- 1School of Medicine, Cheeloo College of Medicine, Shandong University, Ji’nan, China
- 2Center for Reproductive Medicine, Cheeloo College of Medicine, Shandong University, Ji’nan, China
- 3National Research Center for Assisted Reproductive Technology and Reproductive Genetics, Shandong University, Ji’nan, China
- 4Key Laboratory of Reproductive Endocrinology of Ministry of Education, Shandong University, Ji’nan, China
- 5Department of Anesthesiology, Qilu Hospital, Cheeloo College of Medicine, Shandong University, Ji’nan, China
- 6Department of Cardiology, Shandong Provincial Hospital Affiliated to Shandong First Medical University, Ji’nan, China
- 7Department of Cardiology, Shandong Provincial Hospital, Cheeloo College of Medicine, Shandong University, Ji’nan, China
- 8Suzhou Research Institute, Shandong University, Suzhou, China
Background: Women with polycystic ovary syndrome (PCOS) are generally considered to be central obese and at higher risks of metabolic disturbances. Imaging methods are the golden standards for detecting body fat distribution. However, evidence based on magnetic resonance imaging (MRI) and computed tomography (CT) is conflicting. This study systematically reviewed the imaging-based body fat distribution in PCOS patients and quantitatively evaluated the difference in body fat distribution between PCOS and BMI-matched controls.
Methods: PUBMED, EMBASE, and Web of Science were searched up to December 2019, and studies quantitatively compared body fat distribution by MRI, CT, ultrasound, or X-ray absorptiometry (DXA) between women with PCOS and their BMI-matched controls were included. Two researchers independently reviewed the articles, extract data and evaluated the study quality based on Newcastle-Ottawa Scale (NOS).
Results: 47 studies were included in systematic review and 39 were eligible for meta-analysis. Compared to BMI-matched controls, higher accumulations of visceral fat (SMD 0.41; 95%CI: 0.23-0.59), abdominal subcutaneous fat (SMD 0.31; 95%CI: 0.20-0.41), total body fat (SMD 0.19; 95% CI: 0.06-0.32), trunk fat (SMD 0.47; 95% CI: 0.17-0.77), and android fat (SMD 0. 36; 95% CI: 0.06-0.66) were identified in PCOS group. However, no significant difference was identified in all the above outcomes in subgroups only including studies using golden standards MRI or CT to evaluate body fat distribution (SMD 0.19; 95%CI: -0.04-0.41 for visceral fat; SMD 0.15; 95%CI: -0.01-0.31 for abdominal subcutaneous fat). Moreover, meta-regression and subgroup analyses showed that young and non-obese patients were more likely to accumulate android fat.
Conclusions: PCOS women seem to have abdominal fat accumulation when compared with BMI-matched controls. However, MRI- and CT- assessed fat distribution was similar between PCOS and controls, suggesting central obesity may be independent of PCOS. These findings will help us reappraise the relationship between PCOS and abnormal fat deposition and develop specialized lifestyle interventions for PCOS patients.
Systematic Review Registration: PROSPERO, identifier CRD42018102983.
Introduction
Polycystic ovary syndrome (PCOS) is an endocrine disease associated with obesity and multiple metabolic complications, including insulin resistance, diabetes, and cardiovascular diseases (1, 2). According to previous studies, metabolic disturbances in PCOS are partially obesity-related conditions (3). It has been widely acknowledged that obesity aggravates insulin resistance and adverse metabolic outcomes in patients with PCOS (4). However, there are approximately 40–50% of PCOS patients with BMI in the normal range (5). These lean PCOS patients also have increased risks of metabolic dysfunctions and merely losing weight is not a suitable intervention for this population (6). Thus, it is important to investigate whether the body composition and body fat distribution are altered in PCOS patients since different body compositions (that is different percentages of fat, muscle and bone, and body fat mass) may be completely different under the same BMI.
According to the World Health Organization, body fat distribution is another factor that determines the metabolic risks associated with obesity (7). Visceral fat and abdominal subcutaneous fat, which are known as android fat, are recognized to be related to higher risks of metabolic abnormalities such as hypertension and type 2 diabetes, while gluteal or thigh fat, known as gynoid fat, is regarded as a protective fat correlated with low risks of metabolic diseases (8). Different methods can be used to measure fat distribution. Waist circumference (WC), as a conventional clinical measurement of abdominal obesity, has been widely used to estimate central obesity in PCOS patients. A previous meta-analysis showed that women with PCOS had a higher prevalence of central obesity according to WC (9). The golden standard for the measurement of body fat distribution are imaging methods such as magnetic resonance imaging (MRI) and computed tomography (CT). However, studies using these methods to assess fat distribution in women with PCOS showed controversial results. A study using MRI to analyze body composition of women with or without PCOS argued that lean women with PCOS had less visceral fat (10), whereas two other studies including MRI assessment reported no visceral fat accumulation in PCOS women with obesity or insulin resistance (11, 12).
Therefore, it is essential to quantitatively study body fat deposition of PCOS through imaging methods to help us get in-depth knowledge of the fat distribution of women with PCOS. This study systematically reviewed the imaging-based body fat distribution in PCOS patients and quantitatively evaluated the difference between PCOS and BMI-matched controls from 8 aspects: visceral fat, abdominal subcutaneous fat, total body fat, trunk fat, android fat, and gynoid fat. Our findings provide new insights into the fat distribution patterns in PCOS patients, which is of great significance for understanding the etiology of PCOS and guiding lifestyle interventions in clinical practice.
Material and Methods
Search Strategy
Systematic database searches were performed in PUBMED, EMBASE, and Web of Science updated in Dec 2019. The declarations of Preferred Reporting Item for Systematic Reviews and Meta-analyses (PRISMA) were followed. The protocol of this systematic review and meta-analysis was previously registered on PROSPERO (CRD42018102983). We developed the “full text” search strategy based on the combination of the following keywords (subject item plus free items): (polycystic ovary syndrome OR PCOS) AND (body fat distribution OR visceral adipose tissue OR subcutaneous adipose tissue OR central obesity OR android distribution OR gynoid distribution) AND (magnetic resonance OR ultrasound OR computerized tomography OR X-ray). Detailed search strategies were listed in Table S1. Full-text review was implemented after the screening of title and abstract. References of included articles were hand-reviewed to identify the eligible articles.
Inclusion and Exclusion Criteria
The populations being studied in this review were women diagnosed with PCOS and the populations of comparator were BMI-matched control women without PCOS. This review was based on observational studies, therefore interventions were not applicable. The main outcomes in this study were imaging-based body fat distribution including visceral fat, abdominal subcutaneous fat, total body fat, trunk fat, android fat, and gynoid fat in quantity. Moreover, we also included total body fat, trunk fat, android fat, and gynoid fat in percentage as secondary outcomes. Studies that satisfied the following criteria were included in the present meta-analysis: (1) studies that investigated the distribution of body fat including visceral fat, abdominal subcutaneous fat, total body fat (both in quantity and in percentage), android fat (both in quantity and in percentage), gynoid fat (both in quantity and in percentage), and trunk fat (both in quantity and in percentage) between women with PCOS and controls; (2) body fat distribution was measured by standard imaging methods including MRI, CT, ultrasound, and X-ray absorptiometry (DXA); (3) When duplication of same subject population occurred, the most recent study or study with the largest sample was included. Exclusion criteria were: (1) studies that employed testing technologies other than the standard imaging methods such as bioelectrical impedance; (2) studies without BMI adjustment; (3) studies that lack sufficient data to perform quantitative or qualitative analysis. Articles in languages other than English were excluded.
Data Extraction
Two researchers (Zhu SQ and Hu CP) reviewed retrieved studies independently. Baseline characteristics relating to study and its participant (country, ethnicity, design, age, BMI, definition of PCOS, subject number, adjusted confounders, imaging methods, treatments, blind to outcomes, measure region, outcomes) were extracted. The definition of PCOS was defined by Rotterdam criteria, National Institutes of Health (NIH) criteria, the Androgen Excess and PCOS (AE-PCOS) Society criteria, or based on the original articles (13–15). Definitions of obese and non-obese were arbitrarily decided based on original articles due to the heterogeneity of cutoff values. Disagreements were resolved after consensus (Li Y). Regarding the studies with insufficient data and some conference abstracts, the corresponding authors were contacted.
Quality Assessment
Newcastle-Ottawa Scale (NOS) was employed to assess the qualities of the included studies (16). Two researchers (Zhu SQ and Li ZY) independently evaluated the study quality in a blinded manner. Controversies were settled by consultation among co-authors. NOS focuses on three aspects of quality assessment: (1) selection of representative cases and controls; (2) comparability of baseline features; (3) exposure assessment or outcome evaluation. NOS score with minimum 0 and maximum 9, which higher score indicates higher quality.
Statistical Analysis
We evaluated the variation of fat distribution between women with PCOS and controls. The between-study standardized mean difference (SMD) and 95% confidence intervals (CIs) were deployed. SMD>0 represents higher fat distribution in PCOS as compared to that of controls, while SMD<0 indicated the opposite. Between-study heterogeneity was assessed by I2-statistics and Q-test. Random-effects model was used when significant heterogeneity was observed. Subgroup analyses were performed to explore the source of heterogeneity. The impact of a study on the overall effect was assessed through sensitivity analysis. Besides, meta-regression was performed on outcomes that have more than 10 included studies. Publication bias was examined using Egger’s regression test. Two-tailed P < 0.05 was considered as significant. Trim and Filled Analysis was used to further evaluate the variation when significant bias was tested in Egger’s regression test. Statistical analyses were performed using STATA 12.0 (Stata Corporation, College Station, USA).
Results
Search Results
A total of 1284 articles were yielded through electronic search strategy and hand search. After excluding duplicates and screening abstracts based on selection criteria, 122 full-text articles were further assessed for eligibility. After excluding 15 for duplicated datasets, 13 for not using standard imaging methods, 29 for insufficient data, 18 for undesirable controls or outcomes, 47 articles finally remained for qualitative synthesis and 39 were found eligible for meta-analysis (Figure 1).
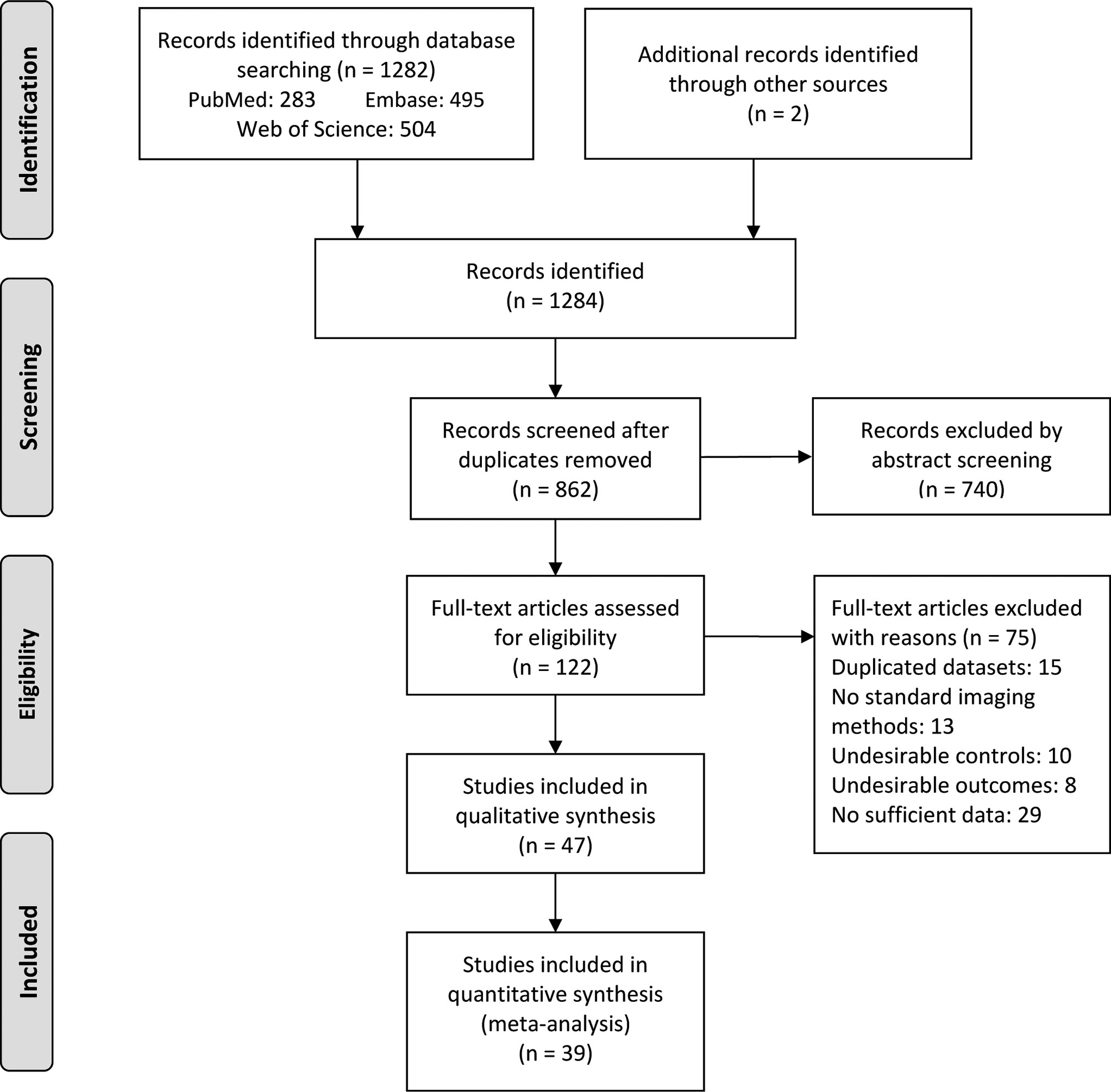
Figure 1 PRISMA flow diagram for study selection. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097.
Study Characteristics
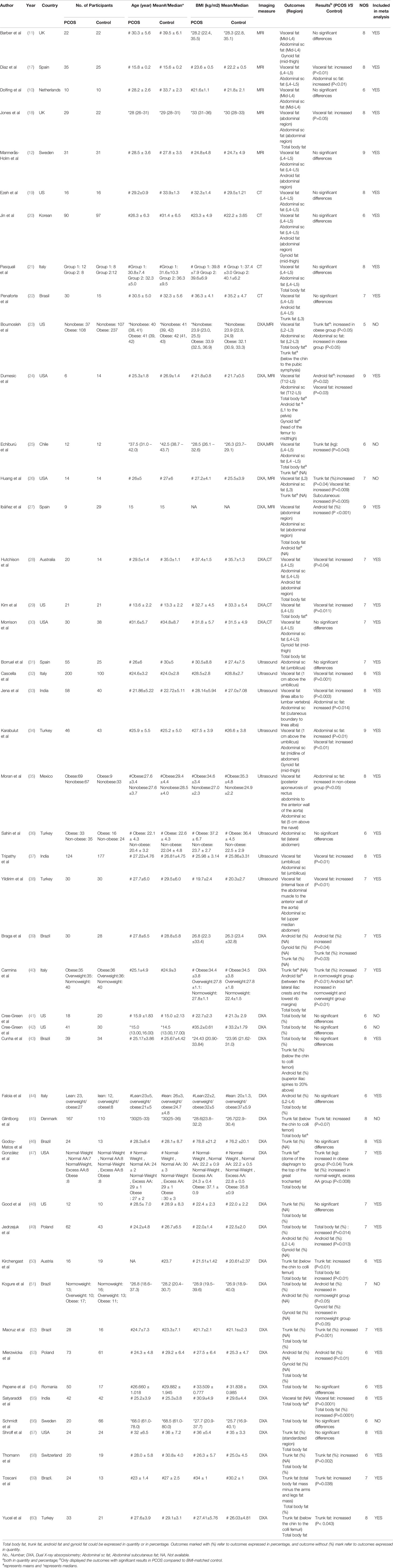
This study included overall 4226 individuals, 2203 with PCOS and 2023 controls (Table 1). BMI was similar between PCOS group and control group in every eligible study. Fat distribution was measured in all study subjects using imaging methods. Among these studies, 10 used MRI (10–12, 17, 18, 23–27), 7 used CT (19–22, 28–30), 8 used ultrasound (31–38), and 30 used DXA (23–30, 39–60) as their measurement indicator. Details of detected areas in each study were displayed in Table 1. The ethnicity of eligible studies varied from Caucasian, Asian, and Mediterranean. Diagnostic criteria of PCOS were adopted NIH, Rotterdam, or AE-PCOS criteria in most of the included studies, and 5 studies describe PCOS definition in their original articles (Table S2).
Methodological Quality
Assessments of study quality were displayed in Table S3. All studies were ranked into medium or high quality, except for one study that was graded low quality (excluded in the meta-analysis). Among all 47 studies, 44 adjusted other confounders such as age, weight, or ethnicity. 35 studies clarified the age stage of participants, of which 25 studies investigated fat distribution in adults. 25 studies stratified participants into specified BMI categories. Overall 43 studies reported no medications or treatment interferences and 11 declared as a blinded study (Table S2). Studies whose data fit the normal distribution and expressed as mean were included in the meta-analysis, whereas studies with data displayed as median were only included in the systematic review.
Visceral Fat and Abdominal Subcutaneous Fat
Overall 24 studies (10–12, 17–30, 32–35, 37, 38, 55) compared the difference of visceral fat between women with PCOS and healthy controls and 21 studies (10–12, 17–22, 24, 27–30, 32–35, 37, 38, 55) were included in meta-analysis. Most studies found no differences in fat distribution between PCOS patients and controls. Huang et al. demonstrated increased visceral fat in PCOS (26), whereas Boumosleh et al. and Echiburú et al. reported similar visceral fat distribution between two groups (23, 25). In the meta-analysis, increased visceral fat accumulation was identified in women with PCOS (SMD 0.41; 95%CI: 0.23-0.59). However, this difference disappeared when imaging methods were restricted to MRI or CT (SMD 0.19; 95%CI: -0.04-0.41) (Figure 2A).
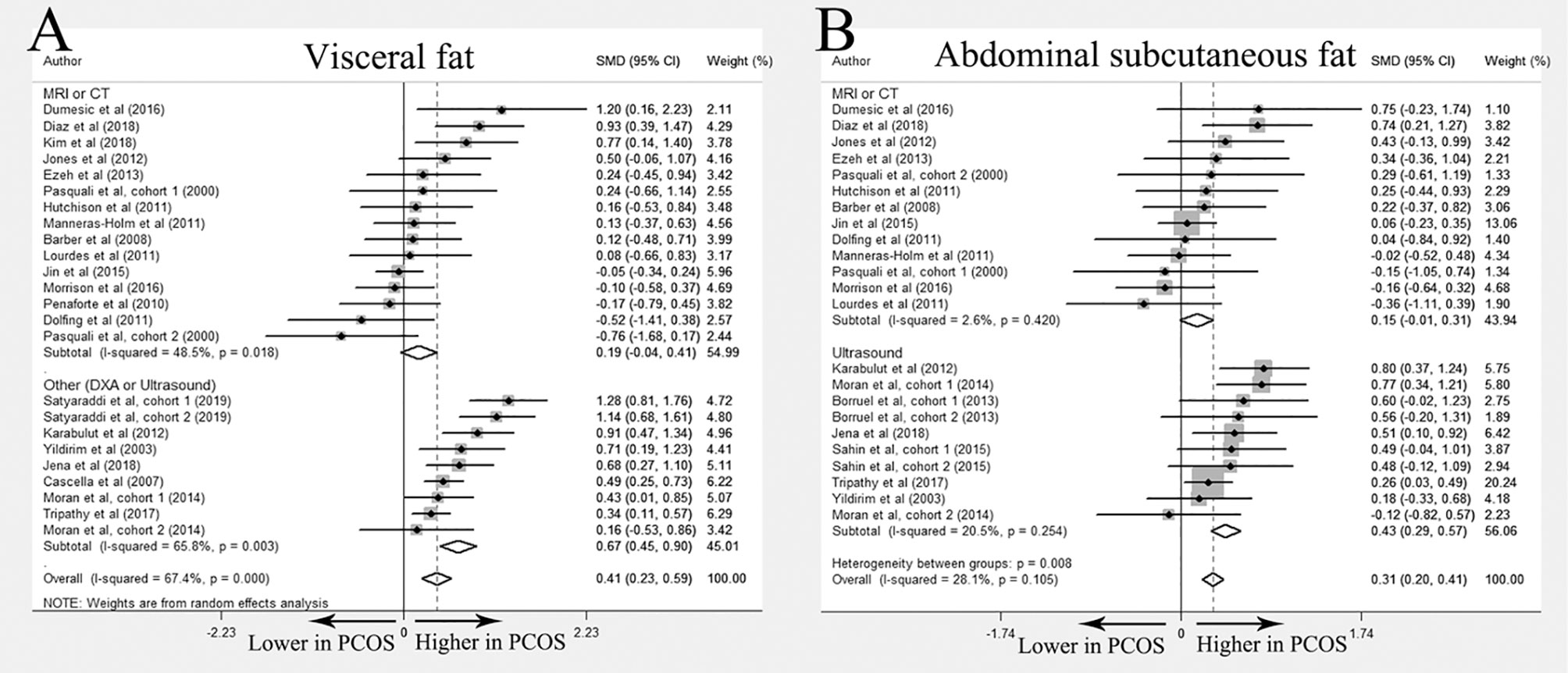
Figure 2 Meta-analysis on visceral fat and abdominal subcutaneous fat: women with PCOS versus BMI-matched healthy controls. Forest plot displayed odds of visceral fat (A) and abdominal subcutaneous fat (B) in subgroups.
Twenty-two studies investigated abdominal subcutaneous fat distribution in PCOS and control groups (10–12, 17–21, 23–28, 30, 31, 33–38). Among them, 5 studies reported elevated abdominal subcutaneous fat accumulation in the PCOS group (17, 23, 33–35), and no significant differences were reported in the remaining studies. Meta-analysis of abdominal subcutaneous fat included 19 studies (10–12, 17–21, 24, 27, 28, 30, 31, 33–38). The results showed that women with PCOS had more abdominal subcutaneous fat than their BMI-matched healthy controls (SMD 0.31; 95%CI: 0.20-0.41). However, similar to the results of visceral fat, no significant differences were found in the subgroup including only studies using MRI or CT (SMD 0.15; 95%CI: -0.01-0.31) (Figure 2B).
Total Body Fat, Trunk Fat, Android Fat and Gynoid Fat
A total of 37 (11, 12, 18, 20–30, 34, 39–60) studies compared the distribution of total body fat, trunk fat, android fat, and gynoid fat between PCOS and BMI-matched control groups, and each outcome was expressed as quantity and percentage. Most of these studies used DXA as the imaging method, especially when describing the percentage of fat distribution. Detailed information about each study was displayed in Table 1. Overall 29 studies (11, 12, 18, 20–22, 24, 27–30, 34, 39, 40, 43, 44, 46–50, 52–55, 57–60) were further included in the meta-analysis. Of them, 23 investigated total body fat distribution (N=14 for quantity; N=11 for percentage); 13 studies trunk fat distribution (N=6 for quantity; N=9 for percentage); 12 studies android fat distribution (N=7 for quantity; N=8 for percentage); and 8 studied gynoid fat distribution (N=5 for quantity; N=4 for percentage).
In the meta-analysis, absolute value of total body fat was elevated in PCOS women but no significant difference was found in the percentage of total body fat between women with PCOS and BMI-matched controls (SMD 0. 19; 95% CI: 0.06-0.32 for quantity; SMD 0.27; 95% CI: -0.14-0.69 for percentage). Increased accumulation of trunk fat (SMD 0.47; 95% CI: 0.17-0.77 for quantity; SMD 0.67; 95% CI: 0.40-0.94 for percentage) and android fat (SMD 0. 36; 95% CI: 0.06-0.66 for quantity; SMD 0.53; 95% CI: 0.12-0.94 for percentage) was identified in women with PCOS. PCOS women also had higher absolute value of gynoid fat (SMD 0. 22, 95% CI: 0. 02-0. 42 for quantity), whereas the percentage of gynoid fat in women with PCOS was comparable to that in healthy controls (SMD -0.07, 95% CI: -0.49-0.35 for percentage). Interestingly, there was no statistically significant difference in all outcomes in MRI or CT subgroup. (Figure 3 and Table 2)
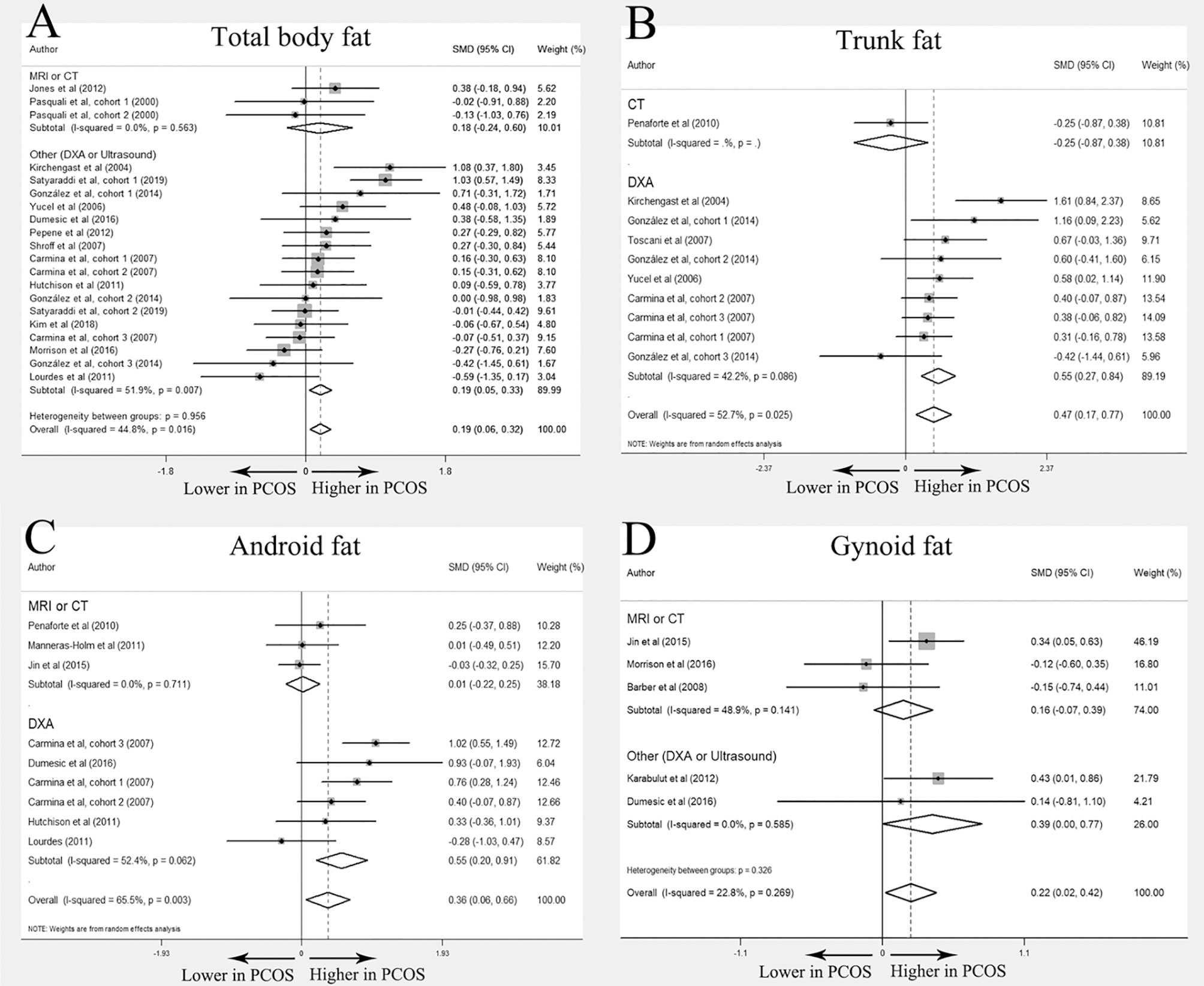
Figure 3 Meta-analysis on total body fat, trunk fat, android fat and gynoid fat (in quantity): women with PCOS versus BMI-matched healthy controls. Forest plot displayed odds of total body fat (A), trunk fat (B), android fat (C) and gynoid fat (D) in subgroups.
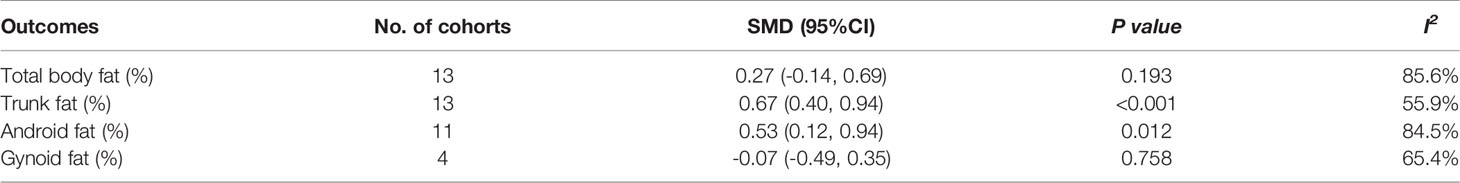
Table 2 Meta-analysis results for total body fat, trunk fat, android fat, and gynoid fat in percentage (%): women with PCOS versus BMI-matched healthy controls.
Meta-Regression and Subgroup Analyses
Unadjusted meta-regression analyses found that age was inversely associated with visceral fat accumulation in PCOS (P<0.05) (Table S4). Subgroup analysis showed that the difference in body fat distribution between PCOS and BMI-matched controls was mainly manifested in non-obese patients. Non-obese PCOS women had elevated accumulation of visceral fat, abdominal subcutaneous fat, total body fat (both in quantity and percentage), trunk fat (in percentage), and android fat (both in quantity and percentage), whereas only trunk fat (in percentage) and android fat (in quantity) were significantly increased in PCOS women with obesity (Tables S5, S6). Moreover, subgroup analyses showed that visceral fat and abdominal subcutaneous fat deposition assessed by MRI and CT were similar between women with PCOS and controls regardless of different ethnicities including Caucasian, Asian, and Mediterranean.
Sensitivity Analyses and Publication Bias
Sensitivity analyses and tests of publication bias verified the robustness of pooled results. No significant variation was introduced in sensitivity analysis for every outcome. Similarly, no significant bias was identified in Egger’s tests and Trim and Filled Analyses (Table S7).
Discussion
This systematic review and meta-analysis initially summarized and compared imaging-based fat distribution between women with PCOS and BMI-matched controls. Higher accumulation of visceral fat, abdominal subcutaneous fat, total body fat, trunk fat, and android fat was observed in women with PCOS, especially non-obese PCOS women. Notably, when imaging method was stratified as the gold standard MRI or CT, there was no difference in fat distribution between women with PCOS and their BMI matched controls.
Based on fat deposition sites and their pathophysiological significance to metabolism, body fat distribution can be generally divided into intra-abdominal/visceral fat (including visceral fat and abdominal subcutaneous fat), upper body fat (including trunk fat and android fat) and lower body fat (gynoid fat). Previous researches have shown that visceral fat and upper body fat are related to higher risks of metabolic disorders such as hypertension and type 2 diabetes, and lower body fat is associated with reduced metabolic risks (61–63). In PCOS, it has been reported that elevated level of testosterone is related to central pattern fat distribution through pro-adipogenic and anti-lipolytic effects, and central obesity in turn aggravates insulin resistance and metabolic complications in women with PCOS (46, 64–66).
Contrary to previous studies which reported an elevated prevalence of central obesity estimated with WC in women with PCOS (9), in this systematic review and meta-analysis, we found that the fat distribution of PCOS patients (including visceral fat, abdominal subcutaneous fat, total body fat, trunk fat, android fat, and gynoid fat) was similar to that of the BMI-matched control group when fat distribution was measured by traditional gold standards MRI or CT. Although these results were inconsistent with the general concept in the field that PCOS patients exhibit visceral fat accumulation, they cannot be simply explained by insufficient sample size since most included studies adopted MRI or CT to evaluate visceral and abdominal subcutaneous fat. Similarly, Mannerås-Holm et al. found in their study that increased abdominal/visceral fat in PCOS women evaluated by waist-to-hip ratio was not supported by MRI, and suggested the need for reassessment of abdominal and visceral fat accumulation in PCOS (12). Moreover, PCOS phenotypes may have impacts on body fat distribution patterns. Aleksandra et al. reported that visceral fat amount was only increased in PCOS phenotype A (hyperandrogenism + oligo/amenorrhea + polycystic ovarian morphology) but not elevated or related to free androgen index in phenotype B (hyperandrogenism + oligo/amenorrhea), C (hyperandrogenism + polycystic ovarian morphology), and D (oligo/amenorrhea + polycystic ovarian morphology), suggesting there are differences in fat distribution between PCOS phenotypes which however is beyond the scope of our study (67). Further studies are therefore needed to clarify the relationship between different PCOS phenotypes and abdominal obesity. Given that PCOS patients with similar BMI or abdominal fat compared to controls also have higher risks of metabolic dysfunctions, our results indicate that central obesity may be independent of PCOS and ectopic fat distribution may not be a dominant reason for high metabolic risks in PCOS (6, 40).
Furthermore, imaging methods may also affect the results. When studies using DXA and ultrasound as measurements were also included in the meta-analysis, the results showed a higher accumulation of total body fat and upper body fat (including visceral fat, abdominal subcutaneous fat, trunk fat, and android fat) in women with PCOS compared to BMI-matched healthy women, which was consistent with previous knowledge. The conflicting results between the different imaging methods may be related to bias from methods. Despite that DXA has been widely used for estimating regional body fat, the results of DXA analysis could be confounded by hydration of lean soft tissue (68). It has been reported that DXA overestimated visceral fat, especially in people who have higher levels of visceral adiposity (69). Therefore, caution should be paid when interpreting the results of DXA in clinical practice.
In meta-regression and subgroup analyses, we found that age was inversely associated with increased visceral fat accumulation in women with PCOS. Similarly, prospective cohort studies have demonstrated that the risks of central obesity and metabolic diseases increased in young PCOS patients but were attenuated in later life (70, 71). However, the underlying mechanism is yet to be elucidated. It is probably due to the protective effect of lifestyle interventions or metformin treatment on PCOS patients. Moreover, subgroup analyses showed that abdominal obesity is more prominent in non-obese patients, suggesting the existence of abnormal body fat distribution in non-obese PCOS phenotype and exercises focused on improving body composition may help prevent and diminish metabolic-related risks in non-obese PCOS women.
This study systematically reviewed the articles that investigated the difference of image-assessed fat distribution between women with PCOS and BMI-matched controls and the robustness of results was verified by sensitivity analyses and tests of publication bias. Through comprehensive subgroup analyses of possible confounders, we found that different imaging methods may be the dominant source of heterogeneity. Given the lack of convenience and efficiency of golden standards MRI and CT, the small sample size is a common problem in studies using MRI or CT to detect fat distribution. This meta-analysis facilitated the integration of these data and found similar abdominal fat distribution between PCOS and BMI-matched healthy controls in MRI or CT subgroup. Despite the above advantages, there are some limitations in this study. Firstly, the main limitation of such an extensive review is that the selection bias in comparator populations. The optimal source of controls should be from the community, but less than half of the included studies met the criteria. In studies that do not use community-based controls, they were more inclined to recruit controls from schools, hospital employees, or medical examiners. This population may be healthier and lead to an overestimation of the difference between PCOS and control groups. Secondly, although details of these possible confounders were extensively extracted from the original studies and displayed in Table 1 and Table S2, subgroup analyses cannot fully explain the heterogeneity. The residual confounding factors may be the definition and phenotype of PCOS, areas measured by imaging methods, lifestyle, and usage of medications. Therefore, further population-based studies with large sample sizes and precise control of confounding factors are still needed.
In conclusion, this systematic review and meta-analysis summarized the current evidence focused on imaging-based body fat distribution in women with PCOS and found similar fat distribution patterns assessed by golden standards MRI or CT between women with PCOS and BMI-matched controls, indicating central obesity may be independent of PCOS and exacerbate metabolic dysregulation in PCOS patients. Moreover, younger patients and non-obese patients were more inclined to accumulate android fat. These results facilitated the understanding of the relationship between PCOS and ectopic fat deposition, and will support the establishment of specialized lifestyle interventions for PCOS patients.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author Contributions
YL and HY designed the study and evaluated the data. SZ, CH, and ZL collected the information and analyzed the data. SZ, ZL, and FS wrote the manuscript. CW and YL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Natural Science Foundation of Jiangsu Province (BK20200223), the Natural Science Foundation of Shandong Province (ZR2020QH051), the Young Scholars Program as well as Fundamental Research Funds (21520079614029) of Shandong University to YL.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Professor Linlin Cui (Center for Reproductive Medicine, Shandong University) for her helpful suggestions while preparing this manuscript.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2021.697223/full#supplementary-material
References
1. Moran LJ, Norman RJ, Teede HJ. Metabolic Risk in PCOS: Phenotype and Adiposity Impact. Trends Endocrinol metabolism: TEM. (2015) 26(3):136–43. doi: 10.1016/j.tem.2014.12.003
2. Anagnostis P, Tarlatzis BC, Kauffman RP. Polycystic Ovarian Syndrome (PCOS): Long-Term Metabolic Consequences. Metabolism (2018) 86:33–43. doi: 10.1016/j.metabol.2017.09.016
3. Chiu WL, Boyle J, Vincent A, Teede H, Moran LJ. Cardiometabolic Risks in Polycystic Ovary Syndrome: Non-Traditional Risk Factors and the Impact of Obesity. Neuroendocrinology (2017) 104(4):412–24. doi: 10.1159/000455233
4. Glueck CJ, Goldenberg N. Characteristics of Obesity in Polycystic Ovary Syndrome: Etiology, Treatment, and Genetics. Metabolism (2019) 92:108–20. doi: 10.1016/j.metabol.2018.11.002
5. Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic Ovary Syndrome: Etiology, Pathogenesis and Diagnosis. Nat Rev Endocrinol (2011) 7(4):219–31. doi: 10.1038/nrendo.2010.217
6. Zhu S, Zhang B, Jiang X, Li Z, Zhao S, Cui L, et al. Metabolic Disturbances in non-Obese Women With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Fertil Steril (2019) 111(1):168–77. doi: 10.1016/j.fertnstert.2018.09.013
7. World Health Organization. The Asia-Pacific Perspective: Redefining Obesity and its Treatment. (2000).
8. Jensen MD. Role of Body Fat Distribution and the Metabolic Complications of Obesity. J Clin Endocrinol Metab (2008) 93(11 Suppl 1):S57–63. doi: 10.1210/jc.2008-1585
9. Lim SS, Davies MJ, Norman RJ, Moran LJ. Overweight, Obesity and Central Obesity in Women With Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Hum Reprod Update (2012) 18(6):618–37. doi: 10.1093/humupd/dms030
10. Dolfing JG, Stassen CM, van Haard PM, Wolffenbuttel BH, Schweitzer DH. Comparison of MRI-Assessed Body Fat Content Between Lean Women With Polycystic Ovary Syndrome (PCOS) and Matched Controls: Less Visceral Fat With PCOS. Hum Reprod (Oxford England) (2011) 26(6):1495–500. doi: 10.1093/humrep/der070
11. Barber TM, Golding SJ, Alvey C, Wass JA, Karpe F, Franks S, et al. Global Adiposity Rather Than Abnormal Regional Fat Distribution Characterizes Women With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2008) 93(3):999–1004. doi: 10.1210/jc.2007-2117
12. Manneras-Holm L, Leonhardt H, Kullberg J, Jennische E, Oden A, Holm G, et al. Adipose Tissue has Aberrant Morphology and Function in PCOS: Enlarged Adipocytes and Low Serum Adiponectin, But Not Circulating Sex Steroids, are Strongly Associated With Insulin Resistance. J Clin Endocrinol Metab (2011) 96(2):E304–311. doi: 10.1210/jc.2010-1290
13. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 Consensus on Diagnostic Criteria and Long-Term Health Risks Related to Polycystic Ovary Syndrome (PCOS). Hum Reprod (Oxford England) (2004) 19(1):41–7. doi: 10.1093/humrep/deh098
14. Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Positions Statement: Criteria for Defining Polycystic Ovary Syndrome as a Predominantly Hyperandrogenic Syndrome: An Androgen Excess Society Guideline. J Clin Endocrinol Metab (2006) 91(11):4237–45. doi: 10.1210/jc.2006-0178
15. Zawadzski JK. Diagnostic Criteria for Polycystic Ovary Syndrome: Towards a Rational Approach. In: Dunaif A, Givens JR, Haseltine FP et al. (eds) Current Issues in Endocrinology and Metabolism. Oxford: Blackwell Scientific (1992). pp. 377–84.
16. Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. (2009). Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm
17. Diaz M, Gallego-Escuredo JM, Lopez-Bermejo A, de Zegher F, Villarroya F, Ibanez L. Low-Dose Spironolactone-Pioglitazone-Metformin Normalizes Circulating Fetuin-A Concentrations in Adolescent Girls With Polycystic Ovary Syndrome. Int J Endocrinol (2018) 2018:4192940. doi: 10.1155/2018/4192940
18. Jones H, Sprung VS, Pugh CJ, Daousi C, Irwin A, Aziz N, et al. Polycystic Ovary Syndrome With Hyperandrogenism Is Characterized by an Increased Risk of Hepatic Steatosis Compared to Nonhyperandrogenic PCOS Phenotypes and Healthy Controls, Independent of Obesity and Insulin Resistance. J Clin Endocrinol Metab (2012) 97(10):3709–16. doi: 10.1210/jc.2012-1382
19. Ezeh U, Pall M, Mathur R, Dey D, Berman D, Chen IY, et al. Effects of Endogenous Androgens and Abdominal Fat Distribution on the Interrelationship Between Insulin and Non-Insulin-Mediated Glucose Uptake in Females. J Clin Endocrinol Metab (2013) 98(4):1541–8. doi: 10.1210/jc.2012-2937
20. Jin CH, Yuk JS, Choi KM, Yi KW, Kim T, Hur JY, et al. Body Fat Distribution and Its Associated Factors in Korean Women With Polycystic Ovary Syndrome. J Obstet Gynaecol Res (2015) 41(10):1577–83. doi: 10.1111/jog.12767
21. Pasquali R, Gambineri A, Biscotti D, Vicennati V, Gagliardi L, Colitta D, et al. Effect of Long-Term Treatment With Metformin Added to Hypocaloric Diet on Body Composition, Fat Distribution, and Androgen and Insulin Levels in Abdominally Obese Women With and Without the Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2000) 85(8):2767–74. doi: 10.1210/jcem.85.8.6738
22. Penaforte FR, Japur CC, Diez-Garcia RW, Chiarello PG. Upper Trunk Fat Assessment and Its Relationship With Metabolic and Biochemical Variables and Body Fat in Polycystic Ovary Syndrome. J Hum Nutr Diet (2011) 24(1):39–46. doi: 10.1111/j.1365-277X.2010.01130.x
23. Boumosleh JM, Grundy SM, Phan J, Neeland IJ, Chang A, Vega GL. Metabolic Concomitants of Obese and Nonobese Women With Features of Polycystic Ovarian Syndrome. J Endocr Soc (2017) 1(12):1417–27. doi: 10.1210/js.2017-00323
24. Dumesic DA, Akopians AL, Madrigal VK, Ramirez E, Margolis DJ, Sarma MK, et al. Hyperandrogenism Accompanies Increased Intra-Abdominal Fat Storage in Normal Weight Polycystic Ovary Syndrome Women. J Clin Endocrinol Metab (2016) 101(11):4178–88. doi: 10.1210/jc.2016-2586
25. Echiburu B, Perez-Bravo F, Galgani JE, Sandoval D, Saldias C, Crisosto N, et al. Enlarged Adipocytes in Subcutaneous Adipose Tissue Associated to Hyperandrogenism and Visceral Adipose Tissue Volume in Women With Polycystic Ovary Syndrome. Steroids (2018) 130:15–21. doi: 10.1016/j.steroids.2017.12.009
26. Huang ZH, Manickam B, Ryvkin V, Zhou XJ, Fantuzzi G, Mazzone T, et al. PCOS Is Associated With Increased CD11c Expression and Crown-Like Structures in Adipose Tissue and Increased Central Abdominal Fat Depots Independent of Obesity. J Clin Endocrinol Metab (2013) 98(1):E17–24. doi: 10.1210/jc.2012-2697
27. Ibáñez L, López-Bermejo A, Díaz M, Marcos MV, de Zegher F. Early Metformin Therapy (Age 8-12 Years) in Girls With Precocious Pubarche to Reduce Hirsutism, Androgen Excess, and Oligomenorrhea in Adolescence. J Clin Endocrinol Metab (2011) 96(8):E1262–7. doi: 10.1210/jc.2011-0555
28. Hutchison SK, Stepto NK, Harrison CL, Moran LJ, Strauss BJ, Teede HJ. Effects of Exercise on Insulin Resistance and Body Composition in Overweight and Obese Women With and Without Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2011) 96(1):E48–56. doi: 10.1210/jc.2010-0828
29. Kim JY, Tfayli H, Michaliszyn SF, Arslanian S. Impaired Lipolysis, Diminished Fat Oxidation, and Metabolic Inflexibility in Obese Girls With Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2018) 103(2):546–54. doi: 10.1210/jc.2017-01958
30. Morrison SA, Goss AM, Azziz R, Raju DA, Gower BA. Peri-Muscular Adipose Tissue may Play a Unique Role in Determining Insulin Sensitivity/Resistance in Women With Polycystic Ovary Syndrome. Hum Reprod (Oxford England) (2017) 32(1):185–92. doi: 10.1093/humrep/dew279
31. Borruel S, Fernandez-Duran E, Alpanes M, Marti D, Alvarez-Blasco F, Luque-Ramirez M, et al. Global Adiposity and Thickness of Intraperitoneal and Mesenteric Adipose Tissue Depots are Increased in Women With Polycystic Ovary Syndrome (PCOS). J Clin Endocrinol Metab (2013) 98(3):1254–63. doi: 10.1210/jc.2012-3698
32. Cascella T, Palomba S, De Sio I, Manguso F, Giallauria F, De Simone B, et al. Visceral Fat Is Associated With Cardiovascular Risk in Women With Polycystic Ovary Syndrome. Hum Reprod (Oxford England) (2008) 23(1):153–9. doi: 10.1093/humrep/dem356
33. Jena D, Choudhury AK, Mangaraj S, Singh M, Mohanty BK, Baliarsinha AK. Study of Visceral and Subcutaneous Abdominal Fat Thickness and Its Correlation With Cardiometabolic Risk Factors and Hormonal Parameters in Polycystic Ovary Syndrome. Indian J Endocrinol Metab (2018) 22(3):321–7. doi: 10.4103/ijem.IJEM_646_17
34. Karabulut A, Yaylali GF, Demirlenk S, Sevket O, Acun A. Evaluation of Body Fat Distribution in PCOS and Its Association With Carotid Atherosclerosis and Insulin Resistance. Gynecol Endocrinol (2012) 28(2):111–4. doi: 10.3109/09513590.2011.589929
35. Moran C, Arriaga M, Arechavaleta-Velasco F, Moran S. Adrenal Androgen Excess and Body Mass Index in Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2015) 100(3):942–50. doi: 10.1210/jc.2014-2569
36. Sahin SB, Durakoglugil T, Ayaz T, Sahin OZ, Durakoglugil E, Sumer F, et al. Evaluation of Para- and Perirenal Fat Thickness and Its Association With Metabolic Disorders in Polycystic Ovary Syndrome. Endocr Pract (2015) 21(8):878–86. doi: 10.4158/EP14435.OR
37. Tripathy P, Sahu A, Sahu M, Nagy A. Ultrasonographic Evaluation of Intra-Abdominal Fat Distribution and Study of Its Influence on Subclinical Atherosclerosis in Women With Polycystic Ovarian Syndrome. Eur J Obstet Gynecol Reprod Biol (2017) 217:18–22. doi: 10.1016/j.ejogrb.2017.08.011
38. Yildirim B. Relation of Intra-Abdominal Fat Distribution to Metabolic Disorders in Nonobese Patients With Polycystic Ovary Syndrome. Fertility Sterility (2003) 79(6):1358–64. doi: 10.1016/S0015-0282(03)00265-6
39. Braga L, Godoy-Matos AF, Siciliano PO, Correa J, Carvalho DP. Is DPP4 Activity Increased in PCOS? Diabetes Metab Syndr (2018) 12(5):673–5. doi: 10.1016/j.dsx.2018.04.032
40. Carmina E, Bucchieri S, Esposito A, Del Puente A, Mansueto P, Orio F, et al. Abdominal Fat Quantity and Distribution in Women With Polycystic Ovary Syndrome and Extent of Its Relation to Insulin Resistance. J Clin Endocrinol Metab (2007) 92(7):2500–5. doi: 10.1210/jc.2006-2725
41. Cree-Green M, Rahat H, Newcomer BR, Bergman BC, Brown MS, Coe GV, et al. Insulin Resistance, Hyperinsulinemia, and Mitochondria Dysfunction in Nonobese Girls With Polycystic Ovarian Syndrome. J Endocr Soc (2017) 1(7):931–44. doi: 10.1210/js.2017-00192
42. Cree-Green M, Bergman BC, Coe GV, Newnes L, Baumgartner AD, Bacon S, et al. Hepatic Steatosis Is Common in Adolescents With Obesity and PCOS and Relates to De Novo Lipogenesis But Not Insulin Resistance. Obes (Silver Spring) (2016) 24(11):2399–406. doi: 10.1002/oby.21651
43. Cunha NBD, Ribeiro CT, Silva CM, Rosa ESA, De-Souza DA. Dietary Intake, Body Composition and Metabolic Parameters in Women With Polycystic Ovary Syndrome. Clin Nutr (Edinburgh Scotland) (2019) 38(5):2342–8. doi: 10.1016/j.clnu.2018.10.012
44. Faloia E, Canibus P, Gatti C, Frezza F, Santangelo M, Garrapa GG, et al. Body Composition, Fat Distribution and Metabolic Characteristics in Lean and Obese Women With Polycystic Ovary Syndrome. J Endocrinol Invest (2004) 27(5):424–9. doi: 10.1007/BF03345285
45. Glintborg D, Petersen MH, Ravn P, Hermann AP, Andersen M. Comparison of Regional Fat Mass Measurement by Whole Body DXA Scans and Anthropometric Measures to Predict Insulin Resistance in Women With Polycystic Ovary Syndrome and Controls. Acta Obstet Gynecol Scand (2016) 95(11):1235–43. doi: 10.1111/aogs.12964
46. Godoy-Matos AF, Vaisman F, Pedrosa AP, Farias ML, Mendonca LM, Pinheiro MF. Central-To-Peripheral Fat Ratio, But Not Peripheral Body Fat, Is Related to Insulin Resistance and Androgen Markers in Polycystic Ovary Syndrome. Gynecol Endocrinol (2009) 25(12):793–8. doi: 10.3109/09513590903015528
47. Gonzalez F, Sia CL, Shepard MK, Rote NS, Minium J. The Altered Mononuclear Cell-Derived Cytokine Response to Glucose Ingestion Is Not Regulated by Excess Adiposity in Polycystic Ovary Syndrome. J Clin Endocrinol Metab (2014) 99(11):E2244–2251. doi: 10.1210/jc.2014-2046
48. Good C, Tulchinsky M, Mauger D, Demers LM, Legro RS. Bone Mineral Density and Body Composition in Lean Women With Polycystic Ovary Syndrome. Fertil Steril (1999) 72(1):21–5. doi: 10.1016/S0015-0282(99)00203-4
49. Jedrzejuk D, Lwow F, Kuliczkowska-Plaksej J, Hirnle L, Trzmiel-Bira A, Lenarcik-Kabza A, et al. Association of Serum Glypican-4 Levels With Cardiovascular Risk Predictors in Women With Polycystic Ovary Syndrome - a Pilot Study. Gynecol Endocrinol (2016) 32(3):223–6. doi: 10.3109/09513590.2015.1110137
50. Kirchengast S, Huber J. Body Composition Characteristics and Fat Distribution Patterns in Young Infertile Women. Fertil Steril (2004) 81(3):539–44. doi: 10.1016/j.fertnstert.2003.08.018
51. Kogure GS, Silva RC, Picchi Ramos FK, Miranda-Furtado CL, Lara LA, Ferriani RA, et al. Women With Polycystic Ovary Syndrome Have Greater Muscle Strength Irrespective of Body Composition. Gynecol Endocrinol (2015) 31(3):237–42. doi: 10.3109/09513590.2014.982083
52. Macruz CF, Lima SM, Salles JE, da Silva GM, Scalissi NM. Assessment of the Body Composition of Patients With Polycystic Ovary Syndrome Using Dual-Energy X-Ray Absorptiometry. Int J Gynaecol Obstet (2017) 136(3):285–9. doi: 10.1002/ijgo.12066
53. Mierzwicka A, Kuliczkowska-Plaksej J, Kolackov K, Bolanowski M. Preptin in Women With Polycystic Ovary Syndrome. Gynecol Endocrinol (2018) 34(6):470–5. doi: 10.1080/09513590.2017.1409715
54. Pepene CE. Evidence for Visfatin as an Independent Predictor of Endothelial Dysfunction in Polycystic Ovary Syndrome. Clin Endocrinol (Oxf) (2012) 76(1):119–25. doi: 10.1111/j.1365-2265.2011.04171.x
55. Satyaraddi A, Cherian KE, Kapoor N, Kunjummen AT, Kamath MS, Thomas N, et al. Body Composition, Metabolic Characteristics, and Insulin Resistance in Obese and Nonobese Women With Polycystic Ovary Syndrome. J Hum Reprod Sci (2019) 12(2):78–84. doi: 10.4103/jhrs.JHRS_2_19
56. Schmidt J, Dahlgren E, Brannstrom M, Landin-Wilhelmsen K. Body Composition, Bone Mineral Density and Fractures in Late Postmenopausal Women With Polycystic Ovary Syndrome - a Long-Term Follow-Up Study. Clin Endocrinol (Oxf) (2012) 77(2):207–14. doi: 10.1111/j.1365-2265.2012.04378.x
57. Shroff R, Kerchner A, Maifeld M, Van Beek EJ, Jagasia D, Dokras A. Young Obese Women With Polycystic Ovary Syndrome Have Evidence of Early Coronary Atherosclerosis. J Clin Endocrinol Metab (2007) 92(12):4609–14. doi: 10.1210/jc.2007-1343
58. Thomann R, Rossinelli N, Keller U, Tirri BF, De Geyter C, Ruiz J, et al. Differences in Low-Grade Chronic Inflammation and Insulin Resistance in Women With Previous Gestational Diabetes Mellitus and Women With Polycystic Ovary Syndrome. Gynecol Endocrinol (2008) 24(4):199–206. doi: 10.1080/09513590801893398
59. Toscani M, Migliavacca R, Sisson de Castro JA, Spritzer PM. Estimation of Truncal Adiposity Using Waist Circumference or the Sum of Trunk Skinfolds: A Pilot Study for Insulin Resistance Screening in Hirsute Patients With or Without Polycystic Ovary Syndrome. Metabolism (2007) 56(7):992–7. doi: 10.1016/j.metabol.2007.03.006
60. Yucel A, Noyan V, Sagsoz N. The Association of Serum Androgens and Insulin Resistance With Fat Distribution in Polycystic Ovary Syndrome. Eur J Obstet Gynecol Reprod Biol (2006) 126(1):81–6. doi: 10.1016/j.ejogrb.2005.11.012
61. Chan JM, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Obesity, Fat Distribution, and Weight Gain as Risk Factors for Clinical Diabetes in Men. Diabetes Care (1994) 17(9):961–9. doi: 10.2337/diacare.17.9.961
62. Seidell JC, Cigolini M, Deslypere JP, Charzewska J, Ellsinger BM, Cruz A. Body Fat Distribution in Relation to Serum Lipids and Blood Pressure in 38-Year-Old European Men: The European Fat Distribution Study. Atherosclerosis (1991) 86(2-3):251–60. doi: 10.1016/0021-9150(91)90221-N
63. Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, et al. Trunk Fat and Leg Fat Have Independent and Opposite Associations With Fasting and Postload Glucose Levels: The Hoorn Study. Diabetes Care (2004) 27(2):372–7. doi: 10.2337/diacare.27.2.372
64. Rosenfield RL, Ehrmann DA. The Pathogenesis of Polycystic Ovary Syndrome (PCOS): The Hypothesis of PCOS as Functional Ovarian Hyperandrogenism Revisited. Endocrine Rev (2016) 37(5):467–520. doi: 10.1210/er.2015-1104
65. Pasquali R, Casimirri F, Venturoli S, Antonio M, Morselli L, Reho S, et al. Body Fat Distribution has Weight-Independent Effects on Clinical, Hormonal, and Metabolic Features of Women With Polycystic Ovary Syndrome. Metabolism (1994) 43(6):706–13. doi: 10.1016/0026-0495(94)90118-X
66. Lim SS, Norman RJ, Davies MJ, Moran LJ. The Effect of Obesity on Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Obes Reviews: an Off J Int Assoc Study Obes (2013) 14(2):95–109. doi: 10.1111/j.1467-789X.2012.01053.x
67. Polak AM, Adamska A, Krentowska A, Łebkowska A, Hryniewicka J, Adamski M, et al. Body Composition, Serum Concentrations of Androgens and Insulin Resistance in Different Polycystic Ovary Syndrome Phenotypes. J Clin Med (2020) 9(3):732. doi: 10.3390/jcm9030732
68. Borga M, West J, Bell JD, Harvey NC, Romu T, Heymsfield SB, et al. Advanced Body Composition Assessment: From Body Mass Index to Body Composition Profiling. J Invest Med: Off Publ Am Fed Clin Res (2018) 66(5):1–9. doi: 10.1136/jim-2018-000722
69. Mohammad A, De Lucia Rolfe E, Sleigh A, Kivisild T, Behbehani K, Wareham NJ, et al. Validity of Visceral Adiposity Estimates From DXA Against MRI in Kuwaiti Men and Women. Nutr Diabetes (2017) 7(1):e238. doi: 10.1038/nutd.2016.38
70. Behboudi-Gandevani S, Ramezani Tehrani F, Hosseinpanah F, Khalili D, Cheraghi L, Kazemijaliseh H, et al. Cardiometabolic Risks in Polycystic Ovary Syndrome: Long-Term Population-Based Follow-Up Study. Fertil Steril (2018) 110(7):1377–86. doi: 10.1016/j.fertnstert.2018.08.046
71. Kazemi Jaliseh H, Ramezani Tehrani F, Behboudi-Gandevani S, Hosseinpanah F, Khalili D, Cheraghi L, et al. Polycystic Ovary Syndrome Is a Risk Factor for Diabetes and Prediabetes in Middle-Aged But Not Elderly Women: A Long-Term Population-Based Follow-Up Study. Fertil Steril (2017) 108(6):1078–84. doi: 10.1016/j.fertnstert.2017.09.004
Keywords: body fat distribution, central obesity, imaging method, polycystic ovary syndrome, systematic review and meta-analysis
Citation: Zhu S, Li Z, Hu C, Sun F, Wang C, Yuan H and Li Y (2021) Imaging-Based Body Fat Distribution in Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. 12:697223. doi: 10.3389/fendo.2021.697223
Received: 30 April 2021; Accepted: 19 August 2021;
Published: 09 September 2021.
Edited by:
Luca Busetto, Università degli Studi di Padova, ItalyReviewed by:
Roberto Mioni, University of Padua, ItalyEvelyn Frias-Toral, Catholic University of Santiago de Guayaquil, Ecuador
Copyright © 2021 Zhu, Li, Hu, Sun, Wang, Yuan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Li, eWFubGkuc2R1QGdtYWlsLmNvbQ==; Haitao Yuan, c2RzbHl5eWh0QDE2My5jb20=
 Shiqin Zhu1,2,3,4
Shiqin Zhu1,2,3,4 Yan Li
Yan Li