
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 29 November 2021
Sec. Clinical Diabetes
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.774567
This article is part of the Research Topic Mechanisms for the Alteration in the Crosstalk among Insulin-Sensitive Tissues View all 8 articles
Aims: We aimed to assess the association between triglyceride–glucose (TyG) index and kidney stones in US adults.
Methods: Data were obtained from the 2007–2014 National Health and Nutrition Examination Survey (NHANES). Participants aged ≥18 years who were not pregnant and provided complete data about TyG index and kidney stones were included in the analysis. Weighted multivariable regression analysis and subgroup analysis were preformed to estimate the independent relationship between TyG index and nephrolithiasis and recurrence.
Results: A total of 20,972 participants were included with the mean TyG index of 8.71 ± 0.72. The prevalence rates of nephrolithiasis and recurrence were 9.30% and 3.17% overall and increased with the higher TyG index tertiles (Nephrolithiasis: Tertile 1, 6.98%; Tertile 2, 9.15%; Tertile 3, 11.98%, p < 0.01; Recurrence: Tertile 1, 1.84%; Tertile 2, 3.27%; Tertile 3, 4.50%, p < 0.01). Each unit increase in TyG index was associated with 12% and 26% higher odds of nephrolithiasis [odds ratio (OR) = 1.12; 95% CI: 1.02–1.22; p = 0.02] and recurrence (OR = 1.26; 95% CI: 1.08–1.46; p < 0.01). Interaction tests indicated no significant effect of gender, age, body mass index, hypertension, and diabetes on this association between TyG index and kidney stones.
Conclusions: Higher TyG index was associated with an increased likelihood of nephrolithiasis and recurrence. Considering TyG index is a reliable indicator of insulin resistance (IR). Treatment and management of IR at a younger age may improve or alleviate the occurrence and recurrence of kidney stones.
Nephrolithiasis is caused by the abnormal accumulation of crystalline substances in the kidney and represents a significant economic and public health burden worldwide (1). The prevalence of nephrolithiasis was estimated to be 7.2%–7.7% globally and showed an increasing tendency with years, with 5%–10% in Europe, 4% in South America, and 1%–19% in Asia, respectively (2, 3). Nephrolithiasis also shows a high recurrence rate of approximately 50% at 10 years (4). In addition, a graded relation was found between kidney stones and the increasing risk of kidney function loss, even end-stage renal disease (5).
Triglyceride–glucose (TyG) index is a logarithmized product of fasting triglyceride and fasting glucose. It has been regarded as a novel and reliable indicator of insulin resistance (IR), a forerunner of type 2 diabetes (6). TyG index is more convenient and easily accessible in clinical practice compared with the plasma insulin in the homeostasis model assessment of IR (7). Previous studies have demonstrated that the elevation of TyG index correlates well with several diseases such as arterial stiffness, coronary artery stenosis, and erectile dysfunction (8–10).
Accumulating evidence showed that IR may increase the risk of nephrolithiasis. Riese and Sakhaee (11) found that formation of uric acid kidney stones appeared to stem on the background of IR, which decreases urinary pH. Hamm (12) reported that IR could also lead to a defect in renal acid excretion and then cause hypocitraturia, which is a significant risk factor for calcium stones. Ando et al. (13) also reported that metabolic syndrome components could increase the risk of kidney stones through IR and subclinical hyperinsulinemia for Japanese women. In addition, increased IR could lower urinary citrate excretion and increase urinary calcium excretion, thus contributing to the formation of calcium stones (14). Since TyG index has been proposed as a marker of IR, it can be speculated that there might be a relationship between TyG index and kidney stones. However, no previous study has assessed the association between TyG index and kidney stones before.
Hence, we explored the association between the TyG index and the likelihood of kidney stones using the 2007–2014 National Health and Nutrition Examination Survey (NHANES) data regarding US men. We hypothesized that higher TyG index was associated with an increased likelihood of kidney stones.
We obtained data from NHANES, a study aimed to evaluate the health and nutrition status of the US population administered by the National Center for Health Statistics (NCHS). The included samples in NHANES have good representativeness because of the stratified multistage probability sampling method adopted in the study design. All NHANES data are publicly available at https://www.cdc.gov/nchs/nhanes/.
Our study was based on four NHANES survey cycles from 2007 to 2014, since only these four cycles include data on both kidney stones (including recurrence of passing kidney stones) and TyG index. A total of 40,617 participants were enrolled at first, after the exclusion of individuals younger than 18 years (n = 15,885), pregnant (n = 247), missing the data on TyG index (n = 2,418), and missing the data on kidney stones (n = 1,095), 20,972 participants were included in our final analysis (Figure 1).
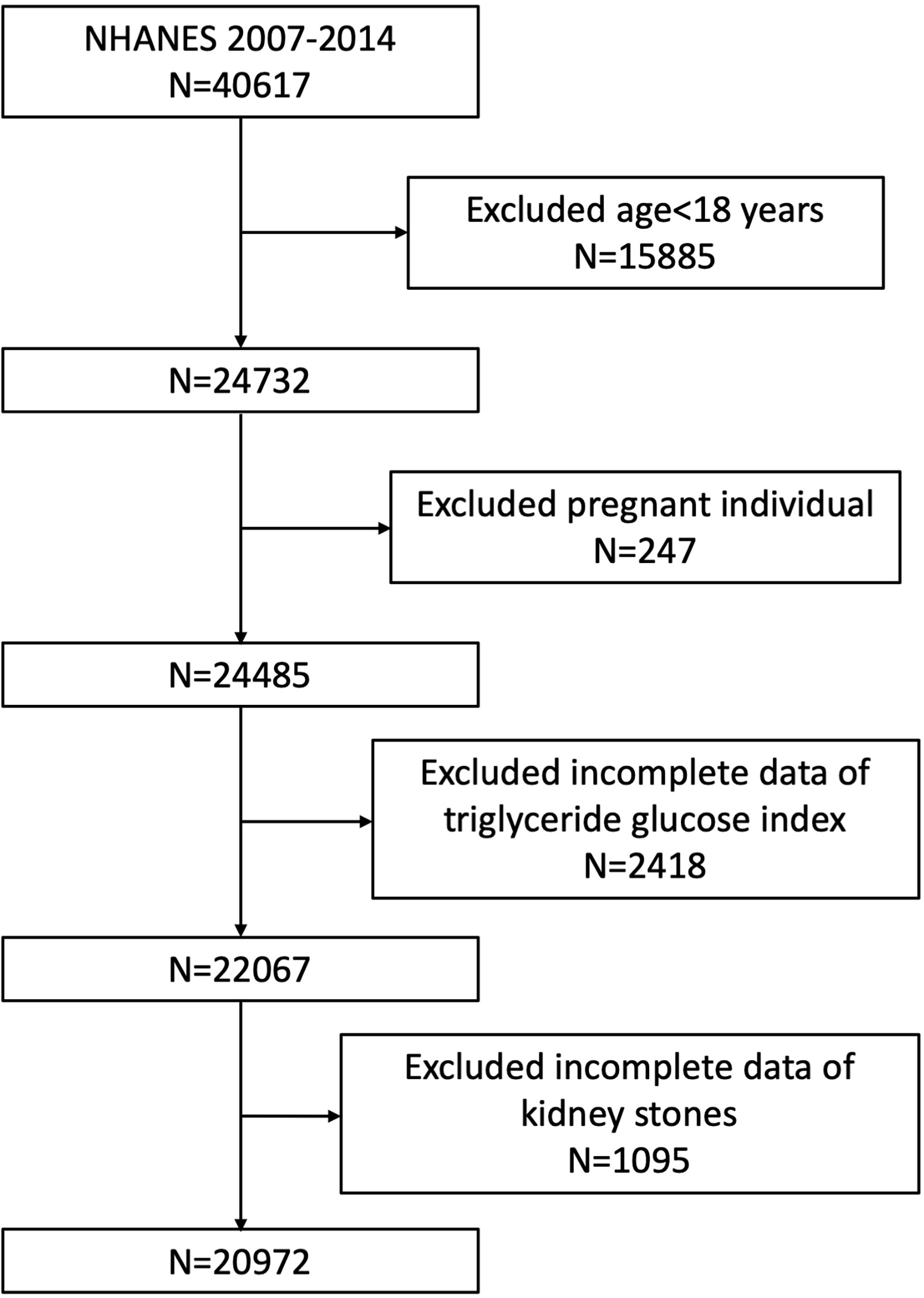
Figure 1 Flowchart of the sample selection from National Health and Nutrition Examination Survey (NHANES) 2007–2014.
The NCHS Research Ethics Review Board granted the human subject approval for the conduction of NHANES, and written informed consent was obtained from each participant.
TyG was designed as an exposure variable. We calculated TyG index as Ln [triglycerides (mg/dl) * fasting glucose (mg/dl)/2] (15). Both the concentrations of triglycerides and fasting glucose were measured by an enzymatic assay using automatic biochemistry analyzer. Serum triglyceride concentration was measured using the Roche Modular P and Roche Cobas 6000 chemistry analyzers. Fasting plasma glucose was measured by the hexokinase-mediated reaction using Roche/Hitachi Cobas C 501 chemistry analyzer.
Two questions about kidney conditions were used to assess the kidney stones, including “Have you/Has sample person (SP) ever has a kidney stone?” and “How many times have you/has SP passes a kidney stone?” Previous studies have validated the accuracy of self-reported kidney stone conditions (16). If a participant answered yes to ever had a kidney stone, he was considered to have nephrolithiasis. An individual who has experienced two or more times of having kidney stones was considered to have a recurrence of passing kidney stones. Both the occurrence of nephrolithiasis and nephrolithiasis recurrence were designed as outcome variables.
Potential covariates that might confound the association between TyG index and kidney stones were summarized in the multivariable-adjusted models. Covariates in our study included gender (male/female), age (years), race, education level, poverty-to-income ratio (PIR), marital status (married or living with a partner/single), alcohol intake (never/up to once a week/2–3 times a week/4–6 times a week/daily of more), physical activity (vigorous/moderate/less than moderate), cholesterol level (mg/dl), body mass index (BMI), smoking status (smoking or not), hypertension, and diabetes. BMI was categorized as <25, 25–29.9, and ≥30 kg/m2, which corresponded to normal weight, overweight, and obese population for all participants. All detailed measurement processes of study variables were publicly available at www.cdc.gov/nchs/nhanes/.
All statistical analyses were conducted according to Centers for Disease Control and Prevention (CDC) guidelines, and an appropriate NHANES sampling weight was applied and accounted for complex multistage cluster survey design in the analysis. Continuous variables were presented as mean with standard deviation, and categorical variables were presented as a percentage. Either a weighted Student’s t-test (for continuous variables) or weighted chi-square test (for categorical variables) was used to evaluate the differences in groups divided by TyG index (tertiles). Multivariate logistic regression models were employed to explore the independent relationship between TyG index and kidney stones in three different models. In model 1, no covariates were adjusted. Model 2 was adjusted for gender, age, and race. Model 3 was adjusted for gender, age, race, education level, poverty-to-income ratio, marital status, alcohol intake, physical activity, cholesterol, BMI, smoking status, hypertension, and diabetes. Smooth curve fitting (penalized spline method) and weighted generalized additive model (GAM) regression were conducted to further assess the nonlinear relationship between TyG index and kidney stones. Subgroup analysis stratified by gender, age, BMI, hypertension, and diabetes was also performed by stratified multivariate regression analysis. In addition, an interaction term was added to test the heterogeneity of associations between the subgroups using log likelihood ratio test model. p < 0.05 was considered statistically significant. All analyses were preformed using Empower software (www.empowerstats.com; X&Y solutions, Inc., Boston, MA, USA) and R version 3.4.3 (http://www.R-project.org, The R Foundation).
Weighted demographic baseline characteristics of included participants were shown in Table 1. A total of 20,972 participants were included in our study, of whom 48.86% were male and 51.54% were female, with the average age of 47.42 ± 16.77 years. The mean of TyG index was 8.71 ± 0.72, and the ranges of TyG index for tertiles 1–3 were 5.87–8.37, 8.38–8.99, and 8.99–13.21, respectively. The average prevalence of nephrolithiasis was 9.30% overall; 6.98%, 9.15%, and 11.98% for Tertile 1, Tertile 2, and Tertile 3. The prevalence of nephrolithiasis recurrence was 3.17% for the whole participants, and participants in higher TyG tertile trended to have higher rates of nephrolithiasis recurrence (Tertile 1, 1.84%; Tertile 2, 3.27%; Tertile 3, 4.50%, p < 0.01).
For nephrolithiasis, a positive association between TyG index and nephrolithiasis was observed. In the fully adjusted model (Model 3), this positive association was still stable (OR = 1.12; 95% CI: 1.02–1.22; p = 0.02), indicating that each unit of increased TyG index was associated with 12% increased risk of nephrolithiasis. We also converted TyG index from a continuous variable to a categorical variable (tertiles) to conduct sensitivity analysis. Compared with the lowest TyG index tertile (Tertile 1), a significant 18% increased likelihood of kidney stones was observed in Tertile 3. However, the difference between Tertile 1 and Tertile 2 did not meet statistical significance (OR = 1.04; 95% CI: 0.89–1.21; p = 0.66) (Table 2).
As for the recurrence of nephrolithiasis, we also observed that increased TyG index was associated with higher odds of nephrolithiasis recurrence (Model 1: OR = 1.58, 95% CI: 1.43–1.75, p < 0.01; Model 2: OR = 1.44, 95% CI: 1.29–1.60, p < 0.01; Model 3: OR = 1.26, 95% CI: 1.08–1.46, p < 0.01). In Model 3 that adjusted for all covariates, our results indicated that each unit of increased TyG index was associated with 26% increased likelihood of nephrolithiasis recurrence. In the sensitivity analysis, the adjusted OR (reference to Tertile 1) was 1.59 (95% CI: 1.20–2.11; p < 0.01) for Tertile 3, suggesting a stable positive relationship between increased TyG index and increased odds of nephrolithiasis recurrence with statistical significance (Table 2).
Weighted generalized additive models and smooth curve fittings were employed to further explore the nonlinear relationship between TyG index and kidney stones. Our results indicated that there was no nonlinear relationship between TyG index with nephrolithiasis (Figure 2). Similar result was observed in the association between TyG index and nephrolithiasis recurrence as well (all p > 0.05), indicating that the relationship between TyG index and kidney stones was linear (Figure 3).
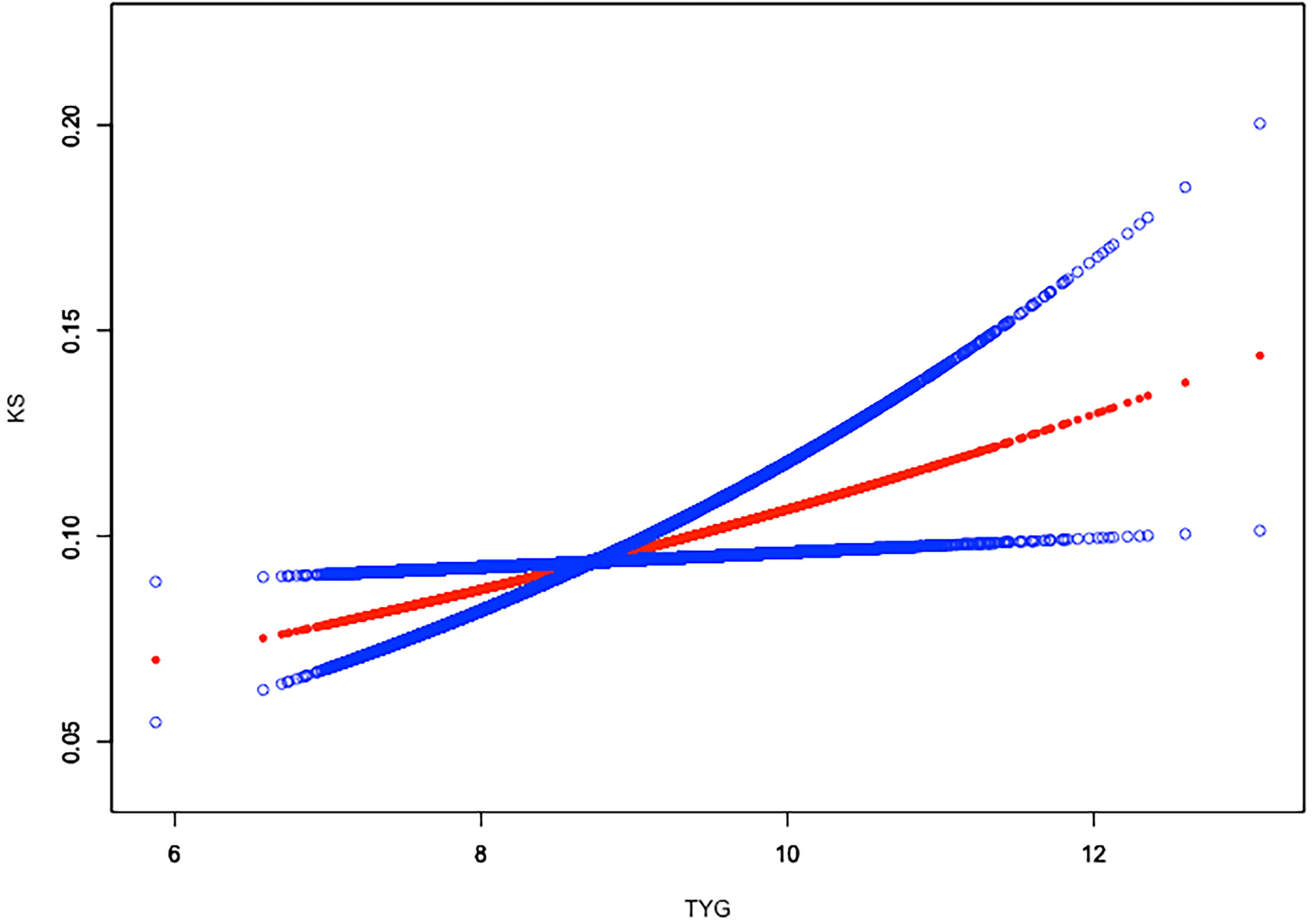
Figure 2 Linear relationship between triglyceride–glucose (TyG) index and kidney stones by the generalized additive model.
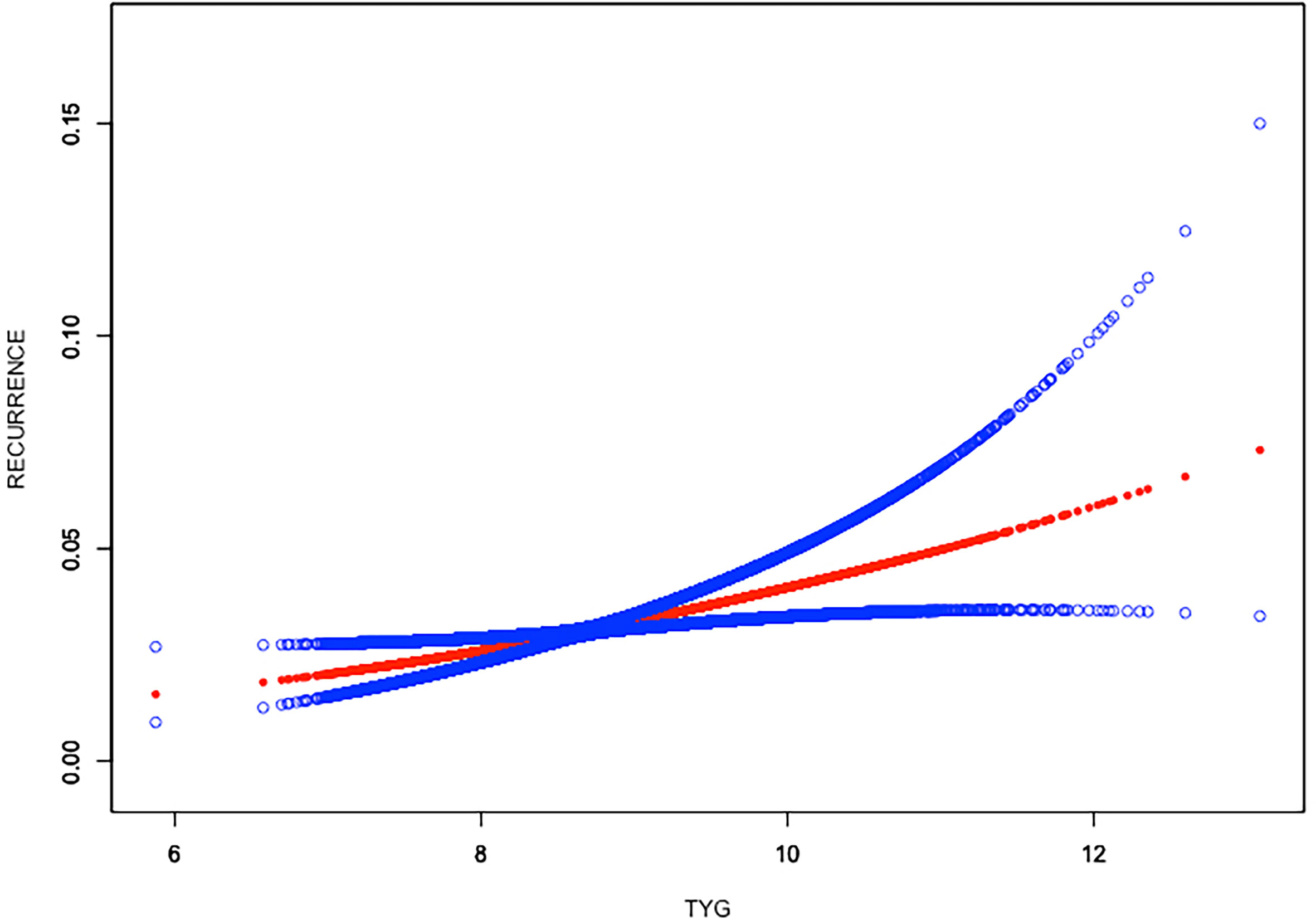
Figure 3 Linear relationship between triglyceride–glucose (TyG) index and the recurrence of kidney stones by the generalized additive model.
Subgroup analysis was performed to evaluate the robustness of association between TyG index and kidney stones. We tested the interactions with gender, age, BMI, hypertension, and diabetes (including both type 1 and type 2 diabetes) as well. However, no correlation with the p for interaction meeting the statistical significance was detected, indicating that there was no dependence on gender, age, BMI, hypertension, and diabetes for this association (all p for interaction >0.05). Our results showed that this positive association of TyG index and kidney stones was similar in populations with different genders, ages, BMIs, and hypertension and diabetes statuses and could be appropriate for various population settings. It was worth noting that we could not further conduct the subgroup analysis stratified by type 1 and type 2 diabetes, since NHANES data did not clarify type 1 or type 2 diabetes in its study design (Table 3).
In this cross-sectional study that included 20,972 adults, we found that higher TyG index was independently associated with increased likelihood of nephrolithiasis and nephrolithiasis recurrence. This association was similar in subgroups stratified by gender, age, BMI, hypertension, and diabetes status. We postulate that treatment and management of IR at a younger age might be beneficial to improve or alleviate the occurrence and recurrence of kidney stones.
To our knowledge, this is the first study assessing the association between TyG index and kidney stones. Previous studies have reported the relationship of kidney stones with several other clinicopathological factors. Maddahi et al. (17) revealed that diet with a higher dietary inflammatory index (DII) could increase the odds of kidney stone formation in stone former men. Zhang et al. (18) also reported that increased intake of pro-inflammatory diet was correlated with higher odds of kidney stone incidence and recurrence. DII scores serve as a tool to evaluate the effect of diet on inflammatory potential. Anti-inflammatory dietary patterns, such as DASH diet and Mediterranean diet, could reduce the level of systemic inflammation (19, 20). Some anti-inflammatory metabolites derived from dietary components, such as short-chain fatty acids and tryptophan and tyrosine metabolites, also play a role in the regulation of inflammation. Tryptamine could reduce fatty acid- and LPS-stimulated production of pro-inflammatory cytokines in macrophages and inhibited the migration of cells toward a chemokine in mice (21). Supplementation with desaminotyrosine, a gut microbiota-derived anti-inflammatory metabolite, was proven to attenuate dextran sodium sulfate-induced mucosal inflammation in a type I interferon signal-dependent manner, thus modulating local and systemic immune homeostasis (22). Commensal gut microflora and dietary fiber also regulated inflammation status through inter-organ signaling, thus playing pathophysiologic roles in obesity, diabetes, and dyslipidemia (23, 24). In contrast, higher-DII diets could enhance the systemic inflammation level and resulted in hypercalciuria, hyperuricosuria, and hypocitraturia, thus leading to the formation of kidney stones. Cohen et al. (25) demonstrated a protective effect of statin intake for the formation of kidney stones. Although the certain mechanism was still unclear, there were signs that anti-inflammatory and antioxidant properties of statins may reduce the occurrence of kidney stones. Increased inflammatory status provided conditions for the formation and deposition of renal tubular crystals, which has been confirmed in a mouse model (26). In addition, statin treatment could lower the inflammatory level, then inhibited the renal crystal retention (27). Inflammation was reported to be positively associated with IR as well (28, 29). At the molecular level, the transition of macrophages from a state of alternative M2 activation maintained by signal transducer and activator of transcription (STAT6) and peroxisome proliferators-activated receptors (PPAR) to a state of classic M1 activation driven by Nuclear Factor-KappaB (NF-KappaB), activator protein-1 (AP1), and other signal-dependent transcription factors that play a key role in innate immunity promotes IR (30, 31). Semins et al. (32) found that the obese population had a higher risk of kidney stone, while as the degree of obesity stratified by BMI increased, the risk stabilized. Previous studies exploring the effect of body size on urine chemistry demonstrated that increasing BMI could enhance the lithogenic risk factors such as decreased urinary volume and citrate concentrations (33). Chronic inflammation in adipose tissue was considered to be a key risk factor for IR and type 2 diabetes in obese individuals. Obesity-induced adipose tissue expansion provides a number of internal signals (such as adipocyte death, hypoxia, and mechanical stress) that could initiate an inflammatory response (34). Since inflammation was associated with IR and kidney stones and TyG index has been regarded as a reliable indicator of IR, a positive association between TyG index and kidney stones could be speculated.
The mechanism underlying the association between TyG index and kidney stones is not clear. A possible explanation to support our results might be that the greater IR represented by higher TyG index could decrease the excretion of urinary citrate (14). Increased level of plasma free fatty acids could be detected in IR patients, which then got into the proximal tubule cells and interfered with the utilization of glutamine, resulting in a reduction in ammoniagenesis (35). Also, it has been proven that insulin could stimulate renal ammonium production from L-glutamine in vitro, representing direct damage of IR to ammoniagenesis (36). In addition, insulin could directly stimulate the Na+/H+ exchangers of proximal renal tubule that play a key role in transporting or ionic trapping of ammonium (37). In summary, the impaired ability of excreting ammonia in patients with IR could lead to hyperacid urine, which is a main risk factor for the formation of uric acid stones. IR could also increase the uptake of citrate in renal tubules and reduce the urinary citrate, which is a main risk factor for the formation of calcium stones. It has been reported that higher IR level also increased urinary calcium excretion, although the mechanism still remains unclear (38). The fact that the TyG index is positively correlated with the IR level may explain the reason why higher TyG index is associated with increased risk of nephrolithiasis.
Previous epidemiological studies demonstrated that obesity, hypertension, and diabetes were risk factors for nephrolithiasis. Taylor et al. (39) revealed that obesity and weight gain aggravated the risk of kidney stone formation, predominantly in females. One study found that hypertension in middle-aged white men could serve as a marked predictor for nephrolithiasis, which may be associated with greater and sustained urinary calcium losses in patients with hypertension (40). Diabetes was also associated with the prevalence of kidney stone disease, and there existed a positive relation between the severity of type 2 diabetes mellitus, which could be explained by the effects of IR on urinary pH and renal transport of ammonium and calcium (41). According to our results of subgroup analysis, the positive association was stable in subgroups stratified by BMI, diabetes, and hypertension, which is consistent with the studies before. In addition, we did not detect any dependence on gender, age, BMI, hypertension, and diabetes for this association (all p for interaction >0.05), suggesting that this positive association may be appropriate for different population settings.
Our study has several strengths. Firstly, our study was based on the data from NHANES and the analyses was conducted considering the appropriate NHANES sample weights. Secondly, we adjusted confounding covariates to ensure our results are reliable and could be applied to a wider range of individuals. However, limitations in our study cannot be ignored. Firstly, the diagnosis of kidney stone was based on personal interview; the recall bias was inevitable. Secondly, since NHANES data did not clarify type 1 or type 2 diabetes in its study design, we could not further evaluate this association for subgroup stratified by type 1 and type 2 diabetes. In addition, since the missing data of covariables were missed randomly and the sample size was large enough to draw a conclusion, we did not use multiple imputation to deal with the missing data, thus it may influence the accuracy. Due to the cross-sectional study design, we could not obtain a causal relationship between TyG and kidney stones.
Higher TyG index was associated with an increased likelihood of kidney stone incidence and recurrence. We postulate that treatment and management of IR at a younger age may improve or alleviate the occurrence and recurrence of kidney stones. However, further large-scale prospective studies are still needed to clarify the precise causality of this relationship.
Publicly available datasets were analyzed in this study. These data can be found here: https://www.cdc.gov/nchs/nhanes/.
The studies involving human participants were reviewed and approved by The NCHS Research Ethics Review Board. The patients/participants provided their written informed consent to participate in this study.
ZQ: data analysis, software, and writing—original draft. JZ: formal analysis and writing—original draft. JG: methodology and software. KC: data analysis. RL: software and funding acquisition. BS: conceptualization, funding acquisition, and writing—reviewing and editing. All authors contributed to the article and approved the submitted version.
This work was supported by the National Natural Science Foundation of China (Grant No. 82000702), the Science and Technology Achievement Transformation Fund of West China Hospital of Sichuan University (Grant No. CGZH19006), the 1.3.5 project for disciplines of excellence from West China Hospital of Sichuan University (Grant No. ZYJC21010), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Grant No. Z2018B10), and Med+ Biomaterial Institute of West China Hospital/West China School of Medicine of Sichuan University (Grant No. ZYME20001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Dr. Yingfei Xu for providing statistical methodology consultation.
1. Yang BY, Lu XL, Li Y, Li YY, Yu DJ, Zhang WW, et al. A Proteomic Network Approach Across the Kidney Stone Disease Reveals Endoplasmic Reticulum Stress and Crystal-Cell Interaction in the Kidney. Oxid Med Cell Longevity (2019) 2019. doi: 10.1155/2019/9307256
2. Abufaraj M, Xu T, Cao C, Waldhoer T, Seitz C, D'Andrea D, et al. Prevalence and Trends in Kidney Stone Among Adults in the USA: Analyses of National Health and Nutrition Examination Survey 2007-2018 Data. Eur Urol Focus (2020). doi: 10.1016/j.euf.2020.08.011
3. Thongprayoon C, Krambeck AE, Rule AD. Determining the True Burden of Kidney Stone Disease. Nat Rev Nephrol (2020) 16:736–46. doi: 10.1038/s41581-020-0320-7
5. Alexander RT, Hemmelgarn BR, Wiebe N, Bello A, Morgan C, Samuel S, et al. Kidney Stones and Kidney Function Loss: A Cohort Study. Bmj-Br Med J (2012) 345. doi: 10.1136/bmj.e5287
6. Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzalez SO, et al. The Product of Triglycerides and Glucose, a Simple Measure of Insulin Sensitivity. Comparison With the Euglycemic-Hyperinsulinemic Clamp. J Clin Endocrinol Metab (2010) 95:3347–51. doi: 10.1210/jc.2010-0288
7. Simental-Mendía LE, Rodríguez-Morán M, Guerrero-Romero F. The Product of Fasting Glucose and Triglycerides as Surrogate for Identifying Insulin Resistance in Apparently Healthy Subjects. Metab Syndrome Related Disord (2008) 6:299. doi: 10.1089/met.2008.0034
8. Lee SB, Ahn CW, Lee BK, Kang S, Nam JS, You JH, et al. Association Between Triglyceride Glucose Index and Arterial Stiffness in Korean Adults. Cardiovasc Diabetol (2018) 17. doi: 10.1186/s12933-018-0692-1
9. Thai PV, Tien HA, Van Minh H, Valensi P. Triglyceride Glucose Index for the Detection of Asymptomatic Coronary Artery Stenosis in Patients With Type 2 Diabetes. Cardiovasc Diabetol (2020) 19. doi: 10.1186/s12933-020-01108-2
10. Yilmaz M, Karaaslan M, Tonyali S, Celik M, Toprak T, Odabas O. Triglyceride-Glucose Index (TyG) Is Associated With Erectile Dysfunction: A Cross-Sectional Study. Andrology (2021) 9:238–44. doi: 10.1111/andr.12904
11. Riese RJ, Sakhaee K. Uric-Acid Nephrolithiasis - Pathogenesis And Treatment. J Urol (1992) 148:765–71. doi: 10.1016/S0022-5347(17)36715-0
13. Ando R, Suzuki S, Nagaya T, Yamada T, Okada A, Yasui T, et al. Impact of Insulin Resistance, Insulin and Adiponectin on Kidney Stones in the Japanese Population. Int J Urol (2011) 18:131–8. doi: 10.1111/j.1442-2042.2010.02690.x
14. Cupisti A, Meola M, D'Alessandro C, Bernabini G, Pasquali E, Carpi A, et al. Insulin Resistance and Low Urinary Citrate Excretion in Calcium Stone Formers. Biomed Pharmacother (2007) 61:86–90. doi: 10.1016/j.biopha.2006.09.012
15. Liu XC, He GD, Lo K, Huang YQ, Feng YQ. The Triglyceride-Glucose Index, an Insulin Resistance Marker, Was Non-Linear Associated With All-Cause and Cardiovascular Mortality in the General Population. Front Cardiovasc Med (2021) 7:9. doi: 10.3389/fcvm.2020.628109
16. Curhan GC, Willett WC, Rimm EB, Stampfer MJ. A Prospective-Study Of Dietary Calcium And Other Nutrients And The Risk Of Symptomatic Kidney-Stones. N Engl J Med (1993) 328:833–8. doi: 10.1056/NEJM199303253281203
17. Maddahi N, Yarizadeh H, Aghamir SMK, Alizadeh S, Yekaninejad MS, Mirzaei K. The Association of Dietary Inflammatory Index With Urinary Risk Factors of Kidney Stones Formation in Men With Nephrolithiasis. BMC Res Notes (2020) 13. doi: 10.1186/s13104-020-05206-y
18. Zhang C, Qiu S, Bian H, Tian B, Wang H, Tu X, et al. Association Between Dietary Inflammatory Index and Kidney Stones in US Adults: Data From the National Health and Nutrition Examination Survey (NHANES) 2007-2016. Public Health Nutr (2021) 1–9. doi: 10.1017/S1368980021000793
19. Saneei P, Hashemipour M, Kelishadi R, Esmaillzadeh A. The Dietary Approaches to Stop Hypertension (DASH) Diet Affects Inflammation in Childhood Metabolic Syndrome: A Randomized Cross-Over Clinical Trial. Ann Nutr Metab (2014) 64:20–7. doi: 10.1159/000358341
20. Chrysohoou C, Panagiotakos DB, Pitsavos C, Das UN, Stefanadis C. Adherence to the Mediterranean Diet Attenuates Inflammation and Coagulation Process in Healthy Adults - The ATTICA Study. J Am Coll Cardiol (2004) 44:152–8. doi: 10.1016/j.jacc.2004.03.039
21. Krishnan S, Ding YF, Saedi N, Choi M, Sridharan GV, Sherr DH, et al. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep (2018) 23:1099–111. doi: 10.1016/j.celrep.2018.03.109
22. Wei YX, Gao J, Kou YB, Liu MN, Meng LY, Zheng XP, et al. The Intestinal Microbial Metabolite Desaminotyrosine Is an Anti-Inflammatory Molecule That Modulates Local and Systemic Immune Homeostasis. FASEB J (2020) 34:16117–28. doi: 10.1096/fj.201902900RR
23. Singh N, Gurav A, Sivaprakasam S, Brady E, Padia R, Shi HD, et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity (2014) 40:128–39. doi: 10.1016/j.immuni.2013.12.007
24. Chavez-Talavera O, Tailleux A, Lefebvre P, Staels B. Bile Acid Control of Metabolism and Inflammation in Obesity, Type 2 Diabetes, Dyslipidemia, and Nonalcoholic Fatty Liver Disease. Gastroenterology (2017) 152:1679. doi: 10.1053/j.gastro.2017.01.055
25. Cohen AJ, Adamsky MA, Nottingham CU, Pruitt J, Lapin B, Wang CH, et al. Impact of Statin Intake on Kidney Stone Formation. Urology (2019) 124:57–61. doi: 10.1016/j.urology.2018.01.029
26. Taguchi K, Okada A, Hamamoto S, Iwatsuki S, Naiki T, Ando R, et al. Proinflammatory and Metabolic Changes Facilitate Renal Crystal Deposition in an Obese Mouse Model of Metabolic Syndrome. J Urol (2015) 194:1787–96. doi: 10.1016/j.juro.2015.07.083
27. Tsujihata M, Momohara C, Yoshioka I, Tsujimura A, Nonomura N, Okuyama A. Atorvastatin Inhibits Renal Crystal Retention in a Rat Stone Forming Model. J Urol (2008) 180:2212–7. doi: 10.1016/j.juro.2008.07.024
28. Wu H, Ballantyne CM. Metabolic Inflammation and Insulin Resistance in Obesity. Circ Res (2020) 126:1549–64. doi: 10.1161/CIRCRESAHA.119.315896
29. Shoelson SE, Lee J, Goldfine AB. Inflammation and Insulin Resistance. J Clin Invest (2006) 116:1793–801. doi: 10.1172/JCI29069
30. Olefsky JM, Glass CK. Macrophages, Inflammation, and Insulin Resistance. Annu Rev Physiol (2010) 72:219–46. doi: 10.1146/annurev-physiol-021909-135846
31. Kojta I, Chacińska M, Błachnio-Zabielska A. Obesity, Bioactive Lipids, and Adipose Tissue Inflammation in Insulin Resistance. Nutrients (2020) 12:517–75. doi: 10.3390/nu12051305
32. Semins MJ, Shore AD, Makary MA, Magnuson T, Johns R, Matlaga BR. The Association of Increasing Body Mass Index and Kidney Stone Disease. J Urol (2010) 183:571–5. doi: 10.1016/j.juro.2009.09.085
33. Duffey BG, Pedro RN, Kriedberg C, Weiland D, Melquist J, Kramuddin S, et al. Lithogenic Risk Factors in the Morbidly Obese Population. J Urol (2008) 179:1401–6. doi: 10.1016/j.juro.2007.11.072
34. Zatterale F, Longo M, Naderi J, Raciti GA, Desiderio A, Miele C, et al. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front Physiol (2019) 10:1607. doi: 10.3389/fphys.2019.01607
35. Vinay P, Lemieux G, Cartier P, Ahmad M, Baverel G. Effect Of Fatty-Acids On Renal Ammoniagenesis In In Vivo And In Vitro Studies. Am J Physiol (1976) 231:880–7. doi: 10.1152/ajplegacy.1976.231.3.880
36. Chobanian MC, Hammerman MR. Insulin Stimulates Ammoniagenesis In Canine Renal Proximal Tubular Segments. Am J Physiol (1987) 253:F1171–7. doi: 10.1152/ajprenal.1987.253.6.F1171
37. Klisic J, Hu MC, Nief V, Reyes L, Fuster D, Moe OW, et al. Insulin Activates Na+/H+ Exchanger 3: Biphasic Response and Glucocorticoid Dependence. Am J Physiology-Renal Physiol (2002) 283:F532–9. doi: 10.1152/ajprenal.00365.2001
38. Kerstetter J, Caballero B, Obrien K, Wurtman R, Allen L. Mineral Homeostasis In Obesity - Effects Of Euglycemic Hyperinsulinemia. Metab-Clin Exp (1991) 40:707–13. doi: 10.1016/0026-0495(91)90088-E
39. Taylor EN, Stampfer MJ, Curhan GC. Obesity, Weight Gain, and the Risk of Kidney Stones. Jama-J Am Med Assoc (2005) 293:455–62. doi: 10.1001/jama.293.4.455
40. Cappuccio FP, Siani A, Barba G, Mellone MC, Russo L, Farinaro E, et al. A Prospective Study of Hypertension and the Incidence of Kidney Stones in Men. J Hypertens (1999) 17:1017–22. doi: 10.1097/00004872-199917070-00019
Keywords: insulin resistance, triglyceride–glucose index, kidney stones, NHANES, cross-sectional study
Citation: Qin Z, Zhao J, Geng J, Chang K, Liao R and Su B (2021) Higher Triglyceride–Glucose Index Is Associated With Increased Likelihood of Kidney Stones. Front. Endocrinol. 12:774567. doi: 10.3389/fendo.2021.774567
Received: 14 September 2021; Accepted: 08 November 2021;
Published: 29 November 2021.
Edited by:
Amalia Gastaldelli, National Research Council (CNR), ItalyReviewed by:
Claudia Torino, Italian National Research Council, ItalyCopyright © 2021 Qin, Zhao, Geng, Chang, Liao and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baihai Su, aW1zYmhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.