- 1University of Pittsburgh Physicians, University of Pittsburgh Medical Center, Pittsburgh, PA, United States
- 2Mario Lemieux Center for Blood Cancers, University of Pittsburgh Medical Center (UPMC) Hillman Cancer Center, Pittsburgh, PA, United States
- 3Department of Sciences, Sewickley Academy, Pittsburgh, PA, United States
- 4UPMC Hillman Cancer Center, Magee Women’s Hospital, Pittsburgh, PA, United States
Aromatase inhibitors (AIs) are a key component in the chemoprevention and treatment of hormone receptor-positive (HR+) breast cancer. While the addition of AI therapy has improved cancer-related outcomes in the management of HR+ breast cancer, AIs are associated with musculoskeletal adverse effects known as the aromatase inhibitor-associated musculoskeletal syndrome (AIMSS) that limit its tolerability and use. AIMSS is mainly comprised of AI-associated bone loss and arthralgias that affect up to half of women on AI therapy and detrimentally impact patient quality of life and treatment adherence. The pathophysiology of AIMSS is not fully understood though has been proposed to be related to estrogen deprivation within the musculoskeletal and nervous systems. This review aims to characterize the prevalence, risk factors, and clinical features of AIMSS, and explore the syndrome’s underlying mechanisms and management strategies.
Introduction
HR+ breast cancer is the most common subtype of breast cancer and a significant cause of cancer-related death in women (1). Endocrine therapy targeting aromatase has an important role in the primary chemoprevention of HR+ breast cancer in high-risk postmenopausal women and is integral to the management of HR+ breast cancer in the adjuvant and metastatic setting to prevent recurrence and control progression of disease, respectively.
Aromatase is a cytochrome p450 enzyme encoded by the CYP19A1 gene that converts androgens to estrogens, specifically converting testosterone and androstenedione to aromatic estrogens estradiol and estrone, respectively (2). Aromatase facilitated production of estrogens primarily occurs in the ovaries of premenopausal women, whereas in postmenopausal women it takes place in peripheral tissues, particularly adipose tissue. Aromatase has been found to be expressed in numerous tissues including placenta, the central nervous system, bone, muscle, testis, prostate, adrenals, and skin facilitating pleiotropic roles in the body (3). In HR+ breast cancer, aromatase is frequently overexpressed in breast endothelial cells and the surrounding stroma leading to local estrogen synthesis within the tumor microenvironment, thus stimulating cancer growth through estrogen receptor activation (4). Lifetime exposure to estrogen correlates with breast cancer risk by promoting carcinogenesis and the proliferation of neoplastic breast tissue (5, 6). The essential role of aromatase in estrogen production makes it an ideal target for endocrine therapy in the management of HR+ breast cancer.
The advent of third generation aromatase inhibitors (AIs) has played a significant role in advancing the endocrine chemoprevention and treatment of HR+ breast cancer. At present, there are three AIs in common practice. Anastrozole and letrozole are nonsteroidal AIs that competitively inhibit aromatase, whereas exemestane is a steroidal AI that irreversibly binds and inhibits aromatase (3, 7, 8). Clinical trials have demonstrated a role for AIs in breast cancer chemoprevention and have been shown to reduce the rate of breast cancer development in high-risk women. In the MAP.3 trial, high-risk postmenopausal women received either exemestane or placebo (9). After a median follow up of 35 months, there was a 53% reduction in the incidence of all breast cancer and a 65% reduction in invasive breast cancer. The International Breast Cancer Intervention Study II (IBIS-II) randomized high-risk postmenopausal women with no breast cancer to receive either anastrozole or placebo and found a significant reduction in incidence for all breast cancer (53%), including invasive HR+ breast cancer (58%) and ductal carcinoma in situ (70%) in the anastrozole group (10). A second analysis at 131 months identified continuing reduction in breast cancer with anastrozole (11). AIs have also demonstrated clinical superiority over tamoxifen in postmenopausal women and have established themselves as standard of care in both the adjuvant and metastatic setting (12–14). Multiple trials have demonstrated improved outcomes in postmenopausal women who switched from tamoxifen to an AI for extended endocrine therapy in the adjuvant setting (15–19). Adjuvant AI therapy is presently recommended for five years to prevent recurrence though AI therapy extended to 10 years can provide further disease-free survival in certain high-risk individuals (20, 21). Moreover, growing evidence has supported the role of AIs in premenopausal women at high risk for disease recurrence when combined with ovarian function suppression (22–24). Over two decades of clinical experience, AI therapy has proven itself to be a major achievement in breast cancer endocrine therapy.
While generally considered to have a well-tolerated side effect profile (25), AIs have been recognized to cause musculoskeletal symptoms resulting in diminished quality of life and frequent discontinuation of therapy (26). Musculoskeletal symptoms of AIs that have been described include arthralgias, myalgias, joint stiffness, and tendinopathy (27, 28). Furthermore, AIs appear to augment bone mineral density decline observed during menopause (29). The constellation of these musculoskeletal symptoms has become known as the AI-associated musculoskeletal syndrome (AIMSS). Given the established benefits of AI treatment, this review aims to summarize the current knowledge about the prevalence, pathophysiology, and management strategies for AIMSS.
Prevalence and Associated Risk Factors
The reported prevalence of AIMSS varies widely in the literature but is estimated to occur in one-third to one-half of women utilizing AIs (30–32). One meta-analysis involving 21 studies with 13,177 participants found a prevalence of arthralgia in women on AI therapy ranging from 20-74% with a pooled estimate of 46% (30). Even in the preventative setting where AIs have demonstrated a significant reduction in breast cancer incidence, there was an increased frequency of grade 2 or higher symptoms of AIMSS including arthralgias, myalgias, joint stiffness, and carpal tunnel syndrome (9, 33). Whereas the reported AIMSS prevalence varies considerably, the true prevalence has been suggested to be higher than initially appreciated in early clinical trials (31, 34).
AIMSS frequently leads to early discontinuation and nonadherence of AI therapy in a significant proportion of patients, which in turn has been associated with breast cancer recurrence and increased all-cause mortality (35). One study demonstrated that breast cancer patients who discontinued endocrine therapy had a 20% recurrence of their disease compared to 11% of women who completed their recommended treatment (36). Among women on AI therapy for risk reduction in early-stage breast cancer, one study estimates that nearly a quarter of patients discontinue therapy at a median time of 6 months due to AIMSS (37). While many patients are able to tolerate a switch to a different AI, the majority find insufficient relief and forgo continued AI therapy. Among patients who switched to a different AI due to adverse effects in this study, only 38% of patients were able to tolerate a second AI for a median of 13 months. Within the gynecologic oncology literature, one retrospective study found similar rates of AIMSS symptoms in women on AI therapy at equivalent doses for primarily advanced stage ovarian or uterine cancer, though only 5.0% of patients discontinued AI therapy due adverse effects (38). The authors of this study hypothesized that the gynecologic oncology sample was more likely to continue AI therapy since their sample population had more advanced disease on average than in breast cancer adjuvant therapy studies, however data is comparatively limited in the gynecologic oncology setting.
The prevalence of musculoskeletal symptoms has distinguished AIs from other endocrine therapies used in treatment of HR-positive breast cancer. Tamoxifen and AIs share numerous side effects including menopausal vasomotor and vulvovaginal symptoms. Compared to AIs, tamoxifen is associated with increased risk for venous thromboembolism, cerebrovascular accident, and endometrial cancer, though is relatively spared from musculoskeletal pain and has protective effects on bone mineralization (39). The significance of AIMSS is highlighted by the fact that arthralgias and bone health are among the most common reasons women switch from an AI to tamoxifen (40). Gonadotropin-releasing hormone (GnRH) agonists, most commonly used for ovarian function suppression in premenopausal women, are known to cause bone mineral loss predisposing to osteoporosis but uncommonly provoke musculoskeletal pain (41, 42). Moreover, fulvestrant, a selective estrogen receptor down-regulator (SERD) approved for use in advanced and metastatic hormone receptor positive breast cancer (43, 44), has an incidence of joint disorders comparable to or less than AIs (45–47) and its effects on bone density are not well defined (48).
Studies exploring risk factors for AIMSS have yielded inconsistent findings. Among the largest studies to identify correlative risk factors were retrospective analyses of the Arimidex Tamoxifen Alone or in Combination (ATAC) trial and the Intergroup Exemestane Study (IES) trial which discovered an association between AIMSS and body weight. ATAC and IES similarly found that BMI >30 and weight > 80 kg, respectively, correlated with increased risk for AIMSS (47, 49), with the association between obesity and AIMSS further supported by other study data (50). However, there may be a distinction in risk between overweight and obese. One cross-sectional survey found that women with BMI 25-30 had significantly fewer joint symptoms than both women with BMI >30 and BMI <25, indicating that being overweight but not obese may be protective (35). In contrast to these results, a number of smaller studies did not find BMI to be a significant risk factor (32, 51, 52). Some explanations for the associations with obesity have been proposed. Obesity and increased adipose tissue are known to be associated with increased aromatase activity and increased estrogen levels. A study of 44 women on AI therapy found that BMI correlated with higher baseline estradiol and estrone sulfate was associated with incomplete though still effective suppression of estrogen with AI therapy (52). Baseline estradiol and estrone sulfate levels in patients with BMI>35 were nearly three times that of women with BMI <25 and obese patients had more significant absolute decreases in estrogen levels compared to patients in lower BMI categories. The gravity of decrease in estrogen has been proposed as a possible mechanism for higher AIMSS risk in obese patients (47). Obesity is also a notable independent risk factor for osteoarthritis and carpal tunnel syndrome and thus may contribute to musculoskeletal symptoms through mechanisms irrespective of AI therapy (53–55) The exact relationship of AIMSS with obesity remains poorly understood.
Some data indicate that perimenopausal women are at higher risk for AIMSS than women who have been postmenopausal for a longer duration. A prospective and cross-sectional study found that women whose last menstrual period was within 5 years had higher rates of arthralgias while on AIs (32, 52). One proposed explanation argues that women who have more recently reached menopause have higher residual circulating estrogen and that AI therapy results in a more precipitous decline in estrogen from their baseline (52). Moreover, both the ATAC and IES trials found that prior hormone replacement therapy was a significant risk factor for musculoskeletal symptoms (35, 47, 49). This supports the notion that AIMSS onset may be related to the absolute drop and rate of change in estrogen levels from baseline.
Prior use of tamoxifen has not been clearly identified as a risk factor for AIMSS. Studies have been discordant with varying data suggesting prior tamoxifen use decreases risk (34, 56), increases risk (57), or is not associated with AIMSS (32, 58).
Results correlating AIMSS with taxane chemotherapy have similarly been mixed. Patients who received taxanes as part of their chemotherapy regimen were estimated to have four times the risk of developing AI-associated joint pain and stiffness in a cross-sectional survey (30, 34), and the implication of taxanes was supported by the ATAC trial, which reported higher rates of AIMSS in women who received chemotherapy (48). The association between AIMSS and taxanes however has not been well replicated in other studies (32, 49–51, 57–61). Taxane administration is recognized as an independent risk factor for arthralgias and myalgias further making the relationship with AIMSS unclear (62, 63). It remains unknown if taxanes and AIs have a synergistic role with regard to the development of arthralgia pain.
A recent systematic review and meta-analysis has looked at the protective role CDK4/6 inhibitors may provide against the development of AIMSS. CDK4/6 inhibitors are a novel class of drugs utilized in the treatment of metastatic HR+ breast cancer. The meta-analysis of 13 phase III trials involving patients on combined AI and CDK4/6 versus AI monotherapy demonstrated arthralgias in 1-47% of patients receiving AI monotherapy compared to a rate of 5.8-33.3% in those on combined treatment (64). Similarly lower rates were observed when comparing the AI monotherapy group to combination AI and CDK4/6 inhibitors for myalgias (2-23.7% vs 4.8-11.9%), back pain (7-32.9% vs 2.9-8.5%), and bone pain (7-32.9% vs 2.9-8.5%). Although promising, larger trials will be needed to clarify the role of CDK4/6 inhibitors in the mitigation of AIMSS.
Interestingly, it has been proposed that AIMSS may be a marker of improved outcomes with respect to decreased recurrence rates and improved disease-free and overall survival (65–68). While this association has not been consistently observed in all trials (49, 69), there has been speculation regarding this observation. It has been suggested that patients reporting adverse effects are more adherent to therapy (70) or alternatively that patients with AIMSS are benefiting from greater reductions in estrogen levels with enhanced efficacy against HR+ breast cancer (71).
Manifestations of AIMSS
The musculoskeletal adverse events associated with AIMSS can be primarily classified into two major groups of 1) AI-induced bone loss and 2) AI-induced arthralgias (AIA). The clinical features and pathogenesis of these categories will be elaborated on in the following sections.
AI-Induced Bone Loss
Several studies have established that treatment with AIs results in loss of bone density and increased fracture risk (71–73). A meta-analysis of seven randomized controlled trials (RCTs) involving 30,023 patients demonstrated that longer duration of AI use was associated with increased odds of developing bone fractures (OR = 1.47, 95% CI = 1.34 to 1.61, P <.001) (74). One potential mechanism is related to the hypoestrogenic state induced by AIs. In postmenopausal women, aromatase regulates estrogen which plays a role in the modulation of bone mass. As previously stated, AIs decrease estrogen concentrations by blocking aromatase (75). In post‐menopausal women, anastrozole, letrozole and exemestane lower the serum levels of estrogen by 81–94%, 88–98% and 52–72%, respectively (76).
At the cellular level, bone metabolism is a balance between osteoblastic and osteoclastic activity (Figure 1). Estrogen decreases the osteoblastic production of resorptive cytokines such as receptor activator of nuclear factor kappa B ligand (RANKL), colony‐stimulating factor‐1 (CSF-1), Interleukin-1 (IL-1), and tumor necrosis factor (TNF) resulting in an increase in osteoblast activity (77). Simultaneously, estrogen increases the production of osteoprotegerin (OPG) that is vital in inhibiting osteoclastogenesis and bone resorption. RANKL attaches to its receptor on the osteoclast surface and promotes cell differentiation, whereas OPG prevents this by binding to RANKL directly. Thus, in the absence of estrogen these mechanisms are hindered. Furthermore, estrogen deficiency is concomitantly associated with higher production of TNFα and RANKL by T cells and monocytes, resulting in increased bone resorption (78).
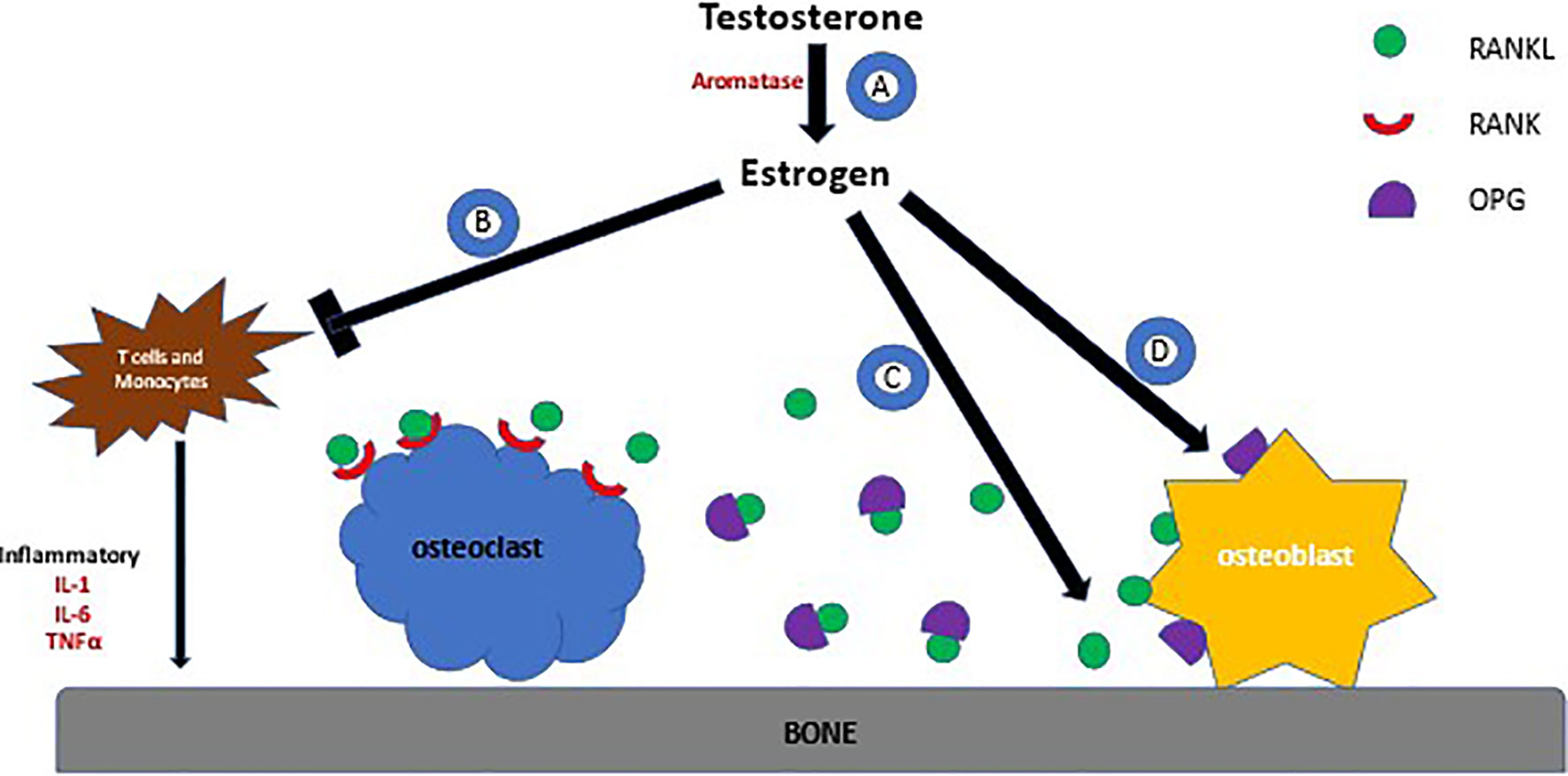
Figure 1 Effects of Estrogen on Bone Metabolism. (A) Testosterone is metabolized to make estrogen by aromatase. (B) Estrogen prevents the production of cytokines by T cells and monocyctes that are responsible for bone resporption (C) Estrogen decreases production of RANKL by osteoblasts, a protein that binds to the RANK receptor on the surface of osteoclasts. RANK stimulates the osteoclast to adhere to bone, activating bone resorption (D) OPG is produced by osteoblasts and acts as a decoy receptor for RANKL, thus preventing osteoclastsic activity.
Recent evidence suggests that single-nucleotide polymorphisms (SNPs) in certain genes lead to AI-induced bone loss. In postmenopausal ER-positive breast cancer, SNPs in the genes encoding the estrogen receptors (ESR1 and ESR2), the expression of aromatase (CYP19A1), and CYP11A1 were predictors of decreased bone density (79). Additionally, a case-cohort genome-wide association study (GWAS) using samples from 1071 patients identified three SNPs in or near six genes (CTSZ, SLMO2, ATP5E, TRAM2, TRAM14A, MAP4K4) that were associated with increased risk of bone fractures in those receiving AIs (80). These genes displayed estrogen-dependent induction and their knockdown increased the expression of genes implicated in the development of osteoporosis.
Aromatase Inhibitor-Induced Arthralgias
AIA is a common manifestation occurring in patients undergoing treatment with AI. Although there is no widely accepted definition for AIA a review proposed one that requires patients to meet all major criteria and at least three minor criteria to be met (Table 1) (81).
The AIA syndrome consists of symmetrical joint pain and stiffness most commonly affecting hands, wrists, knees, ankles, and shoulders, but can also involve central joints of the axial spine and pelvis. Other symptoms include decreased grip strength, myalgias, and extra-articular presentations such as carpal tunnel syndrome and trigger finger (82). A meta-analysis of twenty-one studies involving 13,000 patients showed the prevalence of AIA ranging from 20-74% (30). Although AIA can occur at any time after initiating AI, the median time to onset is approximately 6 weeks with peak symptoms at 6 months (59). Intolerance to AI therapy causes 20-30% of patients to discontinue treatment early, with 75% of them citing arthralgias as the primary reason (34). The arthralgias are typically relieved with discontinuation of AI therapy and recur with resumption of the AI.
Although not well understood, several mechanisms have been proposed for the development of AIA. Similar to AI-induced bone loss, estrogen deprivation has been proposed as a cause for the development of arthralgias. Aromatase is expressed in synovial cells and chondrocytes in articular cartilage (83). The estrogen produced here impacts chondrocyte formation through interactions with cytokines, adhesion molecules, and cell growth factors. Declining levels of estrogen result in increased production of proinflammatory cytokines such as interleukin-6 (IL-6) and IL-1 in articular chondrocytes resulting in joint pain and swelling (84). The chondroprotective role of estrogen was demonstrated in ovariectomized rats that demonstrated resorption of subchondral bone and accelerated degeneration of articular cartilage four weeks after surgery (85). With hormone replacement therapy, cartilage erosions were significantly reduced. Similarly, studies have demonstrated that hormone replacement with conjugated equine estrogens result in decreased joint pain, pain severity, and joint swelling in postmenopausal women (86).
SNPs in several genes have been linked to the development of AIA and myalgias. In one study of 254 BC patients on AI, increased musculoskeletal pain was associated with the SNP rs2073618 in OPG (87). In the B-ABLE cohort study, 343 women with AIA were found to have SNPs in the CYP17A1 (gene encoding for enzymes involved in estrogen metabolism) and VDR and CYP27B1 (genes encoding for enzymes involved in vitamin D signaling) (88). In another randomized clinical trial an inherited genetic variant involving an SNP in ESR1 (rs9322336) was associated with increased risk of musculoskeletal toxicity-related exemestane discontinuation (HR 5.0 (95% CI 2.1–11.8), p<0.0002) (89). Additionally, a study with 1049 women on AI were found to have an SNP (rs11648233) within the HSD17B2 gene in the estrogen pathway which significantly increased odds of developing AIA (OR 2.21, 95% CI 1.55–3.16) (90).
Other possible mechanisms responsible for AIA relate to the effects of estrogen on the pain pathway. Aromatase and estrogen receptors are expressed in the brain and the spinal cord kappa-opioid analgesic system (91). Estrogen have anti-nociceptive properties therefore deprivation can decrease pain thresholds, which leads to increased pain perception in women treated with AIs. In one animal model, ovariectomized rats who were treated with letrozole demonstrated significantly reduced pain thresholds compared to those not treated (92). Furthermore, examination of the dorsal root ganglia of rats treated with letrozole long-term showed increased excitability of the small and medium-diameter sensory neurons, providing evidence that AIs can augment the pain response.
Joint stiffness and tenosynovitis of the hands or feet in the absence of systemic inflammation or autoimmune disease is a common presentation of AIMSS (93). Studies have demonstrated characteristic radiologic changes demonstrating the inflammatory changes associated with AIA (94, 95). In a study involving 12 women reporting severe AIA, ultrasound and MRI showed increased fluid in the tendon sheath surrounding the digital flexor tendons, thickening of the tendon sheath, and increased intra-articular fluid (96). Similar findings were reported in a larger study where musculoskeletal sonography demonstrated increased tendon thickness (p<0.001) and higher rates of joint effusions (p<0.033) in patients receiving AIs (97).
Another proposed mechanism is that AI therapy can lead to the induction of the autoimmune system. From the rheumatology literature, estrogen appears to play a key role in the pathogenesis of autoimmune disease. In preclinical studies, animal models of human rheumatoid arthritis induced an increase in proinflammatory cytokines IFN-γ and IL-12 and decreased levels of anti-inflammatory cytokines IL-4 and IL-10 secretion when treated with anastrozole (98). AI treatment has additionally been shown to suppress differentiation of naïve T cells to Tregs which is important in preventing the autoimmune response and stimulation of CD4+ T cells involved in the inflammatory response. Thus, AI treatment results in inflammatory changes by the cytokine-induced activation of fibroblasts, macrophages, and monocytes in the joint microenvironment and the CD4+ T cell infiltration into the synovial membrane (99). Similarly, clinical studies in breast cancer patients treated with 6 months of letrozole found a significant Treg cell down-regulation (100). Further implication of a AI induced autoimmune response is that discontinuation of AI treatment results in decreased arthralgias greater than two-third of patients and decrease in autoimmune markers including ANA and RF (101).
Other preclinical studies have looked at the role of aromatase in the development of autoimmune disease. In one study, aromatase-knockout (ARKO) mice developed inflammatory changes in salivary and lacrimal glands similar to human Sjogren’s syndrome which was further exacerbated by the administration of exemestane (102). The glands of the ARKO mice had a higher infiltration of white adipose tissue which expressed increased levels of proinflammatory cytokines and macrophages compared to the wild-type mice. These results suggest that aromatase may have a role in the pathogenesis of autoimmune disease such as Sjogren syndrome. Another potential biological pathway involves SNPs that created an estrogen response element near the 3’ end of the T-cell leukemia 1A (TCL1A) gene and was associated with increased musculoskeletal pain in women on adjuvant AI for breast cancer (103). Estradiol increases expression of TCL1A. The SNP increased expression of TCL1A in an estrogen-dependent manner which resulted in upregulation of IL-17RA expression and downregulated the expression of IL-17, IL-12, IL-12RB2 and IL-1R2. IL-17 is a key driver in the T-helper type 1 and 17 immune pathway in patients with autoimmune disease (104). Therefore the E2-dependent regulation of cytokine and cytokine receptor expression mediated by TCL1A might help explain the association of TCL1A and AIMSS. However, a small clinical study of 198 postmenopausal HR+ breast cancer patients was not able to find an association between patients with AIMSS and TCL1A polymorphisms (105).
Aromatase Inhibitors: Similarities and Differences
In contrast to the first two generations of AIs, the third generation AIs demonstrate high potency by inhibiting ≥98% aromatase activity in vivo(106) and have been consistently found to suppress plasma estrogen levels >90% (107). Letrozole has been recognized to be more potent than anastrozole and exemestane at prescribed doses, though the clinical significance of this difference remains unclear (108–114). A randomized cross-over study of 12 patients found that letrozole 2.5 mg daily suppressed plasma levels of estrone, estradiol, and estrone sulfate greater than anastrozole 1 mg daily (114), and a subsequent study by the same researchers demonstrated that letrozole also suppressed estrone, estradiol, and estrone sulfate levels greater than anastrozole in breast cancer tissue in addition to plasma levels (115). Evidence of letrozole’s greater potency was further supported by a randomized cross-over study of 54 patients demonstrating significantly lower estradiol and estrone sulfate plasma levels in patients receiving letrozole versus anastrozole (116). In contrast to letrozole and anastrozole, there are limited data directly comparing the potency of exemestane’s estrogen suppressive effects with that of anastrozole and letrozole. Early studies showed that exemestane suppressed whole body aromatase activity and plasma levels of estrone, estradiol, and estrone sulfate in vivo to a similar degree as anastrozole and letrozole, though it was not compared directly with anastrozole or letrozole (115). A recent cross-over trial using exemestane and letrozole in the neoadjuvant setting discovered higher estrogen activity in serum samples during exemestane therapy than during letrozole therapy in 21 out of 26 patients, suggesting that letrozole suppresses estrogen greater than exemestane (117).
Letrozole’s modest but greater potency for estrogen deprivation has not been evidently associated with more severe AIMSS. A single-blind, crossover trial of 72 women whose HR+ breast cancer progressed on tamoxifen were randomized to either letrozole or anastrozole for four weeks and then crossed over to the other AI for another 4 weeks found significantly less reported joint pain in the letrozole arm than the anastrozole arm (3% vs 11%; p=0.025) and a greater preference for continued treatment with letrozole among participants (p<0.01) (118). Furthermore, the Articular Tolerance of Letrozole (ATOLL) study was a non-randomized prospective study that aimed to assess the effect of switching to a difference AI therapy after intolerable musculoskeletal symptoms. The study followed 179 patients who discontinued anastrozole due to AIMSS and were switched to letrozole after a 1-month anastrozole washout period. After 6 months, 71.5% of patients remained on letrozole while 28.5% of patients discontinued it due to persistent AIMSS. While AIMSS remained highly prevalent with reported rates of arthralgia (73.9%), myalgia (21%), arthritis (15.9%), tendinitis (14%), and polyalgic syndrome (12.7%), fewer musculoskeletal symptoms were reported than when on anastrozole and patients had significantly improved pain and QoL survey results after switching to letrozole. A shorter period of time until discontinuation of anastrozole was predictive of letrozole discontinuation (p=0.04). The ATOLL study showed that patients may be able to tolerate AI therapy longer if switched from anastrozole to letrozole though the study was not designed to clearly answer if letrozole or anastrozole are more likely to cause AIMSS.
A series of studies have demonstrated no difference in AIMSS between anastrozole and letrozole. One randomized multicenter trial of 713 women with advanced breast cancer found no significant differences in tolerability or safety between anastrozole and letrozole (119). The Anastrozole Versus Letrozole: Investigation into Quality of Life and Tolerability (ALIQUOT) study was an open-label crossover trial involving 181 HR+ breast cancer patients randomized to 12 weeks of letrozole followed by 12 weeks of anastrozole or vice versa designed to detect differences in adverse effects (116). In the trial, AIMSS were significantly associated with the second 12-week period in both arms indicating that the time from starting an AI rather than the difference between letrozole or anastrozole best explained joint pain development. Letrozole and anastrozole had comparable tolerability with similar rates of joint pain and quality of life measurements. Moreover, the Femara Versus Anastrozole Clinical Evaluation (FACE) trial, a randomized, open-label trial comparing adjuvant letrozole to anastrozole in node-positive HR+ breast cancer for 5 years or until disease recurrence, demonstrated similar rates of AIMSS with grade 3-4 adverse events of arthralgia 3.9% and 3.3% and myalgia 0.8% and 0.7% in letrozole and anastrozole arms, respectively (120). Additionally, one retrospective review of 141 patients in Japan comparing letrozole to anastrozole found no difference in frequency or time to onset of joint symptoms, though the anastrozole group had a shorter time to onset of painless joint symptoms (p=0.022), such as joint stiffness or decreased joint motion (121).
Data exploring comparative differences in joint pain between exemestane use with other AIs is limited and did not reveal significant differences (122, 123). While there is a paucity of literature comparing joint symptoms between exemestane against anastrozole and letrozole, studies exploring the comparative effects of exemestane on bone turnover and bone mineral density are more robust. Exemestane has been hypothesized to have less adverse effects on bone density than the non-steroidal AIs anastrozole and letrozole. Exemestane’s steroidal structure affords it distinct endocrine properties. The exemestane metabolite 17-hydroexemestane exhibits androgenic activity suggested to stimulate bone formation. One animal model showed that exemestane decreased serum markers of bone turnover pyridinoline and osteocalcin and resulted in greater bending strength and trabecular bone volume in ovariectomized rats (124). In human biomarker studies, serum procollagen type 1 N-terminal propeptide and urine N-telopeptide, markers of bone formation, were significantly increased in patients on exemestane compared to a non-steroidal AI (125, 126). However, some studies have not demonstrated significant differences in markers of bone turnover between exemestane, anastrozole, and letrozole arms (127). There is evidence that exemestane use results in less BMD loss compared to letrozole and anastrozole at 24 months of therapy (128, 129), though it remains to be seen if fewer patients on exemestane develop fractures. The MA.27 trial comparing exemestane with anastrozole in the adjuvant setting identified less reported osteoporosis in patients randomized to exemestane, 31% vs 35% in the exemestane and the anastrozole arm (p=0.001), respectively (130), however further analysis within the MA.27 cohort showed no significant differences in BMD between exemestane and anastrozole at 24 months (130). While exemestane results in BMD loss in postmenopausal women with breast cancer (131), it may cause slower BMD loss than non-steroidal AIs.
To our knowledge, there is no head-to-head study comparing anastrozole, letrozole, and exemestane designed to explore differences in the manifestations and severity of AIMSS in patients on AI therapy. Such a trial would help clarify differences in AI therapy by helping to answer if letrozole’s more potent estrogen suppression leads to greater severity of AIMSS symptoms and if exemestane’s steroidal structure results in fewer fractures. With greater understanding of the mechanisms of AIMSS, HR+ breast cancer therapies with fewer musculoskeletal symptoms can be developed. Advancements in pharmacologic research increase optimism for the design of more tolerable therapies targeting aromatase that spare musculoskeletal tissues from toxicities of estrogen deprivation (128, 132).
Management of AIMSS
Pharmacological Therapy
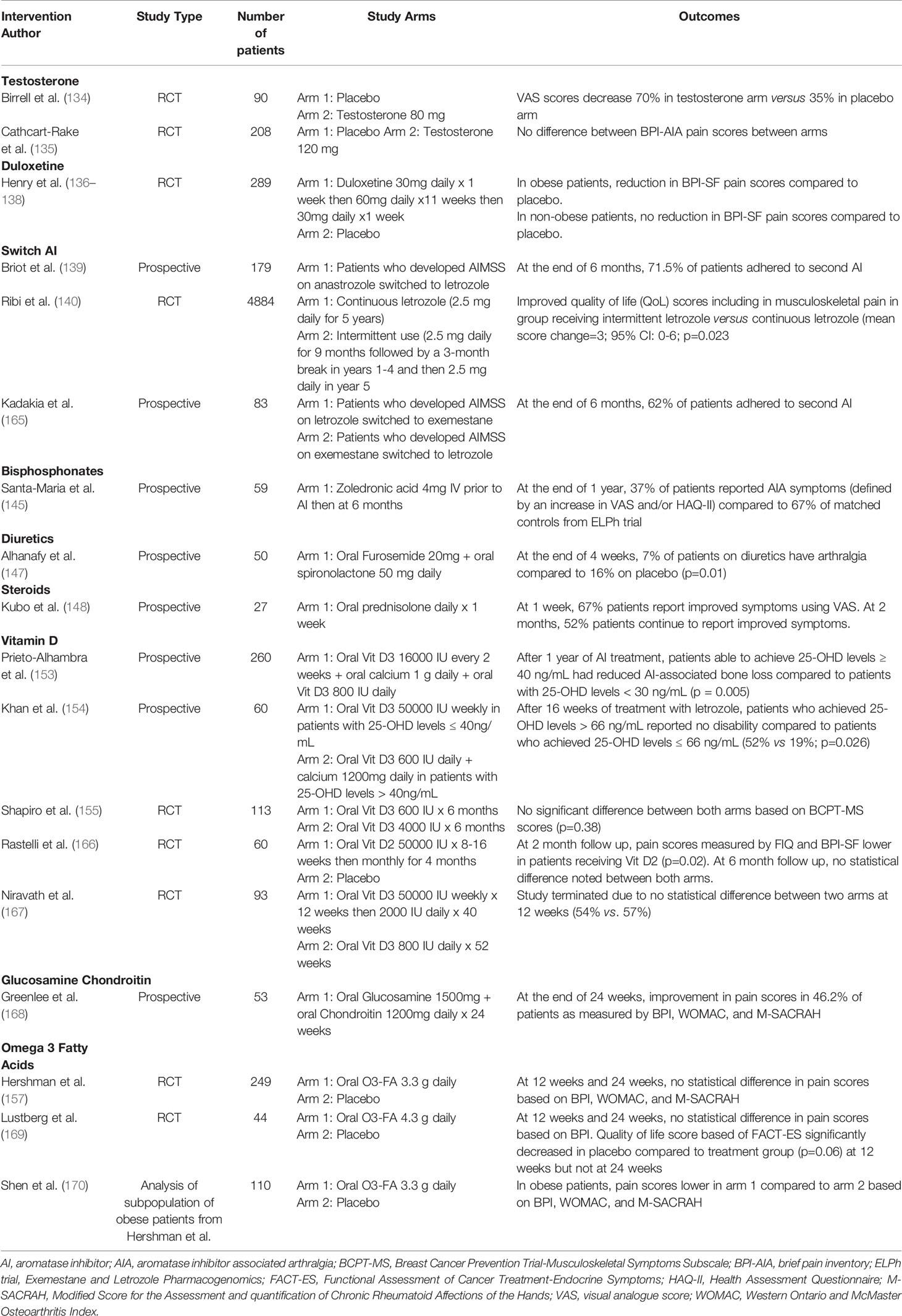
Nonsteroidal anti-inflammatory drugs and acetaminophen can be used for pain control in the short-term. Opioids should not be used in the management of pain. There is no single mitigation strategy that has been found to be effective for AIMSS. Several pharmacological and complementary modalities have been studied with mixed results (Table 2).
Testosterone
Testosterone and dihydrotestosterone, have an anti-inflammatory effect on joints minimizing joint pain and damage (133). In a phase II trial, ninety women on adjuvant anastrozole were randomized to receive placebo, 40mg of testosterone undecanoate, or 80mg of testosterone undecanoate (134). Patients receiving testosterone reported pain reduction at 3 months with 43% in the testosterone 40mg group (p=0.06) and 70% in the testosterone 80mg group (p=0.04). Additionally, testosterone levels stabilized within a physiologic range at 3 months and did not result in significantly increased estradiol concentrations, an important finding considering that HR+ breast cancers also express androgen receptors. In the subsequent A221102 trial, 208 postmenopausal women experiencing moderate-to-severe arthralgias while taking adjuvant AI were randomized to receive testosterone or placebo (135). Two thirds of patients reported improvement in joint pain at 3 months although there was no significant difference between the two groups. The discrepancy between the two studies may partially be explained by the fact that the second study utilized topical testosterone gel that may not achieve the same systemic concentrations as the oral formulation.
Duloxetine
Duloxetine is a serotonin-norepinephrine reuptake inhibitor used to treat depression and chronic pain conditions. In a randomized control trial, 300 patients with AIMSS received either duloxetine (30 mg daily for 1 week, 60 mg for week 2-11, and then 30 mg daily for week 12) or placebo (136). At week 6 of treatment, patients receiving duloxetine had a clinically significant decrease in joint pain score (> 2 points) compared to placebo (68% versus 49%; p=0.003). By 12 weeks, the average joint score was 0.82 points lower in the duloxetine-treated group compared to placebo. However, once the drug was discontinued, the pain scores were equivalent in the two arms, suggesting that duloxetine exerts an analgesic effect rather than a reversal in the disease process. In addition, rates of adverse events were higher in the group receiving duloxetine (78% versus 50%). Although duloxetine decreases AIMSS, benefit is only derived for the treatment duration and the adverse effects may make it intolerable for some patients. However, in the SWOGS1202 randomized control trial, 289 were randomized to receive duloxetine versus placebo (137). Patients receiving duloxetine were more likely to report more beneficial than placebo (73.3% vs 41.8%, respectively; 95% CI for difference = 15.4-47.2 percentage points). Further sub-analysis revealed that obese patients (BMI ≥ 30 kg/m2) obtained more analgesic benefits than the non-obese with a significant reduction in pain scores obese (-2.73 vs -1.64 points; P = .003). Conversely, in the nonobese patients, the reduction in the mean average pain score was similar in the 2 cohorts (-2.46 vs -2.34 points; P = .75) (138).
Switching AIs or Intermittent Dosing of AIs
Another clinical approach suggests benefit from alternating between AI agents within the same class. In the prospective, non-randomized ATOLL trial, 179 patients who had discontinued anastrozole due to musculoskeletal symptoms received letrozole (139). At the end of the 6-month period, 72% of patients continued the letrozole with the remaining discontinuing treatment secondary to severe joint pain. Furthermore, among patients who continued letrozole, 74% continued to experience arthralgias, myalgias, arthritis, or tendinitis. The phase III SOLE trial investigated the intermittent use versus continuous use of letrozole. Postmenopausal women who had completed 4-6 years of adjuvant endocrine therapy and were clinically disease free of breast cancer were randomized to receive either continuous letrozole (2.5 mg daily for 5 years) or intermittent use (2.5 mg daily for 9 months followed by a 3-month break in years 1-4 and then 2.5 mg daily in year 5) (140). Although the primary outcome of disease-free survival (DFS) did not improve between the groups, patients receiving intermittent letrozole reported better quality of life (QoL) scores including in musculoskeletal pain (mean score change=3; 95% CI: 0-6; p=0.023) (141). The rates of adherence of letrozole were similar between both groups which could be the result of enrolling patients who had previously tolerated 4-6 years of endocrine therapy. Therefore, they may not have experienced significant AIMSS leading to discontinuation of the drug. These results suggest that intermittent administration of AIs can result in increased tolerability of the drug without significantly affecting disease outcomes.
Bisphosphonates and Denosumab
Bisphosphonates have an integral role in the treatment of osteoporosis given their ability to decrease bone loss and increase bone density. Several interdisciplinary cancer and bone societies have developed an algorithm for the management of AI-associated bone loss (142). In all patients initiating AI treatment, lifestyle changes such as diet rich in calcium, weight-bearing exercise, limitation in alcohol and smoking cessation are recommended. All patients should be monitored for fracture risk and bone marrow density (BMD) every 1-2 years. Risk factors associated with increased fracture risk should be identified which include age > 65 years, T-score < 1.5, smoking, BMI<24, family history of hip fractures, personal history of fragility fracture above age 50, and oral glucocorticoid use >6 months. For patients with a T-score < -2.0 and/or more than two of the above risk factors is present, an anti-resorptive agent, such as bisphosphonates or denosumab, should be utilized. In an observational cohort study involving 36,472 breast cancer patients, fracture risk in AI users was > 40% compared to tamoxifen (143). However, amongst patients on AIs at high risk of fracture, bisphosphonate-treated patients had an HR 0.73 [95% CI, 0.51 to 1.04] and SHR 0.69 [95% CI, 0.48 to 0.98] for fractures compared to those not on concomitant bisphosphonates. In the ABCSG-18 trial, AI-treated patients who received denosumab for 3 years showed a lower incidence of fractures and a significant increase of the femoral neck and lumbar spine BMD compared to patients receiving placebo (144).
Based on the efficacy of bisphosphonates in decreasing bone loss, studies have investigated their role in the management of AIA. In the single-arm Zoledronic Acid Prophylaxis (ZAP) trial, 59 postmenopausal breast cancer patients received zoledronic acid concomitantly with letrozole for six months (145). Compared to historical controls from the Exemestane and Letrozole Pharmacogenomics (ELPh) trial, there was a significant decrease in AIA within the zoledronic acid group (AIA incidence: 37% versus 67%; p<0.001).
Diuretics
A retrospective analysis of women treated with adjuvant AI therapy showed that women who were on chronic diuretic treatment for heart disease or hypertension were less likely to have symptoms of arthralgia, muscular or skeletal stiffness (6.97% versus 15.85%, P = 0.01), suggesting that fluid retention within joints may play a role in AI-induced arthralgia (146). In a phase II trial, 50 women were randomized to receive diuretic or placebo for 4 weeks (147). After the treatment period, the modified Western Ontario and McMaster Universities osteoarthritis (WOMAC) index for lower limb improved significantly (6.0 v 10; P < 0.001), in addition to improvement in the mean WOMAC stiffness score (2.3 v 3.9; P = 0.002), the mean WOMAC functional score (8.7 v 15; P < 0.001), and the total WOMAC score (17 v 29; P < 0.001).
Steroids
In a single-arm study, 27 patients with AIMSS were administered 5 mg of prednisolone daily for one week (148). Pain scores improved in 67% of patients at one week with one half of patients still reporting analgesic benefit at two months follow-up. Further studies need to be done to examine the long-term benefit of this intervention in these patients.
Vitamin D Supplementation
Patients receiving AIs are frequently vitamin D deficient most likely due to the role estrogen has in the activation of vitamin D and its receptor (149). Vitamin D is essential for calcium absorption and bone mineralization, which is positively associated with bone mineral density. A number of studies have suggested the importance of vitamin D in improving muscle strength and function (150–152). Therefore, correcting a vitamin D deficient state may aid in alleviating musculoskeletal symptoms associated with AI-treatment. In a prospective cohort study involving 290 breast cancer patients received 800 IU of vitamin D3 daily (16000 IU of vitamin D3 every 2 weeks given in addition to those with baseline 25(OH)D concentration <30 ng/ml) (153). Although there was an increase in joint pain in the cohort based on visual analog scale (VAS) for joint pain (mean 1.16 points SD 2.66; P < 0.001), the increase was significantly less (p=0.02) in those patients who were able to reach 25(OH)D concentrations of ≥40 ng/ml. Similar findings were reported in a cohort study that demonstrated differences in pain scores between patients receiving vitamin D3 supplementation who achieved 25(OH)D concentrations of ≥ 66 ng/ml (19% versus 52%; p=0.026) (154). Conversely, in a recent trial that randomized breast cancer patients experiencing AIMSS to receive either 600IU D3 or 4000 IU D3, there was no statistical significance in the primary endpoint which was a change in AIMSS score from baseline (155). Another important issue is that certain breast cancer subtypes express VDR and long term clinical data will be needed to ensure that vitamin D supplementation does not have a negative therapeutic impact. Further research is needed to examine the utility of vitamin D supplementation as a way to ameliorate AIMSS.
Omega-3 Fatty Acids
Omega-3 fatty acids (O3-FA) has demonstrated efficacy in decreasing joint pain, the number of swollen joints, and the use of NSAIDs in patients with inflammatory arthritis, prompting an investigation with regard to its role in AIMSS management (156). In the SWOG S0927 trial, 262 women were randomized to receive O3-FA versus placebo for 24 weeks. At the end of the study period, AIMSS symptoms as measured with the Brief Pain Inventory-Short Form (BPI-SF) decreased in the O3-FA group compared to placebo group (mean score=2.22 versus 1.81; p<0.001) (157). A post-hoc analysis demonstrated a significant improvement in pain scores in obese patients (BMI≥ 30kg/m2) receiving O3-FA versus placebo; a difference absent among non-obese patients (BMI < 30kg/m2). These findings may be attributed to the anti-inflammatory effect of O3-FAs on adipose tissue, which is thought to be a source of inflammatory mediators.
Complementary Therapy
Physical Activity
Exercise can have multiple benefits in the management of AIMSS. Similar to its role in osteoporosis, exercise can help increase bone density (158). Moreover, it can increase the circulation of body fluid to tissues and increase the volume of skeletal muscle making physical activity easier (159). Exercise may also increase the pain threshold by improving the range of motion and muscle strength of patients experiencing musculoskeletal symptoms (160). An effective exercise program refers for the prevention and improvement of AIMSS includes aerobic exercise, resistance exercise, or a combination of both. It can involve activities such as yoga, walking, and swimming. Exercise intensity should be guided by an exercise trainer at a safe and comfortable pace or no more than 80% of heart rate reserve. A meta-analysis of 9 studies involving 743 participants evaluated the effect of aerobic exercise in alleviating AIMSS. The duration of interventions ranged from 6 weeks to 12 months with at least 120 minutes/week of exercise prescribed. Aerobic exercise was performed in all studies, five of which included resistance exercise. Results demonstrated an improvement in scores of pain (p=0.006), stiffness (p=0.01), and grip strength (p=0.002) and an overall improvement in quality of life, regardless of the form of exercise program undertaken (161). Another form of exercise providing relief was yoga. In a study, 95 breast cancer patients on AI or tamoxifen were randomized to undergo standard care versus 4 weeks of yoga. At baseline, the AI group reported higher levels of myalgias and arthralgias; however, amongst those in the yoga group, there was improved scores at the end of the intervention period with 90% reporting reduced severity of symptoms (162).
Acupuncture
Acupuncture is a non-pharmacologic modality used in the treatment of a variety of pain conditions. Small trials have investigated its role in the treatment of AIMSS. In a RCT, 226 patients were randomized to true acupuncture, sham acupuncture, or no treatment for 6 weeks (163). The BPI-WP at the end of the study period demonstrated a decrease by 2 points in the true acupuncture group compared to 1 point in the other two groups. The pain-reporting guidelines describe the clinical meaning of an individual’s response, with studies suggesting that a reduction of 2 points on an 11-point scale (or 30%) represents a clinically significant improvement (164). In this study, 58% of patients in the true acupuncture group reported at least a 2-point improvement compared to 33% in the sham group and 31% in the no treatment group. Currently, a RCT investigating a novel treatment of auricular point acupuncture as a low-cost intervention in the management of AIMSS (NCT03697200) is underway.
Future Directions
Despite the central role of AIs in the chemoprevention and management of HR+ breast cancer in postmenopausal women, significant musculoskeletal side effects have led to premature discontinuation of therapy in patients. AIMSS encompasses bone loss, arthralgias, and autoimmune rheumatologic disease. Although there have been several mechanisms proposed for AIMSS, it remains challenging to predict which patients will develop musculoskeletal symptoms. In the B-ABLE study (NCT03811509), patients are randomized to receive bisphosphonates or denosumab concurrently with AI. The aim of this trial is to identify the deleterious effects of AIs on bone microarchitecture by dual energy x-ray absorptiometry (DEXA), lumbar spine Rx, Trabecular Bone Score (TBS) and bone mineral strength (BMSi) for early identification of patients at risk for developing AI-bone loss. Evaluation of cartilage degradation markers (C-telopeptide II, Procollagen type 2 N-terminal propeptide) may provide valuable information to identify both patients at risk, and further serve as objective markers for monitoring the development of AIMSS. Recently, there has been growing interest in potential genetic determinants leading to AIMSS. An active cohort study involving 1000 participants is underway to further elucidate the role of other SNPs in cytochrome P450 enzymes (CYP), glucuronosyltransferases (UGT), Vitamin D, serotonin and other receptors that may be associated with discontinuation of treatment due to the development of severe AIMSS (NCT01824836). The primary goal is to develop a gene signature that can help identify patients at risk for developing severe AIMSS. Additionally, there are a number of ongoing trials focusing on potential AIMSS management strategies including auriculotherapy (NCT03096041), physical therapy (NCT04560699), curcumin (NCT03865992), cannabidiol (NCT04754399), and tai chi (NCT04716920). Ultimately, a deeper understanding of the underlying mechanisms of AIMSS is needed to address and develop effective therapeutic strategies for the musculoskeletal adverse effects of AI therapy.
Author Contributions
All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Howlader N, Altekruse SF, Li CI, Chen VW, Clarke CA, Ries LA, et al. US Incidence of Breast Cancer Subtypes Defined by Joint Hormone Receptor and HER2 Status. J Natl Cancer Inst (2014) 106(5):dju055. doi: 10.1093/jnci/dju055
2. Subramanian A, Salhab M, Mokbel K. Oestrogen Producing Enzymes and Mammary Carcinogenesis: A Review. Breast Cancer Res Treat (2008) 111(2):191–202. doi: 10.1007/s10549-007-9788-0
3. Santen RJ, Brodie H, Simpson ER, Siiteri PK, Brodie A. History of Aromatase: Saga of an Important Biological Mediator and Therapeutic Target. Endocr Rev (2009) 30(4):343–75. doi: 10.1210/er.2008-0016
4. Zhao H, Zhou L, Shangguan AJ, Bulun SE. Aromatase Expression and Regulation in Breast and Endometrial Cancer. J Mol Endocrinol (2016) 57(1):R19–33. doi: 10.1530/JME-15-0310
5. Russo J, Russo IH. The Role of Estrogen in the Initiation of Breast Cancer. J Steroid Biochem Mol Biol (2006) 102(1-5):89–96. doi: 10.1016/j.jsbmb.2006.09.004
6. Lippman ME, Krueger KA, Eckert S, Sashegyi A, Walls EL, Jamal S, et al. Indicators of Lifetime Estrogen Exposure: Effect on Breast Cancer Incidence and Interaction With Raloxifene Therapy in the Multiple Outcomes of Raloxifene Evaluation Study Participants [Published Correction Appears in J Clin Oncol 2002 Mar 1;20(5):1430]. J Clin Oncol (2001) 19(12):3111–6. doi: 10.1200/JCO.2001.19.12.3111
7. Nabholtz JM, Mouret-Reynier MA, Durando X, Van Praagh I, Al-Sukhun S, Ferriere JP, et al. Comparative Review of Anastrozole, Letrozole and Exemestane in the Management of Early Breast Cancer. Expert Opin Pharmacother (2009) 10(9):1435–47. doi: 10.1517/14656560902953738
8. Tomao F, Spinelli G, Vici P, Pisanelli GC, Cascialli G, Frati L, et al. Current Role and Safety Profile of Aromatase Inhibitors in Early Breast Cancer. Expert Rev Anticancer Ther (2011) 11(8):1253–63. doi: 10.1586/era.11.96
9. Goss PE, Ingle JN, Alés-Martínez JE, Cheung AM, Chlebowski RT, Wactawski-Wende J, et al. Exemestane for Breast-Cancer Prevention in Postmenopausal Women [Published Correction Appears in N Engl J Med. N Engl J Med (2011) 364(25):2381–91. doi: 10.1056/NEJMoa1103507
10. Cuzick J, Sestak I, Cawthorn S, Hamed H, Holli A, Howell K, et al. Tamoxifen for Prevention of Breast Cancer: Extended Long-Term Follow-Up of the IBIS-I Breast Cancer Prevention Trial. Lancet Oncol (2015) 16(1):67–75. doi: 10.1016/S1470-2045(14)71171-4
11. Cuzick J, Sestak I, Forbes JF, Dowsett M, Cawthorn S, Mansel RE, et al. Use of Anastrozole for Breast Cancer Prevention (IBIS-II): Long-Term Results of a Randomised Controlled Trial [Published Correction Appears in Lancet. Lancet (2020) 395(10218):117–22. doi: 10.1016/S0140-6736(19)32955-1
12. Pineda-Moncusí M, Garcia-Giralt N, Diez-Perez A, Tusquets I, Servitja S, Albanell J, et al. Thromboembolic, Cardiovascular and Overall Mortality Risks of Aromatase Inhibitors, Compared With Tamoxifen Treatment: An Outpatient-Register-Based Retrospective Cohort Study. Ther Adv Med Oncol (2020) 12:1758835920909660. doi: 10.1177/1758835920909660
13. Gibson L, Lawrence D, Dawson C, Bliss J. Aromatase Inhibitors for Treatment of Advanced Breast Cancer in Postmenopausal Women. Cochrane Database Syst Rev (2009) 2009(4):CD003370. doi: 10.1002/14651858.CD003370.pub3
14. Rydén L, Heibert Arnlind M, Vitols S, Höistad M, Ahlgren J. Aromatase Inhibitors Alone or Sequentially Combined With Tamoxifen in Postmenopausal Early Breast Cancer Compared With Tamoxifen or Placebo - Meta-Analyses on Efficacy and Adverse Events Based on Randomized Clinical Trials. Breast (2016) 26:106–14. doi: 10.1016/j.breast.2016.01.006
15. Mamounas EP, Jeong JH, Wickerham DL, Smith RE, Ganz PA, Land SR, et al. Benefit From Exemestane as Extended Adjuvant Therapy After 5 Years of Adjuvant Tamoxifen: Intention-to-Treat Analysis of the National Surgical Adjuvant Breast and Bowel Project B-33 Trial. J Clin Oncol (2008) 26(12):1965–71. doi: 10.1200/JCO.2007.14.0228
16. Jakesz R, Greil R, Gnant M, Schmid M, Kwasny W, Kubista E, et al. Extended Adjuvant Therapy With Anastrozole Among Postmenopausal Breast Cancer Patients: Results From the Randomized Austrian Breast and Colorectal Cancer Study Group Trial 6a [Published Correction Appears in J Natl Cancer Inst. J Natl Cancer Inst (2007) 99(24):1845–53. doi: 10.1093/jnci/djm246
17. Boccardo F, Rubagotti A, Guglielmini P, Fini A, Paladini G, Mesiti M, et al. Switching to Anastrozole Versus Continued Tamoxifen Treatment of Early Breast Cancer. Updated Results of the Italian Tamoxifen Anastrozole (ITA) Trial. Ann Oncol (2006) 17 Suppl 7:vii10–4. doi: 10.1093/annonc/mdl941
18. Goss PE, Ingle JN, Martino S, Robert NJ, Muss HB, Piccart MJ, et al. Randomized Trial of Letrozole Following Tamoxifen as Extended Adjuvant Therapy in Receptor-Positive Breast Cancer: Updated Findings From NCIC CTG MA.17. J Natl Cancer Inst (2005) 97(17):1262–71. doi: 10.1093/jnci/dji250
19. Jakesz R, Jonat W, Gnant M, Mittlboeck M, Greil R, Tausch C, et al. Switching of Postmenopausal Women With Endocrine-Responsive Early Breast Cancer to Anastrozole After 2 Years' Adjuvant Tamoxifen: Combined Results of ABCSG Trial 8 and ARNO 95 Trial. Lancet (2005) 366(9484):455–62. doi: 10.1016/S0140-6736(05)67059-6
20. Goss PE, Ingle JN, Pritchard KI, Robert NJ, Muss H, Gralow J, et al. Extending Aromatase-Inhibitor Adjuvant Therapy to 10 Years. N Engl J Med (2016) 375(3):209–19. doi: 10.1056/NEJMoa1604700
21. Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year Risks of Breast-Cancer Recurrence After Stopping Endocrine Therapy at 5 Years. N Engl J Med (2017) 377(19):1836–46. doi: 10.1056/NEJMoa1701830
22. Francis PA, Pagani O, Fleming GF, Walley BA, Colleoni M, Láng I, et al. Tailoring Adjuvant Endocrine Therapy for Premenopausal Breast Cancer. N Engl J Med (2018) 379(2):122–37. doi: 10.1056/NEJMoa1803164
23. Pagani O, Francis PA, Fleming GF, Walley BA, Viale G, Colleoni M, et al. Absolute Improvements in Freedom From Distant Recurrence to Tailor Adjuvant Endocrine Therapies for Premenopausal Women: Results From TEXT and SOFT. J Clin Oncol (2020) 38(12):1293–303. doi: 10.1200/JCO.18.01967
24. Regan MM, Francis PA, Pagani O, Fleming GF, Walley BA, Viale G, et al. Absolute Benefit of Adjuvant Endocrine Therapies for Premenopausal Women With Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Early Breast Cancer: TEXT and SOFT Trials. J Clin Oncol (2016) 34(19):2221–31. doi: 10.1200/JCO.2015.64.3171
25. Arimidex, Tamoxifen, Alone or in Combination Trialists' Group, Buzdar A, Howell A, Cuzick J, Wale C, Distler W, et al. Comprehensive Side-Effect Profile of Anastrozole and Tamoxifen as Adjuvant Treatment for Early-Stage Breast Cancer: Long-Term Safety Analysis of the ATAC Trial. Lancet Oncol (2006) 7(8):633–43. doi: 10.1016/S1470-2045(06)70767-7
26. Cella D, Fallowfield L, Barker P, Cuzick J, Locker G, Howell A, et al. Quality of Life of Postmenopausal Women in("Arimidex", Tamoxifen, Alone or in Combination) Trial After Completion of 5 Years' Adjuvant Treatment for Early Breast Cancer. Breast Cancer Res Treat (2006) 100(3):273–84. doi: 10.1007/s10549-006-9260-6
27. Condorelli R, Vaz-Luis I. Managing Side Effects in Adjuvant Endocrine Therapy for Breast Cancer. Expert Rev Anticancer Ther (2018) 18(11):1101–12. doi: 10.1080/14737140.2018.1520096
28. Laroche F, Coste J, Medkour T, Cottu PH, Pierga JY, Lotz JP, et al. Classification of and Risk Factors for Estrogen Deprivation Pain Syndromes Related to Aromatase Inhibitor Treatments in Women With Breast Cancer: A Prospective Multicenter Cohort Study. J Pain (2014) 15(3):293–303. doi: 10.1016/j.jpain.2013.11.004
29. Gaillard S, Stearns V. Aromatase Inhibitor-Associated Bone and Musculoskeletal Effects: New Evidence Defining Etiology and Strategies for Management. Breast Cancer Res (2011) 13(2):205. doi: 10.1186/bcr2818
30. Beckwée D, Leysen L, Meuwis K, Adriaenssens N. Prevalence of Aromatase Inhibitor-Induced Arthralgia in Breast Cancer: A Systematic Review and Meta-Analysis. Support Care Cancer (2017) 25(5):1673–86. doi: 10.1007/s00520-017-3613-z
31. Presant CA, Bosserman L, Young T, Vakil M, Horns R, Upadhyaya G, et al. Aromatase Inhibitor-Associated Arthralgia and/ or Bone Pain: Frequency and Characterization in non-Clinical Trial Patients. Clin Breast Cancer (2007) 7(10):775–8. doi: 10.3816/CBC.2007.n.038
32. Mao JJ, Stricker C, Bruner D, Xie S, Bowman MA, Farrar JT, et al. Patterns and Risk Factors Associated With Aromatase Inhibitor-Related Arthralgia Among Breast Cancer Survivors. Cancer (2009) 115(16):3631–9. doi: 10.1002/cncr.24419
33. Cuzick J, Sestak I, Forbes JF, Dowsett M, Knox J, Cawthorn S, et al. Anastrozole for Prevention of Breast Cancer in High-Risk Postmenopausal Women (IBIS-II): An International, Double-Blind, Randomised Placebo-Controlled Trial.Lancet (2014) 22:1041–8383. doi: 10.1016/S0140-6736(13)62292-8
34. Crew KD, Greenlee H, Capodice J, Raptis G, Brafman L, Fuentes D, et al. Prevalence of Joint Symptoms in Postmenopausal Women Taking Aromatase Inhibitors for Early-Stage Breast Cancer. J Clin Oncol (2007) 25(25):3877–83. doi: 10.1200/JCO.2007.10.7573
35. Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early Discontinuation and non-Adherence to Adjuvant Hormonal Therapy are Associated With Increased Mortality in Women With Breast Cancer. Breast Cancer Res Treat (2011) 126(2):529–37. doi: 10.1007/s10549-010-1132-4
36. Collin LJ, Cronin-Fenton DP, Ahern TP, Goodman M, McCullough LE, Waller LA, et al. Early Discontinuation of Endocrine Therapy and Recurrence of Breast Cancer Among Premenopausal Women. Clin Cancer Res (2021) 27(5):1421–8. doi: 10.1158/1078-0432.CCR-20-3974
37. Henry NL, Azzouz F, Desta Z, Li L, Nguyen AT, Lemler S, et al. Predictors of Aromatase Inhibitor Discontinuation as a Result of Treatment-Emergent Symptoms in Early-Stage Breast Cancer. J Clin Oncol (2012) 30(9):936–42. doi: 10.1200/JCO.2011.38.0261
38. Bell SG, Dalton L, McNeish BL, Fang F, Henry NL, Kidwell KM, et al. Aromatase Inhibitor Use, Side Effects and Discontinuation Rates in Gynecologic Oncology Patients. Gynecol Oncol (2020) 159(2):509–14. doi: 10.1016/j.ygyno.2020.08.015
39. Osborne CK. Tamoxifen in the Treatment of Breast Cancer. N Engl J Med (1998) 339(22):1609–18. doi: 10.1056/NEJM199811263392207
40. Kwan ML, Roh JM, Laurent CA, Lee J, Tang L, Hershman D, et al. Patterns and Reasons for Switching Classes of Hormonal Therapy Among Women With Early-Stage Breast Cancer. Cancer Causes Control (2017) 28(6):557–62. doi: 10.1007/s10552-017-0888-9
41. Shapiro CL. Osteoporosis: A Long-Term and Late-Effect of Breast Cancer Treatments. Cancers (Basel) (2020) 12(11):3094. doi: 10.3390/cancers12113094
42. Mirza F, Canalis E. Management of Endocrine Disease: Secondary Osteoporosis: Pathophysiology and Management. Eur J Endocrinol (2015) 173(3):R131–51. doi: 10.1530/EJE-15-0118
43. Lee CI, Goodwin A, Wilcken N. Fulvestrant for Hormone-Sensitive Metastatic Breast Cancer. Cochrane Database Syst Rev (2017) 1(1):CD011093. doi: 10.1002/14651858.CD011093.pub2
44. Robertson JFR, Bondarenko IM, Trishkina E, Dvorkin M, Panasci L, Manikhas A, et al. Fulvestrant 500 Mg Versus Anastrozole 1 Mg for Hormone Receptor-Positive Advanced Breast Cancer (FALCON): An International, Randomised, Double-Blind, Phase 3 Trial. Lancet (2016) 388(10063):2997–3005. doi: 10.1016/S0140-6736(16)32389-3
45. Vergote I, Robertson JF. Fulvestrant is an Effective and Well-Tolerated Endocrine Therapy for Postmenopausal Women With Advanced Breast Cancer: Results From Clinical Trials. Br J Cancer (2004) 90 Suppl 1(Suppl 1):S11–4. doi: 10.1038/sj.bjc.6601631
46. Al-Mubarak M, Sacher AG, Ocana A, Vera-Badillo F, Seruga B, Amir E. Fulvestrant for Advanced Breast Cancer: A Meta-Analysis. Cancer Treat Rev (2013) 39(7):753–8. doi: 10.1016/j.ctrv.2013.03.004
47. Sestak I, Cuzick J, Sapunar F, Eastell R, Forbes JF, Bianco AR, et al. Risk Factors for Joint Symptoms in Patients Enrolled in the ATAC Trial: A Retrospective, Exploratory Analysis. Lancet Oncol (2008) 9(9):866–72. doi: 10.1016/S1470-2045(08)70182-7
48. Yardley DA. Pharmacologic Management of Bone-Related Complications and Bone Metastases in Postmenopausal Women With Hormone Receptor-Positive Breast Cancer. Breast Cancer (Dove Med Press) (2016) 8:73–82. doi: 10.2147/BCTT.S97963
49. Mieog JS, Morden JP, Bliss JM, Coombes RC, van de Velde CJ, IES Steering Committee. Carpal Tunnel Syndrome and Musculoskeletal Symptoms in Postmenopausal Women With Early Breast Cancer Treated With Exemestane or Tamoxifen After 2-3 Years of Tamoxifen: A Retrospective Analysis of the Intergroup Exemestane Study. Lancet Oncol (2012) 13(4):420–32. doi: 10.1016/S1470-2045(11)70328-X
50. Borrie AE, Rose FA, Choi YH, Perera FE, Read N, Sexton T, et al. Genetic and Clinical Predictors of Arthralgia During Letrozole or Anastrozole Therapy in Breast Cancer Patients. Breast Cancer Res Treat (2020) 183(2):365–72. doi: 10.1007/s10549-020-05777-1
51. Kanematsu M, Morimoto M, Honda J, Nagao T, Nakagawa M, Takahashi M, et al. The Time Since Last Menstrual Period is Important as a Clinical Predictor for non-Steroidal Aromatase Inhibitor-Related Arthralgia. BMC Cancer (2011) 11:436. doi: 10.1186/1471-2407-11-436
52. Folkerd EJ, Dixon JM, Renshaw L, A'Hern RP, Dowsett M. Suppression of Plasma Estrogen Levels by Letrozole and Anastrozole is Related to Body Mass Index in Patients With Breast Cancer. J Clin Oncol (2012) 30(24):2977–80. doi: 10.1200/JCO.2012.42.0273
53. Jiang L, Xie X, Wang Y, Wang Y, Lu Y, Tian T, et al. Body Mass Index and Hand Osteoarthritis Susceptibility: An Updated Meta-Analysis. Int J Rheum Dis (2016) 19(12):1244–54. doi: 10.1111/1756-185X.12895
54. Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The Incidence of Co-Morbidities Related to Obesity and Overweight: A Systematic Review and Meta-Analysis. BMC Public Health (2009) 9:88. doi: 10.1186/1471-2458-9-88
55. Shiri R, Pourmemari MH, Falah-Hassani K, Viikari-Juntura E. The Effect of Excess Body Mass on the Risk of Carpal Tunnel Syndrome: A Meta-Analysis of 58 Studies. Obes Rev (2015) 16(12):1094–104. doi: 10.1111/obr.12324
56. Horimoto Y, Saito M, Kasumi F. Arthralgia in 329 Patients Taking Aromatase Inhibitors. Breast Care (Basel) (2009) 4(5):319–23. doi: 10.1159/000236050
57. Park JY, Lee SK, Bae SY, Kim J, Kim MK, Kil WH, et al. Aromatase Inhibitor-Associated Musculoskeletal Symptoms: Incidence and Associated Factors. J Korean Surg Soc (2013) 85(5):205–11. doi: 10.4174/jkss.2013.85.5.205
58. Henry NL, Giles JT, Ang D, Mohan M, Dadabhoy D, Robarge J, et al. Prospective Characterization of Musculoskeletal Symptoms in Early Stage Breast Cancer Patients Treated With Aromatase Inhibitors. Breast Cancer Res Treat (2008) 111(2):365–72. doi: 10.1007/s10549-007-9774-6
59. Menas P, Merkel D, Hui W, Lawton J, Harper A, Carro G. Incidence and Management of Arthralgias in Breast Cancer Patients Treated With Aromatase Inhibitors in an Outpatient Oncology Clinic. J Oncol Pharm Pract (2012) 18(4):387–93. doi: 10.1177/1078155211434853
60. Moscetti L, Agnese Fabbri M, Sperduti I, Mohan M, Dadabhoy D, Robarge J, et al. Adjuvant Aromatase Inhibitor Therapy in Early Breast Cancer: What Factors Lead Patients to Discontinue Treatment? . Tumori (2015) 101(5):469–73. doi: 10.5301/tj.5000376
61. Chien HC, Kao Yang YH, Kwoh CK, Chalasani P, Wilson DL, Lo-Ciganic WH. Aromatase Inhibitors and Risk of Arthritis and Carpal Tunnel Syndrome Among Taiwanese Women With Breast Cancer: A Nationwide Claims Data Analysis. J Clin Med (2020) 9(2):566. doi: 10.3390/jcm9020566
62. Asthana R, Zhang L, Wan BA, Gallo-Hershberg D, Giotis A, Pasetka M, et al. Pain Descriptors of Taxane Acute Pain Syndrome (TAPS) in Breast Cancer Patients-a Prospective Clinical Study. Support Care Cancer (2020) 28(2):589–98. doi: 10.1007/s00520-019-04845-7
63. Fenlon D, Addington-Hall JM, O'Callaghan AC, Clough J, Nicholls P, Simmonds P. A Survey of Joint and Muscle Aches, Pain, and Stiffness Comparing Women With and Without Breast Cancer. J Pain Symptom Manage (2013) 46(4):523–35. doi: 10.1016/j.jpainsymman.2012.10.282
64. Andrikopoulou A, Fiste O, Liontos M, Dimopoulos MA, Zagouri F. Aromatase and CDK4/6 Inhibitor-Induced Musculoskeletal Symptoms: A Systematic Review. Cancers (Basel) (2021) 13(3):465. doi: 10.3390/cancers13030465
65. Fontein DB, Seynaeve C, Hadji P, Hille ET, van de Water W, Putter H, et al. Specific Adverse Events Predict Survival Benefit in Patients Treated With Tamoxifen or Aromatase Inhibitors: An International Tamoxifen Exemestane Adjuvant Multinational Trial Analysis. J Clin Oncol (2013) 31(18):2257–64. doi: 10.1200/JCO.2012.45.3068
66. Huober J, Cole BF, Rabaglio M, Giobbie-Hurder A, Wu J, Ejlertsen B, et al. Symptoms of Endocrine Treatment and Outcome in the BIG 1-98 Study. Breast Cancer Res Treat (2014) 143(1):159–69. doi: 10.1007/s10549-013-2792-7
67. Hadji P, Kieback DG, Tams J, Hasenburg A, Ziller M. Correlation of Treatment-Emergent Adverse Events and Clinical Response to Endocrine Therapy in Early Breast Cancer: A Retrospective Analysis of the German Cohort of TEAM. Ann Oncol (2012) 23(10):2566–72. doi: 10.1093/annonc/mds055
68. Cuzick J, Sestak I, Cella D, Fallowfield L, ATAC Trialists' Group. Treatment-Emergent Endocrine Symptoms and the Risk of Breast Cancer Recurrence: A Retrospective Analysis of the ATAC Trial. Lancet Oncol (2008) 9(12):1143–8. doi: 10.1016/S1470-2045(08)70259-6
69. Stearns V, Chapman JA, Ma CX, Ellis MJ, Ingle JN, Pritchard KI, et al. Treatment-Associated Musculoskeletal and Vasomotor Symptoms and Relapse-Free Survival in the NCIC CTG MA.27 Adjuvant Breast Cancer Aromatase Inhibitor Trial. J Clin Oncol (2015) 33(3):265–71. doi: 10.1200/JCO.2014.57.6926
70. Yoo TK, Jang MJ, Lee E, Moon HG, Noh DY, Han W. Endocrine Treatment-Related Symptoms and Patient Outcomes in Breast Cancer: A Meta-Analysis. J Breast Cancer (2018) 21(1):37–44. doi: 10.4048/jbc.2018.21.1.37
71. Eastell R, Adams JE, Coleman RE, Howell A, Hannon RA, Cuzick J, et al. Effect of Anastrozole on Bone Mineral Density: 5-Year Results From the Anastrozole, Tamoxifen, Alone or in Combination Trial 18233230. J Clin Oncol (2008) 26(7):1051–7. doi: 10.1200/JCO.2007.11.0726
72. De Placido S, Gallo C, De Laurentiis M, Bisagni G, Arpino G, Sarobba MG, et al. Adjuvant Anastrozole Versus Exemestane Versus Letrozole, Upfront or After 2 Years of Tamoxifen, in Endocrine-Sensitive Breast Cancer (FATA-GIM3): A Randomised, Phase 3 Trial. Lancet Oncol (2018) 19(4):474–85. doi: 10.1016/S1470-2045(18)30116-5
73. Rabaglio M, Sun Z, Price KN, Castiglione-Gertsch M, Hawle H, Thürlimann B, et al. Bone Fractures Among Postmenopausal Patients With Endocrine-Responsive Early Breast Cancer Treated With 5 Years of Letrozole or Tamoxifen in the BIG 1-98 Trial. Ann Oncol (2009) 20(9):1489–98. doi: 10.1093/annonc/mdp033
74. Amir E, Seruga B, Niraula S, Carlsson L, Ocaña A. Toxicity of Adjuvant Endocrine Therapy in Postmenopausal Breast Cancer Patients: A Systematic Review and Meta-Analysis. J Natl Cancer Inst (2011) 103(17):1299–309. doi: 10.1093/jnci/djr242
75. Henry NL, Giles JT, Stearns V. Aromatase Inhibitor-Associated Musculoskeletal Symptoms: Etiology and Strategies for Management. Oncol (Williston Park) (2008) 22(12):1401–8.
76. Buzdar AU, Robertson JF, Eiermann W, Nabholtz JM. An Overview of the Pharmacology and Pharmacokinetics of the Newer Generation Aromatase Inhibitors Anastrozole, Letrozole, and Exemestane. Cancer (2002) 95(9):2006–16. doi: 10.1002/cncr.10908
77. Miki Y, Suzuki T, Hatori M, Igarashi K, Aisaki KI, Kanno J, et al. Effects of Aromatase Inhibitors on Human Osteoblast and Osteoblast-Like Cells: A Possible Androgenic Bone Protective Effects Induced by Exemestane. Bone (2007) 40(4):876–87. doi: 10.1016/j.bone.2006.11.029
78. D'Amelio P, Grimaldi A, Di Bella S, Brianza SZM, Cristofaro MA, Tamone C, et al. Estrogen Deficiency Increases Osteoclastogenesis Up-Regulating T Cells Activity: A Key Mechanism in Osteoporosis. Bone (2008) 43(1):92–100. doi: 10.1016/j.bone.2008.02.017
79. Napoli N, Rastelli A, Ma C, Yarramaneni J, Vattikutti S, Moskowitz G, et al. Genetic Polymorphism at Val80 (Rs700518) of the CYP19A1 Gene is Associated With Aromatase Inhibitor Associated Bone Loss in Women With ER + Breast Cancer. Bone (2013) 55(2):309–14. doi: 10.1016/j.bone.2013.04.021
80. Liu M, Goss PE, Ingle JN, Kubo M, Furukawa Y, Batzler A, et al. Aromatase Inhibitor-Associated Bone Fractures: A Case-Cohort GWAS and Functional Genomics. Mol Endocrinol (2014) 28(10):1740–51. doi: 10.1210/me.2014-1147
81. Niravath P. Aromatase Inhibitor-Induced Arthralgia: A Review. Ann Oncol (2013) 24(6):1443–9. doi: 10.1093/annonc/mdt037
82. Tenti S, Correale P, Cheleschi S, Fioravanti A, Pirtoli L. Aromatase Inhibitors-Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. Int J Mol Sci (2020) 21(16):5625. doi: 10.3390/ijms21165625
83. Richmond RS, Carlson CS, Register TC, Shanker G, Loeser RF. Functional Estrogen Receptors in Adult Articular Cartilage: Estrogen Replacement Therapy Increases Chondrocyte Synthesis of Proteoglycans and Insulin-Like Growth Factor Binding Protein 2. Arthritis Rheum (2000) 43(9):2081–90. doi: 10.1002/1529-0131(200009)43:92081::AID-ANR203.0.CO;2-I
84. Le Bail J, Liagre B, Vergne P, Bertin P, Beneytout J, Habrioux G. Aromatase in Synovial Cells From Postmenopausal Women. Steroids (2001) 66(10):749–57. doi: 10.1016/s0039-128x(01)00104-0
85. Xu X, Li X, Liang Y, Ou Y, Huang J, Xiong J, et al. Estrogen Modulates Cartilage and Subchondral Bone Remodeling in an Ovariectomized Rat Model of Postmenopausal Osteoarthritis. Med Sci Monit (2019) 25:3146–53. doi: 10.12659/MSM.916254
86. Chlebowski RT, Cirillo DJ, Eaton CB, Stefanick ML, Pettinger M, Carbone LD, et al. Estrogen Alone and Joint Symptoms in the Women's Health Initiative Randomized Trial. Menopause (2013) 20(6):600–8. doi: 10.1097/GME.0b013e31828392c4
87. Lintermans A, Van Asten K, Jongen L, Van Brussel T, Laenen A, Verhaeghe J, et al. Genetic Variant in the Osteoprotegerin Gene is Associated With Aromatase Inhibitor-Related Musculoskeletal Toxicity in Breast Cancer Patients. Eur J Cancer (2016) 56:31–6. doi: 10.1016/j.ejca.2015.12.013
88. Garcia-Giralt N, Rodríguez-Sanz M, Prieto-Alhambra D, Servitja S, Torres-Del Pliego E, Balcells S, et al. Genetic Determinants of Aromatase Inhibitor-Related Arthralgia: The B-ABLE Cohort Study. Breast Cancer Res Treat (2013) 140(2):385–95. doi: 10.1007/s10549-013-2638-3
89. Henry NL, Skaar TC, Dantzer J, Li L, Kidwell K, Gersch C, et al. Genetic Associations With Toxicity-Related Discontinuation of Aromatase Inhibitor Therapy for Breast Cancer. Breast Cancer Res Treat (2013) 138(3):807–16. doi: 10.1007/s10549-013-2504-3
90. Romero SAD, Su HI, Satagopan J, Li QS, Seluzicki CM, Dries A, et al. Clinical and Genetic Risk Factors for Aromatase Inhibitor-Associated Arthralgia in Breast Cancer Survivors. Breast (2020) 49:48–54. doi: 10.1016/j.breast.2019.10.008
91. Lintermans A, Neven P. Safety of Aromatase Inhibitor Therapy in Breast Cancer. Expert Opin Drug Saf (2015) 14(8):1201–11. doi: 10.1517/14740338.2015.1053458
92. Robarge JD, Duarte DB, Shariati B, Wang R, Flockhart DA, Vasko MR. Aromatase Inhibitors Augment Nociceptive Behaviors in Rats and Enhance the Excitability of Sensory Neurons. Exp Neurol (2016) 281:53–65. doi: 10.1016/j.expneurol.2016.04.006
93. Singer O, Cigler T, Moore AB, Levine AB, Hentel K, Belfi L, et al. Defining the Aromatase Inhibitor Musculoskeletal Syndrome: A Prospective Study. Arthritis Care Res (Hoboken) (2012) 64(12):1910–8. doi: 10.1002/acr.21756
94. Lintermans A, Van Calster B, Van Hoydonck M, Pans S, Verhaeghe J, Westhovens R, et al. Aromatase Inhibitor-Induced Loss of Grip Strength is Body Mass Index Dependent: Hypothesis-Generating Findings for its Pathogenesis. Ann Oncol (2011) 22(8):1763–9. doi: 10.1093/annonc/mdq699
95. Lintermans A, Laenen A, Van Calster B, Pans S, Verhaeghe J, Westhovens R, et al. Prospective Study to Assess Fluid Accumulation and Tenosynovial Changes in the Aromatase Inhibitor-Induced Musculoskeletal Syndrome: 2-Year Follow-Up Data. Ann Oncol (2013) 24(2):350–5. doi: 10.1093/annonc/mds290
96. Morales L, Pans S, Paridaens R, Westhovens R, Timmerman D, Verhaeghe J, et al. Debilitating Musculoskeletal Pain and Stiffness With Letrozole and Exemestane: Associated Tenosynovial Changes on Magnetic Resonance Imaging. Breast Cancer Res Treat (2007) 104(1):87–91. doi: 10.1007/s10549-006-9394-6
97. Dizdar O, Ozçakar L, Malas FU, Harputluoglu H, Bulut N, Aksoy S, et al. Sonographic and Electrodiagnostic Evaluations in Patients With Aromatase Inhibitor-Related Arthralgia. J Clin Oncol (2009) 27(30):4955–60. doi: 10.1200/JCO.2008.20.5435
98. Wang J, Zhang Q, Jin S, Feng M, Kang X, Zhao S, et al. Immoderate Inhibition of Estrogen by Anastrozole Enhances the Severity of Experimental Polyarthritis. Exp Gerontol (2009) 44(6-7):398–405. doi: 10.1016/j.exger.2009.03.003
99. Zarkavelis G, Kollas A, Kampletsas E, Vasiliou V, Kaltsonoudis E, Drosos A, et al. Aromatase Inhibitors Induced Autoimmune Disorders in Patients With Breast Cancer: A Review. J Adv Res (2016) 7(5):719–26. doi: 10.1016/j.jare.2016.04.001
100. Generali D, Bates G, Berruti A, Brizzi MP, Campo L, Bonardi S, et al. Immunomodulation of FOXP3+ Regulatory T Cells by the Aromatase Inhibitor Letrozole in Breast Cancer Patients. Clin Cancer Res (2009) 15(3):1046–51. doi: 10.1158/1078-0432.CCR-08-1507
101. Laroche M, Seniow M, Roché H, Ruyssen-Witrand A. Arthralgia Associated With Autoimmune Abnormalities Under Aromatase Inhibitor Therapy: Outcome After Cessation of Treatment. J Rheumatol (2016) 43(10):1945–6. doi: 10.3899/jrheum.160254
102. Iwasa A, Arakaki R, Honma N, Ushio A, Yamada A, Kondo T, et al. Aromatase Controls Sjögren Syndrome-Like Lesions Through Monocyte Chemotactic Protein-1 in Target Organ and Adipose Tissue-Associated Macrophages. Am J Pathol (2015) 185(1):151–61. doi: 10.1016/j.ajpath.2014.09.006
103. Liu M, Wang L, Bongartz T, Hawse JR, Markovic SN, Schaid DJ, et al. Aromatase Inhibitors, Estrogens and Musculoskeletal Pain: Estrogen-Dependent T-Cell Leukemia 1A (TCL1A) Gene-Mediated Regulation of Cytokine Expression. Breast Cancer Res (2012) 14(2):R41. doi: 10.1186/bcr3137
104. Toh ML, Kawashima M, Hot A, Miossec P, Miossec P. Role of IL-17 in the Th1 Systemic Defects in Rheumatoid Arthritis Through Selective IL-12Rbeta2 Inhibition. Ann Rheum Dis (2010) 69(8):1562–7. doi: 10.1136/ard.2009.111757
105. Umamaheswaran G, Kadambari D, Muthuvel SK, Kumar NAN, Dubashi B, Aibor Dkhar S, et al. Polymorphisms of T- Cell Leukemia 1A Gene Loci are Not Related to the Development of Adjuvant Letrozole-Induced Adverse Events in Breast Cancer. PloS One (2021) 16(3):e0247989. doi: 10.1371/journal.pone.0247989
106. Geisler J, Lønning PE. Aromatase Inhibition: Translation Into a Successful Therapeutic Approach. Clin Cancer Res (2005) 11(8):2809–21. doi: 10.1158/1078-0432.CCR-04-2187
107. Geisler J. Differences Between the non-Steroidal Aromatase Inhibitors Anastrozole and Letrozole–of Clinical Importance? Br J Cancer (2011) 104(7):1059–66. doi: 10.1038/bjc.2011.58
108. Geisler J, Haynes B, Anker G, Dowsett M, Lønning PE. Influence of Letrozole and Anastrozole on Total Body Aromatization and Plasma Estrogen Levels in Postmenopausal Breast Cancer Patients Evaluated in a Randomized, Cross-Over Study. J Clin Oncol (2002) 20(3):751–7. doi: 10.1200/JCO.2002.20.3.751
109. Geisler J, Helle H, Ekse D, Duong NK, Evans DB, Nordbø Y, et al. Letrozole is Superior to Anastrozole in Suppressing Breast Cancer Tissue and Plasma Estrogen Levels. Clin Cancer Res (2008) 14(19):6330–5. doi: 10.1158/1078-0432.CCR-07-5221
110. Geisler J. Differences Between the non-Steroidal Aromatase Inhibitors Anastrozole and Letrozole–of Clinical Importance? . Br J Cancer (2011) 104(7):1059–66. doi: 10.1038/bjc.2011.58
111. Lønning PE. The Potency and Clinical Efficacy of Aromatase Inhibitors Across the Breast Cancer Continuum. Ann Oncol (2011) 22(3):503–14. doi: 10.1093/annonc/mdq337
112. Folkerd EJ, Dixon JM, Renshaw L, A'Hern RP, Dowsett M. Suppression of Plasma Estrogen Levels by Letrozole and Anastrozole is Related to Body Mass Index in Patients With Breast Cancer. J Clin Oncol (2012) 30(24):2977–80. doi: 10.1200/JCO.2012.42.0273
113. Dixon JM, Renshaw L, Young O, Murray J, Macaskill EJ, McHugh M, et al. Letrozole Suppresses Plasma Estradiol and Estrone Sulphate More Completely Than Anastrozole in Postmenopausal Women With Breast Cancer. J Clin Oncol (2008) 26(10):1671–6. doi: 10.1200/JCO.2007.13.9279
114. Bahrami N, Chang G, Kanaya N, Sauer T, Park D, Loeng M, et al. Changes in Serum Estrogenic Activity During Neoadjuvant Therapy With Letrozole and Exemestane. J Steroid Biochem Mol Biol (2020) 200:105641. doi: 10.1016/j.jsbmb.2020.105641
115. Geisler J, King N, Anker G, Ornati G, Di Salle E, Lønning PE, et al. In Vivo Inhibition of Aromatization by Exemestane, a Novel Irreversible Aromatase Inhibitor, in Postmenopausal Breast Cancer Patients. Clin Cancer Res (1998) 4(9):2089–93.
116. Dixon JM, Renshaw L, Langridge C, Young OE, McHugh M, Williams L, et al. Anastrozole and Letrozole: An Investigation and Comparison of Quality of Life and Tolerability. Breast Cancer Res Treat (2011) 125(3):741–9. doi: 10.1007/s10549-010-1091-9
117. Bahrami N, Chang G, Kanaya N, Sauer T, Park D, Loeng M, et al. Changes in Serum Estrogenic Activity During Neoadjuvant Therapy With Letrozole and Exemestane. J Steroid Biochem Mol Biol (2020) 200:105641. doi: 10.1016/j.jsbmb.2020.105641
118. Thomas R, Godward S, Makris A, Bloomfield D, Moody AM, Williams M. Giving Patients a Choice Improves Quality of Life: A Multi-Centre, Investigator-Blind, Randomised, Crossover Study Comparing Letrozole With Anastrozole. Clin Oncol (R Coll Radiol) (2004) 16(7):485–91. doi: 10.1016/j.clon.2004.06.023
119. Rose C, Vtoraya O, Pluzanska A, Davidson N, Gershanovich M, Thomas R, et al. An Open Randomised Trial of Second-Line Endocrine Therapy in Advanced Breast Cancer. Comparison of the Aromatase Inhibitors Letrozole and Anastrozole. Eur J Cancer (2003) 39(16):2318–27. doi: 10.1016/s0959-8049(03)00630-0
120. Smith I, Yardley D, Burris H, De Boer R, Amadori D, McIntyre K, et al. Comparative Efficacy and Safety of Adjuvant Letrozole Versus Anastrozole in Postmenopausal Patients With Hormone Receptor-Positive, Node-Positive Early Breast Cancer: Final Results of the Randomized Phase III Femara Versus Anastrozole Clinical Evaluation (FACE) Trial. J Clin Oncol (2017) 35(10):1041–8. doi: 10.1200/JCO.2016.69.2871
121. Morimoto Y, Sarumaru S, Oshima Y, Tsuruta C, Watanabe K. Joint Symptoms Associated With Anastrozole and Letrozole in Patients With Breast Cancer: A Retrospective Comparative Study. J Pharm Health Care Sci (2017) 3:25. doi: 10.1186/s40780-017-0095-6
122. Iwata H, Masuda N, Ohno S, Rai Y, Sato Y, Ohsumi S, et al. A Randomized, Double-Blind, Controlled Study of Exemestane Versus Anastrozole for the First-Line Treatment of Postmenopausal Japanese Women With Hormone-Receptor-Positive Advanced Breast Cancer. Breast Cancer Res Treat (2013) 139(2):441–51. doi: 10.1007/s10549-013-2573-3
123. Goss PE, Ingle JN, Pritchard KI, Ellis MJ, Sledge GW, Budd GT, et al. Exemestane Versus Anastrozole in Postmenopausal Women With Early Breast Cancer: NCIC CTG MA.27–a Randomized Controlled Phase III Trial. J Clin Oncol (2013) 31(11):1398–404. doi: 10.1200/JCO.2012.44.7805
124. Goss PE, Qi S, Josse RG, Pritzker KP, Mendes M, Hu H, et al. The Steroidal Aromatase Inhibitor Exemestane Prevents Bone Loss in Ovariectomized Rats. Bone (2004) 34(3):384–92. doi: 10.1016/j.bone.2003.11.006
125. Goss PE, Hadji P, Subar M, Abreu P, Thomsen T, Banke-Bochita J. Effects of Steroidal and Nonsteroidal Aromatase Inhibitors on Markers of Bone Turnover in Healthy Postmenopausal Women. Breast Cancer Res (2007) 9(4):R52. doi: 10.1186/bcr1757
126. Oesterreich S, Henry NL, Kidwell KM, Van Poznak CH, Skaar TC, Dantzer J, et al. Associations Between Genetic Variants and the Effect of Letrozole and Exemestane on Bone Mass and Bone Turnover. Breast Cancer Res Treat (2015) 154(2):263–73. doi: 10.1007/s10549-015-3608-8
127. McCloskey EV, Hannon RA, Lakner G, Fraser WD, Clack G, Miyamoto A, et al. Effects of Third Generation Aromatase Inhibitors on Bone Health and Other Safety Parameters: Results of an Open, Randomised, Multi-Centre Study of Letrozole, Exemestane and Anastrozole in Healthy Postmenopausal Women. Eur J Cancer (2007) 43(17):2523–31. doi: 10.1016/j.ejca.2007.08.029
128. Caciolla J, Bisi A, Belluti F, Rampa A, Gobbi S. Reconsidering Aromatase for Breast Cancer Treatment: New Roles for an Old Target. Molecules (2020) 25(22):5351. doi: 10.3390/molecules25225351
129. Kamdem LK, Xi J, Clark BL, Gregory BJ, Kidwell KM, Storniolo AM, et al. Exemestane may be Less Detrimental Than Letrozole to Bone Health in Women Homozygous for the UGT2B17*2 Gene Deletion. Breast Cancer Res Treat (2019) 175(2):297–303. doi: 10.1007/s10549-019-05158-3
130. Goss PE, Hershman DL, Cheung AM, Ingle JN, Khosla S, Stearns V, et al. Effects of Adjuvant Exemestane Versus Anastrozole on Bone Mineral Density for Women With Early Breast Cancer (MA.27B): A Companion Analysis of a Randomised Controlled Trial. Lancet Oncol (2014) 15(4):474–82. doi: 10.1016/S1470-2045(14)70035-X
131. Geisler J, Lønning PE, Krag LE, Løkkevik E, Risberg T, Hagen AI, et al. Changes in Bone and Lipid Metabolism in Postmenopausal Women With Early Breast Cancer After Terminating 2-Year Treatment With Exemestane: A Randomised, Placebo-Controlled Study. Eur J Cancer (2006) 42(17):2968–75. doi: 10.1016/j.ejca.2006.07.005
132. Ghosh D, Lo J, Egbuta C. Recent Progress in the Discovery of Next Generation Inhibitors of Aromatase From the Structure-Function Perspective. J Med Chem (2016) 59(11):5131–48. doi: 10.1021/acs.jmedchem.5b01281
133. Schmidt M, Naumann H, Weidler C, Schellenberg M, Anders S, Straub RH. Inflammation and Sex Hormone Metabolism. Ann N Y Acad Sci (2006) 1069:236–46. doi: 10.1196/annals.1351.021
134. Birrell S, Tilley W. Testosterone Undecanoate Treatment Reduces Joint Morbidities Induced by Anastrozole Therapy in Postmenopausal Women With Breast Cancer: Results of a Double-Blind, Randomized Phase II Trial. (2009), San Antonio Breast Cancer Symposium (SABCS) (09 Dec 2009 - 13 Dec 2009 : San Antonio, Texas. doi: 10.1158/0008-5472.SABCS-09-804.
135. Cathcart-Rake E, Novotny P, Leon-Ferre R, Le-Rademacher J, Storrick EM, Adjei AA, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Testosterone for Treatment of Postmenopausal Women With Aromatase Inhibitor-Induced Arthralgias: Alliance Study A221102. Support Care Cancer (2021) 29(1):387–96. doi: 10.1007/s00520-020-05473-2
136. Henry NL, Unger JM, Schott AF, Fehrenbacher L, Flynn PJ, Prow DM, et al. Randomized, Multicenter, Placebo-Controlled Clinical Trial of Duloxetine Versus Placebo for Aromatase Inhibitor-Associated Arthralgias in Early-Stage Breast Cancer: SWOG S1202. J Clin Oncol (2018) 36(4):326–32. doi: 10.1200/JCO.2017.74.6651
137. Schnell PM, Lustberg MB, Henry NL. Adverse Events and Perception of Benefit From Duloxetine for Treating Aromatase Inhibitor-Associated Arthralgias. JNCI Cancer Spectr (2021) 5(2):pkab018. doi: 10.1093/jncics/pkab018
138. Henry NL, Unger JM, Till C, Schott AF, Crew KD, Lew DL, et al. Association Between Body Mass Index and Response to Duloxetine for Aromatase Inhibitor-Associated Musculoskeletal Symptoms in SWOG S1202. Cancer (2019) 125(12):2123–9. doi: 10.1002/cncr.32024
139. Briot K, Tubiana-Hulin M, Bastit L, Kloos I, Roux C. Effect of a Switch of Aromatase Inhibitors on Musculoskeletal Symptoms in Postmenopausal Women With Hormone-Receptor-Positive Breast Cancer: The(Articular Tolerance of Letrozole) Study. Breast Cancer Res Treat (2010) 120(1):127–34. doi: 10.1007/s10549-009-0692-7
140. Colleoni M, Luo W, Karlsson P, Chirgwin J, Aebi S, Jerusalem G, et al. Extended Adjuvant Intermittent Letrozole Versus Continuous Letrozole in Postmenopausal Women With Breast Cancer (SOLE): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol (2018) 19(1):127–38. doi: 10.1016/S1470-2045(17)30715-5
141. Ribi K, Luo W, Colleoni M, Karlsson P, Chirgwin J, Aebi S, et al. Quality of Life Under Extended Continuous Versus Intermittent Adjuvant Letrozole in Lymph Node-Positive, Early Breast Cancer Patients: The SOLE Randomised Phase 3 Trial. Br J Cancer (2019) 120(10):959–67. doi: 10.1038/s41416-019-0435-4
142. Hadji P, Aapro MS, Body JJ, Gnant M, Brandi ML, Reginster JY, et al. Management of Aromatase Inhibitor-Associated Bone Loss (AIBL) in Postmenopausal Women With Hormone Sensitive Breast Cancer: Joint Position Statement of the IOF, CABS, ECTS, IEG, ESCEO IMS, and SIOG. J Bone Oncol (2017) 7:1–12. doi: 10.1016/j.jbo.2017.03.001
143. Pineda-Moncusí M, Garcia-Giralt N, Diez-Perez A, Servitja S, Tusquets I, Prieto-Alhambra D, et al. Increased Fracture Risk in Women Treated With Aromatase Inhibitors Versus Tamoxifen: Beneficial Effect of Bisphosphonates. J Bone Miner Res (2020) 35(2):291–7. doi: 10.1002/jbmr.3886
144. Paschou SA, Augoulea A, Lambrinoudaki I. Bone Health Care in Women With Breast Cancer. Hormones (Athens) (2020) 19(2):171–8. doi: 10.1007/s42000-019-00164-y
145. Santa-Maria CA, Bardia A, Blackford AL, Snyder C, Connolly RM, Fetting JH, et al. A Phase II Study Evaluating the Efficacy of Zoledronic Acid in Prevention of Aromatase Inhibitor-Associated Musculoskeletal Symptoms: The ZAP Trial. Breast Cancer Res Treat (2018) 171(1):121–9. doi: 10.1007/s10549-018-4811-1
146. Xepapadakis G, Ntasiou P, Koronarchis D, Koufoudakis D, Panousis D, Grosomanidis D, et al. New Views on Treatment of Aromatase Inhibitors Induced Arthralgia. Breast (2010) 19(3):249–50. doi: 10.1016/j.breast.2010.03.031
147. Alhanafy AM, Labeeb A, Khalil A. The Role of Diuretics in Treatment of Aromatase Inhibitors Induced Musculoskeletal Symptoms in Women With non Metastatic Breast Cancer. Asian Pac J Cancer Prev (2018) 19(12):3525–31. doi: 10.31557/APJCP.2018.19.12.3525
148. Kubo M, Onishi H, Kuroki S, Okido M, Shimada K, Yokohata K, et al. Short-Term and Low-Dose Prednisolone Administration Reduces Aromatase Inhibitor-Induced Arthralgia in Patients With Breast Cancer. Anticancer Res (2012) 32(6):2331–6.
149. Nogues X, Servitja S, Peña MJ, Prieto-Alhambra D, Nadal R, Mellibovsky L, et al. Vitamin D Deficiency and Bone Mineral Density in Postmenopausal Women Receiving Aromatase Inhibitors for Early Breast Cancer. Maturitas (2010) 66(3):291–7. doi: 10.1016/j.maturitas.2010.03.012
150. Latham NK, Anderson CS, Lee A, Bennett DA, Moseley A, Cameron ID, et al. A Randomized, Controlled Trial of Quadriceps Resistance Exercise and Vitamin D in Frail Older People: The Frailty Interventions Trial in Elderly Subjects (FITNESS). J Am Geriatr Soc (2003) 51(3):291–9. doi: 10.1046/j.1532-5415.2003.51101.x
151. Sato Y, Iwamoto J, Kanoko T, Satoh K. Low-Dose Vitamin D Prevents Muscular Atrophy and Reduces Falls and Hip Fractures in Women After Stroke: A Randomized Controlled. Cerebrovasc Dis (2005) 20(3):187–92. doi: 10.1159/000087203
152. Gerdhem P, Ringsberg KA, Obrant KJ, Akesson K. Association Between 25-Hydroxy Vitamin D Levels, Physical Activity, Muscle Strength and Fractures in the Prospective Population-Based OPRA Study of Elderly Women. Osteoporos Int (2005) 16(11):1425–31. doi: 10.1007/s00198-005-1860-1
153. Prieto-Alhambra D, Javaid MK, Servitja S, Arden NK, Martinez-García M, Diez-Perez A, et al. Vitamin D Threshold to Prevent Aromatase Inhibitor-Induced Arthralgia: A Prospective Cohort Study. Breast Cancer Res Treat (2011) 125(3):869–78. doi: 10.1007/s10549-010-1075-9
154. Khan QJ, Kimler BF, Reddy PS, Sharma P, Klemp JR, Nydegger JL, et al. Randomized Trial of Vitamin D3 to Prevent Worsening of Musculoskeletal Symptoms in Women With Breast Cancer Receiving Adjuvant Letrozole. VITAL trial. Breast Cancer Res Treat (2017) 166(2):491–500. doi: 10.1007/s10549-017-4429-8
155. Shapiro AC, Adlis SA, Robien K, Kirstein MN, Liang S, Richter SA, et al. Randomized, Blinded Trial of Vitamin D3 for Treating Aromatase Inhibitor-Associated Musculoskeletal Symptoms (AIMSS. Breast Cancer Res Treat (2016) 155(3):501–12. doi: 10.1007/s10549-016-3710-6
156. Goldberg RJ, Katz J. A Meta-Analysis of the Analgesic Effects of Omega-3 Polyunsaturated Fatty Acid Supplementation for Inflammatory Joint Pain. Pain (2007) 129(1-2):210–23. doi: 10.1016/j.pain.2007.01.020
157. Hershman DL, Unger JM, Crew KD, Awad D, Dakhil SR, Gralow J, et al. Randomized Multicenter Placebo-Controlled Trial of Omega-3 Fatty Acids for the Control of Aromatase Inhibitor-Induced Musculoskeletal Pain: SWOG S0927. J Clin Oncol (2015) 33(17):1910–7. doi: 10.1200/JCO.2014.59.5595
158. Troy KL, Mancuso ME, Butler TA, Johnson JE. Exercise Early and Often: Effects of Physical Activity and Exercise on Women's Bone Health. Int J Environ Res Public Health (2018) 15(5):878. doi: 10.3390/ijerph15050878
159. Church TS, Earnest CP, Skinner JS, Blair SN. Effects of Different Doses of Physical Activity on Cardiorespiratory Fitness Among Sedentary, Overweight or Obese Postmenopausal Women With Elevated Blood Pressure: A Randomized Controlled Trial. JAMA (2007) 297(19):2081–91. doi: 10.1001/jama.297.19.2081
160. Schmitz KH, Ahmed RL, Troxel A, Cheville A, Smith R, Lewis-Grant L, et al. Weight Lifting in Women With Breast-Cancer-Related Lymphedema. N Engl J Med (2009) 361(7):664–73. doi: 10.1056/NEJMoa0810118
161. Lu G, Zheng J, Zhang L. The Effect of Exercise on Aromatase Inhibitor-Induced Musculoskeletal Symptoms in Breast Cancer Survivors :a Systematic Review and Meta-Analysis. Support Care Cancer (2020) 28(4):1587–96. doi: 10.1007/s00520-019-05186-1
162. Peppone LJ, Janelsins MC, Kamen C, Mohile SG, Sprod LK, Gewandter JS, et al. The Effect of YOCAS©® Yoga for Musculoskeletal Symptoms Among Breast Cancer Survivors on Hormonal Therapy. Breast Cancer Res Treat (2015) 150(3):597–604. doi: 10.1007/s10549-015-3351-1
163. Hershman DL, Unger JM, Greenlee H, Capodice JL, Lew DL, Darke AK, et al. Effect of Acupuncture vs Sham Acupuncture or Waitlist Control on Joint Pain Related to Aromatase Inhibitors Among Women With Early-Stage Breast Cancer: A Randomized Clinical Trial. JAMA (2018) 320(2):167–76. doi: 10.1001/jama.2018.8907
164. Dworkin RH, Turk DC, McDermott MP, Peirce-Sandner S, Burke LB, Cowan P, et al. Interpreting the Clinical Importance of Group Differences in Chronic Pain Clinical Trials: IMMPACT Recommendations. Pain (2009) 146(3):238–44. doi: 10.1016/j.pain.2009.08.019
165. Kadakia KC, Kidwell KM, Seewald NJ, Snyder CF, Flockhart DA, Carpenter JS, et al. Crossover From One Aromatase Inhibitor (AI) to Another in the Exemestane and Letrozole Pharmacogenetics (Elph) Trial 2016. ASCO Annu Meeting Proc (2007) 297(19):2081–91. doi: 10.1200/jco.2016.34.3_suppl.158
166. Rastelli AL, Taylor ME, Gao F, Armamento-Villareal R, Jamalabadi-Majidi S, Napoli N, et al. Vitamin D and Aromatase Inhibitor-Induced Musculoskeletal Symptoms (AIMSS): A Phase II, Double-Blind, Placebo-Controlled, Randomized Trial. Breast Cancer Res Treat (2011) 129(1):107–16. doi: 10.1007/s10549-011-1644-6
167. Niravath P, Hilsenbeck SG, Wang T, Jiralerspong S, Nangia J, Pavlick A, et al. Randomized Controlled Trial of High-Dose Versus Standard-Dose Vitamin D3 for Prevention of Aromatase Inhibitor-Induced Arthralgia. Breast Cancer Res Treat (2019) 177(2):427–35. doi: 10.1007/s10549-019-05319-4
168. Greenlee H, Crew KD, Shao T, Kranwinkel G, Kalinsky K, Maurer M, et al. Phase II Study of Glucosamine With Chondroitin on Aromatase Inhibitor-Associated Joint Symptoms in Women With Breast Cancer. Support Care Cancer (2013) 21(4):1077–87. doi: 10.1007/s00520-012-1628-z
169. Lustberg MB, Orchard TS, Reinbolt R, Andridge R, Pan X, Belury M, et al. Randomized Placebo-Controlled Pilot Trial of Omega 3 Fatty Acids for Prevention of Aromatase Inhibitor-Induced Musculoskeletal Pain. Breast Cancer Res Treat (2018) 167(3):709–18. doi: 10.1007/s10549-017-4559-z
Keywords: aromatase inhibitor, aromatase inhibitor-associated musculoskeletal syndrome, breast cancer, aromatase inhibitor-induced arthralgia, aromatase inhibitor-induced bone loss
Citation: Hyder T, Marino CC, Ahmad S, Nasrazadani A and Brufsky AM (2021) Aromatase Inhibitor-Associated Musculoskeletal Syndrome: Understanding Mechanisms and Management. Front. Endocrinol. 12:713700. doi: 10.3389/fendo.2021.713700
Received: 23 May 2021; Accepted: 12 July 2021;
Published: 27 July 2021.
Edited by:
Bruno M. Simões, The University of Manchester, United KingdomReviewed by:
Kenji Ohe, Fukuoka University, JapanDouglas Yee, University of Minnesota Twin Cities, United States
Copyright © 2021 Hyder, Marino, Ahmad, Nasrazadani and Brufsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Adam M. Brufsky, YnJ1ZnNreWFtQHVwbWMuZWR1
 Tara Hyder
Tara Hyder Christopher C. Marino
Christopher C. Marino Sasha Ahmad3
Sasha Ahmad3 Azadeh Nasrazadani
Azadeh Nasrazadani