- Department of Endocrinology and Internal Medicine, Faculty of Medicine, Medical University of Gdańsk, Gdańsk, Poland
The ongoing coronavirus disease 2019 (COVID-19) pandemic forced a change in the way we provide medical treatment. Endocrinology in the era of COVID-19 had to transform and reduce its vast potential to the absolute necessities. Medical professionals needed to update their clinical practice to provide their patients as much support and as little harm as possible in these increasingly difficult times. International expert statements were published to offer guidance regarding proper care. It was suggested to simplify the diagnostic scheme of hypercortisolemia and to modify the approach to treatment. Hypercortisolemic patients with COVID-19 and iatrogenic hypercortisolemia due to glucocorticoid use are important clinical scenarios – we aimed to provide a cohesive summary of issues to consider.
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic forced a change in the way we provide medical treatment. Overburdened healthcare systems struggled to carry out urgent medical procedures, not to mention meticulous, time-consuming diagnostics typical for advanced endocrine services. Endocrinology in the era of COVID-19 had to transform and reduce its vast potential.
The pandemic started in December 2019 in the city of Wuhan localized in Central China. It was first reported to the World Health Organization (WHO) in late December of 2019. The WHO declared the new disease a global public health threat of international concern in late January 2020, and in mid-March 2020 marked it as a pandemic. Clinicians and researchers started seeking for potential salvage drugs. Glucocorticoids – especially dexamethasone – gained worldwide attention due to the overall mortality reduction in oxygen-depending patients in the RECOVERY trial (1).
Axial symptoms of COVID-19 include high fever and respiratory distress. Involvement of multiple organs and systems was observed, including cardiovascular, neurological, psychiatric, and gastrointestinal signs and symptoms (2). In most cases, the clinical course of the disease is nonsevere [up to 84% of cases (3)]. Nevertheless, certain groups, such as the elderly, immunocompromised, and patients with chronic illness, are more at risk of developing the severe form of COVID-19.
Hypercortisolemia was a complex and often confusing problem even in the pre-COVID-19 times. Though fulminant cases of Cushing’s syndrome are generally difficult to overlook due to their distinctive clinical and laboratory features, mild autonomous hypercortisolemia or cyclic Cushing’s syndrome can be more challenging to diagnose (4, 5). Most cases of neoplastic hypercortisolemia are ACTH-dependent and arise from hormonal activity of pituitary tumors overproducing corticotropin (ACTH, adrenocorticotropic hormone). Other backgrounds include primary adrenal tumors, and ectopic ACTH secretion (EAS) seen in, for example, bronchial carcinoid or small cell lung cancer (6). Sometimes patients appear cushingoid and/or exhibit laboratory changes typical for hypercortisolemia without underlying neoplasia – such cases may be explained by non-neoplastic/physiological hypercortisolemia (previously referred to as pseudo-Cushing’s syndrome), factitious disorder, or iatrogenic glucocorticoid excess (4, 7).
Patients suffering from hypercortisolemia tend to develop a wide range of metabolic complications, including impaired glucose metabolism [diabetes in 32% of adult patients, impaired glucose tolerance in additional 30.6% (8)], arterial hypertension [80-95% adults (9, 10)], hypercoagulability, and immunodeficiency (11), all of which overlap with abnormalities previously reported as detrimental in COVID-19. As final outcomes in hypercortisolemic individuals are tied with the level of circulating cortisol, optimal control of hormonal hyperactivity remains a staple of care, and in many patients secondary comorbidities resolve once cortisol excess is eliminated. Typically, surgery is the basic modality of treatment, with additional radiotherapeutic and pharmacological options available. Nonetheless, special circumstances of the COVID-19 pandemic forced alternative, not always simpler, approaches to follow-up and treatment.
Diagnostic Algorithm of Hypercortisolemia in the COVID-19 Era
Early in the course of the pandemic, experts issued a number of statements updating current clinical approach (12–17). Expert opinion on hypercortisolemia focused mainly on: i. quick and efficient triage of patients into low-risk and high-risk groups, ii. urgent care delivery in moderate and severe cases of hypercortisolemia, iii. avoidance of non-essential diagnostic procedures and hospital visits for high-risk patients, iv. deferral of unnecessary diagnostics in milder cases modo watch and wait, v. alternative approach to establishing diagnosis and following with treatment, vi. optimal control over comorbidities, vii. prioritization of medical treatment over surgery whenever necessary, viii. development of well-functioning telemedicine and consultation networks, ix. extensive patient education (12).
Patients with Cushing’s syndrome, especially the ones with fulminant and uncontrolled disease, should be considered chronically immunocompromised and metabolically unstable, and therefore require swift care (17). Such individuals have a significant risk of contracting COVID-19 and a range of secondary comorbidities puts them in a high risk group if they become infected (17, 18). It is crucial for them to adhere to the rules and regulations regarding personal safety, self-isolation, and rigorously hygienic lifestyle (12). High-risk patients should avoid unnecessary hospital/clinic visits, and their medical providers should accommodate them with convenient teleconsultations whenever necessary (19). Blood sampling in a medical facility should be limited to a rational minimum. Basic clinical parameters that are easy to check at home, such as blood pressure or capillary glucose, together with clinical evaluation, could help with day-to-day monitoring of the disease. If cases are ubiquitous and/or mild, their in-depth investigation could be reasonably postponed by 3-6 months (12).
Updated diagnostic and therapeutic approach was discussed in detail by Newell-Price et al. and general trends of proposed changes are displayed in Figure 1 (12). Early assessment of plasma ACTH can help to determine the source of hypercortisolemia (ACTH-dependent vs. ACTH-independent). Experts state that in severely symptomatic hypercortisolemia even a single measurement of serum cortisol concentration exceeding 1000 nmol/l (37 µg/dl) could confirm the diagnosis, if only pathologies provoking severe physiological stress are excluded. Similarly, diagnosis of hypercortisolemia is highly likely if urinary free cortisol (UFC) exceeds the upper limit of the norm at least five times. Though the usual first line of tests includes late-night salivary cortisol, during the pandemic this step should be omitted due to possible contamination of the material with SARS CoV-2 copies (12).
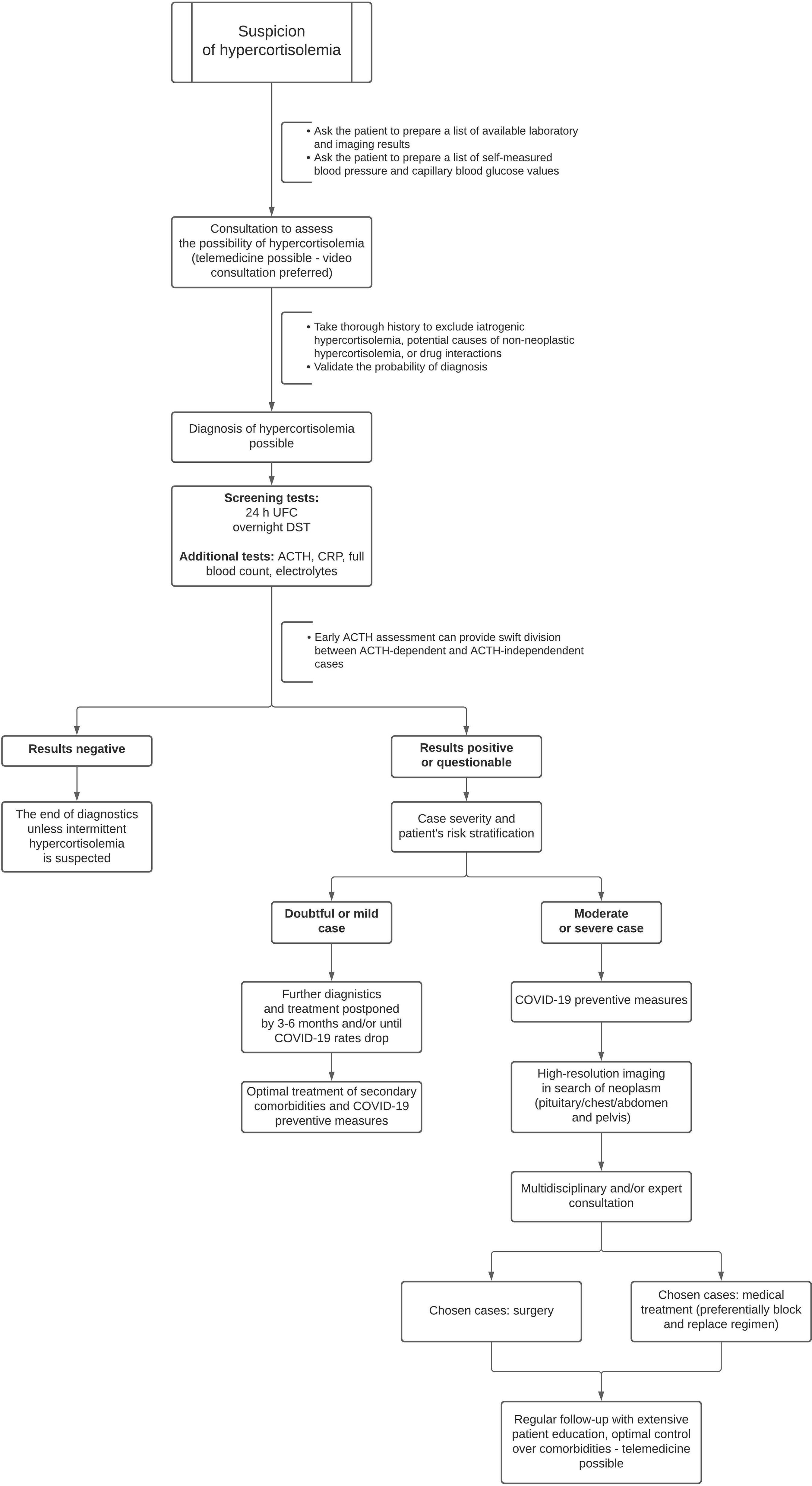
Figure 1 Modified approach to assessment of newly suspected hypercortisolemia in the era of COVID-19 based on the expert guidelines. UFC, urinary free cortisol; DST, dexamethasone suppression test; ACTH, adrenocorticotropic hormone; corticotropin; CRP, C-reactive protein.
Medical Treatment of Hypercortisolemia and Secondary Comorbidities
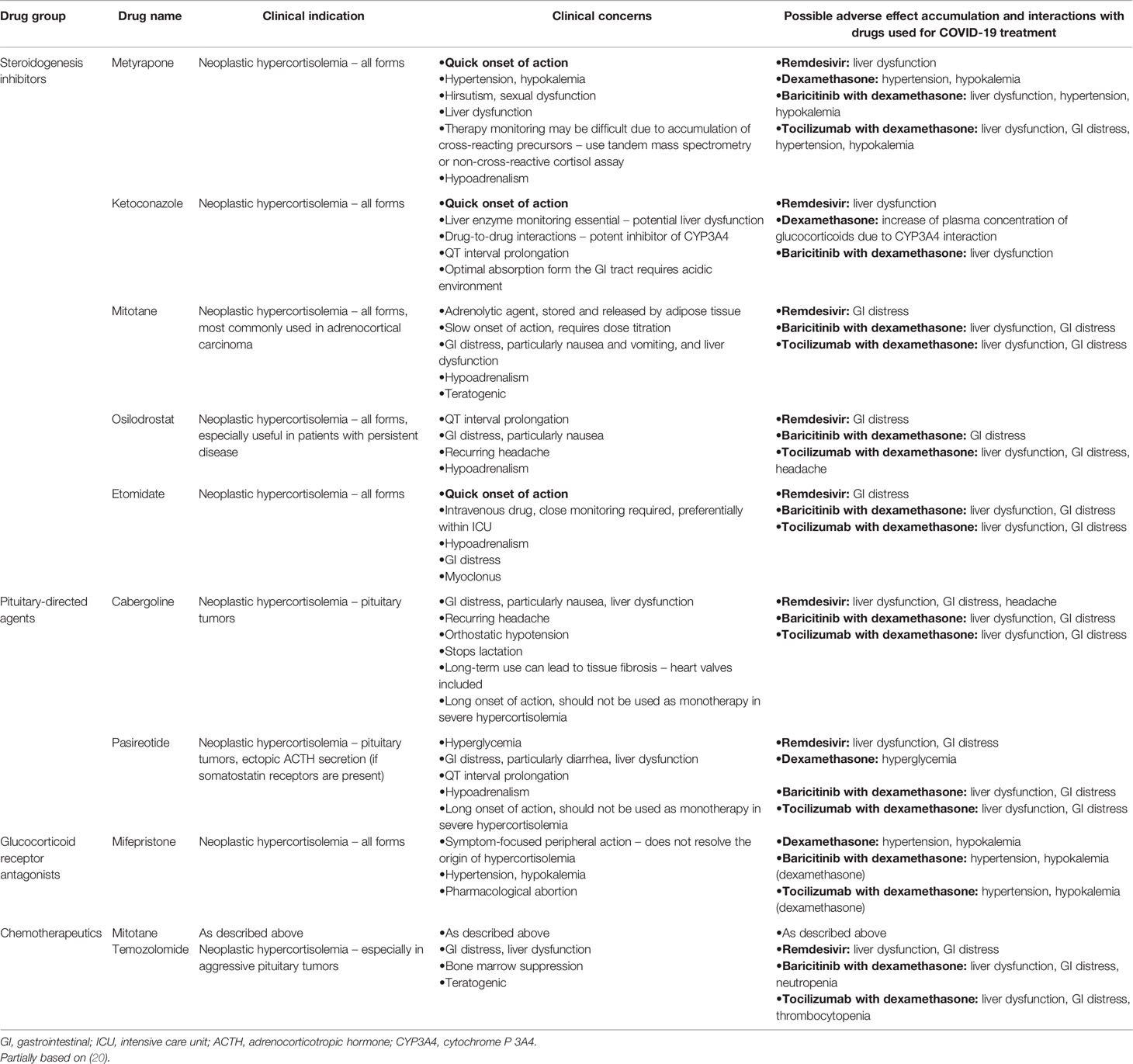
Steady drop in circulating cortisol levels together with optimal control over secondary comorbidities should be the primary goals of medical treatment. Physicians can choose between steroidogenesis inhibitors, pituitary- and/or ectopic-source- directed agents, glucocorticoid receptor antagonists, and chemotherapeutics. Medications should be chosen based on their onset of action, safety profile, local availability, monitoring possibility, and the cause of hypercortisolemia. Block-and-replace therapy with steroidogenesis inhibitors and replacement glucocorticoids (and mineralocorticoids whenever necessary) might be introduced de novo in steroidogenesis-inhibitor naïve patients, and previously established efficient pharmacological regimens should not be routinely changed, unless it is a switch to block-and-replace therapy to prevent hypoadrenalism (12). Possible interactions between drugs used in Cushing’s syndrome and approved in COVID-19 by the WHO are listed in Table 1 (21–25). We might be lacking extensive information about potential side effects of some approved drugs such as casirivimab/imdevimab or sotrovimab – due to their novelty (26, 27). Medications showing no clear benefit from treatment – like chloroquine or hydroxychloroquine – were recommended against (21, 28).
Hypertension secondary to hypercortisolemia is common (up to 80-95% of hypercortisolemic adults) and multifactorial (9, 10). Its severity is tied to the length of exposure and degree of cortisol excess (29). Mineralocorticoid-like action of glucocorticoids, vascular remodeling, local disruption of synthesis of vasoactive agents, insulin resistance, sleep apnea, and catecholamine hypersensitivity play a detrimental role in development of hypertension (10, 29, 30). In many cases hypertension fades once hormonal stabilization is reached (30). Lifestyle changes, such as weight loss, smoking cessation, and physical activity, remain a staple in non-pharmacological treatment. Due to disturbed renin-angiotensin-aldosterone (RAA) system and progressive vascular and cardiac remodeling, angiotensin-converting enzyme inhibitors (ACE-Is) and angiotensin receptor blockers (ARBs) are perceived the drugs of choice by many physicians (29, 31). Virtually any antihypertensive medication could find its place in the treatment of hypercortisolemia-induced hypertension, however some may be preferred over others due to their cardioprotective properties or potency to wash out or spare serum potassium (10, 29, 31). At the beginning of the pandemic, certain controversies arose around ACE-Is and ARBs since ACE2 receptors were identified as entrance points for the virus. Nonetheless, currently available data proves a high profile of safety of these groups in COVID-19 and advises their continuous use (21, 32–35). Metyrapone can further exacerbate hypertension (Table 1) (29).
Up to 60% of hypercortisolemic patients develop glucose metabolism impairment (8). Postprandial hyperglycemia is especially common and blood glucose levels tend to peak in the afternoon or early evening (36). Multiple mechanisms are involved, with hepatic and skeletal muscle glucose homeostasis disruption, disturbed insulin secretion, and insulin resistance (37, 38). Optimal glycemic control can be achieved after resolution of hypercortisolemia. General pharmacological management does not necessarily differ from that proposed for non-hypercortisolemic patients (38, 39). A whole variety of drugs, including metformin, sulfonylureas, acarbose, dipeptidyl peptidase-4 (DPP4) inhibitors, insulin, and others, can be used (38). A potential of dipeptidyl peptidase 4 (DPP4) inhibitors to negatively affect clinical course of COVID-19 was disputed at the beginning of the pandemic. However, prolonged observation showed that DPP4 inhibitor use reduces COVID-19 mortality among diabetic patients (40, 41) and that these drugs should not be routinely withdrawn (42–44). Pasireotide may further provoke hyperglycemia (Table 1) (45).
Additional steps in medical treatment include antithrombotic prophylaxis (17, 46, 47) and antibiotic prophylaxis with trimethoprim+sulfamethoxazole to prevent opportunistic Pneumocystis jiroveci infection in severe cases of hypercortisolemia (12, 18, 48).
Surgical Treatment of Hypercortisolemia
Typical approach to corticotropinomas revolves around surgical excision by an experienced pituitary surgeon (49, 50). Nevertheless, current concerns highlight the risk of transsphenoidal surgery amidst the pandemic for both surgical teams and patients (12, 17, 51). Aerosol formation throughout the procedure could potentially lead to further spread of the disease (12, 17). As most pituitary masses are rather stagnant and display a relatively small growth potential, it was suggested that patients could receive medical treatment as a bridge therapy while awaiting surgery (Table 1) (12, 17). Nonetheless, certain scenarios, such as risk of vision loss, highly aggressive tumors creating a significant mass effect, or fulminant hypercortisolemia responding poorly to medical treatment, should trigger appropriate actions, surgery included (12, 17). In cases of rare aggressive macroadenomas, alternative approaches minimizing the risk of droplet formation, like supraorbital craniotomy, can be considered (12, 52). Patients should be screened for SARS CoV-2 infection before the surgery, and their anti-COVID-19 vaccination status should be checked. Besides the aforementioned pituitary tumors, adrenocortical carcinoma and EAS call for urgent surgical care due to their malignant behavior and often fast progression.
Radiotherapy in Hypercortisolemia
If the tumor growth and/or hormonal activity can be no longer managed by debulking surgeries or medical therapies, or when residual mass shows features typical for an aggressive neoplasm, pituitary radiotherapy can be introduced. Stereotactic radiosurgery (SRS) or fractioned external beam radiotherapy (EBRT) can be optimal options (53). However, radiotherapy sessions require subsequent hospital visits and may put the patient at risk of in-hospital COVID-19 transmission.
Telemedicine
Telemedicine became essential and effective in providing medical care, especially in at-risk population, those with active COVID-19, and/or those attending follow-up visits (19). Videoconferences can facilitate visual evaluation. Patients should be taught proper techniques of blood pressure and capillary blood glucose measurement. If steroidogenesis inhibitors are started, individuals should be informed about the possibility of developing hypocortisolism and learn about its clinical picture. Education regarding stress-dosing of glucocorticoids (preferably also in written form) should be offered; patients should be equipped with a dose of parenteral glucocorticoids and instructed how to apply it. If the block-and-replace tactic is chosen, its background should be discussed in detail.
Special Circumstance: Hypercortisolemic Patient With COVID-19
To our best knowledge, cases of patients suffering from endogenous hypercortisolemia and COVID-19 were rarely reported (20, 54, 55), therefore many considerations may be only hypothesized. Hypercortisolemia induces persistent low-grade inflammation and immunosuppression. Immune dysfunction in Cushing’s syndrome originates from defective immune reaction and regulation, as well as immune cell apoptosis (18). Natural immune barriers, such as for example skin, can be disrupted. Hypercortisolemia needs to be treated urgently whenever a life-threatening infection ensues (56). Viral infections in the course of Cushing’s syndrome are often severe and prolonged (18, 57). Initial signs and symptoms of COVID-19 in this group of patients can be misleading, and the typical combination of fever and dyspnea may be absent (17, 54). Therefore, other features like diarrhea, anosmia, dysgeusia, and cough should be assessed (17). If antiviral drugs are started, it is suggested that immunocompromised patients may require prolonged therapy (17, 57–60). Hypertension and diabetes, common sequelae of hypercortisolemia, are known negative prognostic factors in COVID-19, therefore optimal treatment should be introduced early (17). Secondary infections may not generate typical signs and symptoms as well. Infected individuals should be carefully and methodically assessed for superimposed secondary infections and treated accordingly. Opportunistic pathogens are not uncommon in Cushing’s syndrome (18, 57). Whenever a superimposed infection is suspected, routine laboratory essays such as CRP, lactate, procalcitonin, leukocyte count, urinalysis, blood/urine/sputum samples should be obtained, with an optional chest X-ray or CT if indicated. Frail skin should be inspected in search of wounds – possible gates for infection (57). The degree of hypercortisolism can predict the final outcomes, with severe hypercortisolism putting patients at risk the most (61).
Immunocompromise can be reversed only once hypercortisolemia is sufficiently treated, and if the disease-specific drugs are continued, physicians should pay special attention to possible drug-to-drug interactions, risk of organ damage, and side effects(Table 1) (20). Effective therapeutic schemes should not be routinely changed (12) unless serious concerns ensue. Routine screening of disease activity in the course of an acute illness is challenging: corticosteroids are physiological stress hormones and their levels rise as a part of the “fight or flight” response. In the acute phase of infection, we suggest using simple indicators of disease control, such as clinical state of the patient, blood pressure, heart rate, serum/capillary glucose, serum electrolytes, and total blood count, instead of more elaborate and perhaps misleading targets. Patients using steroidogenesis inhibitors can eventually develop hypoadrenalism and clinical suspicion of such scenario was already reported in a patient with Cushing’s disease and COVID-19 from Italy (20). Simple parameters such as blood pressure, heart rate, capillary glucose, and serum electrolytes can be used as predictors of possible hypoadrenalism. If suspicion of hypoadrenalism seems valid, stress dosing of glucocorticoids should be introduced, with intravenous infusion readily available at site (12). If the patient’s condition seems stable and ambulatory treatment remains the preferred option, “sick day rules” should be strictly followed. If a particular need for hormonal assessment exists, in our opinion UFC might be helpful as it provides integrated information about cortisol excess over a period of 24 hours contrary to serum cortisol measurement covering a single point in time. Metyrapone or exogenous steroids can alter the results of hormonal tests owing to assay interactions (Table 1).
Hypercortisolemia, acute inflammatory disorders, and immobility are all well recognized factors promoting clot formation. Therefore, antithrombotic prophylaxis should be introduced (17, 47), and deep vein thrombosis and/or pulmonary embolism should be adequately treated if only they occur.
As Cushing’s syndrome leads to often dramatic catabolism, sarcopenia, truncal obesity, and osteoporosis, it seems probable that the patients might experience respiratory fatigue and require higher doses of oxygen and/or ventilation support earlier on than their non-hypercortisolemic counterparts.
Vitamin D became known as a natural immunomodulatory compound (62); hence, its levels should be promptly assessed in COVID-19 patients and supplementation should be started if necessary. Vitamin D level was linked to clinical outcomes in COVID-19 (63). General nutrition status which is commonly hampered in hypercortisolemic subjects can be tied to final outcomes as well – the higher the nutrition risk, the worse the prognosis in COVID-19 (64). This, together with concerns over balanced diabetic diet whenever indicated, require a consultation from an experienced dietician. Sarcopenia and osteoporosis might worsen due to transient immobility, decreased physical activity, and possibly detrimental effect of proinflammatory cytokines (58–60) and require urgent rehabilitation. Post-COVID-19 recovery of hypercortisolemic patients may be long and challenging; some patients may experience the debilitating post-acute COVID-19 syndrome (57).
COVID-19 survivors often experience various kinds of psychological and/or psychiatric trauma (65), posttraumatic stress disorder included (66), and hypercortisolemic patients are especially prone to mental distress (67); a consultation with an experienced mental health specialist might be beneficial.
To sum it up, we suggest i. close monitoring of possible signs and symptoms of COVID-19, ii. continuation of cortisol-lowering therapy if only possible, iii. optimal control over secondary comorbidities, iv. prevention of thromboembolic events, v. early assessment of superimposed infections, often opportunistic, vi. nutritional assessment and early dietary intervention, with special regard for vitamin D, vii. early detection of signs and symptoms of hypoadrenalism, viii. support of a multidisciplinary team.
Special Circumstance: Iatrogenic Hypercortisolemia
The RECOVERY trial proved efficacy of synthetic steroid dexamethasone in improving the outcomes in COVID-19 patients requiring oxygen therapy or mechanical ventilation (1). The proposed regimen consisted of 6 mg of oral or intravenous dexamethasone once daily for up to 10 days (1). Other steroids, such as hydrocortisone or methylprednisolone, were investigated with similar results (68, 69). Physicians must vary prolonged treatment with glucocorticoids, especially in high doses, to prevent the development of iatrogenic hypercortisolemia. Whenever glucocorticoids are used, the approach should focus on using the lowest effective doses for the shortest time possible.
Iatrogenic hypercortisolemia can take the same toll on the body as endogenous steroid excess, with identical complications including hypertension, glucose metabolism impairment, clot formation, infections, sarcopenia, osteoporosis, and mental distress. Secondary comorbidities should start to fade once steroids are discontinued, but before that happens each disease should be properly treated. If the risk of glucocorticoid-induced osteoporosis is high, vitamin D supplementation should be started (70). Proper physical activity/rehabilitation and nutrition can help to resolve sarcopenia (71, 72).
Glucocorticoid cessation requires special attention, especially if the drugs were used for a long time, due to the risk of adrenal gland atrophy. Slow reduction of glucocorticoid doses and early detection of features typical for adrenal insufficiency are essential. The post-acute COVID-19 syndrome shares some characteristics with hypoadrenalism [for example fatigue, tachycardia, and mood changes (73, 74)] and physicians must make sure to distinguish between the two. Simple biochemical investigation including serum electrolytes, total blood count, glucose, cortisol, and plasma ACTH could aid the diagnosis. Adrenal insufficiency can be lethal, therefore proper investigation should never be omitted.
Our suggestions for this subgroup of patients include: i. glucocorticoids used in the lowest effective doses for the shortest time possible; ii. evaluation for signs and symptoms of hypoadrenalism after discontinuation of glucocorticoids; iii. optimal control over secondary comorbidities; iv. prevention of thromboembolic events; v. early assessment for superimposed infections; vi. optimal rehabilitation and nutrition, with special regard for vitamin D status.
Summary
COVID-19 destabilized global healthcare and forced medical professionals to provide treatment in previously uncommon manners. Multiple expert opinions were published early in the course of the pandemic to help with proper care. The proposed diagnostic algorithm of hypercortisolemia was simplified. It became essential to identify cases requiring urgent medical attention and to offer watchful waiting to mild or doubtful cases. Medical treatment of hypercortisolemia and secondary comorbidities became especially important. Hypercortisolemic patients with COVID-19 and the possibility of iatrogenic hypercortisolemia due to prolonged glucocorticoid use should be given special attention. Hopefully, in the wake of new treatment options for COVID-19 and widespread vaccination programs, the pandemic will finally come to an end.
Author Contributions
AB – concept of the manuscript, literature review, drafting of the manuscript. RŚ-S – literature review, drafting of the manuscript, critical review of the manuscript. KS – critical review of the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in Hospitalized Patients With Covid-19. N Engl J Med (2021) 384:693–704. doi: 10.1056/NEJMOA2021436
2. Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of Comorbidities and Its Effects in Coronavirus Disease 2019 Patients: A Systematic Review and Meta-Analysis. Int J Infect Dis (2020) 94:91–5. doi: 10.1016/j.ijid.2020.03.017
3. Guan W, Ni Z, Hu Y, Liang W, Ou C, He J, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med (2020) 382:1708–20. doi: 10.1056/NEJMoa2002032
4. Nieman LK. Diagnosis of Cushing’s Syndrome in the Modern Era. Endocrinol Metab Clin North Am (2018) 47:259–73. doi: 10.1016/j.ecl.2018.02.001
5. Świątkowska-Stodulska R, Berlińska A, Stefańska K, Kłosowski P, Sworczak K. Cyclic Cushing’s Syndrome – A Diagnostic Challenge. Front Endocrinol (Lausanne) (2021) 12(658429):331. doi: 10.3389/fendo.2021.658429
6. Ejaz S, Vassilopoulou-Sellin R, Busaidy NL, Hu MI, Waguespack SG, Jimenez C, et al. Cushing Syndrome Secondary to Ectopic Adrenocorticotropic Hormone Secretion: The University of Texas MD Anderson Cancer Center Experience. Cancer (2011) 117:4381–9. doi: 10.1002/cncr.26029
7. Findling JW, Raff H. Differentiation of Pathologic/Neoplastic Hypercortisolism (Cushing’s Syndrome) From Physiologic/Non-Neoplastic Hypercortisolism (Formerly Known as Pseudo-Cushing’s Syndrome). Eur J Endocrinol (2017) 176:R205–16. doi: 10.1530/EJE-16-0946
8. Biering H, Knappe G, Gerl H, Lochs H. Diabetes-Haufigkeit Bei Akromegalie Und Cushing-Syndrom. Acta Med Austriaca (2000) 27:27–31. doi: 10.1046/j.1563-2571.2000.200106.x
9. Magiakou MA, Smyrnaki P, Chrousos GP. Hypertension in Cushing’s Syndrome. Best Pract Res Clin Endocrinol Metab (2006) 20:467–82. doi: 10.1016/j.beem.2006.07.006
10. Cicala MV, Mantero F. Hypertension in Cushing’s Syndrome: From Pathogenesis to Treatment. In: Neuroendocrinology. Karger Publishers (2010). p. 44–9. doi: 10.1159/000314315
11. Nieman LK, Biller BMK, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The Diagnosis of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2008) 93:1526–40. doi: 10.1210/jc.2008-0125
12. Newell-Price J, Nieman LK, Reincke M, Tabarin A. Endocrinology In The Time Of Covid-19: Management of Cushing’s Syndrome. Eur J Endocrinol (2020) 183:G1–7. doi: 10.1530/EJE-20-0352
13. Arlt W, Baldeweg SE, Pearce SHS, Simpson HL. Endocrinology in the TIME of COVID-19: Management of Adrenal Insufficiency. Eur J Endocrinol (2020) 183:G25–32. doi: 10.1530/EJE-20-0361
14. Boelaert K, Edward Visser W, Taylor PN, Moran C, Léger J, Persani L. Endocrinology in the Time of COVID-19: Management of Hyperthyroidism and Hypothyroidism. Eur J Endocrinol (2020) 183:G33–9. doi: 10.1530/EJE-20-0445
15. Wake DJ, Gibb FW, Kar P, Kennon B, Klonoff DC, Rayman G, et al. Remodelling Diabetes Services and Emerging Innovation. Eur J Endocrinol (2020) 183:G67–77. doi: 10.1530/EJE-20-0377
16. Casey RT, Valk GD, Schalin-Jäntti C, Grossman AB, Thakker RV. Clinical Management of Neuroendocrine Neoplasms (NENs). Eur J Endocrinol (2020) 183:G79–88. doi: 10.1530/EJE-20-0424
17. Pivonello R, Ferrigno R, Isidori AM, Biller BMK, Grossman AB, Colao A. COVID-19 and Cushing’s Syndrome: Recommendations for a Special Population With Endogenous Glucocorticoid Excess. Lancet Diabetes Endocrinol (2020) 8:654–6. doi: 10.1016/S2213-8587(20)30215-1
18. Hasenmajer V, Sbardella E, Sciarra F, Minnetti M, Isidori AM, Venneri MA. The Immune System in Cushing’s Syndrome. Trends Endocrinol Metab (2020) 31:655–69. doi: 10.1016/J.TEM.2020.04.004
19. Ceccato F, Voltan G, Sabbadin C, Camozzi V, Merante Boschin I, Mian C, et al. Tele-Medicine Versus Face-to-Face Consultation in Endocrine Outpatients Clinic During COVID-19 Outbreak: A Single-Center Experience During the Lockdown Period. J Endocrinol Invest (2020) 1:1689–98 doi: 10.1007/s40618-020-01476-2
20. Beretta F, Dassie F, Parolin M, Boscari F, Barbot M, Busetto L, et al. Practical Considerations for the Management of Cushing’s Disease and COVID-19: A Case Report. Front Media S.A (2020) 11:554. doi: 10.3389/fendo.2020.00554
21. COVID-19 Treatment Guidelines Panel. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Available at: https://www.covid19treatmentguidelines.nih.gov/ (Accessed July 17, 2021).
22. Veeravalli V, Dash RP, Thomas JA, Babu RJ, Madgula LMV, Srinivas NR. Critical Assessment of Pharmacokinetic Drug–Drug Interaction Potential of Tofacitinib, Baricitinib and Upadacitinib, the Three Approved Janus Kinase Inhibitors for Rheumatoid Arthritis Treatment. Drug Saf 2020 438 (2020) 43:711–25. doi: 10.1007/S40264-020-00938-Z
23. Berlińska A, Świątkowska-Stodulska R, Sworczak K. Factors Affecting Dexamethasone Suppression Test Results. Exp Clin Endocrinol Diabetes (2020) 128:667–71. doi: 10.1055/a-1017-3217
24. Kumar D, Trivedi N. Disease-Drug and Drug-Drug Interaction in COVID-19: Risk and Assessment. BioMed Pharmacother (2021) 139:111642. doi: 10.1016/J.BIOPHA.2021.111642
25. Gandhi Z, Mansuri Z, Bansod S. Potential Interactions of Remdesivir With Pulmonary Drugs: A Covid-19 Perspective. Sn Compr Clin Med (2020) 2:1707–8. doi: 10.1007/S42399-020-00462-2
26. Statement on Anti-SARS-CoV-2 Monoclonal Antibodies EUA | COVID-19 Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-anti-sars-cov-2-monoclonal-antibodies-eua/ (Accessed July 17, 2021).
27. Statement on Casirivimab Plus Imdevimab EUA | COVID-19 Treatment Guidelines. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/statement-on-casirivimab-plus-imdevimab-eua/ (Accessed July 17, 2021).
28. Manivannan E, Karthikeyan C, Moorthy NSHN, Chaturvedi SC. The Rise and Fall of Chloroquine/Hydroxychloroquine as Compassionate Therapy of COVID-19. Front Pharmacol (2021) 0:584940. doi: 10.3389/FPHAR.2021.584940
29. Barbot M, Ceccato F, Scaroni C. The Pathophysiology and Treatment of Hypertension in Patients With Cushing’s Syndrome. Front Endocrinol (Lausanne) (2019) 10:321. doi: 10.3389/fendo.2019.00321
30. Sacerdote A, Weiss K, Tran T, Noor BR, McFarlane SI. Hypertension in Patients With Cushing’s Disease: Pathophysiology, Diagnosis, and Management. Curr Hypertens Rep (2005) 7:212–8. doi: 10.1007/s11906-005-0013-4
31. Isidori AM, Graziadio C, Paragliola RM, Cozzolino A, Ambrogio AG, Colao A, et al. The Hypertension of Cushing’s Syndrome: Controversies in the Pathophysiology and Focus on Cardiovascular Complications. J Hypertens (2015) 33:44–60. doi: 10.1097/HJH.0000000000000415
32. Position Statement of the ESC Council on Hypertension on ACE-Inhibitors and Angiotensin Receptor Blockers. Available at: https://www.escardio.org/Councils/Council-on-Hypertension-(CHT)/News/position-statement-of-the-esc-council-on-hypertension-on-ace-inhibitors-and-ang (Accessed July 17, 2021).
33. Sriram K, Insel PA. Risks of ACE Inhibitor and ARB Usage in COVID-19: Evaluating the Evidence. Clin Pharmacol Ther (2020) 108:236–41. doi: 10.1002/cpt.1863
34. Kow CS, Zaidi STR, Hasan SS. Cardiovascular Disease and Use of Renin-Angiotensin System Inhibitors in COVID-19. Am J Cardiovasc Drugs (2020) 20:217–21. doi: 10.1007/s40256-020-00406-0
35. Rico-Mesa JS, White A, Anderson AS. Outcomes in Patients With COVID-19 Infection Taking ACEI/ARB. Curr Cardiol Rep (2020) 22:31. doi: 10.1007/S11886-020-01291-4
36. Kuo T, McQueen A, Chen T-C, Wang J-C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv Exp Med Biol (2015) 872:99–126. doi: 10.1007/978-1-4939-2895-8_5
37. Pivonello R, De Leo M, Vitale P, Cozzolino A, Simeoli C, De Martino MC, et al. Pathophysiology of Diabetes Mellitus in Cushing’s Syndrome. In: Neuroendocrinology. Karger Publishers (2010). p. 77–81. doi: 10.1159/000314319
38. Barbot M, Ceccato F, Scaroni C. Diabetes Mellitus Secondary to Cushing’s Disease. Front Endocrinol (Lausanne) (2018) 9:284. doi: 10.3389/fendo.2018.00284
39. Munir A, Newell-Price J. Management of Diabetes Mellitus in Cushing’s Syndrome. In: Neuroendocrinology (2010) p. 82–5. doi: 10.1159/000314316
40. Mirani M, Favacchio G, Carrone F, Betella N, Biamonte E, Morenghi E, et al. Impact of Comorbidities and Glycemia at Admission and Dipeptidyl Peptidase 4 Inhibitors in Patients With Type 2 Diabetes With COVID-19: A Case Series From an Academic Hospital in Lombardy, Italy. Diabetes Care (2020) 43:3042–9. doi: 10.2337/DC20-1340
41. Solerte SB, D’Addio F, Trevisan R, Lovati E, Rossi A, Pastore I, et al. Sitagliptin Treatment at the Time of Hospitalization Was Associated With Reduced Mortality in Patients With Type 2 Diabetes and COVID-19: A Multicenter, Case-Control, Retrospective, Observational Study. Diabetes Care (2020) 43:2999–3006. doi: 10.2337/DC20-1521
42. Scheen AJ. DPP-4 Inhibition and COVID-19: From Initial Concerns to Recent Expectations. Diabetes Metab (2021) 47:101213. doi: 10.1016/j.diabet.2020.11.005
43. Noh Y, Oh IS, Jeong HE, Filion KB, Yu OHY, Shin JY. Association Between Dpp-4 Inhibitors and Covid-19–Related Outcomes Among Patients With Type 2 Diabetes. Diabetes Care (2021) 44:e64–6. doi: 10.2337/dc20-1824
44. Fadini GP, Morieri ML, Longato E, Bonora BM, Pinelli S, Selmin E, et al. Exposure to Dipeptidyl-Peptidase-4 Inhibitors and COVID-19 Among People With Type 2 Diabetes: A Case-Control Study. Diabetes Obes Metab (2020) 22:1946–50. doi: 10.1111/dom.14097
45. Silverstein JM. Hyperglycemia Induced by Pasireotide in Patients With Cushing’s Disease or Acromegaly. Pituitary (2016) 19:536–43. doi: 10.1007/s11102-016-0734-1
46. Boscaro M, Sonino N, Scarda A, Barzon L, Fallo F, Sartori MT, et al. Anticoagulant Prophylaxis Markedly Reduces Thromboembolic Complications in Cushing’s Syndrome. J Clin Endocrinol Metab (2002) 87:3662–6. doi: 10.1210/jcem.87.8.8703
47. Nieman LK, Biller BMK, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2015) 100:2807–31. doi: 10.1210/jc.2015-1818
48. Alexandraki KI, Grossman AB. Therapeutic Strategies for the Treatment of Severe Cushing’s Syndrome. Drugs (2016) 76:447–58. doi: 10.1007/s40265-016-0539-6
49. Raverot G, Burman P, McCormack A, Heaney A, Petersenn S, Popovic V, et al. European Society of Endocrinology Clinical Practice Guidelines for the Management of Aggressive Pituitary Tumours and Carcinomas. Eur J Endocrinol (2018) 178:G1–24. doi: 10.1530/EJE-17-0796
50. Ciric I, Ragin A, Baumgartner C, Pierce D. Complications of Transsphenoidal Surgery: Results of a National Survey, Review of the Literature, and Personal Experience. Neurosurgery (1997) 40:225–37. doi: 10.1097/00006123-199702000-00001
51. Mitchell RA, King JAJ, Goldschlager T, Wang YY. Impact of COVID-19 on Pituitary Surgery. ANZ J Surg (2020) 90:963–4. doi: 10.1111/ans.15959
52. Reisch R, Perneczky A, Filippi R. Surgical Technique of the Supraorbital Key-Hole Craniotomy. Surg Neurol (2003) 59 (3):223–7. doi: 10.1016/S0090-3019(02)01037-6
53. Petersenn S. Management of Aggressive Pituitary Tumors-A 2019 Update. Horm Metab Res (2019) 51:755–64. doi: 10.1055/a-1060-1883
54. Belaya Z, Golounina O, Melnichenko G, Tarbaeva N, Pashkova E, Gorokhov M, et al. Clinical Course and Outcome of Patients With ACTH-Dependent Cushing’s Syndrome Infected With Novel Coronavirus Disease-19 (COVID-19): Case Presentations. Endocrine (2021) 72:12–9. doi: 10.1007/S12020-021-02674-5
55. Yuno A, Kenmotsu Y, Takahashi Y, Nomoto H, Kameda H, Cho KY, et al. Successful Management of a Patient With Active Cushing’s Disease Complicated With Coronavirus Disease 2019 (COVID-19) Pneumonia. Endocr J (2021) 68:477–84. doi: 10.1507/ENDOCRJ.EJ20-0613
56. Nieman LK, Biller BMK, Findling JW, Murad MH, Newell-Price J, Savage MO, et al. Clinical Practice Guideline: Treatment of Cushing’s Syndrome: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2015) 100:2807. doi: 10.1210/JC.2015-1818
57. Fareau GG, Vassilopoulou-Sellin R. Hypercortisolemia and Infection. Infect Dis Clin North Am (2007) 21:639–57. doi: 10.1016/j.idc.2007.06.001
58. Hughes S, Troise O, Donaldson H, Mughal N, Moore LSP. Bacterial and Fungal Coinfection Among Hospitalized Patients With COVID-19: A Retrospective Cohort Study in a UK Secondary-Care Setting. Clin Microbiol Infect (2020) 26:1395–9. doi: 10.1016/j.cmi.2020.06.025
59. Scheffel RS, Dora JM, Weinert LS, Aquino V, Maia AL, Canani LH, et al. Invasive Fungal Infections in Endogenous Cushing’s Syndrome. Infect Dis Rep (2010) 2:9–10. doi: 10.4081/idr.2010.e4
60. Sieswerda E, de Boer MGJ, Bonten MMJ, Boersma WG, Jonkers RE, Aleva RM, et al. Recommendations for Antibacterial Therapy in Adults With COVID-19 – An Evidence Based Guideline. Clin Microbiol Infect (2021) 27:61–6. doi: 10.1016/j.cmi.2020.09.041
61. Sarlis NJ, Chanock SJ, Nieman LK. Cortisolemic Indices Predict Severe Infections in Cushing Syndrome Due to Ectopic Production of Adrenocorticotropin1. J Clin Endocrinol Metab (2000) 85:42–7. doi: 10.1210/jcem.85.1.6294
62. Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, et al. Mechanisms in Endocrinology: Vitamin D and COVID-19. Eur J Endocrinol (2020) 183:R133–47. doi: 10.1530/EJE-20-0665
63. Mazziotti G, Lavezzi E, Brunetti A, Mirani M, Favacchio G, Pizzocaro A, et al. Vitamin D Deficiency, Secondary Hyperparathyroidism and Respiratory Insufficiency in Hospitalized Patients With COVID-19. J Endocrinol Investig 2021 (2021), 1–9. doi: 10.1007/S40618-021-01535-2
64. Zhao X, Li Y, Ge Y, Shi Y, Lv P, Zhang J, et al. Evaluation of Nutrition Risk and Its Association With Mortality Risk in Severely and Critically Ill COVID-19 Patients. J Parenter Enter Nutr (2021) 45:32–42. doi: 10.1002/JPEN.1953
65. Talevi D, Socci V, Carai M, Carnaghi G, Faleri S, Trebbi E, et al. Mental Health Outcomes of the Covid-19 Pandemic. Riv Psichiatr (2020) 55:137–44. doi: 10.1708/3382.33569
66. Xiao S, Luo D, Xiao Y. Survivors of COVID-19 Are at High Risk of Posttraumatic Stress Disorder. Glob Heal Res Policy 2020 51 (2020) 5:1–3. doi: 10.1186/S41256-020-00155-2
67. Pivonello R, De Martino MC, De Leo M, Simeoli C, Colao A. Cushing’s Disease: The Burden of Illness. Endocr 2016 561 (2016) 56:10–8. doi: 10.1007/S12020-016-0984-8
68. Fatima SA, Asif M, Khan KA, Siddique N, Khan AZ. Comparison of Efficacy of Dexamethasone and Methylprednisolone in Moderate to Severe Covid 19 Disease. Ann Med Surg (2020) 60:413–6. doi: 10.1016/j.amsu.2020.11.027
69. Sterne JAC, Murthy S, Diaz JV, Slutsky AS, Villar J, Angus DC, et al. Association Between Administration of Systemic Corticosteroids and Mortality Among Critically Ill Patients With COVID-19: A Meta-Analysis. JAMA - J Am Med Assoc (2020) 324:1330–41. doi: 10.1001/jama.2020.17023
70. Mazziotti G, Formenti AM, Adler RA, Bilezikian JP, Grossman A, Sbardella E, et al. Glucocorticoid-Induced Osteoporosis: Pathophysiological Role of GH/IGF-I and PTH/VITAMIN D Axes, Treatment Options and Guidelines. Endocr 2016 543 (2016) 54:603–11. doi: 10.1007/S12020-016-1146-8
71. Kirwan R, McCullough D, Butler T, de Heredia FP, Davies IG, Stewart C. Sarcopenia During COVID-19 Lockdown Restrictions: Long-Term Health Effects of Short-Term Muscle Loss. GeroScience 2020 426 (2020) 42:1547–78. doi: 10.1007/S11357-020-00272-3
73. Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-Acute COVID-19 Syndrome. Nat Med 2021 274 (2021) 27:601–15. doi: 10.1038/s41591-021-01283-z
Keywords: hypercortisolemia, cushing’s syndrome, cushing’s disease, coronavirus disease 2019, COVID-19, severe acute respiratory syndrome coronavirus 2, SARS CoV-2, iatrogenic hypercortisolemia
Citation: Berlińska A, Świątkowska-Stodulska R and Sworczak K (2021) Old Problem, New Concerns: Hypercortisolemia in the Time of COVID-19. Front. Endocrinol. 12:711612. doi: 10.3389/fendo.2021.711612
Received: 18 May 2021; Accepted: 10 August 2021;
Published: 05 October 2021.
Edited by:
Jeff M. P. Holly, University of Bristol, United KingdomReviewed by:
Gherardo Mazziotti, University of Milan, ItalyDimitra Argyro Vassiliadi, University General Hospital Attikon, Greece
Copyright © 2021 Berlińska, Świątkowska-Stodulska and Sworczak. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Agata Berlińska, YWdhdGEuYmVybGluc2thQGd1bWVkLmVkdS5wbA==
†These authors have contributed equally to this work
 Agata Berlińska
Agata Berlińska Renata Świątkowska-Stodulska
Renata Świątkowska-Stodulska Krzysztof Sworczak
Krzysztof Sworczak