
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Endocrinol., 16 August 2021
Sec. Obesity
Volume 12 - 2021 | https://doi.org/10.3389/fendo.2021.706914
Background: Sodium-glucose-cotransporter-2 (SGLT2) inhibitors have proven to be effective in improving glycemic control and lowering body weight in patients with type 2 diabetes mellitus. However, the efficacy and safety on weight loss in adults with overweight or obesity but not diabetes remain unclear. In this article, we aimed to identify the efficacy and safety of SGLT2 inhibitors in adults with overweight or obesity but not diabetes in randomized controlled studies (RCTs).
Methods: We searched for RCTs concerning SGLT2 inhibitors in adults with overweight or obesity but not diabetes in Medline (Ovid SP), Embase (Ovid SP), Cochrane Central Register of Controlled Trials (Ovid SP), and ClinicalTrials.gov up to February 2021. The primary outcomes were changes in body weight and body mass index (BMI). Trial sequential analysis (TSA) was used to test the reliability of the primary outcomes. We analyzed the data using Review Manager 5.3 and pooled data to calculate the mean differences (MDs) or the relative risk (RR). We assessed the evidence quality of evidence of outcomes according to GRADE.
Results: Six randomized controlled trials involving 872 individuals were included in the meta-analysis. Compared to the placebo group, the SGLT2 inhibitors group had statistically significant reductions in absolute changes in body weight (MD: -1.42 kg, 95% CI: -1.70 to -1.14; P<0.00001) and BMI (MD: -0.47 kg/m2, 95% CI: -0.63 to -0.31; P<0.00001) in SGLT2 inhibitors group, as indicated by TSA. However, no significant benefits were observed in the SGLT2 inhibitors group in terms of waist circumference (MD: -1.34 cm, 95%CI: -2.75 to 0.07; Z=1.86, P=0.06) compared with the placebo group. The GRADE profiles indicated very low-quality evidence for body weight change and low-quality evidence for BMI change. SGLT2 inhibitors were generally safe and well tolerated.
Conclusion: SGLT2 inhibitors could be used in selected adults with overweight and obesity but not diabetes if they are at low risk of genital infection and urinary infection. Further studies are warranted to confirm the efficacy and safety of SGLT2 inhibitors in adults with overweight or obesity but not diabetes for long-term weight management.
Systematic Review Registration: [https://www.crd.york.ac.uk/prospero/#loginpage], identifier [PROSPERO, CRD42021252931]
Overweight and obesity are major risk factors for several diseases, such as hypertension, dyslipidaemia, type 2 diabetes, cardiovascular disease, osteoarthritis, obstructive sleep apnoea, fatty liver disease cancers and other diseases (1, 2). Moderate weight loss (5% of body weight) can improve glycaemic control and insulin homeostasis and mitigate cardiovascular risk factors associated with overweight and obesity (3). In 2016, the World Health Organization reported that more than 1.9 billion adults were affected by overweight, of whom 650 million adults were affected by obesity (4), and that over 2.8 million deaths were attributable to overweight or obesity per year. The issues once considered specific to developed countries are now also on the rise in developing countries, especially in urban settings, which require additional healthcare interventions (5).
The management of overweight and obesity is challenging but imperative. Clinical practice guidelines have recommended lifestyle interventions such as diet, exercise, and behavioural modification for weight management. Bariatric surgery and/or pharmacological treatment have also been recommended based on lifestyle interventions. Although bariatric surgery is an effective treatment option, it is invasive, relatively expensive, available only to a limited population, and may be associated with adverse consequences. Weight loss medications for obesity include phentermine, topiramate/phentermine, lorcaserin, orlistat, naltrexone/bupropion and liraglutide, often with some side effects for long-term use (6).
Sodium-glucose transporter 2 (SGLT2) inhibitors are a novel class of oral therapeutic medications that have been approved for the treatment of type 2 diabetes mellitus by the Food and Drug Administration (FDA) (7). SGLT2 is mostly expressed in the renal proximal convoluted tubule. Its inhibition leads to a decreased renal threshold for glucose excretion (RTG) and increased urinary glucose excretion (UGE), resulting in mild diuresis and a net caloric loss. SGLT2 inhibitors have been shown to be successful in improving glycaemic control and lowering body weight (8). A great deal of evidence has indicated that SGLT-2 inhibitors have strong effects on body weight in patients with diabetes mellitus and can be used as potential agents for obesity management (9, 10). However, the efficacy and safety of SGLT2 inhibitors therapy in adults with overweight or obesity but not diabetes remain unknown.
Therefore, we conducted a systematic review and meta-analysis of SGLT2 inhibitors in randomized controlled trials (RCTs) to assess whether SGLT2 inhibitors could lead to weight loss in adults with overweight or obesity but not diabetes.
This systematic review and meta-analysis was written in accord with the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) (11). This systematic review was registered on International Prospective Register of Systematic Review (PROSPERO, CRD42021252931).
An extensive search for RCTs in Medline (Ovid SP), Embase (Ovid SP), Cochrane Central Register of Controlled Trials (Ovid SP), for studies published from the creation time of databases until February 20th, 2021, using the keywords: “Sodium-Glucose Transporter 2”, “sodium glucose cotransporter 2 inhibitors”, “canagliflozin”, “dapagliflozin”, “empagliflozin”, “ipragliflozin”, “tofogliflozin”, “luseogliflozin”, “sergliflozin”, “remogliflozin”, “ertugliflozin”, “sotagliflozin”, “overweight”, “obesity” and “obese” (Supplementary Information 2). ClinicalTrial.gov was screened for potentially eligible studies. The reference lists of relevant published researches investigating the use of SGLT2 inhibitors in non-diabetes with overweight or obesity were also reviewed for potentially relevant studies. We contacted authors by email if the full-text was not available or if the outcomes were not enough.
We included studies meeting the following criteria: (1) Participants: adults with overweight or obesity but not diabetes undergoing SGLT2 inhibitors based on the study definition; (2) Interventions/comparisons: using SGLT2 inhibitors as a monotherapy and placebo as the control. All included participants received standardized advice on diet and physical activity throughout the trial; (3) Outcomes: reporting one of the primary outcomes of interest, namely body weight and body mass index (BMI). Weight loss ≥ 5%, Waist circumference (WC), Hip circumference (HC), Waist/hip ratio (W/H) and adverse events were secondary outcomes. The adverse events included general adverse events and serious adverse events; (4) Study design: randomized controlled trials (RCTs) limited to the English language without restrictions of study size, follow-up length or publication year. No ethical approval and no contact with individual patients were required. The exclusion criteria were as follows: (1) including participants with pregnant; (2) animal experiments; (3) studies published in a language other than Chinese or English; (4) published as abstract only; (5) including patients with prediabetes.
All retrieved literatures were identified by two independent reviewers (HZ and ML) and data were extracted by a pre-defined form. Any discrepancies were resolved by discussion with a 3rd reviewer (NS) when necessary. We extracted the following data: (1) the last name of the first author, publication year; (2) sample size, follow-up length, intervention and comparison; (3) the characteristics of participants’ age, gender, country; body weight; BMI; (4) outcomes and (5) funding. SGLT2 inhibitors with diverse doses were separated to several trials. If a study contained more than one SGLT-2 inhibitors or more than one dose of SGLT-2 inhibitors, we separated it to different trials, one of which only included one of SGLT-2 inhibitors group with only one dose.
Two independent reviewers (HZ and ML) assessed the risk of bias of the included studies according to the Cochrane Handbook for Systematic Reviews of Interventions (version 5.1), and the disagreement were resolved by consulting the 3rd reviewer (NS). We assessed the quality of the included studies concerning 7 aspects including random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, and selective reporting and other bias (12). Each of them was judged as low, high or unclear risk. Grading of Recommendations Assessment, Development and Evaluation (GRADE) (13, 14) tool was used to assess the evidence quality and provide evidence for future guidelines, concerning inconsistency, indirectness, imprecision, and other bias.
All data were analyzed by Revman software (version 5.3; Cochrane Collaboration). Trial sequential analysis (TSA) (version 0.9.5.10 Beta; Copenhagen Trial Unit, Copenhagen, Denmark) was used for assessing the risk of type I and II errors, quantifying the statistical reliability of data in the meta-analysis and control this potential risk. TSA was conducted for primary outcome. An overall 5% type I error was maintained with a power of 80%. All dichotomous data were calculated as a relative risks (RR) with accompanying 95% confidence interval (CI), while all continuous data were calculated as a mean difference (MD) with accompanying 95% CI. Chi-squared test and I2 statistic was used to assess the degree of statistical heterogeneity. When statistical heterogeneity occurred (P value <0.10, I2 >25%), a random-effect model was used and possible sources of heterogeneity were explored, otherwise, a fixed-effects model was used. Subgroup analyses according to the drugs of SGLT2 inhibitors were pursued. Publication bias was assessed using funnel plots and Egger’ s test (meta package in R v4.1.0). A sensitivity analysis was conducted removing a single study at a time in an iterated manner and using different pooling methods.
As illustrated in Figure 1, a total of 1150 studies were identified, among which 6 were from the ClinicalTrial.gov. Owing to repetition, 283 studies were omitted. After screening the titles and abstracts, 845 studies were excluded, and 22 potentially eligible studies were reviewed by full-text. Full-text reviewed excluded 16 studies (Supplementary Information 2). Eventually, six studies involving 872 participants (15–20) were included in the final meta-analysis and all of the included studies were reported in English.
The characteristics of the included studies are reported in Table 1. All six studies (15–20) focused on adults with overweight or obesity but not diabetes. Participants in these studies came from four regions, including the United States, Puerto Rico, the United Kingdom, Denmark. Five studies (16–18, 20) were randomized, placebo-controlled studies, and one (19) was a pilot trial. Of these, 2 RCTs (16, 18) (n=720) evaluated canagliflozin (50 to 300 mg once daily), 2 RCTs (15, 17) (n=86) evaluated dapagliflozin (10 mg once daily), 2 RCTs (19, 20) (n=45) evaluated sergliflozin (500 to 1000 mg three times daily), and 1 RCT (19) (n=21) evaluated remogliflozin (250 mg three times daily), while all control groups were placebo. The follow-up periods ranged from 2 to 26 weeks. The mean ages of the included individuals ranged from 18.0 to 61.4 years old. The proportion of men was reported to be 21.25% (143 to 673). The mean body weight varied from 68.0 to 105.0 kg.
Two studies (15, 16) were assessed low risk for random sequence generation bias, while four (17–20) were unclear. Four studies (16–18, 20) were assessed low risk bias for blinding of participants and personnel, while two (15, 19) was high risk bias. The assessment results of quality were shown in Supplementary Figures 1, 2.
No publication bias was found from Egger’s test (t = −0.08, P = 0.939), and the funnel plot showed a symmetric distribution (Figure 2).
Six studies displayed body weight change (15–20). One study reported that participants in the dapagliflozin group had reduced body weight by −1.1 kg (95% CI: −2.6 to 0.3) compared with the control (15). Others (16–20) were summarized for meta-analysis. Compared to placebo, SGLT2 inhibitors were associated with a statistically significant reduction in body weight (MD: 1.42 kg, 95% CI: -1.70 to -1.14; Z=9.98, P<0.00001), which was homogeneous (I2 = 0%, P = 0.80) (Figure 3). This was very low-quality evidence that was downgraded one level for risk of bias, one level for indirectness and one level for imprecision (Table 2).
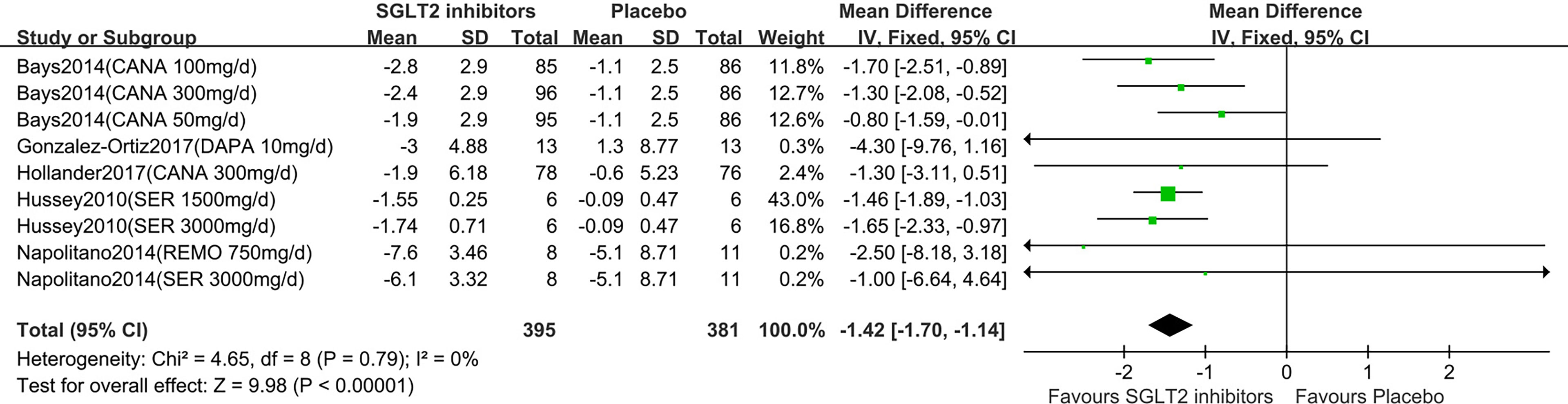
Figure 3 Changes of body weight in patients receiving SGLT2 inhibitors versus placebo. CI, confidence interval; IV, inverse variance; SD, standardized deviation.
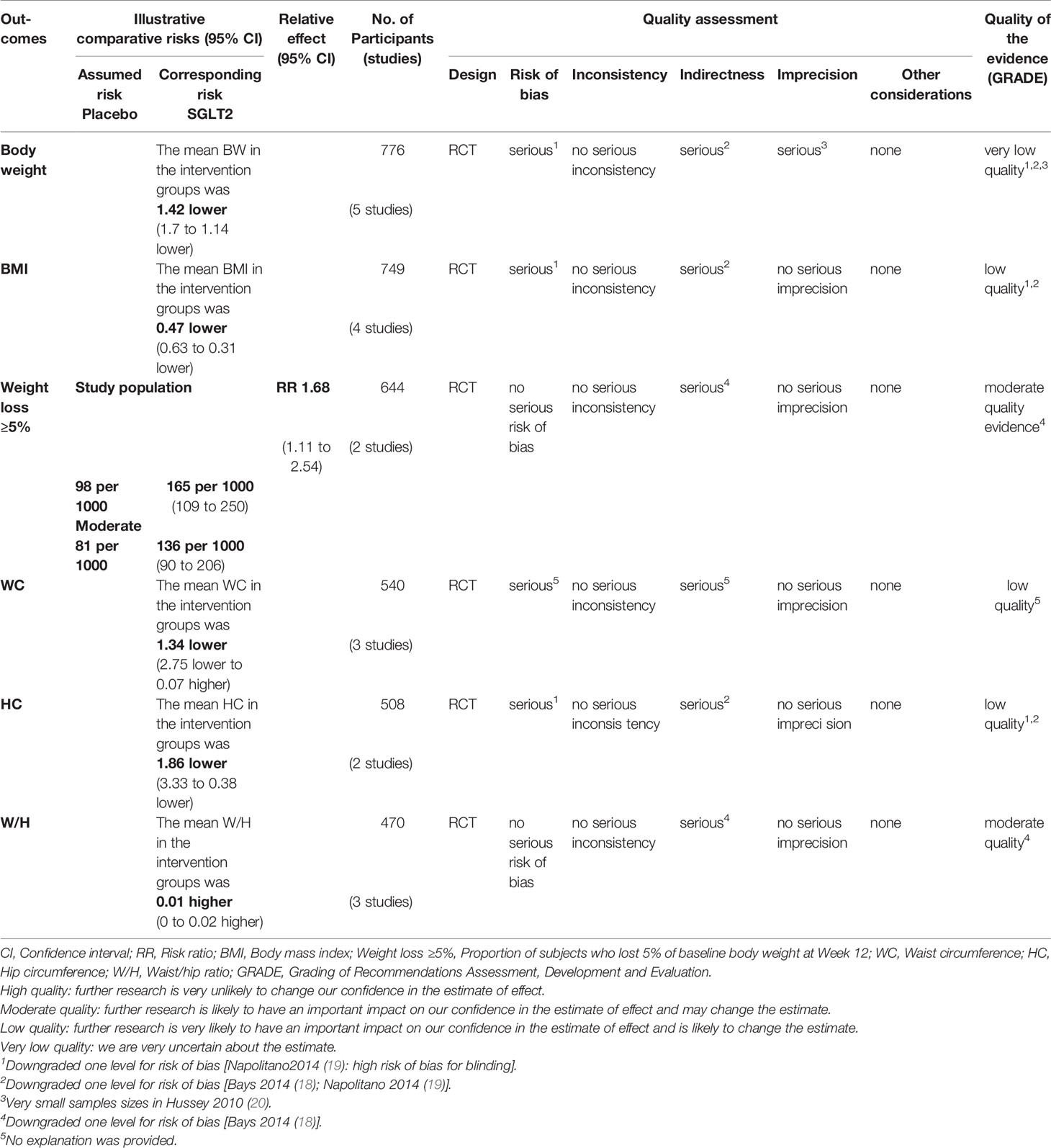
Table 2 The GRADE profiles: SGLT2 inhibitors compared to placebo in overweight or obese individuals without diabetes.
In the subgroup analyses of SGLT2 inhibitors, we found that the individuals in the canagliflozin and sergliflozin groups had a statistically significant reduction in body weight compared to those in the placebo group (MD: -1.26 kg, 95% CI: -1.70 to -0.82; MD: -1.51 kg, 95% CI: -1.87 to -1.15, respectively), while the individuals in the dapagliflozin and remogliflozin groups had no statistically significant reduction in body weight compared to those in the placebo group (MD: -4.30 kg, 95% CI: -9.76 to 1.16; MD: -2.50 kg, 95% CI: -8.18 to 3.18, respectively). TSA showed that the pooled results (Z curve) crossed the conventional boundary of benefit and reach the required information size (RIS=316). It confirmed that the SGLT2 inhibitors could significantly lowered body weight (Supplementary Figure 3).
Five studies displayed BMI change (15–19). One study reported that participants in the dapagliflozin group had reduced BMI by −0.3 kg/m2 (95%CI: −0.8 to 0.1) compared with the control (15). Others (16–19) were summarized for meta-analysis. Compared to placebo, SGLT2 inhibitors were associated with a statistically significant reduction in BMI (MD: -0.47 kg/m2, 95%CI: -0.63 to -0.31;Z=5.92, P<0.00001), which was homogeneous (I2 = 0%, P = 0.85) (Figure 4). This was low quality evidence that was downgraded one level for risk of bias and one level for indirectness (Table 2).
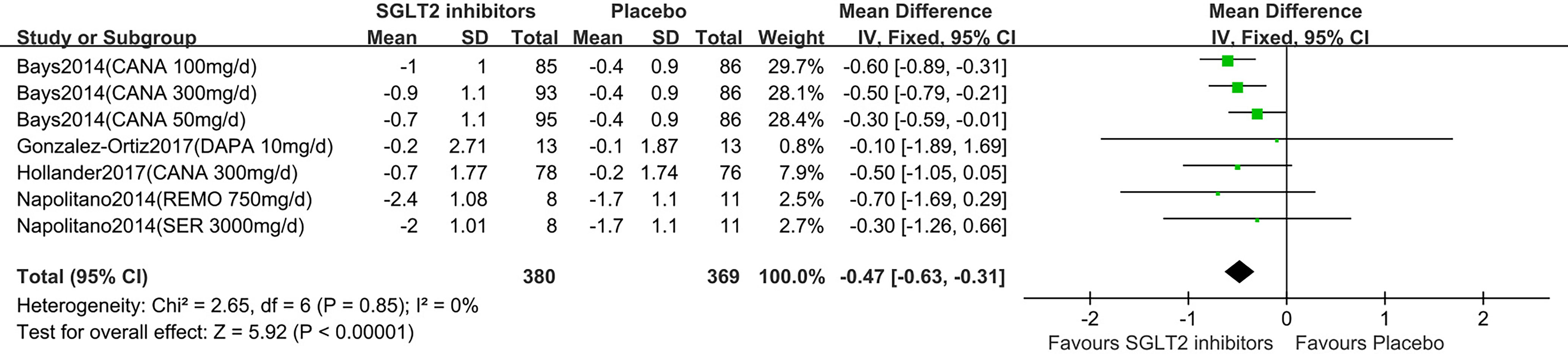
Figure 4 Changes of BMI in patients receiving SGLT2 inhibitors versus placebo. BMI, body mass index; CI, confidence interval; IV, inverse variance; SD, standardized deviation.
In the subgroup analyses of SGLT2 inhibitors, we found that the individuals in the canagliflozin groups had a statistically significant reduction in BMI compared to those in the placebo group (MD: -0.47 kg/m2, 95%CI: -0.63 to -0.31), while the individuals in the dapagliflozin, remogliflozin and sergliflozin groups had no statistically significant reduction in BMI (MD: -0.10 kg/m2, 95% CI: -1.89 to 1.69; MD: -0.70 kg/m2, 95% CI: -1.69 to 0.29; MD: -0.30 kg/m2; 95% CI: -1.26 to 0.66, respectively).
TSA showed that the pooled results (Z curve) crossed the conventional boundary of benefit and reach the required information size (RIS=218). It confirmed that the SGLT2 inhibitors could significantly lowered BMI (Supplementary Figure 4).
Two studies (16, 18) were summarized for meta-analysis. Compared to placebo, SGLT2 inhibitors were associated with a statistically significant greater in the proportion of individuals achieved weight loss over 5% (RR: 1.68, 95% CI: 1.11 to 2.54; Z=2.46, P=0.01), and it was homogeneous (I2 = 0%, P = 0.49) (Figure 5). This was moderate quality evidence that was downgraded one level for indirectness (Table 2).
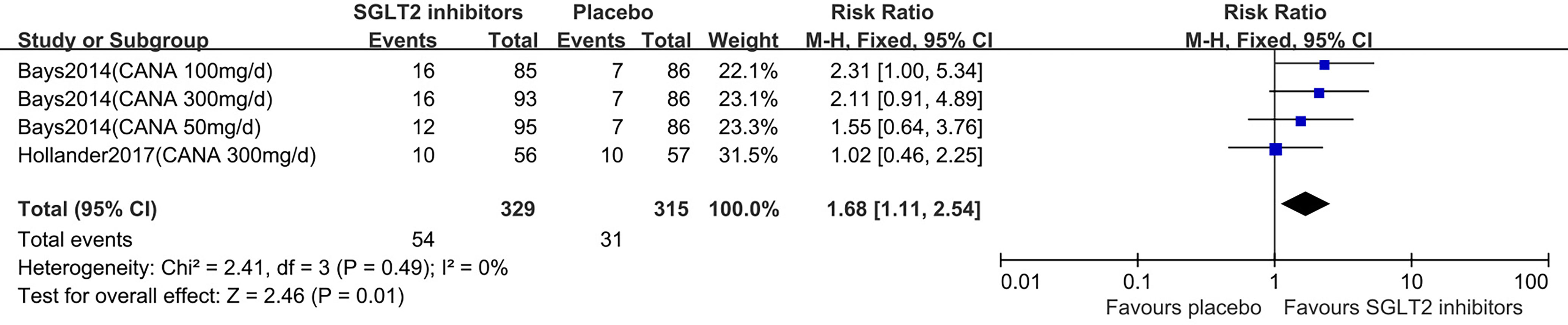
Figure 5 Changes of weight loss ≥5% in patients receiving SGLT2 inhibitors versus placebo. CI, confidence interval; M-H, Mantel-Haenszel.
Five studies displayed waist circumference change (15–19). One study reported that participants in the dapagliflozin group had reduced waist circumference by −2.4 cm (95% CI: −4.8 to 0.0) compared with control (15). The others (16–19) were summarized for meta-analysis. Compared to placebo, SGLT2 inhibitors were not associated with a statistically significant reduction in waist circumference (MD: -1.34 cm, 95% CI: -2.75 to 0.07; Z=1.86, P=0.06), which was homogeneous (I2 = 0%, P = 0.77) (Figure 6). This was low quality evidence that was downgraded one level for risk of bias and one level for indirectness (Table 2).
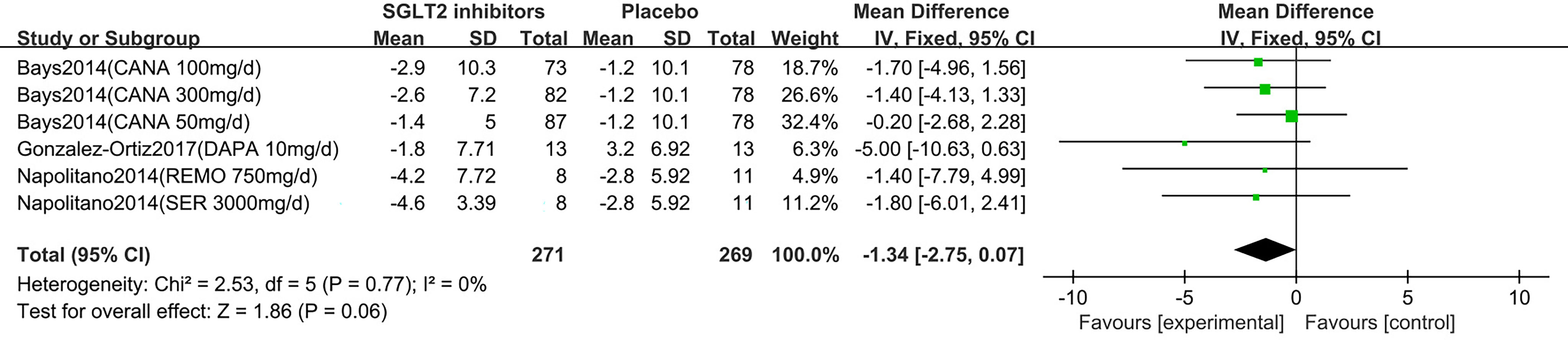
Figure 6 Changes of waist circumference in patients receiving SGLT2 inhibitors versus placebo. CI, confidence interval; IV, inverse variance; SD, standardized deviation.
In the subgroup analyses of SGLT2 inhibitors, we found that the individuals in the canagliflozin, dapagliflozin, remogliflozin and sergliflozin groups had no statistically significant reduction in waist circumference compared to those in the placebo group (MD: -0.97 cm, 95% CI: -2.57 to 0.63; MD: -5.00 cm, 95% CI: -10.63 to 0.63; MD: -1.40 cm, 95% CI: -7.79 to 4.99; MD: -1.80 cm, 95% CI: -6.01 to 2.41, respectively).
Two (18, 19) studies were summarized for meta-analysis. As shown in Figure 7, compared to placebo, SGLT2 inhibitors were associated with a statistically significant reduction in hip circumference (MD: -1.86 cm, 95% CI: -3.33 to -0.38; Z=2.46, P=0.01), which was homogeneous (I2 = 0%, P = 0.56). This was low quality evidence that was downgraded one level for risk of bias and one level for indirectness (Table 2).
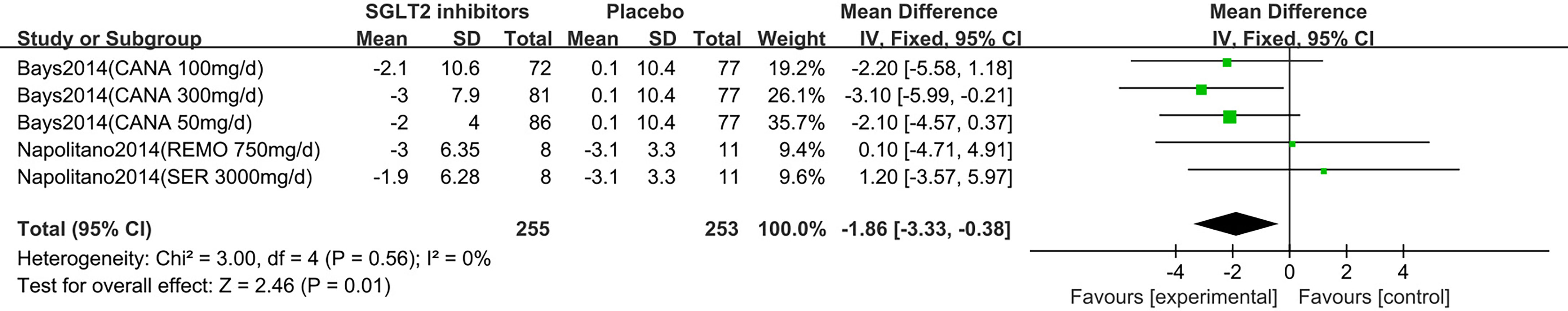
Figure 7 Changes of hip circumference in patients receiving SGLT2 inhibitors versus placebo. CI, confidence interval; IV, inverse variance; SD, standardized deviation.
In the subgroup analyses of SGLT2 inhibitors, we found that individuals in the canagliflozin group had a statistically significant reduction in hip circumference compared to those in the placebo group (MD: -2.45 cm, 95% CI: -4.09 to -0.80), while the individuals in the remogliflozin and sergliflozin groups had no statistically significant reduction in hip circumference (MD: 0.10 cm, 95% CI: -4.71 to 4.91; MD: 1.20 cm, 95% CI: -3.57 to 5.97, respectively).
Two studies displayed waist/hip ratio change (15, 18). One study reported that participants in dapagliflozin group had on average reduced waist/hip ratio (MD: −0.02, 95% CI −0.04 to 0.01) compared with control (15). One study (18) was summarized for meta-analysis. It is no significant difference of waist/hip ratio was observed between SGLT2 inhibitors and control group (MD: 0.01, 95% CI: -0.00 to 0.02; Z=1.88, P=0.06), which was homogeneous (I2 = 0%, P = 0.70) (Figure 8). This was moderate quality evidence that was downgraded one level for indirectness (Table 2).
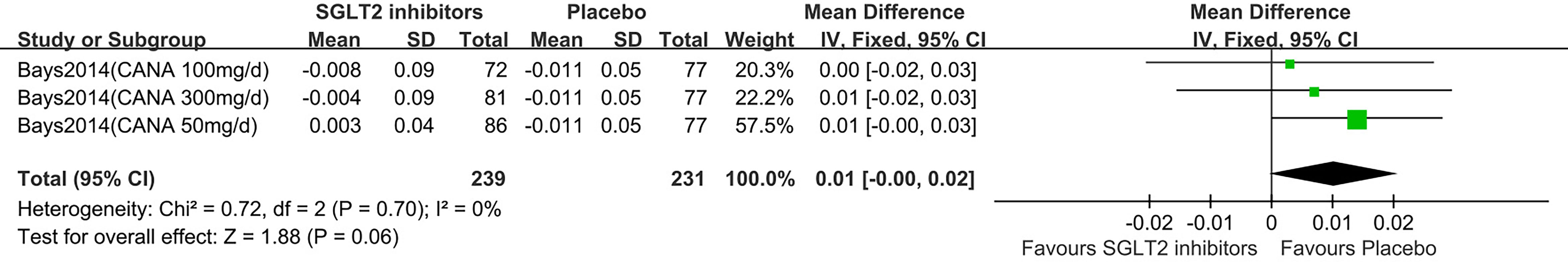
Figure 8 Changes of waist hip ratio in patients receiving SGLT2 inhibitors versus placebo. CI, confidence interval; IV, inverse variance; SD, standardized deviation.
SGLT2 inhibitors were generally well tolerated. Few serious adverse events were observed and none were considered related to study drug. It was shown that SGLT2 inhibitors increased the number of participants who withdrew or dropped out from studies (Table 3). Hypoglycemia, urinary tract infection, or sinusitis observed with SGLT2 inhibitors were similar to those reported in the placebo group. However, individuals assigned to SGLT2 inhibitors treatment suffered more genital mycotic infection, vulvovaginal mycotic infection and nausea than those in the placebo group. No adverse events of fractures, diabetic ketoacidosis (DKA) and cardiovascular safety were reported in the studies included here.
SGLT2 inhibitors, including canagliflozin, dapagliflozin, empagliflozin, and so on, have proven efficacy when used to treat type 2 diabetes, and all of them were considered effective in reducing body weight (10). Weight loss not only can reduce the risk of cardiovascular disease and endocrine metabolism disease, but also improved the fertility. A prospective cohort study in Boston indicated short term weight loss (3kg) was related to higher MII oocytes yield in women with obesity or overweight undergoing assisted reproductive technology (21). This meta-analysis involving 872 individuals showed that SGLT2 inhibitors may reduce body weight in adults with overweight or obesity but not diabetes, and the result is similar to that of a previous meta-analysis, which showed a reduction in body weight (MD: 1.74 kg, 95% CL: -2.03 to -1.45) compared with placebo in diabetes. Additionally, SGLT2 inhibitors also seemed helpful in reducing BMI but had no beneficial effects on waist circumference control compared with placebo. The subgroup analyses suggested that the weight reduction effect of canagliflozin and sergliflozin may not act in a dose-response manner.
SGLT2 inhibitors were generally well tolerated in previous studies in the type 2 diabetes population (9, 10). The major adverse reactions were genital mycotic infection and urinary infection (16, 18), and they were considered mild to moderate in severity. Compared to placebo, SGLT2 inhibitors were associated with meaningful differences in the incidences of genital mycotic infection and nausea, and particular attention was given to the higher rates of vulvovaginal mycotic infection in women in the SGLT2 inhibitors group, which may be attributable to increased UGE resulting in an increase in vulvovaginal Candida growth (22). No treatment-related fractures or DKA were reported in any group. Reporting of cardiovascular safety was also absent in the included studies, and SGLT2 inhibitors were recently proven to reduce the risk of heart failure in patients with type 2 diabetes by the EMPA-REG OUTCOME study and CANAS study (23, 24).
The exact mechanisms by which SGLT2 inhibitors reduce body weight are not completely understood. Recent clinical studies indicated that the weight loss effect observed with SGLT2 inhibitors contributed to the increased energy loss via urinary glucose excretion and mild osmotic diuresis (9, 19). Treatment with SGLT2 inhibitors could also alter body composition through energy loss and osmotic drain, which were associated with fat mass (19). Cefalu and colleagues (25) illustrated that the weight loss observed with canagliflozin in T2DM was mainly due to a reduction in fat mass, with a slightly greater loss of visceral versus subcutaneous fat. The reduction in the leptin-adiponectin ratio with remogliflozin has been reported to improve the metabolic health of adults with overweight or obesity but not diabetes, proposing this as an additional mechanism of body weight reduction with SGLT2 inhibitors (19, 26). Furthermore, inhibition of SGLT2 triggered glycogen depletion signals in the liver and activated the liver-brain-adipose axis, resulting in PKA activation in adipocytes, thereby inducing fat reduction and weight loss (27). Further study is required to confirm the potential mechanisms.
A study by Sarich and colleagues (28) showed that canagliflozin increased 24 h urinary glucose excretion in a dose-dependent manner and reduced body weight but was not associated with meaningful changes in plasma glucose or insulin levels in adults with obesity but not diabetes. The adverse events were transient and mild, with no reported hypoglycaemia. Lundkvist and colleagues (29, 30) reported a study of adults with obesity but not diabetes who received dapagliflozin 10 mg once daily and exenatide 2 mg once weekly, acquiring a mean weight loss of −4.5 kg after 24 weeks and −5.7 kg after an additional open-label 28 weeks, and the weight loss was largely due to the reduction in subcutaneous and visceral abdominal adipose tissue. This treatment also had a greater effect on glycaemic control, prediabetes prevalence and SBP. This suggests a potential role for the prevention of T2D and cardiovascular disease in this population.
Although some reviews or meta-analyses have been published before (31–33), this is the first study focusing on the efficacy and safety of SGLT2 inhibitors in adults with overweight or obesity but not diabetes. The retrieved RCTs had mild-to-moderate risks of all biases, and the heterogeneity between each included study was not significant. However, clinical heterogeneity may also exist, including the use of varying types and dosages of SGLT-2 inhibitors among the studies or other baseline and clinical characteristics of the individuals recruited. For example, the majority of the study population was women. Therefore, TSA was used to test the reliability of our study, controlling the potential risk.
There are still certain limitations of this analysis. The major limitation is that we only included six studies with small sample sizes and short follow-up periods, the sample sizes of the included studies ranged from 18 to 376, and the follow-up periods ranged from 2 to 26 weeks, potentially leading to unstable estimates of treatment effects. Additionally, the discontinuation rate was high due to some safety issues. Thirdly, This meta-analysis showed SGLT2 inhibitors can reduce average 1.42kg body weight in adults with overweight or obesity but not diabetes, but the clinical and prognostic benefit of weight change were limited base on the previous studies (34–36). Thus, larger sample sizes with longer durations of observation are needed to clarify the long-term benefits and risks of SGLT2 inhibitors in the treatment of adults with overweight or obesity but not diabetes. Moreover, we were unable to analyse whether the following factors would result in changes in outcomes. For example, the energy intake compensation that occurs in individuals without type 2 diabetes treated with SGLT2 inhibitors over a longer duration have yet to be considered. Only monotherapy and placebo-controlled RCTs were included in this study, and the differences among individual SGLT2 inhibitors cannot be compared due to the lack of head-to-head studies. All the included studies received industry funding, which may produce bias in the results.
In conclusion, SGLT2 inhibitors reduced body weight with statistical significance in adults with overweight or obesity but not diabetes. Given the limited weight reduction and potential harms, selected people may consider SGLT2 inhibitors as an alternative treatment for weight loss in addition to lifestyle intervention when they are at low risk of genital infection and urinary infection. Real world surveillance of the use of SGLT2 inhibitors in people with overweight and obesity but not diabetes is warranted for further information of their effectiveness and safety.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Design: NS and SL. Conduct/data collection: HZ, ML, and NS. Analysis: HZ, ML, and SZ. Writing manuscript: HZ, ML, SL, QS, YZ, SZ, and NS. All authors contributed to the article and approved the submitted version.
This study did not receive any grants or funds. Na Su was supported by grants from Health Commission Program (grant number 2020-111) and 1.3.5 Project for Disciplines of Excellence, West China Hospital, Sichuan University (grant number 2018HXFH048).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.706914/full#supplementary-material
Supplementary Figure 1 | Risk of bias graph of all included studies (n=6).
Supplementary Figure 2 | Risk of bias summary of all included studies (n=6).
Supplementary Figure 3 | Trial sequential analysis of body weight change. RIS, Required information size.
Supplementary Figure 4 | Trial sequential analysis of BMI change. RIS, Required information size; BMI, Body mass index.
Supplementary Information 1 | Medline Searching strategy.
Supplementary Information 2 | Rational of excluding studies.
Supplementary Table 1 | PRISMA Checklist for the Meta-analysis.
1. Li S, Nemeth I, Donnelly L, Hapca S, Zhou K, Pearson ER. Visit-To-Visit HbA1c Variability Is Associated With Cardiovascular Disease and Microvascular Complications in Patients With Newly Diagnosed Type 2 Diabetes. Diabetes Care (2020) 43:426–32. doi: 10.2337/dc19-0823
2. Zhou YL, Zhang YG, Zhang R, Zhou YL, Li N, Wang MY, et al. Population Diversity of Cardiovascular Outcome Trials and Real-World Patients With Diabetes in a Chinese Tertiary Hospital. Chin Med J (2021) 134:1317–23. doi: 10.1097/CM9.0000000000001407
3. Klein S, Sheard NF, Pi-Sunyer X, Daly A, Wylie-Rosett J, Kulkarni K, et al. Weight Management Through Lifestyle Modification for the Prevention and Management of Type 2 Diabetes: Rationale and Strategies: A Statement of the American Diabetes Association, the North American Association for the Study of Obesity, and the American Society for Clinical Nutrition. Diabetes Care (2004) 27:2067–73. doi: 10.1093/ajcn/80.2.257
4. World Health Organization. Obesity and Overweight (2021). Available at: http://www.who.int/mediacentre/factsheets/fs311/en/ (Accessed Feb 27, 2021).
5. World Health Organization. Obesity (2021). Available at: http://www.who.int/gho/ncd/risk_factors/obesity_text/en/ (Accessed Feb 27, 2021).
6. Apovian CM, Aronne LJ, Bessesen DH, McDonnell ME, Murad MH, Pagotto U, et al. Pharmacological Management of Obesity: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab (2015) 100:342–62. doi: 10.1210/jc.2014-3415
7. U.S. FOOD & DRUG ADMINISTRATION. Sodium-Glucose Cotransporter-2 (SGLT2) Inhibitors (2021). Available at: https://www.fda.gov/drugs/drugsafety/postmarketdrugsafetyinformationforpatientsandproviders/ucm446852.htm (Accessed Feb 27, 2021).
8. Heerspink HJL, de Zeeuw D, Wie L, Leslie B, List J. Dapagliflozin a Glucose-Regulating Drug With Diuretic Properties in Subjects With Type 2 Diabetes. Diabetes Obes Metab (2013) 15:853–62. doi: 10.1111/dom.12127
9. El Masri D, Ghosh S, Jaber LA. Safety and Efficacy of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibitors in Type 1 Diabetes: A Systematic Review and Meta-Analysis. Diabetes Res Clin Pract (2018) 137:83–92. doi: 10.1016/j.diabres.2018.01.004
10. Liu J, Li L, Li S, Wang Y, Qin X, Deng K, et al. Sodium-Glucose Co-Transporter-2 Inhibitors and the Risk of Diabetic Ketoacidosis in Patients With Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Obes Metab (2020) 22:1619–27. doi: 10.1111/dom.14075
11. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ (2015) 350:g7647. doi: 10.1136/bmj.g7647
12. Higgins JPT, Green S, Collaboration TC. Cochrane Handbook for Systematic Reviews of Interventions (2018). Available at: http://handbook.cochrane.org (Accessed Feb 27, 2018).
13. Langer G, Meerpohl JJ, Perleth M, Gartlehner G, Kaminski-Hartenthaler A, Schunemann H. GRADE Guidelines: 1. Introduction - GRADE Evidence Profiles and Summary of Findings Tables. Z Evid Fortbild Qual Gesundhwes (2012) 106:357–68. doi: 10.1016/j.zefq.2012.05.017
14. Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso-Coello P, Rind D, et al. GRADE Working Group. GRADE Guidelines: 7. Rating the Quality of Evidence—Inconsistency. J Clin Epidemiol (2011) 64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017
15. Færch K, Blond MB, Bruhn L, Amadid H, Vistisen D, Clemmensen KKB, et al. The Effects of Dapagliflozin, Metformin or Exercise on Glycaemic Variability in Overweight or Obese Individuals With Prediabetes (the PRE-D Trial): A Multi-Arm, Randomised, Controlled Trial. Diabetologia (2021) 64:42–55. doi: 10.1007/s00125-020-05306-1
16. Hollander P, Bays HE, Rosenstock J, Frustaci ME, Fung A, Vercruysse F, et al. Coadministration of Canagliflozin and Phentermine for Weight Management in Overweight and Obese Individuals Without Diabetes: A Randomized Clinical Trial. Diabetes Care (2017) 40:632–9. doi: 10.2337/dc16-2427
17. Gonzalez-Ortiz M, Grover-Paez F, Diaz-Cruz C, de JP-LA, Lopez-Murillo LD, Martinez-Abundis E. Dapagliflozin Administration on Visceral Adiposity, Blood Pressure and Aortic Central Pressure in Overweight Patients Without Type 2 Diabetes. Minerva Med (2017) 108:384–6. doi: 10.23736/S0026-4806.17
18. Bays HE, Weinstein R, Law G, Canovatchel W. Canagliflozin: Effects in Overweight and Obese Subjects Without Diabetes Mellitus. Obes (Silver Spring) (2014) 22:1042–9. doi: 10.1002/oby.20663
19. Napolitano A, Miller S, Murgatroyd PR, Hussey E, Dobbins RL, Bullmore ET, et al. Exploring Glycosuria as a Mechanism for Weight and Fat Mass Reduction. A Pilot Study With Remogliflozin Etabonate and Sergliflozin Etabonate in Healthy Obese Subjects. J Clin Transl Endocrinol (2014) 1:e3–8. doi: 10.1016/j.jcte.2013.12.001
20. Hussey EK, Dobbins RL, Stoltz RR, Stockman NL, O’Connor-Semmes RL, Kapur A, et al. Multiple-Dose Pharmacokinetics and Pharmacodynamics of Sergliflozin Etabonate, a Novel Inhibitor of Glucose Reabsorption, in Healthy Overweight and Obese Subjects: A Randomized Double-Blind Study. J Clin Pharmacol (2010) 50:636–46. doi: 10.1177/0091270009352185
21. Jorge EC, Shelley E, Daniela SC, Diane LW, Thomas LT, John CP, et al. Body Mass Index and Short-Term Weight Change in Relation to Treatment Outcomes in Women Undergoing Assisted Reproduction. Fertil Steril (2012) 98:109–16. doi: 10.1016/j.fertnstert.2012.04.012
22. Nyirjesy P, Zhao Y, Ways K, Usiskin K. Evaluation of Vulvovaginal Symptoms and Candida Colonization in Women With Type 2 Diabetes Mellitus Treated With Canagliflozin, a Sodium Glucose Co-Transporter 2 Inhibitor. Curr Med Res Opin (2012) 28:1173–8. doi: 10.1185/03007995.2012.697053
23. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med (2015) 373:2117–28. doi: 10.1056/NEJMoa1504720
24. Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med (2017) 377:644–57. doi: 10.1056/NEJMc1712572
25. Cefalu WT, Leiter LA, Yoon K-H, Arias P, Niskanen L, Xie J, et al. Efficacy and Safety of Canagliflozin Versus Glimepiride in Patients With Type 2 Diabetes Inadequately Controlled With Metformin (CANTATA-SU): 52 Week Results From a Randomised, Double-Blind, Phase 3 Non-Inferiority Trial. Lancet (2013) 382:941–50. doi: 10.1016/S0140-6736(13)60683-2
26. Zeng L, Ye Z, Li Y, Zhou YL, Shi QY, Hu T, et al. Different Lipid Parameters in Predicting Clinical Outcomes in Chinese Statin-Naïve Patients After Coronary Stent Implantation. Front Cardiovasc Med (2021) 8:638663. doi: 10.3389/fcvm.2021.638663
27. Sawada Y, Izumida Y, Takeuchi Y, Aita Y, Wada N, Li E, et al. Effect of Sodium-Glucose Cotransporter 2 (SGLT2) Inhibition on Weight Loss Is Partly Mediated by Liver-Brain-Adipose Neurocircuitry. Biochem Biophys Res Commun (2017) 493:40–5. doi: 10.1016/j.bbrc.2017.09.081
28. Sha S, Devineni D, Ghosh A, Polidori D, Chien S, Wexler D, et al. Canagliflozin, A Novel Inhibitor of Sodium Glucose Co-Transporter 2, Dose Dependently Reduces Calculated Renal Threshold for Glucose Excretion and Increases Urinary Glucose Excretion in Healthy Subjects. Diabetes Obes Metab (2011) 13:669–72. doi: 10.1111/j.1463-1326.2011.01406.x
29. Lundkvist P, Sjostrom CD, Amini S, Pereira MJ, Johnsson E, Eriksson JW. Dapagliflozin Once-Daily and Exenatide Once-Weekly Dual Therapy: A 24-Week Randomized, Placebo-Controlled, Phase II Study Examining Effects on Body Weight and Prediabetes in Obese Adults Without Diabetes. Diabetes Obes Metab (2017) 19:49–60. doi: 10.1111/dom.12779
30. Lundkvist P, Pereira MJ, Katsogiannos P, Sjostrom CD, Johnsson E, Eriksson JW. Dapagliflozin Once Daily Plus Exenatide Once Weekly in Obese Adults Without Diabetes: Sustained Reductions in Body Weight, Glycaemia and Blood Pressure Over 1 Year. Diabetes Obes Metab (2017) 19:1276–88. doi: 10.1111/dom.12954
31. Ding L, Sun B, Xiao XH. Comparing the Efficacy and Safety of Glucagon-Like Peptide 1 Receptor Agonists with Sodium-Glucose Cotransporter 2 Inhibitors for Obese Type 2 Diabetes Patients Uncontrolled on Metformin: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Int J Endocrinol (2020) 28:1626484. doi: 10.1155/2020/1626484
32. Guo M, Gu J, Teng F, Chen J, Ma X, Chen Q, et al. The Efficacy and Safety of Combinations of SGLT2 Inhibitors and GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes or Obese Adults: A Systematic Review and Meta-Analysis. Endocrine (2020) 67:294–304. doi: 10.1007/s12020-019-02175-6
33. Uneda K, Kawai Y, Yamada T, Kinguchi S, Azushima K, Kanaoka T, et al. Systematic Review and Meta-Analysis for Prevention of Cardiovascular Complications Using GLP-1 Receptor Agonists and SGLT-2 Inhibitors in Obese Diabetic Patients. Sci Rep (2021) 11:10166. doi: 10.1038/s41598-021-89620-7
34. Andrea MB, Robert AS, Fanchao Y, Elvis AC, Lauren MS, Bret HG. Individual Response Variation in the Effects of Weight Loss and Exercise on Insulin Sensitivity and Cardiometabolic Risk in Older Adults. Front Endocrinol (Lausanne) (2020) 11:632. doi: 10.3389/fendo.2020.00632
35. Bethany BG, Frederick LB, Haiying C, Mace C, John MJ, et al. Effect of Improved Fitness Beyond Weight Loss on Cardiovascular Risk Factors in Individuals With Type 2 Diabetes in the Look AHEAD Study. Eur J Prev Cardiol (2014) 21:608–17. doi: 10.1177/2047487312462823
Keywords: SGLT2 inhibitors, obesity, overweight, non-diabetic adults, meta-analysis
Citation: Zheng H, Liu M, Li S, Shi Q, Zhang S, Zhou Y and Su N (2021) Sodium-Glucose Co-Transporter-2 Inhibitors in Non-Diabetic Adults With Overweight or Obesity: A Systematic Review and Meta-Analysis. Front. Endocrinol. 12:706914. doi: 10.3389/fendo.2021.706914
Received: 08 May 2021; Accepted: 26 July 2021;
Published: 16 August 2021.
Edited by:
Massimiliano Caprio, Università telematica San Raffaele, ItalyReviewed by:
Evelyn Frias-Toral, Catholic University of Santiago de Guayaquil, EcuadorCopyright © 2021 Zheng, Liu, Li, Shi, Zhang, Zhou and Su. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Na Su, em95YTE1OUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.