- 1University of Trieste, Trieste, Italy
- 2Institute for Maternal and Child Health IRCCS “Burlo Garofolo”, Trieste, Italy
Neuroimaging is a key tool in the diagnostic process of various clinical conditions, especially in pediatric endocrinology. Thanks to continuous and remarkable technological developments, magnetic resonance imaging can precisely characterize numerous structural brain anomalies, including the pituitary gland and hypothalamus. Sometimes the use of radiological exams might become excessive and even disproportionate to the patients’ medical needs, especially regarding the incidental findings, the so-called “incidentalomas”. This unclarity is due to the absence of well-defined pediatric guidelines for managing and following these radiological findings. We review and summarize some indications on how to, and even if to, monitor these anomalies over time to avoid unnecessary, expensive, and time-consuming investigations and to encourage a more appropriate follow-up of brain MRI anomalies in the pediatric population with endocrinological conditions.
Introduction
The widespread use of brain magnetic resonance imaging (MRI) has put at our disposal incredibly accurate images that give us the chance to improve our diagnostic competence. However, this widespread use of neuroimaging has led to a remarkable increase of incidental radiological findings, the so-called “incidentalomas”, which may generate interpretation uncertainty for radiologists and clinicians (1, 2). Here we describe two clinical cases to exemplify this context.
Clinical Scenario 1
A boy was referred for short stature at the age of 5 years. His height was -2.3 standard deviation score (SDS), his growth rate was -2.6 SDS, and his bone age was 3 years. His previous medical history was unremarkable. Two stimulation tests were performed with arginine and insulin, and peak values of growth hormone (GH) were pathological (<8 ng/mL) in both, supporting the diagnosis of GH deficiency (GHD). No other pituitary deficits were found on laboratory tests. A brain MRI under sedation was performed before starting treatment, highlighting the presence of “pituitary stalk interruption syndrome”. An annual MRI follow-up was suggested, and the patient underwent a brain MRI every year for 7 consecutive years, through which the radiological finding remained stable.
Clinical Scenario 2
A girl was referred for precocious puberty at the age of 7 years. On physical examination, Tanner stages B2, Ph2, and A2-3 were observed. Her growth rate was +6 SDS, and her bone age was 9 years. A pelvic ultrasound showed pubertal changes in the uterus and ovaries, and a GnRH stimulation test showed a pathological level of LH peak (> 5 ng/mL) and an LH/FSH ratio >1, confirming the diagnosis of central precocious puberty (CPP). A brain MRI showed the presence of an arachnoid cyst in the parietal lobe, with dimensions of 2 x 3 cm, not compressing nor close to the ventricular system. An annual MRI follow-up was suggested. The patient underwent a brain scan for 4 consecutive years, through which the radiological finding did not show any variation.
Discussion
Brain MRI is mandatory in many pediatric endocrinological conditions to detect anatomic anomalies and rule out neoplastic lesions (3, 4).
In two of the most frequent conditions in pediatric endocrinology, GHD (prevalence 1:4.000-1:10.000) and CPP (prevalence 1:5.000-1:10.000) (5), brain MRI is requested once stimulation tests confirm the clinical diagnosis (3, 4). Nevertheless, other rare endocrine conditions (such as Cushing syndrome, hyperprolactinemia, gigantism, central hypo- and hyperthyroidism, and diabetes insipidus) unquestionably require a brain MRI in their diagnostic work-up.
In GHD, brain MRI may show characteristic anatomic pituitary abnormalities that can explain the endocrine disorder (such as anterior pituitary dysplasia, pituitary stalk interruption syndrome [PSIS], Rathke cleft cyst [RCC], empty sella, etc.), but can also detect possible tumoral lesions (such as craniopharyngioma or Langerhans cell histiocytosis) (6).
In CPP, the international guidelines state that neuroimaging is mandatory in all males and in females under six years of age since both these groups have a higher chance of presenting brain lesions causing early pubertal development (such as hamartoma, astrocytoma/glioma, germinoma) (7–9). Conversely, MRI is still controversial in females aged 6-8 years: the incidence of cranial abnormalities in this age group is minimal (10–12), even if a possible late cancer diagnosis cannot be excluded by clinical and biochemical parameters only (13).
In both GHD and CPP, identifying anomalies and malformations during brain MRI, including “incidentalomas”, is not infrequent (9, 14). Starting from the clinical scenarios we described, we realized that unrelated brain anomalies, incidentalomas, and anatomic anomalies represent a real problem in the pediatric population. Thus, we asked ourselves whether it was necessary to routinely monitor all these findings after their identification or not, based on the current medical literature. Avoiding unnecessary tests on pediatric patients is not just a matter of cost, both for the health system and the patients’ families, but also of physical and psychological stress for the patients and their families. The image acquisition process for brain MRI lasts from 20 to 30 minutes, and procedural sedation is usually needed in children younger than 7 years of age. Furthermore, unnecessary investigations and tests may postpone the medical evaluation of other patients in real need of consult.
We performed a narrative review of the literature, which was limited to the pediatric population, to identify the most common brain abnormalities in patients with endocrinological conditions who require an MRI scan. We then conducted subsequent research to determine the current management for every specific MRI abnormal finding. In doing so, we decided to include exclusively papers that described cohorts of patients, excluding single case reports or international guidelines on the matter. For rare conditions, if more than one cohort study was identified, we reported the ones with the largest population. The selection of papers was limited to the English language. The following three electronic databases were searched: Pubmed, Scielo, and Scopus. As search string, we used a combination of the condition’s name plus pediatric/children plus management/guidelines/follow up or, alternatively, the condition’s name plus pediatric/children plus MRI/magnetic resonance. We also searched for pediatric guidelines referring to the overall topic of brain incidentalomas via the database above reported.
Remarkably, despite the increasing relevance of the matter, we found out that there was a general lack of guidelines on incidentalomas and MRI brain abnormalities in the pediatric population. While the medical literature has vastly discussed this topic in the adult population, only a few studies currently support pediatricians’ decisions on managing these conditions. For example, a 2011 paper by the Endocrine Society formulated some practical guidelines for managing pituitary incidentalomas in adults but clearly stated that they do not apply to the pediatric population (15). On the other hand, most papers on the pediatric population either describe small sample populations or analyze only a single subtype of incidentaloma without considering the remaining anomalies, missing the big picture. The works by Souteiro et al. and Shareef et al. represent the two most extensive studies (2, 16). The first one identified 41 incidentalomas in a cohort of pediatric patients who underwent brain imaging for various reasons, primarily headache. Pituitary hypertrophy was the most common finding (29.3%), followed by arachnoid cysts (17.1%), pituitary adenomas (14.6%), and RCC (12.2%). Remarkably, 56.1% of these patients underwent a radiological reevaluation, but none of them presented dimensional progression (2). The second one described 31 incidental lesions, among which RCC was the most frequent (67.7%), followed by cystic pituitary lesions (19.4%) and microadenomas (12.9%). Only 5 patients had a radiological reevaluation, and lesion growth was never documented (16).
According to the available evidence, we divided the radiological findings into two main groups:
a) Findings with definite or possible clinical or anatomical relevance, i.e., conditions involving the sellar region that might affect the endocrine system function. These include:
- Adenohypophysis hypoplasia
- Pituitary stalk interruption syndrome (PSIS)
- Ectopic neurohypophysis
- Empty sella, complete or partial
- Rathke cleft cysts (RCC)
- Pituitary adenomas
- Craniopharyngiomas
Overall, these findings have been identified more frequently in GHD than CPP (3, 17). Arnold Chiari type I malformation, i.e., the descent of the cerebellar tonsils through the foramen magnum, cannot be included in this group since it does not affect the sellar region. Still, it often has clinical relevance for the patient (18, 19).
Only three findings can potentially grow over time in this category: RCC, pituitary adenomas, and craniopharyngiomas (20–22). There is evidence that craniopharyngioma may progress from a longstanding RCC via a transitional stage of extensive squamous metaplasia (23).
The remaining lesions cannot progress and thus do not need radiological follow-up. For example, in the first clinical scenario, no further MRIs should have been performed in a patient with PSIS.
b) Findings without any relevance that lack a correlation with the endocrinological condition under study. This group contains the so-called “incidentalomas”, such as:
- Arachnoid cysts
- Epiphyseal cysts
- Choroid plexus cysts
- Vascular anomalies
- Increased hypophyseal volume
Various studies have followed these findings throughout the years with subsequent MRI (6). However, due to the lack of standardized protocols on how to, and even if to, monitor them, and to a general misunderstanding on their ability to evolve or not, it is not infrequent that these lesions can be troublesome to deal with, leading to a relevant number of pointless tests.
Among incidentalomas, only arachnoid cysts may be worth further radiological evaluations since they can grow to a large size if the retention of cerebrospinal fluid progresses through time. However, a radiological follow-up is recommended only in the presence of a large cyst or if the lesion is located close to the ventricular system, posing a risk of hydrocephalus (24). Therefore, in the second clinical scenario we described, no other exams were needed.
We propose a summary table to manage the most frequent brain anomalies found in pediatric patients affected by GHD and CPP that is now in use in our Institute (Table 1) (2, 18, 24–59). This table is provided only as a guide and does not give absolute indications.
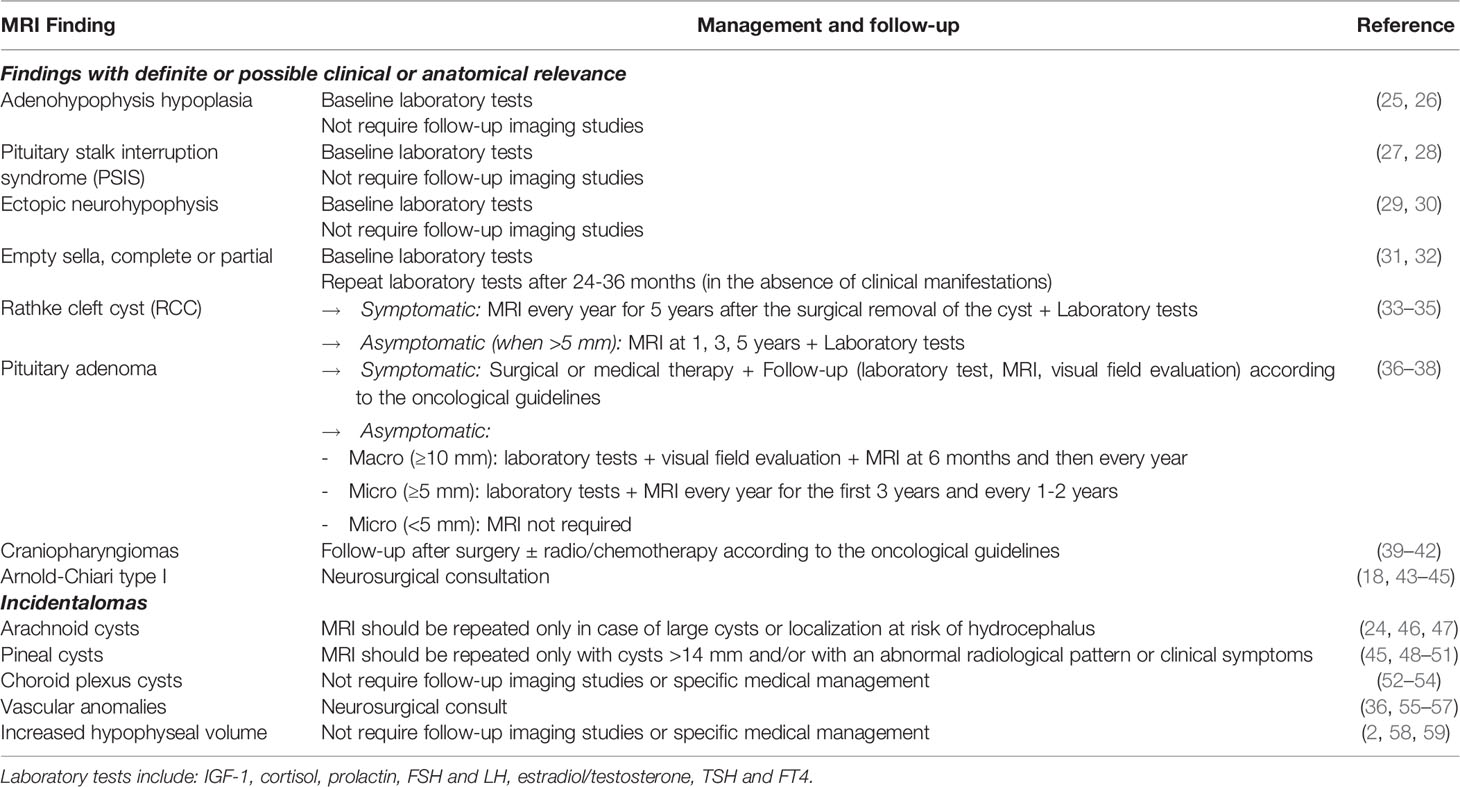
Table 1 Suggested management of the most frequent brain anomalies found in pediatric patients affected by growth hormone deficiency (GHD) and central precocious puberty (CPP).
Overall, there are a few elements that we would like to remark on.
First, all the indications reported in the table refer to brain MRI findings that are not causing any neurological manifestation. In the presence of signs or symptoms suggestive of endocranial hypertension, such as headache, vomit, and arterial hypertension with bradycardia, an MRI evaluation is always warranted, regardless of a history of previous brain findings incidental or not.
Another critical element is that the pituitary gland volume should always be compared to the normal dimensions per age and sex of the patient. In our personal experience, we deal with various inappropriate endocrinological referrals due to misinterpretation of pituitary gland dimensions, especially coming from centers lacking pediatric radiologists. The most typical context is the identification of an enlarged pituitary gland in a teenager who underwent brain MRI because of recurrent headaches but in which no possible underlying elements were found. Therefore, before starting a complete examination of the pituitary gland functionality with a laboratory test and scheduling a follow-up brain MRI, we suggest evaluation of the initial imaging study by a radiologist with expertise in pediatric neuro-imaging.
A third clarification must be made on vascular anomalies. In this case, a neurosurgical consult is necessary because their follow-up and management depend primarily on their correct classification. For example, while cerebral developmental venous anomalies are managed conservatively (56), arteriovenous malformations are usually treated surgically or endoscopically because of their high risk of rupture in the pediatric population (55). A similar approach must also be chosen for asymptomatic Arnold-Chiari type 1 malformations since the timing of their radiological follow-up is currently discussed among specialists, even if the management is known to be conservative (60).
Conclusions
The purpose of this manuscript is not to underestimate the possible relevance of incidental MRI findings in the diagnostic process of GHD and CPP. Our goal was to highlight that, based on the current literature, most of the follow-up brain MRIs are probably not required in these incidentalomas and that only a few neuroimaging findings are worth subsequent investigations.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
FB, MM, and FM performed the literature search and wrote the manuscript. EB critically reviewed the manuscript for important intellectual content. GT conceptualized the paper and reviewed and revised the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Dai C, Kang J, Liu X, Yao Y, Wang H, Wang R. How to Classify and Define Pituitary Tumors: Recent Advances and Current Controversies. Front Endocrinol (Lausanne) (2021) 12:604644. doi: 10.3389/fendo.2021.604644
2. Souteiro P, Maia R, Santos-Silva R, Figueiredo R, Costa C, Belo S, et al. Pituitary Incidentalomas in Paediatric Age Are Different From Those Described in Adulthood. Pituitary (2019) 22(2):124–8. doi: 10.1007/s11102-019-00940-4
3. Growth Hormone Research Society. Consensus Guidelines for the Diagnosis and Treatment of Growth Hormone (GH) Deficiency in Childhood and Adolescence: Summary Statement of the GH Research Society. GH Research Society. J Clin Endocrinol Metab (2000) 85(11):3990–3. doi: 10.1210/jcem.85.11.6984
4. Eugster EA. Treatment of Central Precocious Puberty. J Endocr Soc (2019) 3(5):965–72. doi: 10.1210/js.2019-00036
5. Bellotto E, Monasta L, Pellegrin MC, Bossini B, Tamaro G, Conte MS, et al. Pattern and Features of Pediatric Endocrinology Referrals: A Retrospective Study in a Single Tertiary Center in Italy. Front Pediatr (2020) 8:580588. doi: 10.3389/fped.2020.580588
6. Xu C, Zhang X, Dong L, Zhu B, Xin T. MRI Features of Growth Hormone Deficiency in Children With Short Stature Caused by Pituitary Lesions. Exp Ther Med (2017) 13(6):3474–8. doi: 10.3892/etm.2017.4377
7. Merke DP, Cutler GB Jr. Evaluation and Management of Precocious Puberty. Arch Dis Child (1996) 75(4):269–71. doi: 10.1136/adc.75.4.269
8. Latronico AC, Brito VN, Carel JC. Causes, Diagnosis, and Treatment of Central Precocious Puberty. Lancet Diabetes Endocrinol (2016) 4(3):265–74. doi: 10.1016/S2213-8587(15)00380-0
9. Aguirre RS, Eugster EA. Central Precocious Puberty: From Genetics to Treatment. Best Pract Res Clin Endocrinol Metab (2018) 32(4):343–54. doi: 10.1016/j.beem.2018.05.008
10. Cantas-Orsdemir S, Garb JL, Allen HF. Prevalence of Cranial MRI Findings in Girls With Central Precocious Puberty: A Systematic Review and Meta-Analysis. J Pediatr Endocrinol Metab (2018) 31(7):701–10. doi: 10.1515/jpem-2018-0052
11. Kaplowitz P, Bloch C. Section on Endocrinology, American Academy of Pediatrics. Evaluation and Referral of Children With Signs of Early Puberty. Pediatrics (2016) 137(1):e20153732. doi: 10.1542/peds.2015-3732
12. Pedicelli S, Alessio P, Scirè G, Cappa M, Cianfarani S. Routine Screening by Brain Magnetic Resonance Imaging Is Not Indicated in Every Girl With Onset of Puberty Between the Ages of 6 and 8 Years. J Clin Endocrinol Metab (2014) 99(12):4455–61. doi: 10.1210/jc.2014-2702
13. Mogensen SS, Aksglaede L, Mouritsen A, Sørensen K, Main KM, Gideon P, et al. Pathological and Incidental Findings on Brain MRI in a Single-Center Study of 229 Consecutive Girls With Early or Precocious Puberty. PloS One (2012) 7(1):e29829. doi: 10.1371/journal.pone.0029829
14. Maghnie M, Lindberg A, Koltowska-Häggström M, Ranke MB. Magnetic Resonance Imaging of CNS in 15,043 Children With GH Deficiency in KIGS (Pfizer International Growth Database). Eur J Endocrinol (2013) 168(2):211–7. doi: 10.1530/EJE-12-0801
15. Freda PU, Beckers AM, Katznelson L, Molitch ME, Montori VM, Post KD, et al. Pituitary Incidentaloma: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2011) 96(4):894–904. doi: 10.1210/jc.2010-1048
16. Shareef M, Nasrallah MP, AlArab N, Atweh LA, Zadeh C, Hourani R. Pituitary Incidentalomas in Paediatric Population: Incidence and Characteristics. Clin Endocrinol (Oxf) (2021) 94(2):269–76. doi: 10.1111/cen.14353
17. Soriano-Guillén L, Argente J. Central Precocious Puberty, Functional and Tumor-Related. Best Pract Res Clin Endocrinol Metab (2019) 33(3):101262. doi: 10.1016/j.beem.2019.01.003
18. Hidalgo JA, Tork CA, Varacallo M. Arnold Chiari Malformation. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2021). Available at: https://www.ncbi.nlm.nih.gov/books/NBK431076/.
19. Kular S, Cascella M. Chiari I Malformation. In: StatPearls. Treasure Island FL: StatPearls Publishing (2021).
20. Güneş A, Özbal Güneş S. The Neuroimaging Features of Rathke's Cleft Cysts in Children With Endocrine-Related Diseases. Diagn Interv Radiol (2020) 26(1):61–7. doi: 10.5152/dir.2019.19352
21. Keil MF, Stratakis CA. Pituitary Tumors in Childhood: Update of Diagnosis, Treatment and Molecular Genetics. Expert Rev Neurother (2008) 8(4):563–74. doi: 10.1586/14737175.8.4.563
22. Pan J, Qi S, Liu Y, Lu Y, Peng J, Zhang X, et al. Growth Patterns of Craniopharyngiomas: Clinical Analysis of 226 Patients. J Neurosurg Pediatr (2016) 17(4):418–33. doi: 10.3171/2015.7.PEDS14449
23. Alomari AK, Kelley BJ, Damisah E, Marks A, Hui P, DiLuna M, et al. Craniopharyngioma Arising in a Rathke's Cleft Cyst: Case Report. J Neurosurg Pediatr (2015) 15(3):250–4. doi: 10.3171/2014.11.PEDS14370
24. Hall S, Smedley A, Sparrow O, Mathad N, Waters R, Chakraborty A, et al. Natural History of Intracranial Arachnoid Cysts. World Neurosurg (2019) 126:e1315–20. doi: 10.1016/j.wneu.2019.03.087
25. Yadav P, Singhal S, Chauhan S, Harit S. MRI Evaluation of Size and Shape of Normal Pituitary Gland: Age and Sex Related Changes. J Clin Diagn Res (2017) 11(12):TC01–4. doi: 10.7860/JCDR/2017/31034.10933
26. Di Iorgi N, Allegri AE, Napoli F, Bertelli E, Olivieri I, Rossi A, et al. The Use of Neuroimaging for Assessing Disorders of Pituitary Development. Clin Endocrinol (Oxf) (2012) 76(2):161–76. doi: 10.1111/j.1365-2265.2011.04238.x
27. Vergier J, Castinetti F, Saveanu A, Girard N, Brue T, Reynaud R. Diagnosis of Endocrine Disease: Pituitary Stalk Interruption Syndrome: Etiology and Clinical Manifestations. Eur J Endocrinol (2019) 181(5):R199–209. doi: 10.1530/EJE-19-0168
28. Wang Q, Hu Y, Li G, Sun X. Pituitary Stalk Interruption Syndrome in 59 Children: The Value of MRI in Assessment of Pituitary Functions. Eur J Pediatr (2014) 173(5):589–95. doi: 10.1007/s00431-013-2214-1
29. Ybarra M, Hafiz R, Robinson ME, von Oettingen JE, Bui H, Saint-Martin C. A New Imaging Entity Consistent With Partial Ectopic Posterior Pituitary Gland: Report of Six Cases. Pediatr Radiol (2020) 50(1):107–15. doi: 10.1007/s00247-019-04502-5
30. Chen S, Léger J, Garel C, Hassan M, Czernichow P. Growth Hormone Deficiency With Ectopic Neurohypophysis: Anatomical Variations and Relationship Between the Visibility of the Pituitary Stalk Asserted by Magnetic Resonance Imaging and Anterior Pituitary Function. J Clin Endocrinol Metab (1999) 84(7):2408–13. doi: 10.1210/jcem.84.7.5849
31. Chiloiro S, Giampietro A, Bianchi A, Tartaglione T, Capobianco A, Anile C, et al. Diagnosis of Endocrine Disease: Primary Empty Sella: A Comprehensive Review. Eur J Endocrinol (2017) 177(6):R275–85. doi: 10.1530/EJE-17-0505
32. Ekhzaimy AA, Mujammami M, Tharkar S, Alansary MA, Al Otaibi D. Clinical Presentation, Evaluation and Case Management of Primary Empty Sella Syndrome: A Retrospective Analysis of 10-Year Single-Center Patient Data. BMC Endocr Disord (2020) 20(1):142. doi: 10.1186/s12902-020-00621-5
33. Mahdi ES, Webb RL, Whitehead MT. Prevalence of Pituitary Cysts in Children Using Modern Magnetic Resonance Imaging Techniques. Pediatr Radiol (2019) 49(13):1781–7. doi: 10.1007/s00247-019-04479-1
34. Trifanescu R, Ansorge O, Wass JA, Grossman AB, Karavitaki N. Rathke's Cleft Cysts. Clin Endocrinol (Oxf) (2012) 76(2):151–60. doi: 10.1111/j.1365-2265.2011.04235.x
35. Culver SA, Grober Y, Ornan DA, Patrie JT, Oldfield EH, Jane JA Jr, et al. A Case for Conservative Management: Characterizing the Natural History of Radiographically Diagnosed Rathke Cleft Cysts. J Clin Endocrinol Metab (2015) 100(10):3943–8. doi: 10.1210/jc.2015-2604
36. Thaker VV, Lage AE, Kumari G, Silvera VM, Cohen LE. Clinical Course of Nonfunctional Pituitary Microadenoma in Children: A Single-Center Experience. J Clin Endocrinol Metab (2019) 104(12):5906–12. doi: 10.1210/jc.2019-01252
37. Derrick KM, Gomes WA, Gensure RC. Incidence and Outcomes of Pituitary Microadenomas in Children With Short Stature/Growth Hormone Deficiency. Horm Res Paediatr (2018) 90(3):151–60. doi: 10.1159/000489456
38. Steele CA, MacFarlane IA, Blair J, Cuthbertson DJ, Didi M, Mallucci C, et al. Pituitary Adenomas in Childhood, Adolescence and Young Adulthood: Presentation, Management, Endocrine and Metabolic Outcomes. Eur J Endocrinol (2010) 163(4):515–22. doi: 10.1530/EJE-10-0519
39. Drapeau A, Walz PC, Eide JG, Rugino AJ, Shaikhouni A, Mohyeldin A, et al. Pediatric Craniopharyngioma. Childs Nerv Syst (2019) 35(11):2133–45. doi: 10.1007/s00381-019-04300-2
40. Jensterle M, Jazbinsek S, Bosnjak R, Popovic M, Zaletel LZ, Vesnaver TV, et al. Advances in the Management of Craniopharyngioma in Children and Adults. Radiol Oncol (2019) 53(4):388–96. doi: 10.2478/raon-2019-0036
42. Amayiri N, Spitaels A, Zaghloul M, Figaji A, Cavalheiro S, Muller HL, et al. SIOP PODC-Adapted Treatment Guidelines for Craniopharyngioma in Low- and Middle-Income Settings. Pediatr Blood Cancer (2020) 13:e28493. doi: 10.1002/pbc.28493
43. Entezami P, Gooch MR, Poggi J, Perloff E, Dupin M, Adamo MA. Current Management of Pediatric Chiari Type 1 Malformations. Clin Neurol Neurosurg (2019) 176:122–6. doi: 10.1016/j.clineuro.2018.12.007
44. Davidson L, Phan TN, Myseros JS, Magge SN, Oluigbo C, Sanchez CE, et al. Long-Term Outcomes for Children With an Incidentally Discovered Chiari Malformation Type 1: What Is the Clinical Significance? Childs Nerv Syst (2021) 37(4):1191–7. doi: 10.1007/s00381-020-04980-1
45. Brener A, Kozyrev DA, Shiran SI, Azoulay E, Pratt LT, Precel R, et al. Incidental Findings on Brain Magnetic Resonance Imaging (MRI) in Pediatric Endocrine Patients. Endocr Pract (2020) 26(10):1105–14. doi: 10.4158/EP-2020-0208
46. Al-Holou WN, Yew AY, Boomsaad ZE, Garton HJ, Muraszko KM, Maher CO. Prevalence and Natural History of Arachnoid Cysts in Children. J Neurosurg Pediatr (2010) 5(6):578–85. doi: 10.3171/2010.2.PEDS09464
47. Maher CO, Piatt JH Jr. Section on Neurologic Surgery, American Academy of Pediatrics. Incidental Findings on Brain and Spine Imaging in Children. Pediatrics (2015) 135(4):e1084–96. doi: 10.1542/peds.2015-0071
48. Fakhran S, Escott EJ. Pineocytoma Mimicking a Pineal Cyst on Imaging: True Diagnostic Dilemma or a Case of Incomplete Imaging? AJNR Am J Neuroradiol (2008) 29(1):159–63. doi: 10.3174/ajnr.A0750
49. Jussila MP, Olsén P, Salokorpi N, Suo-Palosaari M. Follow-Up of Pineal Cysts in Children: Is It Necessary? Neuroradiology (2017) 59(12):1265–73. doi: 10.1007/s00234-017-1926-8
50. Barboriak DP, Lee L, Provenzale JM. Serial MR Imaging of Pineal Cysts: Implications for Natural History and Follow-Up. AJR Am J Roentgenol (2001) 176(3):737–43. doi: 10.2214/ajr.176.3.1760737
51. Al-Holou WN, Maher CO, Muraszko KM, Garton HJ. The Natural History of Pineal Cysts in Children and Young Adults. J Neurosurg Pediatr (2010) 5(2):162–6. doi: 10.3171/2009.9.PEDS09297
52. Riebel T, Nasir R, Weber K. Choroid Plexus Cysts: A Normal Finding on Ultrasound. Pediatr Radiol (1992) 22(6):410–2. doi: 10.1007/BF02013498
53. Shah N. Prenatal Diagnosis of Choroid Plexus Cyst: What Next? J Obstet Gynaecol India (2018) 68(5):366–8. doi: 10.1007/s13224-017-1047-7
54. Hung KL, Liao HT. Neonatal Choroid Plexus Cysts and Early Childhood Developmental Outcome. J Formos Med Assoc (2002) 101(1):43–7.
55. San Millán Ruíz D, Gailloud P. Cerebral Developmental Venous Anomalies. Childs Nerv Syst (2010) 26(10):1395–406. doi: 10.1007/s00381-010-1253-4
56. El-Ghanem M, Kass-Hout T, Kass-Hout O, Alderazi YJ, Amuluru K, Al-Mufti F, et al. Arteriovenous Malformations in the Pediatric Population: Review of the Existing Literature. Interv Neurol (2016) 5(3-4):218–25. doi: 10.1159/000447605
57. Kosnik-Infinger L, Carroll C, Greiner H, Leach J, Mangano FT. Management of Cerebral Cavernous Malformations in the Pediatric Population: A Literature Review and Case Illustrations. J Neurosurg Sci (2015) 59(3):283–94.
58. Chanson P, Daujat F, Young J, Bellucci A, Kujas M, Doyon D, et al. Normal Pituitary Hypertrophy as a Frequent Cause of Pituitary Incidentaloma: A Follow-Up Study. J Clin Endocrinol Metab (2001) 86(7):3009–15. doi: 10.1210/jcem.86.7.7649
59. Sari S, Sari E, Akgun V, Ozcan E, Ince S, Saldir M, et al. Measures of Pituitary Gland and Stalk: From Neonate to Adolescence. J Pediatr Endocrinol Metab (2014) 27(11-12):1071–6. doi: 10.1515/jpem-2014-0054
Keywords: incidentaloma, growth hormone deficiency, central precocious puberty, incidental radiological finding, follow-up
Citation: Baldo F, Marin M, Murru FM, Barbi E and Tornese G (2022) Dealing With Brain MRI Findings in Pediatric Patients With Endocrinological Conditions: Less Is More? Front. Endocrinol. 12:780763. doi: 10.3389/fendo.2021.780763
Received: 21 September 2021; Accepted: 20 December 2021;
Published: 12 January 2022.
Edited by:
Sasha R. Howard, Queen Mary University of London, United KingdomReviewed by:
Alan David Rogol, University of Virginia, United StatesNikolina Kyprianou, Birmingham Children’s Hospital, United Kingdom
Copyright © 2022 Baldo, Marin, Murru, Barbi and Tornese. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maura Marin, bWF1cmFfbWFyaW5AbWUuY29t
 Francesco Baldo
Francesco Baldo Maura Marin1*
Maura Marin1* Egidio Barbi
Egidio Barbi Gianluca Tornese
Gianluca Tornese