
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Endocrinol. , 17 September 2021
Sec. Obesity
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.744710
This article is part of the Research Topic Non-Alcoholic Steatohepatitis (NASH) – Metabolic Contributors and Therapeutic Targets View all 25 articles
 Jiang Deng1
Jiang Deng1 Yonghong Zhang2
Yonghong Zhang2 Limei Bu3
Limei Bu3 Haitao Shi4
Haitao Shi4 Hailing Tang5
Hailing Tang5 Shenhao Wang4
Shenhao Wang4 Qian Wang6
Qian Wang6 Shuangsuo Dang1
Shuangsuo Dang1 Ming Li4†
Ming Li4† Zhiyi Han2†
Zhiyi Han2† Xiaolan Lu3,4*†
Xiaolan Lu3,4*†Background and Aims: There are few studies on non-obese fatty liver disease, the aims of this study was to analyze its prevalence, popular trends, and associated and predictive factors, so as to provide reference for its prevention and treatment.
Methods: Individuals with complete data of body mass index, sex, age, and abdominal ultrasound in Karamay Central Hospital from 2009 to 2016 were selected to analyze the prevalence and popular trends of non-obese fatty liver disease (body mass index <24 kg/m2), and associated and predictive factors.
Results: Between 2009 and 2016, a total of 191,555 medical check-ups were included. The prevalence of non-obese fatty liver disease increased from 1.9% to 5.1% among general medical examinants (P<0.001), increased from 4.6% to 11.7% in non-obese individuals (P<0.001). Compared with the non-obese control group, the levels of age, body mass index, blood pressure, fasting blood glucose, triglycerides, total cholesterol and uric acid in the non-obese fatty liver group were higher (P<0. 05). Even among non-obese subjects, elevated body mass index was associated with a 0.63-fold increased risk for non-obese fatty liver disease (P<0.001, odds ratio=1.63, 95% confidence interval 1.54-1.72) for every one-unit increase in body mass index. The most common abnormal indicator of non-obese fatty liver disease was elevated triglycerides (44.2%), which was also the best predictor of non-obese fatty liver disease (area under the curve =0.795) in non-obese physical examinators.
Conclusions: The prevalence of non-obese fatty liver disease was high and increasing rapidly in Karamay. Triglycerides is the best predictor of non-obese fatty liver in non-obese physical examinators.
In the past decade, the prevalence of fatty liver disease (FLD) has increased significantly (1–3). In China, non-alcoholic fatty liver disease (NAFLD) is more common than alcoholic FLD, rising from 18% to 29.2% (4), and its prevalence has been consistent with the prevalence of metabolic diseases such as type 2 diabetes mellitus and obesity. According to Chinese standards, the prevalence of being overweight and being obese in adults are 34.3% and 16.4% (5), respectively, and the prevalence of NAFLD in obese individuals is as high as 60%-90% (6). Although the association between high body mass index (BMI) and FLD has received widespread attention (7), there are few studies on non-obese FLD.
Some of the few existing relevant studies are mostly meta-analyses based on the general population, providing a good picture of the global distribution of non-obese FLD (8, 9). However, these studies did not provide the popular trends of non-obese FLD, nor did they propose how to screen it from a large number of people by simple indicators. To address the gaps on popular trends of non-obese FLD over the past decade, describe its characteristics, and identify its indicators in low-resource settings, a long-term observation on a fixed population is necessary.
As such, this study analyzed the laboratory data and physical examination data of Karamay Central Hospital from 2009 to 2016. Different from other studies that focus on the prevalence and outcome of non-obese FLD, this study is the first to analyze the popular trends and predictors of non-obese FLD, with more emphasis on its screening and prevention.
A total of 191,555 medical check-ups in Karamay Central Hospital from 2009 to 2016 were analyzed. The city of Karamay, located in northwest China, is an economically developed city that produces oil, with a permanent population of approximately 0.4 million. Karamay Central Hospital is the only large-scale comprehensive hospital in the city with a wide physical examination coverage. It services local residents and employees who consider the medical examiner as a representative of the area.
Individuals with complete data of BMI, sex, age, and abdominal ultrasound in Karamay Central Hospital from 2009 to 2016 were selected to analyze the popular trends of non-obese FLD. Non-obese individuals with complete data of BMI, gender, age, abdominal ultrasound, systolic blood pressure (SBP), diastolic blood pressure (DBP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), total bilirubin (TBIL), fasting blood glucose (FBG), triglycerides (TG), total cholesterol (TC), and blood uric acid (UA) were selected for analysis independent influencing and predictive factors of non-obese FLD in 2016. This study was approved by the Ethics Committee of Karamay Central Hospital.
According to China’s criteria for diagnosing overweight and obesity, the diagnosis of FLD in a patient with BMI < 24 kg/m2 is considered non-obese FLD. According to the standards of Karamay Central Hospital, ALT >40 U/L, AST >40 U/L, TC >5.69 mmol/L, TG >1.83 mmol/L, FBG >6.2 mmol/L, and TBIL >17.2 U mol/L are elevated in individuals with non-obese FLD.
Counting data were expressed as ratios, Chi-square exact test was used for comparison. The continuous variables were tested by the t-test or the Mann-Whitney U test. Logistic regression was used for multivariate analysis. The receiver operating characteristic (ROC) curve was used to evaluate the diagnostic efficacy of some meaningful indicators, including BMI, FBG, SBP, TC, ALT and TG, and the differences were considered statistically significant at P < 0.05. The IBM SPSS 23.0 Statistic Software (SPSS Inc., Armonk, NY, USA) was used for all statistical analyses.
Between 2009 and 2016, a total of 191,555 medical check-ups were included. The prevalence of non-obese FLD increased from 1.9% to 5.1%, showing a 1.7-fold increase (P<0.001). More specifically, prevalence increased from 1.9% to 5.4% in males and from 1.8% to 4.8% in females. In general physical examination subjects, the prevalence of non-obese FLD showed no consistent difference between men and women, sometimes higher in men, and sometimes the difference was not statistically significant (Table 1).
In the non-obese examinees group, the prevalence of non-obese FLD increased from 2009 to a peak in 2012 before decreasing. From 2009 to 2016, the prevalence of non-obese FLD increased from 4.6% to 11.7%, showing a 1.5-fold increase (P<0.001). More specifically, prevalence increased 2.1 times, from 5.8% to 17.8%, in males, and 1.5 times, from 3.1% to 7.9%, in females. The prevalence was statistically higher in males than in females (P<0.001) (Table 2).
In 2016, complete data of BMI, sex, age, and abdominal ultrasound was obtained for 40,232 individuals, and the prevalence of FLD was 31.9% (12834/40232), prevalence was higher in males than in females (P<0.001), with 39.3% (9171/23338) males and 21.7% (3663/16894) females diagnosed with FLD. The prevalence of FLD in non-obese, overweight, and obese subjects was 9.0%, 39.6%, and 70.8%, respectively, and the differences were statistically significant (P<0.001) (Table 3).
The prevalence of non-obese FLD were 5.3%, 13.6%, and 16.5% in individuals aged <45 years, 45-55 years, and >55 years, respectively, and the differences were statistically significant (P<0.001). Before the age of 55, prevalence was higher in men. However, it was significantly higher in post-menopausal women than in men of the same age group(P<0.001) (Table 4).
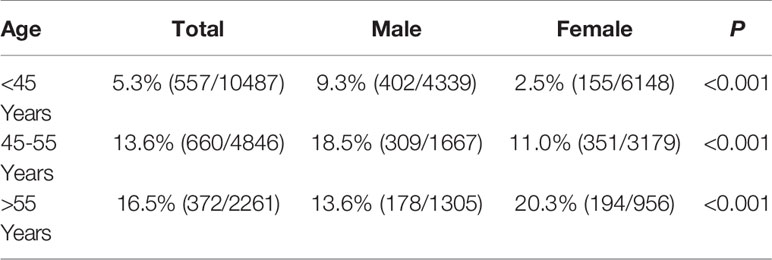
Table 4 Prevalence of non-obese FLD among non-obese physical examinees of different age groups in 2016 [%(n+/n)].
In 2016, 33,195 individuals with complete data of abdominal ultrasound, age, sex, BMI, SBP, DBP, ALT, FBG, TC, TG, UA, and TBIL had physical examinations in Karamay Central Hospital. Among them, 14,375 were considered non-obese. Compared with the non-obese control group, the levels of age, BMI, systolic blood pressure, diastolic blood pressure, ALT, TBIL, FBG, TG, TC and UA in the non-obese fatty liver group were higher (P<0. 05) (Table 5).
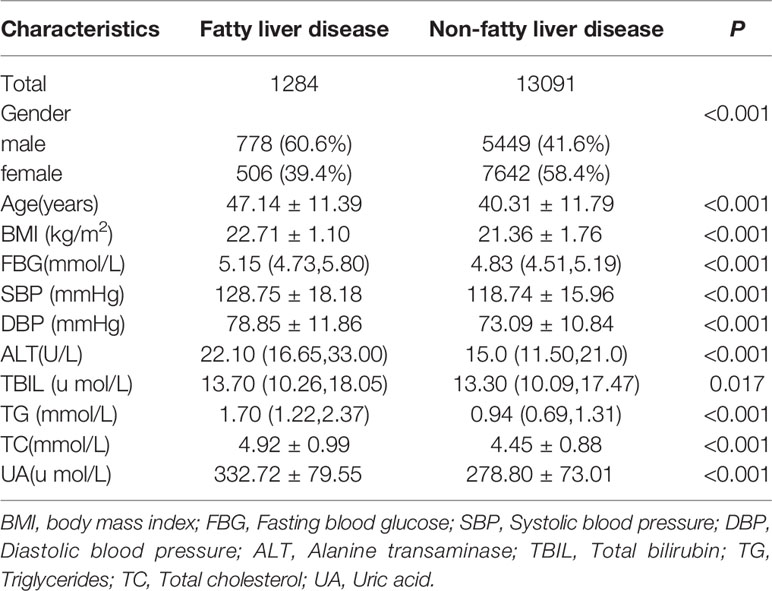
Table 5 Comparison of baseline data of the non-FLD group and the non-obese FLD group among the non-obese physical examinees in 2016.
AST/ALT >2 was found in 23 (1.8%) of 1,284 non-obese individuals, and AST >40 U/L was found in eight of them. ALT/AST >1 was detected in 793 individuals (61.8%), and 199 of them had ALT > 40 U/L. Based on these preliminary calculations, the main form of FLD seen in the hospital was NAFLD, with alcoholic FLD rarely diagnosed.
Characteristics of 1,284 individuals with non-obese FLD were analyzed, and the abnormal rates, shown in decreasing levels, were as follows: increased TG in 567 individuals (44.2%), increased TBIL in 374 individuals (29.1%), increased SBP in 330 individuals (25.7%), increased TC in 271 individuals (21.1%), increased DBP in 217 individuals (16.9%), increased FBG in 216 individuals (16.8%), increased ALT in 212 individuals (16.5%), and increased UA in 166 individuals (12.9%). Normal TC, TG, SBP, DBP, FBG, and UA were only seen in 380 individuals (29.6%), and only 257 of them had normal ALT and TBIL.
Logistic regression analysis showed that sex, age, BMI, FBG, SBP, ALT, TC, TG, and UA were independent influencing factors for the occurrence of non-obese FLD in non-obese physical examinees in Karamay Central Hospital in 2016 (P<0. 05). In non-obese individuals, elevated BMI was associated with a 0.63-fold increased risk for non-obese FLD (P<0. 001, odds ratio [OR]=1.63, 95% confidence interval [CI]: 1.54-1.72) for every one-unit increase in BMI (Table 6).
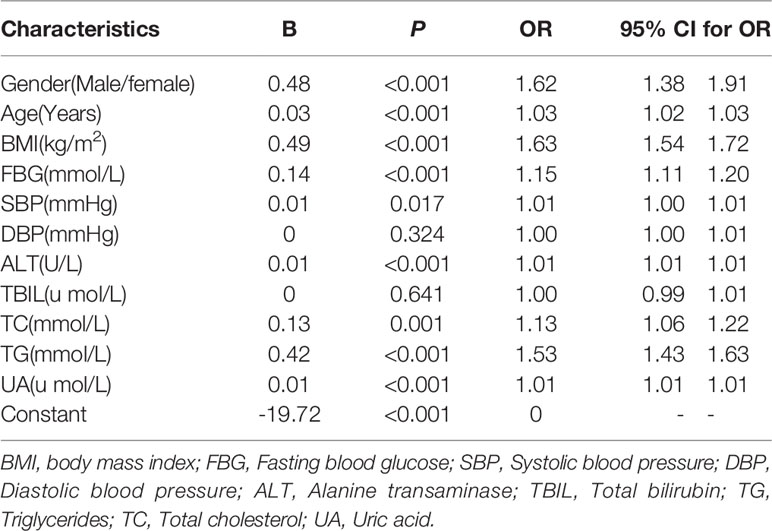
Table 6 Logistic regression analysis of non-obese fatty liver disease in 2016 non-obese physical examination subjects.
BMI, FBG, SBP, ALT, TC, and TG were analyzed by the ROC curve. Results showed that the area under the curve (AUC) value of triglycerides was the highest, which was 0.795, illustrating that TG had the best diagnostic efficiency. The maximum Youden index was taken as the critical value, and the sensitivity and specificity determined at this time were 74.1% and 72.2%, respectively. In addition, the AUC value of TG combined with ALT was 0.815.
Therefore, considering the absence of physical examination and/or imaging and based on a large volume of physical indicators analyzed, TG is the best indicator in screening non-obese FLD among non-obese physical examinees. Table 7 and Figure 1.
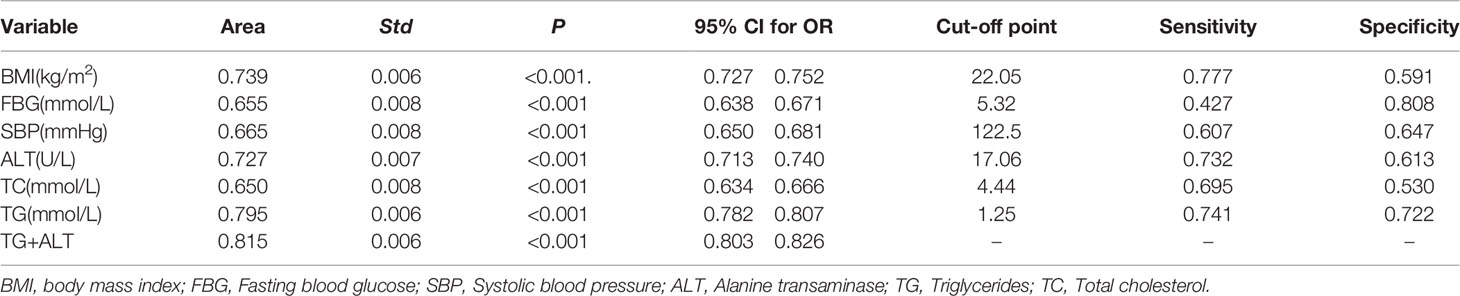
Table 7 The ROC curve was used to evaluate the diagnostic efficacy of index in the diagnosis of non-obese fatty liver disease.
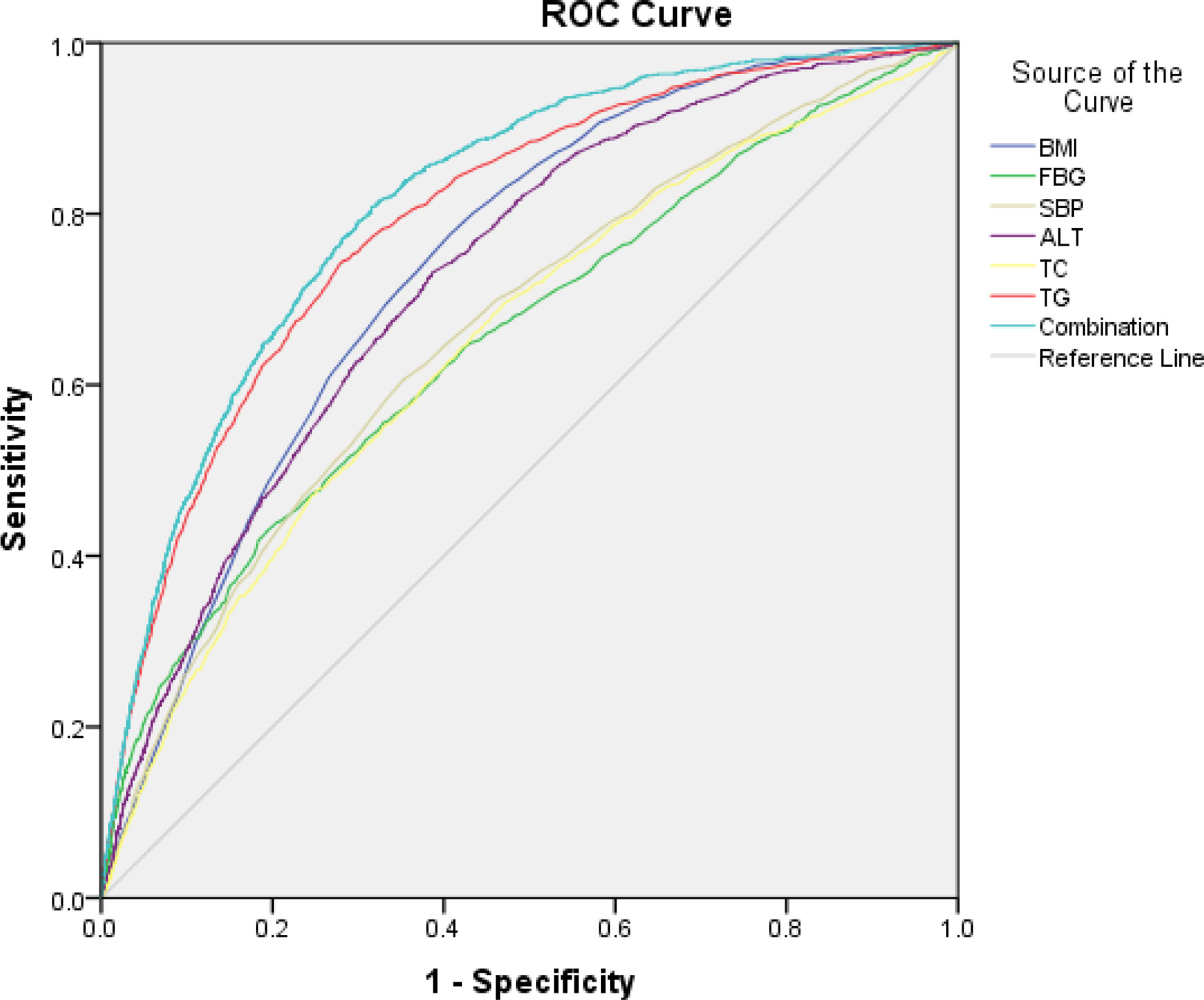
Figure 1 The ROC curve was used to evaluate the diagnostic efficacy of index in the diagnosis of non-obese FLD.
This study shows that, between 2009 and 2016, the prevalence of non-obese FLD increased from 1.9% to 5.1% in general medical examiner, the prevalence of non-obese FLD increased from 4.6% to 11.7% in the non-obese examinees group. A meta-analysis from China showed that the prevalence of NAFLD in the non-obese population was 10.8% (95% CI: 9.0%-12.6%) (4). Another meta-analysis from 14 countries showed that the global prevalence of lean NAFLD was 4.1% (95% CI: 3.4%-4.8%) in the general population and 9.7% (95% CI: 7.7%-11.8%) in the lean population (8). These figures are consistent with the results of our study. In 2020, a meta-analysis covering 24 countries and regions showed that the overall proportion of non-obese NAFLD in the global NAFLD population was 40.8% (95% CI: 36.6-45.1). In addition, the prevalence of non-obese NAFLD in the general population was 12.1% (95% CI: 9.3-15.6) (9), which is much higher than the prevalence determined in other studies (1.9%-5.1%). The differences may be related to race, genetic polymorphism (PNPLA3, rs738409, etc.), regional differences, differences in diagnostic criteria, and other factors. Despite having the same BMIs, distribution of body fat varies by region and ethnicity; Asians store more visceral or abdominal fat at lower BMIs, which puts them at a higher risk for the disease (10). There is substantial evidence that Chinese may have a higher percentage of body fat and therefore have both a higher cardiovascular risk and all-cause mortality than Caucasians at the same BMIs (5).
The prevalence of lean FLD varies greatly among different ethnic groups and regions. In the overall population, the prevalence of lean NAFLD is the highest in Asia (4.8%), which is followed by Oceania (3.5%), North America (3.1%), and Europe (2.2%). The highest prevalence is seen in China (5.5%) (95% CI: 2.5-8.5%) and the lowest prevalence is in the United States (3.1%) (95% CI: 2.3-3.8%) (8). Heterogeneity in study area, study time, and study subjects are some factors that may cause these differences. Compared to the prevalence of lean NAFLD based on health screening (4.5%, 95% CI: 3.9 5.2%) and community-based studies (3.0%, 95% CI: 0.7 5.3%), population-based studies (5.7%, 95% CI: 3.6 7.7%) showed the highest prevalence (8).
Non-obese FLD can occur in non-obese individuals who have a normal BMI but have recently gained weight or increased their waist circumference. Some obese people lose a lot of weight too fast, leading to a large amount of fat decomposition, as the liver’s ability to process free fatty acids is limited, a large amount of fat accumulates in the liver, resulting in FLD. Obese people may also take a vegetarian diet to lose weight, reducing protein intake and subsequently lacking apolipoprotein, triglycerides cannot be transferred out of the liver without apolipoprotein, leading to the formation of malnutrition FLD (6). Other causes of FLD include uneven distribution of body fat, high visceral fat, and metabolic disorders.
BMI alone cannot be used as a preliminary screening index. Compared to subcutaneous fat and BMI, visceral obesity is a better indicator for developing NAFLD in non-obese individuals. Additionally, waist-to-hip ratio may actually be more likely to identify abdominal obesity than BMI. Besides these, neck circumference and body fat analysis can also be used to screen non-obese FLD (11). There are also studies that showed how the sagittal abdominal diameter (SAD) can reflect the ability of abdominal fat more accurately than waist circumference and BMI, especially in young and non-obese individuals. SAD exhibited a stronger correlation with risk factors of metabolic syndrome compared to waist circumference, waist-to-hip ratio, and BMI. As such, the combined diagnostic power of multiple indicators may be higher than that of a single indicator (12–14).
The risk for FLD differs among age groups and sex. Genetic factors and excessive nutrition are important reasons in diagnosing FLD in children (15). For adults, FLD is affected by hormones, lifestyle, work stress, genetics, and other factors (4). The prevalence of FLD in women increased significantly across time, the reasons may be related to the decline of ovarian function, the decrease of estrogen level, and the increased risk of metabolic diseases. Conversely, this increasing trend is not completely consistent in men. With increasing age, the body’s hormone level changes, activity decreases, and lipid metabolism function declines. Current research makes it clear that as age increases, airframe fat turnover evidently drops. In fact, in a male not following an abstinent diet, weight increases by 20% on average (16, 17). This may also be related to work and life stress.
Compared with the non-obese control group, the levels of age, BMI, systolic blood pressure, diastolic blood pressure, ALT, TBIL, FBG, TG, TC and UA in the non-obese fatty liver group were higher (P<0. 05). The results are consistent with previous studies based on the general population and non-obese individuals (4, 8, 18, 19). NAFLD is a manifestation of metabolic syndrome involving the liver (6), and metabolic abnormalities therefore increase the risk of FLD. For the general population, metabolic abnormalities such as high BMI and triglycerides are the important independent risk factors associated with the occurrence of FLD. Although people with lean NAFLD may have better metabolic syndrome-related indicators and a lower incidence of metabolic complications compared to overweight and obese individuals with NAFLD (8, 20), lean FLD remains to be associated with a higher risk for metabolic disorders (21, 22). This study showed that in 1,284 people with non-obese FLD, the most common abnormal metabolic index was high triglycerides (44.2%), and its AUC value was the highest. Combined the results of logistic regression and ROC curve, it is difficult to identify FLD based on BMI in non-obese physical examinees who lacked diagnostic equipment or did not have access to imaging. Additionally, triglycerides appeared to be the best predictor of diagnosing FLD in non-obese physical examinees.
In addition to metabolic and genetic factors, muscle atrophy and loss of muscle strength (23, 24), unhealthy eating patterns (i.e., High cholesterol and fructose intake) (25), and changes in intestinal flora are also important influencing factors that do not only promote the occurrence of non-obese FLD but also aggravate its progression. Studies have shown that the decrease of butyrate-producing Eubacterium may play an important role in the development of NAFLD in non-obese individuals (26). In non-obese individuals, Ruminococcaceae and Veillonellaceae were the predominant microbiota associated with severe hepatic fibrosis (27). In a prospective cohort study of 307 individuals from Hong Kong, Leung et al, reported that non-obese individuals (23.5%) had a lower incidence of metabolic syndrome and a lower NAFLD activity score (28). Although non-obese NAFLD individuals have healthier metabolic profiles and less advanced fibrosis, their prognosis may be worse than obese NAFLD patients (25, 29). Results of a cohort study of 646 patients with biopsy-confirmed NAFLD showed that lean NAFLD patients were older, and had lower transaminase levels, lower fibrosis stage, and lower prevalence of non-alcoholic steatohepatitis than those with higher BMI. At a mean follow-up time of 19.9 years (0.4-40 years), compared with overweight patients, patients with lean NAFLD had no increased risk for overall mortality (hazard ratio [HR]=1.06, P=0.73) but had an increased risk for severe liver disease (HR=2.69, P=0.007) (20). Additionally, cardiovascular events are a major factor affecting the prognosis of patients with NAFLD, and all patients with NAFLD should therefore be assessed for the risk of cardiovascular events (6) to reduce the incidence of complications and adverse outcomes.
This was a large single-center retrospective study. Subjective data, such as history of alcohol consumption and diabetes, are hard to come by. Objective data such as fasting glucose and BMI were used to reflect the baseline data of patients. Unlike other studies, this study did not follow clinical outcomes, nor were visceral fat levels measured. In China and some other places, the main cause of end-stage liver disease is viral liver disease—not FLD (6), there were only 14 cases of cirrhosis and 4 cases of liver fibrosis among the 40,232 people who underwent ultrasound examination in 2016. Moreover, early FLD is a curable disease, more research should focus on attention, screening, prevention and treatment (22, 30). Large-scale measurements of visceral fat to determine the risk of developing non-obese FLD are difficult and impractical in terms of resources, which can instead be allocated to the prevention and treatment of non-obese FLD for better outcomes. Therefore, the focus of this study is to analyze the popular trends of non-obese FLD to attract people’s attention, and then analyze the characteristics of non-obese FLD, and find a simple but effective screening method, which can be applied to a large number of people.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
This study was approved by the Ethics Committee of Karamay Central Hospital.
JD, ZH, and XL conceived and designed the study. JD, YZ, LB, HS, HT, SD, and ML collected the data. JD analyzed the data. All authors contributed to the article and approved the submitted version. JD, ML, ZH, and XL wrote the manuscript.
1. Key Discipline Construction Project of Pudong Health and Family Planning Commission of Shanghai (Grant No. PWZxk 2017-27); 2. This study was supported by National Key Research and Development Program of China during the 13th Five-Year Plan Period (2018YFC1311504); 3.This work was supported by Talents Training Program of Pudong Hospital affiliated to Fudan University (YJRCJJ201801).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Thanks to the staff of Karamay Central Hospital for their help in this study.
FLD, fatty liver disease; NAFLD, non-alcoholic fatty liver disease; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; AST, aspartate aminotransferase; ALT, alanine aminotransferase; TBIL, total bilirubin; FPG,fasting blood glucose; TG, triglycerides; TC, total cholesterol; UA,uric acid; ROC,receiver operating characteristic curve; OR, odds ratio; AUC, area under the curve; CI, confidence interval; SAD, sagittal abdominal diameter; HR, hazard ratio.
1. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology (2016) 64(1):73–84. doi: 10.1002/hep.28431
2. Wang XJ, Malhi H. Nonalcoholic Fatty Liver Disease. Ann Intern Med (2018) 169(9):Itc65–itc80. doi: 10.7326/aitc201811060
3. Tomic D, Kemp WW, Roberts SK. Nonalcoholic Fatty Liver Disease: Current Concepts, Epidemiology and Management Strategies. Eur J Gastroenterol Hepatol (2018) 30(10):1103–15. doi: 10.1097/meg.0000000000001235
4. Zhou F, Zhou J, Wang W, Zhang XJ, Ji YX, Zhang P, et al. Unexpected Rapid Increase in the Burden of NAFLD in China From 2008 to 2018: A Systematic Review and Meta-Analysis. Hepatology (2019) 70(4):1119–33. doi: 10.1002/hep.30702
5. Pan XF, Wang L, Pan A. Epidemiology and Determinants of Obesity in China. Lancet Diabetes Endocrinol (2021) 9(6):373–92. doi: 10.1016/s2213-8587(21)00045-0
6. National Workshop on Fatty Liver and Alcoholic Liver Disease. Chinese Society of Hepatology, Chinese Medical Association Fatty Liver Expert Committee, Chinese Medical Doctor Association.Guidelines of Prevention and Treatment for Nonalcoholic Fatty Liver Disease: A 2018 Update. Chin J Hepatol (2018) 26(03):195–203. doi: 10.3760/cma.j.issn.1007-3418.2018.03.008
7. Fan JG, Kim SU, Wong VW. New Trends on Obesity and NAFLD in Asia. J Hepatol (2017) 67(4):862–73. doi: 10.1016/j.jhep.2017.06.003
8. Lu FB, Zheng KI, Rios RS, Targher G, Byrne CD, Zheng MH. Global Epidemiology of Lean non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. J Gastroenterol Hepatol (2020) 35(12):2041–50. doi: 10.1111/jgh.15156
9. Ye Q, Zou B, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global Prevalence, Incidence, and Outcomes of Non-Obese or Lean Non-Alcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Lancet Gastroenterol Hepatol (2020) 5(8):739–52. doi: 10.1016/s2468-1253(20)30077-7
10. Caleyachetty R, Barber TM, Mohammed NI, Cappuccio FP, Hardy R, Mathur R, et al. Ethnicity-Specific BMI Cutoffs for Obesity Based on Type 2 Diabetes Risk in England: A Population-Based Cohort Study. Lancet Diabetes Endocrinol (2021) 9(7):419–26. doi: 10.1016/s2213-8587(21)00088-7
11. Ha Y, Seo N, Shim JH, Kim SY, Park JA, Han S, et al. Intimate Association of Visceral Obesity With Non-Alcoholic Fatty Liver Disease in Healthy Asians: A Case-Control Study. J Gastroenterol Hepatol (2015) 30(11):1666–72. doi: 10.1111/jgh.12996
12. Yim JY, Kim D, Lim SH, Park MJ, Choi SH, Lee CH, et al. Sagittal Abdominal Diameter Is a Strong Anthropometric Measure of Visceral Adipose Tissue in the Asian General Population. Diabetes Care (2010) 33(12):2665–70. doi: 10.2337/dc10-0606
13. Ohrvall M, Berglund L, Vessby B. Sagittal Abdominal Diameter Compared With Other Anthropometric Measurements in Relation to Cardiovascular Risk. Int J Obes Relat Metab Disord (2000) 24(4):497–501. doi: 10.1038/sj.ijo.0801186
14. Møller G, Ritz C, Kjølbæk L, Vuholm S, Korndal SK, Larsen TM, et al. Sagittal Abdominal Diameter and Waist Circumference Appear to be Equally Good as Identifiers of Cardiometabolic Risk. Nutr Metab Cardiovasc Dis (2021) 31(2):518–27. doi: 10.1016/j.numecd.2020.09.032
15. Zdanowicz K, Białokoz-Kalinowska I, Lebensztejn DM. Non-Alcoholic Fatty Liver Disease in Non-Obese Children. Hong Kong Med J (2020) 26(5):459–62. doi: 10.12809/hkmj198361
16. Arner P, Bernard S, Appelsved L, Fu KY, Andersson DP, Salehpour M, et al. Adipose Lipid Turnover and Long-Term Changes in Body Weight. Nat Med (2019) 25(9):1385–9. doi: 10.1038/s41591-019-0565-5
17. Palmisano BT, Zhu L, Eckel RH, Stafford JM. Sex Differences in Lipid and Lipoprotein Metabolism. Mol Metab (2018) 15:45–55. doi: 10.1016/j.molmet.2018.05.008
18. Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat Rev Gastroenterol Hepatol (2018) 15(1):11–20. doi: 10.1038/nrgastro.2017.109
19. Eshraghian A, Nikeghbalian S, Geramizadeh B, Kazemi K, Shamsaeefar A, Malek-Hosseini SA. Characterization of Biopsy Proven non-Alcoholic Fatty Liver Disease in Healthy Non-Obese and Lean Population of Living Liver Donors: The Impact of Uric Acid. Clin Res Hepatol Gastroenterol (2020) 44(4):572–8. doi: 10.1016/j.clinre.2019.09.002
20. Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Risk for Development of Severe Liver Disease in Lean Patients With Nonalcoholic Fatty Liver Disease: A Long-Term Follow-Up Study. Hepatol Commun (2018) 2(1):48–57. doi: 10.1002/hep4.1124
21. Zou ZY, Wong VW, Fan JG. Epidemiology of Nonalcoholic Fatty Liver Disease in Non-Obese Populations: Meta-Analytic Assessment of Its Prevalence, Genetic, Metabolic, and Histological Profiles. J Dig Dis (2020) 21(7):372–84. doi: 10.1111/1751-2980.12871
22. Li Y, Zheng R, Li J, Feng S, Wang L, Huang Z. Association Between Triglyceride Glucose-Body Mass Index and Non-Alcoholic Fatty Liver Disease in the Non-Obese Chinese Population With Normal Blood Lipid Levels: A Secondary Analysis Based on a Prospective Cohort Study. Lipids Health Dis (2020) 19(1):229. doi: 10.1186/s12944-020-01409-1
23. Shida T, Oshida N, Suzuki H, Okada K, Watahiki T, Oh S, et al. Clinical and Anthropometric Characteristics of Non-Obese Non-Alcoholic Fatty Liver Disease Subjects in Japan. Hepatol Res (2020) 50(9):1032–46. doi: 10.1111/hepr.13543
24. Kashiwagi K, Takayama M, Fukuhara K, Shimizu-Hirota R, Chu PS, Nakamoto N, et al. A Significant Association of Non-Obese Non-Alcoholic Fatty Liver Disease With Sarcopenic Obesity. Clin Nutr ESPEN (2020) 38:86–93. doi: 10.1016/j.clnesp.2020.05.025
25. Chrysavgis L, Ztriva E, Protopapas A, Tziomalos K, Cholongitas E. Nonalcoholic Fatty Liver Disease in Lean Subjects: Prognosis, Outcomes and Management. World J Gastroenterol (2020) 26(42):6514–28. doi: 10.3748/wjg.v26.i42.6514
26. Iwaki M, Kessoku T, Ozaki A, Kasai Y, Kobayashi T, Nogami A, et al. Gut Microbiota Composition Associated With Hepatic Fibrosis in Non-Obese Patients With Non-Alcoholic Fatty Liver Disease. J Gastroenterol Hepatol (2021) 36(8):2275–84. doi: 10.1111/jgh.15487
27. Lee G, You HJ, Bajaj JS, Joo SK, Yu J, Park S, et al. Distinct Signatures of Gut Microbiome and Metabolites Associated With Significant Fibrosis in non-Obese NAFLD. Nat Commun (2020) 11(1):4982. doi: 10.1038/s41467-020-18754-5
28. Leung JC, Loong TC, Wei JL, Wong GL, Chan AW, Choi PC, et al. Histological Severity and Clinical Outcomes of Nonalcoholic Fatty Liver Disease in Nonobese Patients. Hepatology (2017) 65(1):54–64. doi: 10.1002/hep.28697
29. Cruz A, Bugianesi E, George J, Day CP, Liaquat H, Charatcharoenwitthaya P, et al. 379 Characteristics and Long-Term Prognosis of Lean Patients With Nonalcoholic Fatty Liver Disease. Gastroenterology (2014) 146(5):S–909. doi: 10.1016/S0016-5085(14)63307-2
Keywords: obese, fatty liver disease, prevalence, risk factor, predictor
Citation: Deng J, Zhang Y, Bu L, Shi H, Tang H, Wang S, Wang Q, Dang S, Li M, Han Z and Lu X (2021) The Prevalence, Popular Trends, and Associated and Predictive Factors of Non-Obese Fatty Liver Disease. Front. Endocrinol. 12:744710. doi: 10.3389/fendo.2021.744710
Received: 20 July 2021; Accepted: 10 August 2021;
Published: 17 September 2021.
Edited by:
Magdalene K. Montgomery, The University of Melbourne, AustraliaReviewed by:
Aleksandra Klisic, Primary Health Care Center Podgorica, MontenegroCopyright © 2021 Deng, Zhang, Bu, Shi, Tang, Wang, Wang, Dang, Li, Han and Lu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaolan Lu, eGlhb2xhbl9sdUAxNjMuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.