
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 07 January 2022
Sec. Endocrinology of Aging
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.805244
This article is part of the Research Topic The Relationship Between Sarcopenia and Metabolic Diseases, Its Formation Mechanism and Intervention Means View all 6 articles
 Jun Muratsu1,2*
Jun Muratsu1,2* Kei Kamide3,4
Kei Kamide3,4 Takashi Fujimoto1
Takashi Fujimoto1 Yasushi Takeya3,4
Yasushi Takeya3,4 Ken Sugimoto5
Ken Sugimoto5 Yoshiaki Taniyama4
Yoshiaki Taniyama4 Atsuyuki Morishima1
Atsuyuki Morishima1 Katsuhiko Sakaguchi1
Katsuhiko Sakaguchi1 Yuji Matsuzawa1
Yuji Matsuzawa1 Hiromi Rakugi4
Hiromi Rakugi4Background: Adipokine dysregulation is a key feature of insulin resistance and a metabolic syndrome associated with obesity. Low adiponectin levels are associated with higher risks of cardiovascular diseases (CVD). However, high adiponectin levels have also been associated with increased all-cause and cardiovascular mortality in the elderly. This adiponectin paradox has yet to be clarified, which has hindered our understanding of the biological role of adiponectin. Adipokine dysregulation and insulin resistance are also associated with energy-deprivation conditions, such as frailty in old age. The objective of this study was to investigate the association between plasma adiponectin and insulin resistance using the homeostasis model assessment for insulin resistance (HOMA-IR) classified by age. In particular, we sought to determine the factors of the subjects associated with both high adiponectin levels and HOMA-IR (H-adiponectin/H-HOMA) and high adiponectin levels and low HOMA-IR (H-adiponectin/L-HOMA).
Methods: The eligible subjects in this cross-sectional study were 33,216 individuals who had undergone health checkups at the Physical Checkup Center of Sumitomo Hospital between April 2008 and December 2018. After excluding 26,371 individuals who were under 60 years old, 529 who had been taking medications for diabetes mellitus, and 690 with missing data, the present study included 5,673 (3,467 males, 2,206 females) subjects with no missing data. The relationship between serum adiponectin levels and HOMA-IR was assessed using logistic regression models adjusted by clinically relevant factors.
Results: In the multivariable logistic regression analysis, age and low BMI were shown to positively correlate with the characteristics of H-adiponectin/H-HOMA. In females, systolic blood pressure was also shown to be an associated factor.
Conclusion: In conclusion, this study showed that aging or a low BMI may contribute to high adiponectin levels and insulin resistance.
Adiponectin, an adipocyte-derived cytokine, reduces levels of blood free fatty acids (FFAs) and has been associated with improved lipid profiles, better glycemic control, and reduced inflammation in diabetic patients (1). Conversely, adiponectin has also been associated with increased risks of diabetes and hypertension (2–5). A number of observations suggest that adiponectin deficiency plays a role in the development of insulin resistance and subsequent type 2 diabetes (6), with lower adiponectin levels more closely related to the degree of insulin resistance and hyperinsulinemia than to the degree of adiposity and glucose intolerance (7). Therefore, in general, a high adiponectin level is associated with a favorable cardiovascular disease (CVD) risk profile (8–10). However, the relationship between adiponectin levels and CVD is not consistent, with some studies reporting that high adiponectin levels are associated with increased all-cause and CVD mortality (11, 12). It has been hypothesized that this discrepancy may be related to the aging process in humans, and previous reports have noted that aging may be associated with elevated serum adiponectin levels in healthy adults, despite the higher CVD risk in elderly individuals (13–17). Furthermore, there is the possibility that high serum adiponectin may be associated with frailty in the elderly (18). Since frailty and sarcopenia can lead to insulin resistance in these individuals, insulin resistance could be a key player in the complex relationship among serum adiponectin levels, aging, and CVD, which is referred to as “the adiponectin paradox” (19). The aim of this study was to clarify the underlying mechanisms in the adiponectin paradox using health checkup data from a large sample size, which included assessment of serum adiponectin levels by age stratification and with a focus on insulin resistance.
The eligible subjects in this cross-sectional study were 33,216 individuals who had undergone health checkups at the Physical Checkup Center of Sumitomo Hospital between April 2008 and December 2018 (Figure 1). The health checkup program is designed to detect diseases at early stages. After excluding 26,371 individuals under 60 years old, 529 who had been taking medications for diabetes mellitus, and 690 with missing data, the present study included 5,673 (3,467 males, 2,206 females) subjects with no missing data. We excluded individuals who had been taking medications for diabetes mellitus because it had been previously reported that the antidiabetic drug pioglitazone is associated with high adiponectin levels (20).
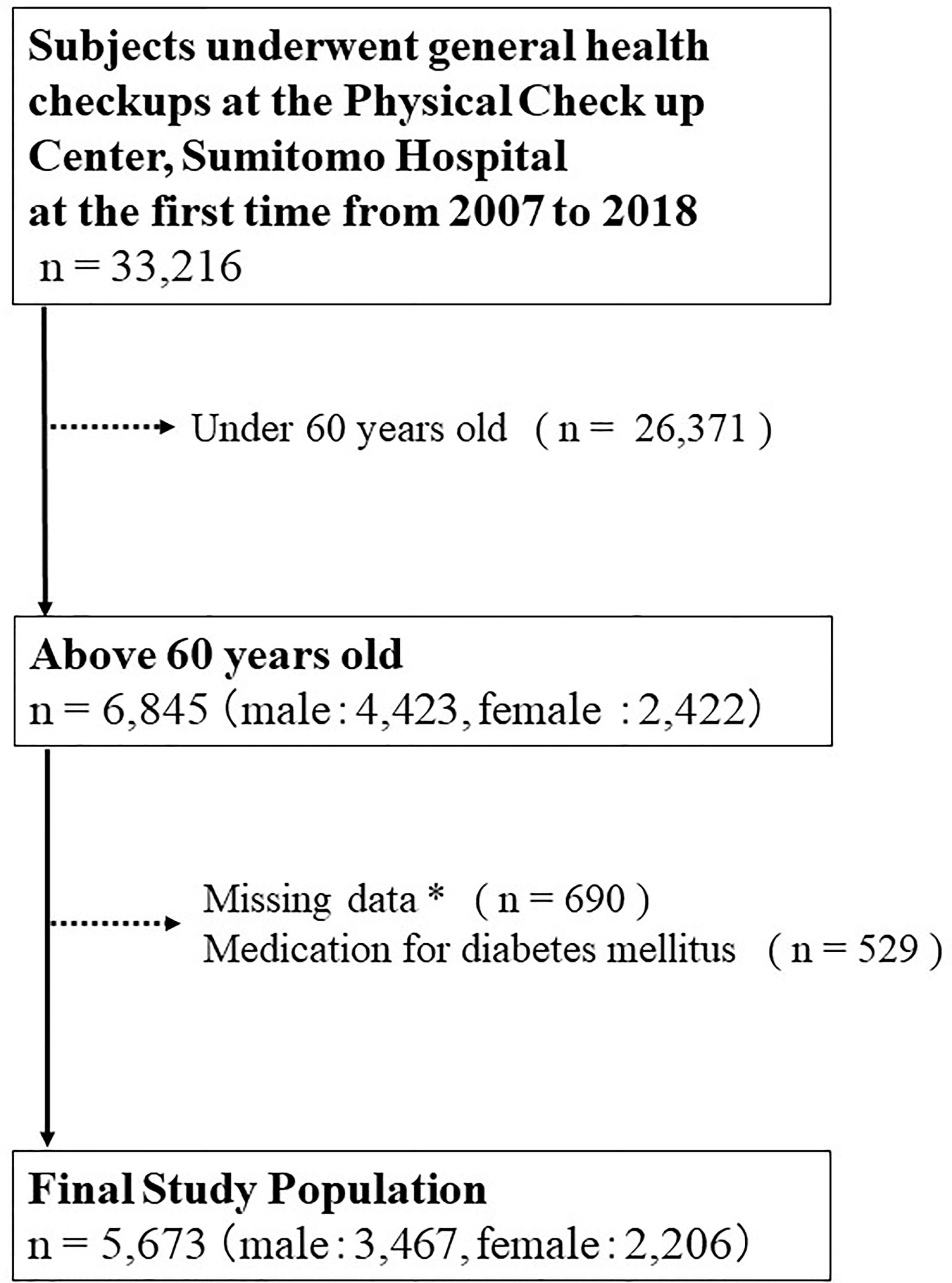
Figure 1 Inclusion and exclusion processes of the present study. * Including age, sex, body mass index, waist circumference, systolic blood pressure, diastolic blood pressure, hemoglobin, aspartate transaminase, alanine aminotransferase, albumin, total cholesterol, triglyceride, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, fasting blood sugar level, creatinine, uric acid, estimated glomerular filtration rate (e-GFR), hemoglobin A1c, insulin, adiponectin, smoking habits, alcohol consumption per day, medical history of hypertension, diabetes mellitus, dyslipidemia, stroke, hyperuricemia or coronary artery disease.
The study was approved by the human ethics committees of Sumitomo Hospital and was conducted according to the principles of the Declaration of Helsinki (approval No. 2021-38). Written informed consent to provide medical information and blood samples was obtained before the checkup examinations from all participants, and each subject had the right to refuse the use of their results.
The subjects’ height and weight were measured while they were wearing light underwear and without shoes, and their waist circumferences were measured at the level of the umbilicus. Blood pressures were measured using an automatic sphygmomanometer (BP-203RVIII; Colin, Tokyo, Japan) while the subjects were sitting. Information on their medical histories was evaluated using standardized self-administered questionnaires and interviews by doctors. Laboratory data and adiponectin levels were measured after overnight fasting. In Japan, the measurement method for HbA1c has changed from JDS to NGSP. Therefore, all HbA1c (JDS) data were converted from JDS to NGSP according to the guidelines of the Japan Diabetes Society as follows: HbA1c (NGSP) (%) = 1.02 × HbA1c (JDS) (%) + 0.25% (21). To calculate eGFR, the Japanese formula was used (eGFR [mL/min/1.73 m2] = 194 × serum creatinine [mg/dL] −1.094 × age [year] − 0.287 × 0.739 [for females]) (22). Serum adiponectin levels, which included both low-molecular weight (LMW) isoforms (approximately 30-70 kDa) and high-molecular weight (HMW) isoforms (12-, 18-mers), were measured by a latex particle-enhanced turbidimetric immunoassay (human adiponectin latex kit; Otsuka Pharmaceutical Co. Ltd, Tokyo, Japan) as previously reported (23). Adiponectin is inversely correlated with visceral adiposity (13), and it has been demonstrated that hypoadiponectinaemia is associated with insulin resistance (24). On the other hand, the homeostasis model assessment for insulin resistance (HOMA-IR) is often used in clinical practice as an index of insulin resistance and is 104 calculated as follows: HOMA-IR = fasting blood sugar (mg/dL) × IRI/405 (25, 26). HOMA-IR reflects both the presence and extent of any insulin resistance and is a terrific way of revealing the dynamic between fasting blood sugar and responsive insulin. Theoretically, if the level of adiponectin is high, HOMA-IR will be low. However, it is possible for subjects to have both high adiponectin and HOMA-IR, and our aim was to determine the factors that give rise to this.
Comparisons between two groups were analyzed using an unpaired t-test or the Mann-Whitney U test for continuous variables and the χ2 test for categorical variables. The level of serum adiponectin is affected by sex, body fat mass, several pathological factors or therapeutic interventions, and possibly age (13). Since the values of adiponectin levels differ depending on sex, the median values were calculated and divided into four groups: high adiponectin + high HOMA-IR (H-adiponectin/H-HOMA); high adiponectin + low HOMA-IR (H-adiponectinL-HOMA); low adiponectin + high HOMA-IR (L-adiponectin/H-HOMA); and low adiponectin + low HOMA-IR (L-adiponectin/L- HOMA). These groups were used to assess the potential role of adiponectin in aging. The median levels of adiponectin were 7.9 and 13.2 μg/mL for males and female, respectively, while median HOMA-IR values for males and females were 1.32 and 1.18. We defined adiponectin levels above 7.9 μg/mL in males and 13.2 μg/mL in females as high (H-adiponectin). Conversely, adiponectin levels less than 7.9 μg/mL in males and 13.2 μg/mL in females were defined as low (L-adiponectin). In addition, we defined HOMA-IR levels above 1.32 in males and 1.18 in females as high (H-HOMA) and levels less than 1.32 in males and 1.18 in females as low (L-HOMA). To assess the characteristics of the subjects who were H-adiponectin/H-HOMA, their odds ratios were calculated in an adjusted multivariable logistic regression model and divided by sex. In addition, the association of age with H-adiponectin/H-HOMA and L-adiponectin/H-HOMA was assessed in three subgroups with BMI values of <22.0, 22.0-24.9, and ≥25 kg/m2, and by sex. Each odds ratio value indicated a degree of influence on H-adiponectin/H-HOMA. P-values < 0.05 were considered to be statistically significant. Categorical variables are expressed as numbers (percentages), and continuous variables are shown as means ± standard deviation or medians (interquartile range), as appropriate. All statistical analyses were performed using Stata version 14.2 (Stata Corp, http://www.stata.com).
Baseline clinical characteristics of this study are shown in Table 1. The 5,673 subjects included 3,467 males and 2,206 females, with average ages of 66 and 67, respectively. Medical histories of hypertension, hyperuricemia, or coronary artery disease were shown to be significantly higher in males than females. On the other hand, a medical history of dyslipidemia was shown to be significantly higher in females. The females also showed a higher prevalence of anemia and lower BMI values, while males had lower levels of total cholesterol. The median levels of adiponectin were 7.9 and 13.2 μg/mL for males and females, respectively, while median HOMA-IR values for males and females were 1.32 and 1.18. Based on these results, we divided all subjects into the H-adiponectin/H-HOMA, H-adiponectin/L-HOMA, L-adiponectin/H-HOMA, and L-adiponectin/L-HOMA groups. For males, these groups comprised 599 (17.3%), 1,135 (32.7%), 1,146 (33.1%), and 587 (16.9%) subjects, respectively, while the corresponding groups for females comprised 396 (18.0%), 695 (31.5%), 708 (32.1%), and 407 (18.5%) subjects (Figure 2). In order to clarify the characteristics associated with H-adiponectin/H-HOMA, we compared the subjects in the H-adiponectin/H-HOMA and H-adiponectin/L-HOMA groups (Tables 2A, 2B) and found that the H-adiponectin/H-HOMA subjects were older. However, body weight, BMI, waist circumference, and hemoglobin levels were significantly lower in the H-adiponectin/H-HOMA group. With regard to the subjects’ medical histories, no significant differences were seen between the subjects in the two groups.
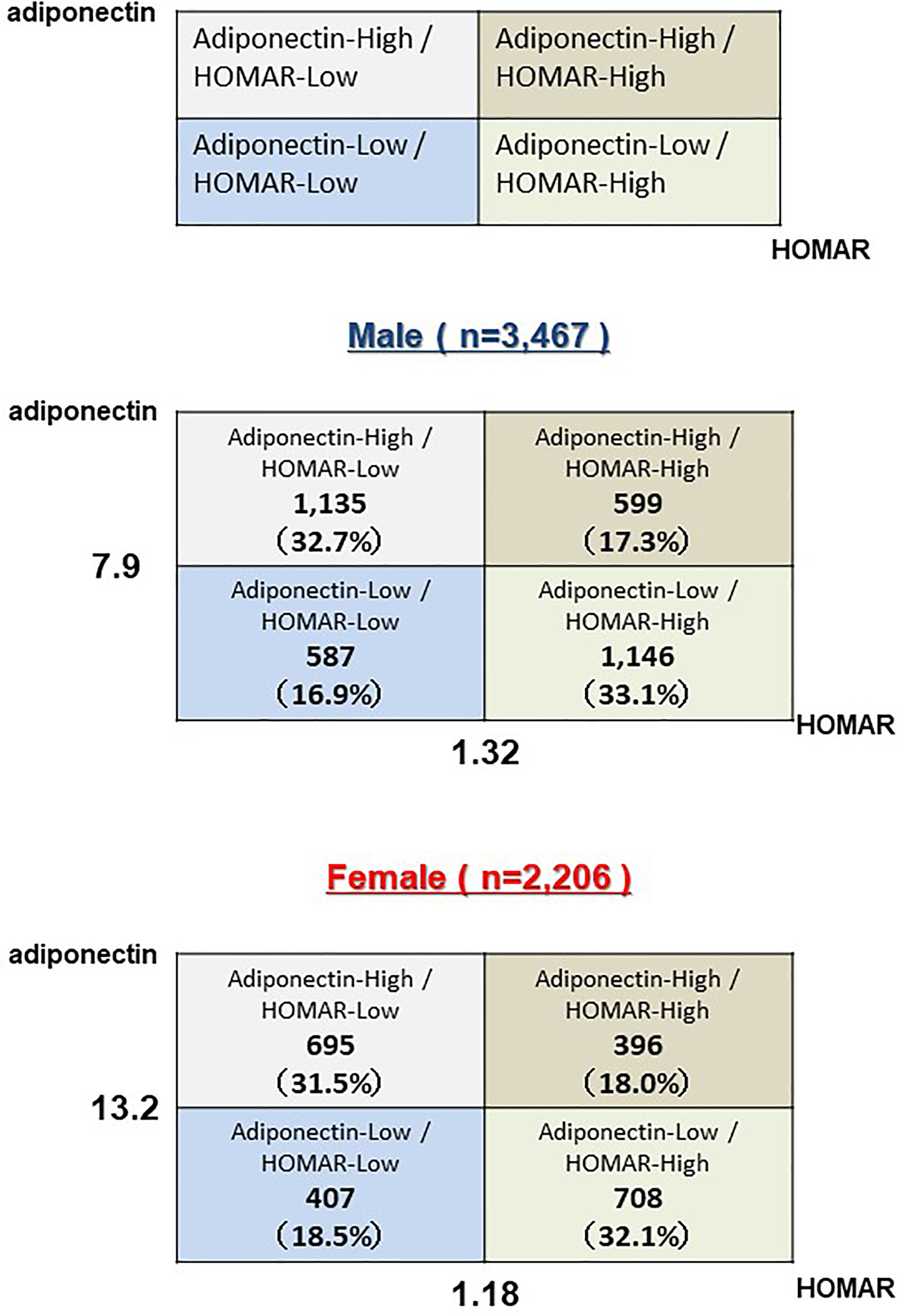
Figure 2 Subjects above 60 years old were divided into four groups (H-adiponectin/H-HOMA, H-adiponectin/L-HOMA, L-adiponectin/H-HOMA, L-adiponectin/L-HOMA) by median adiponectin and HOMA-IR values and by sex.
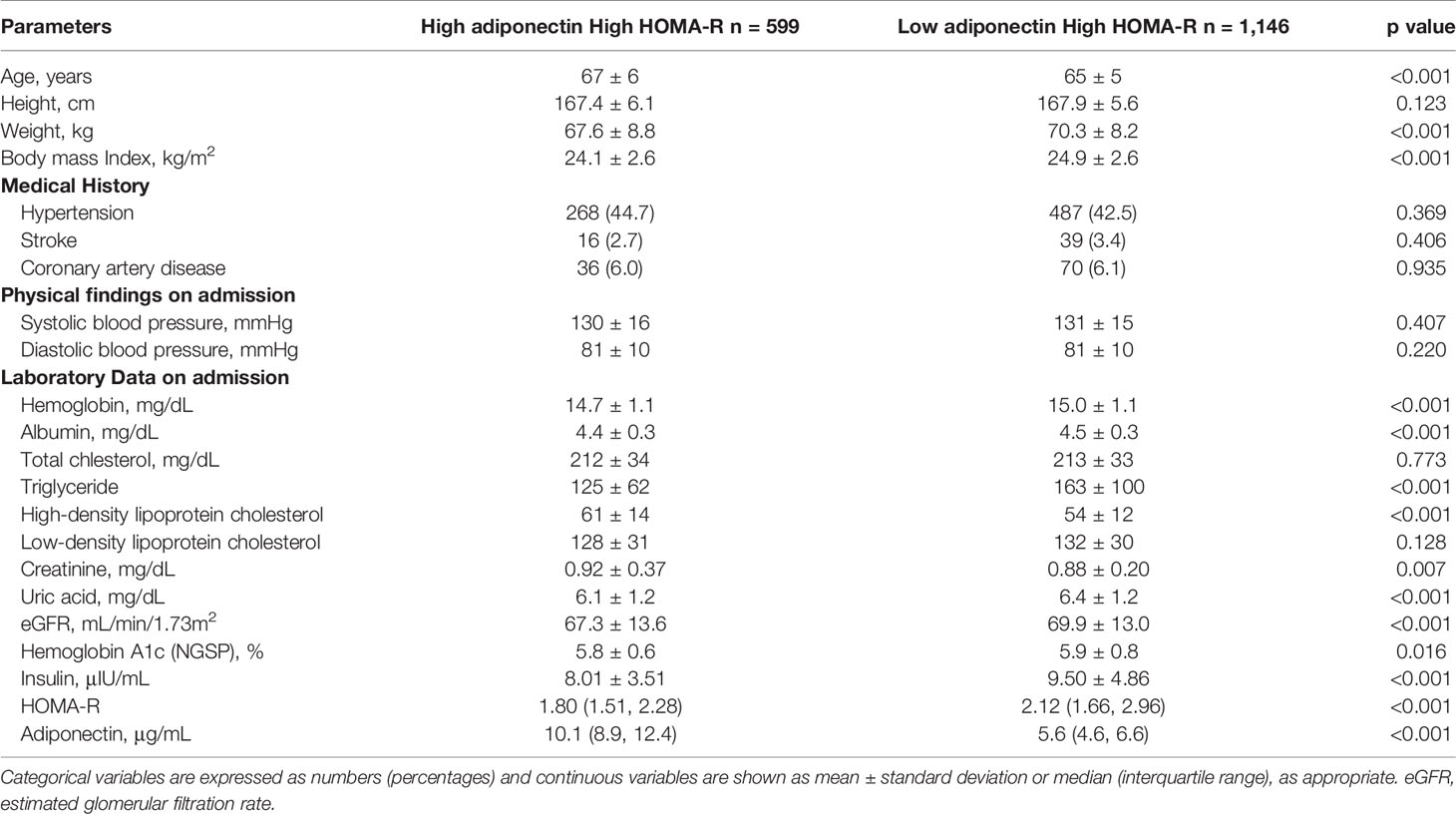
Table 2A Clinical and biological characteristics of high HOMA-R 1,745 males stratified by H-adiponectin/H-HOMA and H-adiponectin/L-HOMA.
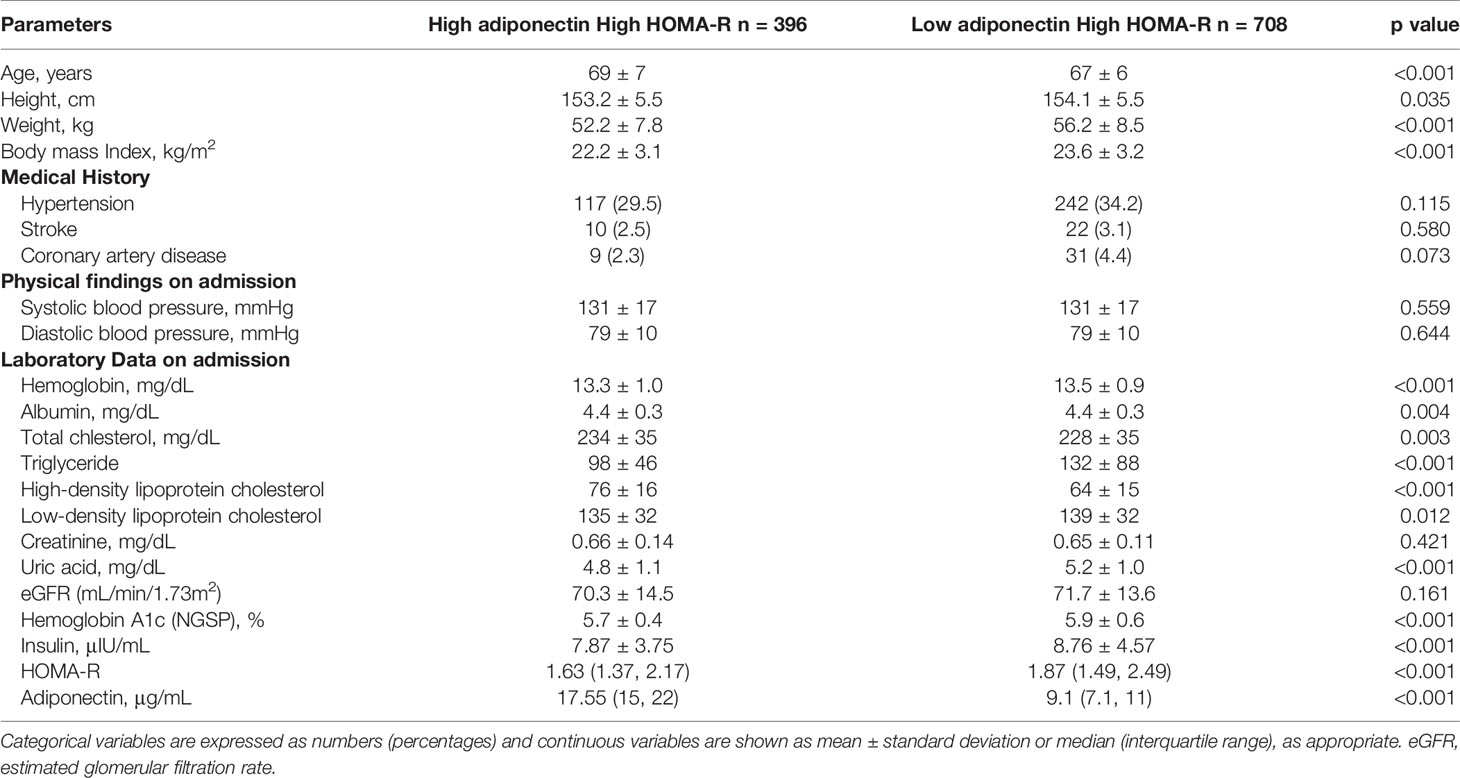
Table 2B Clinical and biological characteristics of high HOMA-R 1,104 females stratified by H-adiponectin/H-HOMA and H-adiponectin/L-HOMA.
To investigate the factors associated with H-adiponectin/H-HOMA, we obtained odds ratios with an adjusted multivariable logistic regression model (Table 3) and found age and BMI to be associated factors. In females, systolic blood pressure also showed a positive correlation. Renal function affects adiponectin levels (27). However, decreased levels of eGFR were shown in many old peoples.

Table 3 Logistic multivariable regression analysis for the combination of high adiponectin and high HOMA-R.
Therefore, in logistic multivariable regression model, eGFR were adjusted as confounding factor. In addition, we performed additional analysis without renal disease and/or eGFR < 60 mL/min/1.73m2 cases. Age and BMI showed a positive correlation as the previous analysis included kidney disease in Supplement Table A.
To clarify the effect of BMI on the association between age and H-adiponectin/H-HOMA, logistic regression analysis was performed after dividing the subjects based on BMI levels. Interestingly, age was positively correlate in BMI <25 kg/m2 (Table 4). On the other hand, to clarify the effect of age on the association between BMI and H-adiponectin/H-HOMA, logistic regression analysis was performed after dividing the subjects based on age. Both men and women with age < 70 years old showed a positive correlation with BMI, although men with age ≥ 75 years old also showed a positive correlation (Supplement Table B).
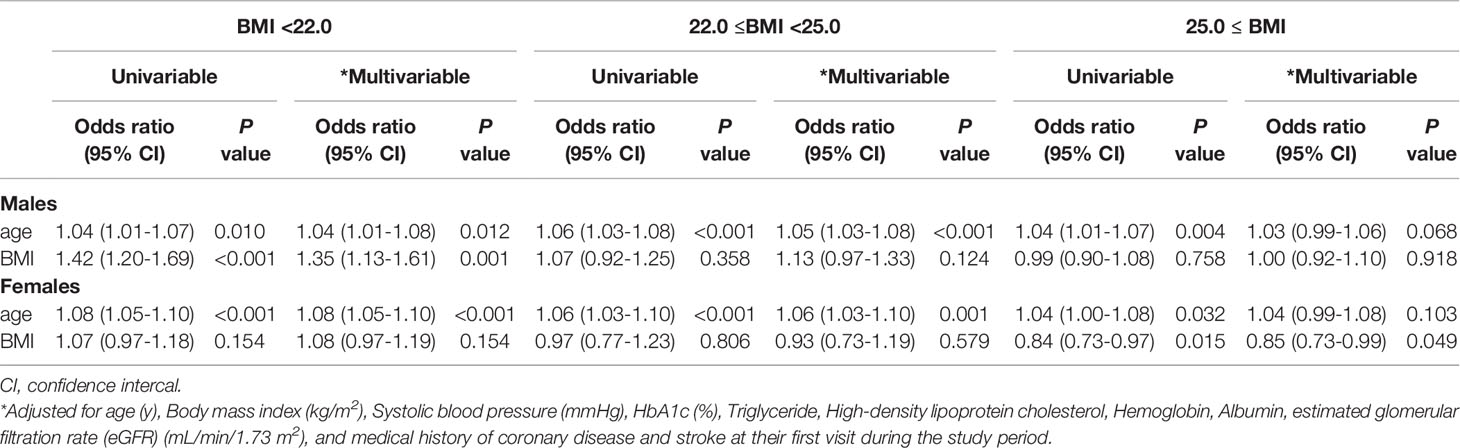
Table 4 Logistic multivariable regression analysis for High adiponectin and High HOMA-R in 5,673 subjects (3,467 males and 2,206 females) stratified by body mass index.
Previous studies have reported that age is associated with elevated serum adiponectin levels in healthy adults, despite the higher cardiovascular risk in elderly individuals (13–17, 28). In animal studies, mice with fat-specific disruption of the insulin receptor gene (FIRKO mice) are reported to have decreased adiposity, lower fasting insulin levels, and an extended lifespan. In addition, FIRKO mice also have elevated serum adiponectin levels (29, 30). Several reports have indicated the occurrence of hyper-adiponectinemia in centenarians and an inverse correlation between adiponectin and HOMA-IR (31).
However, the mechanisms underlying the age-related increase in serum adiponectin is unclear. While several reports have examined the relationship between adiponectin and age in association with visceral fat (13, 15), renal function (14, 16, 32), or sex hormones (14, 28), it remains unclear whether HOMA-IR is associated with serum adiponectin levels in the elderly. On the basis of these findings, age-related changes in serum adiponectin should be taken into account when metabolic changes are evaluated using serum adiponectin levels in clinical practice.
This study revealed that H-adiponectin/H-HOMA may be significantly associated with aging. In addition, our results showed that subjects with H-adiponectin/H-HOMA may be undernourished when compared with H-aiponectin/L-HOMA subjects. Age was shown to positively correlate with BMI values <25 kg/m2 but not with values ≥25 kg/m2 (Table 4). It has been reported that a low BMI can reflect reduced muscle mass (33, 34). On the other hand, both men and women with age < 70 years old showed a positive correlation with BMI, although men with age ≥ 75 years old also showed a positive correlation. This indicated obesity was mainly associated with the combination of high levels of adiponectin and insulin resistance below 70 years old. However, above 70 years old, other factors may strongly be associated with the combination of high levels of adiponectin and insulin resistance. Taken together, this suggest that adiponectin-independent pathways may also be involved in insulin resistance in the elderly.
A previous report examined 44 subjects using the euglycemic hyperinsulinemic glucose clamp test and observed atherosclerotic change by ultrasonography of the heart and carotid arteries. There was a significant negative correlation between aging and insulin sensitivity, while a significant increase in left ventricular mass index and carotid wall thickening accompanied by insulin resistance were shown in non-elderly subjects but not in elderly subjects. From these results, it was concluded that aging decreases insulin sensitivity even in essential hypertensive subjects and that insulin resistance does not affect the progression of cardiac hypertrophy or atherosclerosis in elderly subjects with essential hypertension (18). This, however, does not rule out the possibility that atherosclerosis is involved in insulin resistance in elderly people. In our logistic multivariable study, neither atherosclerotic disease, stroke, nor coronary artery disease were significantly associated with H-adiponectin/H-HOMA. Instead, we found that aging, undernutrition, and sarcopenia may contribute to H-adiponectin/H- HOMA. Indeed, a recent study with subjects aged approximately 83 years indicated that higher plasma adiponectin levels are associated with frailty status in older Japanese adults in the general population (35). Furthermore, a previous report found that centenarians have increased adiponectin levels and that adiponectin levels are inversely correlated with BMI, waist circumference, and the percentage of body fat. The researchers also found that two common variants of adiponectin gene ADIPOQ are associated with higher adiponectin levels and longevity (36). It has also been reported that serum adiponectin may protect against sarcopenia (37) and that adiponectin up-regulates the phosphatidylinositol 30-kinase-AKT pathway, which promotes muscle protein synthesis and prevents muscle protein degradation (38). Adipokine dysregulation is associated with wasting syndromes such as cachexia and sarcopenia, suggesting that adipose endocrine function is essential for maintaining whole-body energy homeostasis, which is indispensable for a multitude of physiological functions under both energy excess and deprivation conditions (39). Since our study subjects were aged 60 years and older, aging may affect insulin resistance from around 60 years old.
In the previous cohort study, the association of adiponectin with the factors studied was strikingly similar for men and women. Sex differences in circulating adiponectin levels in older adults cannot be explained by sex hormone regulation (40). Our study showed that systolic blood pressure was significantly associated with H-adiponectin/H-HOMA in women. Some reports indicated that high adiponectin levels failed to protect against the development of hypertension in menopausal women. Involvement of adiponectin in autoimmune complex with loss of antioxidative-antiatherogenic properties may be underlying (41). In addition, the other reports showed that in late postmenopausal women with normal renal function, high adiponectin level is associated with favorable lipid profiles (42). Our study showed that HDL-C was positively associated with H-adiponectin/H-HOMA in women. There was possibility that HDL-C may be compensatory or protective for H-adiponectin/H-HOMA in postmenopausal women. Previous reports indicated that trunk fat and leg fat were oppositely associated with adiponectin in older men and women (43). Further studies are needed about fat distribution associated with H-adiponectin/H-HOMA.
The present study had several limitations. First, lifestyle behaviors and medical histories were evaluated using a self-administered questionnaire, and participants may have overstated the healthiness of their lifestyles (44). Further evaluation of these factors based on an established questionnaire are necessary. Second, income, social participation, working style, physical activity level, and education level were not included as correction factors. Third, although standardized health examinations were conducted, the present study included subjects who underwent health checkups at a single center in Japan and included mostly Japanese subjects. Previously, there were some reports about difference of adiponectin levels among race-ethnicity (45–47). Thus, the generalizability of results need to be verified in multi-center study. Fourth, other terms not included in our multivariable logistic regression model may also be associated with H-adiponectin/H-HOMA. Finally, this study is the epidemiological study to speculate the biological mechanism. We are not able to do the basic experiments because of clinical hospital and health-checkup center. We hope the basic experiments could be performed to clarify the molecular mechanism underlining adiponectin paradox phenomena after this epidemiological study.
This study indicated that high adiponectin levels and insulin resistance may be associated with aging or low nutrition status. In addition, a mechanism of insulin resistance independent from adiponectin may be linked to low nutrition status or sarcopenia. Since renal function deteriorates withaging and insulin resistance is induced by low nutrition status or muscle loss, these factors could result in the overall effect of high adiponectin levels and insulin resistance in elderly people. Based on these findings, when we clinically evaluate adiponectin levels of elderly subjects, it is necessary to pay attention to their nutrition status and/or sarcopenia. A further longitudinal study is necessary to clarify the role of adiponectin in elderly people.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
The study was approved by the human ethics committees of Sumitomo Hospital and was conducted according to the principles of the Declaration of Helsinki (approval No. 2021-38). The patients/participants provided their written informed consent to participate in this study.
JM and KK planned this study and interpreted the data. JM, TF, AM, KaS and YM recruited and collected clinical cases. JM conducted the statistical analysis of relevant data. JM and KK participated in the writing and modification of the article. YaT, KeS, YoT, YM and HR critically revised the manuscript. All authors have read and approved the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.805244/full#supplementary-material
1. Mantzoros CS, Li T, Manson JE, Meigs JB, Hu FB. Circulating Adiponectin Levels Are Associated With Better Glycemic Control, More Favorable Lipid Profile, and Reduced Inflammation in Women With Type 2 Diabetes. J Clin Endocrinol Metab (2005) 90(8):4542–8. doi: 10.1210/jc.2005-0372
2. Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin Levels and Risk of Type 2 Diabetes: A Systematic Review and Meta-Analysis. JAMA (2009) 302(2):179–88. doi: 10.1001/jama.2009.976
3. Obata Y, Yamada Y, Takahi Y, Baden MY, Saisho K, Tamba S, et al. Relationship Between Serum Adiponectin Levels and Age in Healthy Subjects and Patients With Type 2 Diabetes. Clin Endocrinol (Oxf) (2013) 79(2):204–10. doi: 10.1111/cen.12041
4. Kashiwagi R, Yamada Y, Ito Y, Mitsui Y, Sakaue T, Iwamoto R, et al. Increase in Adiponectin Level Prevents the Development of Type 2 Diabetes in Japanese Men With Low Adiponectin Levels. J Endocr Soc (2018) 2(7):753–64. doi: 10.1210/js.2018-00033
5. Baden MY, Yamada Y, Takahi Y, Obata Y, Saisho K, Tamba S, et al. Association of Adiponectin With Blood Pressure in Healthy People. Clin Endocrinol (Oxf) (2013) 78(2):226–31. doi: 10.1111/j.1365-2265.2012.04370.x
6. Kadowaki T, Yamauchi T, Kubota N, Hara K, Ueki K, Tobe K. Adiponectin and Adiponectin Receptors in Insulin Resistance, Diabetes, and the Metabolic Syndrome. J Clin Invest (2006) 116(7):1784–92. doi: 10.1172/JCI29126
7. Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in Obesity and Type 2 Diabetes: Close Association With Insulin Resistance and Hyperinsulinemia. J Clin Endocrinol Metab (2001) 86(5):1930–5. doi: 10.1210/jcem.86.5.7463
8. Pischon T, Girman CJ, Hotamisligil GS, Rifai N, Hu FB, Rimm EB. Plasma Adiponectin Levels and Risk of Myocardial Infarction in Men. JAMA (2004) 291(14):1730–7. doi: 10.1001/jama.291.14.1730
9. Han SH, Quon MJ, Kim JA, Koh KK. Adiponectin and Cardiovascular Disease: Response to Therapeutic Interventions. J Am Coll Cardiol (2007) 49(5):531–8. doi: 10.1016/j.jacc.2006.08.061
10. Sattar N, Wannamethee G, Sarwar N, Tchernova J, Cherry L, Wallace AM, et al. Adiponectin and Coronary Heart Disease: A Prospective Study and Meta-Analysis. Circulation (2006) 114(7):623–9. doi: 10.1161/CIRCULATIONAHA.106.618918
11. Wannamethee SG, Whincup PH, Lennon L, Sattar N. Circulating Adiponectin Levels and Mortality in Elderly Men With and Without Cardiovascular Disease and Heart Failure. Arch Intern Med (2007) 167(14):1510–7. doi: 10.1001/archinte.167.14.1510
12. Laughlin GA, Barrett-Connor E, May S, Langenberg C. Association of Adiponectin With Coronary Heart Disease and Mortality: The Rancho Bernardo Study. Am J Epidemiol (2007) 165(2):164–74. doi: 10.1093/aje/kwk001
13. Cnop M, Havel PJ, Utzschneider KM, Carr DB, Sinha MK, Boyko EJ, et al. Relationship of Adiponectin to Body Fat Distribution, Insulin Sensitivity and Plasma Lipoproteins: Evidence for Independent Roles of Age and Sex. Diabetologia (2003) 46(4):459–69. doi: 10.1007/s00125-003-1074-z
14. Isobe T, Saitoh S, Takagi S, Takeuchi H, Chiba Y, Katoh N, et al. Influence of Gender, Age and Renal Function on Plasma Adiponectin Level: The Tanno and Sobetsu Study. Eur J Endocrinol (2005) 153(1):91–8. doi: 10.1530/eje.1.01930
15. Koh SJ, Hyun YJ, Choi SY, Chae JS, Kim JY, Park S, et al. Influence of Age and Visceral Fat Area on Plasma Adiponectin Concentrations in Women With Normal Glucose Tolerance. Clin Chim Acta (2008) 389(1-2):45–50. doi: 10.1016/j.cca.2007.11.017
16. Kruger IM, Huisman HW, Schutte AE. The Relationship Between Adiponectin, Ageing and Renal Function in a Bi-Ethnic Sample. Regul Pept (2011) 169(1-3):58–63. doi: 10.1016/j.regpep.2011.04.003
17. Cohen SS, Gammon MD, Signorello LB, North KE, Lange EM, Fowke JH, et al. Serum Adiponectin in Relation to Body Mass Index and Other Correlates in Black and White Women. Ann Epidemiol (2011) 21(2):86–94. doi: 10.1016/j.annepidem.2010.10.011
18. Kamide K, Rakugi H, Nagano M, Nakano N, Ohishi M, Higaki J, et al. Influence of Aging on Progression of Cardiovascular Complications Associated With Insulin Resistance in Patients With Essential Hypertension. Hypertens Res (1997) 20(2):127–32. doi: 10.1291/hypres.20.127
19. Woodward L, Akoumianakis I, Antoniades C. Unravelling the Adiponectin Paradox: Novel Roles of Adiponectin in the Regulation of Cardiovascular Disease. Br J Pharmacol (2017) 174(22):4007–20. doi: 10.1111/bph.13619
20. Maeda N, Takahashi M, Funahashi T, Kihara S, Nishizawa H, Kishida K, et al. PPARgamma Ligands Increase Expression and Plasma Concentrations of Adiponectin, an Adipose-Derived Protein. Diabetes (2001) 50(9):2094–9. doi: 10.2337/diabetes.50.9.2094
21. Committee of the Japan Diabetes Society on the Diagnostic Criteria of Diabetes M, Seino Y, Nanjo K, Tajima N, Kadowaki T, Kashiwagi A, et al. Report of the Committee on the Classification and Diagnostic Criteria of Diabetes Mellitus. J Diabetes Investig (2010) 1(5):212–28. doi: 10.1111/j.2040-1124.2010.00074.x
22. Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, et al. Revised Equations for Estimated GFR From Serum Creatinine in Japan. Am J Kidney Dis (2009) 53(6):982–92. doi: 10.1053/j.ajkd.2008.12.034
23. Nishimura A, Sawai T. Determination of Adiponectin in Serum Using a Latex Particle-Enhanced Turbidimetric Immunoassay With an Automated Analyzer. Clin Chim Acta (2006) 371(1-2):163–8. doi: 10.1016/j.cca.2006.03.008
24. Spranger J, Kroke A, Mohlig M, Bergmann MM, Ristow M, Boeing H, et al. Adiponectin and Protection Against Type 2 Diabetes Mellitus. Lancet (2003) 361(9353):226–8. doi: 10.1016/S0140-6736(03)12255-6
25. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis Model Assessment: Insulin Resistance and Beta-Cell Function From Fasting Plasma Glucose and Insulin Concentrations in Man. Diabetologia (1985) 28(7):412–9. doi: 10.1007/BF00280883
26. Shashaj B, Luciano R, Contoli B, Morino GS, Spreghini MR, Rustico C, et al. Reference Ranges of HOMA-IR in Normal-Weight and Obese Young Caucasians. Acta Diabetol (2016) 53(2):251–60. doi: 10.1007/s00592-015-0782-4
27. Fujita H, Morii T, Koshimura J, Ishikawa M, Kato M, Miura T, et al. Possible Relationship Between Adiponectin and Renal Tubular Injury in Diabetic Nephropathy. Endocr J (2006) 53(6):745–52. doi: 10.1507/endocrj.K06-016
28. Adamczak M, Rzepka E, Chudek J, Wiecek A. Ageing and Plasma Adiponectin Concentration in Apparently Healthy Males and Females. Clin Endocrinol (Oxf) (2005) 62(1):114–8. doi: 10.1111/j.1365-2265.2004.02182.x
29. Bluher M, Kahn BB, Kahn CR. Extended Longevity in Mice Lacking the Insulin Receptor in Adipose Tissue. Science (2003) 299(5606):572–4. doi: 10.1126/science.1078223
30. Trujillo ME, Scherer PE. Adipose Tissue-Derived Factors: Impact on Health and Disease. Endocr Rev (2006) 27(7):762–78. doi: 10.1210/er.2006-0033
31. Bik W, Baranowska-Bik A, Wolinska-Witort E, Martynska L, Chmielowska M, Szybinska A, et al. The Relationship Between Adiponectin Levels and Metabolic Status in Centenarian, Early Elderly, Young and Obese Women. Neuro Endocrinol Lett (2006) 27(4):493–500.
32. Hosokawa Y, Yamada Y, Obata Y, Baden MY, Saisho K, Ihara A, et al. Relationship Between Serum Cystatin C and Serum Adiponectin Level in Type 2 Diabetic Patients. Clin Exp Nephrol (2012) 16(3):399–405. doi: 10.1007/s10157-011-0571-5
33. Dulloo AG, Jacquet J, Solinas G, Montani JP, Schutz Y. Body Composition Phenotypes in Pathways to Obesity and the Metabolic Syndrome. Int J Obes (Lond) (2010) 34(Suppl 2):S4–17. doi: 10.1038/ijo.2010.234
34. Heymsfield SB, Scherzer R, Pietrobelli A, Lewis CE, Grunfeld C. Body Mass Index as a Phenotypic Expression of Adiposity: Quantitative Contribution of Muscularity in a Population-Based Sample. Int J Obes (Lond) (2009) 33(12):1363–73. doi: 10.1038/ijo.2009.184
35. Nagasawa M, Takami Y, Akasaka H, Kabayama M, Maeda S, Yokoyama S, et al. High Plasma Adiponectin Levels Are Associated With Frailty in a General Old-Old Population: The Septuagenarians, Octogenarians, Nonagenarians Investigation With Centenarians Study. Geriatr Gerontol Int (2018) 18(6):839–46. doi: 10.1111/ggi.13258
36. Atzmon G, Pollin TI, Crandall J, Tanner K, Schechter CB, Scherer PE, et al. Adiponectin Levels and Genotype: A Potential Regulator of Life Span in Humans. J Gerontol A Biol Sci Med Sci (2008) 63(5):447–53. doi: 10.1093/gerona/63.5.447
37. Inoue A, Cheng XW, Huang Z, Hu L, Kikuchi R, Jiang H, et al. Exercise Restores Muscle Stem Cell Mobilization, Regenerative Capacity and Muscle Metabolic Alterations via Adiponectin/AdipoR1 Activation in SAMP10 Mice. J Cachexia Sarcopenia Muscle (2017) 8(3):370–85. doi: 10.1002/jcsm.12166
38. Zhou Q, Du J, Hu Z, Walsh K, Wang XH. Evidence for Adipose-Muscle Cross Talk: Opposing Regulation of Muscle Proteolysis by Adiponectin and Fatty Acids. Endocrinology (2007) 148(12):5696–705. doi: 10.1210/en.2007-0183
39. Unger RH. Longevity, Lipotoxicity and Leptin: The Adipocyte Defense Against Feasting and Famine. Biochimie (2005) 87(1):57–64. doi: 10.1016/j.biochi.2004.11.014
40. Laughlin GA, Barrett-Connor E, May S. Sex-Specific Determinants of Serum Adiponectin in Older Adults: The Role of Endogenous Sex Hormones. Int J Obes (Lond) (2007) 31(3):457–65. doi: 10.1038/sj.ijo.0803427
41. Onat A, Aydin M, Can G, Koroglu B, Karagoz A, Altay S. High Adiponectin Levels Fail to Protect Against the Risk of Hypertension and, in Women, Against Coronary Disease: Involvement in Autoimmunity? World J Diabetes (2013) 4(5):219–25. doi: 10.4239/wjd.v4.i5.219
42. Matsui S, Yasui T, Keyama K, Tani A, Kato T, Uemura H, et al. High Adiponectin Level in Late Postmenopausal Women With Normal Renal Function. Clin Chim Acta (2014) 430:104–8. doi: 10.1016/j.cca.2013.12.037
43. Snijder MB, Flyvbjerg A, Stehouwer CD, Frystyk J, Henry RM, Seidell JC, et al. Relationship of Adiposity With Arterial Stiffness as Mediated by Adiponectin in Older Men and Women: The Hoorn Study. Eur J Endocrinol (2009) 160(3):387–95. doi: 10.1530/EJE-08-0817
44. Stockwell T, Zhao J, Sherk A, Rehm J, Shield K, Naimi T. Underestimation of Alcohol Consumption in Cohort Studies and Implications for Alcohol's Contribution to the Global Burden of Disease. Addiction (2018) 113(12):2245–9. doi: 10.1111/add.14392
45. Gardener H, Crisby M, Sjoberg C, Hudson B, Goldberg R, Mendez AJ, et al. Serum Adiponectin in Relation to Race-Ethnicity and Vascular Risk Factors in the Northern Manhattan Study. Metab Syndr Relat Disord (2013) 11(1):46–55. doi: 10.1089/met.2012.0065
46. Arslanian S, El Ghormli L, Bacha F, Caprio S, Goland R, Haymond MW, et al. Adiponectin, Insulin Sensitivity, Beta-Cell Function, and Racial/Ethnic Disparity in Treatment Failure Rates in TODAY. Diabetes Care (2017) 40(1):85–93. doi: 10.2337/dc16-0455
Keywords: adiponectin, insulin resistance, aging, body mass index, old peoples
Citation: Muratsu J, Kamide K, Fujimoto T, Takeya Y, Sugimoto K, Taniyama Y, Morishima A, Sakaguchi K, Matsuzawa Y and Rakugi H (2022) The Combination of High Levels of Adiponectin and Insulin Resistance Are Affected by Aging in Non-Obese Old Peoples. Front. Endocrinol. 12:805244. doi: 10.3389/fendo.2021.805244
Received: 30 October 2021; Accepted: 15 December 2021;
Published: 07 January 2022.
Edited by:
Jinbo Hu, First Affiliated Hospital of Chongqing Medical University, ChinaReviewed by:
Noha M. Mesbah, Suez Canal University, EgyptCopyright © 2022 Muratsu, Kamide, Fujimoto, Takeya, Sugimoto, Taniyama, Morishima, Sakaguchi, Matsuzawa and Rakugi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Muratsu, bXVyYXRzdUBjZ3QubWVkLm9zYWthLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.