- The Reproduction Center, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Objective: To compare the cumulative live birth rate (CLBR) of the progestin-primed ovarian stimulation (PPOS) protocol with that of the flexible GnRH antagonist protocol in patients with poor prognosis diagnosed per the POSEIDON criteria.
Methods: This was a retrospective cohort study. Low-prognosis women who underwent IVF/ICSI at the Reproductive Center of Third Affiliated Hospital of Zhengzhou University between January 2016 and January 2019 were included according to the POSEIDON criteria. The CLBR was the primary outcome of interest. The secondary outcome measures were the numbers of oocytes retrieved, 2PN embryos, available embryos and time to live birth.
Results: A total of 1329 women met the POSEIDON criteria for analysis. For POSEIDON group 1, the dosage of gonadotropin (Gn) was higher in the PPOS group than in the GnRH antagonist group (2757.3 ± 863.1 vs 2419.2 ± 853.1, P=0.01). The CLBR of the PPOS protocols was 54.4%, which was similar to the rate of 53.8% in the GnRH antagonist group. For POSEIDON group 2, the number of available embryos was higher in the PPOS group (2.0 ± 1.7 vs 1.6 ± 1.4, P=0.02) than in the GnRH antagonist group. However, the CLBRs of the two groups were similar (18.1% vs 24.3%, P=0.09). For POSEIDON groups 3 and 4, there were no statistically significant differences in the number of oocytes retrieved, 2PN, available embryos or CLBR between the two protocols. After adjustments for confounding factors, the CLBR remained consistent with the unadjusted rates. In the POSEIDON group 1 population, the GnRH antagonist protocols had a shorter time to live birth (P=0.04).
Conclusion: For low-prognosis patients diagnosed per the POSEIDON criteria, the CLBR of PPOS protocols is comparable to that of GnRH antagonist protocols. In the POSEIDON group 1 population, the GnRH antagonist protocols resulted in a shorter time to live birth.
Introduction
Controlled ovarian stimulation (COS) is a key step in assisted reproductive technology (ART). In routine clinical practice with COS-assisted pregnancy, up to 9%~24% of patients have poor ovarian response (POR) (1). Diagnosis, treatment and fertility assistance for these patients have always been the focus of the field of reproduction, but these issues continue to be challenging. At present, the Bologna criteria, discussed and formulated by the European Society of Human Embryology and Reproduction and the American Society of Reproductive Medicine in 2011, are the most widely used standards in clinical practice (2). This standard facilitates predictions of and consultations regarding clinical outcomes, but it still has certain limitations. Specifically, questions regarding the heterogeneity of these criteria to select homogenous populations for clinical trials have been raised (3–5). Due to the multiple ways in which the Bologna criteria can be met, the baseline characteristics and prognoses of patients are quite different. This heterogeneity is related to differences in underlying causes and may lead to differences in intervention effects. Therefore, the Bologna criteria definition may affect the clinical diagnosis and treatment of specific subpopulations. The POSEIDON criteria were proposed by the POSEIDON group in 2016; they consider ovarian biomarkers, the number of oocytes obtained, the age-related embryo aneuploidy rate and ovarian sensitivity to exogenous gonadotropin (Gn) (6). ‘Low-prognosis women’ are categorized into four groups based on female age, ovarian reserve indicators and the ovarian response to Gn during previous COS. Therefore, the POSEIDON criteria can improve the homogeneity and comparability of clinical research studies, decrease the dilution of potential treatment effects, and provide a more significant guide for formulating clinical protocols for low-prognosis women (6, 7). The development of individualized COS protocols according to different populations is critical to the outcome of assisted pregnancy, especially for patients with low prognosis. Therefore, it is necessary to explore suitable ovulation induction programs for people with low prognosis.
Gonadotropin-releasing hormone (GnRH) antagonist is the routine ovulation stimulation protocol (8, 9). There are a large number of randomized controlled, prospective and retrospective studies on GnRH antagonist regimens that fully prove the effectiveness and safety of GnRH antagonist regimens (10–13). However, it has been reported that GnRH antagonist protocols have a 0.34% to 8.0% chance of failing to control the LH surge, and increased age and diminished ovarian reserve are the main risk factors (14–17). In 2015, Kuang et al. (18) proposed a new COS protocol named progestin-primed ovarian stimulation (PPOS), which has advantages in terms of its effectiveness for suppressing the LH surge as well as its oral administration. Additionally, clinical effectiveness and safety have been demonstrated in women with polycystic ovary syndrome (PCOS), normal ovarian response and POR (19–22). To our knowledge, there have been no studies comparing PPOS and GnRH antagonist protocols according to the POSEIDON criteria, which would be meaningful for the formulation of individualized clinical programs. Therefore, the purpose of this study was to explore the cumulative live birth rate (CLBR) of patients with low prognosis diagnosed per the POSEIDON criteria by comparing patients receiving PPOS protocols with those receiving GnRH antagonist protocols.
Materials and Methods
Study Design and Population
This was a retrospective cohort study approved by the review board of the Third Affiliated Hospital of Zhengzhou University. For this study, we included women with a low prognosis who underwent IVF/ICSI at the Reproductive Center of the Third Affiliated Hospital of Zhengzhou University between January 2016 and January 2019 according to the POSEIDON criteria. All women were categorized into four groups according to the POSEIDON criteria (6).
➤POSEIDON group 1: Female age <35 years; ovarian biomarkers showing antral follicle count (AFC)≥5 and/or AMH≥1.2 ng/ml; ovarian response measured as oocytes retrieved < 10.
➤POSEIDON group 2: Female age ≥35 years; ovarian biomarkers showing AFC≥5 and/or AMH≥1.2 ng/ml; ovarian response measured as oocytes retrieved < 10.
➤POSEIDON group 3: Female age <35 years; ovarian biomarkers showing AFC<5 and/or AMH<1.2 ng/ml.
➤POSEIDON group 4: Female age ≥35 years; ovarian biomarkers showing AFC<5 and/or AMH<1.2 ng/ml.
Cycles with adenomyosis, uterine malformations, endometrial polyps, preimplantation genetic testing (PGT) and donor oocytes were excluded. To reduce the confounding impact caused by multiple enrollments of patients, patients were enrolled only once.
Controlled Ovarian Hyperstimulation Protocols
Previous COS Protocols for the POSEIDON Groups 1 and 2
For the POSEIDON groups 1 and 2, the previous COS protocol was the routine GnRH agonist (GnRH-a) protocol, which was divided into the early follicular phase GnRH-a protocol and the mid-luteal phase GnRH-a protocol. The specific protocols have been carefully described in our previous research (23, 24).
Progestin-Primed Ovarian Stimulation (PPOS)
For the PPOS protocol, COS was initiated on the second or third day of the menstrual cycle. Patients were administered 6 mg of medroxyprogesterone acetate (MPA) (Beijing Zhong Xin Pharmaceutical, China) combined with human menopausal gonadotropin (hMG) (Anhui Fengyuan Pharmaceutical, China) at a dose of 150 to 300 IU/day depending on maternal age, body mass index (BMI), AMH and basal AFC. Then, follicle growth was monitored by vaginal ultrasound combined with serum hormone analysis 4 days later. If necessary, the dose of hMG was adjusted according to follicle development. When the diameter of the dominant follicle was greater than 20 mm or when at least three follicles reached 18 mm, the final stage of trigger ovulation was performed with triptorelin (100 μg) (Ferring International Center SA, Germany) and 2000 IU of human chorionic gonadotropin (hCG) (Lizhu Pharmaceutical Trading, China), followed by oocyte pickup 36 hours later.
GnRH Antagonist
In the flexible GnRH antagonist group, hMG (Anhui Fengyuan Pharmaceutical, China) (150 to 300 IU/day) was initiated on the second or third day of the menstrual cycle. Injection of GnRH antagonist at 0.25 mg/day commenced once the diameter of the dominant follicle reached 14 mm and was continued up to the trigger day. The dose of hMG was adjusted according to the follicle response. As soon as the diameter of the dominant follicle was greater than 20 mm or when at least three follicles reached 18 mm, ovulation induction was co-triggered with triptorelin (100 μg) (Ferring International Center SA, Germany) and 2000 IU hCG (Lizhu Pharmaceutical Trading, China). Oocyte retrieval was performed 36 hours later. Conventional IVF or ICSI was performed based on the sperm quality.
Embryo Transfer and Endometrial Preparation Protocols
For PPOS protocols, whole embryos were frozen. For the flexible GnRH antagonist group, three days after oocyte retrieval, one or two embryos were transferred under monitoring by abdominal ultrasound or single blastocyst transfer was carried out five days after oocyte retrieval. For cases with severe ovarian hyperstimulation syndrome (OHSS), an endometrial thickness ≤7 mm, progesterone levels ≥2 ng/ml on the hCG trigger day and the presence of uterine fluid, we canceled the fresh embryo transfer, cryopreserved all embryos and subsequently performed frozen embryo transfer (FET). Endometrial preparation for FET was performed by means of the natural cycle for women with regular menstrual cycles and spontaneous ovulation; artificial/induced ovulation cycle for women with irregular menstrual cycles; and downregulation + an artificial cycle for women with endometriosis. Follicle and endometrial scanning was performed by vaginal ultrasound, and embryo or blastocyst transfer was performed using abdominal ultrasound after 3 or 5 days of endometrial development with luteosterone. Routine corpus luteum support, namely, oral dydrogesterone (2 times daily, 10 mg once) (Abbott Co. America) and intravaginal administration of 90 mg of a progesterone sustained-release vaginal gel (Merck Co. Germany), was given. Corpus luteum support was continued at least until 55 days after transfer if pregnancy occurred.
Outcome Measures and Definition
The primary outcome measure was CLBR, defined as at least one live birth resulting from one aspirated ART cycle, including all cycles in which fresh and/or frozen embryos were transferred, until one delivery with a live birth occurred or until all embryos were used, whichever occurred first. The delivery of a singleton, twin, or other multiple was registered as one delivery (25). We used a conservative approach to assume the CLBR, which means that couples who discontinued treatment would have zero change in conceiving. The observation and follow-up time was 2 years.
The secondary outcome measures were the number of oocytes retrieved, number of 2PN embryos and number of available embryos. The time to live birth is also an observation indicator of this study and is defined as the time from the start of COS to live birth in this cycle (months).
Statistical Analysis
All statistical management and analyses were performed using SPSS software, version 22.0.
The one-sample K-S test was used to check for normality. Continuous variables with abnormal distributions are expressed as the mean ± SD, and the Student’s t-test was used to assess between-group differences. Categorical variables are represented as the number of cases (n) and percentage (%). The means from chi-square analyses were used to assess the differences between groups with Fisher’s exact test when necessary. For CLBR, binary logistic regression was used to adjust for the baseline characteristics. Adjusted odds ratios (AORs) with 95% confidence intervals (CIs) were calculated. P<0.05 was considered to be statistically significant.
Results
Study Population
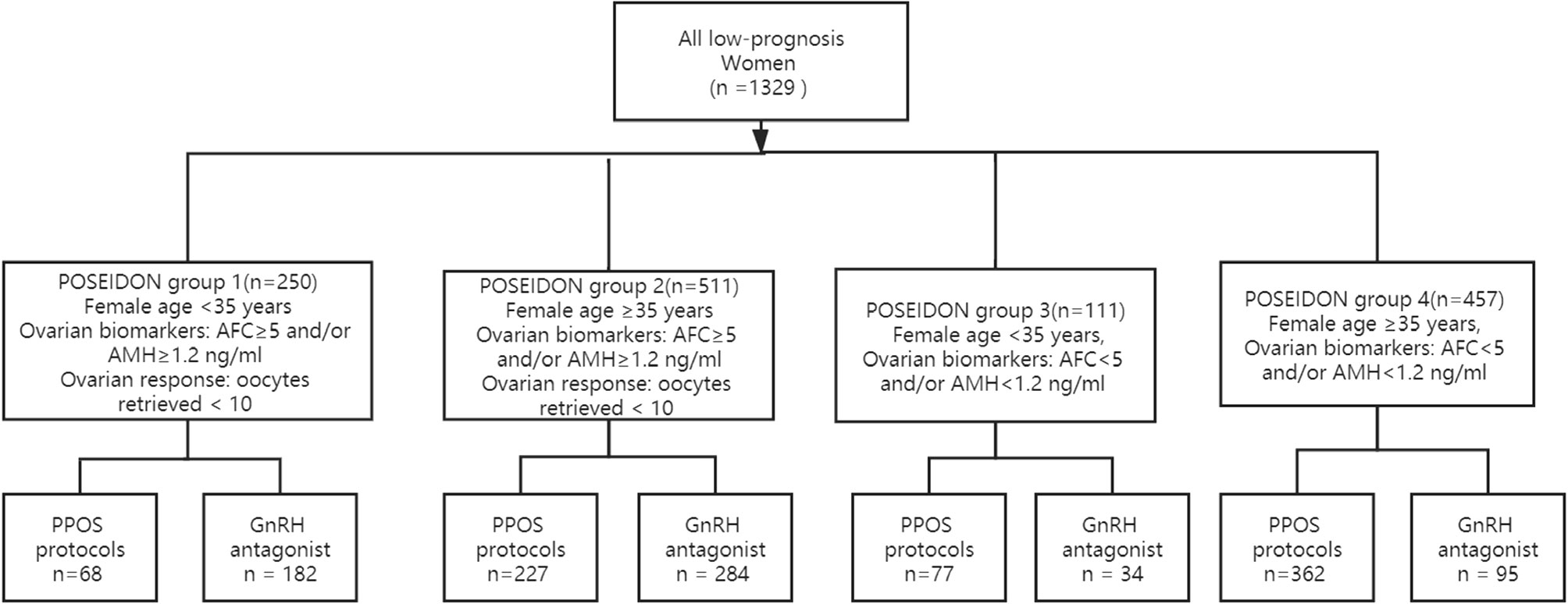
In total, 1329 women met the POSEIDON criteria from January 2016 to January 2019 in our reproductive center; 734 women underwent PPOS protocols and 595 women underwent GnRH antagonist protocols. Two hundred fifty women met the criteria for POSEIDON 1 group, 511 women for POSEIDON 2 group, 111 women for POSEIDON 3 group, and 457 women for POSEIDON 4 group. The flowchart of the participants is presented in Figure 1.
Baseline Characteristics
Table 1 shows the baseline and cycle characteristics of low-prognosis women stratified according to the POSEIDON criteria. Briefly, maternal age and paternal age were higher in POSEIDON groups 2 and 4 than in POSEIDON groups 1 and 3. The AMH level and AFC were higher in groups 1 and 2 than in groups 3 and 4. A total of 55.2% (734/1329) of the women underwent PPOS protocols and 44.8% (595/1329) underwent GnRH antagonist protocols. The baseline and cycle characteristics of the POSEIDON groups 1 and 2 from previous cycles are described in Supplemental Table 1.
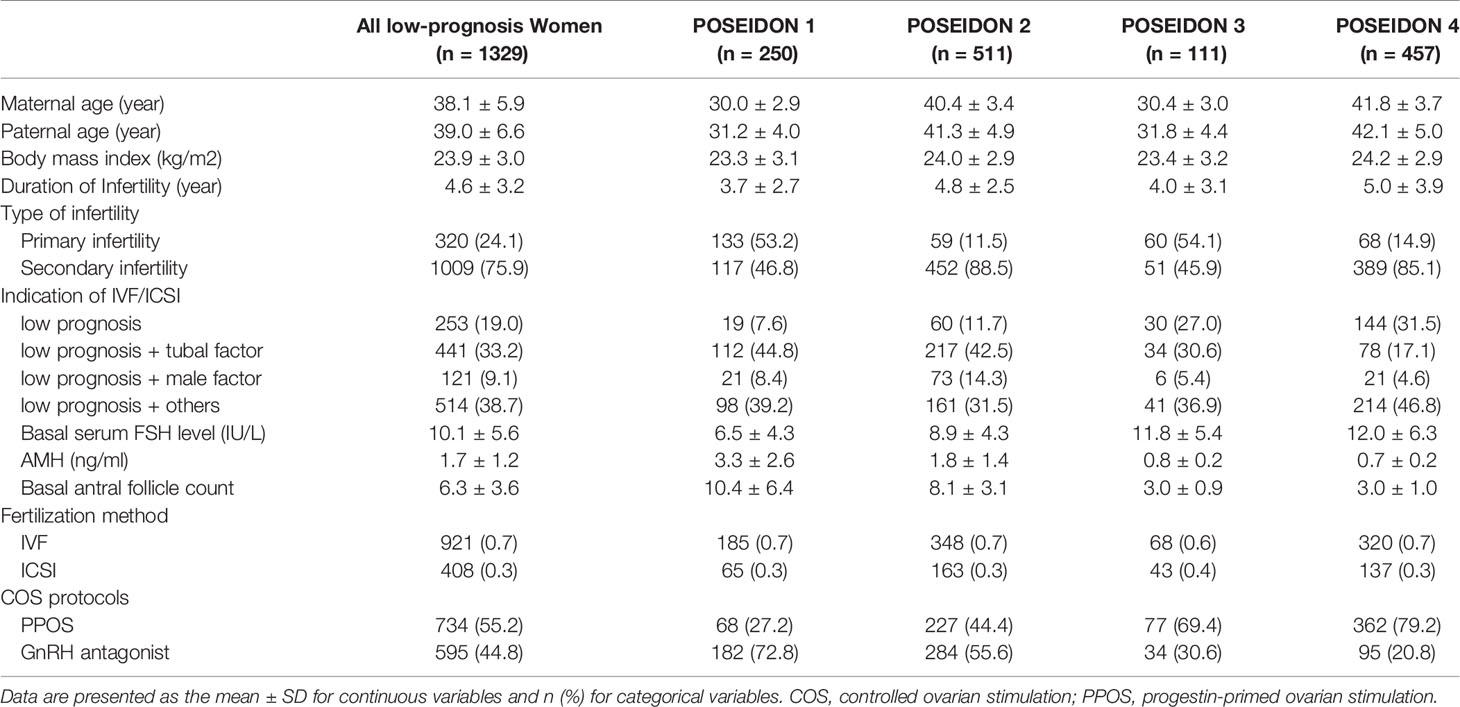
Table 1 Baseline and cycle characteristics of low-prognosis women stratified according to the POSEIDON criteria.
Reproductive Outcomes
The detailed reproductive outcomes are shown in Table 2. For POSEIDON group 1, the Gn dosage was higher in the PPOS protocols than in the GnRH antagonist group (2757.3 ± 863.1 vs 2419.2 ± 853.1, P=0.01). The serum hormone levels (including LH, E2 and P) on the trigger day, number of oocytes retrieved, number of 2PN embryos and number of available embryos were comparable between the two groups. The CLBR in the PPOS group was 54.4%, similar to the 53.8% observed in the GnRH antagonist group. For POSEIDON group 2, the number of available embryos was higher in the PPOS group (2.0 ± 1.7 vs 1.6 ± 1.4, P=0.02) than in the GnRH antagonist group. However, the CLBRs were similar between the two groups (18.1% vs 24.3%, P=0.09). For POSEIDON groups 3 and 4, there were no statistically significant differences in Gn dosage; duration of ovarian stimulation; LH, E2, and P values on the trigger day; number of oocytes retrieved; number of 2PN embryos; number of available embryos; number of embryo transfer cycles that reached live birth or the end of observation or CLBR between the PPOS and GnRH antagonist groups. In POSEIDON group 1, the time to live birth of the GnRH antagonist protocol was significantly shorter than that of the PPOS protocol (P=0.04). In the other three groups, although the time for the GnRH antagonist to reach live birth was shorter than that of the PPOS protocols, the difference was not statistically significant.
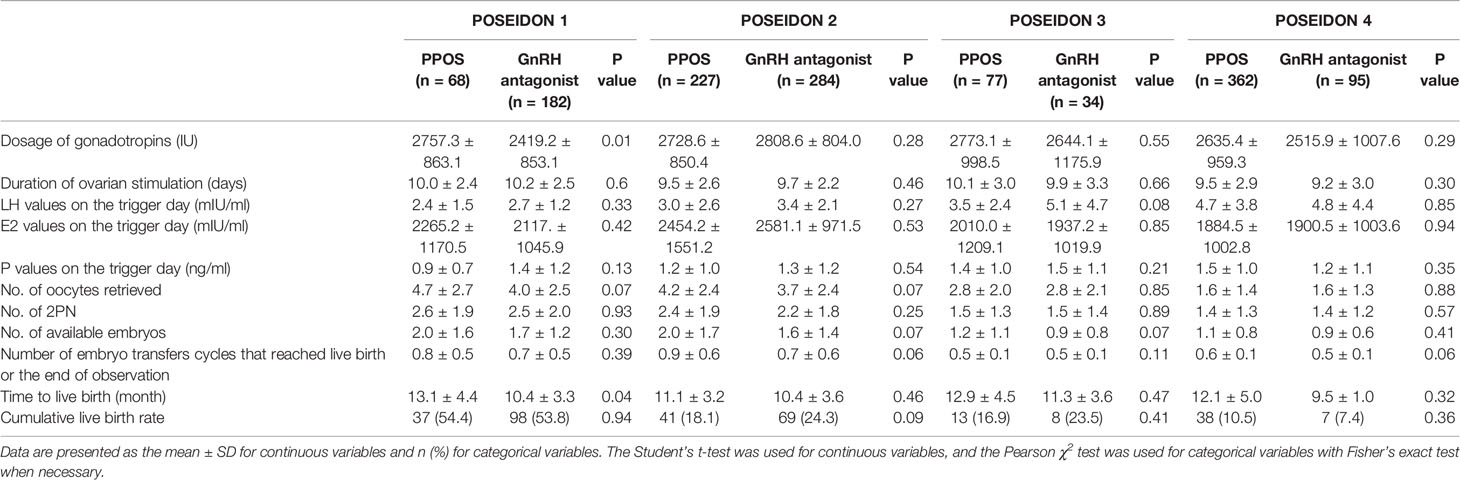
Table 2 Clinical and pregnancy outcomes between PPOS and GnRH antagonist protocols of low-prognosis women.
Regarding the main outcome measures, namely, the CLBR, we conducted a binary logistic regression analysis with adjustments for confounding factors. These factors included maternal age, BMI, duration of infertility, type of infertility (primary/secondary), infertility diagnosis (low prognosis/low prognosis+tubal factor/low prognosis+male factor/low prognosis+others) and AFC. The AOR values with their 95% CIs are presented in Table 3. After adjustments for confounding factors, the CLBR remained consistent with the unadjusted rates, and the rates were comparable between the PPOS and GnRH antagonist groups of patients with low prognosis. For POSEIDON groups 2 and 4, maternal age was associated with the CLBR.
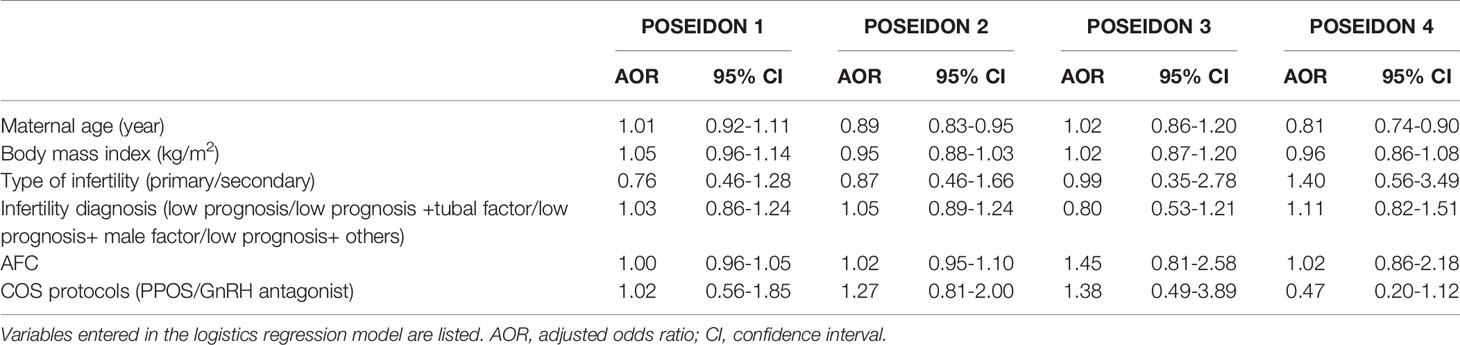
Table 3 Binary logistic regression analysis to account for confounding variables affecting the cumulative live birth rate.
Discussion
In summary, the CLBRs of the PPOS and GnRH antagonist groups were comparable among women with low prognosis per the POSEIDON criteria. For POSEIDON group 2, the number of available embryos was higher in the PPOS group. At the same time, we also analyzed the time to live birth for the two COS protocols. Since GnRH antagonists have the opportunity for fresh embryo transfer, overall, the GnRH antagonist regimen has a shorter time to live birth than the PPOS protocols, but there was a significant difference between the two groups in only POSEIDON 1. In the other three groups, there was no significant difference in the time to live birth between the two COS protocols. This also requires further clinical studies with large samples.
Comparisons With Other Reports
To our knowledge, regarding the comparison of PPOS protocols and GnRH antagonist protocols, there are 11 studies, including four RCTs (20, 22, 26, 27), two prospective studies (28, 29) and five retrospective cohort studies (19, 21, 30–32). The populations in these studies had different characteristics, including PCOS, POR, normal ovarian response and donor oocyte cycles. The progestins used in the studies were mainly MPA and dydrogesterone, and one study used desogestrel. A recent meta-analysis showed that the duration of stimulation, Gn consumption and oocyte yield were similar to those of the PPOS and GnRH antagonist protocols (33). In our study, for POSEIDON group 2, the number of available embryos was higher in the PPOS group; in the other groups, the number of available embryos was not significantly different. A total of five studies, including 1016 women, explored the clinical pregnancy rate, which was similar to the rates in the PPOS and GnRH antagonist groups (RR: 1.12, 95% CI: 0.91 to 1.38) (19, 21, 26–28, 33). The live birth rate (LBR) per embryo transfer cycle (RR: 1.36, 95% CI: 0.88 to 2.11) was similar to those of the PPOS and GnRH antagonist protocols (19, 26, 33). Only one study, which included 318 young women with regular menstrual cycles, explored the CLBR between the PPOS and GnRH antagonist protocols. The CLBRs were 70.6% and 68.7%, respectively, and there was no significant difference. To our knowledge, only one study explored PPOS and GnRH antagonist protocols in women with POR, and that study included 340 poor responders who met the Bologna criteria and were randomly divided into two groups for analysis. The LBRs of the PPOS and GnRH antagonist groups were similar (21.8% vs. 18.2%, RR: 1.25, 95% CI: 0.73-2.13, P>0.05) (26). To our knowledge, the POSEIDON criteria are more meaningful for the formulation of individualized clinical protocols. Therefore, this study examined the population according to the POSEIDON criteria, and the classification was more specific and detailed, which can provide more reference for clinical decision-making and treatment. In our study, the main concern was the CLBR, which contributes to the comprehensive evaluation of ovulation induction methods and laboratory techniques to provide better data support for the formulation of treatment strategies. In our study, the CLBRs of the two protocols for patients with low prognosis diagnosed per the POSEIDON criteria were comparable. Overall, the GnRH antagonist regimen had a shorter time to live birth than the PPOS protocols, though there was only a significant difference between the two groups at Poseidon 1.
Strengths and Limitations
To our knowledge, this is the first study to compare the CLBRs of PPOS and GnRH protocols in patients with low prognosis per the POSEIDON criteria. Compared with the Bologna criteria formulated in 2011, the POSEIDON criteria proposed in 2016 provide a stronger basis for the formulation of clinical protocols and individualized fertility treatments. Moreover, this study used the CLBR as the final observation index to more completely evaluate the entire ovulation induction cycle. However, this study also has certain limitations. First, this study was a retrospective cohort study, and there was interference from confounding factors. To reduce the influence of important confounding factors, this study used logistic regression analysis for adjustments. Second, due to the influence of the PPOS protocols on endometrial receptivity in the uterus, it is necessary to freeze all embryos and perform freeze-thaw embryo transfer in the subsequent cycle. Since the electronic medical record system of this reproductive center cannot display a patient’s specific expenses, this study did not analyze the specific medical expenses.
Future Prospects
Regarding future research directions, the following points are worth considering. First, a larger sample size or a large, well-designed multicenter RCT study is needed. The second point concerns the economic potency ratio, which is also an important aspect that needs to be considered in the choice of the plan because MPA drugs in the PPOS protocol are more convenient and cheaper to take orally. However, due to the impact of MPA on the receptivity of the endometrium, fresh cycle transfer cannot be performed. The resulting embryo freezing costs, preservation costs, and round-trip expenses need to be further evaluated and analyzed in future research. The third and most important aspect is the offspring safety of the two protocols. Since the birth of ART technology, offspring and perinatal safety have been the focus and difficult to research. Treatment with a GnRH antagonist is a routine ovulation stimulation protocol and has been used to prevent premature LH surge since the 1990s (8, 9). There are a large number of randomized controlled, prospective and retrospective studies on GnRH antagonist regimens that fully prove the effectiveness and safety of these regimens (10–13). Regarding the PPOS protocols, although it has not been applied for a long time, studies have shown that it can achieve exact effectiveness and safety in different populations (19–22). However, due to the short application time of the protocols and the limited amount of data, the long-term safety of offspring deserves further study.
Conclusion
In conclusion, for low prognosis patients diagnosed per the POSEIDON criteria, the CLBRs of the PPOS and GnRH antagonist protocols were comparable. For POSEIDON group 2, the number of available embryos was higher in the PPOS group. In the POSEIDON group 1 population, the GnRH antagonist protocols had a shorter time to live birth. Comparison of these two effective COS protocols requires further randomized controlled studies with large samples.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics committee of The Third Affiliated Hospital of Zhengzhou University. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
MD and YG designed the study and selected the population to be included and excluded. JZ and ZL performed the data extraction and analysis. EL, JL, and XL reviewed the data. MD and JZ drafted this article. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge the patients who participated in the study. We also thank American Journal Experts for their professional manuscript editing services.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.705264/full#supplementary-material
Supplementary Table 1 | Baseline and cycle characteristics of the Poseidon 1 and 2 groups from previous cycles.
References
1. Polyzos NP, Devroey P. A Systematic Review of Randomized Trials for the Treatment of Poor Ovarian Responders: Is There Any Light at the End of the Tunnel? Fertil Steril (2011) 96:1058–61.e7. doi: 10.1016/j.fertnstert.2011.09.048
2. Ferraretti AP, La Marca A, Fauser BC, Tarlatzis B, Nargund G, Gianaroli L. ESHRE Consensus on the Definition of 'Poor Response' to Ovarian Stimulation for In Vitro Fertilization: The Bologna Criteria. Hum Reprod (2011) 26:1616–24. doi: 10.1093/humrep/der092
3. Ferraretti AP, Gianaroli L. The Bologna Criteria for the Definition of Poor Ovarian Responders: Is There a Need for Revision? Hum Reprod (2014) 29:1842–5. doi: 10.1093/humrep/deu139
4. Papathanasiou A. Implementing the ESHRE 'Poor Responder' Criteria in Research Studies: Methodological Implications. Hum Reprod (2014) 29:1835–8. doi: 10.1093/humrep/deu135
5. Venetis CA. The Bologna Criteria for Poor Ovarian Response: The Good, the Bad and the Way Forward. Hum Reprod (2014) 29:1839–41. doi: 10.1093/humrep/deu138
6. Alviggi C, Andersen CY, Buehler K, Conforti A, De Placido G, Esteves SC, et al. A New More Detailed Stratification of Low Responders to Ovarian Stimulation: From a Poor Ovarian Response to a Low Prognosis Concept. Fertil Steril (2016) 105:1452–3. doi: 10.1016/j.fertnstert.2016.02.005
7. Malchau SS, Henningsen AA, Forman J, Loft A, Nyboe Andersen A, Pinborg A. Cumulative Live Birth Rate Prognosis Based on the Number of Aspirated Oocytes in Previous ART Cycles. Hum Reprod (2019) 34:171–80. doi: 10.1093/humrep/dey341
8. Hall JE, Brodie TD, Badger TM, Rivier J, Vale W, Conn PM, et al. Evidence of Differential Control of FSH and LH Secretion by Gonadotropin-Releasing Hormone (GnRH) From the Use of a GnRH Antagonist.. J Clin Endocrinol Metab (1988) 67:524–31. doi: 10.1210/jcem-67-3-524
9. Hall JE, Whitcomb RW, Rivier JE, Vale WW, Crowley WF Jr. Differential Regulation of Luteinizing Hormone, Follicle-Stimulating Hormone, and Free Alpha-Subunit Secretion From the Gonadotrope by Gonadotropin-Releasing Hormone (GnRH): Evidence From the Use of Two GnRH Antagonists. J Clin Endocrinol Metab (1990) 70:328–35. doi: 10.1210/jcem-70-2-328
10. Yang R, Guan Y, Perrot V, Ma J, Li R. Comparison of the Long-Acting GnRH Agonist Follicular Protocol With the GnRH Antagonist Protocol in Women Undergoing In Vitro Fertilization: A Systematic Review and Meta-Analysis. Adv Ther (2021) 38(5):2027–37. doi: 10.1007/s12325-020-01612-7
11. Lambalk CB, Banga FR, Huirne JA, Toftager M, Pinborg A, Homburg R, et al. GnRH Antagonist Versus Long Agonist Protocols in IVF: A Systematic Review and Meta-Analysis Accounting for Patient Type. Hum Reprod Update (2017) 23:560–79. doi: 10.1093/humupd/dmx017
12. Toftager M, Bogstad J, Bryndorf T, Løssl K, Roskær J, Holland T, et al. Risk of Severe Ovarian Hyperstimulation Syndrome in GnRH Antagonist Versus GnRH Agonist Protocol: RCT Including 1050 First IVF/ICSI Cycles. Hum Reprod (2016) 31:1253–64. doi: 10.1093/humrep/dew051
13. Toftager M, Bogstad J, Løssl K, Prætorius L, Zedeler A, Bryndorf T, et al. Cumulative Live Birth Rates After One ART Cycle Including All Subsequent Frozen-Thaw Cycles in 1050 Women: Secondary Outcome of an RCT Comparing GnRH-Antagonist and GnRH-Agonist Protocols. Hum Reprod (2017) 32:556–67. doi: 10.1093/humrep/dew358
14. Cheung LP, Lam PM, Lok IH, Chiu TT, Yeung SY, Tjer CC, et al. GnRH Antagonist Versus Long GnRH Agonist Protocol in Poor Responders Undergoing IVF: A Randomized Controlled Trial. Hum Reprod (2005) 20:616–21. doi: 10.1093/humrep/deh668
15. Zhang D, Xia L, Xu H, Chen Q, Jin B, Zhang A, et al. Flexible Low-Dose GnRH Antagonist Protocol Is Effective in Patients With Sufficient Ovarian Reserve in IVF. Front Endocrinol (2018) 9:767. doi: 10.3389/fendo.2018.00767
16. Reichman DE, Zakarin L, Chao K, Meyer L, Davis OK, Rosenwaks Z. Diminished Ovarian Reserve Is the Predominant Risk Factor for Gonadotropin-Releasing Hormone Antagonist Failure Resulting in Breakthrough Luteinizing Hormone Surges in In Vitro Fertilization Cycles. Fertil Steril (2014) 102:99–102. doi: 10.1016/j.fertnstert.2014.04.010
17. Borm G, Mannaerts B. Treatment With the Gonadotrophin-Releasing Hormone Antagonist Ganirelix in Women Undergoing Ovarian Stimulation With Recombinant Follicle Stimulating Hormone is Effective, Safe and Convenient: Results of a Controlled, Randomized, Multicentre Trial. Eur Orgalutran Study Group Hum Reprod (2000) 15:1490–8. doi: 10.1093/humrep/15.7.1490
18. Kuang Y, Chen Q, Fu Y, Wang Y, Hong Q, Lyu Q, et al. Medroxyprogesterone Acetate is an Effective Oral Alternative for Preventing Premature Luteinizing Hormone Surges in Women Undergoing Controlled Ovarian Hyperstimulation for In Vitro Fertilization. Fertil Steril (2015) 104:62–70.e3. doi: 10.1016/j.fertnstert.2015.03.022
19. Huang J, Xie Q, Lin J, Lu X, Wang N, Gao H, et al. Neonatal Outcomes and Congenital Malformations in Children Born After Dydrogesterone Application in Progestin-Primed Ovarian Stimulation Protocol for IVF: A Retrospective Cohort Study. Drug Des Dev Ther (2019) 13:2553–63. doi: 10.2147/dddt.s210228
20. Wang N, Zhu Q, Ma M, Liang Z, Tao Y, Wang Y. Comparison of a Progestin-Primed Ovarian Stimulation Protocol With a Flexible GnRH Antagonist Protocol in Patients With Polycystic Ovary Syndrome Who are Participating in an IVF Programme: Study Protocol for a Randomised Controlled Trial. BMJ Open (2020) 10:e038153. doi: 10.1136/bmjopen-2020-038153
21. Xiao ZN, Peng JL, Yang J, Xu WM. Flexible GnRH Antagonist Protocol Versus Progestin-Primed Ovarian Stimulation (PPOS) Protocol in Patients With Polycystic Ovary Syndrome: Comparison of Clinical Outcomes and Ovarian Response. Curr Med Sci (2019) 39:431–6. doi: 10.1007/s11596-019-2055-x
22. Beguería R, García D, Vassena R, Rodríguez A. Medroxyprogesterone Acetate Versus Ganirelix in Oocyte Donation: A Randomized Controlled Trial. Hum Reprod (2019) 34:872–80. doi: 10.1093/humrep/dez034
23. Zhang J, Du M, Li Z, Wang L, Hu J, Zhao B, et al. Fresh Versus Frozen Embryo Transfer for Full-Term Singleton Birth: A Retrospective Cohort Study. J Ovarian Res (2018) 11:59. doi: 10.1186/s13048-018-0432-x
24. Zhang J, Du M, Sun L. Supraphysiological Estradiol Levels on the hCG Trigger Day are Associated With SGA for Singletons Born From Fresh Embryo Transfer. J Dev Orig Health Dis (2021) 11:1–8. doi: 10.1017/s2040174421000234
25. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The International Glossary on Infertility and Fertility Care, 2017. Fertil Steril (2017) 108:393–406. doi: 10.1016/j.fertnstert.2017.06.005
26. Chen Q, Chai W, Wang Y, Cai R, Zhang S, Lu X, et al. Progestin vs. Gonadotropin-Releasing Hormone Antagonist for the Prevention of Premature Luteinizing Hormone Surges in Poor Responders Undergoing In Vitro Fertilization Treatment: A Randomized Controlled Trial. Front Endocrinol (2019) 10:796. doi: 10.3389/fendo.2019.00796
27. Eftekhar M, Hoseini M, Saeed L. Progesterone-Primed Ovarian Stimulation in Polycystic Ovarian Syndrome: An RCT. Int J Reprod Biomed (2019) 17:671–6. doi: 10.18502/ijrm.v17i9.5103
28. Iwami N, Kawamata M, Ozawa N, Yamamoto T, Watanabe E, Moriwaka O, et al. New Trial of Progestin-Primed Ovarian Stimulation Using Dydrogesterone Versus a Typical GnRH Antagonist Regimen in Assisted Reproductive Technology. Arch Gynecol Obstet (2018) 298:663–71. doi: 10.1007/s00404-018-4856-8
29. Mathieu d'Argent E, Ferrier C. Outcomes of Fertility Preservation in Women With Endometriosis: Comparison of Progestin-Primed Ovarian Stimulation Versus Antagonist Protocols. J Ovarian Res (2020) 13(1):18. doi: 10.1186/s13048-020-00620-z
30. Martínez F, Rodriguez-Purata J. Ovarian Response in Oocyte Donation Cycles Under LH Suppression With GnRH Antagonist or Desogestrel Progestin: Retrospective and Comparative Study. Gynecol Endocrinol (2019) 35(10):884–9. doi: 10.1080/09513590.2019.1604662
31. Yildiz S, Turkgeldi E, Angun B, Eraslan A, Urman B, Ata B. Comparison of a Novel Flexible Progestin Primed Ovarian Stimulation Protocol and the Flexible Gonadotropin-Releasing Hormone Antagonist Protocol for Assisted Reproductive Technology. Fertil Steril (2019) 112:677–83. doi: 10.1016/j.fertnstert.2019.06.009
32. Turkgeldi E, Yildiz S, Cekic SG, Shakerian B. Effectiveness of the Flexible Progestin Primed Ovarian Stimulation Protocol Compared to the Flexible GnRH Antagonist Protocol in Women With Decreased Ovarian Reserve. Hum Fertil (Camb) (2020) 16:1–7. doi: 10.1080/14647273.2020.1794060
Keywords: progestin-primed ovarian stimulation, GnRH antagonist, low prognosis, cumulative live birth rate, controlled ovarian hyperstimulation
Citation: Du M, Zhang J, Li Z, Liu X, Li J, Liu W and Guan Y (2021) Comparison of the Cumulative Live Birth Rates of Progestin-Primed Ovarian Stimulation and Flexible GnRH Antagonist Protocols in Patients With Low Prognosis. Front. Endocrinol. 12:705264. doi: 10.3389/fendo.2021.705264
Received: 05 May 2021; Accepted: 27 August 2021;
Published: 13 September 2021.
Edited by:
Yanping Kuang, Shanghai Jiao Tong University, ChinaReviewed by:
Ariel Weissman, Wolfson Medical Center, IsraelBiljana Popovic Todorovic, University Hospital Brussels, Belgium
Copyright © 2021 Du, Zhang, Li, Liu, Li, Liu and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yichun Guan, Z3VhbnlpY2h1bjE2MTZAMTI2LmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Mingze Du
Mingze Du Junwei Zhang†
Junwei Zhang† Yichun Guan
Yichun Guan