- 1Unit of Andrology and Reproductive Medicine & Centre for Male Gamete Cryopreservation, Department of Medicine, University of Padova, Padova, Italy
- 2Department of Medicine, Clinical Nutrition Unit, University of Padova, Padova, Italy
- 3Department of Women and Children’s Health, University of Padua, Padua, Italy
- 4Unit of Obstetrics and Gynecology, Madonna della Navicella Hospital, Venice, Italy
Content: Dietary supplements (DS) for male infertility marketed in Italy were evaluated for composition, concentration of ingredients, and recommended daily dose. A systematic review of literature identified ingredients potentially effective on sperm parameters and their minimal effective daily dose (mED).
Objective: This study was conducted in order to critically evaluate the composition and efficacy of DS marketed in Italy.
Design, Setting, and Participants: This was a systematic review of randomized controlled trials.
Evidence Acquisition: A formula allowed us to classify the expected efficacy of each DS, based on composition. Each DS was scored and included into three classes of expected efficacy: high, low, and none.
Evidence Synthesis: Among 24 supplements, 3 (12.5%) fall in high, 9 (37.5%) in lower, and 12 (50.0%) in no expected efficacy class. DS composition showed 36 substances, 18 with no literature on male fertility and 18 showing positive effect on sperm parameters, thus considered potentially active ingredients (PAI). All DS were mixtures of ingredients, containing from 2 to 17 different substances. Fifteen supplements (65.2%) contained at least 1 ingredient without evidence of efficacy and 21 formulations had PAI dosed below mED. Some PAI were associated to the improvement of specific sperm parameters.
Conclusions: DS were usually blends of many substances that are frequently employed at negligible dose or without any evidence of efficacy on male reproduction. Some ingredients have been demonstrated to be effective on specific sperm parameters by RCTs. We report a list of ingredients with potential efficacy on specific sperm parameters, aimed to allow a tailored use of DS.
Patient Summary: The market of DS for male infertility offers products with potential efficacy in the improvement of sperm parameters but also many with uncertain effects. Based on current scientific literature, our study can help in the choice of DS that are more likely to be effective on specific sperm alterations, so providing the best supplementation for each patient.
Introduction
Male factor infertility accounts the most varied and multifactorial causes (1, 2). Besides idiopathic infertility (up to 25% of cases), organic causes range from genital tract infections/inflammation, hormonal alterations, varicocele, and genetic problems (3–5). Many recent studies emphasized the role of other risk factors such as incorrect lifestyles, malnutrition, and abuse substances (6, 7). The hypothesis is that these conditions, inducing an elevation of radical oxygen and nitrogen species, might impair spermatogenesis, both directly, through an alteration of a redox status, and indirectly, interfering with the hypothalamic–pituitary–testicular axis (8–10). A recent survey performed by American urologists on clinical practice in the treatment of idiopathic male infertility showed that 64.9% of caregivers use dietary supplements (DS), empirically in the face of a lack of recommendations on the guidelines for the use of these products (11). The European Food Safety Authority (EFSA) stated that “supplements aren’t intended to treat or prevent diseases in humans or to modify physiological functions, but only to support specific physiological functions” (12). Anyway, all meta-analyses and guidelines citing the use of DS for male infertility advise to carefully evaluate the causes of infertility, as well as to accurately assess nutritional status (11, 13).
The term “nutraceutical” is not defined by law and products that fall into this category are mainly contained in the DS (14, 15). In recent years, a growing use of nutraceutical products has been recorded among men seeking fertility or complaining other andrological problems (16, 17). DS are widely available on the market, even if a proven efficacy has not yet been demonstrated for most of them. Despite many authors showing the positive effects of some substances on semen parameters and fertility outcomes (18–20), many others reported the lack of efficacy and even potential side effects (16, 20). We recently summarized the state-of-the-art of single ingredients currently present in the DS marketed to improve sperm parameters (17). The main conclusion was that some DS were mixtures of ingredients with uncertain or unreported benefits and contained substances with very low dosage.
Despite these attempts to clarify the specific role of each ingredient, several confounding factors make difficult and still empirical the choice of the proper formulation for each patient at the right time, in a perspective of personalized medicine. This is due to several issues: i) prescribers rarely know the nutritional state of the patient before administering a nutraceutical product; ii) it is still unclear which infertile patient may have beneficial effects from nutraceutical substances; iii) many supplements are available as mixtures of different substances, confounding the effects of individual components; iv) in different products, the same substance is often present at different doses; and vi) patients who are likely to have a condition of oxidative stress could find benefit from the use of substances with an antioxidant effect.
However, it still incompletely known which molecule reaches the testis, at which dose, and sometimes the molecular mechanism of action (21).
The aim of this study was to evaluate the potential efficacy of each DS using an adapted version of the scoring system by the American Heart Association. In particular, the effect of any ingredients was evaluated according to semen alterations.
Evidence Acquisition
Systematic Review of Literature
We performed the present review following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement (22).
On the website of the Italian Ministry of Health, we found 23 supplements marketed for male infertility (23). Two investigators (GP and GG) performed a systematic electronic search on Google Scholar, Embase, MEDLINE, and Cochrane Register of Controlled Trials since 2000 until September 30, 2021. The following search strategy was used: (“name of each active ingredient” AND (“supplements” OR “nutraceuticals”) AND (“fertility” OR “male reproduction” OR “semen parameters”). The references of retrieved articles together with the proceedings of relevant conferences were hand-searched in order to identify other potentially eligible studies for inclusion in the analysis missed by the initial search or any unpublished data. The literature search, assessment of inclusion and exclusion criteria, quality of studies, and extraction of data were independently undertaken and verified by two investigators (AG and FF-P). The results were then compared, and in case of discrepancies, a consensus was reached with the involvement of a third senior investigator (AV). There was no language restriction applied.
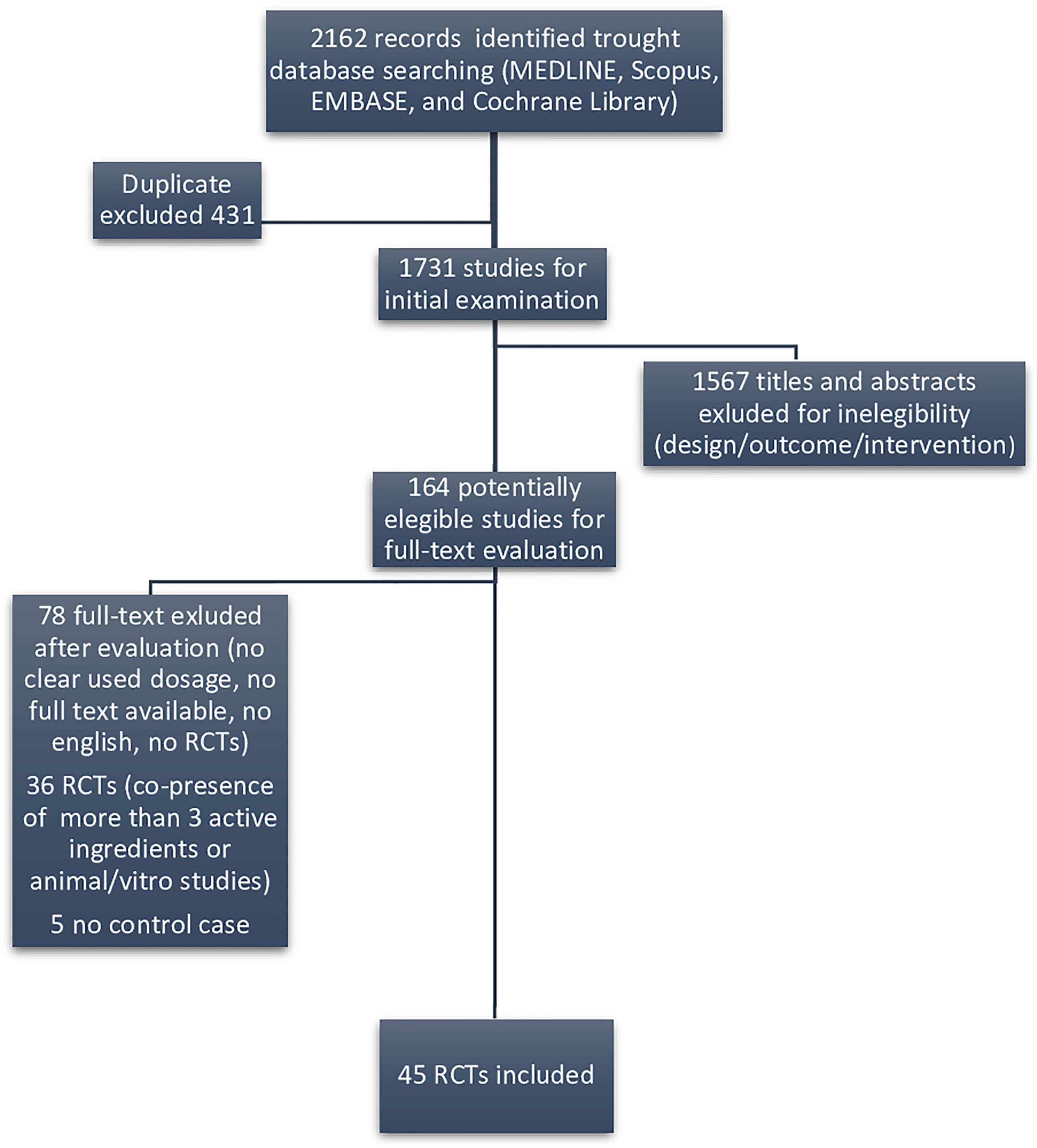
We considered as eligible only randomized clinical trials (RCTs) evaluating substances included in DS marketed for male infertility. Figure 1 displays the flow diagram of the selection of eligible papers.
To ascertain the certainty of evidence, the Grading of Recommendations, Assessment, Development and Evaluations (GRADE) framework was used (24).
Definition of Potential Active Ingredients and Minimal Effective Daily Dose
Selected articles allowed us to identify potential active ingredients (PAI) that were substances with a reported efficacy on at least one sperm parameter (sperm count, motility, morphology, DNA damage, etc.). The minimal effective daily dose (mED) was considered as the lowest effective dose reported in RCTs for each PAI, able to improve at least one sperm parameter.
Formula to Score DS
In our previous paper (17), we suggested a formula derived from the study by Kuchakulla et al. (20) to evaluate the possible efficacy of DS based on their composition. We included both papers evaluating effective and ineffective ingredients in the improvement of sperm parameters aimed to better weigh the possible efficacy of each single molecule. The used formula was conceived as follows: ingredients contained in each supplement were classified into four categories (A, B, C, or D) based on the reported efficacy and suggested daily dose. An ingredient was assigned to category A if multiple randomized control trials showed a net positive impact, level B if just one positive RCT was found, level C if multiple RCTs showed opposing results in an indeterminate outcome, and level D if RCT showed no or even negative effect. Once a category was designated to each ingredient, a score was assigned: A = 5, B = 3, C = 1, D = −1. Subsequently, the scores were designated to each of the supplements depending on their respective composition: briefly, the score of each ingredient constituting the supplement (i.e., A = 5, B = 3, C = 1, D = −1) was summed.
Then this score was weighted for the total number of ingredients in the supplement (N). Finally, in order to reward those supplements with only class A and B ingredients, the relative score was multiplied by the number of class A ingredients plus half the number of class B ingredients, finally resulting in the final score of the supplement.
Given the distribution of the scores resulted in three main clusters, we classified DS into three categories, resembling the potential efficacy of the ingredients: high expected efficacy (corrected score ≥ 3), low expected efficacy (1 > corrected score < 3), and no expected efficacy (corrected score ≤ 1). To obtain the whole list of DS, actually marketed in Italy for male infertility, we referred to the register available on the website of the Italian Ministry of Health, updated to 01/03/2020 [23].
Evidence Synthesis
Among the 1,731 studies for initial examination, we identified 164 eligible papers in our systematic literature search following the exclusion of duplicate publication, after screening of the title and the abstract sections of the paper. Among those, we found that only 45 RCTs reporting their efficacy on sperm parameters were retrieved as full text, in order to be included in the systematic review (Figure 1).
DS Evaluation Based on Literature and Results
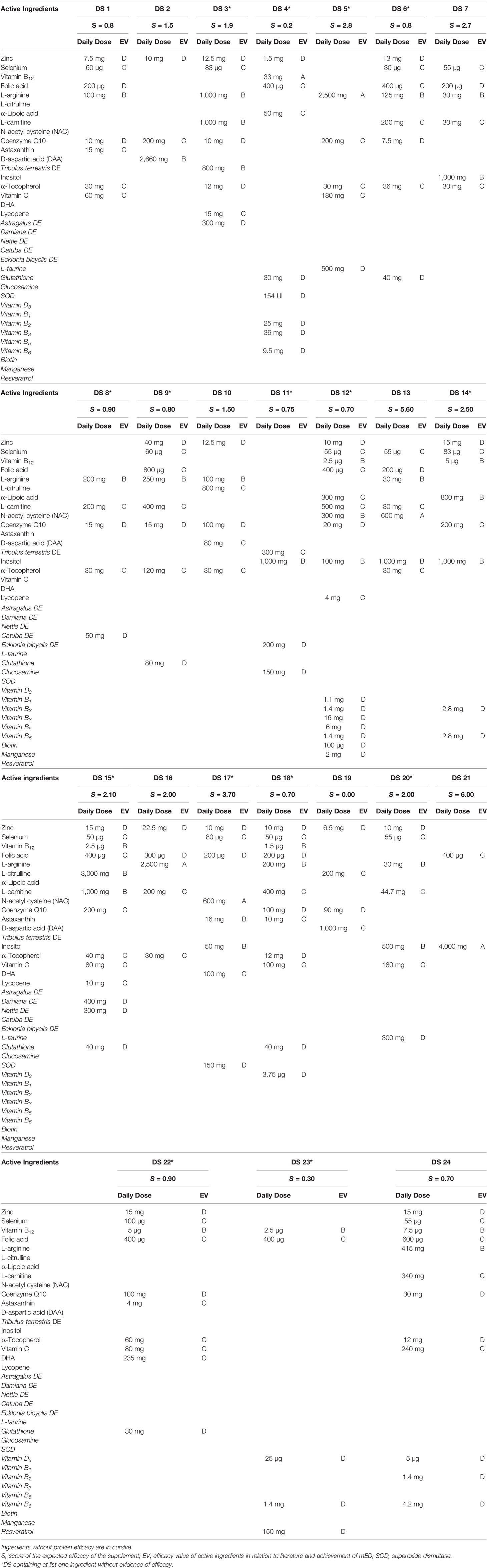
The analysis of literature allowed us to evaluate the potential efficacy of ingredients. We found 18 ingredients active on at least one sperm parameter and other 18 substances without any evidence. The list of PAI, references, effect on evaluated sperm parameters, and employed daily doses are summarized in Table 1. In the last column, the mED with demonstrated efficacy of each substance is reported. In some studies, marked with an asterisk, the employed daily dose of ingredients (zinc and α-tocopherol) exceeded the UL.
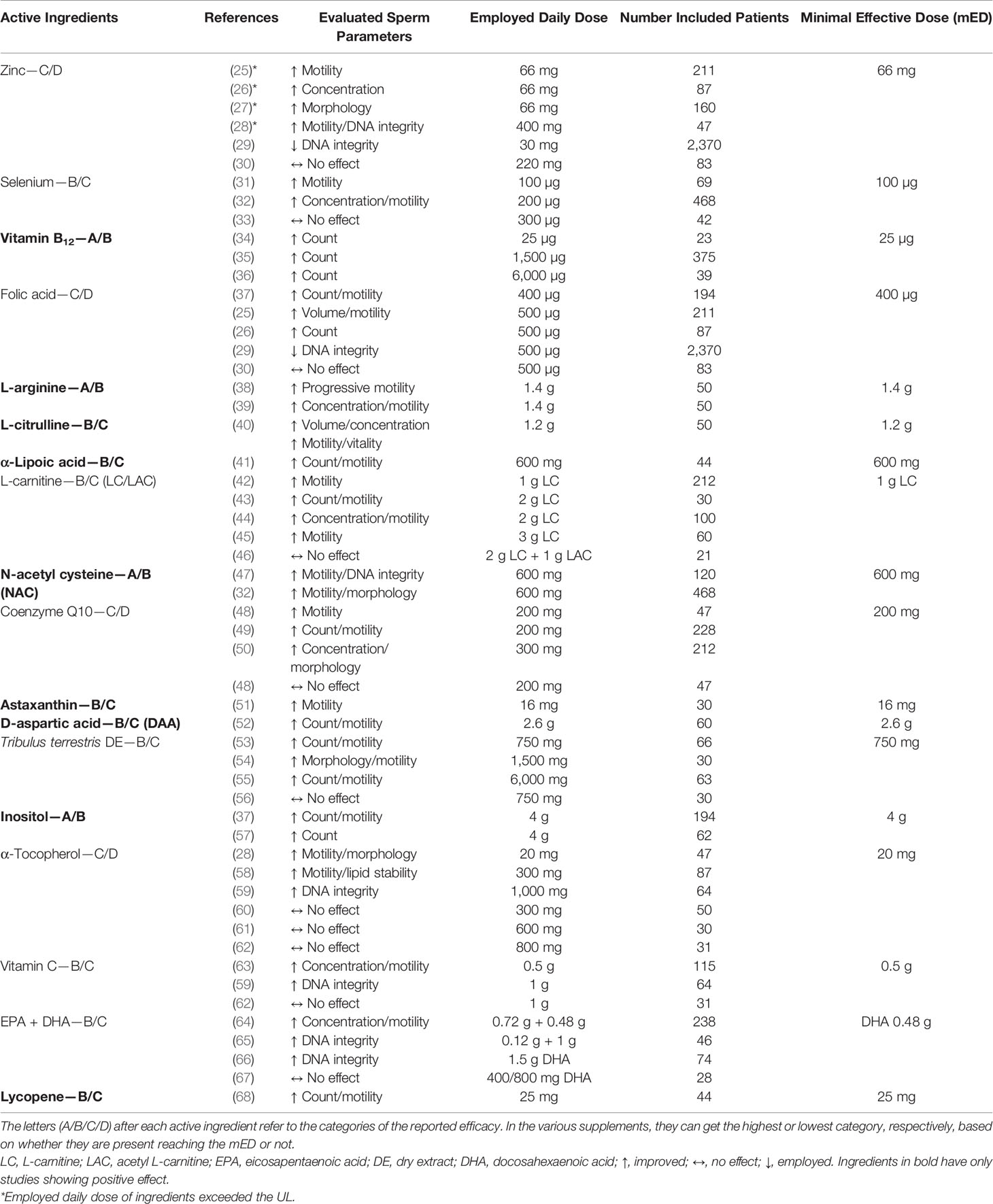
Table 1 Active ingredients with evidence of potential efficacy, class of expected efficacy, references, efficacy on sperm parameters, employed daily dose, and minimal effective dose (mED).
Excluding astaxanthin, D-aspartic acid, and lycopene, each having just one reference, the evidence of efficacy of the other ingredients was supported by at least two RCTs. Half of the PAI had at least one study showing no or even negative effects on sperm parameters. Regarding zinc, folic acid, CQ10, and α-tocopherol, we found more than one study showing no or even negative effects.
In particular, for α-tocopherol, we found six studies: three showed a positive effect and three showed no effect. Nine ingredients (evidenced in bold in Table 1) were evaluated only in studies showing a positive effect. However, five substances of this group had just one paper supporting their efficacy.
The characteristics of the 23 DS are summarized in Table 2. In this table, compositions, recommended daily dose for each ingredient, grade of evidence, and score of efficacy are reported for each DS. A total of 36 ingredients were used by manufacturers in the DS. All the evaluated supplements were mixtures ranging from 2 up to 17 substances. Fifteen out of 23 supplements (65.2%), marked with an asterisk, contained at least 1 ingredient without evidence of efficacy on sperm parameters (evidence by cursive in Table 2). In 21 formulations, there were ingredients dosed below mED. In particular, one supplement (DS 12) counted 17 ingredients, of which 7 were without proven efficacy and 13 dosed below mED. Two DS (2 and 21) contained only ingredients with demonstrated efficacy and satisfying mED. DS 9 had the zinc dosed at the UL (40 mg/day). Among the DS, the most used ingredient was zinc, followed by selenium, arginine, coenzyme Q, folic acid, and carnitine. These six molecules were used in more than 60% of formulations.
In Figure 2, the distribution of supplements divided into three classes of expected efficacy based on their corrected scores is reported. Three out of 23 supplements (12.5%) resulted in the higher efficacy group and 9 (37.5%) in the lower efficacy group. The remaining 12 DS (50.0%) were expected to have no efficacy. In Table 3, active ingredients and DS were grouped based on the supposed efficacy on specific seminal targets (count, total motility, morphology, or DNA integrity). The most part of the ingredients showed a positive effect on sperm count and total motility. Interestingly, zinc showed evidence of efficacy on all the sperm parameters here considered. In the lower part of the table, DS were grouped in relation to their expected efficacy on specific sperm parameters, matching the corrected score and contained ingredients.
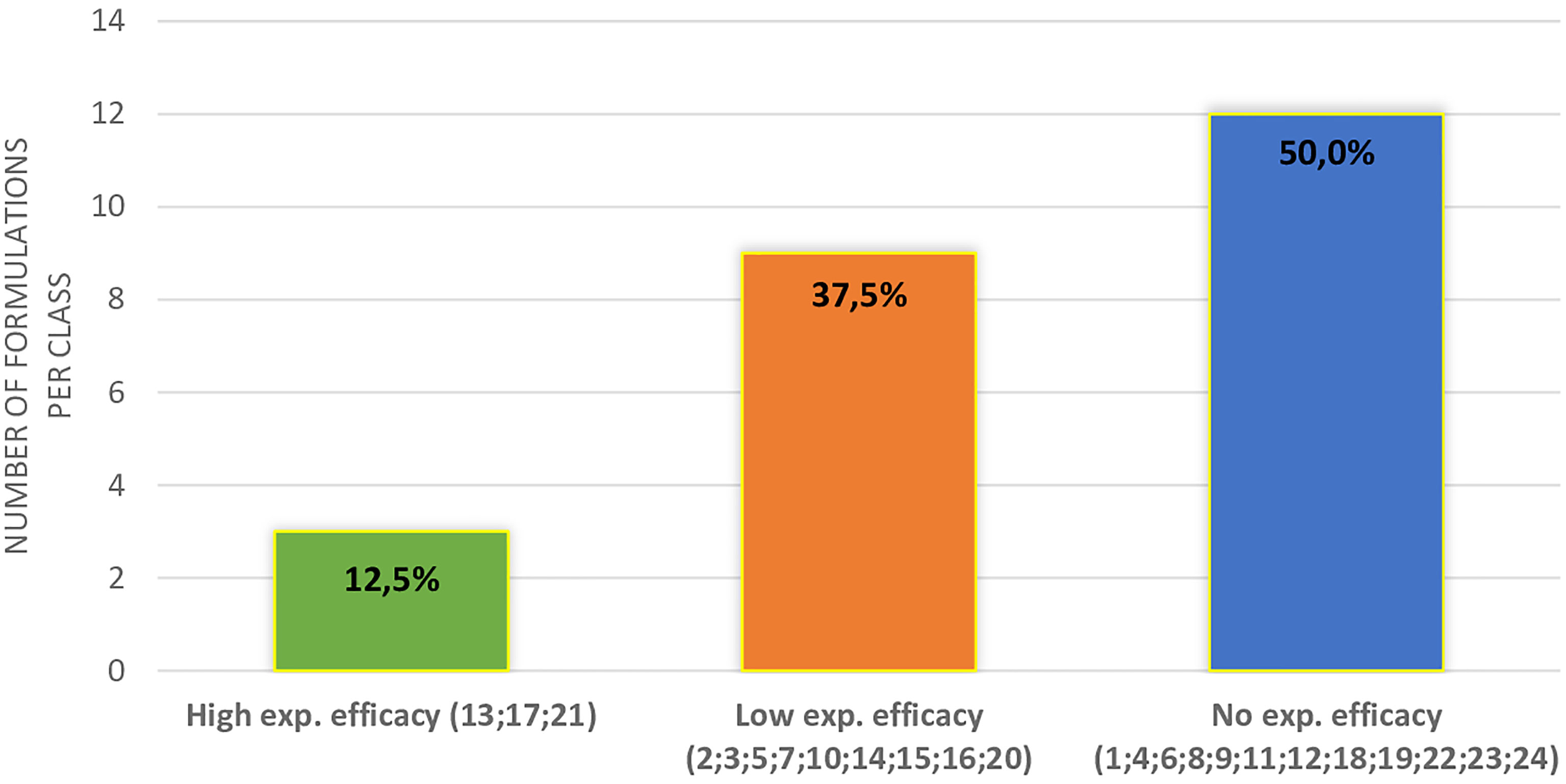
Figure 2 Distribution of different supplements (indicated by the number) in classes of expected efficacy.
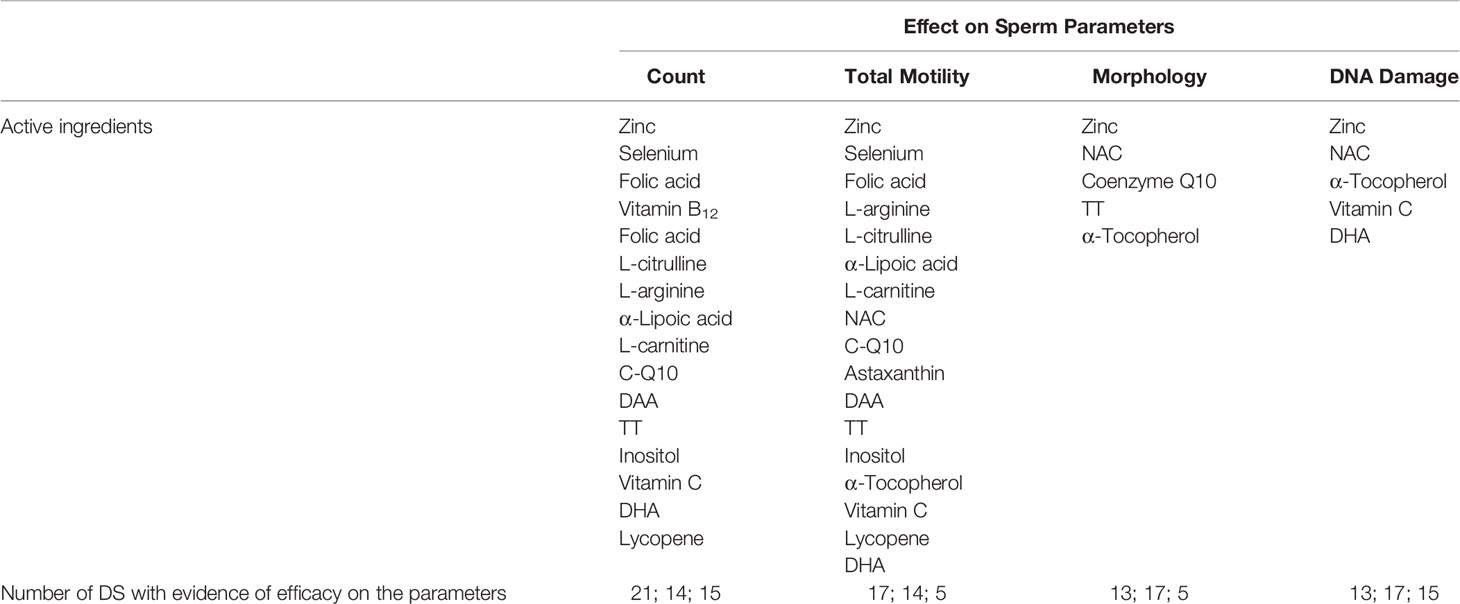
Table 3 Effect of active ingredients on specific sperm parameters (in the lower part of the table, DS with higher expected efficacy on each sperm parameter are listed).
Discussion
The use of supplements in the enhancement of male fertility is as interesting and promising topic as it is still much debated. However, most trials used unsatisfactory methodology due to lack of a control group, lack of randomized and placebo-controlled well-designed study, use of many different doses of ingredients, evaluation of different outcomes, small number of included patients, etc. In fact, in the trials concerning male infertility, there is too much focus on the seminological characteristics without taking into consideration the female outcomes and also the pregnancy rate, which should always be our target (69, 70). Moreover, many clinical studies were sponsored and analyzed mixtures of many ingredients without evidence of clinical efficacy. However, these supplements are frequently prescribed in clinical practice to infertile patients, and sometimes, subjects seeking fertility spontaneously purchase these products since medical prescription is unnecessary (70). In 2019, the Italian market of supplements generated 3.7 billion euros, with an increase of +4.3% compared to 2018 (71).
In this study, we developed a new approach to evaluate DS marketed for male infertility. By a literature review, aimed to understand the clinical efficacy of each ingredient in the improvement of sperm parameters, we developed a new formula able to weigh the potential efficacy of DS. In a previous study (70), we used a similar approach to evaluate the DS for female infertility. Using the same formula, here, we included only RCTs both showing positive, none, and even negative effects of each ingredient. Moreover, we related the potential efficacy of substances on each sperm parameter. Using this method, we observed that the composition of DS marketed in Italy for male infertility is little supported by scientific evidence. In fact, most products evaluated in this study contained a huge number of ingredients, up to 17 different substances in the same supplement, frequently below the mED. A relevant issue consists in the presence of many ingredients at low dosages and/or without any evidence of efficacy. Some substances without evidence of efficacy in the literature, such as vitamin D3, taurine, B group vitamins, glucosamine, glutathione, various enzymes, and non-standardized dry herbal extracts, were widely present in DS for male infertility (69, 70). This evidence reflects the lack of knowledge in this field of reproductive medicine by manufacturers.
Several studies demonstrated that some substances such as omega 3 fatty acid, lycopene, and Tribulus have a positive effect on sperm parameters (sperm motility, number, morphology, DNA integrity, and mitochondrial function) (72–74). However, the mechanism of actions was documented for selenium (75), zinc (76, 77), and carnitine (78), but just supposed for other ingredients or still unknown in most cases. The synergistic effect of more ingredients can also be supposed. Therefore, the benefit that is obtained following the use of DS, which are always combinations of substances, is likely to be given by an overall synergistic effect. For example, the combinations of vitamin B12 and folic acid could have a positive effect on DNA integrity, able to improve homocysteine metabolism (79, 80).
Some ingredients, in particular amino acids, or their derivatives such as arginine and carnitine, were present in most DS aimed to improve sperm parameters and ameliorate spermatogenic process. However, these substances need to be administered at doses close to grams to be effective (81, 82). In contrast, our data demonstrate that ingredients are used even in doses 10 times below mED. In the case of arginine, the dosage should take into account the incomplete intestinal absorption and the liver metabolism which strongly reduce its bioavailability in the reproductive system (83, 84).
Using the new formula, proposed here, we scored, for the first time, the possible efficacy of each DS based on the number of ingredients, their reported or no reported efficacy, their effective or no effective dose, and their level of action at the seminal level. Only a few DS (3 products) resulted in the highest scoring level, while most products (21 DS) fell in the intermediate or lower level. This observation should be taken into account by manufacturers in order to align more and more the DS market according to scientific evidence to conceive more effective DS formulations. Despite actual literature could help to conceive better DS, it is largely insufficient to better drive clinicians on the tailored choice of the DS regarding the specific alteration of an infertile patient. This gap derives from the lack of accuracy in the diagnosis of infertility cause, observed in most clinical studies. In fact, their design often does not consider the cause that underlies sperm abnormalities. It is well known that the same sperm alteration can be induced by different causes (85). For example, a reduction of sperm motility is related both to testicular impairment, semen infections, or inflammation of accessory glands or varicocele (86). The same example can be performed for sperm count, morphology, DNA integrity, and other sperm parameters (87). For this reason, RCTs should be performed in populations of infertile patients stratified according to the etiology of infertility and not only based on seminal parameters. Moreover, to reach an effective dietary supplementation, aimed to improve sperm parameters, the mechanism of action and the effective dose of each ingredient should be clearly elucidated (88–90). With this aim, many and well-designed in-vitro and in-vivo studies are needed.
The choice to include in this review only evidence from RCTs was made in order to minimize bias in the critical evaluation of DS, in order to have for each active ingredient analyzed studies that have reported a positive effect but also studies that have shown a negative or no effect.
The main limitation of this study in the restricted focus of research on DS based on the Italian market, and the most analyzed RCTs are based on combinations of more than one substance, so it cannot be excluded that what is obtained is the result of an interaction (both positive and negative) between the different substances used together.
Conclusions
In the light of our findings, we raise three final considerations: i) the Italian market of DS for male infertility offers products with potential efficacy in the improvement of sperm parameters but also many with uncertain effects; ii) the actual literature is poor of well-designed studies on PAI investigating their mechanisms of action and effective dose in different pathological conditions; and iii) based on current literature, our study can help in the choice of DS and PAI that are more likely to be effective on specific sperm alterations.
Our critical analysis suggests a rational strategy for a tailored use of DS in male infertility.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
AG, GP, and FF-P contributed to the concept/design of the research and acquisition/analysis of the literature data. AG, GP, and FF-P equally contributed to and drafted the manuscript. AN contributed to the concept and performed the data analyses. LT, AV, GG, and CF critically revised the paper for important intellectual content. All authors revised and approved the final manuscript and agreed to be fully accountable for ensuring the integrity and accuracy of the work. All authors had full access to all the data in the study and took responsibility for the integrity of the study and the accuracy of the analysis.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Dr. Marco Ghezzi for his helpful discussion.
References
1. Winters BR, Walsh T. The Epidemiology of Male Infertility. Urol Clin North Am (2014) 41:195–204. doi: 10.1016/j.ucl.2013.08.006
2. Dawson EB, Harris WA, Powell LC. Relationship Between Ascorbic Acid and Male Fertility. World Rev Nutr Diet (1990) 62:1–26. doi: 10.1159/000417532
3. ISS. Istituto Superiore Di Sanità. Available at: http://old.iss.it/rpma/ (Accessed January 3, 2021).
4. Salas-Huetos A, Bulló M, Salas-Salvadó J. Dietary Patterns, Foods and Nutrients in Male Fertility Parameters and Fecundability: A Systematic Review of Observational Studies. Hum Reprod Update (2017) 23:371–89. doi: 10.1093/humupd/dmx006
5. Simon L, Murphy K, Shamsi MB, Liu L, Emery B, Aston KI, et al. Paternal Influence of Sperm DNA Integrity on Early Embryonic Development. Hum Reprod (2014) 29:2402–12. doi: 10.1093/humrep/deu228
6. Lotti F, Maggi M. Ultrasound of the Male Genital Tract in Relation to Male Reproductive Health. Hum Reprod Update (2015) 21:56–83. doi: 10.1093/humupd/dmu042
8. Ferlin A, Foresta C. New Genetic Markers for Male Infertility. Curr Opin Obstet Gynecol (2015) 26:193–8. doi: 10.1097/GCO.0000000000000061
9. Alahmar AT. Role of Oxidative Stress in Male Infertility: An Updated Review. J Hum Reprod Sci (2019) 12:4. doi: 10.4103/jhrs.JHRS_150_18
10. Walczak JR, Wolski JK, Slowikowska HJ. The Role of Oxidative Stress and Antioxidants in Male Fertility. Cent European J Urol (2013) 66:60–7. doi: 10.5173/ceju.2013.01.art19
11. Cui T, Kovell RC, Brooks DC, Terlecki RP. A Urologist’s Guide to Ingredients Found in Top-Selling Nutraceuticals for Men’s Sexual Health. J Sex Med (2015) 12:2105–17. doi: 10.1111/jsm.13013
12. EFSA. European Food Safety Authority (2021). Available at: http://data.europa.eu/eli/reg/2006/1924/2012-11-29/ (Accessed February 1, 2021).
13. Calogero AE, Aversa A, La Vignera S, Corona G, Ferlin A. The Use of Nutraceuticals in Male Sexual and Reproductive Disturbances: Position Statement From the Italian Society of Andrology and Sexual Medicine (SIAMS). J Endocrinol Invest (2017) 40:1389–97. doi: 10.1007/s40618-017-0699-6
14. EUR-Lex.Internet. Available at: https://eurlex.europa.eu/legalcontent/EN/ALL/?uri=celex%3A32002L0046 (Accessed August 2, 2021).
15. U.S Food & Drug Administration. Dietary Supplements Guidance Documents & Regulatory Information. Available at: https://www.fda.gov/food/guidance-documents-regulatory-information-topic-food-and-dietary-supplements/dietary-supplements-guidance-documents-regulatory-information (Accessed December 3, 2021).
16. Henkel R, Sandhu IS, Agarwal A. The Excessive Use of Antioxidant Therapy: A Possible Cause of Male Infertility? Andrologia (2019) 51:0303–4569. doi: 10.1111/and.13162
17. Garolla A, Petre GC, Francini-Pesenti F, De Toni L, Vitagliano A, Di Nisio A, et al. Dietary Supplements for Male Infertility: A Critical Evaluation of Their Composition. Nutrients (2020) 12:1472. doi: 10.3390/nu12051472
18. Salas HA, Rosique EN, Becerra TN, Vizmanos B, Bulló M, Salas SJ. The Effect of Nutrients and Dietary Supplements on Sperm Quality Parameters: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Adv Nutr (2018) 9:833–48. doi: 10.1093/advances/nmy057
19. Sanagoo S, Oskouei BS, Abdollahi NG, Salehi PH, Hazhir N, Farshbaf KA. Effect of Tribulus Terrestris L. @ on Sperm Parameters in Men With Idiopathic Infertility: A Systematic Review. Complement Ther Med (2019) 42:95–103. doi: 10.1016/j.ctim.2018.09.015
20. Kuchakulla M, Soni Y, Patel P, Parekh N, Ramasamy R. A Systematic Review and Evidence-Based Analysis of Ingredients in Popular Male Fertility Supplements. Urology (2019) 136:133–41. doi: 10.1016/j.urology.2019.11.007
21. Majzoub A, Agarwal A. Antioxidant Therapy in Idiopathic Oligoasthenoteratozoospermia. Indian J Urol (2017) 33:207–14. doi: 10.4103/iju.IJU_15_17
22. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (2021) 372:71. doi: 10.1136/bmj.n71
23. Italian Ministry of Health. Available at: http://www.salute.gov.it/portale/temi/p26.jsp?id=3668&area=Alimenti%20particolari%20e%20integrat ri&menu=registri (Accessed October 3, 2021).
24. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE Guidelines: 1. Introduction-GRADE Evidence Profiles and Summary of Findings Tables. Clin Epidemiol (2011) 64:383–94. doi: 10.1016/j.jclinepi.2010.04.026
25. Wong WWY, Merkus HHMW, Thomas CM, Menkveld R, Zielhuis GA, Steegers TRP. Effects of Folic Acid and Zinc Sulfate on Male Factor Subfertility: A Double-Blind, Randomized, Placebo-Controlled Trial. Fertil Steril (2002) 77:491–8. doi: 10.1016/S0015-0282(01)03229-0
26. Ebisch IM, Pierik FH, De Jong FH, Thomas CM, Steegers-Theunissen RP. Does Folic Acid and Zinc Sulphate Intervention Affect Endocrine Parameters and Sperm Characteristics in Men? Int J Androl (2003) 29:339–45. doi: 10.1111/j.1365-2605.2005.00598.x
27. Azizollahi G, Azizollahi S, Babaei H, Kianinejad M, Baneshi MR, Nematollahi-mahani SN. Effects of Supplement Therapy on Sperm Parameters, Protamine Content and Acrosomal Integrity of Varicocelectomized Subjects. J Assist Reprod Genet (2013) 30:593–9. doi: 10.1007/s10815-013-9961-9
28. Omu A, Al-Azemi MK, Kehinde EO, Anim JT, Oriowo MA, Mathew TC. Indications of the Mechanisms Involved in Improved Sperm Parameters by Zinc Therapy. Med Princ Pract (2008) 17:108–16. doi: 10.1159/000112963
29. Schisterman EF, Sjaarda LA, Clemons T, Carrell DT, Perkins NJ, Johnstone E, et al. Effect of Folic Acid and Zinc Supplementation in Men on Semen Quality and Live Birth Among Couples Undergoing Infertility Treatment: A Randomized Clinical Trial. JAMA (2020) 323:35–48. doi: 10.1001/jama.2019.18714
30. Raigani M, Yaghmaei B, Amirjannti N, Lakpour N, Akhondi MM, Zeraati H. The Micronutrient Supplements, Zinc Sulphate and Folic Acid, Did Not Ameliorate Sperm Functional Parameters in Oligoasthenoteratozoospermic Men. Andrologia (2014) 469:956–62. doi: 10.1111/and.12180
31. Scott R, MacPherson A, Yates RW, Hussain B, Dixon J. The Effect of Oral Selenium Supplementation on Human Sperm Motility. Br J Urol (1998) 82:76–80. doi: 10.1046/j.1464-410x.1998.00683.x
32. Safarinejad MR, Safarinejad S. Efficacy of Selenium and/or N-Acetyl-Cysteine for Improving Semen Parameters in Infertile Men: A Double-Blind, Placebo Controlled, Randomized Study. J Urol (2009) 181:741–51. doi: 10.1016/j.juro.2008.10.015
33. Hawkes WC, Alkan Z, Wong K. Selenium Supplementation Does Not Affect Testicular Selenium Status or Semen Quality in North American Men. J Androl (2009) 30:525–33. doi: 10.2164/jandrol.108.006940
34. Goodhope CD. The Treatment of Oligospermia With Stilbestrol and Vitamin B. Fertil Steril (1961) 12:469–73. doi: 10.1016/S0015-0282(16)34271-6
35. Kumamoto Y, Maruta H, Ishigami J, Kamidono S, Orikasa S, Kimura M, et al. Clinical Efficacy of Mecobalamin in the Treatment of Oligozoospermia—Results of Double-Blind Comparative Clinical Study. Hinyokika Kiyo (1988) 34:1109–32.
36. Moriyama H, Nakamura K, Sanda N, Fujiwara E, Seko S, Yamazaki A, et al. Studies on the Usefulness of a Long-Term, High-Dose Treatment of Methylcobalamin in Patients With Oligozoospermia. Hinyokika Kiyo (1987) 33:151–6.
37. Calogero AE, Gullo G, La Vignera S, Condorelli RA, Vaiarelli A. Myoinositol Improves Sperm Parameters and Serum Reproductive Hormones in Patients With Idiopathic Infertility: A Prospective Double-Blind Randomized Placebo-Controlled Study. Andrology (2015) 3:491–5. doi: 10.1111/andr.12025
38. Stanislavov R, Nikolova V, Rohdewald P. Improvement of Seminal Parameters With Prelox: A Randomized, Double-Blind, Placebo-Controlled, Cross-Over Trial. Phytother Res (2009) 23:297–302. doi: 10.1002/ptr.2592
39. Nikolova V, Stanislavov R, Vatev I, Nalbanski B, Pŭnevska M. Sperm Parameters in Male Idiopathic Infertility After Treatment With Prelox. Akush Ginekol (Sofiia) (2007) 46:7–12.
40. Stanislavov R, Rohdewald P. Sperm Quality in Men Is Improved by Supplementation With a Combination of L-Arginine, L-Citrullin, Roburins and Pycnogenol®. Minerva Urol Nefrol (2014) 66:217–23.
41. Haghighian HK, Haidari F, Mohammadi-Asl J, Dadfar M. Randomized, Triple-Blind, Placebo-Controlled Clinical Trial Examining the Effects of Alpha-Lipoic Acid Supplement on the Spermatogram and Seminal Oxidative Stress in Infertile Men. Fertil Steril (2015) 104:318–24. doi: 10.1016/j.fertnstert.2015.05.014
42. Mehni MN, Ketabchi AA, Hosseini E. Combination Effect of Pentoxifylline and L-Carnitine on Idiopathic Oligoasthenoteratozoospermia. Iran J Reprod Med (2014) 12:817–24.
43. Peivandi S, Abasali K, Narges M. Effects of L-Carnitine on Infertile Men’s Spermogram; a Randomised Clinical Trial. J Reprod Infertil (2010) 10:331.
44. Lenzi A, Lombardo F, Sgrò P, Salacone P, Caponecchia L, Dondero F, et al. Use of Carnitine Therapy in Selected Cases of Male Factor Infertility: A Double-Blind Crossover Trial. Fertil Steril (2003) 79:292–300. doi: 10.1016/S0015-0282(02)04679-4
45. Balercia G, Regoli F, Armeni T, Koverech A, Mantero F, Boscaro M. Placebo-Controlled Double-Blind Randomized Trial on the Use of L-Carnitine, L-Acetylcarnitine, or Combined L-Carnitine and L-Acetylcarnitine in Men With Idiopathic Asthenozoospermia. Fertil Steril (2005) 84:662–71. doi: 10.1016/j.fertnstert.2005.03.064
46. Sigman M, Glass S, Campagnone J, Pryor JL. Carnitine for the Treatment of Idiopathic Asthenospermia: A Randomized, Double-Blind, Placebo-Controlled Trial. Fertil Steril (2006) 85:1409–14. doi: 10.1016/j.fertnstert.2005.10.055
47. Ciftci H, Verit A, Savas M, Yeni E, Erel O. Effects of N-Acetylcysteine on Semen Parameters and Oxidative/Antioxidant Status. Urology (2009) 74:73–6. doi: 10.1016/j.urology.2009.02.034
48. Nadjarzadeh A, Sadeghi MR, Amirjannati N, Vafa MR, Motevalian SA, Gohari MR, et al. Coenzyme Q10 Improves Seminal Oxidative Defense But Does Not Effect on Semen Parameters in Idiopathic Oligoasthenoteratozoospermia: A Randomized Double-Blind, Placebo-Controlled Trial. J Endocrinol Invest (2011) 34:224–8. doi: 10.3275/7572
49. Safarinejad MR, Safarinejad SS, Shafiei N, Safarinejad SS. Effects of the Reduced Form of Coenzyme Q10 (Ubiquinol) on Semen Parameters in Men With Idiopathic Infertility: A Double-Blind, Placebo Controlled, Randomized Study. J Urol (2012) 188:526–31. doi: 10.1016/j.juro.2012.03.131
50. Safarinejad MR. Efficacy of Coenzyme Q10 on Semen Parameters, Sperm Function and Reproductive Hormones in Infertile Men. J Urol (2009) 182:237–48. doi: 10.1016/j.juro.2009.02.121
51. Comhaire FH, El Garem Y, Mahmoud A, Eertmans F, Schoonjans F. Combined Conventional/Antioxidant “Astaxanthin” Treatment for Male Infertility: A Double Blind, Randomized Trial. Asian J Androl (2005) 7:257–62. doi: 10.1111/j.1745-7262.2005.00047.x
52. D’Aniello G, Ronsini S T, Notari T, Grieco N, Infante V, D’Angelo N, et al. D-Aspartate, a Key Element for the Improvement of Sperm Quality. Adv Sex Med (2012) 2:47–53.
53. Ismail SB, Bakar MB, Nik Hussain NH, Norhayati MN, Sulaiman SA, Jaafar H, et al. Comparison on the Effects and Safety of Tualang Honey and Tribestaan in Sperm Parameters, Erectile Function and Hormonal Profile Among Oligospermia Males. Evid Based Complement Altern Med (2014) 2014:126138. doi: 10.1155/2014/126138
54. Setiawan L. Tribulus Terrestris L. Extract Improves Spermatozoa Motility and Increases the Efficiency of Acrosome Reaction in Subjects Diagnosed With Oligoastheno-Teratozoospermia. Adv Male Reprod Physiol (1996) 2:105–14.
55. Sellandi TM, Thakar AB, Baghel MS. Clinical Study of Tribulus Terrestris Linn. In Oligozoospermia: A Double Blind Study. Ayu (2012) 33:356–64. doi: 10.4103/0974-8520.108822
56. Roaiah MF, Elkhayat YI, Abd El Salam MA, Din SFG. Prospective Analysis on the Effect of Botanical Medicine (Tribulus Terrestris) on Serum Testosterone Level and Semen Parameters in Males With Unexplained Infertility. J Diet Suppl (2017) 14:25–31. doi: 10.1080/19390211.2016.1188193
57. Gulino FA, Leonardi E, Marilli I, Musmeci G, Vitales G, Leanza V, et al. Effect of Treatment With Myo-Inositol on Semen Parameters of Patients Undergoing an IVF Cycle: In Vivo Study. Gynecol Endocrinol (2016) 32:65–6. doi: 10.3109/09513590.2015.1080680
58. Suleiman S, Eamin Ali M, Zaki Z, El-Malik E, Nasr M. Lipid Peroxidation and Human Sperm Motility: Protective Role of Vitamin E. J Androl (1996) 17:530–7.
59. Greco E, Iacobelli M, Rienzi L, Ubaldi F, Ferrero S, Tesarik J. Reduction of the Incidence of Sperm DNA Fragmentation by Oral Antioxidant Treatment. J Androl (2005) 26:349–53. doi: 10.2164/jandrol.04146
60. Moilanen J, Hovatta O, Lindroth L. Vitamin E Levels in Seminal Plasma can be Elevated by Oral Administration of Vitamin E in Infertile Men. Int J Androl (1993) 16:165–6. doi: 10.1111/j.1365-2605.1993.tb01171.x
61. Kessopoulou E, Powers HJ, Sharma KK, Pearson MJ, Russell JM. A Double-Blind Randomized Placebo Cross-Over Controlled Trial Using the Antioxidant Vitamin E to Treat Reactive Oxygen Species Associated Male Infertility. Fertil Steril (1995) 64:825–31. doi: 10.1016/S0015-0282(16)57861-3
62. Rolf C, Cooper TG, Yeung CH, Nieschlag E. Antioxidant Treatment of Patients With Asthenozoospermia or Moderate Oligoasthenozoospermia With High-Dose Vitamin C and Vitamin E: A Randomized, Placebo-Controlled, Double-Blind Study. Hum Reprod (1999) 14:1028–33. doi: 10.1093/humrep/14.4.1028
63. Cyrus A, Kabir A, Goodarzi D, Moghimi M. The Effect of Adjuvant Vitamin C After Varicocele Surgery on Sperm Quality and Quantity in Infertile Men: A Double Blind Placebo Controlled Clinical Trial. Int Braz J Urol (2015) 41:230–8. doi: 10.1590/S1677-5538.IBJU.2015.02.07
64. Safarinejad MR. Effect of Omega-3 Polyunsaturated Fatty Acid Supplementation on Semen Profile and Enzymatic Anti-Oxidant Capacity of Seminal Plasma in Infertile Men With Idiopathic Oligoasthenoteratospermia: A Double-Blind, Placebo-Controlled, Randomized Study. Andrologia (2011) 43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x
65. Martinez-Soto JC, Domingo JC, Cardobilla LP, Pellicer A, Landeras JEL. Effect of Dietary DHA Supplementation on Sperm DNA Integrity. Fertil Steril (2010) 94:235–6. doi: 10.1016/j.fertnstert.2010.07.914
66. Martínez-Soto JC, Domingo JC, Cordobilla B, Nicolás M, Fernández L, Albero P, et al. Dietary Supplementation With Docosahexaenoic Acid (DHA) Improves Seminal Antioxidant Status and Decreases Sperm DNA Fragmentation. Syst Biol Reprod Med (2016) 62:387–95. doi: 10.1080/19396368.2016.1246623
67. Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Elect of DHA Supplementation on DHA Status and Sperm Motility in Asthenozoospermic Males. Lipids (2000) 35:149–54. doi: 10.1007/BF02664764
68. Nouri M, Amani R, Nasr-Esfahani M, Tarrahi MJ. The Effects of Lycopene Supplement on the Spermatogram and Seminal Oxidative Stress in Infertile Men: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Phytother Res (2019) 33:3203–11. doi: 10.1002/ptr.6493
69. Agarwal A, Finelli R, Selvam MKP, Leisegang K, Majzoub A, Tadros N, et al. A Global Survey of Reproductive Specialists to Determine the Clinical Utility of Oxidative Stress Testing and Antioxidant Use in Male Infertility. World J Mens Health (2021) 39:470–88. doi: 10.5534/wjmh.210025
70. Vitagliano A, Petre GC, Francini-Pesenti F, De Toni L, Di Nisio A, Grande G, et al. Dietary Supplements for Female Infertility: A Critical Review of Their Composition. Nutrients (2021) 13:3552. doi: 10.3390/nu13103552
71. Federsalus. Associazione Nazionale Produttori E Distributori Prodotti Salutistici. Available at: https://www.federsalus.it/il-mercato-degli-integratori-dinamiche-aggiornate-a-marzo-2019/ (Accessed January 4, 2021).
72. Falsig AL, Gleerup CS, Knudsen UB. The Influence of Omega-3 Fatty Acids on Semen Quality Markers: A Systematic PRISMA Review. Andrology (2019) 22:2047–919. doi: 10.1111/andr.12649
73. Gupta NP, Kumar R. Lycopene Therapy in Idiopathic Male Infertility–a Preliminary Report. Int Urol Nephrol (2002) 34:369–72. doi: 10.1023/A:1024483520560
74. Santos HO, Howell S, Teixeira FJ. Beyond Tribulus (Tribulus Terrestris L.): The Effects of Phytotherapics on Testosterone, Sperm and Prostate Parameters. J Ethnopharmacol (2019) 235:392–405. doi: 10.1016/j.jep.2019.02.033
75. Foresta C, Flohé L, Garolla A, Roveri A, Ursini F, Maiorino M. Male Fertility is Linked to the Selenoprotein Phospholipid Hydroperoxide Glutathione Peroxidase. Biol Reprod (2002) 67:967–71. doi: 10.1095/biolreprod.102.003822
76. Foresta C, Garolla A, Cosci I, Menegazzo M, Ferigo M, Gandin V, et al. Role of Zinc Trafficking in Male Fertility: From Germ to Sperm. Hum Reprod (2014) 29:1134–45. doi: 10.1093/humrep/deu075
77. Prasad AS, Mantzoros CS, Beck FW, Hess JW, Brewer GJ. Zinc Status and Serum Testosterone Levels of Healthy Adults. Nutrition (1995) 12:344–8. doi: 10.1016/S0899-9007(96)80058-X
78. Garolla A, Maiorino M, Roverato A, Roveri A, Ursini F, Foresta C. Oral Carnitine Supplementation Increases Sperm Motility in Asthenozoospermic Men With Normal Sperm Phospholipid Hydroperoxide Glutathione Peroxidase Levels. Fertil Steril (2005) 83:355–61. doi: 10.1016/j.fertnstert.2004.10.010
79. Singh K, Jaiswal D. One-Carbon Metabolism, Spermatogenesis, and Male Infertility. Reprod Sci (2013) 20:622–30. doi: 10.1177/1933719112459232
80. Ingles DP, Cruz Rodriguez JB, Garcia H. Supplemental Vitamins and Minerals for Cardiovascular Disease Prevention and Treatment. Curr Cardiol Rep (2020) 22:22. doi: 10.1007/s11886-020-1270-1
81. Vanderhout SM, Rastegar Panah M, Garcia-Bailo B, Grace-Farfaglia P, Samsel K, Dockray J, et al. Nutrition, Genetic Variation and Male Fertility. Transl Androl Urol (2021) 10:1410–31. doi: 10.21037/tau-20-592
82. Balercia G, Moretti S, Vignini A, Magagnini M, Mantero F, Boscaro M, et al. Role of Nitric Oxide Concentrations on Human Sperm Motility. J Androl (2004) 25:245–9. doi: 10.1002/j.1939-4640.2004.tb02784.x
83. Palencia JYP, Saraiva A, Abreu MLT, Zangeronimo MG, Schinckel AP, Pospissil Garbossa CA. Effectiveness of Citrulline and N-Carbamoyl Glutamate as Arginine Precursors on Reproductive Performance in Mammals: A Systematic Review. PloS One (2018) 13:e0209569. doi: 10.1371/journal.pone.0209569
84. Morris SM Jr. Recent Advances in Arginine Metabolism. Curr Opin Clin Nutr Metab Care (2004) 7:45–51. doi: 10.1097/00075197-200401000-00009
85. Vazquez LMH, Verón GL. Myo-Inositol in Health and Disease: Its Impact on Semen Parameters and Male Fertility. Andrology (2020) 8:277–98. doi: 10.1111/andr.12718
86. Kizilay F, Altay B. Evaluation of the Effects of Antioxidant Treatment on Sperm Parameters and Pregnancy Rates in Infertile Patients After Varicocelectomy: A Randomized Controlled Trial. Int J Impot Res (2019) 31:424–31. doi: 10.1038/s41443-018-0109-4
87. Kumar S, Murarka S, Mishra VV, Gautam AK. Environmental & Lifestyle Factors in Deterioration of Male Reproductive Health. Indian J Med Res (2014) 140:29–35.
88. Kamenov Z, Fileva S, Kalinov K, Jannini EA. Evaluation of the Efficacy and Safety of Tribulus Terrestris in Male Sexual Dysfunction? A Prospective, Randomized, Double-Blind, Placebocontrolled Clinical Trial. Maturitas (2017) 99:20–6. doi: 10.1016/j.maturitas.2017.01.011
89. Showell MG, Brown J, Yazdani A, Stankiewicz MT, Hart RJ. Antioxidants for Male Subfertility. Cochrane Database Syst Rev (2011) 19:CD007411. doi: 10.1002/14651858.CD007411.pub2
Keywords: fertility, male reproduction, nutraceuticals, semen parameters, supplements
Citation: Garolla A, Petre GC, Francini-Pesenti F, De Toni L, Vitagliano A, Di Nisio A, Grande G and Foresta C (2022) Systematic Review and Critical Analysis on Dietary Supplements for Male Infertility: From a Blend of Ingredients to a Rationale Strategy. Front. Endocrinol. 12:824078. doi: 10.3389/fendo.2021.824078
Received: 28 November 2021; Accepted: 30 December 2021;
Published: 04 February 2022.
Edited by:
Rosita Angela Condorelli, University of Catania, ItalyReviewed by:
Arcangelo Barbonetti, University of L’Aquila, ItalyFrancesco Lombardo, Sapienza University of Rome, Italy
Copyright © 2022 Garolla, Petre, Francini-Pesenti, De Toni, Vitagliano, Di Nisio, Grande and Foresta. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Garolla, YW5kcmVhLmdhcm9sbGFAdW5pcGQuaXQ=
†These authors share first authorship
 Andrea Garolla
Andrea Garolla Gabriel Cosmin Petre
Gabriel Cosmin Petre Francesco Francini-Pesenti
Francesco Francini-Pesenti Luca De Toni
Luca De Toni Amerigo Vitagliano
Amerigo Vitagliano Andrea Di Nisio1
Andrea Di Nisio1 Giuseppe Grande
Giuseppe Grande Carlo Foresta
Carlo Foresta