- 1Guangdong Provincial Key Laboratory of Malignant Tumor Epigenetics and Gene Regulation, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 2Department of Otolaryngology, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 3Department of Ultrasound, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
- 4Cellular and Molecular Diagnostics Center, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, China
Background: High-volume lymph node metastasis (HVLNM, equal to or more than 5 lymph nodes) is one of the adverse features indicating high recurrence risk in papillary thyroid carcinoma (PTC) and is recommended as one of the indications of completion thyroidectomy for patients undergoing thyroid lobectomy at first. In this study, we aim to develop a preoperative nomogram for the prediction of HVLNMs in the central compartment in PTC (cT1-2N0M0), where preoperative imaging techniques perform poor.
Methods: From October 2016 to April 2021, 423 patients were included, who were diagnosed as PTC (cT1-2N0M0) and underwent total thyroidectomy and prophylactic central compartment neck dissection in our center. Demographic and clinicopathological features were recorded and analyzed using univariate and multivariate logistic regression analysis. A nomogram was developed based on multivariate logistic regression analysis.
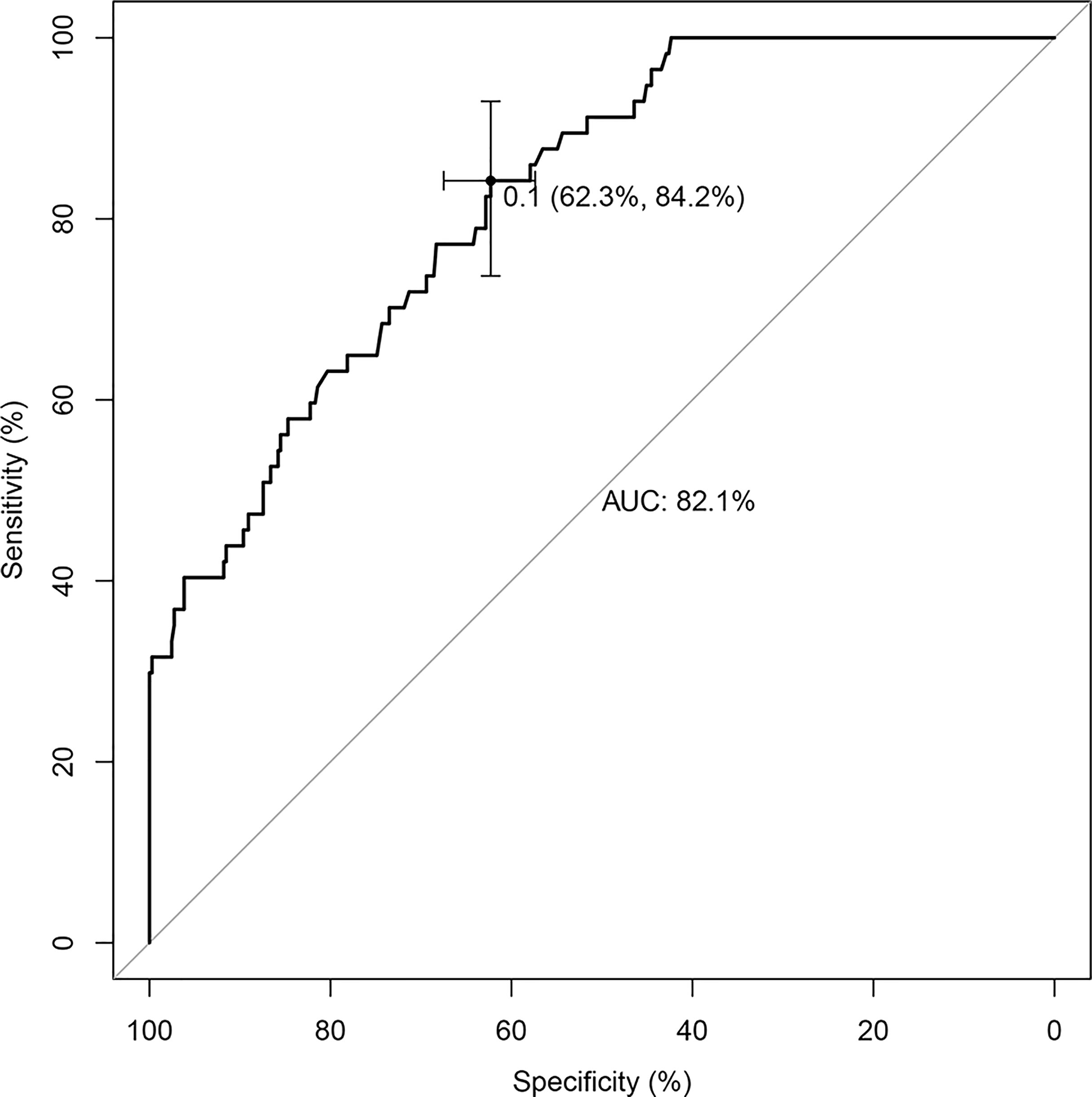
Results: Among the included patients, 13.4% (57 cases) were found to have HVLNMs in the central compartment. Univariate and multivariate logistic regression analysis showed that age (</=35 years vs. >35 years), BRAF with V600E mutated, nodule diameter, and calcification independently predicted HVLNMs in the central compartment. The nomogram showed good discrimination with an AUC of 0.821 (95% CI, 0.768–0.875).
Conclusion: The preoperative nomogram can be used to quantify the probability of HVLNMs in the central compartment and may reduce the reoperation rate after thyroid lobectomy.
Introduction
As several studies indicated that oncologic outcomes of lobectomy are comparable to those of a near-total or total thyroidectomy for low-risk papillary thyroid carcinoma (PTC) (1–3), thyroid lobectomy alone is recommended as an alternative for low-risk PTC (4–6). However, some adverse pathological features, which would upgrade the cases from low risk to intermediate risk or high risk, could only be assessed in postoperative examination (7, 8). Some of these adverse pathological features are recommended as indications of completion thyroidectomy following lobectomy, which may lead to more severe anxiety for the patients and increase expenditures for medical care and public health, compared to one-stage near-total or total thyroidectomy (7, 9). In NCCN clinical practice guidelines in oncology (thyroid carcinoma, version 3.2021), large volume pathological N1a metastasis is recommended as one of the adverse pathological features indicating completion thyroidectomy following lobectomy. It refers to equal to or more than 5 involved lymph nodes or any nodal metastasis >2 mm in largest dimension, which reveals high risk of PTC recurrence (7, 10, 11), whereas the diagnostic performance of imaging techniques, such as ultrasonography (US) or computer tomography, is poor in detecting involved lymph nodes in the central compartment preoperatively (12). In this research, we tried to build a preoperative nomogram to predict high-volume central lymph node metastasis (HVCLNMs, equal to or more than 5 involved lymph nodes in central compartment) to reduce reoperation rate after thyroid lobectomy.
Materials and Methods
Patients
From October 2016 to April 2021, a total of 423 patients undergoing total thyroidectomy and prophylactic central compartment neck dissection in our center were included. Preoperative diagnoses were revealed by US. The most suspicious malignant thyroid nodule with KWAK-TIRADS of 4 to 5 in either thyroid lobe in preoperative US examination was confirmed by means of ultrasound-guided fine-needle aspiration cytologic examination (FNAC) (13).
The eligibility criteria were as follows: (1) unilateral papillary thyroid carcinoma diagnosed in preoperative US and FNAC (2); the largest malignant nodule size </= 4.0 cm in preoperative US examination; and (3) undergoing total thyroidectomy and prophylactic central compartment neck dissection in our center.
The exclusion criteria were (1) bilateral malignant thyroid nodules diagnosed in FNAC; (2) any nodule of contralateral thyroid lobe with KWAK-TIRADS of 4c or 5, even without positive FNAC results; (3) malignant or suspicious malignant thyroid nodules located on isthmus of thyroid gland either with positive FNAC results or with KWAK-TIRADS of 4c or 5 in US; (4) extrathyroidal extension; (5) enlarged lymph nodes on palpation or US scan; (6) clinical evidence of distant metastases such as in the lung and bone; and (7) history of previous neck radiation exposure.
For patients who met all the inclusion criteria but none of the exclusion criteria, the advantages and disadvantages of the two operation procedures, total thyroidectomy and thyroid lobectomy, were explained, and the patients chose either procedure based on preference. Those choosing total thyroidectomy were included in our study.
Demographic features, the BRAF V600E mutation test result, serum thyroperoxidase antibody (TPOAb) and thyroglobulin antibody (TgAb) levels, US, FNAC, and pathological examination results were recorded and analyzed. Hashimoto’s thyroiditis was diagnosed by demonstration of elevated circulating autoantibodies to thyroid antigens and reduced echogenicity on thyroid sonogram (14, 15). In our research, the size of metastatic foci was not taken into consideration in predicting HVCLNMs, as lymph node could be cut perpendicularly, parallelly, or at any angle to the longest dimension of metastatic foci and the diameter of metastatic foci in largest dimension is difficult to obtain actually.
The study was carried out with approval of the Institutional Review Board (IRB) of our hospital (approval No. SYSEC-KY-KS-2020-149).
Ultrasound Characteristics
The ultrasound imaging characteristics of the largest malignant nodule were assessed by two researchers (with 5–10 years of experience), independently. The imaging characteristics included the composition, size, shape (the anteroposterior dimension divided by its transverse dimension, A/T), echogenicity, margins, calcification, and vascularity pattern. Nodule composition was classified as solid or mixed composition. Nodule size refers to the maximum diameter. Shape was classified as A/T </= 1 or A/T > 1. The echogenicity was categorized as hyper-, iso-, hypoechogenicity, or marked hypoechogenicity. Margins were classified as well-circumscribed, irregular, or microlobulated, and the calcification pattern was categorized as noncalcification, microcalcification, macrocalcification, or peripheral (rim) calcification. Microcalcifications were defined as hyperechoic foci that were equal to or less than 1 mm in diameter. When calcifications were larger than 1 mm, they were classified as macrocalcifications. When microcalcifications presented in a nodule, regardless of whether macrocalcifications and/or peripheral (rim) calcifications existed at the same time, they were classified as microcalcifications. The color Doppler flow pattern of a lesion is classified into four types: (1) perinodular vascularity, (2) intranodular vascularity, (3) mixed perinodular and intranodular vascularity, and (4) absence of blood flow. Masses with mixed components were evaluated based on the internal solid components. Whether malignant nodule (diagnosed in FNAC examination) or suspicious malignant nodule (classified as KWAK-TIRADS 4c or 5 in US, but without positive FNAC results) is located at the inferior part (lower third) or superior part (upper third) of the thyroid lobe was also recorded. Discrepancies between the two researchers were resolved by rechecking the images and discussing with another researcher (with more than 20 years of experience).
Identification of BRAF V600E Mutations
The results of BRAF V600E mutation test using preoperative qPCR were recorded.
In preoperative qPCR, DNA extraction was performed on fine-needle cytologic samples using the AmoyDx® FFPE DNA Kit (Amoy Diagnostics Co., Ltd., Xiamen, China) according to the manufacturer’s protocols. The concentration and purity of extracted DNA were assessed by Nanodrop spectrophotometry.
BRAF V600E mutation detection was performed in the Cellular and Molecular Diagnostic Centre of Sun Yan-Sen Memorial Hospital, Sun Yat-Sen University. DNA from the 423 patients was tested using the AmoyDx® BRAF Mutations Detection Kit (Amoy Diagnostics Co., Ltd., Xiamen, China) under the principle of the amplification refractory mutation system (ARMS), detecting BRAF V600E mutation (exon 15). AmoyDx® BRAF Mutations Detection Kit was used to detect BRAF V600E mutation in cytological specimens and tissue specimens in some previous studies (16, 17). Briefly, the PCR was carried out on a 7500 Real‐Time PCR System (Applied Biosystems) according to the manufacturer’s protocol with 10 ng of DNA in each reaction system. The PCR kit allows an LOD (limit of detection) as low as 1% for BRAF V600E (PCR kit instructions). All results were confirmed according to the criterion suggested by the manufacturer.
Statistical Analysis
Qualitative variables were summarized as absolute and relative frequency (percentage). Quantitative variables were summarized as the means and standard deviations (mean ± SD) whenever data proved to be normally distributed; otherwise, the medians and interquartile ranges were used (interquartile range is provided in parentheses within the manuscript).
Logistic regression was used to model the association between each variable and the presence of high-volume lymph node metastases (equal to or more than 5 involved lymph nodes) in the central compartment. Variables with a univariate p-value less than 0.1 were included in multivariate analysis. The multivariable logistic regression model was calculated using likelihood ratio statistics to identify significant and independent variables with forward stepwise model. Thresholds to determine whether variables were enrolled in the model was set as p < 0.05 for variables entering the model, and p > 0.10 for variables rejected, which were set to guard against the possibility of having the program enter and remove the same variable at successive steps (18).
To provide surgeons with a quantitative tool to predict the individual probability of high-volume lymph node metastasis (HVLNM) in the central compartment, we built the diagnostic nomogram using the independent predictors selected by multivariable logistic regression analysis to generate a combined indicator. The ability of the model to discriminate between patients with and without HVCLNMs was assessed using the area under the ROC curve (AUC), also known as the concordance index, whose value ranged from 0.50 to 1.00. A value of 1.00 indicated perfect discrimination, while a value of 0.50 indicated random predictions.
One thousand random bootstrap resamples were used for internal validation of the model to address model overfit and obtain a relatively unbiased evaluation. Calibration curves were plotted to assess the calibration of the diagnostic nomogram, which was assessed by plotting the predicted versus the actual probability of HVCLNMs. The absolute error of the nomogram prediction was measured by the distance between the pairs and the 45° line (19).
All statistical analyses were performed in the rms/pROC package in R version 4.0.0 and SPSS software (version 21.0, Chicago, IL, United States). A p-value of < 0.05 was considered to indicate statistical significance.
Results
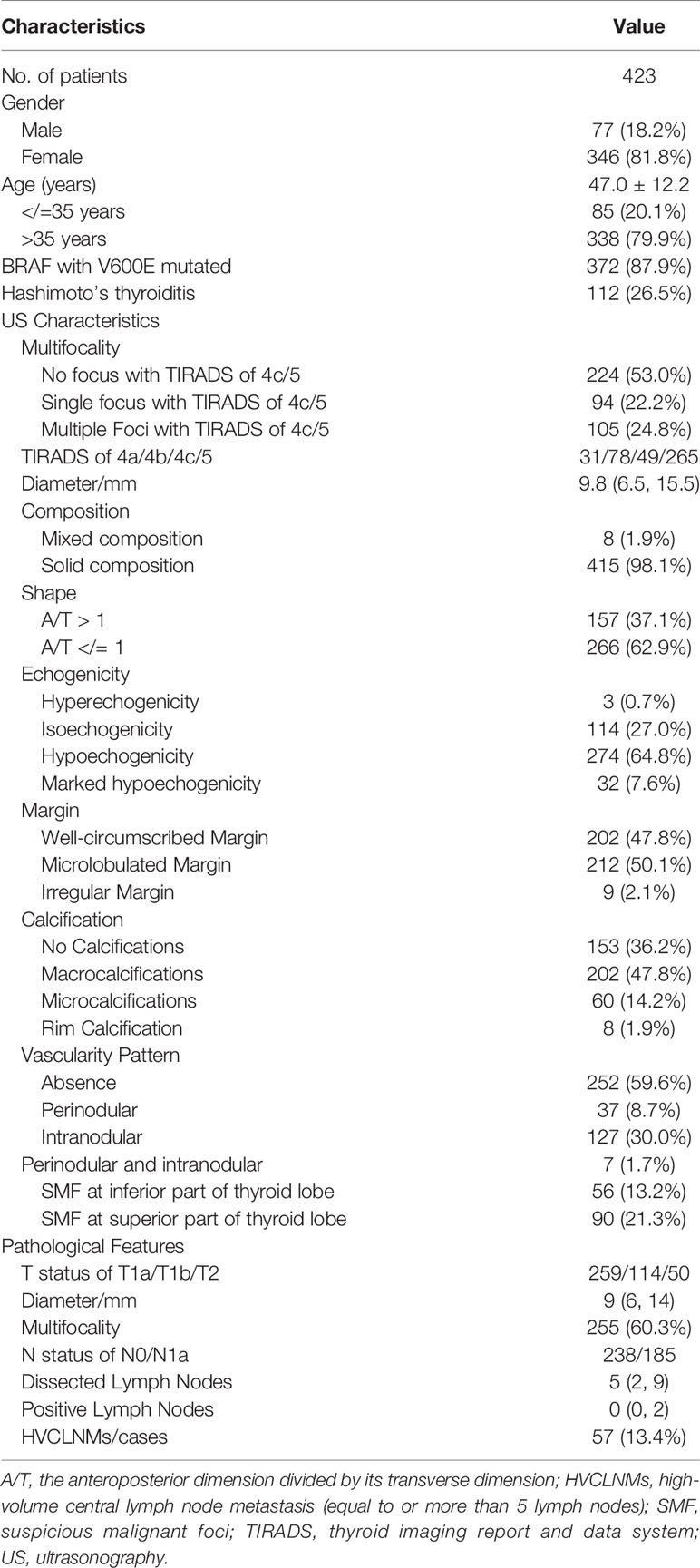
In our study, a total of 423 patients with age of 47.0 ± 12.2 years were included. Among the patients, 77 (18.2%) were male. The BRAF mutation test results showed that 372 (87.9%) had a V600E mutation. A total of 112 (26.5%) patients were diagnosed as Hashimoto’s thyroiditis preoperatively according to circulating autoantibodies and ultrasonic features. The characteristics of the thyroid nodules in ultrasound examination and postoperative pathological features are presented in Table 1. The intra-observer agreements of the two researchers in assessing US characteristics were 0.773–0.966 and 0.839–0.977, respectively, whereas the inter-observer agreements between the two researchers were 0.759–0.947 (Table S1).
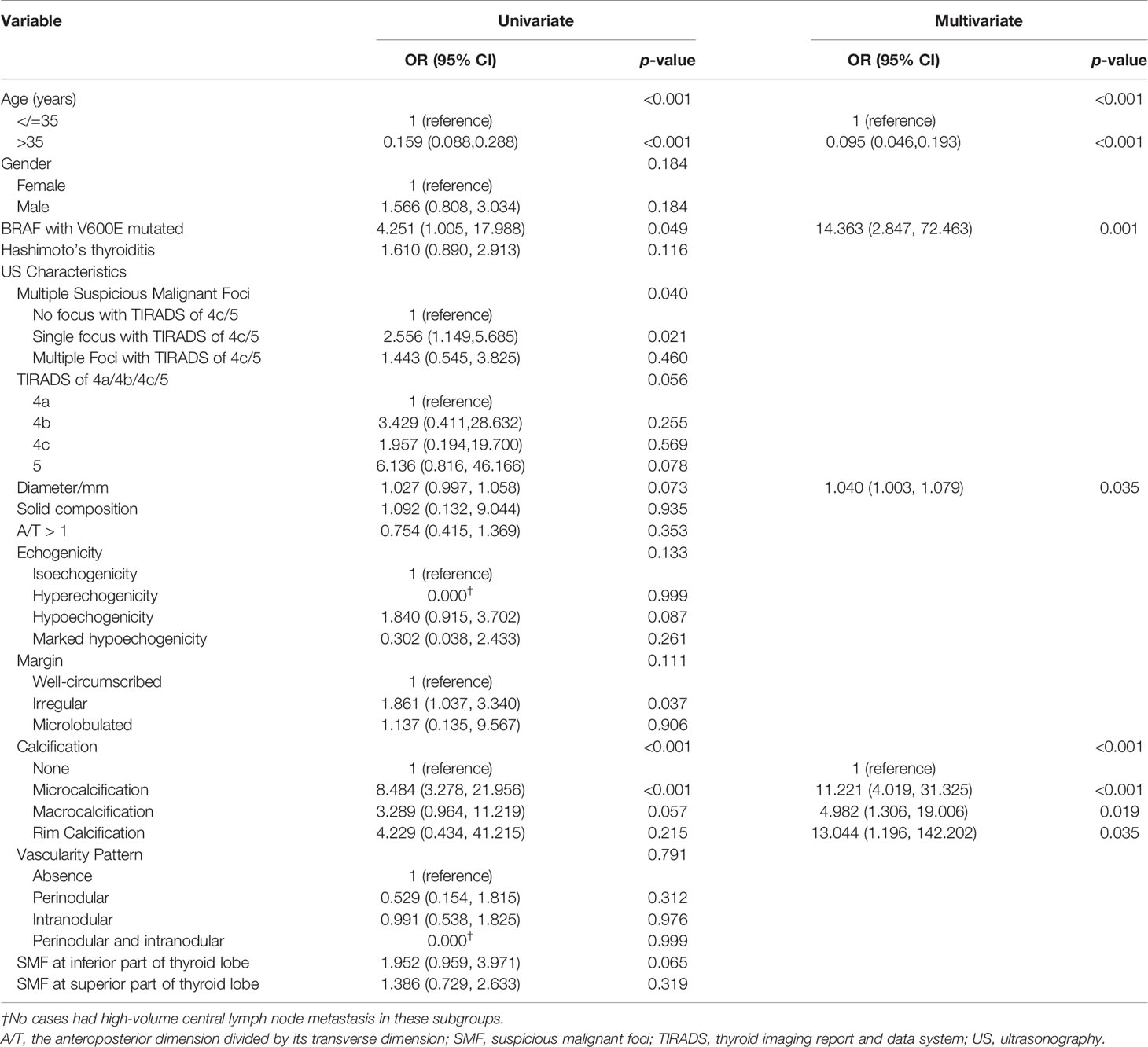
The univariate logistic regression analysis revealed that age, BRAF V600E mutation, multifocality, and calcifications in US examination were significantly associated with HVCLNM. Nodule diameter, TIRADS classification, and suspicious malignant focus at the inferior part of thyroid lobe might be associated with HVCLNMs with a p-value between 0.05 and 0.10. They were all included in multivariate logistic regression analysis. As shown in Table S2 and Supplementary Figure S1, the risk of HVCLNM in patients with PTC decreased dramatically when the patients were older than 35 years. Thus, age was included in multivariate logistic regression analysis as a dichotomous variable (</=35 years vs. >35 years). Multivariate logistic regression analysis showed that age (</=35 years vs. >35 years), BRAF with V600E mutated, nodule diameter, and calcification in US examination independently predicted HVCLNMs (Table 2).
The nomogram that incorporated the independently predictive factors was developed and presented (Figure 1). The mean AUC for the nomogram was 0.821 (95% CI, 0.768–0.875) (Figure 2). Setting a cutoff point of 0.50, sensitivity, specificity, positive predictive value, and negative predictive value were 40.4%, 96.2%, 62.2%, and 91.2%, respectively. The calibration curve of the nomogram presented good agreement between the predicted and observed probability of HVCLNMs, especially for the observed HVCLNMs probability >0.5 (Figure 3).
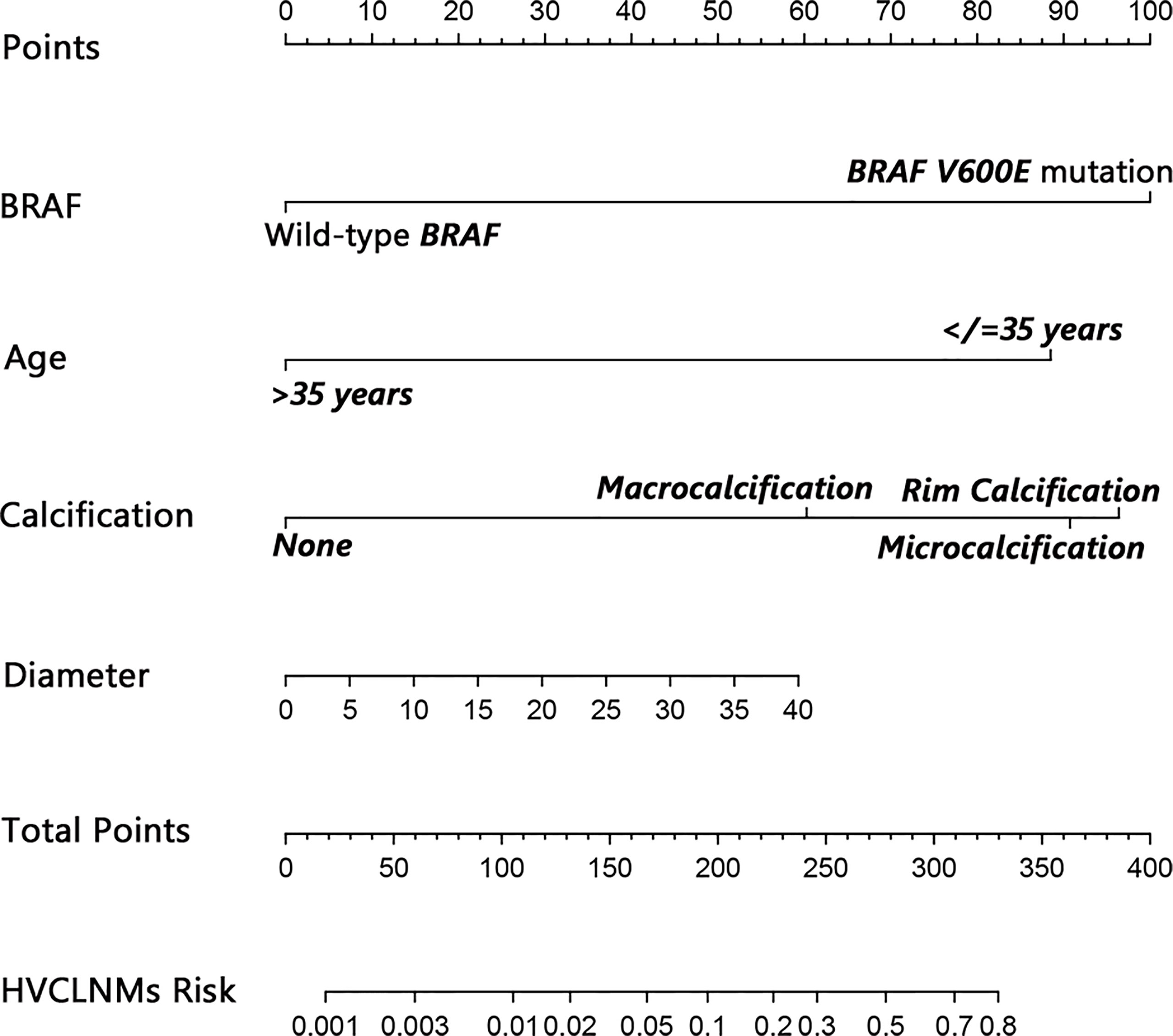
Figure 1 Nomogram to predict high-volume lymph node metastasis in the central compartment (equal to or more than 5 lymph nodes). A 32-year-old (about 87.5 points) patient who has a malignant focus of 20 mm (about 30.0 points) with BRAF V600E mutation (100.0 points) and microcalcification (about 90.0 points) would get a total of about 307.5 points, which implies a near 0.70 risk for HVCLNMs. The bottom line indicating the risk of HVCLNMs is given as logarithm. HVCLNMs, high-volume central lymph node metastasis.
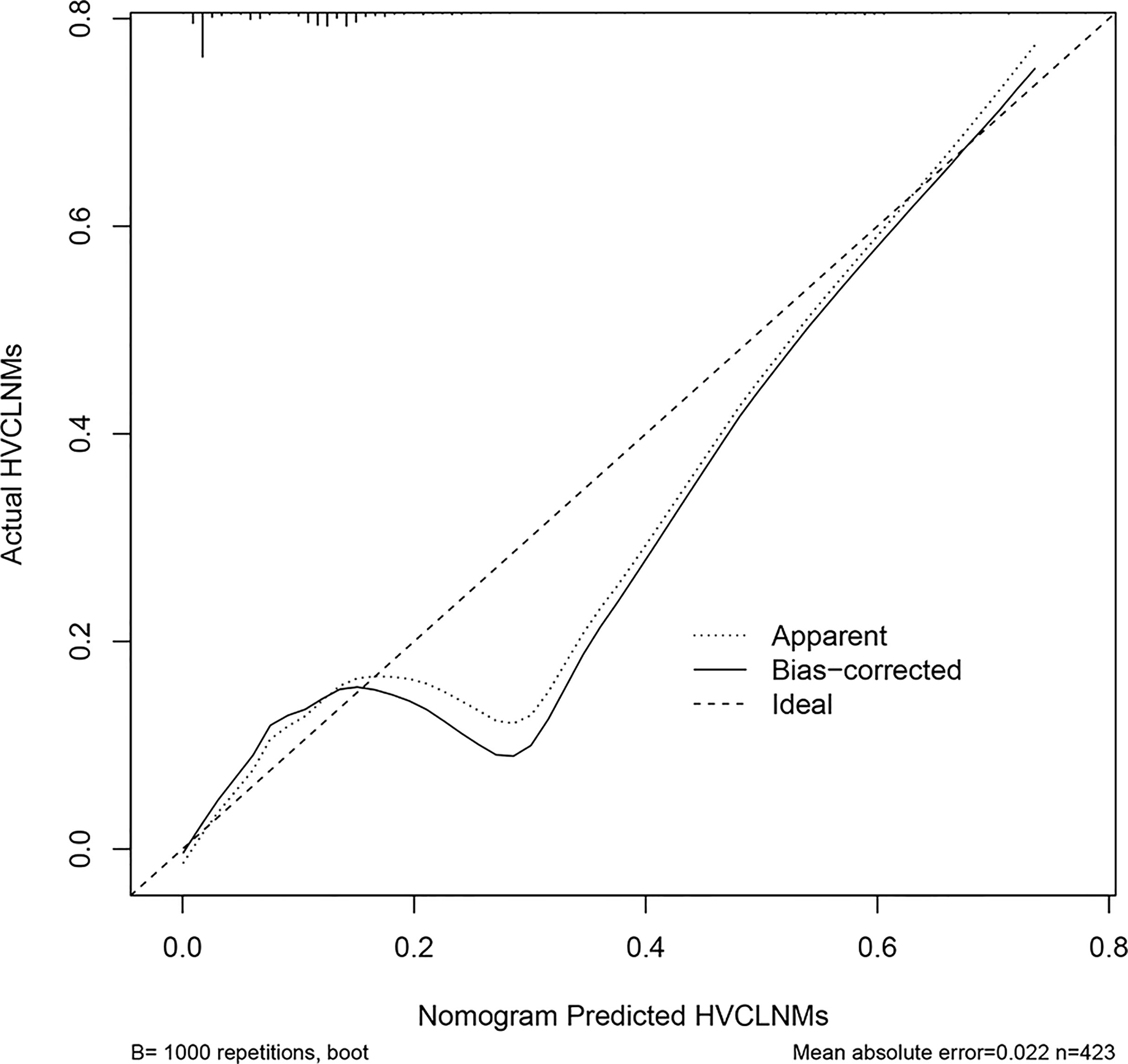
Figure 3 Calibration plot of the nomogram. The x-axis is the nomogram-predicted probability of high-volume central compartment lymph node metastasis (HVCLNMs). The y-axis is the actual probability of HVCLNMs. Dashed line = ideal nomogram; dotted line = apparent predicted accuracy; solid line = calibration estimate from the internally validated model. Perfect prediction would correspond to the dashed line.
Discussion
In the recent two decades, several studies indicated that oncologic outcomes of lobectomy are comparable to those of a near-total or total thyroidectomy for low-risk papillary thyroid carcinoma. Davies et al. analyzed 35,223 cases of PTC confined to the thyroid gland from 1973 to 2005 and found that the prognosis of the patients with either hemithyroidectomy or total thyroidectomy would be the same (1). Bilimoria et al. analyzed 52,173 patients having underwent surgery for PTC from 1985 to 1998 and concluded that extent of surgery (lobectomy versus total thyroidectomy) did not impact recurrence or survival for PTC <1 cm (2). Jonklaas et al. drew a similar conclusion that the extent of thyroid surgery was not associated with overall survival, disease-specific survival, or disease-free survival in stage I patients (3). In the 2015 ATA guidelines, thyroid lobectomy alone was recommended as an alternative for the initial treatment of low-risk PTC (8). A similar opinion was presented in NCCN clinical practice guidelines in oncology (thyroid carcinoma, version 3.2021) (7). However, on account of the unsatisfying diagnostic performance of imaging techniques, some adverse features, which indicate high risk of recurrence and reveal unfavorable prognosis, could only be assessed in postoperative pathological examination. Completion thyroidectomy following lobectomy is recommended to reduce risk of recurrence and improve prognosis for these patients, whereas completion thyroidectomy following lobectomy may lead to increasing complications, severe anxiety of the patients, and extra expenditures for medical care and public health, compared with one-stage surgery (near-total or total thyroidectomy) (9, 20).
HVLNMs (equal to or more than 5 involved lymph nodes) was one of the risk factors indicating high recurrence rate and poor prognosis in several studies (21). Sugitani et al. reported that the patients with > 5 LN metastases demonstrated a significantly higher risk of recurrence (19% versus 8%) (11). Likewise, in the research by Leboulleux et al., the patients with >10 positive LNs (21%) or 6–10 LN metastases (7%) significantly predicted higher 10-year risk of recurrence compared with the patients with <5 LN metastases (3%) (10). In the 2015 ATA guidelines, pathologic N1 with more than 5 involved lymph nodes was one of the criteria to upgrade low-risk PTC to intermediate-risk PTC, which makes risk of structural disease recurrence increase by about 15% (8). Furthermore, HVLNMs is recommended as one of the adverse pathological features indicating completion thyroidectomy following lobectomy in NCCN clinical practice guidelines in oncology (thyroid carcinoma, version 3.2021) (7). However, the diagnostic performance of preoperative US or computer tomography in predicting lymph node metastasis (LNM) in the central compartment is unsatisfactory for now, which is the most frequently involved level in PTC (8, 12, 22). Thus, it may be valuable to develop a predictive model in evaluating the status of lymph nodes and predicting HVLNM in the central compartment, which may help surgeons and patients draw up individual treatment plans preoperatively and reduce reoperation risk. In our research, we developed a nomogram based on preoperative clinical features, in which the AUC was 0.821 (95% CI, 0.768–0.875) in predicting HVCLNMs. These results validate the idea that our nomogram provides good discrimination ability for detecting HVCLNMs in PTC patients (23, 24). Furthermore, the nomogram is easy to use, repeated, and economical, especially in the undeveloped area. In the 2015 ATA guidelines, lateral neck compartmental lymph node dissection was recommended for the patients with biopsy-proven metastatic lateral cervical lymphadenopathy (8). Thus, in this cohort, lateral neck dissection was not performed for the patients. During the follow-up period, the lateral neck lymph node should be actively surveilled for the patients with HVCLNMs. If lateral neck lymph node recurrence increases significantly in patients with HVCLNMs, elective neck dissection should be considered in these cases.
V-raf murine sarcoma viral oncogene homolog B1 (BRAF) mutations play roles in tumor cell proliferation and differentiation (25). BRAF V600E is the most common oncogene in PTC, which is reported to be correlated with some clinicopathological features in some studies (26, 27). In the research by Shi et al., a cohort of 126 patients was retrospectively analyzed and BRAF mutation status was found to be significantly associated with tumor size and LNM (28). A meta-analysis, including 3,437 patients, showed that the BRAF V600E mutation was correlated with some aggressive clinicopathological features, such as tumor multifocality, extrathyroidal extension, lymph node metastases, and advanced stage of PTMC (29). Similarly, Chen et al. reported that BRAF (V600E) mutation was an independent risk factor for central lymph node metastasis (CLNM), together with size </=10mm, microcalcification, internal vascularity, and capsule contact or involvement in preoperative ultrasound examination (30). Although several studies reported the association between BRAF (V600E) mutation and CLNM, no study focused on the predictive value of BRAF (V600E) mutation for HVCLNMs and the assistance in clinical decision-making of thyroidectomy. In our research, positive BRAF V600E mutation in PTC was proved to be able to predict high-volume CLNM independently with an odds ratio of 14.363 (95% CI, 2.847–72.463), which indicated that the preoperative BRAF V600E mutation test is useful in risk stratification of HVCLNMs and could assist in the management of PTC.
Age was reported to be associated with LNM in several studies previously (21, 31–35). Liu et al. included a total of 48,166 PTC and concluded that LNM rate decreased with age, especially women (p < 0.0001) (31). Luo et al. included 1,031 patients with PTMC and reported that male, age (</= 40 years), tumor largest diameter (>/= 5 mm), multifocal, non-uniform echoic distribution, the sum of the maximum diameter of multifocal in a unilateral lobe (>/= 8.5 mm), and tumors in the lower pole location were risk factors of CLNM in papillary thyroid microcarcinoma (PTMC) (32). Another study about PTMC similarly reported that young (<40 years old) and male patients were independent risk factors for large‐volume LNM (>5 metastatic lymph nodes) (21). In our study, the analysis drew a similar conclusion that young age (</=35 years) was an independent risk factor for high-volume CLNM, which could effectively predict HVCLNMs, together with BRAF V600E mutation, nodule diameter, and nodule calcification in US.
Our study has several limitations. Firstly, in our study, we only focused on high-volume CLNM. For the patients with high risk of HVCLNMs, total thyroidectomy should be recommended more strongly. Otherwise, the risk of other adverse features, such as macroscopic multifocal disease and vascular invasion, should be assessed at the same time, which would assist in determining surgical procedure, total thyroidectomy versus thyroid lobectomy, for the patients with low risk of HVCLNMs. Secondly, the nomogram was assessed only using the internal validation method, and this might have some adverse impact on the application in other centers. In the near future, the diagnostic performance of the nomogram may be further improved via multicentral research with a large sample.
Conclusion
The preoperative nomogram performed well in discriminating the patients with or without high-volume CLNM (equal to or more than 5 lymph nodes) in papillary thyroid carcinoma, which is one of the indications for completion thyroidectomy after thyroid lobectomy.
Data Availability Statement
The datasets used during the current study are available in Mendeley Data, V1, doi: 10.17632/sd59nt5dty.1.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Sun Yat-sen Memorial Hospital, Sun Yat-sen University. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author Contributions
PL: Conceptualization, Methodology, Software, Formal analysis, Investigation, Data Curation, and Writing—Original Draft. FL: Methodology, Formal analysis, Investigation, Resources, and Writing—Original Draft. JR: Conceptualization, Formal analysis, Investigation, Resources, Data Curation, and Writing—Original Draft. PH: Validation, Investigation, Writing—Original Draft, and Funding acquisition. JL: Conceptualization, Validation, Investigation, Resources, Writing—Original Draft, and Funding acquisition. RC: Validation, Formal analysis, Investigation, Writing—Original Draft, and Visualization. BL: Methodology, Resources, Writing—Review and Editing, Supervision, and Project administration. NO: Conceptualization, Methodology, Writing—Review and Editing, Supervision, and Project administration. XH: Conceptualization, Methodology, Resources, Data Curation, Writing—Review and Editing, Supervision, and Funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Grants from the Sun Yat-Sen University Clinical Research 5010 Program (Grant 2010008), the National Natural Science Foundation of China (Nos. 81872193, 81702697, and 81903043), and the Natural Science Foundation of Guangdong Province (No. 2018A030310086). The sponsors were not involved in research activities, such as study design, analysis, and interpretation of data.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.753678/full#supplementary-material
Supplementary Figure S1 | Correlation analysis of age at diagnosis and HVCLNMs rate in patients with papillary thyroid carcinoma. Error bars: 95% confidence interval. HVCLNMs, high-volume central lymph node metastasis.
Abbreviations
A/T, the anteroposterior dimension divided by its transverse dimension; AUC, area under receiver operator characteristic curve; CI, confidence interval; CLNM, central lymph node metastasis; FNAC, fine-needle aspiration cytologic examination; HVCLNMs, high-volume central lymph node metastasis (equal to or more than 5 involved lymph nodes) in central compartment; HVLNMs, high-volume lymph node metastasis (equal to or more than 5 lymph nodes); KWAK-TIRADS, thyroid imaging report and data system developed by Kwak et al.; LN, lymph node; LNM, lymph node metastasis; LOD, limit of detection; PTC, papillary thyroid carcinoma; PTMC, papillary thyroid microcarcinoma; ROC, receiver operator characteristic curve; TgAb, thyroglobulin antibody; TIRADS, thyroid imaging report and data system; TPOAb, thyroperoxidase antibody; US, ultrasonography.
References
1. Davies L, Welch HG. Thyroid Cancer Survival in the United States: Observational Data From 1973 to 2005. Arch Otolaryngol Head Neck Surg (2010) 136(5):440–4. doi: 10.1001/archoto.2010.55
2. Bilimoria KY, Bentrem DJ, Ko CY, Stewart AK, Winchester DP, Talamonti MS, et al. Extent of Surgery Affects Survival for Papillary Thyroid Cancer. Ann Surg (2007) 246(3):375–81; discussion 81-4. doi: 10.1097/SLA.0b013e31814697d9
3. Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, et al. Outcomes of Patients With Differentiated Thyroid Carcinoma Following Initial Therapy. Thyroid (2006) 16(12):1229–42. doi: 10.1089/thy.2006.16.1229
4. Erdem E, Gülçelik MA, Kuru B, Alagöl H. Comparison of Completion Thyroidectomy and Primary Surgery for Differentiated Thyroid Carcinoma. Eur J Surg Oncol (2003) 29(9):0–749. doi: 10.1016/j.ejso.2003.08.006
5. Tan MP, Agarwal G, Reeve TS, Barraclough BH, Delbridge LW. Impact of Timing on Completion Thyroidectomy for Thyroid Cancer. Br J Surg (2002) 89(6):802–4. doi: 10.1046/j.1365-2168.2002.02068.x
6. Untch BR, Palmer FL, Ganly I, Patel SG, Michael TR, Shah JP, et al. Oncologic Outcomes After Completion Thyroidectomy for Patients With Well-Differentiated Thyroid Carcinoma. Ann Surg Oncol (2014) 21(4):1374–8. doi: 10.1245/s10434-013-3428-1
7. National Comprehensive Cancer Network. (NCCN) Clinical Practice Guidelines in Oncology. Thyroid Carcinoma, Version 3. Plymouth Meeting, PA: National Comprehensive Cancer Network (2021).
8. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association Management Guidelines for Adult Patients With Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid (2016) 26(1):1–133. doi: 10.1089/thy.2015.0020
9. Rafferty MA, Goldstein DP, Rotstein L, Asa SL, Panzarella T, Gullane P, et al. Completion Thyroidectomy Versus Total Thyroidectomy: Is There a Difference in Complication Rates? An Analysis of 350 Patients. J Am Coll Surg (2007) 205(4):602–7. doi: 10.1016/j.jamcollsurg.2007.05.030
10. Leboulleux S, Rubino C, Baudin E, Caillou B, Hartl DM, Bidart JM, et al. Prognostic Factors for Persistent or Recurrent Disease of Papillary Thyroid Carcinoma With Neck Lymph Node Metastases and/or Tumor Extension Beyond the Thyroid Capsule at Initial Diagnosis. J Clin Endocrinol Metab (2005) 90(10):5723–9. doi: 10.1210/jc.2005-0285
11. Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A Novel Classification System for Patients With PTC: Addition of the New Variables of Large (3 Cm or Greater) Nodal Metastases and Reclassification During the Follow-Up Period. Surgery (2004) 135(2):139–48. doi: 10.1016/S0039-6060(03)00384-2
12. Xing Z, Qiu Y, Yang Q, Yu Y, Liu J, Fei Y, et al. Thyroid Cancer Neck Lymph Nodes Metastasis: Meta-Analysis of US and CT Diagnosis. Eur J Radiol (2020) 129:109103. doi: 10.1016/j.ejrad.2020.109103
13. Kwak JY, Han KH, Yoon JH, Moon HJ, Son EJ, Park SH, et al. Thyroid Imaging Reporting and Data System for US Features of Nodules: A Step in Establishing Better Stratification of Cancer Risk. Radiology (2011) 260(3):892–9. doi: 10.1148/radiol.11110206
14. Farrell E, Heffron C, Murphy M, O'Leary G, Sheahan P. Impact of Lymphocytic Thyroiditis on Incidence of Pathological Incidental Thyroid Carcinoma. Head Neck (2017) 39(1):122–7. doi: 10.1002/hed.24544
15. Caturegli P, De Remigis A, Rose NR. Hashimoto Thyroiditis: Clinical and Diagnostic Criteria. Autoimmun Rev (2014) 13(4-5):391–7. doi: 10.1016/j.autrev.2014.01.007
16. Li X, Li E, Du J, Wang J, Zheng B. BRAF Mutation Analysis by ARMS-PCR Refines Thyroid Nodule Management. Clin Endocrinol (2019) 91(6):834–41. doi: 10.1111/cen.14079
17. Yan C, Huang M, Li X, Wang T, Ling R. Relationship Between BRAF V600E and Clinical Features in Papillary Thyroid Carcinoma. Endocr Connect (2019) 8(7):988–96. doi: 10.1530/EC-19-0246
18. David W, Hosmer J, Lemeshow S, Sturdivant RX. Applied Logistic Regression. 3rd Edition. Hoboken, New Jersey: John Wiley & Sons, Inc (2013).
19. Iasonos A, Schrag D, Raj GV, Panageas KS. How to Build and Interpret a Nomogram for Cancer Prognosis. J Clin Oncol (2008) 26(8):1364–70. doi: 10.1200/JCO.2007.12.9791
20. Gulcelik MA, Kuru B, Dincer H, Camlibel M, Yuksel UM, Yenidogan E, et al. Complications of Completion Versus Total Thyroidectomy. Asian Pac J Cancer Prev (2012) 13(10):5225–8. doi: 10.7314/APJCP.2012.13.10.5225
21. Oh HS, Park S, Kim M, Kwon H, Song E, Sung TY, et al. Young Age and Male Sex Are Predictors of Large-Volume Central Neck Lymph Node Metastasis in Clinical N0 Papillary Thyroid Microcarcinomas. Thyroid (2017) 27(10):1285–90. doi: 10.1089/thy.2017.0250
22. So YK, Son YI, Hong SD, Seo MY, Baek CH, Jeong HS, et al. Subclinical Lymph Node Metastasis in Papillary Thyroid Microcarcinoma: A Study of 551 Resections. Surgery (2010) 148(3):526–31. doi: 10.1016/j.surg.2010.01.003
23. Swets JA. Measuring the Accuracy of Diagnostic Systems. Science (New York NY) (1988) 240(4857):1285–93. doi: 10.1126/science.3287615
24. Greiner M, Pfeiffer D, Smith RD. Principles and Practical Application of the Receiver-Operating Characteristic Analysis for Diagnostic Tests. Prev Vet Med (2000) 45(1-2):23–41. doi: 10.1016/S0167-5877(00)00115-X
25. Kondo T, Ezzat S, Asa SL. Pathogenetic Mechanisms in Thyroid Follicular-Cell Neoplasia. Nat Rev Cancer (2006) 6(4):292–306. doi: 10.1038/nrc1836
26. Xing M. Molecular Pathogenesis and Mechanisms of Thyroid Cancer. Nat Rev Cancer (2013) 13(3):184–99. doi: 10.1038/nrc3431
27. Xing M, Liu R, Liu X, Murugan AK, Zhu G, Zeiger MA, et al. BRAF V600E and TERT Promoter Mutations Cooperatively Identify the Most Aggressive Papillary Thyroid Cancer With Highest Recurrence. J Clin Oncol (2014) 32(25):2718–26. doi: 10.1200/JCO.2014.55.5094
28. Shi CL, Sun Y, Ding C, Lv YC, Qin HD. Correlation Between the BRAF V600E Mutation Status and the Clinicopathologic Features of Papillary Thyroid Carcinoma. Genet Mol Res (2015) 14(3):7377–85. doi: 10.4238/2015.July.3.13
29. Li F, Chen G, Sheng C, Gusdon AM, Huang Y, Lv Z, et al. BRAFV600E Mutation in Papillary Thyroid Microcarcinoma: A Meta-Analysis. Endocr Relat Cancer (2015) 22(2):159–68. doi: 10.1530/ERC-14-0531
30. Chen J, Li XL, Zhao CK, Wang D, Wang Q, Li MX, et al. Conventional Ultrasound, Immunohistochemical Factors and BRAF(V600E) Mutation in Predicting Central Cervical Lymph Node Metastasis of Papillary Thyroid Carcinoma. Ultrasound Med Biol (2018) 44(11):2296–306. doi: 10.1016/j.ultrasmedbio.2018.06.020
31. Liu Y, Wang Y, Zhao K, Li D, Chen Z, Jiang R, et al. Lymph Node Metastasis in Young and Middle-Aged Papillary Thyroid Carcinoma Patients: A SEER-Based Cohort Study. BMC Cancer (2020) 20(1):181. doi: 10.1186/s12885-020-6675-0
32. Luo Y, Zhao Y, Chen K, Shen J, Shi J, Lu S, et al. Clinical Analysis of Cervical Lymph Node Metastasis Risk Factors in Patients With Papillary Thyroid Microcarcinoma. J Endocrinol Invest (2019) 42(2):227–36. doi: 10.1007/s40618-018-0908-y
33. Liu C, Xiao C, Chen J, Li X, Feng Z, Gao Q, et al. Risk Factor Analysis for Predicting Cervical Lymph Node Metastasis in Papillary Thyroid Carcinoma: A Study of 966 Patients. BMC Cancer (2019) 19(1):622. doi: 10.1186/s12885-019-5835-6
34. Ji YB, Yoo HS, Song CM, Park CW, Lee CB, Tae K. Predictive Factors and Pattern of Central Lymph Node Metastasis in Unilateral Papillary Thyroid Carcinoma. Auris Nasus Larynx (2016) 43(1):79–83. doi: 10.1016/j.anl.2015.09.005
Keywords: thyroid papillary carcinoma, lymphatic metastasis, neck, nomograms, reoperation
Citation: Lin P, Liang F, Ruan J, Han P, Liao J, Chen R, Luo B, Ouyang N and Huang X (2021) A Preoperative Nomogram for the Prediction of High-Volume Central Lymph Node Metastasis in Papillary Thyroid Carcinoma. Front. Endocrinol. 12:753678. doi: 10.3389/fendo.2021.753678
Received: 05 August 2021; Accepted: 26 November 2021;
Published: 22 December 2021.
Edited by:
Leandro Luongo Matos, Universidade de São Paulo, BrazilReviewed by:
Ana Kober Leite, Hospital das Clínicas da Faculdade de Medicina da USP, BrazilErivelto Martinho Volpi, Centro de Referencia no Ensino do Diagnóstico por Imagem (CETRUS), Brazil
Copyright © 2021 Lin, Liang, Ruan, Han, Liao, Chen, Luo, Ouyang and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoming Huang, aHhtaW5nQG1haWwuc3lzdS5lZHUuY24=; Nengtai Ouyang, b3V5bnRAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
 Peiliang Lin1,2†
Peiliang Lin1,2† Faya Liang
Faya Liang Ping Han
Ping Han Jianwei Liao
Jianwei Liao Renhui Chen
Renhui Chen Baoming Luo
Baoming Luo Xiaoming Huang
Xiaoming Huang