
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 05 January 2022
Sec. Reproduction
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.773781
This article is part of the Research Topic Polycystic Ovary Syndrome (PCOS): Mechanism and Management View all 35 articles
Aim: To evaluate the association between the apolipoprotein B/A1 ratio (ApoB/ApoA1) and metabolic and endocrine parameters in women with polycystic ovary syndrome (PCOS).
Methods: This study was a secondary analysis of the Acupuncture and Clomiphene for Chinese Women with Polycystic Ovary Syndrome trial (PCOSAct), and 957 subjects with available ApoB and ApoA1 measurements were included. Tests for linear trends and linear regression were used to assess the relation between the ApoB/ApoA1 ratio and metabolic and endocrine parameters. Logistic regression was used to estimate the association between the ratio and risk of metabolic syndrome (MetS) and insulin resistance (IR). The receiver operating characteristics (ROC) curve was used to determine the predictive value of the ApoB/ApoA1 ratio for MetS and IR.
Results: The results showed that the ApoB/ApoA1 ratio was positively associated with waist circumference, systolic blood pressure, total cholesterol, triglycerides, low-density lipoprotein, fasting plasma glucose, fasting insulin, homeostatic model assessment-insulin resistance, high free testosterone, high free androgen index, alanine transferase, aspartate transferase, and higher prevalence of MetS and IR, but was negatively correlated with high-density lipoprotein and sex hormone-binding globulin after adjusting for age and body mass index. Logistic regression showed that compared with the ApoB/ApoA1 ratio in first quartile, those in the fourth quartile demonstrated a higher risk of MetS (OR: 24.48, 95%CI: 8.54–70.15, P trend <0.001) and IR (OR: 1.78, 95%CI: 1.10–2.87, P trend <0.05) after adjusting for confounding factors. ROC curve results showed that the AUCMetS was 0.84 (95%CI: 0.81–0.86) and had 86.8% sensitivity and 70.3% specificity with a threshold value of 0.64, and the AUCIR was 0.68 (95%CI: 0.64–0.71) and had 74.3% sensitivity and 58.2% specificity with a threshold value of 0.56.
Conclusions: Increased ApoB/ApoA1 ratio was associated with worse MetS components, IR, and elevated androgen hormones and liver enzymes. The ratio might be a useful tool to screen for MetS and IR in PCOS patients.
Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in reproductive-age women (1), generally presenting as oligo-/amenorrhea, hyperandrogenism (HA), and infertility (2). In addition, PCOS patients often suffer from metabolic abnormalities, namely, overweight or obesity (3), insulin resistance (IR) (4), dyslipidemia (5), non-alcoholic fatty liver disease (NAFLD) (6), metabolic syndrome (MetS) (7), and cardiovascular diseases (CVD) in the long-term (8). IR, which is the impairment of insulin action, plays an intrinsic role in the pathogenesis of PCOS and aggravates the reproductive and metabolic disorders in patients with PCOS (9). It is therefore important to identify a tool to screen for metabolic and endocrine parameters in women with PCOS.
Apolipoproteins are liver-produced proteins that are responsible for lipid transportation and redistribution. Among them, apolipoprotein A1 (ApoA1), a constituent of high-density lipoprotein (HDL), is involved in the process of reverse transport of peripheral cholesterol to the liver and thus has an anti-atherogenic effect (10). Apolipoprotein B (ApoB), which consists of chylomicrons, intermediate-density lipoprotein, very-low-density lipoprotein (VLDL), and low-density lipoprotein (LDL), is responsible for transporting cholesterol to peripheral cells and is a representative example of atherogenic lipoproteins (11). Therefore, the ApoB/ApoA1 ratio might reflect the balance between atherogenic lipoproteins and anti-atherogenic lipoproteins (12). Numerous studies have shown that the ApoB/ApoA1 ratio is strongly associated with MetS (13–16) and might be an independent predictor of IR (17) among different ethnicities. Moreover, the ApoB/ApoA1 ratio is a better predictor of lipoprotein-related risk of cardiovascular disease (CVD) than traditional lipid indexes (18–20), and ApoB and ApoA1 are correlated with creatine kinase (CK) in myocardial infarction (21). Meanwhile, several studies have indicated that the ratio is related to NAFLD (22, 23) and to liver function markers such as alanine transferase (ALT) (24, 25).
To the best of our knowledge, only two published studies have analyzed the association of the ApoB/ApoA1 ratio with endocrine and metabolic characteristics in adults and adolescents with PCOS, respectively (26, 27). Their studies concluded that the ApoB/ApoA1 ratio is strongly connected with IR and MetS, but their results indicated that the mechanisms of PCOS might be different between adolescents and adults. Thus, the relation between ApoB/ApoA1 and the metabolic characteristics in PCOS remains inconclusive.
The aim of this study was to evaluate the associations between the ApoB/ApoA1 ratio and metabolic and endocrine profiles, namely, MetS, IR, androgen hormones, and cardiac and liver enzymes. We also investigated whether the ApoB/ApoA1 ratio could be used as an indicator to predict MetS and IR.
This study was a cross-sectional secondary analysis of the Acupuncture and Clomiphene for Chinese Women with Polycystic Ovary Syndrome Trial (PCOSAct), which was conducted between 2012 and 2015 in mainland China. The clinical trial was registered at chictr.org.cn (ChiCTR-TRC-12002081) and Clinical Trials.gov (NCT01573858). The protocol was approved by all ethics committees at the local study sites. The study protocol and primary manuscript have been published elsewhere (28, 29). A total of 1,000 infertile women with PCOS were recruited in the trial, and the diagnosis of PCOS was based on the modified Rotterdam criteria, namely chronic oligomenorrhea or amenorrhea together with clinical/biochemical hyperandrogenemia and/or polycystic ovarian morphology confirmed by transvaginal ultrasound. The details of the inclusion and exclusion criteria are described in the protocol (28).
All participants underwent a physical examination at the baseline visit: age, height, weight, waist circumference (WC), systolic blood pressure (SBP), and diastolic blood pressure (DBP). Body mass index (BMI) was calculated as weight divided by height squared.
All blood samples at the baseline visit were collected on the third day of the menstrual cycle after a 12-hour overnight fast and were analyzed at the core laboratory of the Heilongjiang University of Chinese Medicine. Biochemical measurements included total cholesterol (TC), triglycerides (TG), LDL, HDL, ApoA1, ApoB, fasting plasma glucose (FPG), fasting insulin (FIN), total testosterone (TT), free testosterone (FT), sex hormone-binding globulin (SHBG), CK, creatine kinase isoenzyme MB (CKMB), lactate dehydrogenase (LDH), ALT, and aspartate transferase (AST). TG and TC were measured by the N-(3-sulfopropyl)-3-methoxy-5-methylaniline method (Wako Diagnostics). HDL and LDL were measured by direct-method assays. ApoA1 and ApoB levels were measured by the polyethylene glycol-enhanced immunoturbidimetric assay (Maker, Chengdu, China). FPG was measured by hexokinase assay (Maker, Chengdu, China). FIN was measured by electro-chemiluminescence immune assay (ECLIA) (Roche Diagnostic, Basel, Switzerland). TT and SHBG were analyzed by chemiluminescence immunoassay (Siemens Diagnostic, Munich, Germany). FT was measured by radioimmunoassay. CK, LDH, ALT, and AST were determined by the IFCC method. CKMB was analyzed by the selective inhibition method. The free androgen index (FAI) was calculated by the formula: FAI = TT (nmol/L)/SHBG (nmol/L) × 100. The homeostasis model assessment of insulin resistance (HOMA-IR) index was calculated by the equation: HOMA-IR = FIN (mIU/ml) × FBG (mmol/L)/22.5 (30). Insulin resistance (IR) status was considered as a HOMA-IR ≥2.69 (31).
MetS was defined by presenting three or more of the following five items (1): WC >88 cm; (2) SBP ≥130 mmHg or DBP ≥85 mmHg; (3) FPG level of 110–126 mg/dl (to convert to millimoles per liter, multiply by 0.0555); (4) TG level ≥150 mg/dl (to convert to millimoles per liter, multiply by 0.0113); and (5) HDL level <50 mg/dl (to convert to millimoles per liter, multiply by 0.0259) (1).
SPSS Statistics (IBM SPSS, Inc., Chicago, IL, USA version 26.0) was used for data analyses. Continuous variables are presented as means ± standard deviations, and categorical variables are presented as frequencies and percentages. Anthropometric and biochemical parameters and the prevalence of MetS and IR in the participants across the ApoB/ApoA1 quartiles were compared using tests for linear trends. Linear regression was used to determine the correlations between the ApoB/ApoA1 ratio and the characteristics of the study population. In addition, multivariable logistic regression analysis was used to calculate the odds ratio (OR) with 95% confidence interval (CI) for the associations between the ApoB/ApoA1 ratio (the independent variable) and MetS and IR status (the dependent variables). Finally, receiver operating characteristic (ROC) curves were used to evaluate the predictive value of the ApoB/ApoA1 ratio for MetS and IR. The area under the curve (AUC) was measured, and the optimal cut-off values of the ApoB/ApoA1 ratio were also calculated by the highest Youden index (sensitivity + specificity − 1) (32). A P‐value <0.05 was considered to be statistically significant.
A total of 957 PCOS patients with available ApoB and ApoA1 measurements were included in the analysis. The ApoB/ApoA1 ratio was calculated and classified into four quartiles (Q1: ≤0.45, n = 240; Q2: 0.46–0.60, n = 239; Q3: 0.61–0.74, n = 239; and Q4 >0.74, n = 239). Among them, 190 women were diagnosed with MetS and 401 women were diagnosed with IR.
The anthropometric and biochemical characteristics across quartiles of ApoB/ApoA1 ratio are summarized in Table 1. Rising trends were observed for age, BMI, WC, SBP, DBP, TC, TG, LDL, FPG, FIN, HOMA-IR, FT, FAI, LDH, ALT, and AST across the ApoB/ApoA1 ratio quartiles (P-trend <0.01 for all), while declining trends were seen for HDL, SHBG, and CKMB across the four groups (P-trend <0.05 for all). The prevalence of MetS in each category of increasing ApoB/ApoA1 ratio in PCOS patients was 1.7, 5.9, 22.6, and 49.4%, respectively. The corresponding prevalence of IR was 22.92, 33.05, 49.79, and 61.92%, respectively. The differences observed across the quartiles of ApoB/ApoA1 ratio for the prevalence of MetS and IR were all statistically significant (P-trend <0.001).

Table 1 Comprehensive metabolic parameters of the included PCOS participants according to quartile of ApoB/ApoA1 ratio.
There was a significant positive correlation between the ApoB/ApoA1 ratio and age, BMI, WC, SBP, DBP, TC, TG, LDL, FPG, FIN, HOMA-IR, TT, FT, FAI, LDH, ALT, and AST (P <0.01 for all), while there was a significant inverse relationship between the ApoB/ApoA1 ratio and SHBG and HDL (P <0.001 for both). After adjusting for age and BMI, the association between the ApoB/ApoA1 ratio and DBP, TT, and LDH disappeared (Table 2).
The associations between the ApoB/ApoA1 ratio and the risk of MetS and IR in PCOS patients are presented in Table 3. A significant association between higher ApoB/ApoA1 ratio and increased risk of MetS and IR was found after adjusting for age, BMI, FT, and FAI. Compared with the lowest ApoB/ApoA1 ratio quartile, patients in the highest quartile had a significantly greater OR for both MetS (OR: 24.48, 95%CI: 8.54–70.15, P-trend <0.001) and IR (OR: 1.78, 95%CI: 1.10–2.87, P trend <0.05).
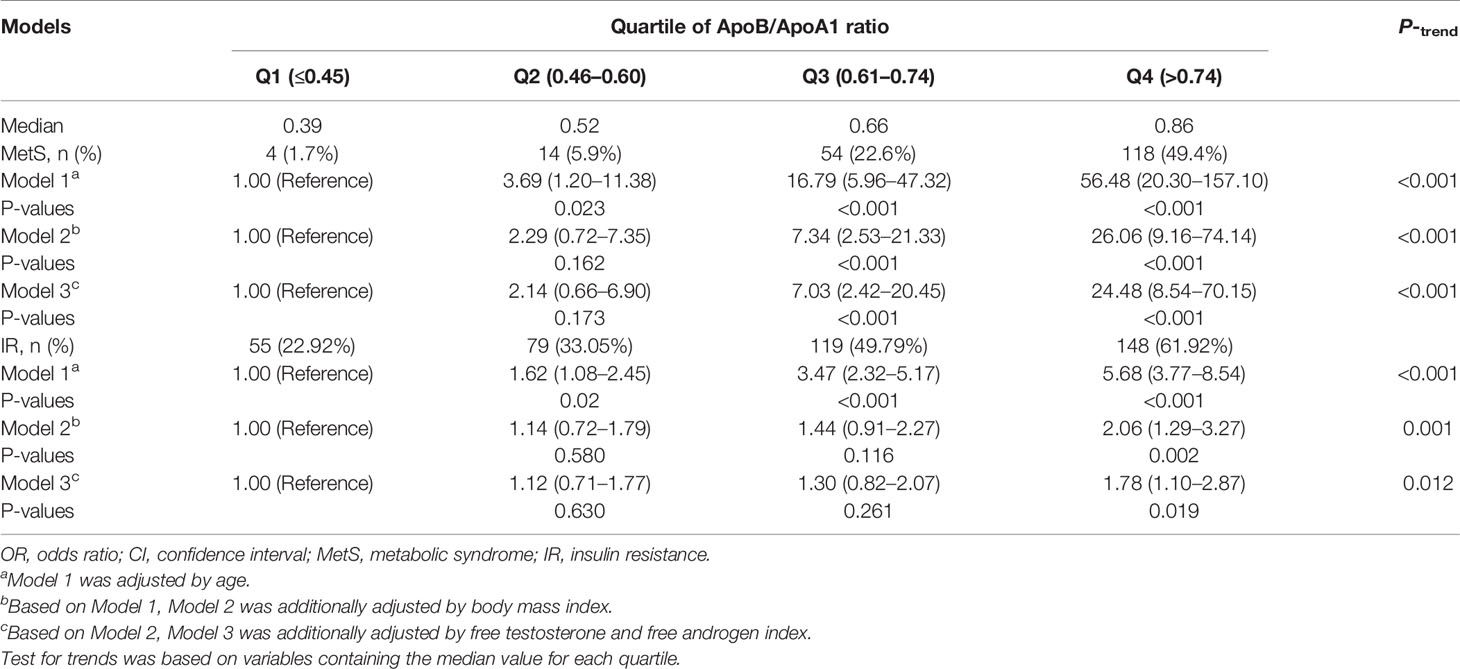
Table 3 Adjusted OR (95% CI) for the associations between the ApoB/ApoA1 ratio and the risk of MetS and IR.
ROC curve analysis of MetS showed that the AUC was 0.84 (95%CI: 0.81–0.86), with a sensitivity of 86.8% and a specificity of 70.3%. The optimal cut-off value of the ApoB/ApoA1 ratio for MetS prediction was 0.64, and the Youden index was 0.57 (Figure 1). For IR, the ApoB/ApoA1 ratio had a 74.3% sensitivity and 58.2% specificity with a threshold value of 0.56, the AUC was 0.68 (95%CI: 0.64–0.71), and the Youden index was 0.33 (Figure 2).
Our results suggest that an increased ApoB/ApoA1 ratio is associated with worse MetS components, IR, and elevated androgen hormones and liver enzymes and that a higher ApoB/ApoA1 ratio is a promising predictor of MetS and IR in PCOS patients.
MetS, a cluster of dysmetabolic factors, namely, central obesity, hypertension, hyperglycemia, and hyperlipidemia, is prevalent in patients with PCOS and confers increased risk of CVD. Prior studies have suggested that the ApoB/ApoA1 ratio is independently associated with MetS and its components in the general populations among various ethnic groups (13–16). Meanwhile, two studies conducted by Yin et al. (26) and Zheng et al. (27) also reported that PCOS patients with MetS have higher ApoB/ApoA1 ratios than those without MetS. In our study, the ApoB/ApoA1 ratio was significantly correlated with WC, TG, HDL, SBP, and FPG, but not with DBP, and the association was independent of age or obesity. In addition, an increased ApoB/ApoA1 ratio is associated with a higher prevalence of MetS. Our results are in line with the above studies, and furthermore our logistic regression results showed that subjects in the fourth quartile of the ApoB/ApoA1 ratio had a 24.48-fold increased risk of MetS than the first quartile even after adjusting for confounding factors. One study conducted on a Korean population showed that the highest ApoB/A1 ratio quartile resulted in an 8.41-fold increased risk for MetS compared to the lowest quartile (13). Recently, Jing et al. (14) and Chou et al. (16) found that the ApoB/ApoA1 ratio is closely associated with MetS in Chinese populations. Their studies reported 5.18-fold and 3.82-fold increased risks of MetS, respectively, among subjects in the 75th quartile of the ApoB/ApoA1 ratio. Compared with the studies mentioned above, we found a 4-fold greater risk. Taken together, these studies suggest that the ApoB/ApoA1 ratio is a more promising indicator of MetS in the PCOS population than in other populations.
IR has been generally recognized to be the link between MetS and PCOS. Previous studies have suggested that the ApoB/ApoA1 ratio is strongly associated with IR in non-diabetic (17) and type 2 diabetic populations (33) after adjusting for confounding factors. Yin et al. (26) and Zheng et al. (27) also found the ApoB/ApoA1 ratio to be significantly associated with IR in Chinese PCOS patients. In our study, the ApoB/ApoA1 ratio showed a strong positive correlation with FPG, FIN, and HOMA-IR after adjusting for age and BMI, which was in agreement with the above results. Furthermore, our regression analysis suggested that the incidence of IR increased across the ApoB/ApoA1 ratio quartiles. The highest quartile of ApoB/ApoA ratio (OR: 1.78; 95%CI: 1.10–2.87, P-trend <0.05) was independently associated with the presence of IR compared to the lowest quartile after controlling for age, BMI, and androgen hormones. Several possible mechanisms may account for the positive association between the ApoB/ApoA1 ratio and IR. The most widely held hypothesis is that insulin-mediated inhibition of lipase activity is reduced under conditions of IR and that excessive free fatty acids produced by lipolysis then flow into the liver resulting in atherogenic dyslipoproteinemia such as the overproduction of ApoB (34). However, this assumption has certain flaws. Taghibiglou et al. (35) confirmed that overproduction of VLDL-ApoB can lead to an increased activity of protein-tyrosine phosphatase-1B (PTP-1B), which can negatively regulate the insulin signaling pathway thus leading to hepatic IR. In turn, chronic exposure of hepatocytes to high concentrations of insulin appeared to increase PTP-1B, accompanied by a marked suppression of ER-60, a cysteine protease involved in ApoB degradation, thus resulting in an increased synthesis and secretion of ApoB in hepatocytes. In addition, both ApoB and IR are related to an inflammatory state, especially to levels of C-reactive protein, and therefore inflammation might play a potential role in mediating the effect of ApoB on IR. These inconsistencies in the mechanisms of ApoB/ApoA1 in regulating IR need to be further elucidated.
HA is the most significant manifestation of PCOS. HA upregulates the activity of hepatic lipase, which plays an important role in the catabolism of HDL particles leading to reduced HDL (36). We found significant positive associations for TT, FT, and FAI with the ApoB/ApoA1 ratio, but negative associations for SHBG. However, the positive association between the ApoB/ApoA1 ratio and TT disappeared after adjusting for age and BMI, suggesting that FAI, which reflects the biological activity of circulating androgen, is a more sensitive indicator than TT for changes in atherogenic apolipoprotein profiles. Previously, Yin et al. (26) reported a higher ApoB/ApoA1 ratio in obese adolescent PCOS patients with high FAI when compared with non-obese subjects with low FAI, and they concluded that FAI might be involved in obesity-related metabolic changes, which was in accordance with our findings. However, contrasting conclusions were reported by Zheng et al. (27). In their study, the positive associations between FT and FAI and the ApoB/ApoA1 ratio were no longer significant after adjusting for age and BMI together, which indicated that obesity might make more of a contribution to the increased ApoB/ApoA1 ratio than FT and FAI in adult PCOS patients. These inconsistent results might be because HA does not reflect all aspects of atherogenic dyslipoproteinemia, and instead these metabolic disturbances appear to be the combined results of obesity, insulin metabolism, and androgen steroid activity.
Our results also showed positive associations between the ApoB/ApoA1 ratio and ALT and AST regardless of age and obesity, which is consistent with previous reports (24, 25). This relationship can be partly explained by the hepato-ovarian axis (37), and numerous studies have indicated that the characteristics of HA in PCOS patients are linked to NAFLD and to elevated liver enzymes (38–40). In addition, apolipoproteins are mainly produced in hepatocytes, and their production is associated with liver function. ALT and AST are both specific markers of liver damage, thus hepatocellular injury might lead to excessive release of ALT and AST along with overproduction of ApoB. However, we did not find any correlation between the ApoB/ApoA1 ratio with CK, CKMB, or LDH, which is inconsistent with a prior study conducted in myocardial infraction patients (21). Our study nevertheless provides some pieces of evidence for the usefulness of the ApoB/ApoA1 ratio in the early prediction of CVD in PCOS patients because the enrolled patients were much younger and with a low chance of CVD. We suggest that prospective studies should be performed in which CVD-related end points such as myocardial infarction or stroke are studied in the long-term follow-up of PCOS patients in order to further evaluate the predictive value of this ratio.
PCOS tends to be associated with more pronounced metabolic disorders than what is seen in the general population. Concerning the long-term health risks, an understanding of the most sensitive risk indicators for early MetS is of great significance. The literature regarding the ApoB/ApoA1 ratio in MetS is limited, especially regarding PCOS. Our results showed that the AUCMetS was 0.84 and had 86.8% sensitivity and 70.3% specificity with a threshold value of 0.64 and a Youden index of 0.57. In addition, we calculated the ROC curve of the ApoB/ApoA1 ratio for IR in PCOS for the first time, and we took an ApoB/ApoA1 ratio of 0.56 as the cut-off point for IR (with a sensitivity of 74.3% and specificity of 58.2%). Therefore, the association between the ApoB/ApoA1 ratio and obesity, blood pressure, glucose, lipid, and IR parameters confirmed the potential role of the ApoB/ApoA1 ratio in the etiology of metabolic disorders and thus in the occurrence and development of MetS. Traditional lipid indexes including TG, TC, and HDL-C are all risk factors for MetS and CVD. However, their levels vary greatly with dietary fat intake and need at least a 12-hour fast for measurement, which makes this an inconvenient measure for use in clinical practice. In contrast, ApoB and ApoA1 have significant advantages for clinical measurement because their testing is standardized, accurate, automated, low-cost, and does not require fasting by the patient. Therefore, our study supports the use of the ApoB/ApoA1 ratio as a biomarker for MetS and IR in PCOS patients.
The major strengths of the study included the large sample size representing the Chinese PCOS population and the consideration of several potential confounding variables. However, some limitations need to be mentioned. There were only PCOS subjects, and no non-PCOS control group was included. It is of great interest to enroll non-PCOS subjects in order to make comparisons and to further elucidate the role of the ApoB/ApoA1 ratio in metabolic abnormalities. In addition, as a secondary analysis based on the PCOSAct, the ApoB/ApoA1 ratio was only available at baseline, and this cross-sectional study is unable to determine causality and any such association should be further confirmed through longitudinal studies in the future.
In conclusion, our results showed that an increased ApoB/ApoA1 ratio was associated with worse MetS components, IR, and elevated androgen hormones and liver enzymes. The ApoB/ApoA1 ratio might therefore be a useful tool for screening for MetS and IR among PCOS patients. Larger studies are needed to confirm these findings before they can be applied in the clinic.
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by the First Affiliated Hospital, Heilongjiang University of Chinese Medicine. The patients/participants provided their written informed consent to participate in this study.
HH, JF, and SZ performed most of the work for the study, wrote the manuscript, and prepared the final version of the manuscript. XW designed and conceived the study. JL took part in the study design and helped in the revision of the manuscript. YW, JC, JG, and YG helped with data collection, statistical analysis, and interpretation. All authors contributed to the article and approved the submitted version.
1. National Key R&D Program of China, Research and Development for Modernization of Chinese Medicine, Evidence-based Evaluation of the Program of Integration of Traditional Chinese and Western Medicine for High Incidence of Gynecological Diseases (No.2019YFC1709500); 2. National Clinical Cooperation Pilot Program of TCM and Western Medicine for Major and Difficult Diseases (National Office of Traditional Chinese Medicine, No.[2018]3), Combined Traditional Chinese and Western Medicine Infertility and Assisted Reproductive Technology; 3. Scientific Research Fund of Heilongjiang University of Chinese Medicine, Study on Acupuncture Combined with Traditional Chinese Medicine Compound to Improve the Live Birth Rate of Patients with In Vitro Fertilization and Embryo Transfer (No. 2019BS09).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Rotterdam ESHRE/ASRM–Sponsored PCOS consensus workshop group. Revised 2003 Consensus on Diagnostic Criteria and Long–Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum Reprod (2004) 19(1):41–7. doi: 10.1093/humrep/deh098
2. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R, et al. Criteria, Prevalence, and Phenotypes of Polycystic Ovary Syndrome. Fertil Steril (2016) 106(1):6–15. doi: 10.1016/j.fertnstert.2016.05.003
3. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, Obesity and Central Obesity in Women With Polycystic Ovary Syndrome: A Systematic Review and Meta–Analysis. Hum Reprod Update (2012) 18(6):618–37. doi: 10.1093/humupd/dms030
4. Moghetti P. Insulin Resistance and Polycystic Ovary Syndrome. Curr Pharm Des (2016) 22(36):5526–34. doi: 10.2174/1381612822666160720155855
5. Wild RA, Rizzo M, Clifton S, Carmina E. Lipid Levels in Polycystic Ovary Syndrome: Systematic Review and Meta–Analysis. Fertil Steril (2011) 95(3):1073–9. e1–11. doi: 10.1016/j.fertnstert.2010.12.027
6. Wu J, Yao XY, Shi RX, Liu SF, Wang XY. A Potential Link Between Polycystic Ovary Syndrome and Non–Alcoholic Fatty Liver Disease: An Update Meta–Analysis. Reprod Health (2018) 15(1):77. doi: 10.1186/s12978-018-0519-2
7. Behboudi–Gandevani S, Amiri M, Bidhendi Yarandi R, Noroozzadeh M, Farahmand M, Dovom MR, et al. The Risk of Metabolic Syndrome in Polycystic Ovary Syndrome: A Systematic Review and Meta–Analysis. Clin Endocrinol (Oxf) (2018) 88(2):169–84. doi: 10.1111/cen.13477
8. Wekker V, van Dammen L, Koning A, Heida KY, Painter RC, Limpens J, et al. Long–Term Cardiometabolic Disease Risk in Women With PCOS: A Systematic Review and Meta–Analysis. Hum Reprod Update (2020) 26(6):942–60. doi: 10.1093/humupd/dmaa029
9. Barber TM, Dimitriadis GK, Andreou A, Franks S. Polycystic Ovary Syndrome: Insight Into Pathogenesis and a Common Association With Insulin Resistance. Clin Med (Lond) (2016) 16(3):262–6. doi: 10.7861/clinmedicine.16-3-262
10. Su X, Peng D. The Exchangeable Apolipoproteins in Lipid Metabolism and Obesity. Clin Chim Acta (2020) 503:128–35. doi: 10.1016/j.cca.2020.01.015
11. Morita SY. Metabolism and Modification of Apolipoprotein B–Containing Lipoproteins Involved in Dyslipidemia and Atherosclerosis. Biol Pharm Bull (2016) 39(1):1–24. doi: 10.1248/bpb.b15-00716
12. Walldius G, Jungner I, Aastveit AH, Holme I, Furberg CD, Sniderman AD, et al. The Apob/Apoa–I Ratio Is Better Than the Cholesterol Ratios to Estimate the Balance Between Plasma Proatherogenic and Antiatherogenic Lipoproteins and to Predict Coronary Risk. Clin Chem Lab Med (2004) 42(12):1355–63. doi: 10.1515/CCLM.2004.254
13. Jung CH, Hwang JY, Yu JH, Shin MS, Bae SJ, Park J-Y, et al. The Value of Apolipoprotein B/A1 Ratio in the Diagnosis of Metabolic Syndrome in a Korean Population. Clin Endocrinol (Oxf) (2012) 77(5):699–706. doi: 10.1111/j.1365-2265.2012.04329.x
14. Jing F, Mao Y, Guo J, Zhang Z, Li Y, Ye Z, et al. The Value of Apolipoprotein B/Apolipoprotein A1 Ratio for Metabolic Syndrome Diagnosis in a Chinese Population: A Cross–Sectional Study. Lipids Health Dis (2014) 13:81. doi: 10.1186/1476-511X-13-81
15. Nurtazina A, Kozhakhmetova D, Dautov D, Shakhanova A, Chattu VK. Apolipoprotein B/A1 Ratio as a Diagnostic Alternative to Triglycerides and HDL–Cholesterol for the Prediction of Metabolic Syndrome Among Hypertensives in Kazakhstan. Diagn (Basel) (2020) 10(8):510. doi: 10.3390/diagnostics10080510
16. Chou YC, Kuan JC, Bai CH, Yang T, Chou WY, Hsieh PC, et al. Predictive Value of Serum Apolipoprotein B/apolipoprotein a–I Ratio in Metabolic Syndrome Risk: A Chinese Cohort Study. Endocrine (2015) 49(2):404–14. doi: 10.1007/s12020-014-0447-z
17. Sierra–Johnson J, Romero–Corral A, Somers VK, Lopez-Jimenez F, Walldius G, Hamsten A, et al. ApoB/Apoa–I Ratio: An Independent Predictor of Insulin Resistance in US Non–Diabetic Subjects. Eur Heart J (2007) 28(21):2637–43. doi: 10.1093/eurheartj/ehm360
18. Sierra–Johnson J, Fisher RM, Romero–Corral A, Somers VK, Lopez-Jimenez F, Öhrvik J, et al. Concentration of Apolipoprotein B Is Comparable With the Apolipoprotein B/apolipoprotein a–I Ratio and Better Than Routine Clinical Lipid Measurements in Predicting Coronary Heart Disease Mortality: Findings From a Multi–Ethnic US Population. Eur Heart J (2009) 30(6):710–7. doi: 10.1093/eurheartj/ehn347
19. Carnevale Schianca GP, Pedrazzoli R, Onolfo S, Colli E, Cornetti E, Bergamasco L, et al. ApoB/Apoa–I Ratio Is Better Than LDL–C in Detecting Cardiovascular Risk. Nutr Metab Cardiovasc Dis (2011) 21(6):406–11. doi: 10.1016/j.numecd.2009.11.002
20. Goswami B, Rajappa M, Mallika V, Kumar S, Shukla DK. Apo–B/apo–AI Ratio: A Better Discriminator of Coronary Artery Disease Risk Than Other Conventional Lipid Ratios in Indian Patients With Acute Myocardial Infarction. Acta Cardiol (2008) 63(6):749–55. doi: 10.2143/AC.63.6.2033393
21. Bausserman LL, Sadaniantz A, Saritelli AL, Martin VL, Nugent AM, Sady SP, et al. Time Course of Serum Amyloid A Response in Myocardial Infarction. Clin Chim Acta (1989) 184(3):297–305. doi: 10.1016/0009-8981(89)90063-6
22. Yang MH, Sung J, Gwak GY. The Associations Between Apolipoprotein B, A1, and the B/A1 Ratio and Nonalcoholic Fatty Liver Disease in Both Normal–Weight and Overweight Korean Population. J Clin Lipidol (2016) 10(2):289–98. doi: 10.1016/j.jacl.2015.11.017
23. Choe YG, Jin W, Cho YK, Chung WG, Kim HJ, Jeon WK, et al. Apolipoprotein B/AI Ratio Is Independently Associated With Non–Alcoholic Fatty Liver Disease in Nondiabetic Subjects. J Gastroenterol Hepatol (2013) 28(4):678–83. doi: 10.1111/jgh.12077
24. Lorenzo C, Hanley AJ, Rewers MJ, Haffner SM. The Association of Alanine Aminotransferase Within the Normal and Mildly Elevated Range With Lipoproteins and Apolipoproteins: The Insulin Resistance Atherosclerosis Study. Diabetologia (2013) 56(4):746–57. doi: 10.1007/s00125-012-2826-4
25. Siddiqui MS, Sterling RK, Luketic VA, Puri P, Stravitz RT, Bouneva I, et al. Association Between High–Normal Levels of Alanine Aminotransferase and Risk Factors for Atherogenesis. Gastroenterology (2013) 145(6):1271–9. e1–3. doi: 10.1053/j.gastro.2013.08.036
26. Yin Q, Chen X, Li L, Zhou R, Huang J, Yang D. Apolipoprotein B/apolipoprotein A1 Ratio Is a Good Predictive Marker of Metabolic Syndrome and Pre–Metabolic Syndrome in Chinese Adolescent Women With Polycystic Ovary Syndrome. J Obstet Gynaecol Res (2013) 39(1):203–9. doi: 10.1111/j.1447-0756.2012.01907.x
27. Zheng J, Yin Q, Cao J, Zhang B. Obesity Contributes More to Increasing ApoB/ApoA1 Ratio Than Hyperandrogenism in PCOS Women Aged 20–38 Years in China. Exp Ther Med (2017) 13(4):1337–42. doi: 10.3892/etm.2017.4094
28. Kuang H, Li Y, Wu X, et al. Acupuncture and Clomiphene Citrate for Live Birth in Polycystic Ovary Syndrome: Study Design of a Randomized Controlled Trial. Evid Based Complement Alternat Med (2013), 527303. doi: 10.1155/2013/527303
29. Wu XK, Stener–Victorin E, Kuang HY, Ma H, Gao J, Xie L, et al. Effect of Acupuncture and Clomiphene in Chinese Women With Polycystic Ovary Syndrome: A Randomized Clinical Trial. JAMA (2017) 317(24):2502–14. doi: 10.1001/jama.2017.7217
30. Wallace TM, Levy JC, Matthews DR. Use and Abuse of HOMA Modeling. Diabetes Care (2004) 27(6):1487–95. doi: 10.2337/diacare.27.6.1487
31. Jayanthi R, Srinivasan AR, Hanifah M, Maran AL. Associations Among Insulin Resistance, Triacylglycerol/High Density Lipoprotein (TAG/HDL Ratio) and Thyroid Hormone Levels–A Study on Type 2 Diabetes Mellitus in Obese and Overweight Subjects. Diabetes Metab Syndr (2017) 11(Suppl 1):S121–6. doi: 10.1016/j.dsx.2016.12.020
32. Biggerstaff BJ. Comparing Diagnostic Tests: A Simple Graphic Using Likelihood Ratios. Stat Med (2000) 19:649–63. doi: 10.1002/(SICI)1097-0258(20000315)19:5<649::AID-SIM371>3.0.CO;2-H
33. Hwang YC, Ahn HY, Kim WJ, Park CY, Park SW. Increased Apob/a–I Ratio Independently Associated With Type 2 Diabetes Mellitus: Cross–Sectional Study in a Korean Population. Diabetes Med (2012) 29(9):1165–70. doi: 10.1111/j.1464-5491.2012.03622.x
34. Sniderman AD, Faraj M. Apolipoprotein B, Apolipoprotein A–I, Insulin Resistance and the Metabolic Syndrome. Curr Opin Lipidol (2007) 18(6):633–7. doi: 10.1097/MOL.0b013e3282f0dd33
35. Taghibiglou C, Rashid–Kolvear F, Van Iderstine SC, Le-Tien H, Fantus IG, Lewis GF, et al. Hepatic Very Low Density Lipoprotein–ApoB Overproduction Is Associated With Attenuated Hepatic Insulin Signaling and Overexpression of Protein–Tyrosine Phosphatase 1B in a Fructose–Fed Hamster Model of Insulin Resistance. J Biol Chem (2002) 277(1):793–803. doi: 10.1074/jbc.M106737200
36. Tikkanen MJ, Nikkilä EA. Regulation of Hepatic Lipase and Serum Lipoproteins by Sex Steroids. Am Heart J (1987) 113(2 Pt 2):562–7. doi: 10.1016/0002-8703(87)90633-8
37. Targher G, Rossini M, Lonardo A. Evidence That Non–Alcoholic Fatty Liver Disease and Polycystic Ovary Syndrome Are Associated by Necessity Rather Than Chance: A Novel Hepato–Ovarian Axis? Endocrine (2016) 51(2):211–21. doi: 10.1007/s12020-015-0640-8
38. Chen MJ, Chiu HM, Chen CL, Yang WS, Yang YS, Ho HN. Hyperandrogenemia Is Independently Associated With Elevated Alanine Aminotransferase Activity in Young Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2010) 95(7):3332–41. doi: 10.1210/jc.2009-2698
39. Vassilatou E, Lafoyianni S, Vryonidou A, Ioannidis D, Kosma L, Katsoulis K, et al. Increased Androgen Bioavailability Is Associated With Non–Alcoholic Fatty Liver Disease in Women With Polycystic Ovary Syndrome. Hum Reprod (2010) 25(1):212–20. doi: 10.1093/humrep/dep380
Keywords: polycystic ovary syndrome, metabolic syndrome, insulin resistance, hyperandrogenism, liver enzyme, apolipoprotein ratio
Citation: He H, Feng J, Zhang S, Wang Y, Li J, Gao J, Cong J, Gong Y and Wu X (2022) The Apolipoprotein B/A1 Ratio is Associated With Metabolic Syndrome Components, Insulin Resistance, Androgen Hormones, and Liver Enzymes in Women With Polycystic Ovary Syndrome. Front. Endocrinol. 12:773781. doi: 10.3389/fendo.2021.773781
Received: 10 September 2021; Accepted: 23 November 2021;
Published: 05 January 2022.
Edited by:
Rong Li, Peking University Third Hospital, ChinaReviewed by:
Alice Albu, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2022 He, Feng, Zhang, Wang, Li, Gao, Cong, Gong and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoke Wu, eGlhb2tld3UyMDAyQHZpcC5zaW5hLmNvbQ==
†These authors shared first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.