- 1Department of Thyroid and Breast Surgery, Chengdu Third People’s Hospital, Chengdu, China
- 2Department of Chemistry, School of Basic Medical Science, North Sichuan Medical College, Nanchong, China
Objective: We aim to assess the accuracy of near infrared autofluorescence in identifying parathyroid gland during thyroid and parathyroid surgery.
Method: A systematic literature search was conducted by using PubMed, Embase, and the Cochrane Library electronic databases for studies that were published up to February 2021. The reference lists of the retrieved articles were also reviewed. Two authors independently assessed the methodological quality and extracted the data. A random-effects model was used to calculate the combined variable. Publication bias in these studies was evaluated with the Deeks’ funnel plots.
Result: A total of 24 studies involving 2,062 patients and 6,680 specimens were included for the meta-analysis. The overall combined sensitivity and specificity, and the area under curve of near infrared autofluorescence were 0.96, 0.96, and 0.99, respectively. Significant heterogeneities were presented (Sen: I2 = 87.97%, Spe: I2 = 65.38%). In the subgroup of thyroid surgery, the combined sensitivity and specificity, and the area under curve of near infrared autofluorescence was 0.98, 0.99, and 0.99, respectively, and the heterogeneities were moderate (Sen: I2 = 59.71%, Spe: I2 = 67.65%).
Conclusion: Near infrared autofluorescence is an excellent indicator for identifying parathyroid gland during thyroid and parathyroid surgery.
Introduction
During thyroid and parathyroid surgery, identification of the parathyroid gland (PG) always relies on the visual judgment and experience of the surgeon (1, 2). Failure to identify the normal PG during thyroid surgery might result in PG damage, devascularization, and inadvertent resection, and thus bring about postoperative hypoparathyroidism. Hypoparathyroidism can lead to poor experience, prolonged hospitalization, and lower quality of life (3–5). On the other hand, failure to identify the abnormal PG during parathyroid surgery for hyperparathyroidism would result in reoperation (6, 7).
In recent decades, several tracers have been reported to assist to identify PG, but they have some disadvantages, such as lack of direct evidence, limitation of instrument and/or invasion (8–10). Indocyanine green angiography was also used to evaluated the function of PG and showed a satisfactory ability to reduce the rate of hypoparathyroidism after thyroid surgery (11–13). The near infrared autofluorescence (NIRAF), a noninvasive, label-free and rapid indicator, was introduced to intraoperatively identify PG during past years (14–17). The NIRAF of PG was first reported by Paras and his colleagues in 2011 and they found that the fluorescence intensity of PG was greater than that of the thyroid and all other tissues in the neck (18). Since then, the number of studies exploring the potential of NIRAF to identify PG during surgery has being increased and the instruments to measure NIRAF were also various (19–21). Some studies suggested that the use of NIRAF improved the early postoperative hypocalcemia rate and increase parathyroid preservation after total thyroidectomy (22, 23). However, the sensitivity of NIRAF was 81%-100% and the specificity ranged from 80% to 100% (14–16). Due to the large variety, we conduct a meta-analysis to assess the accuracy of NIRAF in identifying PG during thyroid and parathyroid surgery.
Methods
Literature Search
Two investigators independently conducted a search by using PubMed, Embase, and the Cochrane Library electronic databases for studies that were published up to 28 February, 2021. The search algorithm was [(Near-infrared) AND (parathyroid)] for PubMed. The following search terms were used in all fields as a search strategy for Embase: 1) parathyroid glands, parathyroid gland, parathyroid, parathyroids; 2) (spectroscopy, near-infrared), near-infrared spectroscopy, near infrared spectroscopy; near infrared, near-infrared. For Cochrane Library electronic databases, the search strategy was the following terms by searching Medical Subject Headings and free word in all field: 1) parathyroid glands, parathyroid gland, parathyroid, parathyroids; 2) (spectroscopy, near-infrared), near infrared spectroscopy, near infrared spectroscopies, near infra-red spectroscopy, near infra-red spectroscopies, near infrared, near infra-red. No restriction was imposed. In addition, we reviewed the reference lists of the retrieved papers and recent reviews.
Study Selection
The first screening was performed based on the title and abstract, and the full-text was then reviewed. A study was included when it met all the following criteria: 1) NIRAF was used to identify PG; 2) the data to calculate the sensitivity and/or specificity were reported; and 3) aforementioned data showed the numbers of PG. Studies were excluded based on the following criteria: 1) Conference Abstract, Review, Case report, Commentary, Discussion and Letter; 2) those in which the fluorescence originated from tracer; 3) those in which the trial was not conducted in human; 4) those which were published in non-English; 5) those from which data could not be collected adequately; and 6) those that the full text of the studies could not be accessed online or by request to the authors.
Data Extraction and Quality Assessment
Data were extracted by two reviewers (Wang B and Zhu CR) using a predefined data extraction form, and any disagreement between reviewers was resolved by consensus. Data were collected as follows: the first author, publication time, type of study, country of origin, study sites and institutes, measurement instrument and method, research period, sample size, the age of patients, disease (the reason for surgery) and surgical method, diagnostic standard and reference standard of PG, data to calculate the sensitivity and/or specificity. The quality of study was assessed with the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) (24).
Statistical Analysis
Combined sensitivity, combined specificity and summary receiver operating characteristic (SROC) curve were used to investigate the accuracy with Stata version 14.0 (Stata Corp LP, College Station, Texas, USA). The quality assessment of study was achieved through Review Manager 5.4. Meta-regression analysis was performed with removing the covariate with the largest P-value one by one by Meta-Disc 1.4. Heterogeneity was quantified statistically with the I2 test. P < 0.1 and I2 > 50% for heterogeneity were considered significant differences. We explored the reasons for heterogeneity by performing subgroup analyses. The measurement instrument, disease type and reference standard were examined as the potential influence factors on the accuracy of NIRAF. Potential publication bias was assessed by the Deeks’ funnel plots. P < 0.05 was considered statistically significant.
Results
Literature Search
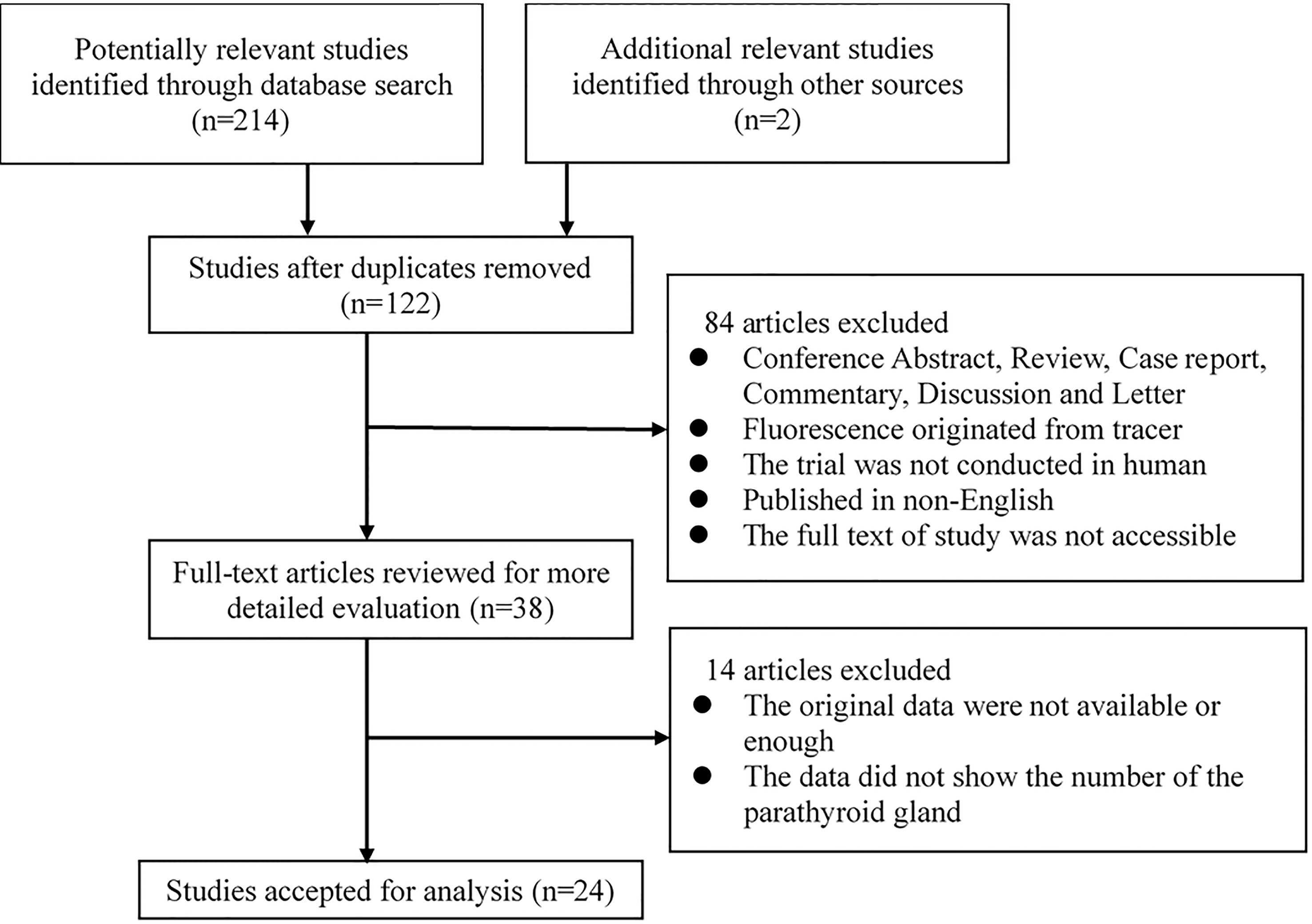
The study selection process is shown in Figure 1. A total of 214 potentially relevant records were identified through searching these databases and 2 other records were added by reviewing the reference lists of the retrieved papers. And one hundred and twenty-two records were retained after duplicates were removed. After screening the titles and abstracts, 84 studies were excluded for various reasons. The remaining 38 studies were assessed via full-text screening, and 14 studies were further excluded. Finally, 24 independent studies were included in the meta-analysis (14–16, 19–21, 25–42).
Study Characteristics
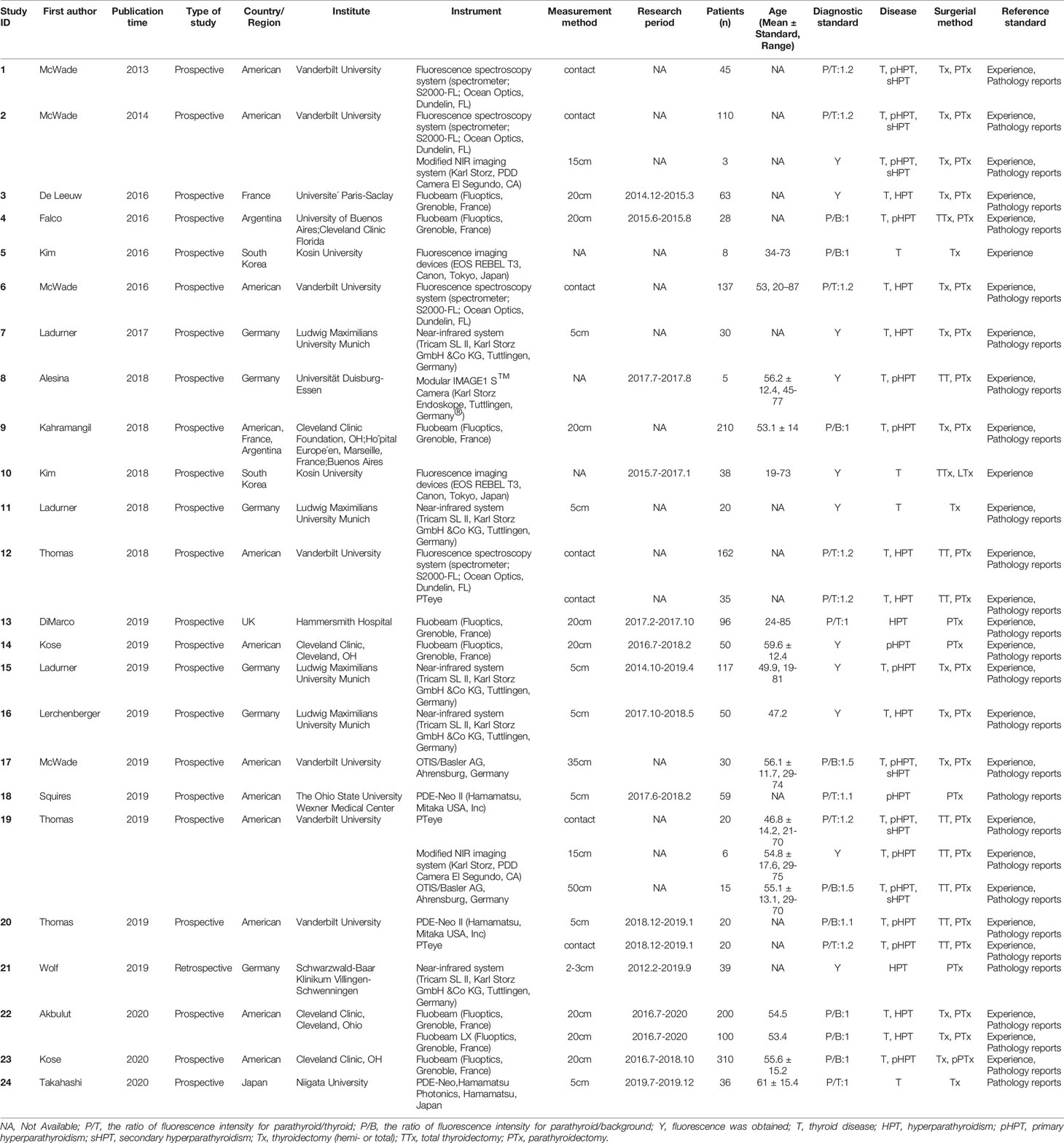
Table 1 summarizes the basic information of the 24 included eligible studies (14–16, 19–21, 25–42). These studies were published between 2013 and 2020 and all of them were prospective studies except one (16). Of the 24 studies, 3 were conducted in Asia, 8 in Europe, 11 in the United States, 1 in Argentina, and 1 in multicenter of American, France and Argentina. According to these studies, 11 types of instrument based on NIRAF were used to identify PG and a total of 2,062 patients and 6,680 specimens were included for analysis. The quality assessment of these studies was shown in Figure 2.
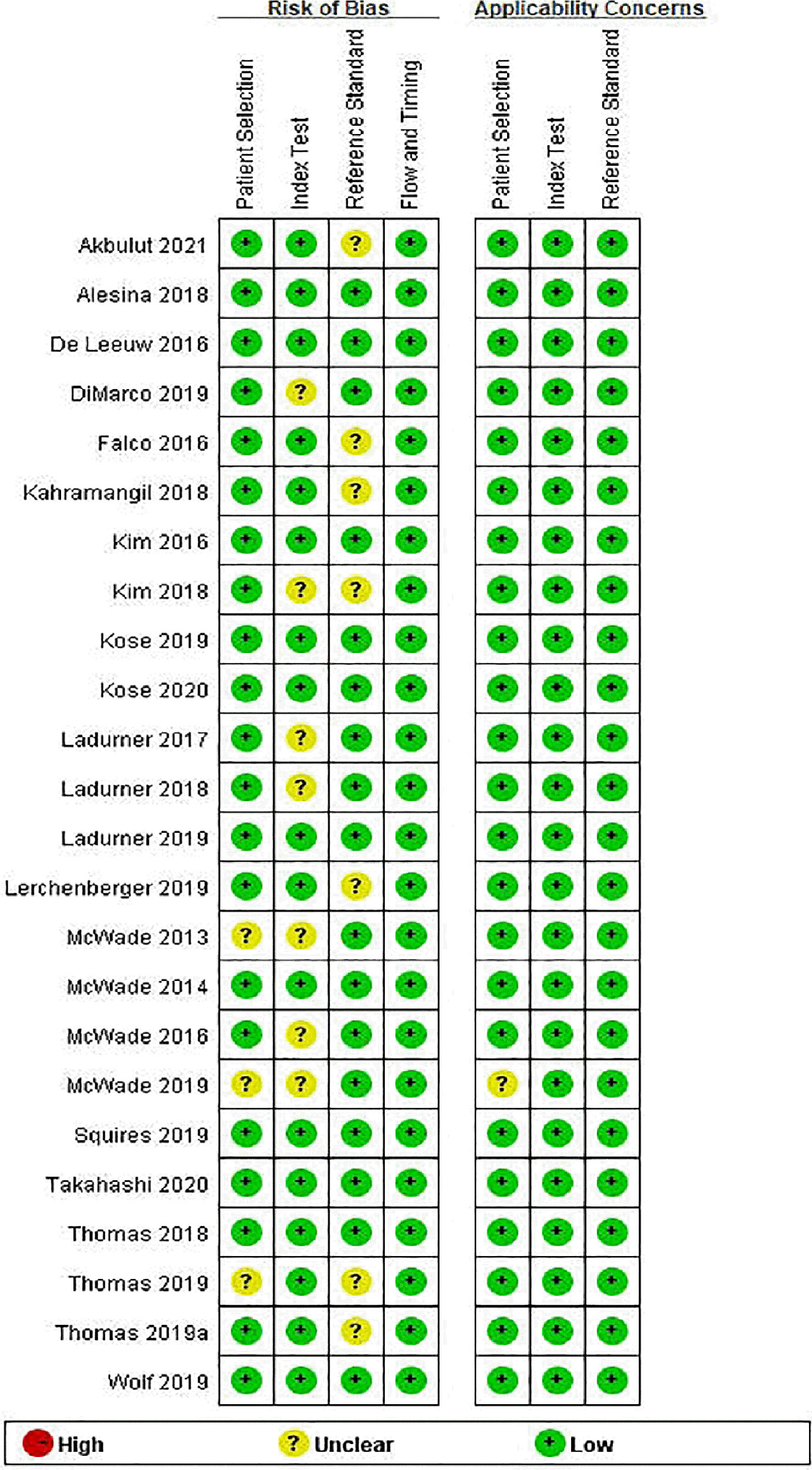
Figure 2 Risk of bias and applicability concerns summary: review authors’ judgements about each domain for each included study.
The Accuracy of NIRAF in Identifying PG
As shown in Figure 3, the overall combined sensitivity and specificity were both 0.96, and the area under curve (AUC) was 0.99. Significant heterogeneities were detected (Sen: I2 = 87.97%, Spe: I2 = 65.38%), but the publication bias did not appear significant as measured by the Deeks’ funnel plot asymmetry tests (p=0.86).
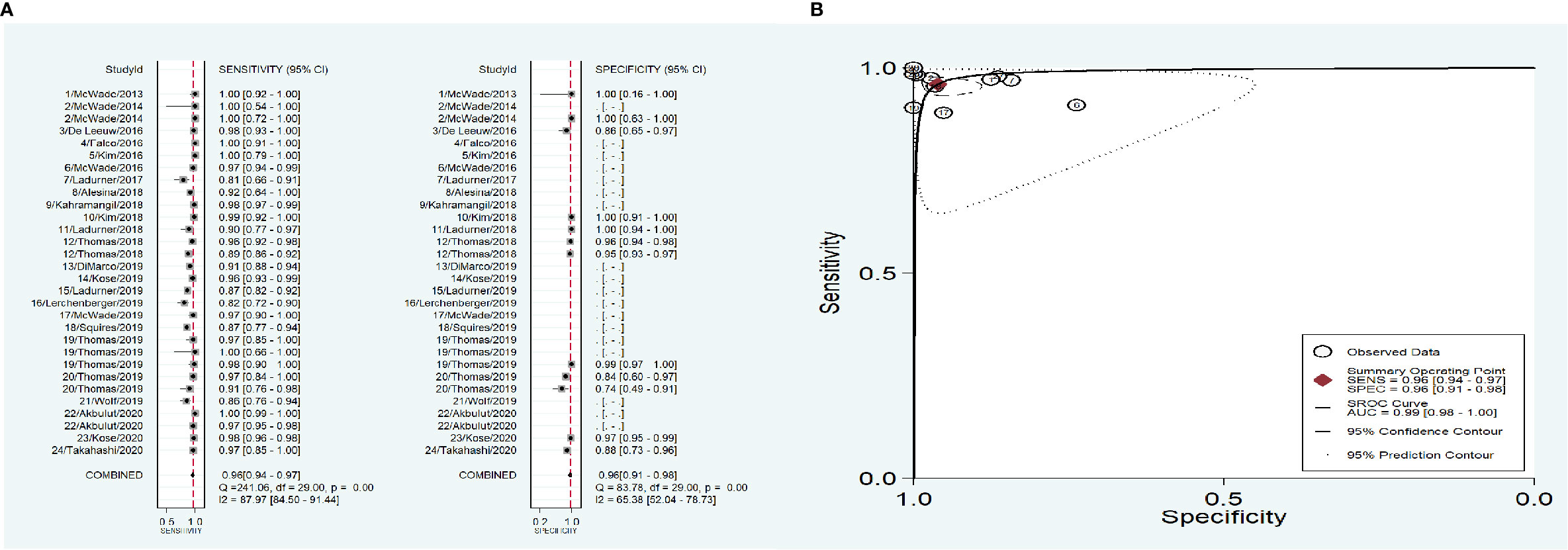
Figure 3 The accuracy of near infrared autofluorescence. (A) forest plot of the included studies. (B) summary receiver operating characteristic (SROC) curve.
To address the heterogeneity, subgroup analysis was performed according to the measurement instrument, disease and reference standard. When the fluorescence spectroscopy system (spectrometer; S2000-FL; Ocean Optics, Dundelin, FL) was used, the combined sensitivity and specificity increased (Sen: 0.97, Spe: 0.98; Figure 4A) and the AUC was also 0.99 (Figure 4B), but the heterogeneities were still significant (Sen: I2 = 97.13%, Spe: I2 = 94.99%; Figure 4A). When the near infrared system (Tricam SL II, Karl Storz GmbH &Co KG, Tuttlingen, Germany) was used, although the heterogeneities decreased, the combined sensitivity was also decreased, and the combined specificity and AUC were not able to be combined because of lack of data (Figures 4C, D). When NIRAF was used during thyroid surgery and parathyroid surgery for primary hyperparathyroidism, the combined specificity decreased, but the combined sensitivity and AUC were same as the overall results and the heterogeneities did not also obviously change (Sen: 0.96, I2 = 86.97%, Spe: 0.95, I2 = 63.86%, AUC=0.99; Figures 5A, B). However, when NIRAF was used to identify normal PG during thyroid surgery, the combined sensitivity and specificity both increased (Sen: 0.98, Spe: 0.99; Figure 5C) and the AUC kept stable (AUC=0.99, Figure 5D), but the heterogeneities decreased (Sen: I2 = 59.71%, Spe: I2 = 67.65%; Figure 5C). When subgroup analysis was performed according to the reference standard, the combined sensitivity, combined specificity and AUC were respectively 0.97, 0.97 and 0.99 in the subgroup of experience (Figures 6A, B), and 0.96, 0.93 and 0.98 in the subgroup of pathology report (Figures 6C, D). The heterogeneities increased in the subgroup of experience (Sen: I2 = 92.88%, Spe: I2 = 68.83%, Figure 6A), while they reduced in the subgroup of pathology report (Sen: I2 = 57.27%, Spe: I2 = 55.15%, Figure 6C).
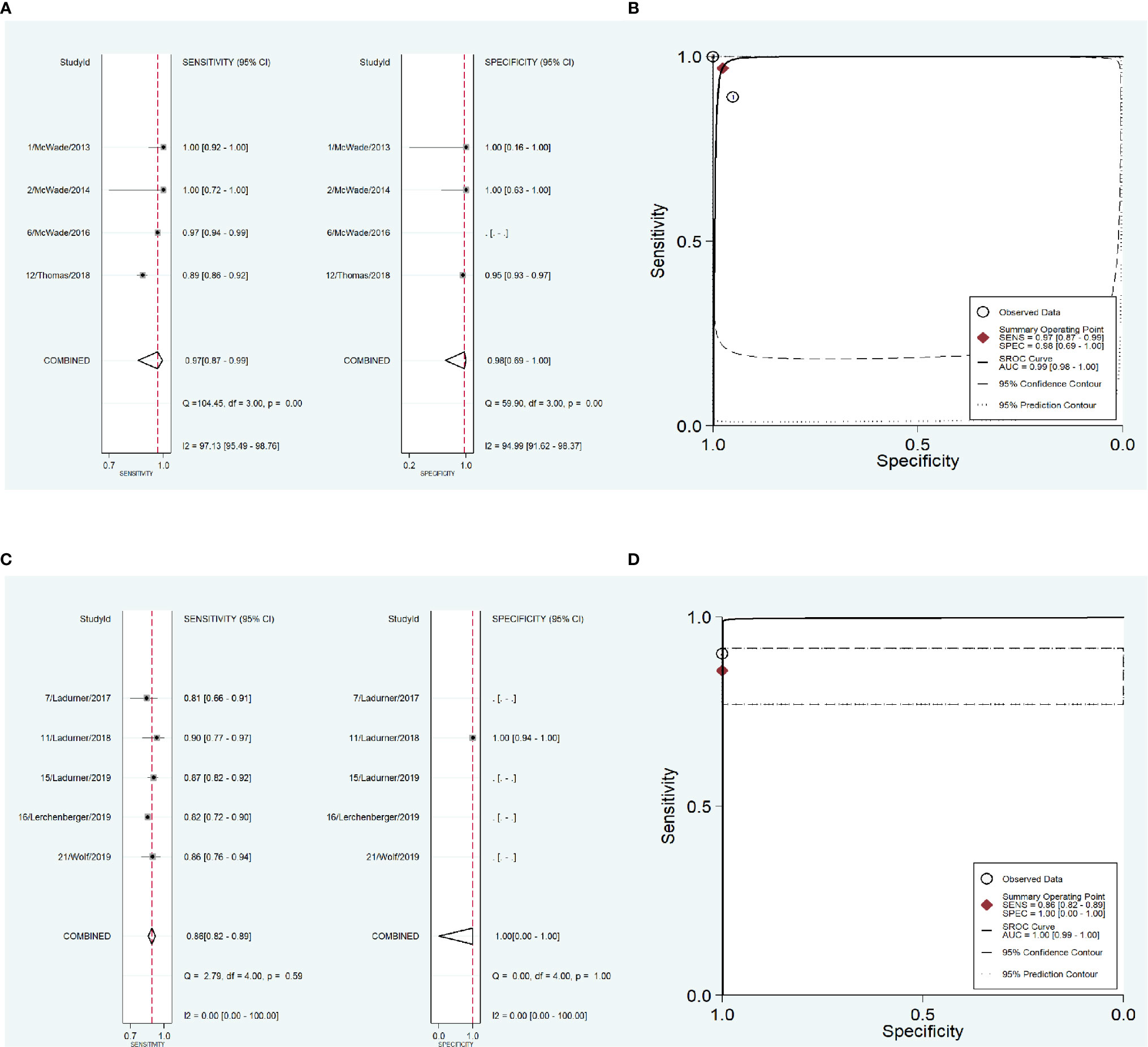
Figure 4 The accuracy of near infrared autofluorescence in the subgroup analysis of the measurement instrument. (A) forest plot of the studies in the subgroup of fluorescence spectroscopy system (spectrometer; S2000-FL; Ocean Optics, Dundelin, FL). (B) summary receiver operating characteristic (SROC) curve in the subgroup of fluorescence spectroscopy system (spectrometer; S2000-FL; Ocean Optics, Dundelin, FL). (C) forest plot of the studies in the subgroup of near infrared system (Tricam SL II, Karl Storz GmbH &Co KG, Tuttlingen, Germany). (D) summary receiver operating characteristic (SROC) curve in the subgroup of near infrared system (Tricam SL II, Karl Storz GmbH &Co KG, Tuttlingen, Germany).
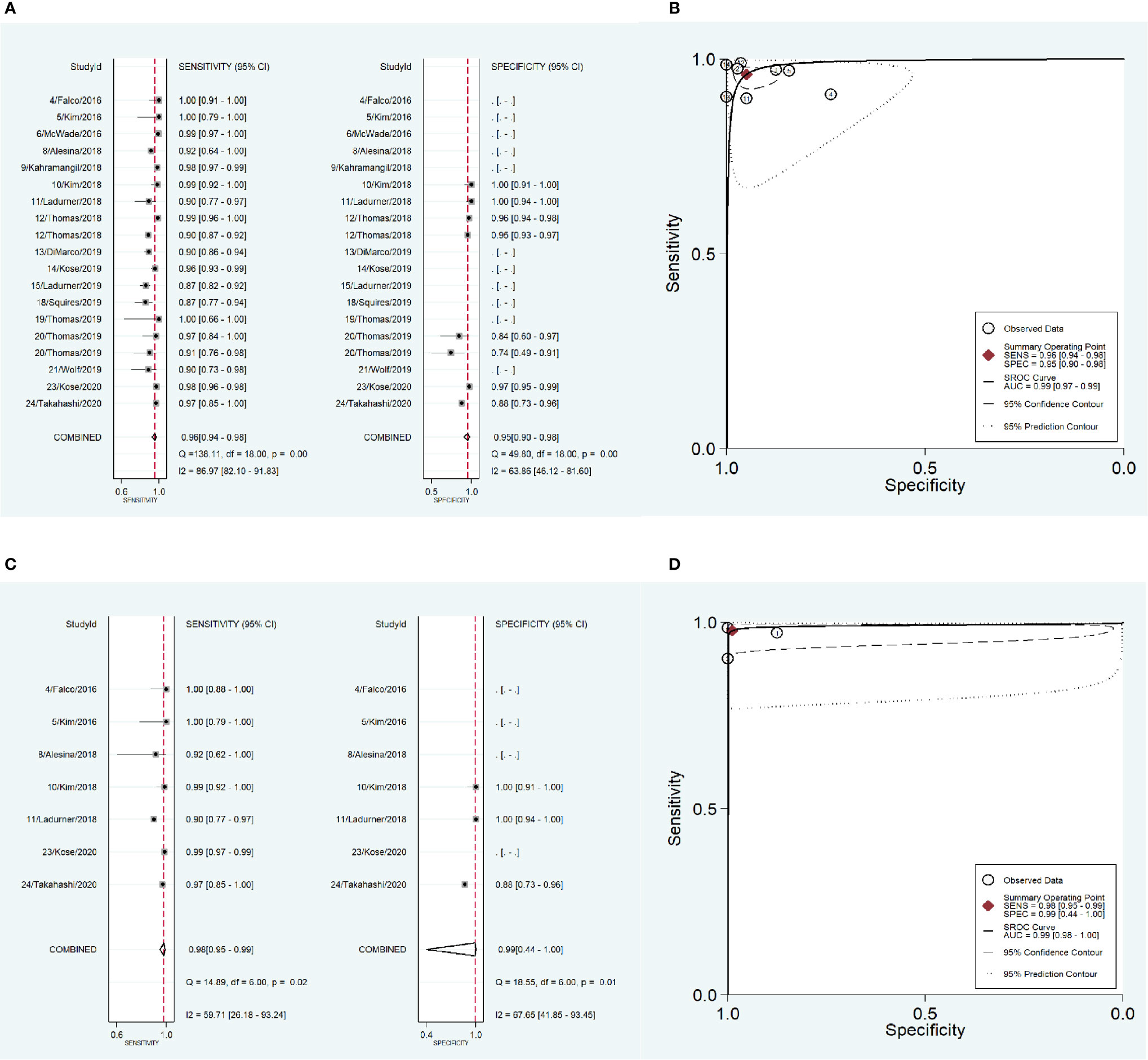
Figure 5 The accuracy of near infrared autofluorescence in the subgroup analysis of the type of disease. (A) forest plot of the studies in the subgroup of primary hyperparathyroidism and thyroid disease. (B) summary receiver operating characteristic (SROC) curve in the subgroup of primary hyperparathyroidism and thyroid disease. (C) forest plot of the studies in the subgroup of thyroid disease. (D) summary receiver operating characteristic (SROC) curve in the subgroup of thyroid disease.
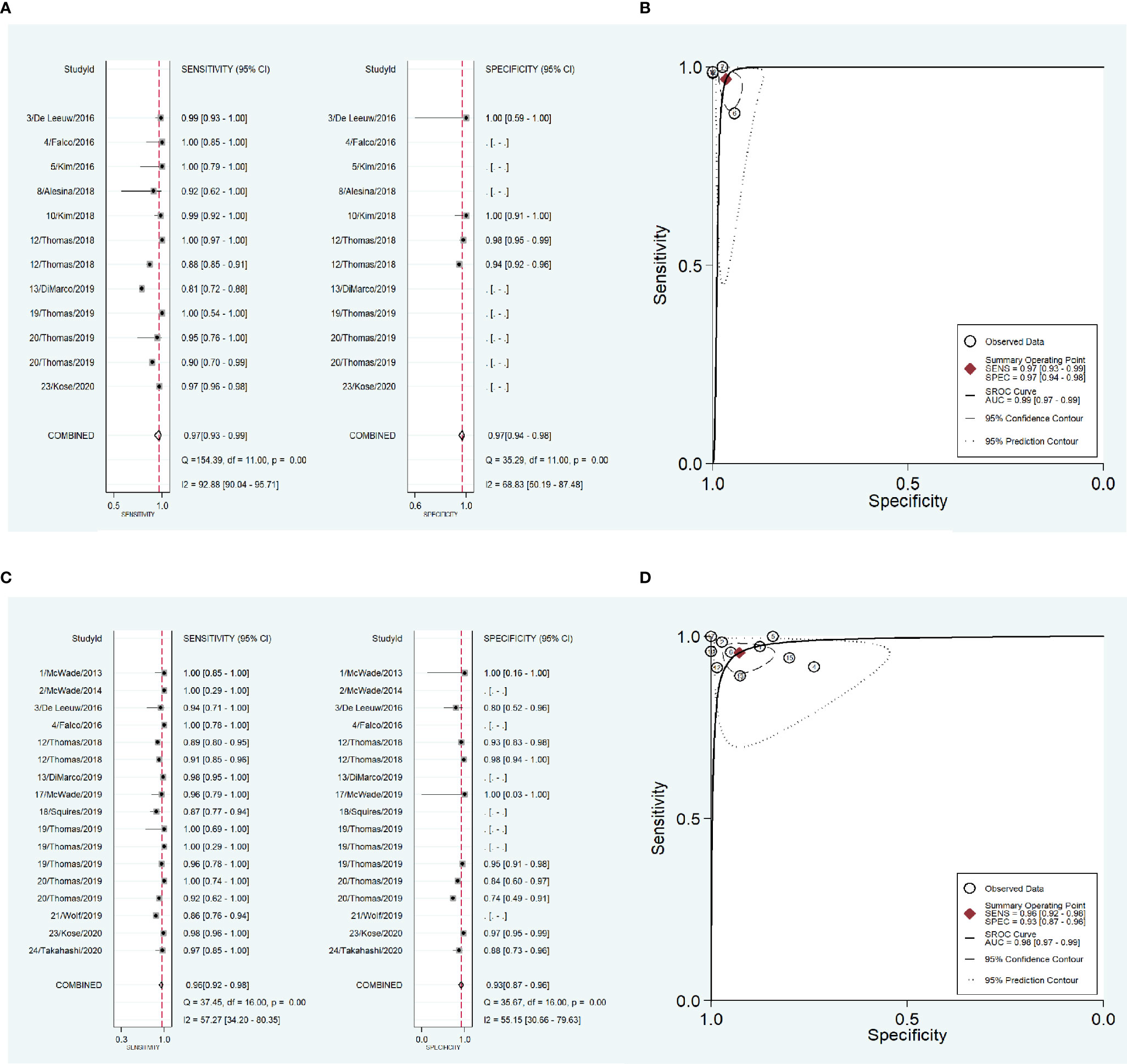
Figure 6 The accuracy of near infrared autofluorescence in the subgroup analysis of the reference standard of the diagnosis of parathyroid gland. (A) forest plot of the studies in the subgroup of experience. (B) summary receiver operating characteristic (SROC) curve in the subgroup of experience. (C) forest plot of the studies in the subgroup of pathology report. (D) summary receiver operating characteristic (SROC) curve in the subgroup of pathology report.
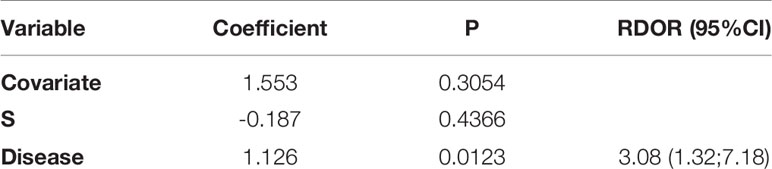
Although the heterogeneities got diminished in the subgroup of near infrared system (Tricam SL II, Karl Storz GmbH &Co KG, Tuttlingen, Germany), thyroid surgery and pathology report, they were still significant. We further performed meta-regression and found that the type of disease was related to heterogeneity (P = 0.01, Table 2).
Discussion
The present meta-analysis demonstrated that NIRAF had a high accuracy in identifying PG during thyroid and parathyroid surgery, and the measurement instrument, status of PG and the reference standard of diagnosis of PG would influence the identification accuracy.
Some studies suggested that the accuracy of NIRAF in identify PG was closed to 100% (30, 41, 42). While, some other researchers got the result that the accuracy was less than 85% (15, 16). The difference in measurement instruments might be a reason for the difference in accuracy and the high heterogeneity across these studies. As described by Solorzano (43), all the approaches for NIRAF detection were based on probe or image, and different approaches had respective advantages and disadvantages, and thus these characteristics of different approaches might result in the difference in the identification accuracy. Due to the lack of data, the subgroup analysis of measurement instrument was only performed in two types of instrument. And we found that the heterogeneity decreased in the subgroup of near infrared system (Tricam SL II, Karl Storz GmbH &Co KG, Tuttlingen, Germany). Di Marco (44) and Solorzano (45) also reviewed the studies that NIRAF was used in thyroid and parathyroid surgery, but they did not perform the meta-analysis.
On account of the subjectivity of experience, the reference standard of diagnosis of PG was also regarded as an influence factor to be performed subgroup analysis. In the subgroup where pathology report was considered as reference standard, the heterogeneity diminished significantly. Although the surgeons in these studies were all senior professional endocrine surgeons, their experience and ability to identify PG might not be still fully consistent.
Surgical method depends on the type of disease, which means that PGs was normal in thyroid surgery and was hyperplastic in parathyroid surgery for primary or/and secondary hyperparathyroidism. When we performed subgroup analysis according to the type of disease, the heterogeneity became moderate in the subgroup of thyroid disease. And the meta-regression analysis also confirmed that the type of disease was significantly related to the heterogeneity. McWade et al. (25) and Kose et al. (34) found that the fluorescence intensity of hyperplastic PG was larger than that of normal PG, while DiMarco (33) and Squires (38) suggested that there was no significant difference in fluorescence intensity between hyperplastic PG and normal PG, but Falco (26) and Aoyama (46) reported that the fluorescence of hyperplastic PG was weaker than that of normal PG. The inconsistent results of comparison of fluorescence intensity between hyperplastic PG and normal PG might affect PG identification and thus cause the heterogeneity. The reason for the difference in fluorescence intensity of hyperplastic PG might be that the fluorescence intensity of PG with primary hyperparathyroidism was stronger than that of PG with secondary hyperparathyroidism and the fluorescence intensity distributed unevenly in hyperplastic PG (47). Wolf and colleagues reported a lower accuracy in identifying PG during surgery for secondary hyperparathyroidism than that during surgery for primary hyperparathyroidism (16). However, we could not verify this result, because the subgroup analyses were not able to be performed in the subgroup of primary and secondary hyperparathyroidism on account of lack of data.
Several limitations existed in this meta-analysis. First, data of sensitivity and specificity could not be collected at the same time in some studies. Second, although we performed subgroup analysis, the heterogeneity did not disappear. Third, some subgroup analyses could not be performed because of lack of eligible data. Fourth, the diagnostic standards were not uniform among these studies, even though no threshold effect was observed in this meta-analysis. Lastly, the exclusion of non-English-language studies might lead to bias.
Conclusion
In conclusion, the NIRAF is an excellent indicator for identifying PG during thyroid and parathyroid surgery, and the accuracy is perfect, especially in thyroid surgery. The ability of NIRAF to preserve PG function during thyroid surgery is worth exploring.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author Contributions
Study conception and design: BW, C-RZ, HL, X-MY, and JW. Acquisition of data: BW and C-RZ. Analysis and interpretation of data: BW, C-RZ, and HL. Drafting of manuscript: BW and C-RZ. Critical revision: BW, C-RZ, HL, X-MY, and JW. All authors contributed to the article and approved the submitted version.
Funding
BW was supported by a nonprofit fund from CHINA HEALTH PROMOTION FOUNDATION. JW was supported by a grant from Scientific Research Fund of the Department of Science and Technology of Chengdu City (2015-HM01-00376-SF) and Science and Technology Program of Science & Technology Department of Sichuan Province (2015JY0190). The funding bodies had no role in the conception of the study, in the collection, analysis, and interpretation of data, in writing the manuscript and in the approval of the publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Chen H, Wang TS, Yen TW, Doffek K, Krzywda E, Schaefer S, et al. Operative Failures After Parathyroidectomy for Hyperparathyroidism: The Influence of Surgical Volume. Ann Surg (2010) 252(4):691–5. doi: 10.1097/SLA.0b013e3181f698df
2. Zarebczan B, Chen H. Influence of Surgical Volume on Operative Failures for Hyperparathyroidism. Adv Surg (2011) 45(1):237–48. doi: 10.1016/j.yasu.2011.03.003
3. Testini M, Gurrado A, Lissidini G, Nacchiero M. Hypoparathyroidism After Total Thyroidectomy. Minerva Chir (2007) 62(5):409–15.
4. Stack BC Jr., Bimston DN, Bodenner DL, Brett EM, Dralle H, Orloff LA. American Association of Clinical Endocrinologists and American College of Endocrinology Disease State Clinical Review: Postoperative Hypoparathyroidism–Definitions and Management. Endocr Pract (2015) 21(6):674–85. doi: 10.4158/EP14462.DSC
5. Puliani G, Hasenmajer V, Sciarra F, Barbagallo F, Sbardella E, Pofi R, et al. Impaired Immune Function in Patients With Chronic Postsurgical Hypoparathyroidism: Results of the EMPATHY Study. J Clin Endocrinol Metab (2021) 106(5):e2215–27. doi: 10.1210/clinem/dgab038
6. Simental A, Ferris RL. Reoperative Parathyroidectomy. Otolaryngol Clin North Am (2008) 41(6):1269–74. doi: 10.1016/j.otc.2008.05.008
7. Cron DC, Kapeles SR, Andraska EA, Kwon ST, Kirk PS, McNeish BL, et al. Predictors of Operative Failure in Parathyroidectomy for Primary Hyperparathyroidism. Am J Surg (2017) 214(3):509–14. doi: 10.1016/j.amjsurg.2017.01.012
8. Zhu J, Tian W, Xu Z, Jiang K, Sun H, Wang P, et al. Expert Consensus Statement on Parathyroid Protection in Thyroidectomy. Ann Transl Med (2015) 3(16):230. doi: 10.3978/j.issn.2305-5839.2015.08.20
9. Pasta V, Monteleone F, Del Vecchio L, Iacobelli S, Urciuoli P, D’Orazi V. Original Technique for Preoperative Preparation of Patients and Intraoperative Localization of Parathyroid Adenomas. G Chir (2015) 36(3):97–100. doi: 10.11138/gchir/2015.36.3.097
10. Papavramidis TS, Anagnostis P, Chorti A, Pliakos I, Panidis S, Koutsoumparis D, et al. Do Near-Infrared Intra-Operative Findings by the Use of Indocyanine Green Correlate With Post-Thyroidectomy Parathyroid Function? - the ICGPREDICT Study. Endocr Pract (2020) 26(9):967–73. doi: 10.4158/EP-2020-0119
11. Sadowski SM, Vidal Fortuny J, Triponez F. A Reappraisal of Vascular Anatomy of the Parathyroid Gland Based on Fluorescence Techniques. Gland Surg (2017) 6(Suppl 1):S30–7. doi: 10.21037/gs.2017.07.10
12. Vidal Fortuny J, Sadowski SM, Belfontali V, Guigard S, Poncet A, Ris Fe, et al. Randomized Clinical Trial of Intraoperative Parathyroid Gland Angiography With Indocyanine Green Fluorescence Predicting Parathyroid Function After Thyroid Surgery. Br J Surg (2018) 105(4):350–7. doi: 10.1002/bjs.10783
13. Triponez F. Re: Evaluation of Parathyroid Glands With Indocyanine Green Fluorescence Angiography After Thyroidectomy. World J Surg (2019) 43(6):1544–5. doi: 10.1007/s00268-019-04967-3
14. De Leeuw F, Breuskin I, Abbaci M, Casiraghi O, Mirghani H, Ben Lakhdar A, et al. Intraoperative Near-Infrared Imaging for Parathyroid Gland Identification by Auto-Fluorescence: A Feasibility Study. World J Surg (2016) 40(9):2131–8. doi: 10.1007/s00268-016-3571-5
15. Ladurner R, Sommerey S, Arabi NA, Hallfeldt KKJ, Stepp H, Gallwas JKS. Intraoperative Near-Infrared Autofluorescence Imaging of Parathyroid Glands. Surg Endosc (2017) 31(8):3140–5. doi: 10.1007/s00464-016-5338-3
16. Wolf HW, Grumbeck B, Runkel N. Intraoperative Verification of Parathyroid Glands in Primary and Secondary Hyperparathyroidism Using Near-Infrared Autofluorescence (Iopa). Updates Surg (2019) 71(3):579–85. doi: 10.1007/s13304-019-00652-1
17. Demarchi MS, Karenovics W, Bedat B, Triponez F. Intraoperative Autofluorescence and Indocyanine Green Angiography for the Detection and Preservation of Parathyroid Glands. J Clin Med (2020) 9(3):830. doi: 10.3390/jcm9030830
18. Paras C, Keller M, White L, Phay J, Mahadevan-Jansen A. Near-Infrared Autofluorescence for the Detection of Parathyroid Glands. J BioMed Opt (2011) 16(6):067012. doi: 10.1117/1.3583571
19. McWade MA, Paras C, White LM, Phay JE, Mahadevan-Jansen A, Broome JT. A Novel Optical Approach to Intraoperative Detection of Parathyroid Glands. Surgery (2013) 154(6):1371–7. doi: 10.1016/j.surg.2013.06.046
20. Kim SW, Song SH, Lee HS, Noh WJ, Oak C, Ahn YC, et al. Intraoperative Real-Time Localization of Normal Parathyroid Glands With Autofluorescence Imaging. J Clin Endocrinol Metab (2016) 101(12):4646–52. doi: 10.1210/jc.2016-2558
21. Akbulut S, Erten O, Gokceimam M, Kim YS, Krishnamurthy V, Heiden K, et al. Intraoperative Near-Infrared Imaging of Parathyroid Glands: A Comparison of First- and Second-Generation Technologies. J Surg Oncol (2021) 123(4):866–71. doi: 10.1002/jso.26336
22. Benmiloud F, Rebaudet S, Varoquaux A, Penaranda G, Bannier M, Denizot A. Impact of Autofluorescence-Based Identification of Parathyroids During Total Thyroidectomy on Postoperative Hypocalcemia: A Before and After Controlled Study. Surgery (2018) 163(1):23–30. doi: 10.1016/j.surg.2017.06.022
23. Benmiloud F, Godiris-Petit G, Gras R, Gillot JC, Turrin N, Penaranda G, et al. Association of Autofluorescence-Based Detection of the Parathyroid Glands During Total Thyroidectomy With Postoperative Hypocalcemia Risk: Results of the PARAFLUO Multicenter Randomized Clinical Trial. JAMA Surg (2020) 155(2):106–12. doi: 10.1001/jamasurg.2019.4613
24. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. Quadas-2: A Revised Tool for the Quality Assessment of Diagnostic Accuracy Studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
25. McWade MA, Paras C, White LM, Phay JE, Solorzano CC, Broome JT, et al. Label-Free Intraoperative Parathyroid Localization With Near-Infrared Autofluorescence Imaging. J Clin Endocrinol Metab (2014) 99(12):4574–80. doi: 10.1210/jc.2014-2503
26. Falco J, Dip F, Quadri P, de la Fuente M, Rosenthal R. Cutting Edge in Thyroid Surgery: Autofluorescence of Parathyroid Glands. J Am Coll Surg (2016) 223(2):374–80. doi: 10.1016/j.jamcollsurg.2016.04.049
27. McWade MA, Sanders ME, Broome JT, Solorzano CC, Mahadevan-Jansen A. Establishing the Clinical Utility of Autofluorescence Spectroscopy for Parathyroid Detection. Surgery (2016) 159(1):193–202. doi: 10.1016/j.surg.2015.06.047
28. Alesina PF, Meier B, Hinrichs J, Mohmand W, Walz MK. Enhanced Visualization of Parathyroid Glands During Video-Assisted Neck Surgery. Langenbecks Arch Surg (2018) 403(3):395–401. doi: 10.1007/s00423-018-1665-2
29. Kahramangil B, Dip F, Benmiloud F, Falco J, de la Fuente M, Verna S, et al. Detection of Parathyroid Autofluorescence Using Near-Infrared Imaging: A Multicenter Analysis of Concordance Between Different Surgeons. Ann Surg Oncol (2018) 25(4):957–62. doi: 10.1245/s10434-018-6364-2
30. Kim SW, Lee HS, Ahn YC, Park CW, Jeon SW, Kim CH, et al. Near-Infrared Autofluorescence Image-Guided Parathyroid Gland Mapping in Thyroidectomy. J Am Coll Surg (2018) 226(2):165–72. doi: 10.1016/j.jamcollsurg.2017.10.015
31. Ladurner R, Al Arabi N, Guendogar U, Hallfeldt K, Stepp H, Gallwas J. Near-Infrared Autofluorescence Imaging to Detect Parathyroid Glands in Thyroid Surgery. Ann R Coll Surg Engl (2018) 100(1):33–6. doi: 10.1308/rcsann.2017.0102
32. Thomas G, McWade MA, Paras C, Mannoh EA, Sanders ME, White LM, et al. Developing a Clinical Prototype to Guide Surgeons for Intraoperative Label-Free Identification of Parathyroid Glands in Real Time. Thyroid (2018) 28(11):1517–31. doi: 10.1089/thy.2017.0716
33. DiMarco A, Chotalia R, Bloxham R, McIntyre C, Tolley N, Palazzo FF. Autofluorescence in Parathyroidectomy: Signal Intensity Correlates With Serum Calcium and Parathyroid Hormone But Routine Clinical Use is Not Justified. World J Surg (2019) 43(6):1532–7. doi: 10.1007/s00268-019-04929-9
34. Kose E, Kahramangil B, Aydin H, Donmez M, Berber E. Heterogeneous and Low-Intensity Parathyroid Autofluorescence: Patterns Suggesting Hyperfunction at Parathyroid Exploration. Surgery (2019) 165(2):431–7. doi: 10.1016/j.surg.2018.08.006
35. Ladurner R, Lerchenberger M, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ. Parathyroid Autofluorescence-How Does it Affect Parathyroid and Thyroid Surgery? A 5 Year Experience. Molecules (2019) 24(14):2560. doi: 10.3390/molecules24142560
36. Lerchenberger M, Al Arabi N, Gallwas JKS, Stepp H, Hallfeldt KKJ, Ladurner R. Intraoperative Near-Infrared Autofluorescence and Indocyanine Green Imaging to Identify Parathyroid Glands: A Comparison. Int J Endocrinol (2019) 2019:e4687951. doi: 10.1155/2019/4687951
37. McWade MA, Thomas G, Nguyen JQ, Sanders ME, Solorzano CC, Mahadevan-Jansen A. Enhancing Parathyroid Gland Visualization Using a Near Infrared Fluorescence-Based Overlay Imaging System. J Am Coll Surg (2019) 228(5):730–43. doi: 10.1016/j.jamcollsurg.2019.01.017
38. Squires MH, Jarvis R, Shirley LA, Phay JE. Intraoperative Parathyroid Autofluorescence Detection in Patients With Primary Hyperparathyroidism. Ann Surg Oncol (2019) 26(4):1142–8. doi: 10.1245/s10434-019-07161-w
39. Thomas G, McWade MA, Nguyen JQ, Sanders ME, Broome JT, Baregamian N, et al. Innovative Surgical Guidance for Label-Free Real-Time Parathyroid Identification. Surgery (2019) 165(1):114–23. doi: 10.1016/j.surg.2018.04.079
40. Thomas G, Squires MH, Metcalf T, Mahadevan-Jansen A, Phay JE. Imaging or Fiber Probe-Based Approach? Assessing Different Methods to Detect Near Infrared Autofluorescence for Intraoperative Parathyroid Identification. J Am Coll Surg (2019) 229(6):596–608 e593. doi: 10.1016/j.jamcollsurg.2019.09.003
41. Kose E, Rudin AV, Kahramangil B, Moore E, Aydin H, Donmez M, et al. Autofluorescence Imaging of Parathyroid Glands: An Assessment of Potential Indications. Surgery (2020) 167(1):173–9. doi: 10.1016/j.surg.2019.04.072
42. Takahashi T, Yamazaki K, Ota H, Shodo R, Ueki Y, Horii A. Near-Infrared Fluorescence Imaging in the Identification of Parathyroid Glands in Thyroidectomy. Laryngoscope (2021) 131(5):1188–93. doi: 10.1002/lary.29163
43. Solorzano CC, Thomas G, Berber E, Wang TS, Randolph GW, Duh QY, et al. Current State of Intraoperative Use of Near Infrared Fluorescence for Parathyroid Identification and Preservation. Surgery (2021) 169(4):868–78. doi: 10.1016/j.surg.2020.09.014
44. Di Marco AN, Palazzo FF. Near-Infrared Autofluorescence in Thyroid and Parathyroid Surgery. Gland Surg (2020) 9(Suppl 2):S136–46. doi: 10.21037/gs.2020.01.04
45. Solorzano CC, Thomas G, Baregamian N, Mahadevan-Jansen A. Detecting the Near Infrared Autofluorescence of the Human Parathyroid: Hype or Opportunity? Ann Surg (2020) 272(6):973–85. doi: 10.1097/SLA.0000000000003700
46. Aoyama M, Takizawa H, Yamamoto K, Inui T, Miyamoto N, Sakamoto S, et al. Effects of Excitation Light Intensity on Parathyroid Autofluorescence With a Novel Near-Infrared Fluorescence Imaging System: Two Surgical Case Reports. Gland Surg (2020) 9(5):1584–9. doi: 10.21037/gs-20-386
Keywords: near infrared, autofluorescence, parathyroid gland, surgery, meta-analysis
Citation: Wang B, Zhu C-R, Liu H, Yao X-M and Wu J (2021) The Accuracy of Near Infrared Autofluorescence in Identifying Parathyroid Gland During Thyroid and Parathyroid Surgery: A Meta-Analysis. Front. Endocrinol. 12:701253. doi: 10.3389/fendo.2021.701253
Received: 27 April 2021; Accepted: 07 June 2021;
Published: 21 June 2021.
Edited by:
Paolo Miccoli, University of Pisa, ItalyReviewed by:
Gabriele Materazzi, University of Pisa, ItalyGianluca Donatini, Centre Hospitalier Universitaire (CHU) de Poitiers, France
Copyright © 2021 Wang, Zhu, Liu, Yao and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jian Wu, d29fZG9jdG9yQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
 Bin Wang
Bin Wang Chun-Rong Zhu
Chun-Rong Zhu Hong Liu
Hong Liu Xin-Min Yao
Xin-Min Yao Jian Wu
Jian Wu