
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol. , 11 November 2021
Sec. Cancer Endocrinology
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.779999
This article is part of the Research Topic New Strategies in the Treatment of Thyroid Carcinoma View all 17 articles
 Cesare Piazza1,2*
Cesare Piazza1,2* Davide Lancini1
Davide Lancini1 Michele Tomasoni1,2
Michele Tomasoni1,2 Anil D’Cruz3
Anil D’Cruz3 Dana M. Hartl4
Dana M. Hartl4 Luiz P. Kowalski5
Luiz P. Kowalski5 Gregory W. Randolph6
Gregory W. Randolph6 Alessandra Rinaldo7
Alessandra Rinaldo7 Jatin P. Shah8,9
Jatin P. Shah8,9 Ashok R. Shaha10
Ashok R. Shaha10 Ricard Simo11
Ricard Simo11 Vincent Vander Poorten12,13
Vincent Vander Poorten12,13 Mark Zafereo14
Mark Zafereo14 Alfio Ferlito15 on behalf of International Head and Neck Scientific Group
Alfio Ferlito15 on behalf of International Head and Neck Scientific GroupAirway involvement by advanced thyroid carcinoma (TC) constitutes a negative prognosticator, besides being a critical clinical issue since it represents one of the most frequent causes of death in locally advanced disease. It is generally agreed that, for appropriate laryngo-tracheal patterns of invasion, (crico-)tracheal resection and primary anastomosis [(C)TRA] is the preferred surgical technique in this clinical scenario. However, the results of long-term outcomes of (C)TRA are scarce in the literature, due to the rarity of such cases. The relative paucity of data prompts careful review of the available relevant series in order to critically evaluate this surgical technique from the oncologic and functional points of view. A systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement on the PubMed, Scopus, and Web of Science databases. English-language surgical series published between January 1985 and August 2021, reporting data on ≥5 patients treated for TC infiltrating the airway by (C)TRA were included. Oncologic outcomes, mortality, complications, and tracheotomy-dependency rates were assessed. Pooled proportion estimates were elaborated for each end-point. Thirty-seven studies were included, encompassing a total of 656 patients. Pooled risk of perioperative mortality was 2.0%. Surgical complications were reported in 27.0% of patients, with uni- or bilateral recurrent laryngeal nerve palsy being the most common. Permanent tracheotomy was required in 4.0% of patients. Oncologic outcomes varied among different series with 5- and 10-year overall survival rates ranging from 61% to 100% and 42.1% to 78.1%, respectively. Five- and 10-year disease specific survival rates ranged from 75.8% to 90% and 54.5% to 62.9%, respectively. Therefore, locally advanced TC with airway invasion treated with (C)TRA provides acceptable oncologic outcomes associated with a low permanent tracheotomy rate. The reported incidence of complications, however, indicates the need for judicious patient selection, meticulous surgical technique, and careful postoperative management.
Advanced resectable (T4a) thyroid cancer (TC) is a relatively uncommon clinical scenario, especially when dealing with differentiated tumors, being reported in just 5-15% of papillary carcinomas (1–4). This condition is associated with a significantly lower long-term survival rate compared to early-stage disease (1, 5, 6), particularly when the macroscopic extra-thyroidal extension involves more than one adjacent anatomical structure (7).
Aerodigestive tract invasion is more often seen in locally recurrent differentiated thyroid carcinoma (DTC) than at initial presentation. On the other hand, 60-70% of patients with such advanced neoplasms will have poorly differentiated or anaplastic carcinomas (8). The most frequently involved neighboring structures (after the strap muscles and recurrent laryngeal nerves [RLN]) are the upper trachea and laryngo-tracheal junction, due to their anatomic contiguity and relationship with the thyroid gland (9, 10), with a reported incidence of invasion of 0.4-0.7% of all TC (11). In descending order of frequency, the fourth and fifth most affected structures are the pharyngo-esophageal conduit and major vessels in the neck (8–10). The source of aerodigestive tract involvement is most frequently the primary tumor, while metastatic lymph nodes are responsible for less than 20% of cases (8).
Airway invasion by TC typically occurs in men (twice more frequently than in females), with a peak incidence in the sixth decade (11) and, usually, involves tumors larger than 3.7 cm (3, 12). Although rare, airway invasion has also been reported in the younger age group, considered to be in the “low-risk” prognostic category (13). Uncontrolled tumor progression in the airway represents one of the most frequent causes of death for TC, especially in the presence of unresectable tumors or loco-regional disease in which complete resection was not achieved (6, 14–16). Thus, in order to increase the chance of cure of these advanced neoplasms invading the airway, the first goal is to achieve a R0 resection within negative margins (5, 17, 18). However, due to the relative paucity of large series on this topic and in the absence of any prospective trials, the indications and comparative outcomes of different surgical techniques for airway management in advanced TC are still a matter of debate. There is general agreement that shaving the tumor off from the laryngo-tracheal axis is acceptable when the lesion involves only its external perichondrium (Shin I according to the classification by Shin et al.) (19), but there is no consensus on the best surgical technique for more extensive tumors (infiltrating the full-thickness of the cartilage [Shin II] or through it into the submucosa [Shin III] or the tracheal lumen [Shin IV]). Essentially, there are two different schools of thought: on one side, window resection with primary or secondary closure of the airway gap by soft tissue local flaps (20, 21) and, on the other, circumferential (crico-)tracheal resection with primary end-to-end anastomosis ([C]TRA) (22). Other groups have tried to design comprehensive, but somewhat cumbersome, algorithms in which both procedures can be performed according to the site, length, and width of airway involvement (8, 23). Head-to-head oncologic comparisons between these two surgical approaches are seriously limited by the low incidence of this condition, the heterogeneity of patients treated, and significant selection biases due to the retrospective nature of the studies. On the other hand, it is possible to objectively analyze postoperative morbidity, complication rates, and quality of life reported in the literature for each type of surgical technique.
The aim of this systematic review was to collect all the available English-language surgical series published between January 1985 and August 2021, reporting data on ≥5 patients treated for TC infiltrating the airway by (C)TRA, to better understand oncologic outcomes, complication rates, and airway-related quality of life.
A systematic review of the literature was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (24). The search was simultaneously conducted on the PubMed, Scopus, and Web of Science online databases, and updated to August 16, 2021. In order to retrieve all the publications dealing with (C)TRA for laryngo-tracheal involvement by TC the query string was composed as (tracheal resection) OR (tracheal involvement) AND (thyroid cancer) OR (thyroid neoplasm) OR (thyroid tumor). The search was conducted by two authors (C.P. and D.L.) who independently assessed the eligibility of the studies by screening article titles and abstracts, and then discussed their inclusion by reading the full-text of the selected works. Discrepancies were clarified by discussion between authors.
The Population/problem Intervention/exposure Comparison, Outcome, and Study design (PICOS) model was adopted for the review (25) (Table 1). The inclusion criteria were as follows: English language, publication from January 1, 1985 to the last day of online search (August 16, 2021), articles including data on (C)TRA for airway involvement by TC and reporting a case series of at least 5 patients. Exclusion criteria were: case reports, case series with less than 5 patients, papers purely describing results of surgical techniques different from (C)TRA (e.g., shaving, window resections, total laryngectomy) or that did not report sufficient data on outcomes and complications and focused on other related issues (e.g., radiological or clinical diagnosis, anesthetic issues, adjuvant treatments). Additionally, papers with duplicated or overlapping data from the same center were excluded, maintaining, when possible, the largest and more recent study among those available. Finally, a case series published by the first author (C.P.) (26), already included in this systematic review, was updated with data of patients treated from the time of the article publication (2016) to date, and their oncologic outcomes updated accordingly.
For each paper included in the systematic review, at the end of the selection process, evaluation of its quality was carried out following the Newcastle-Ottawa Scale (NOS) adapted for cross-sectional, cohort and case-control studies (27). The NOS was considered the evaluation method of choice, based on the recent literature (28, 29). The quality assessment was independently estimated by two different authors (D.L. and M.T.).
Data on study design, number of patients, age, gender, diagnostic work-up, TC histology and degree of airway invasion, length and type of resection, perioperative mortality, surgical complications, rate of patients who remained tracheostomy-dependent after (C)TRA, and oncological outcomes were collected, and a specific database was built.
The primary outcome was proportion of patients who developed a complication, calculated as the number of patients with reported complications divided by the total number of patients treated by (C)TRA for TC. Secondary outcome was the proportion of tracheostomy-dependent patients, defined as the number of patients with long-term tracheostomy dependency divided by the total number of patients treated by (C)TRA.
Meta-analysis of proportions was conducted through a generalized linear mixed model based on logit transformation (30). Pooled analyses are presented as forest plots. For each study, proportions and relative 95% confidence interval (CI) are depicted as gray squares and horizontal lines, respectively. The weight of each study on the overall effect estimate is reported and represented by the square size. The pooled proportion estimate and relative 95% CI, depicted as a diamond, are reported at the bottom of the forest plot. Heterogeneity between studies was assessed with Higgins I2 and τ2 tests (31), defined as low if I2<25%, moderate if between 25-50%, and substantial if >50% (32).
Publication bias was assessed through funnel plot assessment. Statistical analysis was performed with R (version 4.0.5, R foundation for Statistical Computing, Vienna, Austria). Statistical significance was defined as p<0.05.
The initial literature search yielded 1196 titles (525 records came from the PubMed database, 407 from Scopus, and 264 from Web of Science). Among these, 519 articles were excluded because present in two databases, and 147 due to publication in a language other than English. One article (33) was added from other sources, after being identified through the references of other manuscripts. Three-hundred-seventy-three articles were excluded after review of the title, and 48 by the abstract. From the remaining 110 full-text articles, 73 were excluded because they did not meet the eligibility criteria. Finally, 37 papers (3, 26, 33–67) were considered appropriate for the present systematic review (Figure 1, Table 2).
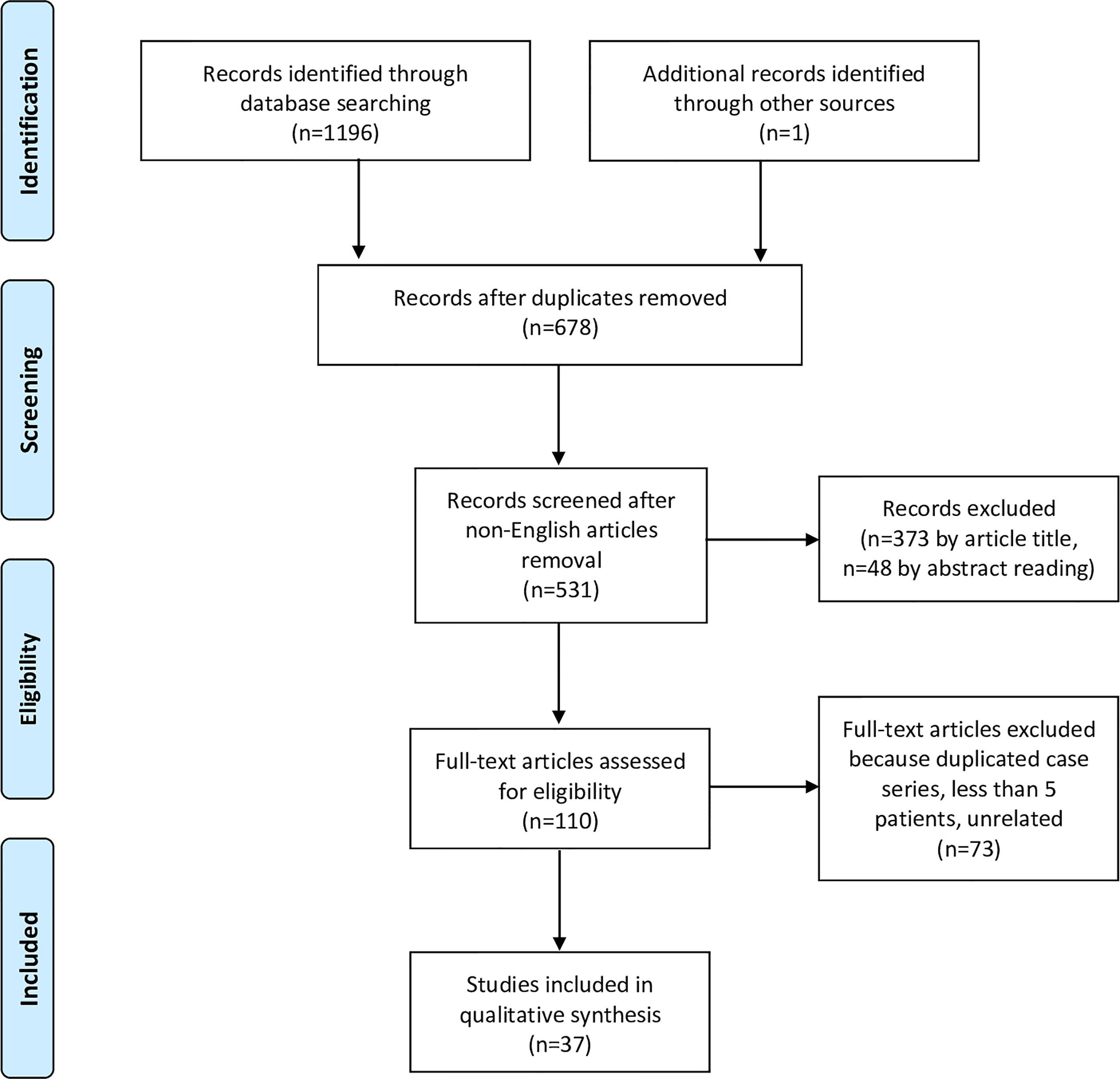
Figure 1 Flowchart showing the study selection process according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.
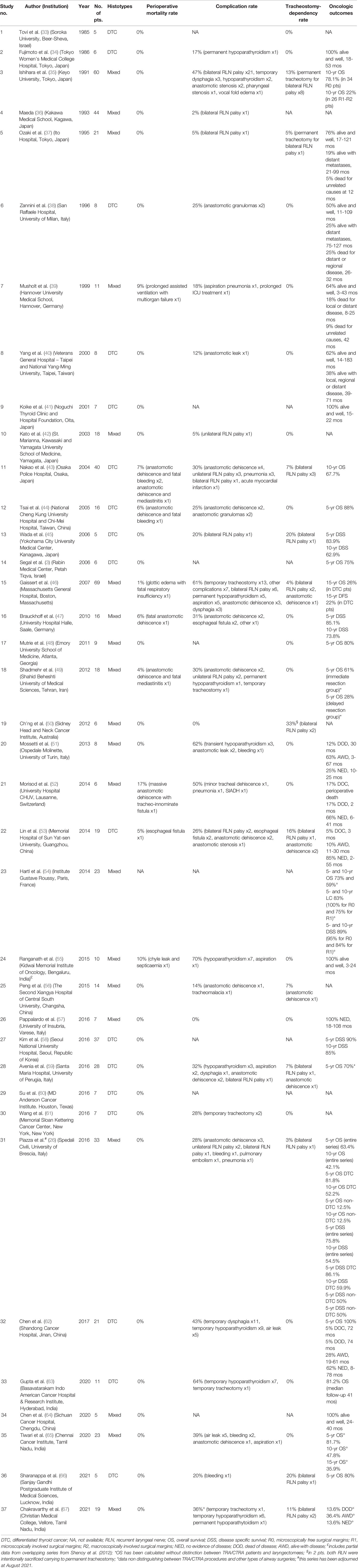
Table 2 Studies included in the systematic review of English-language, non-overlapping, surgical series including ≥5 patients treated by (C)TRA for TC invading the airway between January 1985 and August 2021 (No. of series=37, No. of patients=656). The table reports details about TC histotype, mortality, complications, tracheostomy-dependency, and oncologic outcomes.
According to the NOS adapted for cross-sectional studies (range of the scale, 0-9), the scores ranged from 2 to 7 (median, 5). Detailed scores for each article are reported in Table 3. All included manuscripts were retrospective single institution cross-sectional studies, except for one (42), which was a retrospective bi-institutional case series.
Overall, 656 patients were included in the current systematic review. Gender of patients treated by (C)TRA was detailed in 18 papers (26, 34, 35, 38, 40, 41, 43, 46, 50–53, 55, 57, 58, 62, 64, 66) for a total of 355 patients, of whom 59% were females.
Age of patients was reported in 12 manuscripts (26, 34, 39–41, 50–53, 57, 64, 66) for a total of 121 patients, with a mean of 60 years (range, 20-85).
Seventeen manuscripts reported data on (C)TRA for DTC alone (3, 33, 34, 38, 40, 41, 43–45, 53, 58–63, 66), while 20 for mixed histologies (26, 35–37, 39, 42, 46–52, 54–57, 64, 65, 67). Overall, the distribution of histopathological types in patients treated by (C)TRA was detailed for 376 of them (59%) and their frequency in descending order was as follows: papillary (79%), follicular (7%), poorly differentiated (5%), medullary (2%), Hürtle cell (2%), anaplastic (1%), follicular variant of papillary cancer (1%), metastasis to the thyroid gland from other organs (1%), and rare histotypes such as thyroid squamous cell carcinoma, giant cell carcinoma, and carcinoma with lymphoepithelioma-like pattern (2% all together) (26, 34, 35, 37–40, 42, 44–47, 49–53, 55, 57, 62, 64, 66).
Thirteen articles exclusively described the results of (C)TRA (26, 35, 37, 38, 40, 42, 48, 50–53, 55, 57), while 24 reported data about different treatment strategies also including (C)TRA (3, 33, 34, 36, 39, 41, 43–47, 49, 54, 56, 58–67).
Eight studies did not provide detailed information on the diagnostic work-up employed for detection and assessment of airway invasion by TC (3, 33, 35–37, 42, 45, 51). The remaining 29 manuscripts specified the diagnostic methods utilized (26, 34, 38–41, 43, 44, 46–50, 52–67). Expectedly, neck and chest x-ray for airway invasion assessment were rarely mentioned in studies published after 1996. In contrast, airway endoscopy (either flexible or rigid, under local or general anesthesia) was reported in 100% of the series, computed tomography (CT) in 93%, ultrasonography (US) in 45%, and magnetic resonance (MR) in 32%.
Distinction between types of resection (purely tracheal resection and anastomosis [TRA] or also involving part of the cricoid [CTRA] with consequent thyro-crico-tracheal anastomosis) was reported in 35 papers (3, 26, 33–47, 49–58, 60–67) for a total of 619 patients undergoing 466 (75%) TRA and 153 (25%) CTRA.
Length of resection was reported in 19 papers (26, 33, 34, 37, 38, 40, 42–44, 46, 49–53, 62, 64, 65, 67), usually as range and mean in centimeters or number of removed tracheal rings (for TRA), with associated portions of adjacent cricoid cartilage (for CTRA). In some instances, detailed tables allowed to exactly know the extent of (C)TRA for each patient. However, the length of (C)TRA for 350 patients ranged between 0.5 and 6 cm (mean, 2.5).
The Shin classification was explicitly used to quantify the depth of airway invasion by TC in 10 manuscripts (26, 37, 38, 40, 41, 44, 51, 57, 62, 67), for a total of 148 patients subdivided as follows: Shin I in 12 (8%) patients, Shin II in 35 (24%), Shin III in 39 (26%), and Shin IV in 62 (42%).
Data on perioperative mortality were provided in 36 articles (3, 26, 33–53, 55–67), for a total of 632 patients. The random effects model pooled risk of postoperative mortality was 2.0% (95% CI, 1.0-4.0%), with low heterogeneity. Forest and funnel plots are reported in Figures 2A, B.
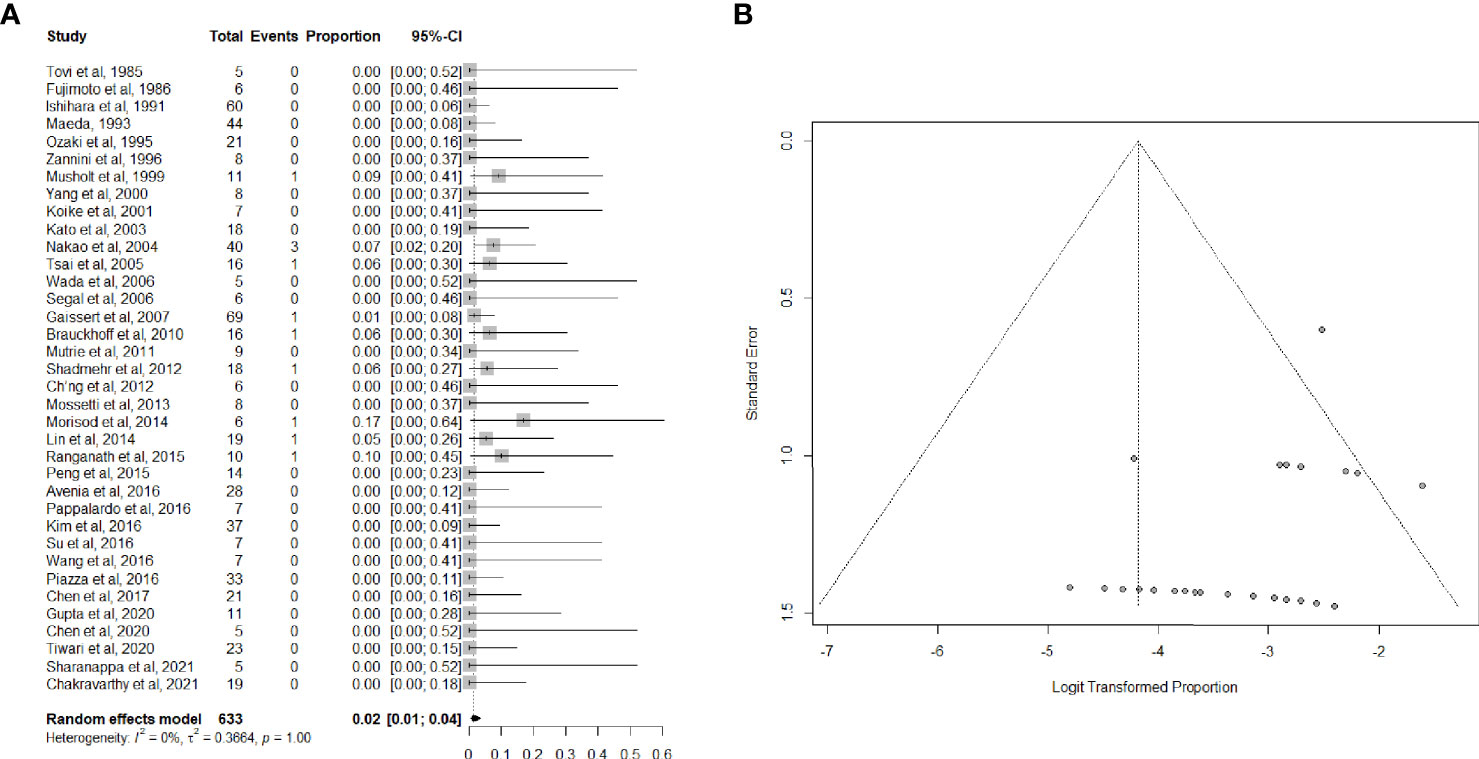
Figure 2 (A) Forest plot showing the pooled analysis of postoperative mortality and (B) relative funnel plot.
Twenty-nine articles (26, 34–40, 42–47, 49–53, 55–57, 59, 61–63, 65–67), including 557 patients, provided data on the proportion of patients suffering from postoperative complications. Complications were mostly bilateral RLN palsy, anastomotic dehiscence, hypoparathyroidism, and pulmonary complications, which are listed in detail in Table 2. The overall summary estimate of the proportion of patients who developed any complication after (C)TRA for TC was 27.0% (95% CI, 20.0-36.0%) (Figures 3A, B). Heterogeneity was high (I2 = 55.0%).
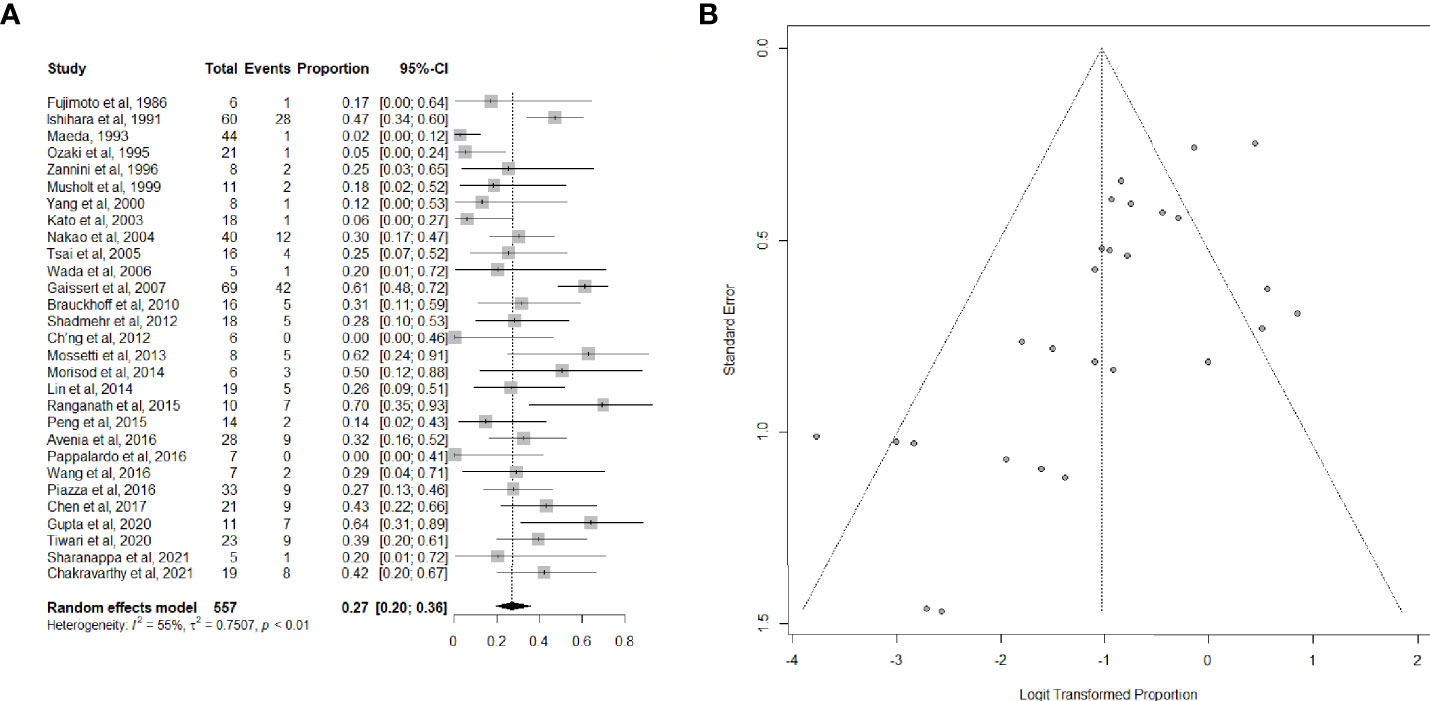
Figure 3 (A) Forest plot showing the pooled analysis of postoperative overall complications and (B) relative funnel plot.
Data on long-term tracheotomy-dependency were reported in 30 studies (26, 33–35, 37–40, 42–53, 55–57, 59, 61–63, 65–67) for a total of 527 patients. The summary estimate of the proportion of patients remaining dependent on tracheotomy after (C)TRA was 4.0% (95%CI, 2.0-8.0%). Heterogeneity of studies was low (Figures 4A, B).
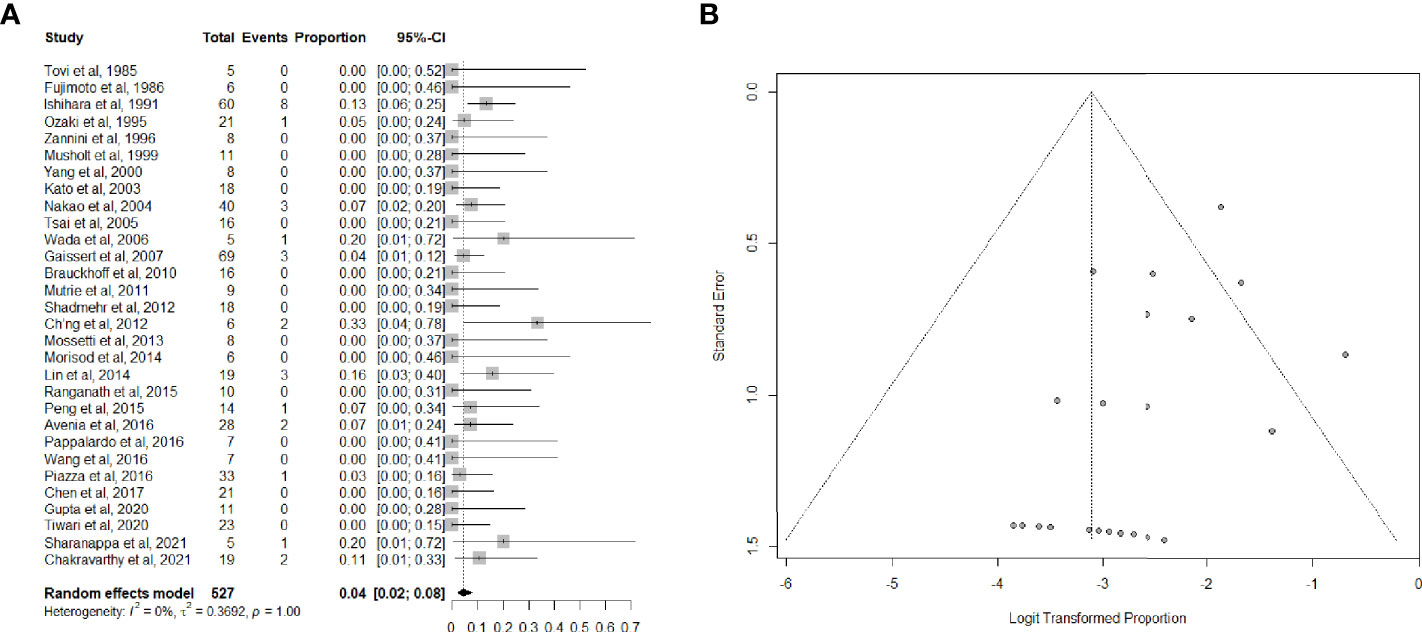
Figure 4 (A) Forest plot showing the pooled analysis of tracheotomy-dependency and (B) relative funnel plot.
Oncological outcomes details were available in 29 articles (3, 26, 34, 35, 37–41, 43–49, 51–55, 57–59, 62–66), as reported in Table 2. Seventeen studies (3, 26, 35, 43–49, 54, 58, 59, 62, 63, 65, 66) provided survival estimates. Five-year overall survival (OS) rate, reported by 11 studies (3, 26, 44, 48, 49, 54, 59, 62, 63, 65, 66), ranged from 61% (49) to 100% (62), whereas only 5 papers (26, 35, 43, 54, 65) reported the 10-year OS rate [ranging between 78.1% (35) and 42.1% (26)], and two studies (46, 65) also provided the 15-year OS rate (26.0% and 35.9%, respectively). Five manuscripts (26, 45, 47, 54, 58) reported 5- and 10-year disease specific survival estimates, which were in the range of 75.8-90% and 54.5-62.9%, respectively.
Specific data on adjuvant radioactive iodine (RAI) were reported by 12 papers (26, 40, 44, 46, 51–54, 62, 63, 65, 66), while 8 (26, 44, 46, 51–53, 62, 66) contained details on the use of postoperative external beam radiotherapy (EBRT). Most patients (86%; 95%CI, 61-96%; I2 = 22%) had been treated with adjuvant RAI, whereas indication to postoperative EBRT was far less common (11%; 95%CI, 4-30%; I2 = 18%). No survival analysis according to adjuvant therapies was conducted by any of the included studies.
When the cartilages or inter-cartilaginous ligaments are penetrated by neoplastic cells up to the level of submucosa, the TC spreads along the cartilaginous framework horizontally (following the inter-cartilaginous blood and lymphatic vessels) and vertically, before fungating into the airway lumen. As a consequence, the endoluminal real tumor extension is frequently more important than what can be seen from the outer surface of the organ (8, 19, 37). This, together with the uncertainty in the precise clinical assessment of the in-depth neoplastic extension within the cartilaginous framework, represents the most important pathological basis for justifying (C)TRA when dealing with tumors penetrating through the crico-tracheal axis, and the most evident limiting factor in supporting a window resection (per se based on the evaluation of the neoplastic extent just at the level of the outer airway surface).
In appropriately selected cases (i.e., for short segment airway involvement not beyond the external perichondrium) and with carefully performed surgery, shaving achieves complete resection and offers local control rates as high as 95-100% (58, 61, 68, 69). On the other hand, shaving for TC with deeper airway involvement, where R0 resection cannot be achieved or confirmed by frozen sections of cartilaginous tissues and possible microscopic penetration of the tumor into the airway submucosa via lymphatics piercing the inter-cartilaginous spaces may remain undetected, local failure with dismal outcomes have been reported in the literature (70, 71). Moreover, while several series included in this systematic review related to patients treated primarily by (C)TRA reported 5-year survival figures >80% (34, 41, 44, 45, 47, 48, 55, 57, 58, 62–66), the complication and mortality rates are considerably higher and survival lower when the procedure is applied as a salvage operation for recurrence after more conservative initial surgery (72, 73).
The same holds true for window resections, which are advocated by some in case of deeper tumor infiltration into the airway (20, 21). Such a surgical procedure is limited in its width and length of resection due to constraints in terms of airway stability, especially if the surgeon attempts a primary closure of the tracheal defect. As a consequence, if pedicled or revascularized myofascial/myoperichondral or skin flaps for tracheo-cutaneous fistula closure (74) are not employed, an R0 resection with negative margins by such a technique is less probable than after (C)TRA.
In case of incidental intraoperative discovery of TC invading the airway deeper than its external perichondrium, with a shaving procedure likely resulting in an R2-R1 resection, the general consensus is to convert the procedure into (C)TRA if it can be safely performed by the surgeon during the same intervention, or abort the procedure and refer the patient to a tertiary center with adequate experience in airway management (22). Clearly, in the best-case scenario, such a therapeutic option should be anticipated by performing the appropriate preoperative diagnostic work-up (including airway endoscopy and detailed cross-sectional imaging studies), referring the patient preoperatively to another team if adequate surgical expertise for (C)TRA is not available.
Invasion of the prevertebral fascia, major cervico-mediastinal blood vessels and/or massive involvement of the thoracic trachea are situations which make TC unresectable, and are categorized as T4b (8, 75). On the other hand, the only contraindications to (C)TRA include: 1) cranio-caudal extent exceeding 5.5 cm (i.e. 11 tracheal rings or cricoid arch plus 9 tracheal rings) (76) even in young patients (while for older ones, shorter airway resections may already represent an issue, with 4 cm of length being demonstrated to significantly increase the incidence of anastomotic dehiscence) (36, 77, 78); 2) major full-thickness esophageal/hypopharyngeal involvement requiring more than shaving of the external muscular layer or limited full-thickness resection with primary closure; and 3) tumor reaching the glottic plane either anteriorly through the crico-thyroid membrane or posteriorly at the level of the crico-arytenoid joint(s) (26). All these factors should be adequately assessed in the preoperative setting, considering that the proximal and distal airway cuts should be performed one tracheal ring above and below the macroscopic invasion site as appreciated from the adventitial side and checked from inside at the level of the airway lumen, with confirmatory mucosal frozen sections as needed. Clearly, these are contraindications for (C)TRA, but such tumor extensions are amenable to more radical surgical procedures, such as pharyngo-laryngo-esophagectomy for extensive invasion of the larynx and/or esophagus. Similarly, more extensive tracheal invasion can be resected making a mediastinal tracheostomy. In such clinical scenarios, a balanced preoperative counseling that may guide patients along the difficult path of choosing between a better quality of life against a higher chance of perioperative complications should be taken into account.
Preoperative bilateral RLN palsy does not represent per se an absolute contraindication to (C)TRA since an R0 resection with postoperative permanent tracheotomy (and usable voice) is always better than both persistent airway disease or total laryngectomy with/without tracheoesophageal voice prosthesis insertion (26). Of note, one should consider that, in case of anterior cricoid arch resection, the ensuing bilateral loss of vocal cords tension for lack of crico-thyroid muscles, if associated with bilateral RLN palsy, may make posterior cordotomy with/without arytenoidectomy useless in the attempt of getting a patent airway without tracheostomy. Last but not least, placing a tracheostomy below the anastomotic line after (C)TRA with bilateral RLN palsy may significantly increase the risk of postoperative complications such as anastomotic dehiscence, stenosis or tracheo-innominate fistula.
Radical comprehensive approaches like (C)TRA, able to maintain a good quality of life, are strongly recommended in patients with DTC even in the presence of a limited burden of asymptomatic distant metastases (8, 26). However, general health status (age, comorbidities, compliance) and willingness to undergo surgery play a prominent role in selecting patients amenable for such a major surgical procedure.
The first endoscopic examination to be performed in every TC patient should include a flexible videolaryngoscopy, even in the absence of an appreciable hoarseness: in fact, finding a unilateral RLN palsy should prompt to the request of more targeted investigations (such as tracheoscopy and cross-sectional imaging studies) to determine and precisely evaluate potential airway involvement, both quantifying its radial (depth) and cranio-caudal extents (22). This also applies to other common signs and symptoms of advanced TC such as hemoptysis, dyspnea, dysphagia, thyroid fixation or clinically enlarged lymph nodes: even though infrequent, these findings should prompt adequate imaging to exclude aerodigestive tract invasion and quantify it for appropriate surgical treatment planning.
Transcutaneous US can detect the depth of airway invasion, reliably distinguishing superficial (Shin I-II) vs. deeper (Shin III-IV) infiltration with a diagnostic accuracy potentially reaching 93% (79–81). However, US is generally considered highly operator-dependent and less reliable for tumors larger than 4 cm or with major intralesional calcifications, as well as with significant retrosternal extension.
CT is considered superior to US and definitely more reproducible for precise three-dimensional assessment of airway invasion (22), with a mean sensitivity, specificity, and accuracy in detecting tracheal invasion of 59.1%, 91.4%, and 83.2%, respectively (82). It is important to emphasize that the CT should be performed with contrast, to give the most precise information. In particular, the most quoted CT diagnostic criteria are tumor in contact for 180° or more of tracheal circumference, deformity of the airway lumen (i.e. indentation due to pressure effect) at the level of such a contact, focal irregularity, thickening or bulging of the mucosal lining and, finally, presence of tumor within the tracheal lumen (82).
MR seems to have lower diagnostic accuracy than US and CT, with a tendency to overestimate the actual depth of airway invasion (83). Others report superior outcomes with MR compared to other imaging techniques (84). However, a tumor-airway contact exceeding 135° of the tracheal circumference seems to efficiently predict some degree of cartilaginous involvement (85).
Laryngo-tracheoscopy allows appreciation of airway invasion when the airway submucosa is reached (Shin III-IV), thus appearing as a subtle localized or diffuse mucosal redness, with elevation, edema, presence of telangiectasias and vascular engorgement, with focal erosions or endoluminal vegetations in the most obvious scenario (41). This is in line with the experience of the first author, who missed Shin II tracheal invasion in 11% of his series by endoscopy and imaging (26). The sensitivity of this tool for tracheal invasion evaluation is, in fact, reported to be around 85%, with a mean underestimation of the actual cranio-caudal tumor extent of an average of 0.8 (maximum 2) tracheal rings compared to postoperative histopathologic specimens (86).
Endobronchial US (EBUS) is the latest imaging technique for assessment of the presence and degree of airway invasion by TC. Recent reports highlight an accuracy significantly higher than those reported by CT and/or MR, with a sensitivity and specificity of 92% and 83%, respectively (84). However, EBUS is still relatively infrequently used in most medical facilities due to some inherent drawbacks such as increased invasiveness, high cost, and limited utility in evaluating tumors infiltrating at the level of the thyroid upper lobe (82).
It would therefore appear that the most adequate diagnostic algorithm for advanced TC with suspicious airway invasion should be based on careful endoscopy of the larynx and trachea, with US and subsequent CT or MR depending to the local facilities and expertise.
Predictors of survival in advanced TC involving the airway may be patient-related (age, gender), tumor-related, and treatment-related. Among tumor-related factors, micro- vs. macroscopic extrathyroidal extension, limited to one vs. multiple organs has been recently demonstrated to play an important role (7). Strap muscles (T3b) and RLN invasion (T4a) have no prognostic influence on survival, but they do affect recurrence in contrast to laryngeal, tracheal, and esophageal involvement which heavily impact both local recurrence and survival rates (9, 87). Tracheal and esophageal invasion (T4a) present no prognostic differences when all tumor tissue can be removed within negative margins (R0 resection). By contrast, invasion of the larynx (T4a) reflects a more aggressive behavior of disease (47), even though no clear distinction is usually made in the literature with respect to the specific anatomic site(s) of TC infiltration. Intuitively, anterior cricoid involvement has a very limited impact on radicality of tumor resection and possibility of organ preservation compared to lateral and/or posterior cricoid infiltration (26). The same holds true when considering invasion of the inferior border of the thyroid laminae compared to transgression of their lateral edges in close proximity with the piriform sinus, or when dealing with a superficial (external perichondrium) vs. a full-thickness thyroid cartilage invasion.
General consensus has been reached on preserving the RLN whenever it is preoperatively functioning, even though encased by tumor, as long as it is not directly infiltrated by TC and all gross disease is removed, adding postoperative adjuvant therapy in the form of RAI or EBRT as indicated (22). Sacrifice of the RLN is generally only justified when preoperatively already non-functioning or when its preservation would inevitably leave behind gross residual disease (R2 resection) (88). In this case, RLN reinnervation by direct suture of healthy stumps at frozen sections, interposition of a nerve graft or suturing the ansa hypoglossi to the distal RLN stump may be considered to maintain vocal muscle tone and improve functional outcomes for voice rehabilitation (89–91).
Tumor histopathological type is a strong predictor of survival in TC invading the aerodigestive tract: the 5-year survival rate in DTC is around 75%, while it declines below 60% in medullary TC, and to 20% in undifferentiated tumors (26, 47).
Among procedure-related prognosticators, the most powerful seems to be R status: a number of series have confirmed better 5-year survival in R0 compared to R2 resections (90-78% vs. 50-35%) (35, 92, 93). Even R1 resections, while apparently presenting similar 5-year survivals (71, 93), in the long run are invariably associated with a higher rate of recurrence (46, 70, 71, 94). Moreover, when comparing immediate R0 resection with R1 resection followed by delayed radicalization, even considering non-organ sparing surgery, 10- and 20-year disease-free survival decrease from 67% and 50% to 7% and 0%, respectively (46). However, the absence of high-quality prospective data does not allow to solve the discrepancy between the above mentioned data and those reported by others, with no statistically significant survival differences between R0 and R1 resections (54, 61).
It should be emphasized at the outset that postoperative RAI and/or EBRT do not replace adequate surgery with R0 resection due to the high failure rates of these adjuvant therapies in controlling residual R2 disease, especially at the level of the airway. Adjuvant therapy by RAI after (C)TRA for T4a DTC is widely used whenever sufficient iodine uptake is demonstrated. However, response to RAI often cannot be established before surgery and is not uniform especially for microscopic residual disease (R1 resection) on the airway surface (95).
Special consideration should be paid to patients who have already received RAI in the past and experience further disease progression, since tolerance to and further potential benefit from RAI is questionable in such a clinical scenario. Moreover, EBRT as initial mode of therapy in TC invading the airway deeper than its cartilaginous framework should not be offered given the limitation it places on wound healing in case of a subsequent (C)TRA (26) and the low probability of appropriate response of bulky TC invading the airway (5). Still controversial is the potential role of adjuvant EBRT after segmental R0 airway resection for DTC, especially when the laryngo-tracheal axis was the only site of macroscopic extrathyroidal extension. Since no survival analysis was available regarding the impact of both RAI and EBRT as adjuvant therapies after (C)TRA, no conclusion on their prognostic role can be withdrawn from the present systematic review.
The most notable limits of our paper are the retrospective nature and relatively low number of patients included in each case series, per se potentially flooded by selection biases and a wide array of geographic, therapeutic, and epidemiologic differences. Lack of details concerning the histotypes and use of adjuvant treatment protocols reduces the possibility to infer their impact on prognosis of patients treated by (C)TRA. Moreover, the evolution in the diagnostic and therapeutic strategies occurred during the long time span of our systematic review must be taken into proper consideration.
The current literature is still devoid of prospective clinical trials addressing optimal management of T4a TC invading the crico-tracheal axis. However, based on the retrospective case series analyzed, even though characterized by the common biases related to the relatively small number of patients recruited in a long period of time, (C)TRA appears to be a reproducible major surgical procedure, which is able to ensure both good oncological outcomes as well as a reasonable chance of laryngeal function preservation for TC invading the trachea deeper than the level of its external perichondrium and less than 5.5 cm in length. However, the non-negligible mortality and complication rates should prompt management of these advanced tumors by skilled surgical teams in tertiary referral centers with the adequate multidisciplinary expertise, after proper diagnostic work-up and patient selection.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
CP, AD’C, DH, LK, GR, AR, JS, AS, RS, VV, MZ, and AF contributed to conception and design of the study. CP, DL, and MT organized the database and performed the statistical analysis. CP, DL, and MT wrote the first draft of the manuscript. CP, AD’C, DH, LK, GR, AR, JS, AS, RS, VV, MZ, and AF wrote the final draft of the manuscript. All authors contributed to the article andapproved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Andersen PE, Kinsella J, Loree TR, Shaha AR, Shah JP. Differentiated Carcinoma of the Thyroid With Extrathyroidal Extension. Am J Surg (1995) 170:467–70. doi: 10.1016/S0002-9610(99)80331-6
2. Hay ID, Thompson GB, Grant CS, Bergstralh EJ, Dvorak CE, Gorman CA, et al. Papillary Thyroid Carcinoma Managed at the Mayo Clinic During Six Decades (1940-1999): Temporal Trends in Initial Therapy and Long-Term Outcome in 2444 Consecutively Treated Patients. World J Surg (2002) 26:879–85. doi: 10.1007/s00268-002-6612-1
3. Segal K, Shpitzer T, Hazan A, Bachar G, Marshak G, Popovtzer A. Invasive Well-Differentiated Thyroid Carcinoma: Effect of Treatment Modalities on Outcome. Otolaryngol Head Neck Surg (2006) 134:819–22. doi: 10.1016/j.otohns.2005.11.040
4. Hotomi M, Sugitani I, Toda K, Kawabata K, Fujimoto Y. A Novel Definition of Extrathyroidal Invasion for Patients With Papillary Thyroid Carcinoma for Predicting Prognosis. World J Surg (2012) 36:1231–40. doi: 10.1007/s00268-012-1518-z
5. Kasperbauer JL. Locally Advanced Thyroid Carcinoma. Ann Otol Rhinol Laryngol (2004) 113:749–53. doi: 10.1177/000348940411300914
6. McCaffrey JC. Aerodigestive Tract Invasion by Well-Differentiated Thyroid Carcinoma: Diagnosis, Management, Prognosis, and Biology. Laryngoscope (2006) 116:1–11. doi: 10.1097/01.MLG.0000200428.26975.86
7. Abraham E, Roshan D, Tran B, Wykes J, Campbell P, Ebrahimi A. The Extent of Extrathyroidal Extension Is a Key Determinant of Prognosis in T4a Papillary Thyroid Cancer. J Surg Oncol (2019) 120:1016–22. doi: 10.1002/jso.25683
8. Brauckhoff M. Classification of Aerodigestive Tract Invasion From Thyroid Cancer. Langenbecks Arch Surg (2014) 399:209–16. doi: 10.1007/s00423-013-1142-x
9. McCaffrey TV, Bergstralh EJ, Hay ID. Locally Invasive Papillary Thyroid Carcinoma: 1940-1990. Head Neck (1994) 16:165–72. doi: 10.1002/hed.2880160211
10. Nixon IJ, Simo R, Newbold K, Rinaldo A, Suarez C, Kowalski LP, et al. Management of Invasive Differentiated Thyroid Cancer. Thyroid (2016) 26:1156–66. doi: 10.1089/thy.2016.0064
11. Kanazawa Y, Takeuchi M, Tateya I, Omori K, Kawakami K. Clinical Epidemiology of Tracheal Invasion From Thyroid Cancer in Japanese Population: Functional Outcomes and Effect of Aging. Cancer Epidemiol (2017) 50:107–12. doi: 10.1016/j.canep.2017.08.011
12. Ortiz S, Rodriguez JM, Soria T, Pérez-Flores D, Pinero A, Moreno J, et al. Extrathyroid Spread in Papillary Carcinoma of the Thyroid: Clinicopathological and Prognostic Study. Otolaryngol Head Neck Surg (2001) 124:261–5. doi: 10.1067/mhn.2001.113141
13. Rosen IB, Bowden J, Luk SC, Simpson JA. Aggressive Thyroid Cancer in Low-Risk Age Population. Surgery (1987) 102:1075–80.
14. Wu HS, Young MT, Ituarte PH, D’Avanzo A, Duh QY, Greenspan FS, et al. Death From Thyroid Cancer of Follicular Cell Origin. J Am Coll Surg (2000) 191:600–6. doi: 10.1016/S1072-7515(00)00731-6
15. Beasley NJ, Walfish PG, Witterick I, Freeman JL. Cause of Death in Patients With Well-Differentiated Thyroid Carcinoma. Laryngoscope (2001) 111:989–91. doi: 10.1097/00005537-200106000-00011
16. Lamartina L, Godbert Y, Nascimento C, Do Cao C, Hescot S, Borget I, et al. Locally Unresectable Differentiated Thyroid Cancer: Outcomes and Perspectives. Endocrine (2020) 69:133–41. doi: 10.1007/s12020-020-02245-0
17. Kowalski LP, Filho JG. Results of the Treatment of Locally Invasive Thyroid Carcinoma. Head Neck (2002) 24:340–4. doi: 10.1002/hed.10058
18. Shaha AR. Implications of Prognostic Factors and Risk Groups in the Management of Differentiated Thyroid Cancer. Laryngoscope (2004) 114:393–402. doi: 10.1097/00005537-200403000-00001
19. Shin DH, Mark EJ, Suen HC, Grillo HC. Pathologic Staging of Papillary Carcinoma of the Thyroid With Airway Invasion Based on the Anatomic Manner of Extension to the Trachea: A Clinicopathologic Study Based on 22 Patients Who Underwent Thyroidectomy and Airway Resection. Hum Pathol (1993) 24:866–70. doi: 10.1016/0046-8177(93)90136-5
20. Ebihara M, Kishimoto S, Hayashi R, Miyazaki M, Shinozaki T, Daiko H, et al. Window Resection of the Trachea and Secondary Reconstruction for Invasion by Differentiated Thyroid Carcinoma. Auris Nasus Larynx (2011) 38:271–5. doi: 10.1016/j.anl.2010.09.003
21. Moritani S. Window Resection for Intraluminal Cricotracheal Invasion by Papillary Thyroid Carcinoma. World J Surg (2017) 41:1812–9. doi: 10.1007/s00268-017-3927-5
22. Shindo ML, Caruana SM, Kandil E, McCaffrey JC, Orloff LA, Porterfield JR, et al. Management of Invasive Well-Differentiated Thyroid Cancer: An American Head and Neck Society Consensus Statement. Head Neck (2014) 36:1379–90. doi: 10.1002/hed.23619
23. Dralle H, Brauckhoff M, Machens A, Gimm O. “Surgical Management of Advanced Thyroid Cancer Invading the Aerodigestive Tract”. In: Clark OH, Duh QY, Kebebew E, editors. Textbook on Endocrine Surgery, 2nd Edition. Philadelphia: Elseviere Saunders (2005). p. 318–33.
24. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med (2009) 151:264–9. doi: 10.7326/0003-4819-151-4-200908180-00135
25. Centre for Reviews and Dissemination. Systematic Reviews: CRD’s Guidance for Undertaking Reviews in Health Care. York: University of York (2006).
26. Piazza C, Del Bon F, Barbieri D, Grazioli P, Paderno A, Perotti P, et al. Tracheal and Crico-Tracheal Resection and Anastomosis for Malignancies Involving the Thyroid Gland and the Airway. Ann Otol Rhinol Laryngol (2016) 125:97–104. doi: 10.1177/0003489415599000
27. Wells G, Shea B, O’Connell D, Peterson J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. Available at: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed February 2021).
28. Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The Methodological Quality Assessment Tools for Preclinical and Clinical Studies, Systematic Review and Meta-Analysis, and Clinical Practice Guideline: A Systematic Review. J Evid Based Med (2015) 8:2–10. doi: 10.1111/jebm.12141
29. Moskalewicz A, Oremus M. No Clear Choice Between Newcastle-Ottawa Scale and Appraisal Tool for Cross-Sectional Studies to Assess Methodological Quality in Cross-Sectional Studies of Health-Related Quality of Life and Breast Cancer. J Clin Epidem (2020) 120:94–103. doi: 10.1016/j.jclinepi.2019.12.013
30. Schwarzer G, Chemaitelly H, Abu-Raddad LJ, Rücker G. Seriously Misleading Results Using Inverse of Freeman-Tukey Double Arcsine Transformation in Meta-Analysis of Single Proportions. Res Synth Methods (2019) 10:476–83. doi: 10.1002/jrsm.1348
31. Higgins JPT, Thompson SG. Quantifying Heterogeneity in a Meta-Analysis. Stat Med (2002) 21:1539–58. doi: 10.1002/sim.1186
32. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring Inconsistency in Meta-Analyses. BMJ (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
33. Tovi F, Goldstein J. Locally Aggressive Differentiated Thyroid Carcinoma. J Surg Oncol (1985) 29:99–104. doi: 10.1002/jso.2930290207
34. Fujimoto Y, Obara T, Ito Y, Kodama T, Yashiro T, Yamashita T, et al. Aggressive Surgical Approach for Locally Invasive Papillary Carcinoma of the Thyroid in Patients Over Forty-Five Years of Age. Surgery (1986) 100:1098–107.
35. Ishihara T, Kobayashi K, Kikuchi K, Kato R, Kawamura M, Ito K. Surgical Treatment of Advanced Thyroid Carcinoma Invading the Trachea. J Thorac Cardiovasc Surg (1991) 102:717–20. doi: 10.1016/S0022-5223(19)36863-1
36. Maeda M. Tracheobronchial Resection for Tumor: Classification and Related Biological Problems. Lung Cancer (1993) 9:191–202. doi: 10.1016/0169-5002(93)90671-J
37. Ozaki O, Sugino K, Mimura T, Ito K. Surgery for Patients With Thyroid Carcinoma Invading the Trachea: Circumferential Sleeve Resection Followed by End-to-End Anastomosis. Surgery (1995) 117:268–71. doi: 10.1016/S0039-6060(05)80200-4
38. Zannini P, Melloni G. Surgical Management of Thyroid Cancer Invading the Trachea. Chest Surg Clin N Am (1996) 6:777–90.
39. Musholt TJ, Musholt PB, Behrend M, Raab R, Scheumann GFW, Klempnauer J. Invasive Differentiated Thyroid Carcinoma: Tracheal Resection and Reconstruction Procedures in the Hands of the Endocrine Surgeons. Surgery (1999) 126:1078–88. doi: 10.1067/msy.2099.102267
40. Yang C-C, Lee C-H, Wang L-S, Huang B-S, Hsu W-H, Huang M-H. Resectional Treatment for Thyroid Cancer With Tracheal Invasion. A Long-Term Follow-Up Study. Arch Surg (2000) 135:704–7. doi: 10.1001/archsurg.135.6.704
41. Koike E, Yamashita H, Noguchi S, Yamashita H, Ohshima A, Watanabe S, et al. Bronchoscopic Diagnosis of Thyroid Cancer With Laryngotracheal Invasion. Arch Surg (2001) 136:1185–9. doi: 10.1001/archsurg.136.10.1185
42. Kato I, Iwatake H, Tsutsumi K, Koizuka I, Suzuki H, Nakamura T. End-To-End Anastomosis in Chronic Tracheal Stenosis. Auris Nasus Larynx (2003) 30:S69–73. doi: 10.1016/S0385-8146(02)00120-7
43. Nakao K, Kurozumi K, Nakahara M, Kido T. Resection and Reconstruction of the Airway in Patients With Advanced Thyroid Cancer. World J Surg (2004) 28:1204–6. doi: 10.1007/s00268-004-7606-y
44. Tsai Y-F, Tseng Y-L, Wu M-H, Hung C-J, Lai W-W, Lin M-Y. Aggressive Resection of the Airway Invaded by Thyroid Carcinoma. Br J Surg (2005) 92:1382–7. doi: 10.1002/bjs.5124
45. Wada N, Nakayama H, Masudo Y, Suganuma N, Rino Y. Clinical Outcomes of Different Modes of Resection in Papillary Thyroid Carcinomas With Laryngotracheal Invasion. Langenbecks Arch Surg (2006) 391:545–9. doi: 10.1007/s00423-006-0106-9
46. Gaissert H, Honings J, Grillo HC, Donahue DM, Wain JC, Wright CD, et al. Segmental Laryngotracheal and Tracheal Resection for Invasive Thyroid Carcinoma. Ann Thorac Surg (2007) 83:1952–9. doi: 10.1016/j.athoracsur.2007.01.056
47. Brauckhoff M, Machens A, Thanh PN, Lorenz K, Schmeil A, Stratmann M, et al. Impact of Extent of Resection for Thyroid Cancer Invading the Aerodigestive Tract on Surgical Morbidity, Local Recurrence, and Cancer-Specific Survival. Surgery (2010) 148:1257–66. doi: 10.1016/j.surg.2010.09.011
48. Mutrie CJ, Eldaif SM, Rutledge CW, Force SD, Grist WJ, Mansour KA, et al. Cervical Tracheal Resection: New Lessons Learned. Ann Thorac Surg (2011) 91:1101–6. doi: 10.1016/j.athoracsur.2010.11.066
49. Shadmehr MB, Farzanegan R, Zangi M, Mohammadzadeh A, Sheikhy K, Pejhan S, et al. Thyroid Cancers With Laryngotracheal Invasion. Eur J Cardiothorac Surg (2012) 41:635–40. doi: 10.1093/ejcts/ezr131
50. Ch’ng S, Palme CE, Wong GL, Brunner M, Ashford B, McGuinness J, et al. Reconstruction of the (Crico)Trachea for Malignancy in the Virgin and Irradiated Neck. J Plast Reconstr Aesthet Surg (2012) 65:1645–53. doi: 10.1016/j.bjps.2012.07.008
51. Mossetti C, Palestini N, Bruna MC, Camandona M, Freddi M, Oliaro A, et al. Segmental Tracheal Resection for Invasive Differentiated Thyroid Carcinoma. Our Experience in Eight Cases. Langenbecks Arch Surg (2013) 398:1075–82. doi: 10.1007/s00423-013-1127-9
52. Morisod B, Monnier P, Simon C, Sandu K. Cricotracheal Resection for Laryngeal Invasion by Thyroid Carcinoma: Our Experience. Eur Arch Otorhinolaryngol (2014) 271:2261–6. doi: 10.1007/s00405-013-2757-9
53. Lin S, Huang H, Liu X, Li Q, Yang A, Zhang Q, et al. Treatments for Complications of Tracheal Sleeve Resection for Papillary Thyroid Carcinoma With Tracheal Invasion. EJSO (2014) 40:176–81. doi: 10.1016/j.ejso.2013.12.008
54. Hartl DM, Zago S, Leboulleux S, Mirghani H, Deandreis D, Baudin E, et al. Resection Margins and Prognosis in Locally Invasive Thyroid Cancer. Head Neck (2014) 36:1034–8. doi: 10.1002/hed.23406
55. Ranganath N, Arathi BHR, Ramamani PV, Gowda VB. Anaesthetic Considerations for Tracheal Resection in Oncological Thyroid Surgeries. Ind J Anaesth (2015) 59:188–90. doi: 10.4103/0019-5049.153043
56. Peng A, Li Y, Yang X, Xiao Z, Tang Q, Wang Q. A Review of the Management and Prognosis of Thyroid Carcinoma With Tracheal Invasion. Eur Arch Otorhinolaryngol (2015) 272:1833–43. doi: 10.1007/s00405-014-3144-x
57. Pappalardo V, La Rosa S, Imperatori A, Rotolo N, Tanda ML, Sessa A, et al. Thyroid Cancer With Tracheal Invasion: A Pathological Estimation. Gland Surg (2016) 5:541–5. doi: 10.21037/gs.2016.10.02
58. Kim H, Jung HJ, Lee SY, Kwon TK, Kim KH, Sung MW, et al. Prognostic Factors of Locally Invasive Well-Differentiated Thyroid Carcinoma Involving the Trachea. Eur Arch Otorhinolaryngol (2016) 273:1919–26. doi: 10.1007/s00405-015-3724-4
59. Avenia N, Vannucci J, Monacelli M, Lucchini R, Polistena A, Santoprete S, et al. Thyroid Cancer Invading the Airway: Diagnosis and Management. Int J Surg (2016) 28 Suppl 1:S75–8. doi: 10.1016/j.ijsu.2015.12.036
60. Su S, Milas ZL, Bhatt N, Roberts D, Clayman GL. Well-Differentiated Thyroid Cancer With Aerodigestive Tract Invasion: Long-Term Control and Functional Outcomes. Head Neck (2016) 38:72–8. doi: 10.1002/hed.23851
61. Wang LY, Nixon IJ, Patel SG, Palmer FL, Tuttle RM, Shaha A, et al. Operative Management of Locally Advanced Differentiated Thyroid Cancer. Surgery (2016) 160:738–46. doi: 10.1016/j.surg.2016.04.027
62. Chen W, Zou S, Wang L, Wu C, Wang Z, Li K, et al. Anastomosis in the Absence of a Suprahyoid Release Following Circumferential Sleeve Resection Is Feasible in Differentiated Thyroid Carcinoma Patients With Tracheal Invasion. Oncol Lett (2017) 14:2822–30. doi: 10.3892/ol.2017.6568
63. Gupta V, Rao C, Raju KVVN, Nemade H, Dasu S, Jayakarthik Y, et al. Tracheal/laryngeal Infiltration in Thyroid Cancer. Indian J Surg Oncol (2020) 11:75–9. doi: 10.1007/s13193-019-00994-7
64. Chen Y-B, Wang Z-H, Fu G-M, Wan Q-X, Li X-J, Chen J. Application of Computer-Aided Design (CAD) and Three-Dimensional (3D) Visualization Technologies in the Diagnosis and Treatment of Refractory Thyroid Tumors. Cancer Manag Res (2020) 12:6887–94. doi: 10.2147/CMAR.S246576
65. Tiwari Y, Krishnamurthy A. Long-Term Outcomes of Differentiated Thyroid Cancers With Tracheal Invasion: A 15-Year Experience. Indian J Cancer (2020) 57:398–404. doi: 10.4103/ijc.IJC_456_19
66. Sharanappa V, Bichoo RA, Mishra A, Pradhan PK, Mishra SK. Circumferential Laryngotracheal Resection in Thyroid Cancer: Experience and Outcome in a Single Center. Indian J Otolaryngol Head Neck Surg (2021). doi: 10.1007/s12070-020-02339-1
67. Chakravarthy NS, Thomas V, Sam TS, Sen S, Cherian AJ, Abraham DT, et al. Laryngotracheal Resection in Thyroid Cancer — Experience From a Single Centre Series of 22 Cases. Indian J Surg Oncol (2021). doi: 10.1007/s13193-021-01407-4
68. Tsukahara K, Sugitani I, Kawabata K. Surgical Management of Tracheal Shaving for Papillary Thyroid Carcinoma With Tracheal Invasion. Acta Otolaryngol (2009) 129:1498–502. doi: 10.3109/00016480902725239
69. Ito Y, Fukushima M, Yabuta T, Tomoda C, Inoue H, Kihara M, et al. Local Prognosis of Patients With Papillary Thyroid Carcinoma Who Were Intra-Operatively Diagnosed as Having Minimal Invasion of the Trachea: A 17-Year Experience in a Single Institute. Asian J Surg (2009) 32:102–8. doi: 10.1016/S1015-9584(09)60019-1
70. Park CS, Suh KW, Min JS. Cartilage-Shaving Procedure for the Control of Tracheal Cartilage Invasion by Thyroid Carcinoma. Head Neck (1993) 15:289–91. doi: 10.1002/hed.2880150403
71. McCarty TM, Kuhn JA, Williams WL Jr, Ellenhorn JD, O’Brien JC, Preskitt JT, et al. Surgical Management of Thyroid Cancer Invading the Airway. Ann Surg Oncol (1997) 4:403–8. doi: 10.1007/BF02305553
72. Grillo HC, Suen HC, Mathisen DJ, Wain JC. Resectional Management of Thyroid Carcinoma Invading the Airway. Ann Thorac Surg (1992) 54:3–9. doi: 10.1016/0003-4975(92)91131-R
73. Brauckhoff M, Meinicke A, Bilkenroth U, Lorenz K, Brauckhoff K, Gimm O, et al. Long-Term Results and Functional Outcome After Cervical Evisceration in Patients With Thyroid Cancer. Surgery (2006) 140:953–9. doi: 10.1016/j.surg.2006.09.001
74. Matsumoto F, Ikeda K. Surgical Management of Tracheal Invasion by Well-Differentiated Thyroid Cancer. Cancers (2021) 13:797. doi: 10.3390/cancers13040797
75. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to Build a Bridge From a Population-Based to a More “Personalized” Approach to Cancer Staging. CA Cancer J Clin (2017) 67:93–9. doi: 10.3322/caac.21388
76. Drevet G, Conti M, Deslauriers J. Surgical Anatomy of the Tracheobronchial Tree. J Thorac Dis (2016) 8:S121–9. doi: 10.3978/j.issn.2072-1439.2016.01.69
77. Maeda M, Nakamoto K, Ohta M, Nakamura K, Nanjo S, Taniguchi K, et al. Statistical Survey of Tracheobronchoplasty in Japan. J Thorac Cardiovasc Surg (1989) 97:402–14. doi: 10.1016/S0022-5223(19)34579-9
78. Piazza C, Del Bon F, Paderno A, Grazioli P, Mangili S, Lombardi D, et al. Complications After Tracheal and Cricotracheal Resection and Anastomosis for Inflammatory and Neoplastic Stensoses. Ann Otol Rhinol Laryngol (2014) 123:798–804. doi: 10.1177/0003489414538764
79. Shimamoto K, Satake H, Sawaki A, Ishigaki T, Funahashi H, Imai T. Preoperative Staging of Thyroid Papillary Carcinoma With Ultrasonography. Eur J Radiol (1998) 29:4–10. doi: 10.1016/S0720-048X(97)00184-8
80. Tomoda C, Uruno T, Takamura Y, Ito Y, Miya A, Kobayashi K, et al. Ultrasonography as a Method of Screening for Tracheal Invasion by Papillary Thyroid Cancer. Surg Today (2002) 35:819–22. doi: 10.1007/s00595-005-3037-0
81. Yamamura N, Fukushima S, Nakao K, Nakahara M, Kurozumi K, Imabun S, et al. Relation Between Ultrasonographic and Histologic Findings of Tracheal Invasion by Differentiated Thyroid Cancer. World J Surg (2002) 26:1071–3. doi: 10.1007/s00268-002-6671-3
82. Seo YL, Yoon DY, Lim KJ, Cha JH, Yun EJ, Choi CS, et al. Locally Advanced Thyroid Cancer: Can CT Help in Prediction of Extrathyroidal Invasion to Adjacent Structures? AJR Am J Roentgenol (2010) 195:240–4. doi: 10.2214/AJR.09.3965
83. Wang JC, Takashima S, Takayama F, Kawakami S, Saito A, Matsushita T, et al. Tracheal Invasion by Thyroid Carcinoma: Prediction Using MR Imaging. AJR Am J Roentgenol (2001) 177:929–36. doi: 10.2214/ajr.177.4.1770929
84. Wakamatsu T, Tsushima K, Yasuo M, Yamazaki Y, Yoshikawa S, Koide N, et al. Usefulness of Preoperative Endobronchial Ultrasound for Airway Invasion Around the Trachea: Esophageal Cancer and Thyroid Cancer. Respiration (2006) 73:651–7. doi: 10.1159/000093160
85. Takashima S, Takayama F, Wang Q, Kawakami S, Saito A, Kobayashi S, et al. Differentiated Thyroid Carcinomas. Prediction of Tumor Invasion With MR Imaging. Acta Radiol (2000) 41:377–83. doi: 10.1080/028418500127345514
86. Koshiishi H, Toriya K, Ozaki O, Ito K, Konaka C, Kato H. Fiberoptic Bronchoscopy in Thyroid Carcinoma With Tracheal Invasion. Diagn Ther Endosc (1998) 4:113–8. doi: 10.1155/DTE.4.113
87. Amit M, Boonsripitayanon M, Goepfert RP, Tam S, Busaidy NL, Cabanillas ME, et al. Extrathyroidal Extension: Does Strap Muscle Invasion Alone Influence Recurrence and Survival in Patients With Differentiated Thyroid Cancer? Ann Surg Oncol (2018) 25:3380–8. doi: 10.1245/s10434-018-6563-x
88. Wu C-W, Dionigi G, Barczynski M, Chiang F-Y, Dralle H, Schneider R, et al. International Neuromonitoring Study Group Guidelines 2018 – Part II: Optimal Recurrent Laryngeal Nerve Management for Invasive Thyroid Cancer – Incorporation of Surgical, Laryngeal, and Neural Electrophysiologic Data. Laryngoscope (2018) 128 Suppl 3:S18–27. doi: 10.1002/lary.27360
89. Yumoto E, Sanuki T, Kumai Y. Immediate Recurrent Laryngeal Nerve Reconstruction and Vocal Outcome. Laryngoscope (2006) 116:1657–61. doi: 10.1097/01.mlg.0000233245.27582.fc
90. Miyauchi A, Inoue H, Tomoda C, Fukushima M, Kihara M, Higashiyama T, et al. Improvement in Phonation After Reconstruction of the Recurrent Laryngeal Nerve in Patients With Thyroid Cancer Invading the Nerve. Surgery (2009) 146:1056–62. doi: 10.1016/j.surg.2009.09.018
91. Simo R, Nixon IJ, Rovira A, Vander Poorten V, Sanabria A, Zafereo M, et al. Immediate Intraoperative Repair of the Recurrent Laryngeal Nerve in Thyroid Surgery. Laryngoscope (2021) 131:1429–35. doi: 10.1002/lary.29204
92. Lipton RJ, McCaffrey TV, van Heerden JA. Surgical Treatments of Invasion of the Upper Aerodigestive Tract by Well-Differentiated Thyroid Carcinoma. Am J Surg (1987) 154:363–7. doi: 10.1016/0002-9610(89)90005-6
93. Czaja JM, McCaffrey TV. The Surgical Management of Laryngotracheal Invasion by Well-Differentiated Papillary Thyroid Carcinoma. Arch Otolaryngol Head Neck Surg (1997) 123:484–90. doi: 10.1001/archotol.1997.01900050030003
94. Nakao K, Kurozumi K, Fukushima S, Nakahara M, Tsujimoto M, Nishida T. Merits and Demerits of Operative Procedure to the Trachea in Patients With Differentiated Thyroid Cancer. Worl J Surg (2001) 25:723–7. doi: 10.1007/s00268-001-0022-7
Keywords: thyroid cancer, airway, surgery, tracheal resection, crico-tracheal resection
Citation: Piazza C, Lancini D, Tomasoni M, D’Cruz A, Hartl DM, Kowalski LP, Randolph GW, Rinaldo A, Shah JP, Shaha AR, Simo R, Vander Poorten V, Zafereo M and Ferlito A (2021) Tracheal and Cricotracheal Resection With End-to-End Anastomosis for Locally Advanced Thyroid Cancer: A Systematic Review of the Literature on 656 Patients. Front. Endocrinol. 12:779999. doi: 10.3389/fendo.2021.779999
Received: 20 September 2021; Accepted: 27 October 2021;
Published: 11 November 2021.
Edited by:
Jose Federico Carrillo, National Institute of Cancerology (INCAN), MexicoReviewed by:
Martin Granados, National Institute of Cancerology (INCAN), MexicoCopyright © 2021 Piazza, Lancini, Tomasoni, D’Cruz, Hartl, Kowalski, Randolph, Rinaldo, Shah, Shaha, Simo, Vander Poorten, Zafereo and Ferlito. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Cesare Piazza, Y2VzYXJlLnBpYXp6YUB1bmlicy5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.