- 1Department of Breast and Thyroid Surgery, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Department of Vascular Surgery, Union Hospital of Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Differentiated thyroid cancer (DTC) is the most common type of thyroid cancer. The 2015 American Thyroid Association (ATA) guidelines recommend that lobectomy is suitable for solitary intrathyroidal DTC (SI-DTC) of 1–4 cm. However, some SI-DTC patients with other high-risk characteristics still have poor prognosis and require more aggressive surgical methods. This study aimed to explore the clinical characteristics that are important for the identification and treatment of high-risk patients with SI-DTC of 1–4 cm.
Methods: The study cohort was obtained from the SEER database, consisting of data between 2004 and 2013. The outcome measures were thyroid carcinoma-specific mortality (CSM) and all-cause mortality (ACM). Patient survival curves were examined using Kaplan–Meier analyses with log-rank tests and Cox proportional hazards regression analyses. Hazard ratios (HRs) were used to show the magnitude of the effect of disease stage on DTC-specific patient mortality.
Results: The study included 55,947 patients with SI-DTC of 1–4 cm and 4,765 patients with DTC >4 cm. Tumor size, surgical approach, age, sex, race, and radiation exposure were independent risk factors for CSM and ACM. SI-DTC patients with female, age ≤45, and 1 cm< tumor size ≤2 cm were at low risk of CSM [HR = 0.014 (0.002–0.115)] and ACM [HR = 0.115 (0.077–0.171)] when stratified by age, sex, and tumor size. Compared to T3 patients, CSM was not significantly different in male patients, age >45, 2 cm< tumor size ≤3 cm [HR = 0.839 (0.414–1.700)] and male patients, age >45, 1 cm< tumor size ≤2 cm [HR = 0.751 (0.410–1.377)]. Furthermore, compared to T3 patients without extrathyroidal extension (ETE) and lymph node metastasis (LNM), more subgroups of SI-DTC of 1–4 cm had a similar prognosis. In addition, patients with SI-DTC of 1–4 cm showed similar rates of CSM and ACM to T3 patients without ETE, LNM, and distant metastasis (DM). Similar results were obtained when we set the age cut-off value as 55 years, according to the 8th edition of AJCC TNM system.
Conclusions: Our study demonstrated that sex, age, and tumor size clearly differentiate SI-DTC of 1–4 cm into low-and high-risk categories. Survival rates were significantly lower in subgroups containing old males with larger tumors compared to younger females with small tumors. Total thyroidectomy may be favored in these high-risk subgroup patients.
Introduction
Thyroid cancer is a frequently encountered endocrine malignancy, and differentiated thyroid cancer (DTC), namely, papillary thyroid cancer (PTC) and follicular thyroid cancer (FTC), are the most common types of thyroid cancer, accounting for about 90% of all thyroid malignancies (1–3). DTC is curable and inert with low mortality, but some patients have an aggressive disease course, such as recurrence (4, 5).
Effective risk stratification based on the evaluation of clinicopathological risk features is important for appropriate treatment of DTC patients to balance treatment, such as surgical benefits and complications (6, 7). This can be referenced in the American Thyroid Association’s guidelines (6th, 7th, and 8th versions) on the management of DTC (5, 8, 9). The 8th version (2015 American Thyroid Association (ATA) guideline) recommended that lobectomy is suitable for solitary intrathyroidal DTC (SI-DTC: without extrathyroidal invasion, lymph node metastasis, and distant metastasis) of tumor size between 1 and 4 cm and is accepted by most clinicians for the treatment of thyroid cancer.
However, the worldwide impact of this recommendation on the treatment of thyroid cancer remains controversial (10–12). Because other high-risk characteristics in patients with SI-DTC may also affect survival, this subgroup may not be suitable for union surgical strategy. Therefore, in this study, we tried to investigate the high-risk patients among the ST-DTC of 1–4 cm and attempted to provide clues for precision treatment of SI-DTC of 1–4 cm.
Materials and Methods
Patient Population
The DTC study cohort was obtained from the Surveillance, Epidemiology, and End Results (SEER) from 18 regions in the United States, consisting of data between 2004 and 2013. The SEER project is a United States population-based cancer registry that began in 1973 and is supported by the National Cancer Institute and the Centers for Disease Control and Prevention. It covers approximately 30% of the population of the United States and contains data across multiple geographic regions on incidence, prevalence, mortality, population-based variables, primary characteristics of the tumor, and other attributes.
Data Collection
Study participants included patients with a diagnosis of DTC, defined by a combination of International Classification of Diseases for Oncology (ICD-O) site code C73.9 (i.e., thyroid) and diagnostic codes of papillary and/or follicular histology. The diagnostic codes used in the study included “papillary carcinoma”, “papillary adenocarcinoma”, “follicular adenocarcinoma”, and “papillary & follicular adenocarcinoma”. Patients without follow-up information were excluded from the study.
Statistical Analyses
Patients were followed up until December 2013. The outcome measures were thyroid carcinoma-specific mortality and all-cause mortality. Patient survival curves were examined using Kaplan–Meier analyses with log-rank tests and Cox proportional hazards regression analyses. Hazard ratios (HRs) were used to show the magnitude of the effect of disease stage on DTC-specific patient mortality, and 95% confidence intervals (CIs) were used to indicate the significance of the risk. All P-values were 2-sided, and P <0.05 was considered significant. Analyses were performed using SPSS version 19.0, Stata/SE version 12 (Stata Corp), and GraphPad Prism version 6 (GraphPad Software Inc.).
Results
Characteristics for Patients With SI-DTC of 0–4 cm
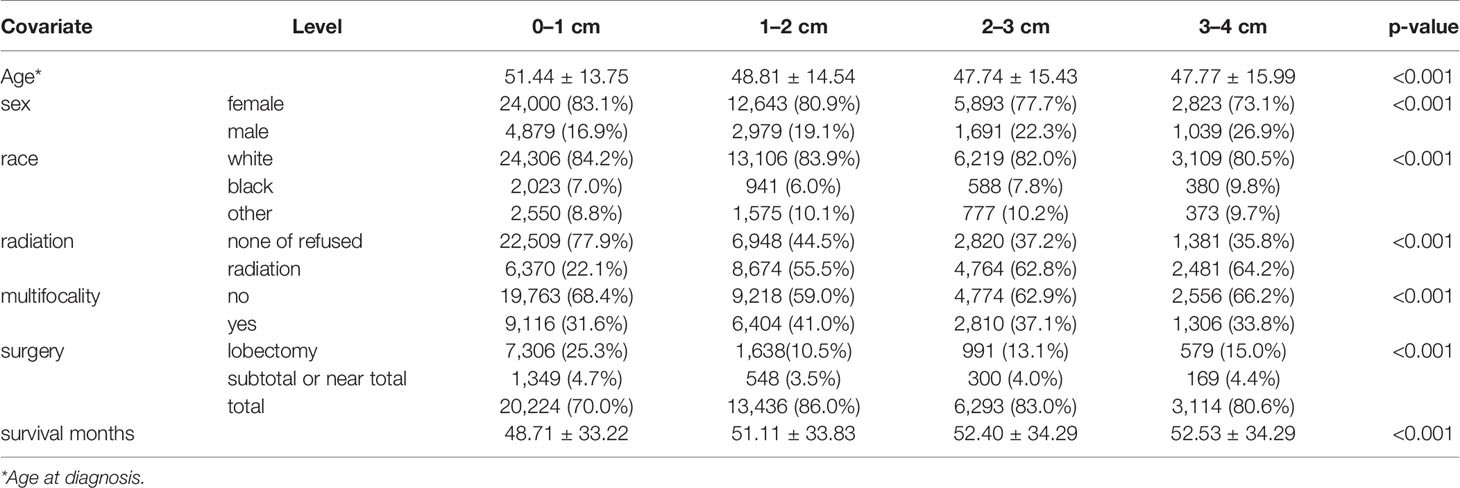
This study assessed the prognosis of 55,947 patients with SI-DTC with tumor size 1–4 cm and 4,765 patients with DTC with tumor size >4 cm. The mean age and follow-up duration according to the different histological subtypes are shown in Table 1. The clinicopathological features of patients with SI-DTC of 0–4 cm were analyzed according to tumor size (Table 1). Age at diagnosis, sex, race, radiation, multifocality, and surgical approaches all showed significant differences among the different tumor size groups.
Risk Factors for Cancer-Specific and All-Cause Mortality
The results of the univariate Cox regression analyses demonstrated that cancer-specific mortality was associated with age, sex, race, tumor size, and treatment approaches (radiation and surgery). Furthermore, all-cause mortality was also found to be associated with age, sex, race, radiation, and surgery. Meanwhile, the multivariate Cox regression model showed that tumor size, surgical approach, age, sex, race, and radiation were independent risk factors for cancer-specific mortality and all-cause mortality (Table 2).
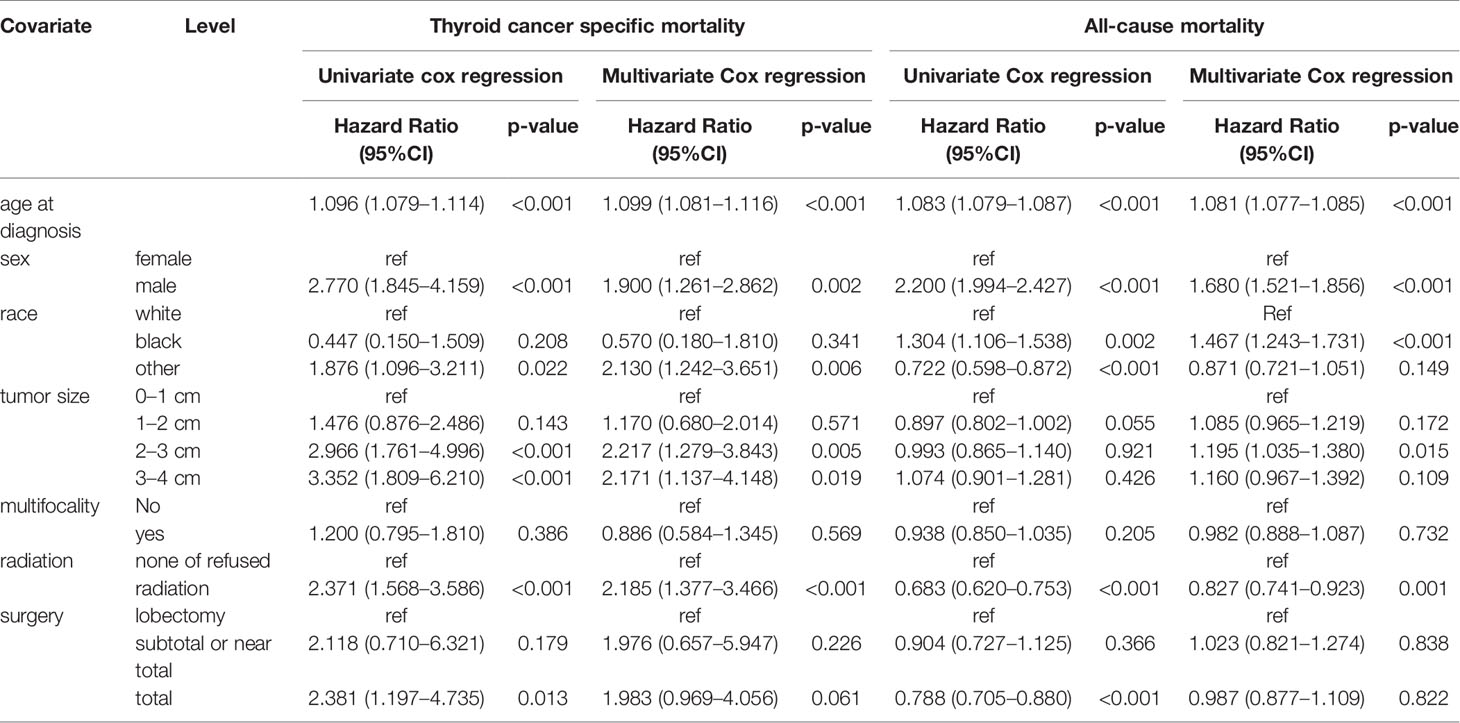
Table 2 Risk factors for survival: Outcome of thyroid cancer specific mortality and all-cause mortality.
Subgroups Analysis With High-Risk Mortality
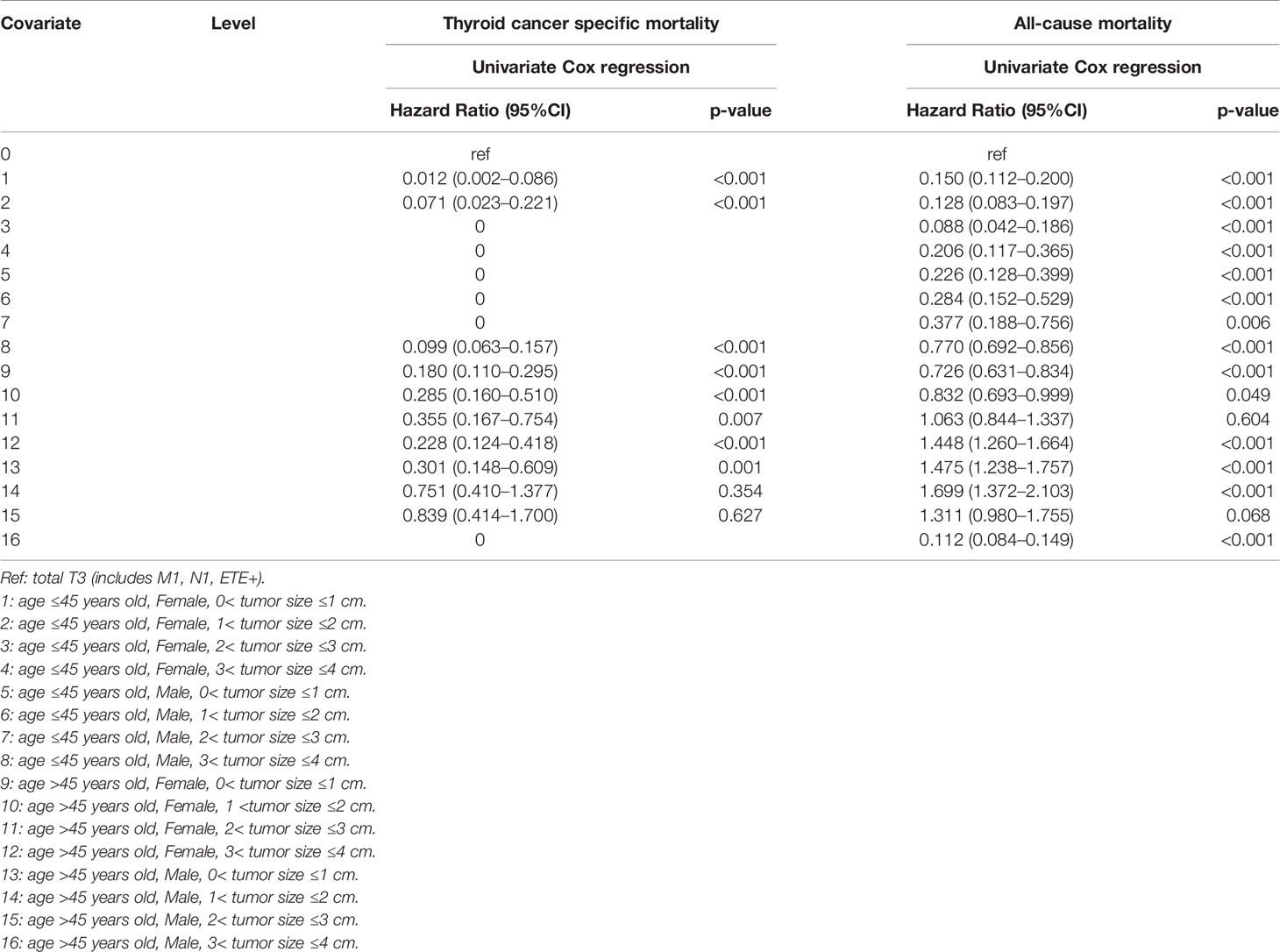
When three common clinical factors, age, sex, and tumor size, were included to select the high-risk mortality of SI-DTC, we found that compared to patients with male sex, age >45 years, and 3 cm< tumor size ≤4 cm, female patients with 1 cm <tumor size ≤3 cm aged ≤45 years, female patients with 0< tumor size ≤3 cm aged >45 years, and male patients with 0< tumor size ≤2 cm aged >45 years were at a lower risk of cancer-specific mortality (Table 3).
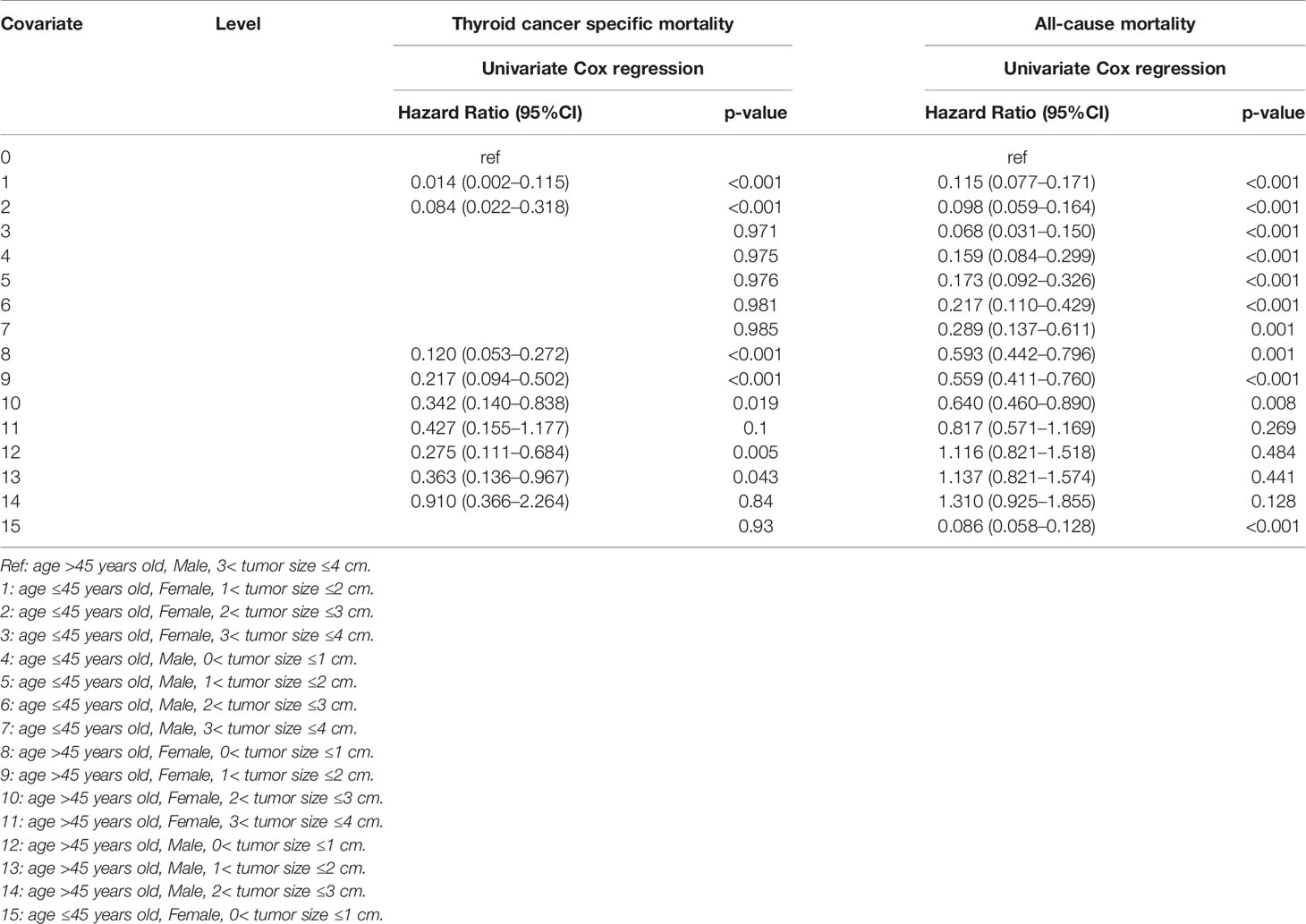
Table 3 Age, sex, tumor size factors for survival: outcome of thyroid cancer specific mortality and all-cause mortality.
Moreover, age ≤45 years was a low-risk factor for all-cause mortality in all patients regardless of sex or tumor size when compared to male patients with age >45 years old and 3 cm< tumor size ≤4 cm. For patients aged >45 years, female sex and 0< tumor size ≤3 cm were low-risk factors for all-cause mortality (Table 3).
Comparison Between SI-DTC With T3 Patients
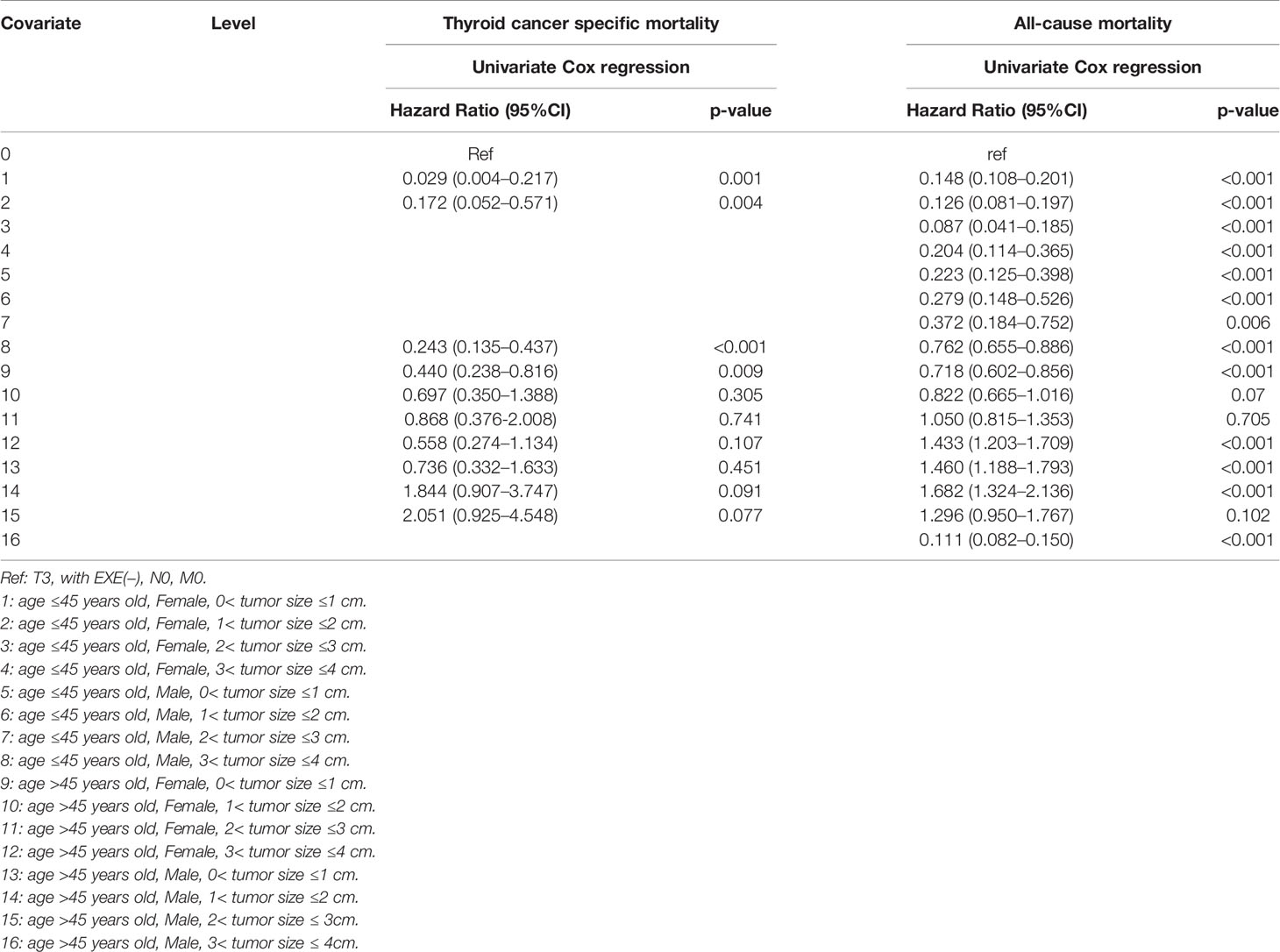
When T3 patients were included for comparison, we found that the cancer-specific mortality showed no significant difference between T3 patients, SI-DTC patients with male sex, age >45 years, and 2 cm< tumor size ≤3 cm (HR: 0.839, 95%CI: 0.414–1.700), and SI-DTC patients with male sex, age >45 years, and 1 cm< tumor size ≤2 cm (HR: 0.751, 95%CI: 0.410–1.377). Similar results were obtained for all-cause mortality (Table 4 and Figure 1).
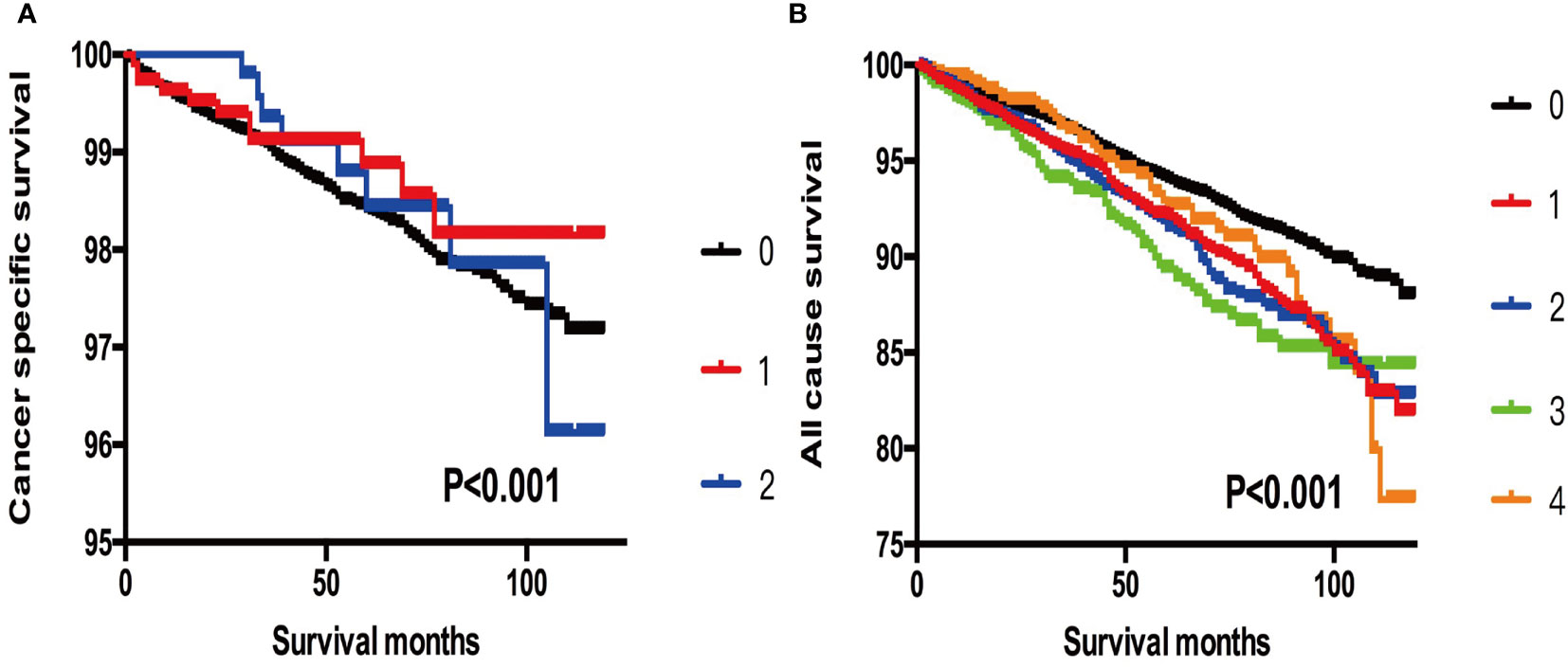
Figure 1 (A) Kaplan-Meier curves among patients stratified by tumor size for cancer-specific mortality (Log rank test, p < 0.001). 0 For patients with thyroid cancer > 4 cm in size, gross extrathyroidal extension (clinical T3), or clinically apparent metastatic disease to nodes (clinical N1) or distant sites (clinical M1). 1 Male, > 45 years old, tumor size 2-3 cm. 2 Male, > 45 years old, tumor size 3-4 cm. (B) Kaplan-Meier curves among patients stratified by tumor size for all-cause mortality (Log rank test, p < 0.001). 0 For patients with thyroid cancer> 4 cm, gross extrathyroidal extension (clinical T3), or clinically apparent metastatic disease to nodes (clinical N1) or distant sites (clinical M1). 1 Male, > 45 years old, tumor size 0-1 cm. 2 Male, > 45 years old, tumor size 1-2 cm. 3 Male, > 45 years old, tumor size 2-3 cm. 4 Male, > 45 years old, tumor size 3-4 cm.
Furthermore, when we selected T3 patients with tumor size >4 cm and excluded T3 patients without ETE and LNM for reference, we found that more subgroups of SI-DTC with a tumor size of 1–4 cm had a similar prognosis (cancer-specific mortality) with T3 patients having a tumor size >4 cm. Similar results were obtained for all-cause mortality (Table 5 and Figure 2).
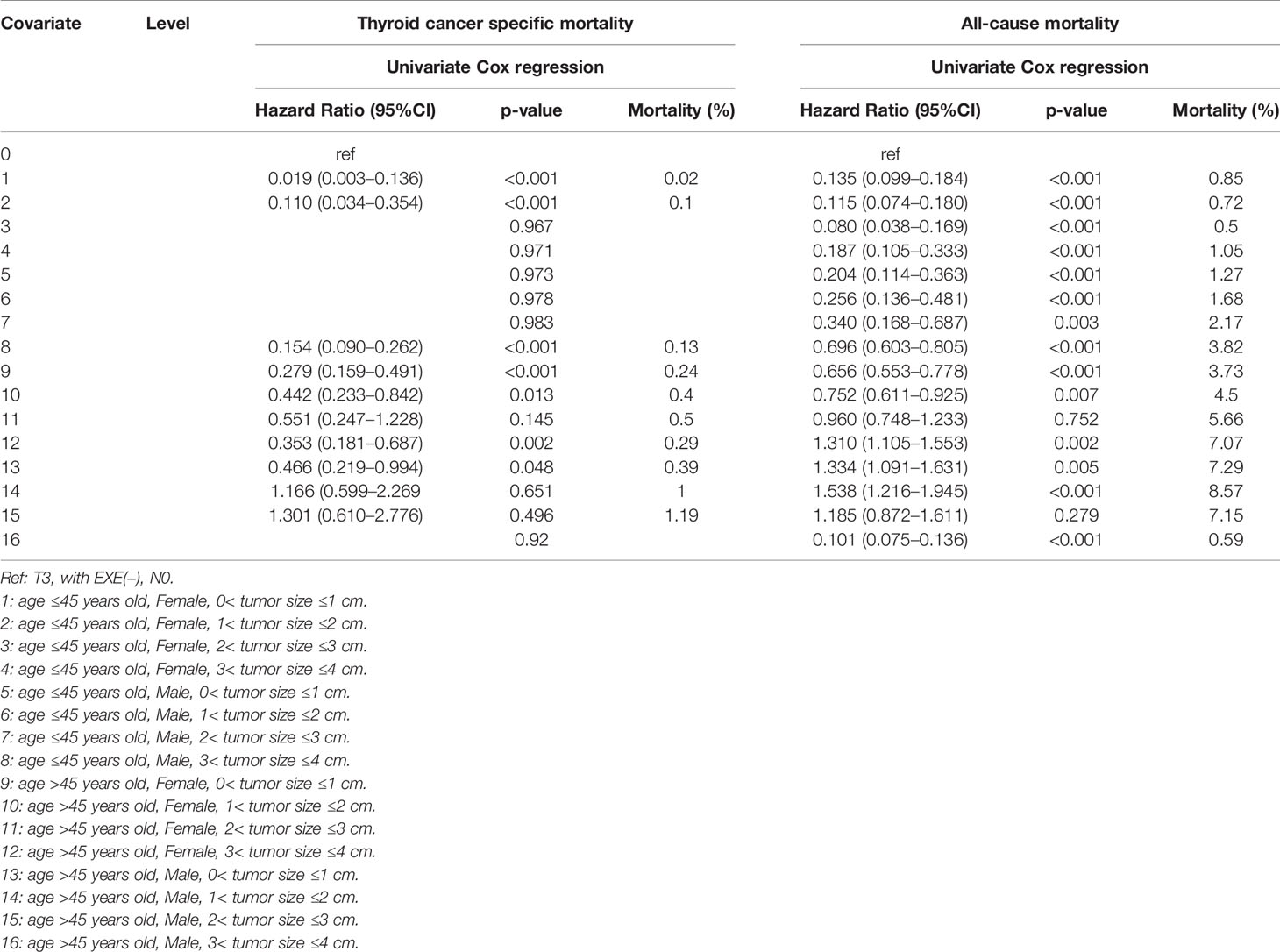
Table 5 Age, T3 and sex, tumor size factors for survival: outcome of thyroid cancer specific mortality and all-cause mortality.
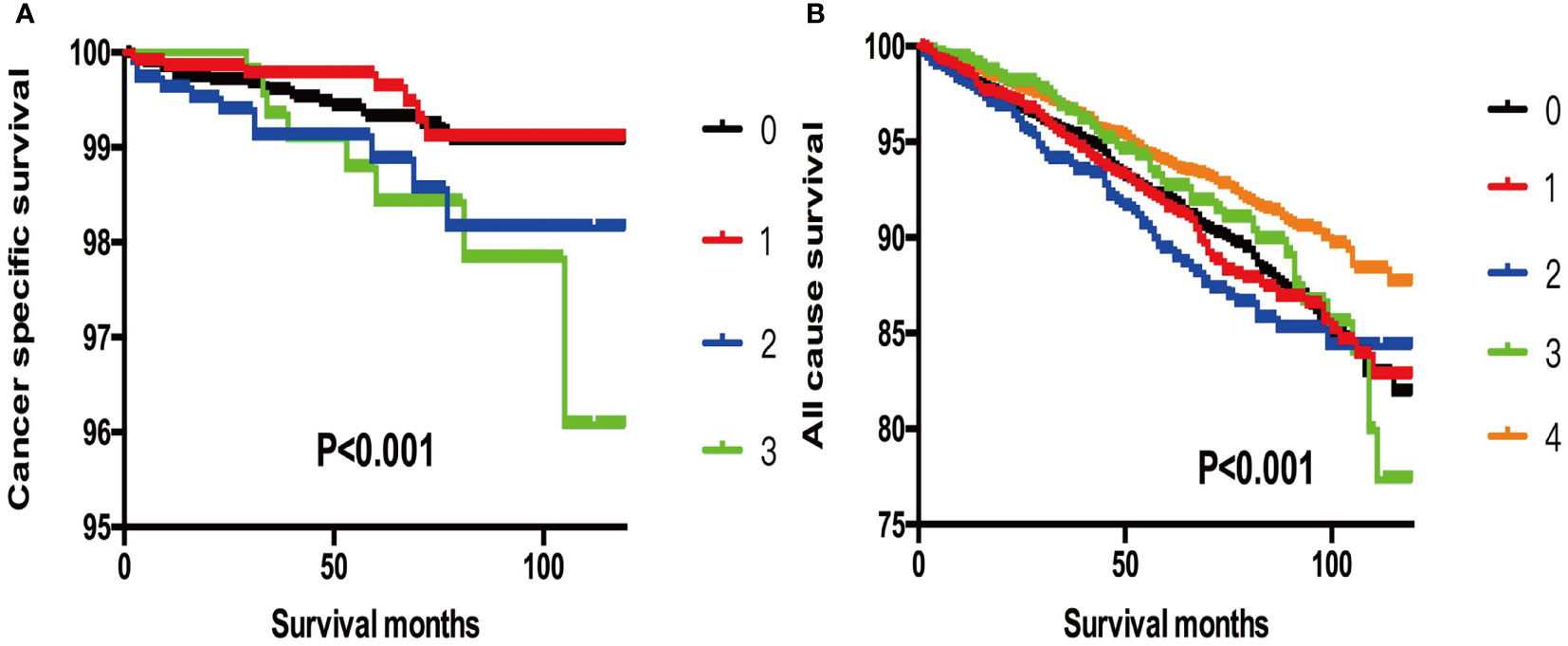
Figure 2 (A) Kaplan-Meier curves among patients stratified by tumor size for cancer-specific mortality (Log rank test, p < 0.001). 0 For patients with thyroid cancer>4 cm and without gross extrathyroidal extension (clinical T3) and no clinically apparent metastatic disease to nodes (clinical N0) or distant sites (clinical M0). 1 Male, > 45 years old, tumor size 1-2 cm. 2 Male, > 45 years old, tumor size 2-3 cm. 3 Male, > 45 years old, tumor size 3-4 cm. (B) Kaplan-Meier curves among patients stratified by tumor size for all-cause mortality (Log rank test, p < 0.001). 0 For patients with thyroid cancer>4 cm and without gross extrathyroidal extension (clinical T3) and no clinically apparent metastatic disease to nodes (clinical N0) or distant sites (clinical M0). 1 Male, > 45 years old, tumor size 0-1 cm. 2 Male, > 45 years old, tumor size 1-2 cm. 3 Male, > 45 years old, tumor size 2-3 cm. 4 Male, > 45 years old, tumor size 3-4 cm.
In addition, when we selected T3 patients without ETE, LNM, and DM as references, we found that more subgroups and patients with SI-DTC with a tumor size of 1–4 cm showed similar rates of cancer-specific mortality and all-cause mortality (Table 6 and Figure 3). Similar results were obtained when we set the age cut-off value as 55 years according to the 8th edition of the American Joint Committee on Cancer (AJCC) Tumor, Node, Metastasis (TNM) system (13) (Tables S1–S3 and Figures S1–S3).
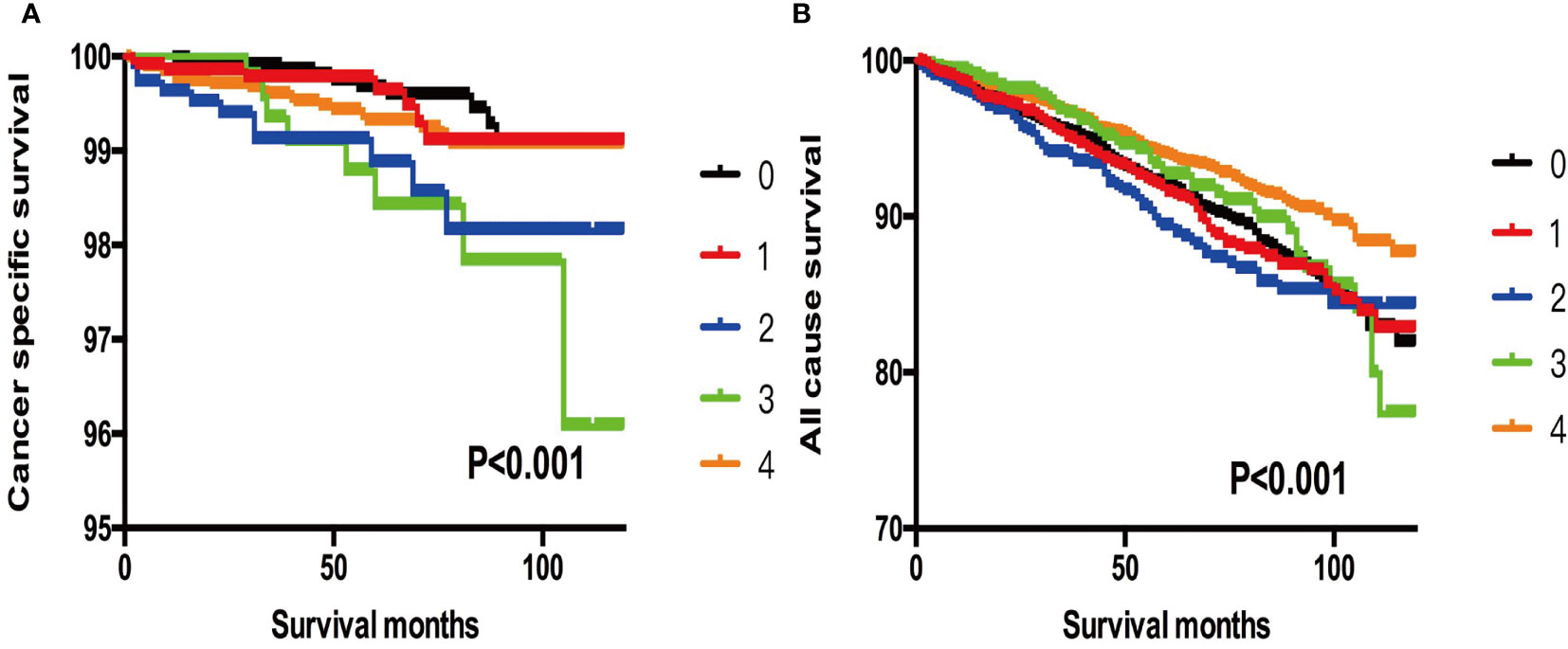
Figure 3 (A) Kaplan-Meier curves among patients stratified by tumor size for cancer-specific mortality (Log rank test, p < 0.001). 0 For patients with thyroid cancer>4 cm and without gross extrathyroidal extension (clinical T3) and no clinically apparent metastatic disease to nodes (clinical N0) and no distant sites (clinical M0). 1 Male, > 45 years old, tumor size 0-1 cm. 2 Male, > 45 years old, tumor size 1-2 cm. 3 Male, > 45 years old, tumor size 2-3 cm. 4 Male, > 45 years old, tumor size 3-4 cm. (B) Kaplan-Meier curves among patients stratified by tumor size for all-cause mortality (Log rank test, p < 0.001). 0 For patients with thyroid cancer>4 cm and without gross extrathyroidal extension (clinical T3) and no clinically apparent metastatic disease to nodes (clinical N0) and no distant sites (clinical M0). 1 Male, > 45 years old, tumor size 0-1 cm. 2 Male, > 45 years old, tumor size 1-2 cm. 3 Male, > 45 years old, tumor size 2-3 cm. 4 Male, > 45 years old, tumor size 3-4 cm.
Discussion
The current trend in the management of DTC has become more conservative. Unilateral lobectomy is now suggested as a viable alternative to total thyroidectomy for patients with SI-DTC 1–4 cm in size based on the ATA 2015 guidelines (4). Although overall prognosis seems to be excellent after lobectomy, some patients that appear to be at low risk, in fact, have high intrinsic risk for poor prognosis and should benefit from an aggressive treatment. Even in papillary thyroid microcarcinoma, cases evaluated as having a low risk of recurrence preoperatively showed intermediate to high-risk disease post-surgery, leading to a higher rate of radioiodine therapy (14).
Lobectomy for low-risk SI-DTC can be beneficial for thyroid function preservation and decreased risk of surgical complications, such as postoperative hypoparathyroidism and recurrent laryngeal nerve injury, according to many reports (15, 16). Despite these advantages, total thyroidectomy is generally accepted for SI-DTC because total thyroidectomy can provide convenience for the use of radioactive iodine in surveillance and postoperative treatment (4). It is easier to detect recurrence early via follow-up and dynamically monitoring serum thyroglobulin levels. In addition, Benjamin et al. reported that nearly half of low-risk DTC patients were found to have tumors in the contralateral lobe and may be more adequate for total thyroidectomy (17).
This study suggested that there were still some patients with high-risk prognosis among the SI-DTC with 1–4 cm, and selecting low-risk patients based on ETE, LNM, and DM was not precise. Our results provided some estimate for this possible need for completion of a total thyroidectomy after an initial lobectomy for these otherwise low-risk patients and can provide a future guide for the precision treatment for patients with SI-DTC of 1–4 cm.
In addition, some patients who underwent lobectomy may be reclassified as intermediate or high risk after surgery based on the histological findings, and Pedro et al. suggested that total thyroidectomy might be a better option for these patients (13). Previous retrospective studies have estimated that 40–60% of patients with low-risk 1–4 cm PTCs, if initially treated with lobectomy, would require a completion thyroidectomy due to high-risk pathological features in postoperative pathology reports (18, 19). These results underscore the importance of preoperative and intraoperative meticulous assessments by the surgeon during lobectomy for SI-PTCs.
Recently, Huang et al. (20) revealed that in tumors larger than 2.0 to 3.0 cm, recurrences of BRAF V600E mutation-positive SI-PTC were comparable with those of counterpart invasive solitary PTC and need more aggressive treatment. However, these adverse features are only apparent on histopathology and cannot be identified preoperatively. If a lobectomy has been performed, a completion thyroidectomy may be required, which may be associated with certain additional risks. It is essential to know the prevalence of these adverse pathological features to enable surgical planning and patient counseling. A recent study stated that for low-risk PTC patients, identification of intraoperative risk factors such as evidence or suspicion of invasion into local structures (namely, muscle, recurrent laryngeal nerve, esophagus, trachea, or other structures) or positive lymph nodes confirmed on intraoperative frozen section analysis can reduce the need for a later completion thyroidectomy in 21% of cases, but not exclude this need completely. Up to 30% of patients would be deemed intermediate or high risk, requiring a second operation (21). Thus, further studies are needed on preoperative risk stratification.
In this study, we included more clinicopathological factors (age, sex, and tumor size) for analysis to evaluate the reclassification of SI-DTC. Considering that age and sex are easily available preoperatively, including these characteristics for analysis may be more reasonable to select high-risk patients with SI-DTC and reconsider the treatment approaches. We found that survival rates were significantly lower in patients with male sex, age >45 years, and larger tumor size when compared to female patients with age ≤45 years and smaller tumor size, indicating that the former group have a poor prognosis and may require a more radical treatment plan. Our findings are consistent with the role of tumor size in the aggressiveness of PTC. Given that cancer-specific mortality occurred frequently in patients with 3–4 cm in size compared to other subgroups, a close preoperative and intraoperative evaluation of tumor size is important in SI-DTC patients.
A common clinical scenario for SI-DTC with 1–4 cm tumor size is that preoperative ultrasonography does not show suspicious LNM and extrathyroidal extension, as preoperative ultrasonography has a limited sensitivity in detecting central LNM; therefore, many patients with SI-DTC may have occult LNM and ETE, which can synergize with other high-risk characteristics, such as male sex and old age, in promoting DTC mortality. If such patients are treated by lobectomy without neck dissection and radioiodine ablation, the mortality risk could be higher. The present study found that the prognosis of SI-DTC was affected by other clinicopathological characteristics such as sex, age, and tumor size; thus, lobectomy for SI-DTC regardless of the specific clinical status seems to be unreasonable.
Some inherent limitations must be considered when interpreting our results. First, all the risk characteristics included clinicopathological features from the SEER database, but other factors such as ultrasonic data, molecular mutations, vascular invasion, and family history were not obtained nor included in our analysis. Second, data regarding recurrence are not captured in the SEER database, and the designation of cancer-specific death is susceptible to overestimation bias, particularly for diseases such as DTC. Furthermore, given the generally favorable prognosis of PTC, the relatively short study period and follow-up period (2010–2013) is a limitation of our analysis.
Conclusion
Our study demonstrated that sex, age, and tumor size clearly differentiate SI-DTC with a tumor size of 1–4 cm into low and high risk categories. Survival rates were significantly lower in subgroups containing males, older patients, and patients with larger tumor size compared to females, younger patients, and patients with smaller tumor size. Therefore, total thyroidectomy may be favored for these high-risk subgroup patients, and more clinicopathological factors should be included for risk stratification in managing patients with SI-DTC with tumors that are 1–4 cm in size.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
SW, LZ, FD, and CL designed the study. FD, LZ, WS, and JM collected and analyzed the data. FD and LZ wrote the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer ZL declared a shared affiliation with the authors to the handling editor at time of review. The reviewer JM declared a shared affiliation with the authors to the handling editor at time of review.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
All authors are grateful to all subjects who participated in this study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.790730/full#supplementary-material
References
1. Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, et al. Cancer Statistics in China, 2015. CA Cancer J Clin (2016) 66(2):115–32. doi: 10.3322/caac.21338
2. Davies L, Hoang JK. Thyroid Cancer in the USA: Current Trends and Outstanding Questions. Lancet Diabetes Endocrinol (2021) 9(1):11–2. doi: 10.1016/S2213-8587(20)30372-7
3. Liu C, Chen T, Zeng W, Wang S, Xiong Y, Liu Z, et al. Reevaluating the Prognostic Significance of Male Gender for Papillary Thyroid Carcinoma and Microcarcinoma: A SEER Database Analysis. Sci Rep (2017) 7(1):11412. doi: 10.1038/s41598-017-11788-8
4. Liu C, Wang S, Zeng W, Guo Y, Liu Z, Huang T. Total Tumour Diameter is Superior to Unifocal Diameter as a Predictor of Papillary Thyroid Microcarcinoma Prognosis. Sci Rep (2017) 7(1):1846. doi: 10.1038/s41598-017-02165-6
5. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
6. Zhang F, Zheng B, Yu X, Wang X, Wang S, Teng W. Risk Factors for Contralateral Occult Carcinoma in Patients With Unilateral Papillary Thyroid Carcinoma: A Retrospective Study and Meta-Analysis. Front Endocrinol (Lausanne) (2021) 12:675643. doi: 10.3389/fendo.2021.675643
7. Luo H, Yan F, Lan L, Ma B, Zhao H, He Y, et al. Ultrasonographic Features, Nodule Size, Capsular Invasion, and Lymph Node Metastasis of Solitary Papillary Carcinoma of Thyroid Isthmus. Front Oncol (2020) 10:558363. doi: 10.3389/fonc.2020.558363
8. Tran CH, Johnston LE, Chang DC, Bouvet M. A Critical Analysis of the American Joint Committee on Cancer (AJCC) Staging System for Differentiated Thyroid Carcinoma in Young Patients on the Basis of the Surveillance, Epidemiology, and End Results (SEER) Registry. Surgery (2012) 152(2):145–51. doi: 10.1016/j.surg.2012.02.015
9. Perrier ND, Brierley JD, Tuttle RM. Differentiated and Anaplastic Thyroid Carcinoma: Major Changes in the American Joint Committee on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin (2018) 68(1):55–63. doi: 10.3322/caac.21439
10. Liu W, Yan X, Cheng R. Continuing Controversy Regarding Individualized Surgical Decision-Making for Patients With 1-4 Cm Low-Risk Differentiated Thyroid Carcinoma: A Systematic Review. Eur J Surg Oncol (2020) 46(12):2174–84. doi: 10.1016/j.ejso.2020.08.014
11. Xu Y, Huang K, Huang P, Ke N, Zeng J, Wang L, et al. Benefits and Harms of Hemithyroidectomy, Total or Near-Total Thyroidectomy in 1-4 Cm Differentiated Thyroid Cancer. Clin Endocrinol (Oxf) (2021) 95(4):668–76. doi: 10.1111/cen.14495
12. Wang X, Zhang C, Srivastava A, Yu W, Liu C, Wei D, et al. Risk Factors That Influence Surgical Decision-Making for Patients With Low-Risk Differentiated Thyroid Cancer With Tumor Diameters of 1-4 Cm. Cancer Manag Res (2020) 12:12423–8. doi: 10.2147/CMAR.S268716
13. Schmid KW, Synoracki S, Dralle H, Wittekind C. Proposal for an Extended pTNM Classification of Thyroid Carcinoma: Commentary on Deficits of the 8th Edition of the TNM Classification. Pathologe (2019) 40(Suppl 1):18–24. doi: 10.1007/s00292-018-0418-x
14. Gao R, Jia X, Liang Y, Fan K, Wang X, Wang Y, et al. Papillary Thyroid Micro Carcinoma: The Incidence of High-Risk Features and Its Prognostic Implications. Front Endocrinol (Lausanne) (2019) 10:74. doi: 10.3389/fendo.2019.00074
15. Liang J, Li Z, Fang F, Yu T, Li S. Is Prophylactic Central Neck Dissection Necessary for Cn0 Differentiated Thyroid Cancer Patients at Initial Treatment? A Meta-Analysis of the Literature. Acta Otorhinolaryngol Ital (2017) 37(1):1–8. doi: 10.14639/0392-100X-1195
16. McMullen C, Rocke D, Freeman J. Complications of Bilateral Neck Dissection in Thyroid Cancer From a Single High-Volume Center. JAMA Otolaryngol Head Neck Surg (2017) 143(4):376–81. doi: 10.1001/jamaoto.2016.3670
17. Papachristos AJ, Glover A, Sywak MS, Sidhu SB. Pros and Cons of Hemi-Thyroidectomy for Low-Risk Differentiated Thyroid Cancer. ANZ J Surg (2021) 91(9):1704–10. doi: 10.1111/ans.16553
18. Murthy SP, Balasubramanian D, Subramaniam N, Nair G, Babu M, Rathod PV, et al. Prevalence of Adverse Pathological Features in 1 to 4 Cm Low-Risk Differentiated Thyroid Carcinoma. Head Neck (2018) 40(6):1214–8. doi: 10.1002/hed.25099
19. DiMarco AN, Wong MS, Jayasekara J, Cole-Clark D, Aniss A, Glover AR, et al. Risk of Needing Completion Thyroidectomy for Low-Risk Papillary Thyroid Cancers Treated by Lobectomy. BJS Open (2019) 3(3):299–304. doi: 10.1002/bjs5.50137
20. Huang Y, Qu S, Zhu G, Wang F, Liu R, Shen X, et al. BRAF V600E Mutation-Assisted Risk Stratification of Solitary Intrathyroidal Papillary Thyroid Cancer for Precision Treatment. J Natl Cancer Inst (2018) 110(4):362–70. doi: 10.1093/jnci/djx227
Keywords: differentiated thyroid cancer, SEER, surgery approaches, tumor size, thyroidectomy
Citation: Dong F, Zhou L, Wang S, Mao J, Liu C and Shi W (2022) Clinical Characteristics-Assisted Risk Stratification for Extent of Thyroidectomy in Patients With 1–4 cm Solitary Intrathyroidal Differentiated Thyroid Cancer. Front. Endocrinol. 12:790730. doi: 10.3389/fendo.2021.790730
Received: 07 October 2021; Accepted: 31 December 2021;
Published: 08 February 2022.
Edited by:
Gianlorenzo Dionigi, University of Milan, ItalyReviewed by:
Zeming Liu, Huazhong University of Science and Technology, ChinaLiang Guo, Wuhan University, China
Jie Ming, Huazhong University of Science and Technology, China
Copyright © 2022 Dong, Zhou, Wang, Mao, Liu and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chunping Liu, bGl1Y3B3aHhoQGh1c3QuZWR1LmNu; Wei Shi, c2hpd2VpaHVzdEAxNjMuY29t
†These authors have contributed equally to this work
 Fang Dong
Fang Dong Lin Zhou
Lin Zhou Shuntao Wang1
Shuntao Wang1