- Department of Endocrinology and Metabolism, NHC Key Laboratory of Diagnosis and Treatment of Thyroid Diseases, Institute of Endocrinology, The First Affiliated Hospital of China Medical University, Shenyang, China
Objective: Thyroid nodules (TNs) are a common thyroid disorder that can be caused by many factors. Several studies have investigated the relationship between TNs and metabolic syndrome (MetS), but the role of sex and age remains controversial. The purpose of this paper was to analyze published data from all relevant studies to reliably estimate the relationship between TNs and MetS.
Methods: Thirteen articles were included in this study; articles were identified by searching for publications until July 2021 in PubMed, EMBASE, the Cochrane Library and the Web of Science. The outcomes are presented as the summary odds ratio (OR) and 95% confidence interval (CI) and the pooled prevalence and 95% CI.
Results: The TNs prevalence was significantly higher in MetS patients than in controls (OR 1.88, 95% CI 1.42-2.50, P < 0.0001) and was independent of sex (male: OR 1.53, 95% CI 1.20-1.94, P = 0.0006; female: OR 1.90, 95% CI 1.54-2.33, P < 0.00001; combined: OR 2.06, 95% CI 1.31-3.25, P = 0.002) and age (< 40 years old: OR 1.62, 95% CI 1.39-1.89, P < 0.0001; 40~50 years old: OR 2.14, 95% CI 1.49-3.08, P < 0.0001;50~60 years old: OR 1.50, 95% CI 1.08-2.07, P = 0. 01; 60 years old: OR 1.70, 95% CI 1.36-2.14, P < 0.00001); the pooled TNs prevalence in MetS patients was 45% (95% CI 36-54%). However, it has not yet been considered that MetS is related to TNs in people with iodine deficiency (OR 3.14, 95% CI 0.92-10.73, P = 0.07).
Conclusion: The meta-analysis results showed a strong correlation between TNs and MetS. Both male and female patients with MetS had an increased TNs prevalence. In addition, the prevalence was independent of age. However, MetS is not considered to be associated with TNs in iodine-deficient populations.
Introduction
Thyroid nodules (TNs) constitute one of the most common clinical thyroid disorders. A recent nationwide cross-sectional survey indicated that the prevalence of TNs in mainland China is as high as 20.43% (1). Approximately 5% of patients have palpable nodules, and up to 70% of patients have nodules that are found incidentally on ultrasonography (2), indicating the high prevalence of occult nodules. In addition, the prevalence of TNs is higher in women, elderly individuals, iodine-deficient individuals and those with radiation exposure than in their counterparts (3–5). Approximately 5%-10% of thyroid nodules are malignant (6, 7). The high prevalence of thyroid nodules may mean an increased possibility of thyroid cancer. Therefore, risk factors associated with TNs have received significant attention in recent years.
A variety of metabolic diseases are considered to be important risk factors for TNs (8–10), but this finding needs to be further validated. Some cross-sectional studies have shown a close association between thyroid disease and metabolic disorders (i.e., metabolic syndrome [MetS] and its components) (11–13). MetS is characterized by a cluster of abnormal metabolic parameters consisting of insulin resistance, central obesity, type 2 diabetes, impaired glucose tolerance, hyperinsulinemia, and dyslipidemia (14). The global prevalence of MetS is between 11.6% and 62.5% (15). One study indicated that the prevalence of MetS in mainland China was 28.6% (11). Studies have found that obesity and insulin resistance are strongly correlated with TNs (10, 16). However, the overall relationship between MetS and TNs is controversial, and whether the relationship is influenced by sex- or age- is still unclear. Some studies found that TNs were significantly related to an increased risk of MetS, and males and elderly people (≥60 years old) with MetS had a higher risk of TNs than females and younger people (<60 years old) (9). However, another study found that in the MetS population over 45 years old, the prevalence of TNs in women was one-third higher than that in men (38.5% versus 26%) (17). In addition, after adjustment for age, smoking and alcohol consumption, MetS was an independent risk factor for TNs in females but not in males (17). A relevant meta-analysis is currently needed to summarize and clarify the potential associations, especially among subgroups with different demographic characteristics.
This study is a comprehensive systematic review and meta-analysis of all published case-control and cross-sectional studies that evaluated the correlation between MetS and TNs and conducted further investigation in sub-analyses according to sex, age, iodine nutrition status, study type and diagnostic criteria.
Methods and Materials
Search Strategy
The criteria for conducting and reporting a meta-analysis of observational studies have been reported in extensive detail elsewhere (18). Two researchers (CZ and XG) independently searched for articles published until July 2021 in the PubMed (http://www.ncbi.nlm.nih.gov/pubmed), Web of Science (http://apps.webofknowledge.com), Cochrane Library (http://www.thecochranelibrary.com) and EMBASE (http://www.embase.com) databases. The search terms were as follows: (‘Nodule, Thyroid’ OR ‘Nodules, Thyroid’ OR ‘Thyroid Nodules’) AND (‘Metabolic Syndromes’ OR ‘Syndrome, Metabolic’ OR ‘Syndromes, Metabolic’). We limited the included studies to those published in English. The search and inclusion criteria were restricted to observational research. We also searched for and screened the full texts of references from relevant original papers to identify potentially eligible publications. The selection process is shown in Figure 1. The retrieved results in.nbib and.ris formats were exported from the databases and collated in a specific library in the reference manager software EndNote® version X9 (Thomson Reuters, New York, USA).
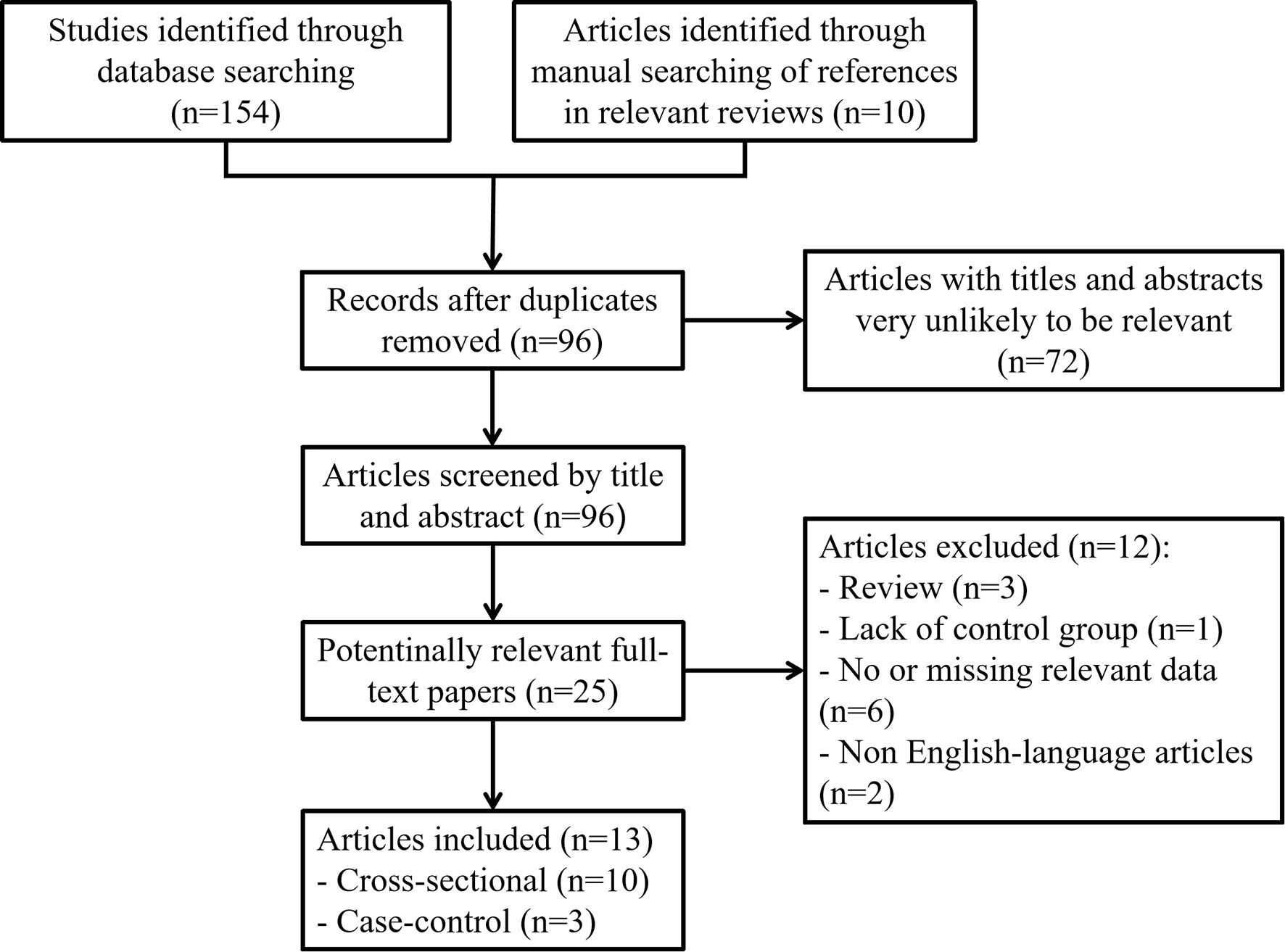
Figure 1 Flow chart showing the detailed inclusion or exclusion procedure. Thirteen independent articles were included in the meta-analysis.
Selection Criteria
We used EndNote® version X9 to exclude duplicate articles. When the title or summary information was not enough to judge eligibility, we read the full text. The study inclusion criteria were as follows: (1) the study design was observational, and the publication language was English; (2) all persons included in the exposure group were MetS patients who had undergone clinical evaluation for MetS; (3) all persons included in the control group were without MetS; (4) the outcomes of interest were TN prevalence in patients with MetS; and (5) sufficient information to calculate the combined prevalence or complete subgroup analysis based on factors such as sex, age and iodine nutrition status. When outcomes in the same population were reported in multiple articles, the most detailed report was included. In contrast, studies were immediately excluded if they met one of the following criteria: (1) the article was a case report, letter, review, editorial, or expert opinion; (2) missing necessary information; (3) missing necessary data to calculate prevalence; or (4) no explicit mention of MetS or diagnostic criteria for TNs.
Data Extraction
The following data were extracted by authors (CZ and XG): first, basic information (first author name, year of publication, location and research design); second, participant characteristics (sample size, number of cases and controls, age, sex, and iodine nutrition status); and third, MetS and TN diagnostic standards. In observational studies, the mean age and body mass index (BMI) in the different sexes were annotated (if any). Two examiners organized the extracted data through Microsoft Excel® spreadsheets, cross-checked the data and independently assessed the quality of each study based on the Newcastle-Ottawa Quality Assessment Scale (19) and the Agency for Healthcare Research and Quality (AHRQ) quality assessment criteria. According to the score for each study, it was classified as low (<4), medium (4-7) or high quality (>8). Any existing differences were discussed and resolved by the two authors until they finally reached a consensus. After assessment, all included studies were found to be of moderate or high quality (shown in Tables S1 and S2).
Statistical Analyses
We used Review Manager version 5.2 software to calculate odds ratios (ORs) and the corresponding 95% confidence intervals (95% CIs) to assess the strength of the association between TNs and the risk of MetS. We used the pooled prevalence and 95% CIs to assess the prevalence of TNs in MetS patients. The significance of the combined OR was determined by the Z test using the Mantel-Haenszel method, and a P value < 0.05 was considered significant. We used the Cochran Q test (P < 0.05 indicated statistical significance) and the I2 statistic to assess heterogeneity. I2 values of 25, 50, and 75% indicated low, moderate, and high heterogeneity, respectively. When a high degree of heterogeneity was found, a random-effects model was used to pool the results; if heterogeneity was low, a fixed-effect model was used to pool the results.
To assess the stability of the results, we performed a sensitivity analysis by omitting one report at a time and recomputing the pooled estimates of the remaining studies. Funnel plots were constructed to assess publication bias.
Results
Characteristics of the Included Studies
First, we searched the PubMed, EMBASE, Cochrane Library and Web of Science databases and searched for references in relevant reviews and found a total of 164 potentially relevant studies. In total, 96 items remained after removing duplicate records. By carefully screening the titles and abstracts, we selected 25 articles for further assessment. After reading the full texts and applying the inclusion and exclusion criteria, a total of 13 original studies were eventually included in the systematic review and meta-analysis (Figure 1). There were two types of studies, including Ten cross-sectional studies and three case-control studies. The basic characteristics of each study included in this meta-analysis are summarized in Table 1.
Meta-Analysis Results
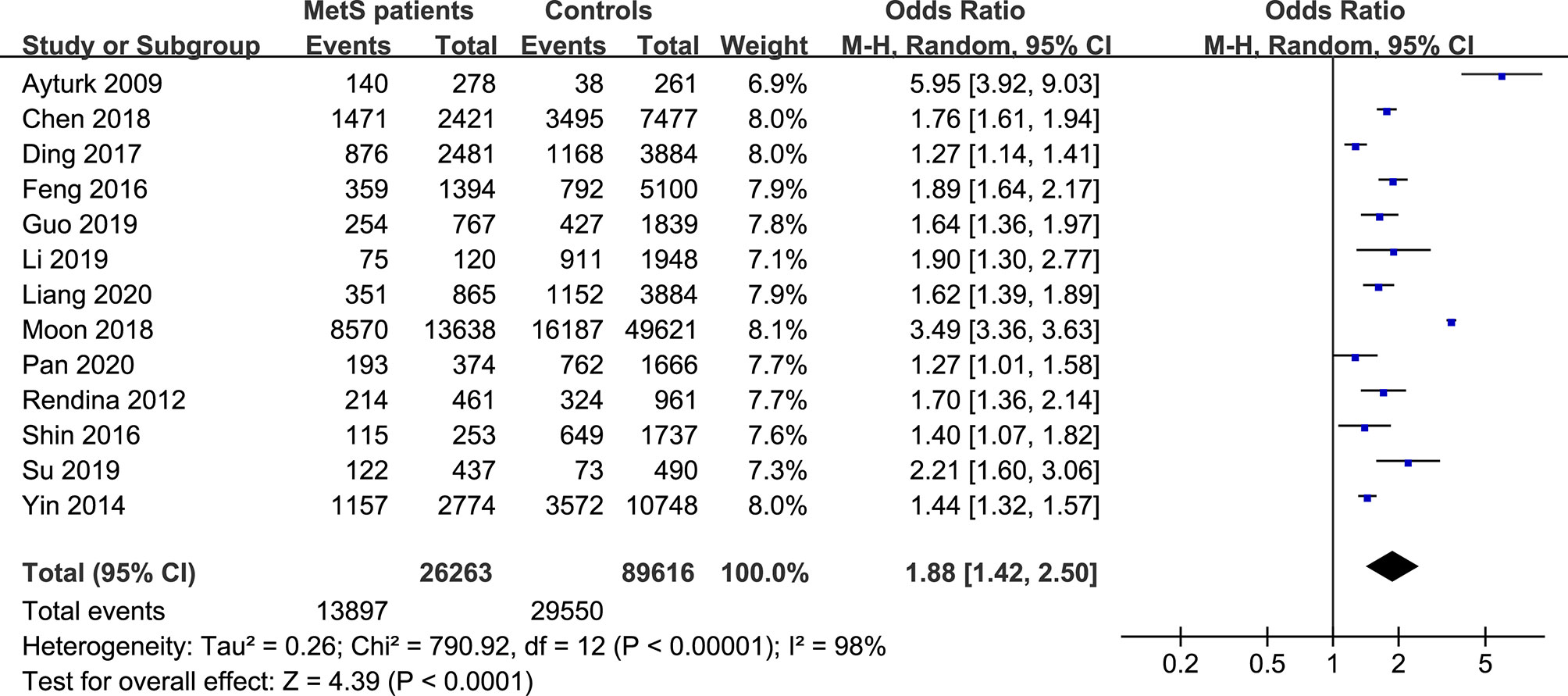
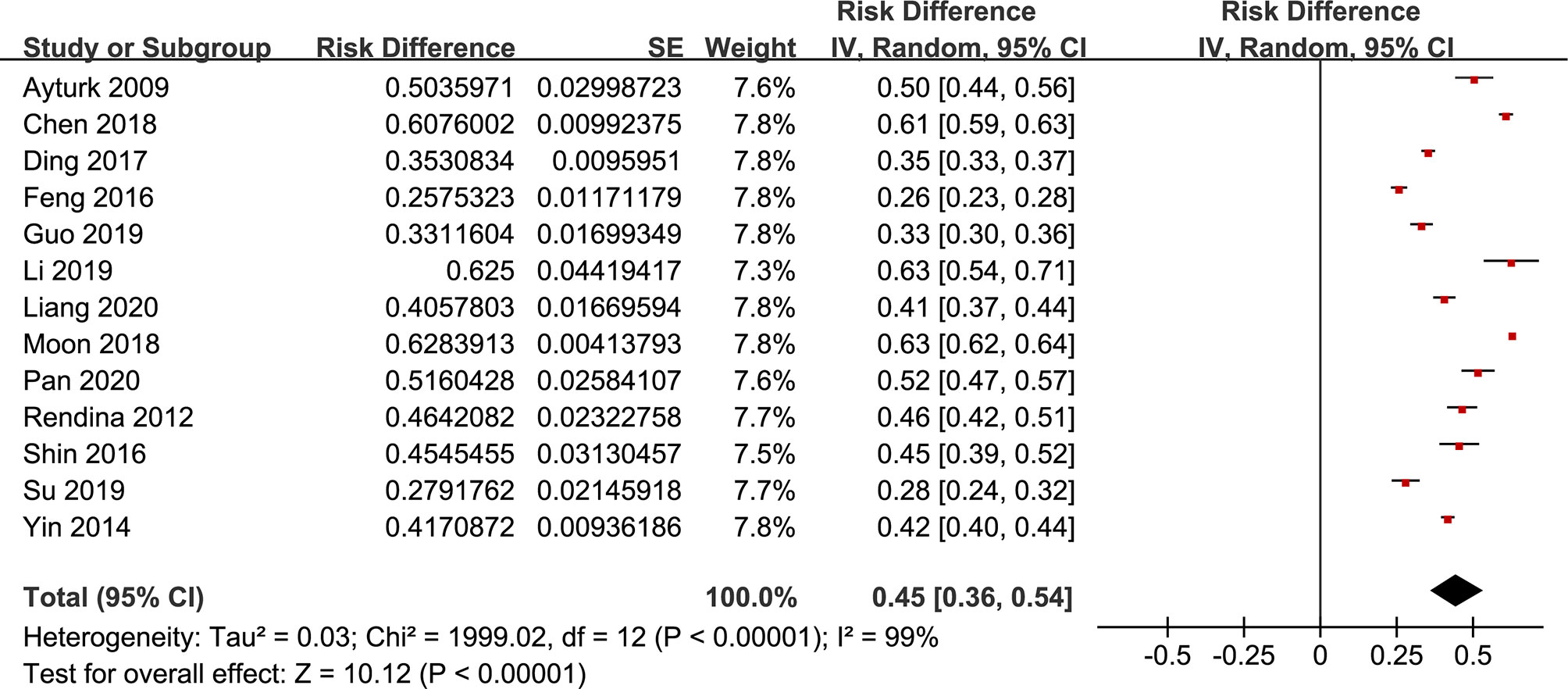
The forest plots of the meta-analysis of TNs and MetS are provided in Figures 2–5. The results of the meta-analysis of the prevalence of TNs in MetS patients versus non-MetS patients (OR 1.88, 95% CI 1.42-2.50, P < 0.0001, I2 = 98%) showed that patients with MetS were more likely to have TNs than controls (Figure 2). The pooled prevalence of TN in MetS patients was 45% (95% CI 36-54%) (Figure 3).
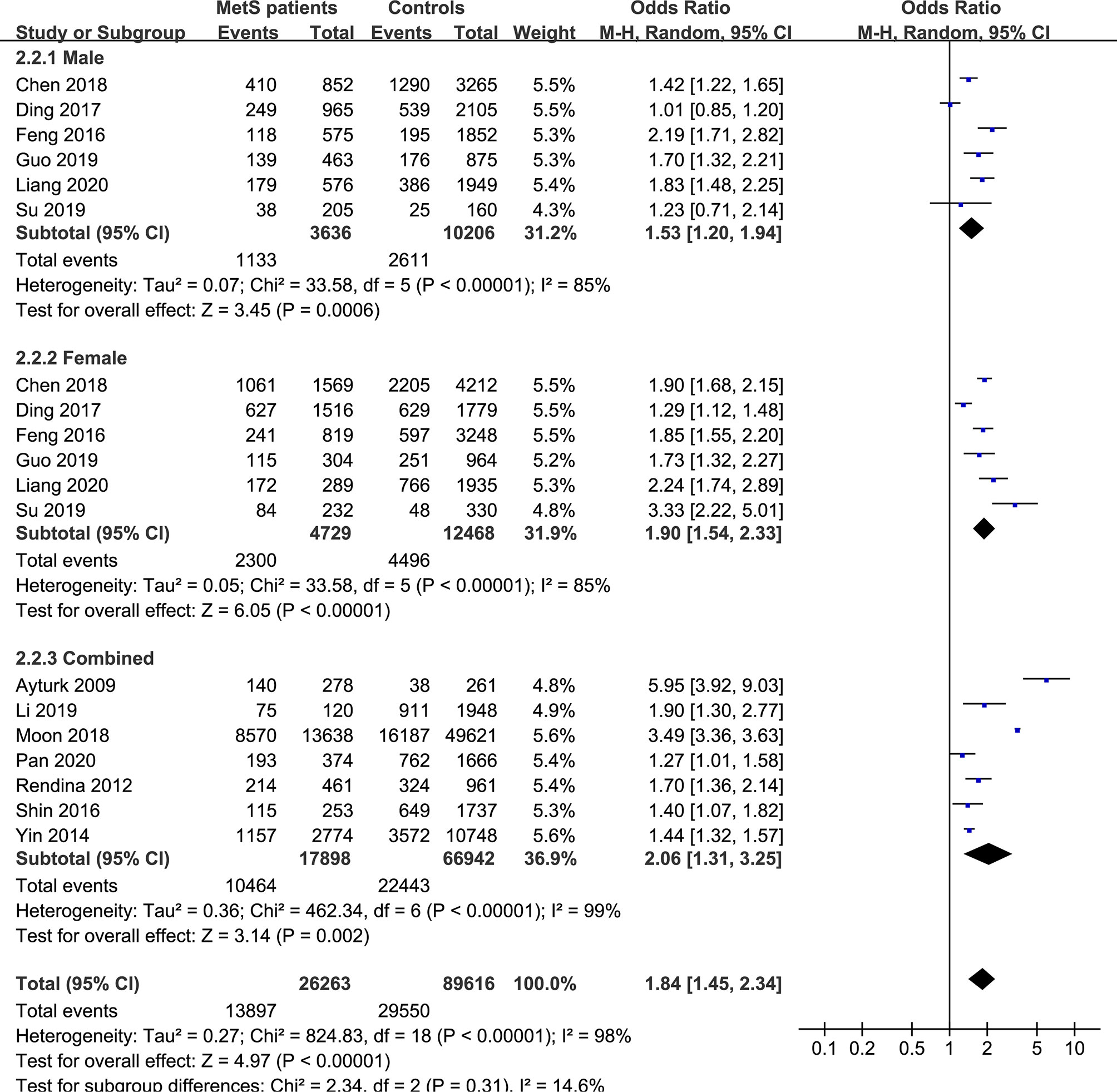
Figure 4 Pooled effect size forest plots of TNs prevalence in MetS patients versus non-MetS patients according to sex (male, female and combined groups).
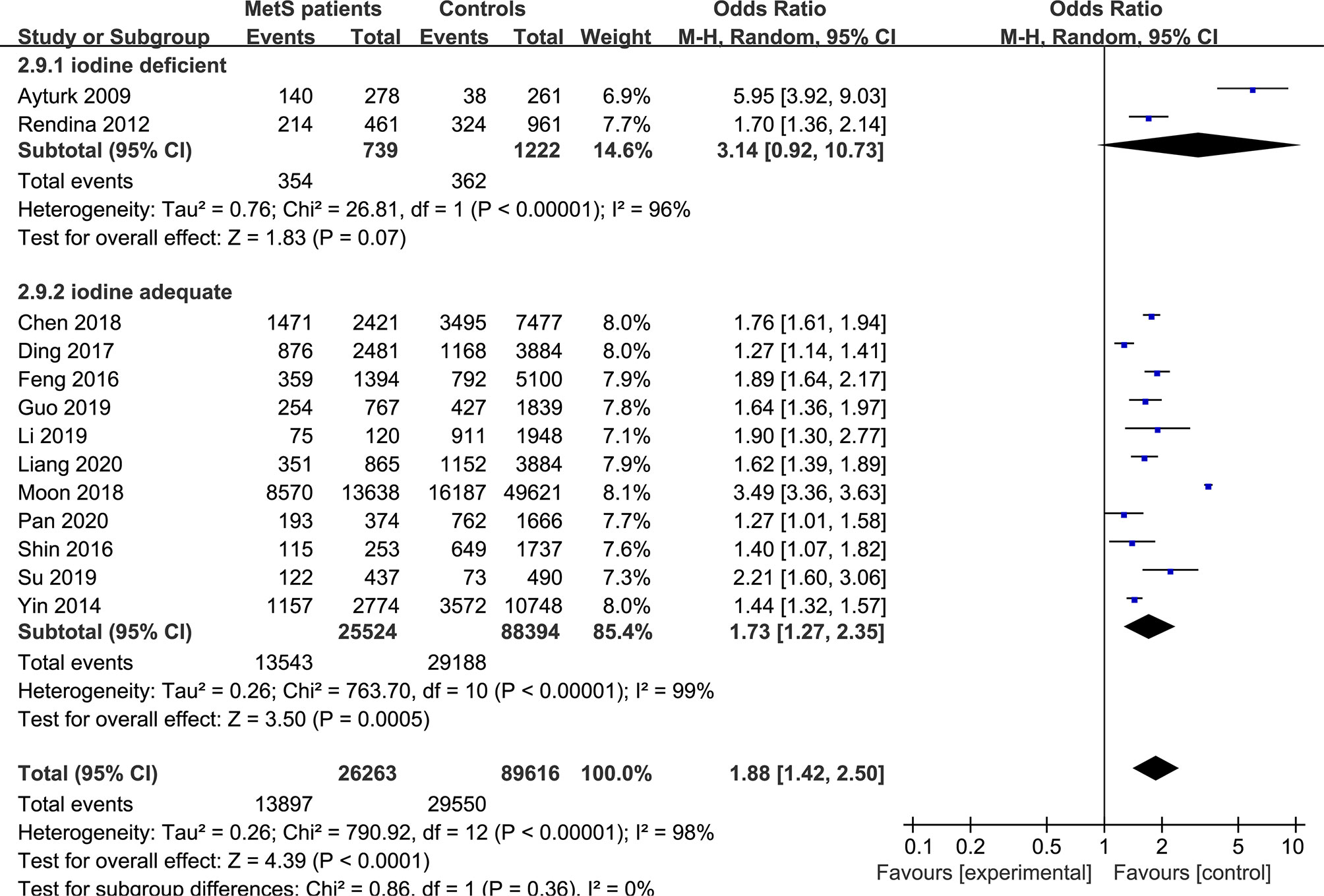
Figure 5 Pooled effect size forest plots of TNs prevalence in MetS patients versus non-MetS patients according to iodine nutrition status (iodine-deficient and iodine-adequate).
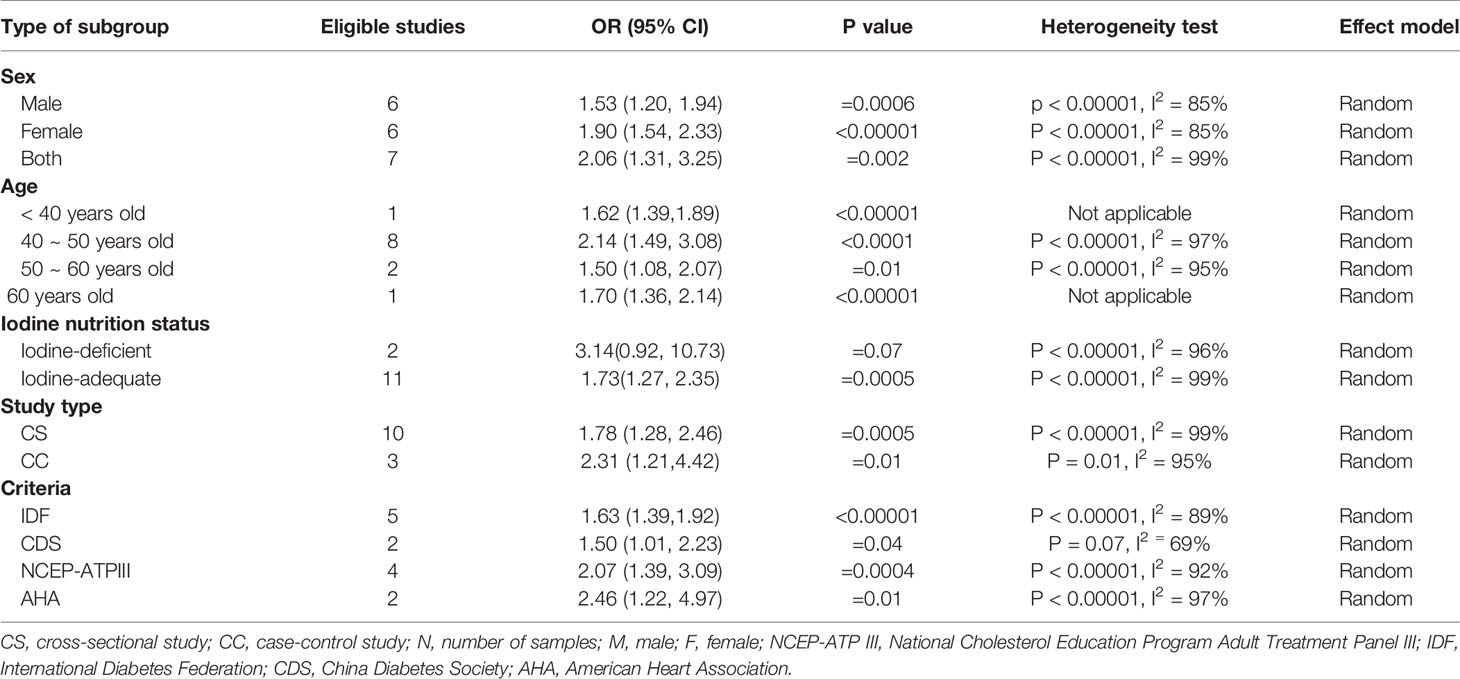
In the subgroup analysis of the prevalence of TNs in MetS patients, the study population was divided into male, female and combined groups. The results showed that the prevalence of TNs was significantly higher in MetS patients than in non-MetS patients (male: OR 1.53, 95% CI 1.20-1.94, P = 0.0006, I2 = 85%; female: OR 1.90, 95% CI 1.54-2.33, P < 0.00001, I2 = 85%; combined: OR 2.06, 95% CI 1.31-3.25, P = 0.002, I2 = 99%) (Figure 4). In addition, we divided the studies into iodine-deficient groups and iodine-adequate groups according to the iodine nutrition status. The results showed that there was a positive relationship between TNs and MetS in the iodine-adequate group (OR 1.73, 95% CI 1.27-2.35, P = 0.0005, I2 = 99%); however, we cannot yet suggest a correlation between TNs and MetS in the iodine-deficient population (OR 3.14, 95% CI 0.92-10.73, P = 0.07, I2 = 96%) (Figure 5). We divided the studies into < 40 years old, 40 ~ 50 years old, 50 ~ 60 years old and ≥60 years old subgroups according to the mean age. The results of this subgroup analysis showed positive associations between TNs and MetS in the < 40 years old (OR 1.62, 95% CI 1,39-1.89, P < 0.00001), 40 ~ 50 years old (OR 2.14, 95% CI 1.49-3.08, P < 0.00001, I2 = 97%), 50 ~ 60 years old (OR 1.50, 95% CI 1.08-2.07, P < 0.00001, I2 = 95%) and ≥60 years old (OR 1.70, 95% CI 1.36-2.14, P < 0.00001) subgroups (Figure 6).
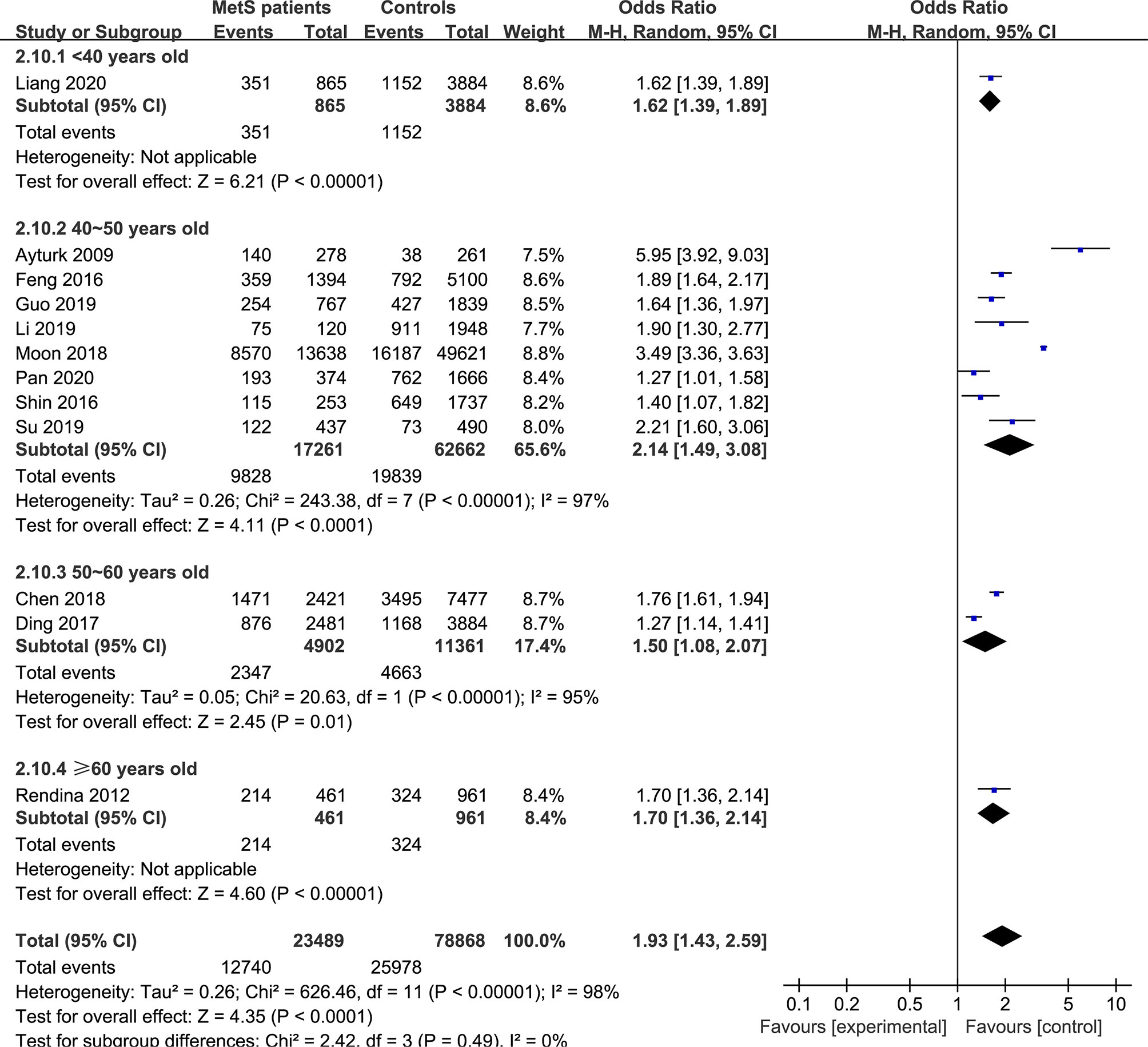
Figure 6 Pooled effect size forest plots of TNs prevalence in MetS patients versus non-MetS patients according to mean age (< 40 years old, 40 ~ 50 years old, 50 ~ 60 years old and ≥60 years old groups).
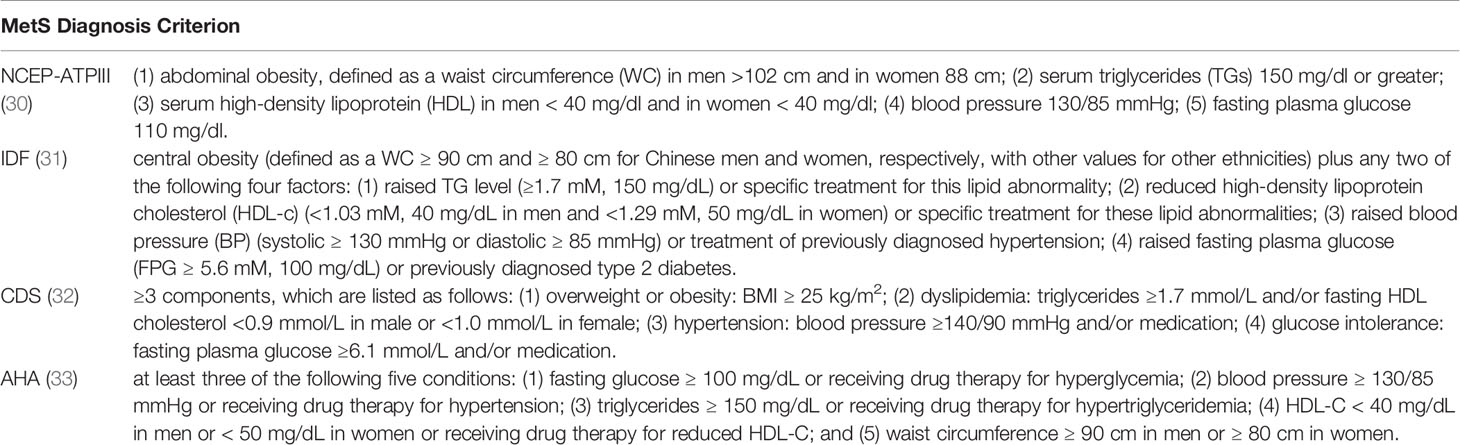
Then, we divided the studies into cross-sectional (CS) and case-control (CC) study subgroups according to the type of study. The results indicated that there was a positive relationship between TNs and MetS in both the CS (OR 1.78, 95% CI 1.28-2.46, P =0.0005, I2 = 99%) and CC (OR 2.31, 95% CI 1.21-4.42, P =0.01, I2 = 95%) groups. We also divided the studies into National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATPIII) (30), International Diabetes Federation (IDF) (31), China Diabetes Society (CDS) (32) and American Heart Association (AHA) (33) subgroups according to the diagnostic criteria for MetS (Table 2). The subgroup analysis results showed that among these subgroups, the prevalence of TNs was higher in the MetS group than in the non-MetS group (NCEP-ATPIII: OR 2.07, 95% CI 1.39-3.09, P = 0.0004, I2 = 92%; IDF: OR 1.63, 95% CI 1.39-1.92, P < 0.00001, I2 = 89%; CDS: OR 1.50, 95% CI 1.01-2.23, P = 0.04, I2 = 92%; AHA: OR 2.46, 95% CI 1.22-4.97, P = 0.01, I2 = 97%) (Table 3).
Sensitivity Analysis and Publication Bias
We next performed sensitivity analyses to determine whether modifying the inclusion criteria for the systematic review and meta-analysis affected the final results. Each study included in this systematic review and meta-analysis was removed to reflect the effect of the individual study on the pooled OR. The corresponding pooled ORs did not change significantly, indicating that our results were stable and plausible.
Heterogeneity was inevitable due to the large number of cross-sectional studies included. Hence, we used a random-effects model to complete the analysis. After conducting multiple subgroup analyses, we found that the correlation between TN and MetS could not yet be considered in the iodine deficiency subgroup. Therefore, differences in iodine nutritional status may explain some of the heterogeneity.
The publication bias in the meta-analysis of the eight included studies is shown in Figure 7, with funnel plots showing publication bias in two small samples of studies.
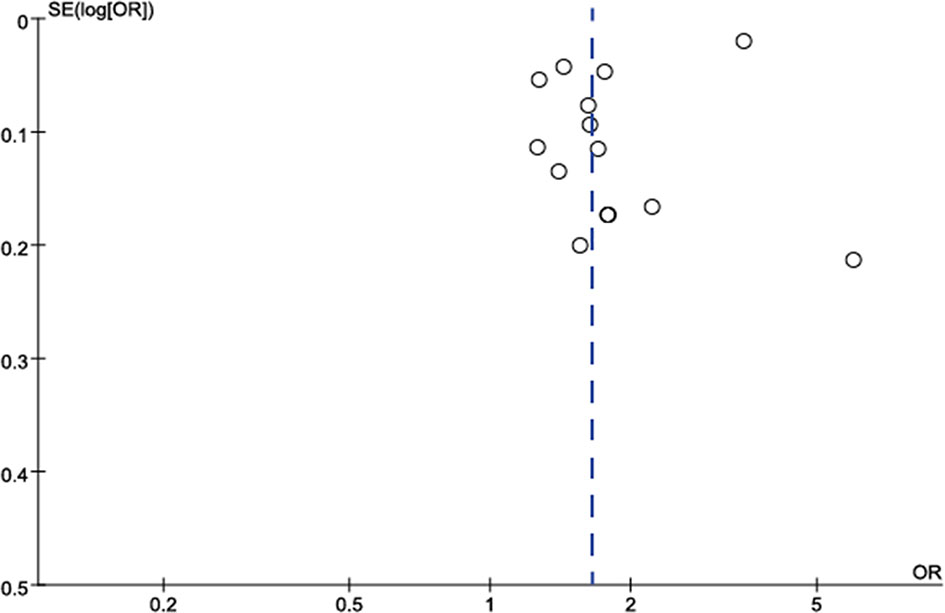
Figure 7 Funnel plot to assess publication bias in studies on the association between TNs and the risk of MetS. Each point represents a separate study for the indicated association. Log OR, natural logarithm of OR. The vertical line represents the effect size.
Discussion
Our meta-analysis quantitatively assessed the association of TNs with MetS by including and evaluating thirteen independent observational studies. We reported that MetS was related to a 1.88-fold increase in the risk of TNs (95% CI 1.42-2.50). The pooled TNs prevalence in MetS patients was 45% (95% CI 36-54%). To our knowledge, this is the first meta-analysis to examine the relationship between TNs and MetS to date.
MetS is a fairly common endocrine syndrome. Since the establishment of the definition by the NCEP-ATPIII in May 2001, it has received much attention, with 81,254 relevant publications in PubMed to date and the establishment of various diagnostic criteria, such as the NCEP-ATPIII, IDF, CDS and AHA criteria. One important question in MetS research is whether MetS is associated with other diseases (34), for example, whether MetS is predictive of coronary heart disease (35). A large cross-sectional study from China, the Thyroid Disorders, Iodine Status and Diabetes (TIDE): a National Epidemiological Survey Project, investigated the prevalence of metabolic and thyroid diseases in 31 Chinese provinces and cities and found a strong correlation between MetS or its components and thyroid dysfunction, thyroid antibodies and urinary iodine concentration (11–13, 16).
TNs is one of the most common thyroid disorders. However, most of the TN patients diagnosed in our clinical practice are asymptomatic and there is no theoretical basis regarding whether TNs have a potential impact on the metabolic index. But we found that there was a strong correlation between TNs and MetS. Therefore, we speculated that metabolic disorders may be a risk factor for thyroid nodules. Some studies have shown that patients with more MetS components are more likely to develop thyroid nodules than patients without or with one MetS component (27). In addition, patients with poorly controlled MetS, especially those with uncontrolled abnormal glucose metabolism, are at higher risk of developing thyroid nodules (27). Studies have shown that insulin resistance is positively correlated with the prevalence of thyroid nodules (10). Insulin resistance may directly activate the proliferation pathway through insulin or insulin-like growth factor 1, regulating the expression of thyroid genes and the proliferation and differentiation of thyroid cells (36). In addition, insulin resistance may also be related to the thyroid vascular structure (37), thereby promoting the formation of thyroid nodules. Whether abnormalities in other metabolic indicators can promote the formation of thyroid nodules through certain channels still needs further research.
Moreover, it is still unclear whether the abovementioned hypothesis will be maintained in subgroups with different demographic characteristics. The two previous studies have reached opposite conclusions at different levels of gender and age (9, 17). Subgroup analyses were used to evaluate the effects of sex and age characteristics on this association. The results of the meta-analysis showed that when MetS patients were analyzed according to sex, the prevalence rates of TNs were increased in men, women and combined groups, and the differences were not significant, indicating that the increased prevalence of MetS in TN patients was independent of sex. The differences were also not significant in the subgroup analysis according to age, so it cannot be assumed that the prevalence of MetS differs among TN patients < 40 years old, 40 ~ 50 years old, 50 ~ 60 years old and ≥60 years old. However, we cannot yet suggest an association between TNs and MetS in the iodine-deficient population. As is known to all, iodine plays a key role in the occurrence and development of thyroid diseases. Too high or too low of iodine consumed by the human body may actually cause the appearance of TNs (38). Multiple thyroid nodules are more common in the case of iodine excess, while single nodules are more common in the case of iodine deficiency (39). In addition to TN, iodine nutrition is also related to the occurrence of MetS. One cross-sectional studies demonstrate a correlation between the urinary iodine concentration (UIC) and metabolic-related diseases which presented as a U-shaped curve (11) and iodine deficiency was a risk factor for hypertension and hypercholesterolemia. The physiological function of iodine in the human body mainly depends on its characteristics as a halogen element. Iodine is highly reactive and is prone to free radical reactions. As an antioxidant, iodine can directly serve as an electron donor to quench reactive oxygen species (ROS). It can also be used as a free radical to iodinate tyrosine and unsaturated fatty acids on cell membranes, reducing their reaction with free radicals (40) to achieve the purpose of anti-oxidation. The reducing properties of iodine can help the production of thyroid hormones, maintain the normal function of the thyroid, and may also play a beneficial role in chronic metabolic diseases related to free radicals (41). Due to the interference of iodine nutrition, our research shows that in iodine-deficient populations, a correlation cannot be considered between TNs and MetS. Furthermore, in subgroups of different study types, MetS is positively correlated with TN; in subgroups of different diagnostic criteria, MetS is also positively correlated with TN.
Heterogeneity is inevitable due to the large number of cross-sectional studies included, so a random effects model was used to complete the analysis. When the Ayturk et al., Ding et al. and Moon et al. studies are removed, we can see that heterogeneity decreases from a high heterogeneity of 99% to a moderate heterogeneity of 65%. Although the heterogeneity is still present, it can also indicate that these studies are the source of most of the heterogeneity. A deeper reading of these study methodologies revealed that Ayturk et al. study restricted the region to iodine-deficient area, Ding et al. study restricted the population to rural people and Moon et al. study was a long-term multicenter study of up to six years. These differences in design may explain the substantial increase in heterogeneity. In addition, sensitivity analyses excluding each study separately showed that none of the studies affected the stability of this meta-analysis.
The main aim of this systematic review and meta-analysis was to elucidate the relationship between TNs and MetS through a rigorous statistical analysis. However, our systematic review and meta-analysis has some limitations. First, the number of relevant articles in this analysis was relatively small, and most of them were observational studies with a cross-sectional design, increasing the heterogeneity. Second, several relevant studies were not included in this systematic review and meta-analysis because they had incomplete original data. Third, our results are based on the data provided in the included articles, whereas a more accurate analysis could be performed if data on individual characteristics were available and adjustment could be performed for other covariates, such as smoking, alcohol consumption, and other lifestyle variables. In conclusion, the results of our meta-analysis suggest that MetS may be associated with an increased risk of TN that is independent of sex or age.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author Contributions
CZ and XG: Conceived and designed the meta-analysis. CZ, XG, and YH: Searched and screened the relevant studies. CZ, XG, and YH: Estimated the quality of the studies. WT and ZS: Responsible for the nationwide cross-sectional study. CZ, XG, and YH: Responsible for the data analysis and manuscript writing. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81970682).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study would not have been possible without the participation of these studies. We should appreciate all of them.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.730279/full#supplementary-material
References
1. Li Y, Teng D, Ba J, Chen B, Du J, He L, et al. Efficacy and Safety of Long-Term Universal Salt Iodization on Thyroid Disorders: Epidemiological Evidence From 31 Provinces of Mainland China. Thyroid (2020) 30(4):568–79. doi: 10.1089/thy.2019.0067
2. Burman KD, Wartofsky L. Clinical Practice. Thyroid Nodules. N Engl J Med (2015) 373(24):2347–56. doi: 10.1056/NEJMcp1415786
3. Gharib H. Changing Concepts in the Diagnosis and Management of Thyroid Nodules. Endocrinol Metab Clin North Am (1997) 26(4):777–800. doi: 10.1016/s0889-8529(05)70282-6
4. Leech JV, Smith LW, Clute HM. Aberrant Thyroid Glands. Am J Pathol (1928) 4(5):481–92. doi: 10.1007/BF01892656
5. Rojeski MT, Gharib H. Nodular Thyroid Disease. Evaluation and Management. N Engl J Med (1985) 313(7):428–36. doi: 10.1056/nejm198508153130707
6. Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-Guided Fine-Needle Aspiration of Thyroid Incidentaloma: Correlation With Pathological Findings. Clin Endocrinol (Oxf) (2004) 60(1):21–8. doi: 10.1046/j.1365-2265.2003.01912.x
7. Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of Malignancy in Nonpalpable Thyroid Nodules: Predictive Value of Ultrasound and Color-Doppler Features. J Clin Endocrinol Metab (2002) 87(5):1941–6. doi: 10.1210/jcem.87.5.8504
8. Liu J, Wang C, Tang X, Fu S, Jing G, Ma L, et al. Correlation Analysis of Metabolic Syndrome and Its Components With Thyroid Nodules. Diabetes Metab Syndr Obes (2019) 12:1617–23. doi: 10.2147/dmso.S219019
9. Guo W, Tan L, Chen W, Fan L, Chen Y, Du C, et al. Relationship Between Metabolic Syndrome and Thyroid Nodules and Thyroid Volume in an Adult Population. Endocrine (2019) 65(2):357–64. doi: 10.1007/s12020-019-01901-4
10. Buscemi S, Massenti FM, Vasto S, Galvano F, Buscemi C, Corleo D, et al. Association of Obesity and Diabetes With Thyroid Nodules. Endocrine (2018) 60(2):339–47. doi: 10.1007/s12020-017-1394-2
11. Jin M, Zhang Z, Li Y, Teng D, Shi X, Ba J, et al. U-Shaped Associations Between Urinary Iodine Concentration and the Prevalence of Metabolic Disorders: A Cross-Sectional Study. Thyroid (2020) 30(7):1053–65. doi: 10.1089/thy.2019.0516
12. Zhang J, Gao Y, Li Y, Teng D, Xue Y, Yan L, et al. The Presence of Serum Tgab Suggests Lower Risks for Glucose and Lipid Metabolic Disorders in Euthyroid General Population From a National Survey. Front Endocrinol (Lausanne) (2020) 11:139. doi: 10.3389/fendo.2020.00139
13. He J, Lai Y, Yang J, Yao Y, Li Y, Teng W, et al. The Relationship Between Thyroid Function and Metabolic Syndrome and Its Components: A Cross-Sectional Study in a Chinese Population. Front Endocrinol (Lausanne) (2021) 12:661160. doi: 10.3389/fendo.2021.661160
14. Kaur J. A Comprehensive Review on Metabolic Syndrome. Cardiol Res Pract (2014) 2014:943162. doi: 10.1155/2014/943162
15. Ranasinghe P, Mathangasinghe Y, Jayawardena R, Hills AP, Misra A. Prevalence and Trends of Metabolic Syndrome Among Adults in the Asia-Pacific Region: A Systematic Review. BMC Public Health (2017) 17(1):101. doi: 10.1186/s12889-017-4041-1
16. He X, Wu D, Hu C, Xu T, Liu Y, Liu C, et al. Role of Metformin in the Treatment of Patients With Thyroid Nodules and Insulin Resistance: A Systematic Review and Meta-Analysis. Thyroid (2019) 29(3):359–67. doi: 10.1089/thy.2017.0707
17. Ding X, Xu Y, Wang Y, Li X, Lu C, Su J, et al. Gender Disparity in the Relationship Between Prevalence of Thyroid Nodules and Metabolic Syndrome Components: The SHDC-CDPC Community-Based Study. Mediators Inflamm (2017) 2017:8481049. doi: 10.1155/2017/8481049
18. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-Analysis of Observational Studies in Epidemiology: A Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. JAMA (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
19. Stang A. Critical Evaluation of the Newcastle-Ottawa Scale for the Assessment of the Quality of Nonrandomized Studies in Meta-Analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
20. Ayturk S, Gursoy A, Kut A, Anil C, Nar A, Tutuncu NB. Metabolic Syndrome and Its Components Are Associated With Increased Thyroid Volume and Nodule Prevalence in a Mild-to-Moderate Iodine-Deficient Area. Eur J Endocrinol (2009) 161(4):599–605. doi: 10.1530/EJE-09-0410
21. Chen Y, Zhu C, Chen Y, Wang N, Li Q, Han B, et al. The Association of Thyroid Nodules With Metabolic Status: A Cross-Sectional SPECT-China Study. Int J Endocrinol (2018) 2018:6853617. doi: 10.1155/2018/6853617
22. Feng S, Zhang Z, Xu S, Mao X, Feng Y, Zhu Y, et al. The Prevalence of Thyroid Nodules and Their Association With Metabolic Syndrome Risk Factors in a Moderate Iodine Intake Area. Metab Syndr Relat Disord (2017) 15(2):93–7. doi: 10.1089/met.2016.0077
23. Liang Q, Yu S, Chen S, Yang Y, Li S, Hu C, et al. Association of Changes in Metabolic Syndrome Status With the Incidence of Thyroid Nodules: A Prospective Study in Chinese Adults. Front Endocrinol (Lausanne) (2020) 11:582. doi: 10.3389/fendo.2020.00582
24. Moon JH, Hyun MK, Lee JY, Shim JI, Kim TH, Choi HS, et al. Prevalence of Thyroid Nodules and Their Associated Clinical Parameters: A Large-Scale, Multicenter-Based Health Checkup Study. Korean J Intern Med (2018) 33(4):753–62. doi: 10.3904/kjim.2015.273
25. Pan Q, Wang Y, Wang G. The Association Between Hyperhomocysteinemia and Thyroid Nodule Prevalence in an Adult Population. Metab Syndr Relat Disord (2020) 18(8):368–72. doi: 10.1089/met.2020.0057
26. Rendina D, De Filippo G, Mossetti G, Zampa G, Muscariello R, Benvenuto G, et al. Relationship Between Metabolic Syndrome and Multinodular non-Toxic Goiter in an Inpatient Population From a Geographic Area With Moderate Iodine Deficiency. J Endocrinol Invest (2012) 35(4):407–12. doi: 10.3275/7842
27. Shin J, Kim MH, Yoon KH, Kang MI, Cha BY, Lim DJ. Relationship Between Metabolic Syndrome and Thyroid Nodules in Healthy Koreans. Korean J Intern Med (2016) 31(1):98–105. doi: 10.3904/kjim.2016.31.1.98
28. Su Y, Zhang YL, Zhao M, Zhang HQ, Zhang X, Guan QB, et al. Association Between Thyroid Nodules and Volume and Metabolic Syndrome in an Iodine-Adequate Area: A Large Community-Based Population Study. Metab Syndr Relat Disord (2019) 17(4):217–22. doi: 10.1089/met.2018.0094
29. Yin J, Wang C, Shao Q, Qu D, Song Z, Shan P, et al. Relationship Between the Prevalence of Thyroid Nodules and Metabolic Syndrome in the Iodine-Adequate Area of Hangzhou, China: A Cross-Sectional and Cohort Study. Int J Endocrinol (2014) 2014:675796. doi: 10.1155/2014/675796
30. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA (2001) 285(19):2486–97. doi: 10.1001/jama.285.19.2486
31. Alberti KG, Zimmet P, Shaw J. The Metabolic Syndrome–a New Worldwide Definition. Lancet (2005) 366(9491):1059–62. doi: 10.1016/s0140-6736(05)67402-8
32. Zhou H, Guo ZR, Yu LG, Hu XS, Xu BH, Liu HB, et al. Evidence on the Applicability of the ATPIII, IDF and CDS Metabolic Syndrome Diagnostic Criteria to Identify CVD and T2DM in the Chinese Population From a 6.3-Year Cohort Study in Mid-Eastern China. Diabetes Res Clin Pract (2010) 90(3):319–25. doi: 10.1016/j.diabres.2010.09.001
33. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and Management of the Metabolic Syndrome: An American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation (2005) 112(17):2735–52. doi: 10.1161/circulationaha.105.169404
34. Cornier MA, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The Metabolic Syndrome. Endocr Rev (2008) 29(7):777–822. doi: 10.1210/er.2008-0024
35. DeBoer MD, Filipp SL, Gurka MJ. Associations of a Metabolic Syndrome Severity Score With Coronary Heart Disease and Diabetes in Fasting vs. non-Fasting Individuals. Nutr Metab Cardiovasc Dis (2020) 30(1):92–8. doi: 10.1016/j.numecd.2019.08.010
36. Tirosh A, Shimon I. Complications of Acromegaly: Thyroid and Colon. Pituitary (2017) 20(1):70–5. doi: 10.1007/s11102-016-0744-z
37. Eggo MC. Molecular Regulation of Thyroid Gland Function. Curr Opin Endocrinol Diabetes Obes (2010) 17(5):396–401. doi: 10.1097/MED.0b013e32833c8942
38. Shan Z, Chen L, Lian X, Liu C, Shi B, Shi L, et al. Iodine Status and Prevalence of Thyroid Disorders After Introduction of Mandatory Universal Salt Iodization for 16 Years in China: A Cross-Sectional Study in 10 Cities. Thyroid (2016) 26(8):1125–30. doi: 10.1089/thy.2015.0613
39. Yu X, Fan C, Shan Z, Teng X, Guan H, Li Y, et al. A Five-Year Follow-Up Study of Goiter and Thyroid Nodules in Three Regions With Different Iodine Intakes in China. J Endocrinol Invest (2008) 31(3):243–50. doi: 10.1007/bf03345597
40. Tseng YL, Latham KR. Iodothyronines: Oxidative Deiodination by Hemoglobin and Inhibition of Lipid Peroxidation. Lipids (1984) 19(2):96–102. doi: 10.1007/bf02534498
Keywords: thyroid nodules, metabolic syndrome, meta-analysis, thyroid, iodine-deficient
Citation: Zhang C, Gao X, Han Y, Teng W and Shan Z (2021) Correlation Between Thyroid Nodules and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 12:730279. doi: 10.3389/fendo.2021.730279
Received: 24 June 2021; Accepted: 31 August 2021;
Published: 16 September 2021.
Edited by:
Trevor Edmund Angell, University of Southern California, United StatesReviewed by:
Gabriela Brenta, Dr. César Milstein Care Unit, ArgentinaCheng Han, Albert Einstein College of Medicine, United States
Copyright © 2021 Zhang, Gao, Han, Teng and Shan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhongyan Shan, Y211c2hhbnpob25neWFuQDE2My5jb20=
 Chenyu Zhang
Chenyu Zhang Xiaotong Gao
Xiaotong Gao Yutong Han
Yutong Han Weiping Teng
Weiping Teng