vagus nerve stimulation modulates the prefrontal cortex in chronic insomnia patients: fMRI study in the first session. Front Neurol (2022) 13:827749. doi: 10.3389/fneur.2022.827749
98. Barch DM, Harms MP, Tillman R, Hawkey E, Luby JL. Early childhood depression, emotion regulation, episodic memory, and hippocampal development. J Abnormal Psychol (2019) 128:81–95. doi: 10.1037/abn0000392
99. Levin RL, Heller W, Mohanty A, Herrington JD, Miller GA. Cognitive deficits in depression and functional specificity of regional brain activity. Cognit Ther Res (2007) 31:211–33. doi: 10.1007/s10608-007-9128-z
101. Rao U, Chen L-A. Characteristics, correlates, and outcomes of childhood and adolescent depressive disorders. Dialogues Clin Neurosci (2009) 11:45–62. doi: 10.31887/DCNS.2009.11.1/urao
102. Wagner S, Müller C, Helmreich I, Huss M, Tadić A. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur Child Adolesc Psychiatry (2015) 24:5–19. doi: 10.1007/s00787-014-0559-2
103. Strawn JR, Aaronson ST, Elmaadawi AZ, Schrodt GR, Holbert RC, Verdoliva S, et al. Treatment-resistant depression in adolescents: Clinical features and measurement of treatment resistance. J Child Adolesc Psychopharmacol (2020) 30:261–6. doi: 10.1089/cap.2020.0008
104. George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, et al. Vagus nerve stimulation: a new tool for brain research and therapy∗. Biol Psychiatry (2000) 47:287–95. doi: 10.1016/S0006-3223(99)00308-X
105. Trevizol AP, Shiozawa P, Taiar I, Soares A, Gomes JS, Barros MD, et al. Transcutaneous vagus nerve stimulation (taVNS) for major depressive disorder: An open label proof-of-Concept trial. Brain Stimul. (2016) 9:453–4. doi: 10.1016/j.brs.2016.02.001
106. Vanderlind WM, Millgram Y, Baskin-Sommers AR, Clark MS, Joormann J. Understanding positive emotion deficits in depression: From emotion preferences to emotion regulation. Clin Psychol Rev (2020) 76:101826. doi: 10.1016/j.cpr.2020.101826
107. Bandelow B, Michaelis S. Epidemiology of anxiety disorders in the 21st century. Dialogues Clin Neurosci (2015) 17:327–35. doi: 10.31887/DCNS.2015.17.3/bbandelow
108. Baxter AJ, Scott KM, Vos T, Whiteford HA. Global prevalence of anxiety disorders: a systematic review and meta-regression. psychol Med (2013) 43:897–910. doi: 10.1017/S003329171200147X
109. Andrews G, Hobbs MJ, Borkovec TD, Beesdo K, Craske MG, Heimberg RG, et al. Generalized worry disorder: a review of DSM-IV generalized anxiety disorder and options for DSM-V. Depression Anxiety (2010) 27:134–47. doi: 10.1002/da.20658
110. Chalmers JA, Quintana DS, Abbott MJ-A, Kemp AH. Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Front Psychiatry (2014) 5:80. doi: 10.3389/fpsyt.2014.00080
111. Friedman BH. An autonomic flexibility–neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol (2007) 74:185–99. doi: 10.1016/j.biopsycho.2005.08.009
112. Peña DF, Childs JE, Willett S, Vital A, McIntyre CK, Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front Behav Neurosci (2014) 8:327. doi: 10.3389/fnbeh.2014.00327
113. Bremner JD, Gurel NZ, Wittbrodt MT, Shandhi MH, Rapaport MH, Nye JA, et al. Application of noninvasive vagal nerve stimulation to stress-related psychiatric disorders. J Person. Med (2020) 10:119. doi: 10.3390/jpm10030119
114. Jacobs GR, Voineskos AN, Hawco C, Stefanik L, Forde NJ, Dickie EW, et al. Integration of brain and behavior measures for identification of data-driven groups cutting across children with ASD, ADHD, or OCD. Neuropsychopharmacol (2021) 46:643–53. doi: 10.1038/s41386-020-00902-6
115. Kushki A, Anagnostou E, Hammill C, Duez P, Brian J, Iaboni A, et al. Examining overlap and homogeneity in ASD, ADHD, and OCD: a data-driven, diagnosis-agnostic approach. Transl Psychiatry (2019) 9:1–11. doi: 10.1038/s41398-019-0631-2
116. Vandewouw MM, Choi E, Hammill C, Arnold P, Schachar R, Lerch JP, et al. Emotional face processing across neurodevelopmental disorders: a dynamic faces study in children with autism spectrum disorder, attention deficit hyperactivity disorder and obsessive-compulsive disorder. Transl Psychiatry (2020) 10:1–12. doi: 10.1038/s41398-020-01063-2
117. Stock S, Uvnäs-Moberg K. Increased plasma levels of oxytocin in response to afferent electrical stimulation of the sciatic and vagal nerves and in response to touch and pinch in anaesthetized rats. Acta Physiologica Scandinavica (1988) 132:29–34. doi: 10.1111/j.1748-1716.1988.tb08294.x
118. Kendrick KM, Guastella AJ, Becker B. Overview of human oxytocin research. In: Hurlemann R, Grinevich V, editors. Behavioral pharmacology of neuropeptides: Oxytocin. current topics in behavioral neurosciences. Cham: Springer International Publishing (2018). doi: 10.1007/7854_2017_19
119. Shamay-Tsoory SG, Abu-Akel A. The social salience hypothesis of oxytocin. Biol Psychiatry (2016) 79:194–202. doi: 10.1016/j.biopsych.2015.07.020
120. Yatawara CJ, Einfeld SL, Hickie IB, Davenport TA, Guastella AJ. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: a randomized clinical crossover trial. Mol Psychiatry (2016) 21:1225–31. doi: 10.1038/mp.2015.162
121. Parker KJ, Oztan O, Libove RA, Sumiyoshi RD, Jackson LP, Karhson DS, et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc Natl Acad Sci (2017) 114:8119–24. doi: 10.1073/pnas.1705521114
122. Le J, Zhang L, Zhao W, Zhu S, Lan C, Kou J, et al. Infrequent intranasal oxytocin followed by positive social interaction improves symptoms in autistic children: A pilot randomized clinical trial. PPS (2022) 91:335–47. doi: 10.1159/000524543
123. Cullen LA, Barlow JH, Cushway D. Positive touch, the implications for parents and their children with autism: an exploratory study. Complement. Therapies Clin Pract (2005) 11:182–9. doi: 10.1016/j.ctcp.2004.12.004
124. Diego MA, Field T, Hernandez-Reif M, Deeds O, Ascencio A, Begert G. Preterm infant massage elicits consistent increases in vagal activity and gastric motility that are associated with greater weight gain. Acta Paediatrica (2007) 96:1588–91. doi: 10.1111/j.1651-2227.2007.00476.x
125. Polanczyk G, de Lima MS, Horta BL, Biederman J, Rohde LA. The worldwide prevalence of ADHD: A systematic review and metaregression analysis. AJP (2007) 164:942–8. doi: 10.1176/ajp.2007.164.6.942
126. Arias AJ, Gelernter J, Chan G, Weiss RD, Brady KT, Farrer L, et al. Correlates of co-occurring ADHD in drug-dependent subjects: Prevalence and features of substance dependence and psychiatric disorders. Addictive Behav (2008) 33:1199–207. doi: 10.1016/j.addbeh.2008.05.003
127. Barkley RA, Fischer M, Smallish L, Fletcher K. Young adult follow-up of hyperactive children: antisocial activities and drug use. J Child Psychol Psychiatry (2004) 45:195–211. doi: 10.1111/j.1469-7610.2004.00214.x
128. Ramos Olazagasti MA, Klein RG, Mannuzza S, Belsky ER, Hutchison JA, Lashua-Shriftman EC, et al. Does childhood attention-Deficit/Hyperactivity disorder predict risk-taking and medical illnesses in adulthood? J Am Acad Child Adolesc Psychiatry (2013) 52:153–162.e4. doi: 10.1016/j.jaac.2012.11.012
129. McCarthy H, Skokauskas N, Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. psychol Med (2014) 44:869–80. doi: 10.1017/S0033291713001037
130. Rubia K. Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front Hum Neurosci (2018) 12:100. doi: 10.3389/fnhum.2018.00100
131. Rubia K, Alegría AA, Brinson H. Brain abnormalities in attention-deficit hyperactivity disorder: a review. Rev Neurol (2014) 58 Suppl 1:S3–16.
132. Dickstein SG, Bannon K, Castellanos FX, Milham MP. The neural correlates of attention deficit hyperactivity disorder: an ALE meta-analysis. J Child Psychol Psychiatry (2006) 47:1051–62. doi: 10.1111/j.1469-7610.2006.01671.x
133. Rubia K. “Cool” inferior frontostriatal dysfunction in attention-Deficit/Hyperactivity disorder versus “Hot” ventromedial orbitofrontal-limbic dysfunction in conduct disorder: A review. Biol Psychiatry (2011) 69:e69–87. doi: 10.1016/j.biopsych.2010.09.023
134. Stevens MC, Pearlson GD, Kiehl KA. An fMRI auditory oddball study of combined-subtype attention deficit hyperactivity disorder. AJP (2007) 164:1737–49. doi: 10.1176/appi.ajp.2007.06050876
135. Keute M, Ruhnau P, Heinze H-J, Zaehle T. Behavioral and electrophysiological evidence for GABAergic modulation through transcutaneous vagus nerve stimulation. Clin Neurophysiol (2018) 129:1789–95. doi: 10.1016/j.clinph.2018.05.026
136. Jin Y, Kong J. Transcutaneous vagus nerve stimulation: A promising method for treatment of autism spectrum disorders. Front Neurosci (2017) 10:609. doi: 10.3389/fnins.2016.00609
137. Lai M-C, Lombardo MV, Baron-Cohen S. Autism. Lancet (2014) 383:896–910. doi: 10.1016/S0140-6736(13)61539-1
138. Porges SW. The polyvagal theory: phylogenetic contributions to social behavior. Physiol Behav (2003) 79:503–13. doi: 10.1016/S0031-9384(03)00156-2
139. Lindgren KA, Folstein SE, Tomblin JB, Tager-Flusberg H. Language and reading abilities of children with autism spectrum disorders and specific language impairment and their first-degree relatives. Autism Res (2009) 2:22–38. doi: 10.1002/aur.63
140. Cummings CM, Caporino NE, Kendall PC. Comorbidity of anxiety and depression in children and adolescents: 20 years after. psychol Bull (2014) 140:816–45. doi: 10.1037/a0034733
141. Avenevoli S, Stolar M, Li J, Dierker L, Ries Merikangas K. Comorbidity of depression in children and adolescents: models and evidence from a prospective high-risk family study. Biol Psychiatry (2001) 49:1071–81. doi: 10.1016/S0006-3223(01)01142-8
142. Sunderland M, Mewton L, Slade T, Baillie AJ. Investigating differential symptom profiles in major depressive episode with and without generalized anxiety disorder: true co-morbidity or symptom similarity? psychol Med (2010) 40:1113–23. doi: 10.1017/S0033291709991590
143. Watson D. Rethinking the mood and anxiety disorders: A quantitative hierarchical model for DSM-V. J Abnormal Psychol (2005) 114:522–36. doi: 10.1037/0021-843X.114.4.522
144. Zbozinek TD, Rose RD, Wolitzky-Taylor KB, Sherbourne C, Sullivan G, Stein MB, et al. Diagnostic overlap of generalized anxiety disorder and major depressive disorder in a primary care sample. Depress Anxiety (2012) 29:1065–71. doi: 10.1002/da.22026
145. Zhang S, He J-K, Meng H, Zhao B, Zhao Y-N, Wang Y, et al. Effects of transcutaneous auricular vagus nerve stimulation on brain functional connectivity of medial prefrontal cortex in patients with primary insomnia. Anat. Rec (2021) 304:2426–35. doi: 10.1002/ar.24785
146. Zhao B, Bi Y, Li L, Zhang J, Hong Y, Zhang L, et al. The instant spontaneous neuronal activity modulation of transcutaneous auricular vagus nerve stimulation on patients with primary insomnia. Front Neurosci (2020) 14:205. doi: 10.3389/fnins.2020.00205
147. Vieira A, Reis AM, Matos LC, Machado J, Moreira A. Does auriculotherapy have therapeutic effectiveness? an overview of systematic reviews. Complement. Therapies Clin Pract (2018) 33:61–70. doi: 10.1016/j.ctcp.2018.08.005
148. Li S, Zhang Z, Jiao Y, Jin G, Wu Y, Xu F, et al. An assessor-blinded, randomized comparative trial of transcutaneous auricular vagus nerve stimulation (taVNS) combined with cranial electroacupuncture vs. citalopram for depression with chronic pain. Front Psychiatry (2022) 13:902450. doi: 10.3389/fpsyt.2022.902450
149. Kaniusas E, Kampusch S, Tittgemeyer M, Panetsos F, Gines RF, Papa M, et al. Current directions in the auricular vagus nerve stimulation II – an engineering perspective. Front Neurosci (2019) 13:772. doi: 10.3389/fnins.2019.00772
150. Badran BW, Brown JC, Dowdle LT, Mithoefer OJ, LaBate NT, Coatsworth J, et al. Tragus or cymba conchae? investigating the anatomical foundation of transcutaneous auricular vagus nerve stimulation (taVNS). Brain Stimul (2018) 11:947–8. doi: 10.1016/j.brs.2018.06.003
151. Burger AM, Verkuil B. Transcutaneous nerve stimulation via the tragus: are we really stimulating the vagus nerve? Brain Stimul.: Basic Transl. Clin Res Neuromodulation (2018) 11:945–6. doi: 10.1016/j.brs.2018.03.018
152. Cristancho P, Cristancho MA, Baltuch GH, Thase ME, O’Reardon JP. Effectiveness and safety of vagus nerve stimulation for severe treatment-resistant major depression in clinical practice after FDA approval: Outcomes at 1 year. J Clin Psychiatry (2011) 72:1376–82. doi: 10.4088/JCP.09m05888blu
153. De Couck M, Cserjesi R, Caers R, Zijlstra WP, Widjaja D, Wolf N, et al. Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Autonomic Neurosci (2017) 203:88–96. doi: 10.1016/j.autneu.2016.11.003
154. Redgrave J, Day D, Leung H, Laud PJ, Ali A, Lindert R, et al. Safety and tolerability of transcutaneous vagus nerve stimulation in humans; a systematic review. Brain Stimul. (2018) 11:1225–38. doi: 10.1016/j.brs.2018.08.010
155. Fallgatter AJ, Ehlis A-C, Ringel TM, Herrmann MJ. Age effect on far field potentials from the brain stem after transcutaneous vagus nerve stimulation. Int J Psychophysiol (2005) 56:37–43. doi: 10.1016/j.ijpsycho.2004.09.007
156. Fallgatter AJ, Neuhauser B, Herrmann MJ, Ehlis A-C, Wagener A, Scheuerpflug P, et al. Far field potentials from the brain stem after transcutaneous vagus nerve stimulation. J Neural Transm (2003) 110:1437–43. doi: 10.1007/s00702-003-0087-6
157. Nonis R, D’Ostilio K, Schoenen J, Magis D. Evidence of activation of vagal afferents by non-invasive vagus nerve stimulation: An electrophysiological study in healthy volunteers. Cephalalgia (2017) 37:1285–93. doi: 10.1177/0333102417717470
158. Polak T, Markulin F, Ehlis A-C, Langer JBM, Ringel TM, Fallgatter AJ. Far field potentials from brain stem after transcutaneous vagus nerve stimulation: optimization of stimulation and recording parameters. J Neural Transm (2009) 116:1237–42. doi: 10.1007/s00702-009-0282-1
159. Yuan H, Silberstein SD. Vagus nerve and vagus nerve stimulation, a comprehensive review: Part I. Headache: J Head Face Pain (2016) 56:71–8. doi: 10.1111/head.12647
160. Ben-Menachem E. Vagus-nerve stimulation for the treatment of epilepsy. Lancet Neurol (2002) 1:477–82. doi: 10.1016/S1474-4422(02)00220-X
161. Müller HHO, Moeller S, Lücke C, Lam AP, Braun N, Philipsen A. Vagus nerve stimulation (VNS) and other augmentation strategies for therapy-resistant depression (TRD): Review of the evidence and clinical advice for use. Front Neurosci (2018) 12:239. doi: 10.3389/fnins.2018.00239
162. Thompson SL, O’Leary GH, Austelle CW, Gruber E, Kahn AT, Manett AJ, et al. A review of parameter settings for invasive and non-invasive vagus nerve stimulation (VNS) applied in neurological and psychiatric disorders. Front Neurosci (2021) 0:709436. doi: 10.3389/fnins.2021.709436
163. Hulsey DR, Riley JR, Loerwald KW, Rennaker RL, Kilgard MP, Hays SA. Parametric characterization of neural activity in the locus coeruleus in response to vagus nerve stimulation. Exp Neurol (2017) 289:21–30. doi: 10.1016/j.expneurol.2016.12.005
164. Kong S-S, Liu J-J, Hwang T-C, Yu X-J, Zhao M, Zhao M, et al. Optimizing the parameters of vagus nerve stimulation by uniform design in rats with acute myocardial infarction. PloS One (2012) 7:e42799. doi: 10.1371/journal.pone.0042799
165. Mridha Z, de Gee JW, Shi Y, Alkashgari R, Williams J, Suminski A, et al. Graded recruitment of pupil-linked neuromodulation by parametric stimulation of the vagus nerve. Nat Commun (2021) 12:1539. doi: 10.1038/s41467-021-21730-2
166. Badran BW, Mithoefer OJ, Summer CE, LaBate NT, Glusman CE, Badran AW, et al. Short trains of transcutaneous auricular vagus nerve stimulation (taVNS) have parameter-specific effects on heart rate. Brain Stimul. (2018) 11:699–708. doi: 10.1016/j.brs.2018.04.004
167. Warren CM, Tona KD, Ouwerkerk L, van Paridon J, Poletiek F, van Steenbergen H, et al. The neuromodulatory and hormonal effects of transcutaneous vagus nerve stimulation as evidenced by salivary alpha amylase, salivary cortisol, pupil diameter, and the P3 event-related potential. Brain Stimul (2019) 12:635–42. doi: 10.1016/j.brs.2018.12.224
168. Frangos E, Ellrich J, Komisaruk BR. Non-invasive access to the vagus nerve central projections via electrical stimulation of the external ear: fMRI evidence in humans. Brain Stimul. (2015) 8:624–36. doi: 10.1016/j.brs.2014.11.018
169. Ventura-Bort C, Wirkner J, Genheimer H, Wendt J, Hamm AO, Weymar M. Effects of transcutaneous vagus nerve stimulation (tVNS) on the P300 and alpha-amylase level: A pilot study. Front Hum Neurosci (2018) 12:202. doi: 10.3389/fnhum.2018.00202
170. Colzato LS, Wolters G, Peifer C. Transcutaneous vagus nerve stimulation (tVNS) modulates flow experience. Exp Brain Res (2018) 236:253–7. doi: 10.1007/s00221-017-5123-0
171. Sellaro R, van Leusden JWR, Tona K-D, Verkuil B, Nieuwenhuis S, Colzato LS. Transcutaneous vagus nerve stimulation enhances post-error slowing. J Cogn Neurosci (2015) 27:2126–32. doi: 10.1162/jocn_a_00851
172. Burger AM, D’Agostini M, Verkuil B, Diest IV. Moving beyond belief: A narrative review of potential biomarkers for transcutaneous vagus nerve stimulation. Psychophysiology (2020) 57:e13571. doi: 10.1111/psyp.13571
173. Borges U, Laborde S, Raab M. Influence of transcutaneous vagus nerve stimulation on cardiac vagal activity: Not different from sham stimulation and no effect of stimulation intensity. PloS One (2019) 14:e0223848. doi: 10.1371/journal.pone.0223848
174. D’Agostini M, Burger AM, Villca Ponce G, Claes S, von Leupoldt A, Van Diest I. No evidence for a modulating effect of continuous transcutaneous auricular vagus nerve stimulation on markers of noradrenergic activity. Psychophysiology (2022) 59:e13984. doi: 10.1111/psyp.13984
175. D’Agostini M, Burger AM, Franssen M, Claes N, Weymar M, von Leupoldt A, et al. Effects of transcutaneous auricular vagus nerve stimulation on reversal learning, tonic pupil size, salivary alpha-amylase, and cortisol. Psychophysiology (2021) 58:e13885. doi: 10.1111/psyp.13885
176. Yu Y, Ling J, Yu L, Liu P, Jiang M. Closed-loop transcutaneous auricular vagal nerve stimulation: Current situation and future possibilities. Front Hum Neurosci (2022) 15:785620. doi: 10.3389/fnhum.2021.785620
177. Napadow V, Edwards RR, Cahalan CM, Mensing G, Greenbaum S, Valovska A, et al. Evoked pain analgesia in chronic pelvic pain patients using respiratory-gated auricular vagal afferent nerve stimulation. Pain Med (2012) 13:777–89. doi: 10.1111/j.1526-4637.2012.01385.x
178. Garcia RG, Lin RL, Lee J, Kim J, Barbieri R, Sclocco R, et al. Modulation of brainstem activity and connectivity by respiratory-gated auricular vagal afferent nerve stimulation (RAVANS) in migraine patients. Pain (2017) 158:1461–72. doi: 10.1097/j.pain.0000000000000930
179. Badran B, Jenkins D, DeVries W, Dancy M, Cook D, Mappin G, et al. Development of closed-loop transcutaneous auricular vagus nerve stimulation (taVNS) as a neurorehabilitation tool. Brain Stimul.: Basic Transl. Clin Res Neuromodulation (2019) 12:523. doi: 10.1016/j.brs.2018.12.721
180. Cook DN, Thompson S, Stomberg-Firestein S, Bikson M, George MS, Jenkins DD, et al. Design and validation of a closed-loop, motor-activated auricular vagus nerve stimulation (MAAVNS) system for neurorehabilitation. Brain Stimul.: Basic Transl. Clin Res Neuromodulation (2020) 13:800–3. doi: 10.1016/j.brs.2020.02.028
181. Badran BW, Jenkins DD, Cook D, Thompson S, Dancy M, DeVries WH, et al. Transcutaneous auricular vagus nerve stimulation-paired rehabilitation for oromotor feeding problems in newborns: An open-label pilot study. Front Hum Neurosci (2020) 14:77. doi: 10.3389/fnhum.2020.00077
182. Keute M, Ruhnau P, Zaehle T. Reply to “Reconsidering sham in transcutaneous vagus nerve stimulation studies”. Clin Neurophysiol (2018) 129:2503–4. doi: 10.1016/j.clinph.2018.09.001
183. Merchant K, Zanos S, Datta-Chaudhuri T, Deutschman CS, Sethna CB. Transcutaneous auricular vagus nerve stimulation (taVNS) for the treatment of pediatric nephrotic syndrome: a pilot study. Bioelectron. Med (2022) 8:1. doi: 10.1186/s42234-021-00084-6
184. Rogers SJ, Lewis H. An effective day treatment model for young children with pervasive developmental disorders. J Am Acad Child Adolesc Psychiatry (1989) 28:207–14. doi: 10.1097/00004583-198903000-00010
185. Reichow B, Wolery M. Comprehensive synthesis of early intensive behavioral interventions for young children with autism based on the UCLA young autism project model. J Autism Dev Disord (2009) 39:23–41. doi: 10.1007/s10803-008-0596-0
186. Zwaigenbaum L, Bauman ML, Choueiri R, Kasari C, Carter A, Granpeesheh D, et al. Early intervention for children with autism spectrum disorder under 3 years of age: Recommendations for practice and research. Pediatrics (2015) 136 Suppl 1:S60–81. doi: 10.1542/peds.2014-3667e
Keywords: transcutaneous auricular vagus nerve stimulation, non-invasive, neural plasticity, pediatric disorders, protocol
Citation: Zhu S, Zhang X, Zhou M, Kendrick KM and Zhao W (2022) Therapeutic applications of transcutaneous auricular vagus nerve stimulation with potential for application in neurodevelopmental or other pediatric disorders. Front. Endocrinol. 13:1000758. doi: 10.3389/fendo.2022.1000758
Received: 22 July 2022; Accepted: 27 September 2022;
Published: 12 October 2022.
Edited by:
Dragos Cretoiu, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Mohd Kaisan Mahadi, National University of Malaysia, MalaysiaCopyright © 2022 Zhu, Zhang, Zhou, Kendrick and Zhao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weihua Zhao, emFyYXpoYW9AdWVzdGMuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.